Abstract
Age-related declines in long- and short-term memory show relationships to decreases in N-methyl-D-aspartate (NMDA) receptor expression, which may involve inflammation. This study was designed to determine effects of an anti-inflammatory drug, ibuprofen, on cognitive function and NMDA receptor expression across aging. Male C57BL/6 mice (ages 5, 14, 20, and 26 months) were fed ibuprofen (375 ppm) in NIH31 diet or diet alone for 6 weeks prior to testing. Behavioral testing using the Morris water maze showed that older mice performed significantly worse than younger in spatial long-term memory, reversal, and short-term memory tasks. Ibuprofen enhanced overall performance in the short-term memory task, but this appeared to be more related to improved executive function than memory. Ibuprofen induced significant decreases over all ages in the mRNA densities for GluN2B subunit, all GluN1 splice variants, and GluN1-1 splice forms in the frontal cortex and in protein expression of GluN2A, GluN2B and GluN1 C2′ cassettes in the hippocampus. GluN1-3 splice form mRNA and C2′ cassette protein were significantly increased across ages in frontal lobes of ibuprofen-treated mice. Ibuprofen did not alter expression of pro-inflammatory cytokines IL-1β and TNFα, but did reduce the area of reactive astrocyte immunostaining in frontal cortex of aged mice. Enhancement in executive function showed a relationship to increased GluN1-3 mRNA and decreased gliosis. These findings suggest that inflammation may play a role in executive function declines in aged animals, but other effects of ibuprofen on NMDA receptors appeared to be unrelated to aging or inflammation.
Keywords: N-Methyl-D-Aspartate receptor, executive function, anti-inflammatory, aging
INTRODUCTION
Increased life expectancy, along with decreased fertility, in most areas of the world is creating a shift in relative populations from younger to older groups (Division, 2015). By 2050, the number of people over 60 years of age is expected to equal those below 15 years of age (Division, 2015). In addition, the number of individuals living longer than 80 years is expected to increase 3-fold by 2050 and seven-fold by 2100 (Division, 2015). Learning and memory declines are some of the earliest cognitive dysfunctions to arise during aging (Albert and Funkenstein, 1992, Park et al., 2002). Severity can range from dementia of Alzheimer’s Disease (AD) to the far more common Age Associated Memory Impairment (AAMI), a milder clinical state that affects the ability to process new and old information (Larrabee and Crook, 1994). AAMI is found in an estimated 40% of humans within the fifth decade of life and 85% of those over age 80 (Larrabee and Crook, 1994). Declines in memory pose a significant problem for individuals in their interactions with their environment, leading to loss of independence and early entry into care facilities (Gilsky, 2007, Okura and Langa, 2011). Due to the impending demographic shift, age-related memory loss will likely become even more prevalent. Preventing or mitigating memory declines with aging will be a significant component to preserving quality of life and independence through old age and in delaying AD symptoms.
In addition to humans, age-associated memory declines have been observed in nonhuman primates (Gallagher and Nicolle, 1993, Gallagher and Rapp, 1997), dogs (Head et al., 1995), and rodents (Gage et al., 1984, Rapp et al., 1987, Barnes, 1988, Pelleymounter et al., 1990). A common finding in these species is that aging negatively affects binding density of N-methyl-D-aspartate (NMDA) receptors in the cerebral cortex and hippocampus (Magnusson et al., 2010). NMDA receptors are one subtype of excitatory glutamate receptor that provide regulatory roles in neurotransmission and spatial memory tasks (Morris et al., 1986, Morris, 1989, Morris and Davis, 1994). In many brain regions, NMDA receptors are important for the initiation of long-term potentiation (LTP), a cellular mechanism believed to underlie some types of memory formation (Mondadori et al., 1989, Morris and Davis, 1994, Lisman et al., 1998). NMDA receptors are more vulnerable to the effects of aging than other glutamate receptors (Magnusson and Cotman, 1993, Magnusson, 1997, 1998) and these changes appear to be associated with age-related spatial memory declines in rodents (Magnusson, 1998, 2001, Zhao et al., 2009, Brim et al., 2013). Targeting the NMDA receptor complex may help in developing treatment options to prevent or improve age-related changes in memory.
NMDA receptors are composed of a combination of the following subunit families: GluN1, GluN2A-D, or GluN3A-B (Kutsuwada et al., 1992, Meguro et al., 1992, Monyer et al., 1992, Ishii et al., 1993). The GluN1 subunit appears to be necessary and sufficient for the formation of functional channels (Kutsuwada et al., 1992, Meguro et al., 1992, Monyer et al., 1992, Ishii et al., 1993). Eight different splice variants of mRNA exist for the GluN1 subunit through alternative splicing of one N-terminal and two C-terminal cassettes, C1 and alternatively C2 or C2′ (Laurie and Seeburg, 1994, Zukin and Bennett, 1995). Studies have found that mRNA for C-terminal splice forms, GluN1-1 (+ C1 and + C2 cassettes) and GluN1-3 (+ C1 and + C2′), show significant declines during aging in several brain regions, even though overall GluN1 mRNA expression is not always significantly affected by aging, suggesting these splice forms are more influenced by aging than the subunit as a whole (Magnusson et al., 2005, Das and Magnusson, 2008).
The four members of the GluN2 family each enhance the activity of the receptor when coupled with GluN1 subunits, conferring different agonist/antagonist affinities and gating behaviors to the receptor (Kutsuwada et al., 1992, Yamazaki et al., 1992). Previous research has shown that, of the NMDA receptor subunits, the GluN2B subunit is most affected by the aging process (Magnusson, 2000, Magnusson et al., 2002). Increasing GluN2B subunit expression throughout multiple brain regions from birth in transgenic mice is beneficial to spatial long-term and delayed short-term memory (Tang et al., 1999, Cao et al., 2007). Enhancing GluN2B expression in old mice enhances spatial long-term memory (Brim et al., 2013).
Increasing evidence suggests that some of the changes in NMDA receptor expression and memory during aging could be due to inflammation. Inflammation can be induced through infection, injury, or oxidative stress. A chronic inflammatory response has been described in brains of patients with Alzheimer’s Disease (AD) (Lim et al., 2000). Aging is also associated with increases in inflammation- and oxidative stress-related molecules (Prolla, 2002), such as IL-1β and TNFα (Blalock et al., 2003), supporting the theory that "inflamm-aging" is a major player in the aging process (Franceschi et al., 2000). Studies also suggest that inflammation could be a factor in middle age or earlier (Karlamangla et al., 2014, Hascup et al., 2016). NMDA receptors have been shown to directly interact with inflammatory mediators such as stress-induced glucocorticoids (Nair and Bonneau, 2006). In old rats, redox sites of NMDA receptors appear to be in a more oxidized state, than seen in young (Bodhinathan et al., 2010), suggesting there is a more oxidized environment in the aged brain. A non-steroidal anti-inflammatory drug, sulindac, was able to improve spatial short-term memory and reverse the effects of normal aging on protein expression of the GluN1 and GluN2B subunits of the NMDA receptor in old rats (Mesches et al., 2004).
In the present study, we addressed the hypothesis that long-term treatment with an anti-inflammatory drug would improve age-related changes in cognitive flexibility and long-term, as well as, short-term memory and reverse age-associated changes in NMDA receptor subunit expression in both middle-aged and aged animals. An equivalent dose of sulindac to that used in the aged rat study (Mesches et al., 2004), however, was not tolerated well by young mice (unpublished observation). Ibuprofen is another non-steroidal anti-inflammatory drug that inhibits both cyclooxygenase (COX)-1 and COX-2 (Van Hecken et al., 2000), similar to sulindac (Boolbol et al., 1996, Davies and Watson, 1997). It has been used successfully in transgenic mouse models for AD. Chronic oral ibuprofen treatment has been shown to suppress plaque pathology, including amyloid deposition, and improve memory in these AD models (Lim et al., 2000, Lim et al., 2001, Yan et al., 2003, Kotilinek et al., 2008, McKee et al., 2008, Choi et al., 2009). A similar dose of 375 ppm ibuprofen was used in the present study.
EXPERIMENTAL PROCEDURES
Animals
A total of 48 male C57BL/6 mice (National Institute on Aging, NIH) from four different age groups (5, 14, 20 and 26 months of age at the end of the study) were used for the study. Animals were randomly assigned to two treatment groups (375 ppm of ibuprofen in NIH31 chow or NIH31 chow alone; N = 6 for each treatment/age group). Advil tablets (200 mg ibuprofen each) and NIH31 pellets (Charles Rivers, Stone Ridge, N.Y.) were crushed, mixed to a concentration of 375 ppm ibuprofen, and repelleted by Research Diets (New Brunswick, N.J.). The ibuprofen dose was obtained from a study by Lim and coworkers, which reduced pathology in an Alzheimer’s disease mouse model (Lim et al., 2000). Animals were fed their corresponding diet ad libitum, prior to and during behavioral testing, for a total of 2 months. Mice were housed under 12 hr light and 12 hr dark cycles. During the two-month total treatment administration, body weight was assessed every 3-4 days. After behavioral testing, animals were euthanized by exposure to CO2 and decapitated. The brains were harvested, frozen rapidly with dry ice, and stored at −80°C until further processing. All experiments were approved by the Oregon State University Institutional Animal Care and Use Committee and conformed to International Guidelines for the ethical use of animals. All efforts were made to minimize suffering and the numbers of animals used.
Behavioral testing
Spatial long-term memory, delayed short-term memory, cognitive flexibility, and cued control task abilities were tested using the Morris water maze as previously described (Das and Magnusson, 2008, Das et al., 2012). A 1.2 m diameter plastic tank was filled with water (16–18°C) that was made an opaque white with non-toxic paint. A platform was placed 1 cm below the surface of the water. Spatial cues consisted of geometric shapes, towels and toys. Cues were situated high up on the walls of both the tank and the room. Seven different platform positions were utilized at different distances from the sides of the tank. A CCD camera, which was attached to the ceiling and centered above the tank, was used to video tape the trials. The “SMART” video tracking system (San Diego Instruments, San Diego, CA, USA) was used to analyze the paths of each mouse during each trial. Entry points were randomly assigned for each trial and the mice were placed in the tank facing the wall.
Acclimation
Days 1-2: Acclimation was performed for 2 days before memory training began. Each mouse swam for 60s in the tank with no platform present. After swimming, the platform was placed in a location that differed from the memory testing location and the mice were taught to sit on the platform for 30 s. This procedure was performed on both days of acclimation.
Spatial long-term memory
Days 3-5: Mice were tested for long-term memory. Eight place trials were conducted each day for 3 days. One probe trial occurred at the end of each day, separated by an hour from the last place trial. Place trials were performed in two four-trial blocks, with a 90-min inter-block interval. The platform remained in the same quadrant (SE) for each place trial and three different start positions were randomly assigned. Place trials consisted of 60 s maximum in the water, 30 s on the platform and 2 min of cage rest. A mouse that did not find the platform within 60 s was led to the platform. Place trials were used as an assessment of the animal’s overall spatial long-term memory. A naive probe trial was performed prior to the first place trial. Probe trials at the end of each day were used to assess the animal’s bias for the learned platform location (Gallagher et al., 1993). For probe trials, the platform was absent and the mouse was allowed to search for 30 s from a randomly assigned start position.
Cognitive Flexibility
Day 6: Mice underwent reversal trials to test cognitive flexibility. Reversal place and probe trials were conducted similar to one day of testing described above, but the platform was placed in the opposite quadrant to that which had been used for long-term memory testing.
Spatial delayed short-term memory
Day 7-13: Mice underwent a spatial delayed short-term memory task (Magnusson et al., 2003). The task consisted of two sessions with a 3 hr inter-session interval. The platform position was changed between each session. Each session was comprised of 4 trials. Each trial consisted of a maximum of 60 s swim time and 30 s platform time. The first trial was a naïve trial (Tnaïve), in which the mouse searched for the new platform position, followed by cage rest for a delay period of 10 min, and then a second trial (Tdelay). Two more trials were conducted with 2-min inter-trial intervals, followed by cage rest until the next session. Delayed short-term memory was assessed using Tnaïve and Tdelay trials. The extra trials per session were performed based on evidence that mice need more trials per session in order to show improvement between trials (Magnusson et al., 2003). These extra trials were excluded from the analysis.
Cued control task
Day 14: Mice were tested in 6 cued trials. Cued trials were used to assess motivation, visual acuity, and physical ability for the task. The platform was visible and had a flag, attached to a 20.3 cm tall stick, upon it. Each cued trial used a unique platform position. Maximum search time allowed was 60 s. All mice were tested at one platform position, then the position was changed for the next trial.
Brain Sectioning
The frozen brain from each animal was divided into two halves in the sagittal plane. One half was sectioned for in situ hybridization with a cryostat. Use of the left or right half of the brain was randomly assigned for each individual. Chilled gelled slides (.5% gelatin solution) were used for collecting brain tissue sections. Horizontal brain sections of 12 μm thickness, from representatives from each age and behavioral group, were placed on each slide and stored at −80°C until used.
In situ hybridization
Antisense oligonucleotides for use in the in situ hybridization assays were commercially prepared (Macromolecular Resources, Colorado State University, Fort Collins, CO). The probe sequences were: GluN2B 5′ CACTGTAGCGGTCACTCTTGAAAGAGAACTTGCCGTACAGGTCGC 3′ GluN1-1 TCCACCCCCGGTGCTCGTGTCTTTGGAGGACCTACGTCTC GluN1-3 GATATCAGTGGGATGGTACTGCGTGTCTTTGGAGGACCTA GluN1-2 TCCACCCCCGGTGCTCTGCAGGTTCTTCCTCCACACGTTC GluN1-pan GCACAGCGGGCCTGGTTCTGGGTTGCGCGAGCGCGACCACCTCGC (Watanabe et al., 1993; Laurie and Seeburg, 1994). Terminal deoxyribonucleotidyl transferase (Invitrogen Corp., Carlsbad, CA) was used to label oligonucleotides with 33P-dATP (Perkin Elmer, Waltham, MA; specific activity: 3000 Ci/mM). Labeled oligonucleotides were then purified in Microspin G-25 columns (Amersham Bioscience, Piscataway, NJ).
In situ hybridization was performed as described previously (Watanabe et al., 1993, Magnusson et al., 2005). For each solution step, gentle rotation was applied, with the exception of the fixation and hybridization steps. Sections on slides were thawed, air-dried, and then placed in solutions in the following order: 4% paraformaldehyde / PBS (pH 7.2; 25 °C) for 15 min for fixation, 2 mg/ml glycine in PBS (pH 7.2; 25 °C) for 20 min, .25% acetic anhydride / .1 M triethanolamine (pH 8.0; 25 °C) for 10 min, and prehybridization solution, consisting of 50% formamide, .1 M Tris–HCl, pH 7.5, 4X SSC (1X SSC=150 mM NaCl and 15 mM sodium citrate), .02% Ficoll, .02% polyvinylpyrrolidone, .02% bovine serum albumin, 2% sarkosyl, and 250 μg/ml salmon testes DNA; (25 °C) for 2 h. Sections were washed for 5 min each in 2× SSC, 70 and 100% ethanol, and air-dried for 15 min. For hybridization, 150 μl of prehybridization solution (containing 10% dextran sulfate and .33 pmol of 33P-labeled oligonucleotide probe) was placed on the slides, covered by parafilm, and incubated in a 42 °C oven, humidified with 5X SSC, for 18 h. Following incubation, coverslips were rinsed off and slides were rinsed in 2X SSC /.1% sarkosyl (25 °C) for 40 min and in .1X SSC / .1% sarkosyl (55 °C) for 2 × 40 min and then air-dried. A 50-fold excess of non-radiolabeled oligonucleotide was added to the hybridization solution on some slides to determine non-specific hybridization. Slides were exposed to Kodak Biomax films for 4-14 days depending on the splice form or subunit, along with a slide containing 14C standards.
A Powerlook 2100 XL scanner (UMAX, Taiwan), Macintosh G4 computer and NIH ImageJ software were used to capture brain and standard images. Four sections for total hybridization and two sections for nonspecific hybridization from each animal were analyzed using quantitative densitometry, with the use of NIH ImageJ software. The mRNA expression was analyzed in deep (cortical layers IV–VI) layers of medial prefrontal cortex (areas containing cingulate, infralimbic, and prelimbic cortices) and lateral prefrontal cortex (deep and superficial (cortical layers II–III) layers of ventral and lateral orbital cortices, granular and agranular insular cortices, secondary and primary motor cortices and the somatosensory cortex (including both primary and secondary somatosensory cortex)). Specific signal was equal to total hybridization minus non-specific hybridization. The 14C standards were used to convert optical density to fmol of labeled 33P-dATP/mm2 tissue (Eakin et al., 1994).
Western blots
The remaining half brains were dissected to obtain prefrontal/frontal cortices and hippocampus. Dissected tissue was biochemically-fractionated as previously described (Dunah and Standaert, 2001) with a few modifications (Das and Magnusson, 2011). The tissue was homogenized with TE buffer (10 mM Tris HCl (pH 7.4), 1 mM EDTA, 1 mM EGTA) plus 320 mM sucrose, with the use of a Dounce homogenizer. The resulting homogenate was centrifuged at 1000 × g for 3 min using the RSR20 rotor in a Savant μSpeedFuge SFR13K refrigerated centrifuge (Thermo Fisher Scientific, Waltham, MA, USA) and the pellet (P1) was discarded. Supernatant (S1) was centrifuged for 11 min at 9000×g, with the use of the same centrifuge, resulting in a crude synaptosomal pellet (P2). P2 was resuspended in TE buffer, sonicated, and protein was determined with Bio-Rad Dc Protein Assay (Bio-Rad Laboratories, Hercules, CA, USA).
Western blotting was performed with the use of sodium dodecyl sulfate - poly acrylamide gel electrophoresis (SDS-PAGE; 7.5%) as previously described (Magnusson et al., 2002). Four different μg loads (1.5, 3, 6 and 12 μg/well) of standards, obtained from crude synaptosomes prepared from combined caudal cortices from four young, untreated mice, were present on each gel. Protein samples, including representatives of each different age/treatment group, were loaded on triplicate gels. Gels were run at 125V for 2 h. Proteins were transferred to Immobilon-FL polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA) at 100V for 90 min at 4°C. Membrane blocking was performed with a 1:1 dilution of LI-COR Odyssey buffer (LI-COR Biosciences, Lincoln, NE, USA): Tris buffered saline (TBS) for 1 hr at room temperature with shaking. Membranes were incubated overnight at 4°C in one of the following primary antibodies: GluN1-pan (Zymed Laboratories, San Francisco, CA, USA), C2 and C2′ cassettes (Novus Biologicals, Littleton, CO, USA), C1 cassette (Sigma Aldrich, St. Louis, MO, USA), and GAPDH (Calbiochem, Merck KGaA, Darmstadt, Germany). Primary antibody dilutions, using 1:1 dilution of LI-COR Odyssey buffer:TBS, were 1:500 for C1, 1:1000 for C2, C2′, 1:3000 for GluN1 and 1:10000 for GAPDH. Following four rinses for five min each in TBS + .1% Tween20, membranes were incubated in fluorescence-based secondary antibody labeled with either Alexa Fluor 680 (1:8000 dilution; LI-COR Biosciences) or IR Dye 800 (1:4000 dilution; Rockland Immunochemicals, Gilbertsville, PA, USA) for one h at room temperature. A LI-COR Odyssey imager was used to scan the bands. Integrated densities from bands of NMDA receptor subunit protein were corrected for loading by division with GAPDH densities from the same lane.
Inflammatory cytokines
Levels of cytokine IL-1β and TNFα in the brain were used as measures of inflammation. A coronal slab of brain tissue, caudal to the frontal cortex and rostral to the hippocampus was used to assess cytokine gene expressions. Total RNA from brain tissue was isolated using TRIzol (Life Technologies, Grand Island, NY) and was reverse transcribed into cDNA using SuperScript III First-Strand Synthesis SuperMix for quantitative real-time polymerase chain reaction (qRT-PCR) (Life Technologies, Grand Island, NY). Real-time PCR was performed using the following PCR primers: mouse IL-1β (forward: 5′-AAGATGAAGGGCTGCTTCCAA-3′, reverse: 5′-TGAAGGAAAAGAAGGTGCT- CATG-3′), TNFα (forward: 5′- CTGTAGCCCACGTCGTAGCA -3′, reverse: 5′- GTGTGGGTGAGGAGCACGTA -3′), and mouse 18S ribosomal RNA (18S) (forward: 5′-CCGCAGCTAGGAATAATGGAAT-3′, reverse: 5′-CGAACCTCCGACTTTCGTTCT-3′). Real-time PCR was performed using Fast SYBR Green Mastermix (Life Technologies, Grand Island, NY) on 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). Serial dilutions of purified plasmid DNA that encoded for each gene of interest were used to generate a standard curve. Data represent the copy number of the gene of interest normalized to the copy number of 18S housekeeping genes.
Immunohistochemistry
Representative horizontal tissue cryostat sections from each animal were processed with the Vectastain avidin-biotin-peroxidase method (Vector Labs, Burlingame, CA) as follows, with 3 rinses in TBS-0.1% Tween 20 following each incubation step. Sections were fixed in 4% paraformaldehyde for .5 hr, treated with .3% hydrogen peroxide for 20 min, and blocked with 5% goat serum for 1 hr. Sections were incubated overnight with a 1:500 dilution of anti-glial fibrillary acidic protein (GFAP) antibody (Abcam, Cambridge, MA) to visualize reactive astrocytes, for 1 hr in secondary biotinylated antibody diluted 1:8 in TBS and 1 hr in avidin-biotin-peroxidase complex (ABC) diluted 1:8 in TBS. Sections were reacted with diaminobenzidine (DAB) and hydrogen peroxide (Sigma-Aldrich, St. Louis, MO) to produce a brown homogeneous reaction product. Immunostaining was assessed with NIH Image J software, by thresholding (Max entropy) and determining the area of staining.
Serum liver enzyme
Alanine aminotransferase (ALT; serum glutamic-pyruvic transaminase) concentrations in serum were determined by the Veterinary Diagnostic Laboratory in the College of Veterinary Medicine at Oregon State University.
Data analysis
Analysis of behavioral testing data was performed as described previously (Magnusson et al., 2003). Cumulative proximity measurements were used to assess performance in the place, reversal, short-term memory and cued trials. Cumulative proximity was obtained from the Smart tracking program, based on methods by Gallagher et al. (Gallagher et al., 1993), and was manually corrected for start position. The computer measured, every .2s during the animal's swim, the animal’s distance from the platform. These distance measurements were then added together to give a cumulative proximity. The cumulative proximity was corrected for start position by calculating the cumulative proximity for an ideal path, which was based on average swim speed and starting point, and subtracting this from the cumulative proximity measurement derived from the tracking system. For the probe trials, the average proximity to the platform was used to assess performance (Gallagher et al., 1993). Proximity measures have been found to be less influenced by swim speed differences than more traditional measures such as latency to reach the platform and are more sensitive to some of the alternative strategies that may not involve place learning (Gallagher et al., 1993, Magnusson, 1998).
Age-related differences in performance in the different behavioral tasks were analyzed separately by repeated measures ANOVA (Age X Treatment X Trial), followed by Fisher's protected post-hoc analysis using Statview software (SAS Institute Inc., Cary, NC). Age-related differences in the different mRNAs, proteins, cytokines, GFAP, and serum ALT were analyzed separately by two- or three-way (for grouped brain regions) ANOVA followed by Fisher's protected post-hoc analysis. Food consumption and weight differences were also analyzed with repeated measures ANOVA.
RESULTS
Behavioral cognitive testing
Age-related changes in spatial long-term memory
To address the hypothesis that inflammation during aging contributes to long-term spatial memory declines, place (platform remaining in the same location) and probe trials in the water maze were examined. Three of the oldest animals (1 ibuprofen-treated and 2 controls) were not able to complete all of the place trials during the first three days of water maze testing. There was a significant difference between animals that completed all place trials and those that had missed trials in average place trial cumulative proximity (p=.05) and a significant interaction between probe trials and missing trial status (p=.003). Only animals that completed all place trials were included in the analysis of place and probe trials for this study.
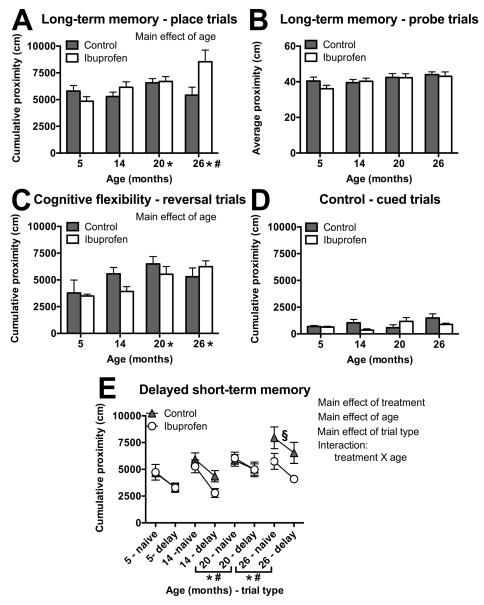
There was no significant main effect of treatment (F(1,35)=3.99, p=.054), but there was an overall effect of age (F(3,35)=3.75, p=.02) on cumulative proximity in place trials for spatial long-term memory. Young animals spent more time closer to the platform (lower cumulative proximity) than the two oldest groups (20- (p=.02) and 26-month olds (p=.003)) and 14-month olds were closer than the 26-month olds (p=.02; Figure 1A), when data was collapsed across treatments and place trials. An age by treatment interaction was observed in place trials (F(3,35)=4.04; p=.01), so individual age groups were analyzed separately for treatment effects. However, the oldest mice treated with ibuprofen only showed near significant (p=.08) greater cumulative proximity scores in the spatial long-term memory place trials, compared to age-matched controls (Figure 1A). There was no significant main effect of treatment (F(3,35)=.51, p=.48) or age (F(1,35)=2.48, p=.07) for average proximity scores in probe trials 0-3 for long-term spatial memory bias (Figure 1B).
Figure 1.
Effects of age and ibuprofen on spatial long-term memory (A-B), cognitive flexibility (C), associative (control) (D), and delayed short-term memory (E) tasks. A,B) Performance in a spatial long-term memory task averaged across all place trials showed significantly higher proximity scores in older versus young age groups (A), but there was no significant effect of age or ibuprofen treatment on performance in probe trials (B). C) The older mice had significantly higher cumulative proximity than younger mice in the reversal trials. D) There was no significant effect of age or ibuprofen treatment on performance in cued control trials. E) Performances in naive and 10 min delay trials for delayed short-term memory averaged across sessions for different adult ages of mice. p < .05 for lower cumulative proximity in ibuprofen-treated mice than control; for increased cumulative proximities with increased age; and lower cumulative proximity in the delayed trials, compared to the naive. The significant age X treatment interaction showed that 26 month old control mice had higher cumulative proximities than the young controls, but the oldest ibuprofen-fed mice performed similarly to ibuprofen-treated young. Two delayed short-term memory sessions were omitted due to missing data for one of the two trials. Note: Higher proximity measures indicate more time spent searching away from the platform location. Symbols indicate p<.05 for differences between older mice and 5 (*) and 14 (#) month olds with data collapsed across treatment and trial type or difference between 26 month control mice and 5 month old controls (§) with data collapsed across trial type, Mean ± SEM, A-B) N = 3-6. C-E) N = 5-6.
Age-related changes in cognitive flexibility
In order to determine whether ibuprofen had any influence on cognitive flexibility or associative memory during aging, we examined reversal trials and cued control trials, respectively, in the water maze. There were no significant effects of treatment (F(3,38)=.28; p=.84), but there was an overall effect of age (F(3,38)=3.53; p=.02) on cumulative proximity in reversal trials within the cognitive flexibility task. The 5-month old group showed significantly lower cumulative proximities than the 20- (p<.01) and 26-month olds (p=.02), when data were collapsed across treatments and reversal trials (Figure 1C). Within the associative memory (control) task, which was used as an assessment of motivation and sensory and motor skills, there was no overall significant main effect of age (F(3,38) = 1.75; p=.17) or treatment (F(3,38) = 2.74; p=.06; Figure 1D).
Effect of age and ibuprofen on delayed short-term memory
We assessed whether ibuprofen could impact delayed short-term memory during aging with the use of a 10-min delay between naive and delay trials and a new platform position each session. Two of the 14 sessions were excluded from analysis due to missing data. There was a significant main effect of treatment (F(1,76)=7.4, p=.008) on performance in delayed short-term memory sessions (Figure 1E), with ibuprofen-treated animals exhibiting lower cumulative proximity scores when data was collapsed across both Tnaïve and Tdelay trials, sessions, and age groups (Figure 1E). There was a significant main effect of trial type (F(1,76)=24.1, p<.0001), with significantly lower cumulative proximities in the Tdelay trials, as compared to the Tnaïve trials, with data collapsed across treatment, sessions, and age (Figure 1E). There was a significant main effect of age (F(3,76)=9.16, p<.0001) on performance across trials Tnaïve and Tdelay and sessions on cumulative proximity in the delayed short-term memory task with the youngest two age groups showing lower cumulative proximities, as compared to the oldest two groups (Figure 1E). Because this effect involved data collapsed across Tnaïve and Tdelay trials, these results were not reflective of short-term memory alone. There was a significant interaction between age and treatment in short-term memory trials (F(3,76)=3.4 p=.02), when data were collapsed across trials and sessions. The 26-month old mice treated with ibuprofen showed significantly lower cumulative proximity scores (cm) than age-matched controls (p=.013) and were not significantly different from the young treatment-matched mice (p=.18), but the age-matched controls had higher cumulative proximity scores than control young (p<.0001), with data collapsed across Tnaïve and Tdelay and sessions (Figure 1E).
Ibuprofen altered mRNA densities for NMDA subunits primarily in frontal cortex
In order to address the question of whether ibuprofen could influence memory-related receptors at the mRNA level, in situ hybridization was performed for NMDA receptor subunits and splice variants. There was no significant effect of age (F(3,39)=.64; p=.60) or treatment (F(1,39)=.09; p=.76) on GluN2A subunit mRNA (Figure 2A, Table 1). In situ hybridization assays showed an overall significant effect of treatment (F(1,39)=5.0; p=.03), with ibuprofen reducing mRNA densities (fmole 33P-dATP/mm2) for the GluN2B subunit, when data was collapsed across age and brain region (Figure 2B,3A; Table 1). There was also a significant interaction between brain regions and treatment (F(5,195)=13.3; p<.0001) on mRNA densities for GluN2B subunit. Ibuprofen treatment significantly reduced GluN2B expression within the lateral frontal superficial and medial and lateral frontal deep brain regions (p<.002) (Figure 2B,3A; Table 1).
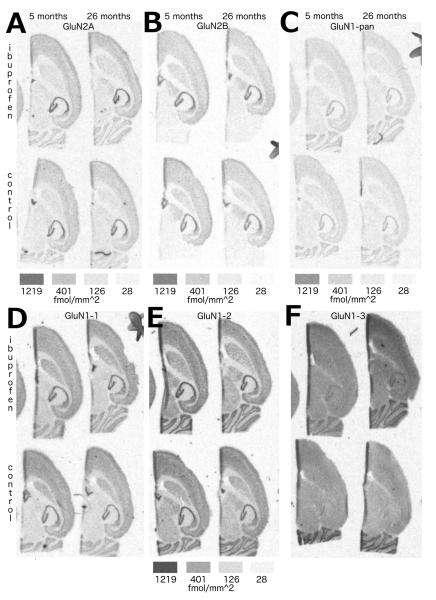
Figure 2.
Effects of ibuprofen on NMDA receptor mRNA expression. Representative autoradiographic images from in situ hybridization of mRNA for NMDA receptor subunits GluN2A (A), GluN2B (B) and GluN1-pan (all GluN1 splice variants; C) and GluN1 splice forms GluN1-1 (D), GluN1-2 (E) and GluN1-3 (F) for ibuprofen (upper sections within each lettered image) and controls (lower sections) groups at 5 (left sections) and 26 (right sections) months of age. Density keys beneath the images indicate the fmol/mm2 of 33P dATP. The density key located below E is representative for D-F.
Table 1.
Effects of ibuprofen on average mRNA densitya for all GluN2A, GluN2B and GluN1 (GluN1-pan) subunits and 3 splice forms of the GluN1 subunit for seven brain regions in 4 different age groups.
| GluN2A | 5 month | 14 month | 20 month | 26 month | ||||
| Brain Region | Control | Ibuprofen | Control | Ibuprofen | Control | Ibuprofen | Control | Ibuprofen |
|
| ||||||||
|
Medial frontal
deep |
233 ± 33 |
236 ± 67 |
251 ± 11 |
239 ± 28 |
235 ± 27 |
230 ± 24 |
192 ± 37 |
206 ± 41 |
|
Lateral frontal
deep |
221 ± 31 |
253 ± 61 |
254 ± 15 |
253 ± 25 |
240 ± 30 |
253 ± 21 |
200 ± 37 |
238 ± 39 |
|
Lateral frontal
superficial |
362 ± 31 |
353 ± 54 |
385 ± 15 |
342 ± 34 |
353 ± 29 |
367 ± 12 |
320 ± 35 |
347 ± 32 |
| Caudate | 64 ± 30 |
50 ± 52 |
104 ± 13 |
76 ± 24 |
83 ± 17 |
75 ± 18 |
39 ± 25 |
40 ± 36 |
|
Dentate granule
cell layer-upper |
1077± 42 |
1037± 69 |
1068± 52 |
1013± 72 |
1091 ±44 |
1015± 60 |
972 ± 37 |
1030± 62 |
| CA3 | 880 ± 50 |
857 ± 59 |
889 ± 44 |
821 ± 77 |
865 ± 47 |
877 ± 41 |
794 ± 52 |
845 ± 32 |
| Cerebellum | 289 ± 28 |
252 ± 51 |
319 ± 20 |
292 ± 30 |
309 ± 28 |
285 ± 25 |
239 ± 39 |
247 ± 38 |
| GluN2B * | 5 month | 14 month | 20 month | 26 month | ||||
| Brain Region | Control | Ibuprofen | Control | Ibuprofen | Control | Ibuprofen | Control | Ibuprofen |
|
| ||||||||
|
Medial
frontal deep * |
398 ± 12 |
326 ± 13 |
357 ± 15 |
324 ± 7 |
364 ± 20 |
307 ± 22 |
355 ± 17 |
317 ± 12 |
|
Lateral
frontal deep * |
339 ± 6 |
291 ± 16 |
327 ± 16 |
278 ± 15 |
324 ± 17 |
282 ± 21 |
290 ± 12 |
290 ± 8 |
|
Lateral
frontal superficial * |
524 ± 24 |
440 ± 20 |
554 ± 19 |
398 ± 16 |
527 ± 7 |
428 ± 20 |
459 ± 28 |
432 ± 11 |
| Caudate | 227 ± 8 |
214 ± 11 |
216 ± 16 |
200 ± 16 |
210 ± 11 |
202 ± 16 |
188 ± 8 |
190 ± 9 |
|
Dentate granule
cell layer-upper |
910 ± 29 |
922 ± 31 |
905 ± 27 |
931 ± 25 |
916 ± 21 |
929 ± 34 |
861 ± 45 |
923 ± 18 |
| CA3 | 873 ± 31 |
881 ± 19 |
870 ± 41 |
880 ± 29 |
867 ± 28 |
890 ±29 |
840 ± 35 |
884 ± 5 |
| GluN1-pan | 5 month | 14 month | 20 month | 26 month | ||||
| Brain Region | Control | Ibuprofen | Control | Ibuprofen | Control | Ibuprofen | Control | Ibuprofen |
|
| ||||||||
|
Medial
frontal deep * |
204 ± 5 |
179 ± 8 |
187 ± 7 |
175 ± 8 |
182 ± 16 |
169 ± 11 |
184 ± 13 |
164 ± 12 |
|
Lateral frontal
deep |
203 ± 7 |
183 ± 8 |
185 ± 6 |
178 ± 9 |
185 ± 14 |
171 ± 8 |
180 ± 16 |
184 ± 3 |
|
Lateral
frontal superficial * |
236 ± 9 |
213 ± 10 |
238 ± 6 |
219 ± 17 |
230 ± 18 |
206 ± 7 |
249 ± 13 |
221 ± 9 |
| Caudate | 111 ± 5 |
105 ± 9 |
99 ± 5 |
97 ± 8 | 87 ± 6 |
89 ± 8 | 90 ± 5 | 90 ± 2 |
|
Dentate granule
cell layer-upper |
520 ± 28 |
538 ± 24 |
539 ± 24 |
558 ± 21 |
510 ± 29 |
526 ± 37 |
513 ± 17 |
535 ± 15 |
| CA3 | 534 ± 21 |
552 ± 16 |
526 ± 19 |
545 ± 10 |
533 ± 37 |
553 ± 14 |
521 ± 17 |
553 ± 27 |
| Cerebellum * | 390 ± 10 |
427 ± 13 |
376 ± 10 |
429 ± 14 |
381 ± 22 |
402 ± 17 |
372 ± 10 |
422 ± 19 |
| GluN1-1 | 5 month | 14 month | 20 month | 26 month | ||||
| Brain Region | Control | Ibuprofen | Control | Ibuprofen | Control | Ibuprofen | Control | Ibuprofen |
|
| ||||||||
|
Medial frontal
deep |
312 ± 22 |
281 ± 39 |
290 ± 35 |
290 ± 22 |
326 ± 20 |
273 ± 28 |
287 ± 33 |
273 ± 34 |
|
Lateral frontal
deep |
295 ± 27 |
270 ± 42 |
270 ± 34 |
262 ± 21 |
297 ± 22 |
263 ± 28 |
268 ± 31 |
264 ± 32 |
|
Lateral
frontal superficial * |
346 ± 28 |
307 ± 30 |
334 ± 30 |
285 ± 32 |
363 ± 19 |
313 ± 23 |
346 ± 28 |
282 ± 21 |
| Caudate | 245 ± 32 |
233 ± 42 |
211 ± 35 |
213 ± 23 |
231 ± 25 |
208 ± 29 |
193 ± 34 |
193 ± 33 |
|
Dentate granule
cell layer-upper |
488 ± 26 |
524 ± 39 |
499 ± 43 |
507 ± 28 |
522 ± 24 |
504 ± 27 |
471 ± 33 |
493 ± 29 |
| CA3 | 444 ± 27 |
444 ± 39 |
425 ± 39 |
452 ± 27 |
464 ± 26 |
462 ± 15 |
407 ± 33 |
423 ± 27 |
| Cerebellum | 271 ± 31 |
282 ± 42 |
231 ± 32 |
280 ± 14 |
286 ± 23 |
302 ± 22 |
238 ± 29 |
266 ± 40 |
| GluN1-2 | 5 month | 14 month | 20 month | 26 month | ||||
| Brain Region | Control | Ibuprofen | Control | Ibuprofen | Control | Ibuprofen | Control |
Ibupro
fen |
|
| ||||||||
|
Medial frontal
deep |
363 ± 31 |
381 ± 36 |
317 ± 28 |
345 ± 22 |
334 ± 22 |
340 ± 28 |
339 ± 33 |
368 ± 38 |
|
Lateral frontal
deep |
355 ± 34 |
376 ± 42 |
314 ± 26 |
349 ± 26 |
333 ± 25 |
341 ± 30 |
333 ± 36 |
381 ± 37 |
|
Lateral frontal
superficial |
380 ± 27 |
384 ± 33 |
330 ± 29 |
338 ± 30 |
367 ± 30 |
376 ± 27 |
363 ± 28 |
408 ± 44 |
| Caudate | 248 ± 32 |
270 ± 44 |
199 ± 26 |
222 ± 30 |
211 ± 30 |
220 ± 34 |
212 ± 39 |
251 ± 32 |
|
Dentate granule
cell layer-upper |
554 ± 23 |
592 ± 46 |
538 ± 21 |
572 ± 32 |
529 ± 25 |
574 ± 31 |
518 ± 40 |
602 ± 45 |
| CA3 | 660 ± 49 |
648 ± 41 |
614 ± 19 |
632 ± 27 |
613 ± 36 |
653 ± 32 |
610 ± 52 |
665 ± 43 |
| Cerebellum | 453 ± 34 |
475 ±45 |
399 ± 27 |
449 ± 28 |
420 ± 26 |
474 ± 44 |
421 ± 30 |
485 ± 46 |
| GluN1-3 | 5 month | 14 month | 20 month | 26 month | ||||
| Brain Region | Control | Ibuprofen | Control | Ibuprofen | Control | Ibuprofen | Control | Ibuprofen |
|
| ||||||||
|
Medial frontal
deep |
147 ± 38 |
216 ± 59 |
124 ± 50 |
211 ± 35 |
130 ± 44 |
167 ± 18 |
133 ± 34 |
144 ± 18 |
|
Lateral frontal
deep |
141 ± 42 |
206 ± 58 |
120 ± 45 |
192 ± 40 |
123 ± 46 |
155 ± 11 |
137 ± 37 |
157 ± 26 |
|
Lateral
frontal superficial * |
133 ± 36 |
240 ± 61 |
84 ± 36 |
160 ± 32 |
90 ± 36 |
165 ± 43 |
95 ± 31 |
159 ± 41 |
| Caudate | 101 ± 36 |
118 ± 53 |
83 ± 40 |
97 ± 28 |
92 ± 41 |
67 ± 18 |
91 ± 41 |
37 ± 13 |
|
Dentate granule
cell layer-upper |
217 ± 50 |
230 ± 64 |
192 ± 73 |
196 ± 30 |
132 ± 44 |
180 ± 23 |
180 ± 48 |
182 ± 25 |
| CA3 | 191 ± 44 |
214 ± 73 |
164 ± 64 |
177 ± 36 |
128 ± 42 |
157 ± 15 |
147 ± 45 |
140 ± 31 |
| Cerebellum | 400 ± 103 |
235 ± 58 |
277 ± 86 |
195 ± 25 |
198 ± 52 |
207 ± 25 |
299 ± 86 |
242 ± 56 |
Main effect of treatment
fmole 33P-dATP/mm2 ± SEM
superficial, cortical layers II-III; deep, cortical layers IV-VI
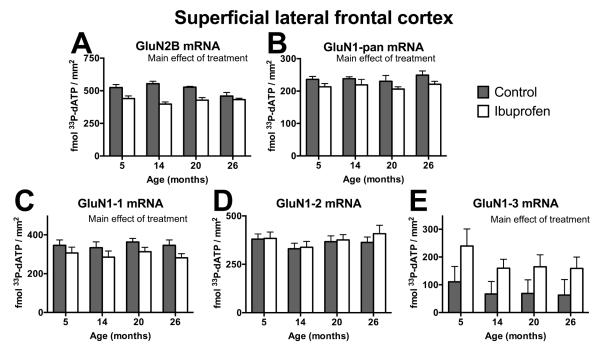
Figure 3.
Effects of ibuprofen on NMDA receptor mRNA expression in superficial lateral frontal cortex. mRNA densities (fmole 33P-dATP/mm2) for all GluN2B (A) and GluN1 (GluN1-pan; B) subunits and for 3 splice forms of the GluN1 subunit, GluN1-1 (C), GluN1-2 (D), and GluN1-3 (E) in the superficial layers (II-III) of the lateral frontal cortex for different ages and treatments. Ibuprofen reduced mRNA expression of GluN2B (A), GluN1-pan (B), and GluN1-1 (C), but increased GluN1-3 mRNA (E) in superficial lateral frontal cortex, with data collapsed across ages (main effect of treatment (p<.05)). Mean ± SEM, N = 5-6.
There was no significant overall main effect of treatment (prange=.18-.71) or age (prange=.49-.72) on mRNA density measurements for GluN1-1, GluN1-2, and GluN1-3 splice forms and all GluN1 splice variants (GluN1-pan) subunit across all brain regions. There was, however, a significant interaction between brain region and treatment for all GluN1 splice variants (GluN1-pan; F(6,234)=6.6; p<.0001) and GluN1-1 (F(6,234)=8.04; p<.0001) and GluN1-3 splice forms (F(6,234)=6.3; p<.0001), but not for the GluN1-2 splice form (F(6,234) =1.9; p=.08; Figure 2C-F, Table 1). Ibuprofen significantly reduced mRNA densities for all GluN1 splice variants (GluN1-pan; p=.008) and the GluN1-1 splice form (p=.009), but increased mRNA density for the GluN1-3 splice form (p=.008) across all ages in the superficial layers of the lateral frontal cortex (Figure 2C-F, 3B-E; Table 1). Ibuprofen also decreased GluN1-pan mRNA in the medial deep layers of the frontal cortex (Figure 2C; Table 1). In the cerebellum, ibuprofen raised mRNA densities for GluN1-pan across all ages (p=.0004; Figure 2C; Table 1).
Ibuprofen had different effects on NMDA receptor proteins in frontal cortex versus hippocampus
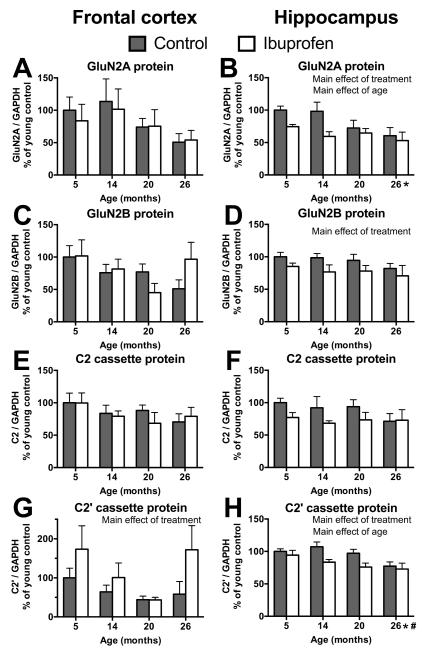
In order to assess whether ibuprofen could influence memory-related proteins in the synaptic environment, Western blotting was performed on crude synaptosomes. There were no significant main effects of age on protein expression of GluN2A (p=.15) and GluN2B (p=.17) subunits or the C2 (p=.39) and C2′ (p=.06) cassettes of the GluN1 subunit in the frontal cortex (Figure 4A,C,E,G, respectively). There was a significant main effect of treatment on protein expression of the C2′ cassette in the frontal cortex (F(1,37)=5; p=.03), with ibuprofen fed mice expressing more C2′ cassettes than those on control diet (Figure 4G).
Figure 4.
Effects of age and ibuprofen treatment on protein expression of NMDA receptor subunits GluN2A (A, B) and GluN2B (C,D) and GluN1 splice cassettes C2 (E,F) and C2′ (G,H) in frontal cortex (A,C,E,G) and hippocampus (B,D,F,H). Protein expression was normalized to GAPDH levels within the same samples. Ibuprofen reduced GluN2A (B) and C2′ cassette (H) proteins in the hippocampus, but increased C2′ cassette protein expression in the frontal cortex (G; main effect of treatment (p<.05)). p<.05 for main effect of age and differences between the oldest mice and 5 (*) and 14 (#) month olds with data collapsed across treatment. Mean ± SEM, N = 4-6.
There was a significant main effect of age on GluN2A subunit (F(1,37)=3.2; p=.03) and C2′ cassette (F(1,38)=4.7; p=.007) protein expressions in the hippocampus, with lower expression with increasing age (Figure 4B, H). There was a significant main effect of treatment on protein expression of GluN2A (F(1,37)=7.4; p<.05) and GluN2B (F(1,37)=6.2; p=.02) subunits and the C2′ (F(1,37)=9; p=.005) cassette of the GluN1 subunit in the hippocampus (Figure 4B,D,H, respectively), but not for the C2 cassette (F(1,37)=3.8; p=.06; Figure 4F). Mice fed ibuprofen had lower expression levels of GluN2A, GluN2B and C2′ cassette proteins in the hippocampus when data was collapsed across age groups.
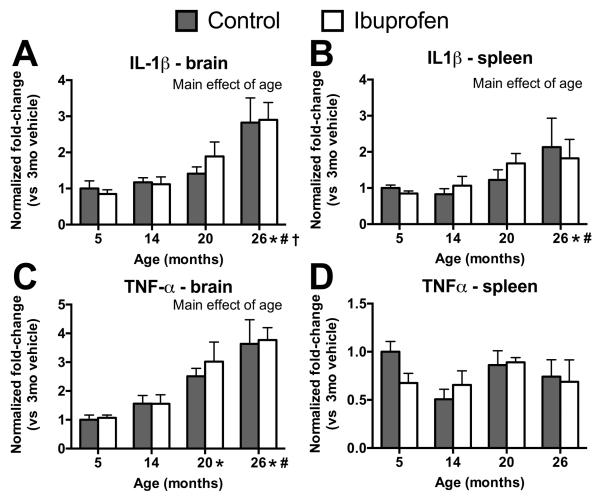
Age, but not ibuprofen, affected brain and spleen pro-inflammatory cytokine mRNA
Because ibuprofen effects appeared to be across ages, pro-inflammatory cytokine mRNA expression was examined in the brain and systemically in the spleen in order to determine whether the effects were related to inflammation. There was no significant effect of treatment on IL-1β mRNA expression in the brain (F(1,38)=.12; p=.73; Figure 5A) or spleen (F(1,38)=.06; p=.81; Figure 5B) or on TNFα mRNA expression in the brain (F(1,38)=.31; p=.58; Figure 5C) or spleen (F(1,38)=.28; p=.60; Figure 5D). There was a significant main effect of age on IL-1β mRNA expression in the brain (F(3,38)=12.22; p<.0001; Figure 5A), as well as the spleen (F(3,38)=3.93; p<.02; Figure 5B) and in TNFα mRNA expression in the brain (F(3,38)=15; p<.0001; Figure 5C), measured as normalized fold change from 5-month old controls, with older animals having greater expression than younger animals.
Figure 5.
Effects of age on pro-inflammatory cytokines within the brain and spleen. Fold changes from 5-month-old controls in IL-1β (A,B) and TNFα (C,D) levels in the brain (A,C) and spleen (B,D) from different ages and treatments. There were significant overall effects of age (p<.02), but no effect of ibuprofen treatment. Symbols indicate p<.05 for differences between older mice and 5 (*), 14 (#), and 20 (†) month olds with data collapsed across treatment. Mean ± SEM. N = 5-6.
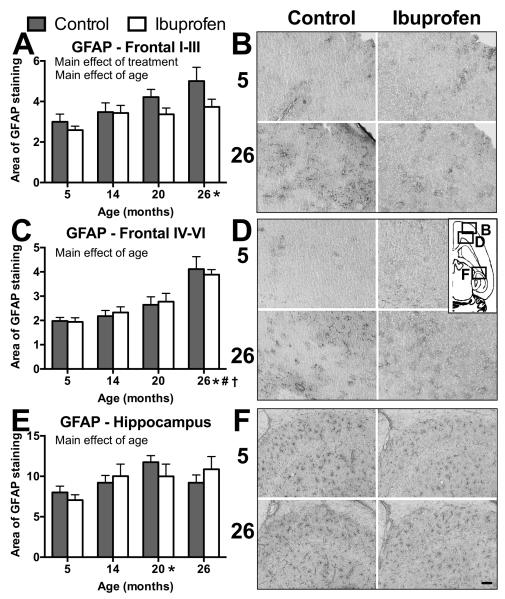
Ibuprofen only reduced astrogliosis in superfical layers of frontal cortex
Changes in reactive astrocytes were assessed with the use of area of GFAP staining in the lateral frontal cortex and hippocampus as another index of inflammation and the anti-inflammatory effects of ibuprofen. There was a significant main effect of age overall in lateral frontal cortex (F(3,39)=12; p<.0001; Figure 6A-D) and across hippocampal CA3 and dentate gyrus regions (F(3,39)=3.3; p=.03; Figure 6E-F) in area of GFAP-positive staining, with older ages exhibiting a greater area of GFAP staining than young. There was a treatment by region interaction (F(1,39)=5.6; p=.02) in the lateral frontal cortex in GFAP staining. The superficial layers (I-III) of lateral frontal cortex showed a significant main effect of treatment (F(3,39)=4.7; p=.04; Figure 6A,B) on area of GFAP staining, with ibuprofen-treated animals showing less widespread GFAP staining. Deep (IV-VI) frontal cortical layers (F(1,39)=.0001; p=.99; Figure 6C,D) and combined hippocampal regions (F(1,39)=.005; p=.94; Figure 6E,F) showed no significant effect of treatment on GFAP staining area.
Figure 6.
The effects of age and ibuprofen on reactive astrocytes. Graphs of the area (A,C,E) and images (B,D,F) of GFAP staining within superficial (I-III; A,B) and deep layers (IV-VI; C,D) of lateral frontal cortex and hippocampus (E,F). B,D,F) Images within each lettered figure are from 5 (upper row) and 26 (lower row) month old mice in control (left column) or ibuprofen-fed (right column) mice. Inset indicates where lettered figure images were taken from on horizontal slice. Ibuprofen reduced GFAP staining in the superficial layers of the lateral frontal cortex (main effect of treatment (p<.05)). p<.05 for main effect of age and differences between older mice and 5 (*), 14 (#), and 20 (†) month olds with data collapsed across treatment. Mean ± SEM. N = 5-6. Bar = 100μm.
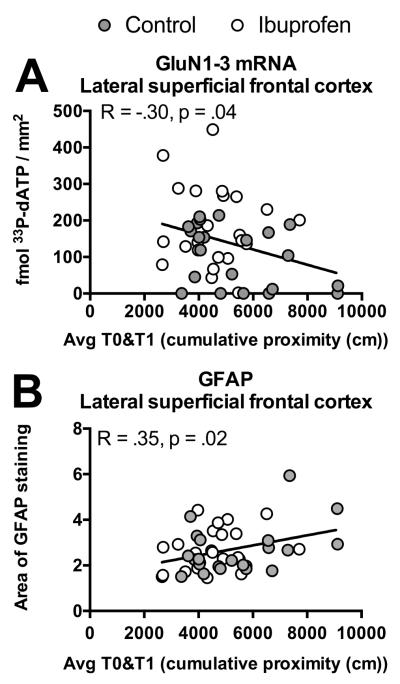
GluN1-3 mRNA and GFAP were related to performance in short-term memory task
The significant changes in the superficial layers of lateral frontal cortex were examined for relationships to the performance across trials in the working memory task. Both GluN1-3 mRNA (p=.04; Figure 7A) and GFAP area (p=.02; Figure 7B) showed significant correlations with the average performance across trials in the working memory task. Interestingly, GluN1-3 mRNA and GFAP area in this region did not show a relationship to each other (p=.86; not shown). None of the other NMDA receptor mRNAs in the superficial frontal cortex or proteins in frontal cortex or hippocampus showed an association with the working memory task (p=.08-.87).
Figure 7.
Relationships between behavior and NMDA receptors or gliosis. Correlation graphs, using all individuals across ages and treatments, showing performance across trials (Avg T0&T1) in the short-term memory task and GluN1-3 mRNA (A) or area of GFAP immunostaining (B) within the lateral superficial frontal cortex.
No evidence of toxicity from ibuprofen treatment
Because of the high dose of ibuprofen used in this study, body weights and liver enzymes in serum were measured for signs of toxicity. There was no significant main effect of treatment on individual mouse weights when averaged across age groups and different weighing days (F(1,39)=2.6; p=.12; Table 2). There was a significant main effect of age (F(3,39)=16; p<.01), with young animals having lower weights compared to older animals, when averaged across treatments and weighing days. There was no significant main effect of age (F(3,39)=.91; p=.45; Table 2) or treatment (F(1,39)=.32; p=.58; Table 2) on serum ALT.
Table 2.
No evidence of effects of ibuprofen on weight or serum liver enzymes.
| 5 months | 14 months | 20 months | 26 months | |||||
|---|---|---|---|---|---|---|---|---|
| Control |
Ibuprofe
n |
Control |
Ibuprofe
n |
Control |
Ibuprofe
n |
Control |
Ibuprofe
n |
|
| Weigh t (gms) * |
27.2±1. 0 |
28.5±0.6 | 34.2±0. 6 |
34.1±1.5 | 35.6±1.2 | 36.6±2.4 | 32.7±0.8 | 34.6±1.6 |
| Seru m ALT (U/L) |
40.8±1. 6 |
45.7±4.9 | 39.5±4. 2 |
38.3±2.4 | 52.3±13. 9 |
46.8±4.9 | 59.4±19. 9 |
46.4±12. 0 |
p < .05 for main effect of age: 14, 20 and 26 month olds had increased weight over the 5 month olds, with data collapsed across treatments.
DISCUSSION
This study showed that a non-steroidal anti-inflammatory drug, ibuprofen, improved overall performance in a short-term spatial memory task, but had no significant effects on long-term memory or cognitive flexibility. Ibuprofen had effects on NMDA receptor subunit expression in both the frontal cortex and hippocampus, but the effects were seen in both young and older mice. There was no effect of ibuprofen on pro-inflammatory cytokines, but there was a small regional reduction in gliosis. Both GluN1-3 mRNA and astrocyte area showed an association with overall performance in the short-term memory task. There was no evidence of toxicity at the dose tested. These findings suggest that inflammation may play a role in performance deficits in a short-term memory task in aged animals, but the effects of ibuprofen on NMDA receptors appeared to be primarily unrelated to aging or inflammation.
The main effect of ibuprofen on behavior was in the delayed short-term spatial memory task. Mice on ibuprofen performed better when both types of trials were considered than controls. In addition, the old control mice performed worse than young across both naive and delay trials, while the ibuprofen-treated old mice were not significantly different from the ibuprofen-treated young. The age differences in the control group were similar to a previous report (Magnusson et al., 2003). Since the performance across all groups on the delay trial was better than the naive trial, there did not appear to be any problem with short-term memory over a 10-min delay in this study. This suggests that the ibuprofen effect is more about the naive trial, particularly in the oldest mice. This could be due to enhanced executive function, such as improved search strategy or greater flexibility between sessions. The lack of significant improvement in the reversal trials, however, argues against greater cognitive flexibility in the ibuprofen-treated mice.
In the spatial long-term memory task, there were signs of reduced learning ability in 20- and 26- month olds across treatment groups in the place learning trials. Similar age-related differences have been observed in previous studies (Magnusson, 1998, 2001). There was no significant effect of ibuprofen on long-term memory in these mice, however, there was a trend for ibuprofen to worsen performance in the oldest mice in the place trials. In probe trials, used to assess the development of a spatial bias, there were no differences in performance for treatment or age, unlike in other studies (Magnusson et al., 2007, Das and Magnusson, 2008, Das and Magnusson, 2011, Brim et al., 2013, Zamzow et al., 2013), but see (Zhao et al., 2009). Some of the oldest mice were unable to complete all of the place trials in this study. The missed trials showed a significant effect on place and probe trial performance, so these mice were omitted from the statistical analysis of long-term memory. However, it is possible that these mice were poor learners, causing them to take more time per trial and tiring out, rather than that they performed poorly due to missing some place learning trials. There was no significant effect of missing place trials on reversal trials, working memory or cued trials, so all animals were included in these analyses.
Reversal trials, used to assess the ability of mice to learn a new platform position, showed that ibuprofen had no effect on the performance of animals compared to the control group. However, there were signs of reduction in cognitive flexibility in the reversal trials at the older ages. Equivalent performance of mice across treatment and age groups in the cued control task, suggested that motivation, sensory and motor skills were not factors that could account for differences observed in the other water maze tasks.
Ibuprofen primarily affected NMDA receptor mRNA expression in the frontal cortex. The mRNAs for both GluN2B and all splice variants of GluN1 combined (GluN1-pan) were reduced in medial and lateral frontal cortex across the ages examined. The GluN1-1 splice form mRNA also showed reductions in the superficial layers of lateral frontal cortex in the ibuprofen group at every age, which may contribute to the declines seen in GluN1-pan. However, ibuprofen did not have the same effect on all NMDA subunit and splice form mRNAs. There was an increase in all ages in the GluN1-3 mRNA in the superficial layers of lateral frontal cortex and in GluN1-pan in the cerebellum in the ibuprofen-fed mice. There was no effect of ibuprofen on GluN2A and GluN1-2 mRNA.
The GluN2B subunit (Magnusson, 2000, Magnusson et al., 2002) and GluN1-1 and GluN1-3 splice variants (Magnusson et al., 2005, Das and Magnusson, 2008) have previously been shown to be selectively vulnerable to the effect of aging in C57BL/6 mice. If inflammation was playing a role during aging on NMDA receptors, it was expected that ibuprofen would improve the expression of GluN2B, all GluN1 subunits as a whole, and the GluN1-1 and GluN1-3 splice variants, particularly in the oldest animals, but not affect GluN1-2 splice variants. The effects of ibuprofen on all ages and the reductions seen in multiple subunits/splice forms that normally show reductions with increased age, suggest that ibuprofen had effects on mRNA for the NMDA receptor that were unrelated to aging. The ibuprofen-related increase in GluN1-3 across ages appeared to be associated with the improved executive function, such as search strategy or flexibility.
The mRNA changes in the GluN2B subunit in the frontal cortex induced by ibuprofen did not manifest as protein changes. However, ibuprofen enhanced the protein expression of the C2′ cassette in the frontal cortex across the age groups. The C2′ cassette is translated from the GluN1-3 mRNA (Zukin and Bennett, 1995), so this enhancement of the protein expression could be due to the mRNA changes. The C2′ cassette is also found in the GluN1-4 splice variants (Zukin and Bennett, 1995), which may explain why there was no significant relationship between the C2′ cassette and executive function. In a previous study, higher levels of C2′ cassettes in the frontal cortex were associated with improved long-term memory in middle-aged mice, but no correlations were examined for averaged Tnaïve and Tdelay trials in the short-term memory task in that study (Das and Magnusson, 2011).
Despite the lack of significant ibuprofen effects on mRNA in the hippocampus, there were changes in the protein expression of NMDA receptor subunits and cassettes in this region. Ibuprofen decreased the expression of GluN2A, GluN2B and the C2′ cassette protein. This effect was across all ages. This suggests that ibuprofen can affect NMDA receptors at the protein level and can affect some proteins in opposite directions, depending on the brain region. In the mouse hippocampus, chronic ibuprofen (6 months) affected the expression of 28 different proteins and seven phosphoproteins, both up- and down-regulation was seen, depending on the protein (Matsuura et al., 2015). Affected proteins that are involved in signal transduction, such as G protein and synapsin-2 phosphoprotein, were down-regulated (Matsuura et al., 2015). The mechanism by which ibuprofen can alter mRNA and protein expression, however, is not yet known.
IL-1β and TNFα cytokines are one of several types of small signaling molecules involved in mediating pro-inflammatory responses (Dinarello, 1994). There was an increase in the pro-inflammatory cytokines IL-1β in both the brain and spleen and in TNFα in the brain with increasing age. Ibuprofen did not alter this expression across aging. Two months duration of sulindac treatment was sufficient to reduce IL-1β in aged rat hippocampus (Mesches et al., 2004). Three months of treatment with ibuprofen reduced IL-1β 33% in the brain of an AD mouse model (Tg2576), but greater reductions were achieved with a six-month exposure (Lim et al., 2000, Lim et al., 2001). It is possible that two months of treatment with ibuprofen was insufficient to reduce cytokines during aging in mice in the present study. The results of the current study, however, suggest that the effects of ibuprofen on behavior and the NMDA receptor were not via effects on cytokines. The improvement of memory by ibuprofen in an AD mouse model also did not appear to be related to cytokine levels (Kotilinek et al., 2008).
A study on the postmortem frontal cortices of Alzheimer disease (AD) and bipolar disorder (BD) patients has shown that increased cyclooxygenase-2 (COX-2) expression, related to increased neuroinflammatory markers like IL-1β, may partly be due to the hypomethylated state of the COX-2 CpG promoter region (Rao et al., 2012). That study proposed that chronic treatment of mood stabilizer and antipsychotic drugs for AD and BD, acting at the cellular level, may provide transient protection by correcting neuroinflammation and synaptic remodeling, but may not provide full recovery by not targeting epigenetic regulation (Rao et al., 2012). This study also suggests that attenuation of COX-2 expression at transcriptional or posttranscriptional levels (as in the ibuprofen mechanism) may not be enough to override the epigenetic mechanism at the COX-2 promoter region (Rao et al., 2012). These findings may explain why cytokine levels may not be altered by ibuprofen, which acts as a prostaglandin inhibitor by targeting COX, if additional epigenetic mechanisms are playing a role in upregulating COX-2 expression through hypomethylation of the COX-2 promoter region. There is also evidence that prolonged COX-2 elevation may lead to cellular changes that are also not altered by non-steroidal anti-inflammatory drugs (Dore et al., 2003).
Several studies have elucidated mechanisms for a subset of NSAIDs, including ibuprofen, in altering protein expression in Alzheimer’s disease (AD) models through pathways outside of cyclooxygenase (COX) inhibition, the principal mode of action of NSAIDs (Weggen et al., 2001, Zhou et al., 2003, Wilkinson et al., 2012). There is evidence demonstrating that ibuprofen is among a subset of NSAIDs capable of decreasing the production of the highly amyloidogenic Aβ42 peptide, which contributes to AD pathology, from a variety of cultured cells (Weggen et al., 2001). This effect was not seen with all NSAIDs and appears to not be mediated by inhibition of COX, but rather by inhibition of Rho guanosine-5′-triphosphatases (GTPases) and alterations in γ-secretase activity (Weggen et al., 2001, Zhou et al., 2003). Inhibition of Rho could also cause destabilization of NMDA receptors in the synaptic membrane, via uncoupling of F-actin. This could have led to the protein reductions in the hippocampal synaptosomes in the current study. Additional studies suggest ibuprofen inhibits nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activation and prevents oxidative damage in a COX-independent manner in an AD mouse model (Wilkinson et al., 2012). The oxidative environment of the aged brain inhibits NMDA receptor function (Bodhinathan et al., 2010), but it is not clear if it also alters the expression of the receptors. NMDA receptors have been implicated in causing oxidative stress in AD (Kamat et al., 2016). It is possible that ibuprofen reduces oxidative damage by decreasing NMDA receptor protein expression. While ibuprofen in this study did not alter expression of pro-inflammatory cytokines downstream of the COX pathway like TNFα and IL-1β, its effects on cognitive functions and NMDA receptor expression may involve mechanisms, such as altering synaptosomal localization of receptors or reducing oxidative stress, that are unrelated to COX inhibition.
Reactive astrocytes are a form of gliosis, which can be induced by many forms of central nervous system injury, including inflammatory cytokines and innate immunity modulators, such as lipopolysaccharides (Yong et al., 1991, Sofroniew and Vinters, 2010). GFAP is not consistently found in healthy astrocytes, but is up-regulated in reactive astrocytes (Sofroniew and Vinters, 2010). Aging is associated with increased levels of GFAP (Goss et al., 1991, Kohama et al., 1995, Ojo et al., 2015). There was an increase in volume of reactive astrocytes, as evidenced by increases in the area of GFAP staining, in both frontal cortex and hippocampus with increasing age in this study. Ibuprofen reduced the gliosis in the superficial layers of the frontal cortex. The expression of GFAP showed a relationship with performance in the short-term memory task, suggesting that the problem with search strategy or flexibility could have been produced by inflammation. This is the same region that showed mRNA changes in several subunits/splice variants of the NMDA receptor. It is possible that reduced inflammation could account for the mRNA changes seen, but there were no significant correlations between gliosis and mRNA expression seen in this study, including with the GluN1-3 splice variants. The effects of ibuprofen on protein expression of the NMDA receptor in the hippocampus also did not appear to be related to gliosis.
Ibuprofen administered to 16 month old Wistar rats for 2 months via gavage, at a similar dose to the current study, produced no enhancement in working memory, but did reduce response latency (Bilgin et al., 2013). There was no effect of ibuprofen on GluN2A or GluN2B expression in hippocampal homogenates, although there was a non-significant trend for reduced GluN2A (Bilgin et al., 2013). Another anti-inflammatory drug, sulindac, improved both working memory and contextual fear conditioning in aged rats (Mesches et al., 2004). Unlike the present study, sulindac reversed the age-related declines in GluN2B and GluN1 in rats and returned IL-1β in aged rat hippocampus to levels similar to young (Mesches et al., 2004). Both ibuprofen and sulindac inhibit COX1 and COX2 (Boolbol et al., 1996, Davies and Watson, 1997, Van Hecken et al., 2000). The differences from the present study, thus, could be due to species or specific drug differences or non-equivalent dosages or dosing methods. They also could be due to using homogenates (Mesches et al., 2004, Bilgin et al., 2013) versus synaptosomes.
The dose of ibuprofen used in this study was a high prescription dose that was effective in AD models (Lim et al., 2000, Lim et al., 2001, Yan et al., 2003, Choi et al., 2009), so there was concern about whether the effects during normal aging were due to ibuprofen toxicity. There were no significant differences between the two treatment groups on body weight across ages, suggesting that mice were eating equivalent amounts of food and were not suffering from any illness. Hepatotoxicity is an occasional side effect of ibuprofen toxicity (Adams et al., 1969, Sternlieb and Robinson, 1978, Rodriguez Gonzalez et al., 2002) and elevations of the liver enzyme ALT have been reported with chronic high-dose treatment (Royer et al., 1984, Rodriguez Gonzalez et al., 2002). There was no evidence of increased liver enzymes in the ibuprofen-treated mice. These weight and ALT results suggest that toxicity was not the cause of the changes in the NMDA receptor associated with ibuprofen treatment.
In conclusion, ibuprofen did not significantly alter memory, but did appear to enhance executive functions, such as search strategy or flexibility. In the outer layers of the frontal cortex, this enhanced cognitive ability showed relationships to reductions in gliosis and increases in GluN1-3 mRNA. However, ibuprofen also had effects on expression of NMDA receptor mRNA and proteins in the frontal cortex and hippocampus, which did not appear to be related to anti-inflammatory or toxic properties of the drug.
HIGHLIGHTS.
Ibuprofen enhanced executive function more than memory in a short-term memory task.
Ibuprofen decreased expression of several NMDA receptor subunits across adult aging.
Ibuprofen increased expression of GluN1-3 splice form mRNA and C2′ cassette protein in frontal lobes regardless of age.
Ibuprofen did not alter expression of pro-inflammatory cytokines, but reduced gliosis in frontal cortex of aged mice.
Enhancement in executive function was related to increased GluN1-3 mRNA and decreased gliosis.
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health grant AG016322 to K.R.M, Oregon Agricultural Experiment Station Hatch fund to C.P.W. and OSU CHAR Life Scholar fellowship to A.M-L.
GLOSSARY
- AAMI
Age-associated memory impairment
- AD
Alzheimer's disease
- ALT
Alanine aminotransferase (serum glutamic-pyruvic transaminase)
- ANOVA
analysis of variance
- dATP
deoxyadenosine triphosphate
- DNA
deoxyribonucleic acid
- GFAP
glial fibrillary acidic protein
- GluN
glutamate receptor subunit, N-methyl-D-aspartate specific
- IL-1β
interleukin-1beta
- mRNA
messenger ribonucleic acid
- NMDA
N-methyl-D-aspartate
- P2
crude synaptosome pellet
- PBS
Phosphate-buffered saline
- PCR
polymerase chain reaction
- Tdelay
Second trial after a 10 minute delay in a working memory task
- Tnaïve
First naive trial in each session in a working memory task
- TNFα
tumor necrosis factor alpha
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adams SS, Bough RG, Cliffe EE, Lessel B, Mills RF. Absorption, distribution and toxicity of ibuprofen. Toxicology and applied pharmacology. 1969;15:310–330. doi: 10.1016/0041-008x(69)90032-5. [DOI] [PubMed] [Google Scholar]
- Albert MS, Funkenstein HH. The effects of age: Normal variation and its relation to disease. In: Asbury AK, et al., editors. Diseases of the Nervous System: Clinical Neurobiology. Saunders; Philadelphia: 1992. pp. 598–611. [Google Scholar]
- Barnes CA. Aging and the physiology of spatial memory. Neurobiol Aging. 1988;9:563–568. doi: 10.1016/s0197-4580(88)80114-3. [DOI] [PubMed] [Google Scholar]
- Bilgin OO, Doguc DK, Altuntas I, Sutcu R, Delibas N. Effects of subchronic treatment with ibuprofen and nimesulide on spatial memory and NMDAR subunits expression in aged rats. Iran J Pharm Res. 2013;12:877–885. [PMC free article] [PubMed] [Google Scholar]
- Blalock EM, Chen KC, Sharrow K, Herman JP, Porter NM, Foster TC, Landfield PW. Gene microarrays in hippocampal aging: statistical profiling identifies novel processes correlated with cognitive impairment. J Neurosci. 2003;23:3807–3819. doi: 10.1523/JNEUROSCI.23-09-03807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodhinathan K, Kumar A, Foster TC. Intracellular redox state alters NMDA receptor response during aging through Ca2+/calmodulin-dependent protein kinase II. J Neurosci. 2010;30:1914–1924. doi: 10.1523/JNEUROSCI.5485-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boolbol SK, Dannenberg AJ, Chadburn A, Martucci C, Guo XJ, Ramonetti JT, Abreu-Goris M, Newmark HL, Lipkin ML, DeCosse JJ, Bertagnolli MM. Cyclooxygenase-2 overexpression and tumor formation are blocked by sulindac in a murine model of familial adenomatous polyposis. Cancer research. 1996;56:2556–2560. [PubMed] [Google Scholar]
- Brim BL, Haskell R, Awedikian R, Ellinwood NM, Jin L, Kumar A, Foster TC, Magnusson KR. Memory in aged mice is rescued by enhanced expression of the GluN2B subunit of the NMDA receptor. Behav Brain Res. 2013;238:211–226. doi: 10.1016/j.bbr.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Cui Z, Feng R, Tang YP, Qin Z, Mei B, Tsien JZ. Maintenance of superior learning and memory function in NR2B transgenic mice during ageing. Eur J Neurosci. 2007;25:1815–1822. doi: 10.1111/j.1460-9568.2007.05431.x. [DOI] [PubMed] [Google Scholar]
- Choi J, Jenkins B, Carreras I, Kaymakcalan S, Cormier K, Kowall N, Dedeoglu A. Anti-inflammatory treatment in AD mice protects against neuronal pathology. Exp Neurol. 2009 doi: 10.1016/j.expneurol.2009.07.032. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SR, Jensen R, Kelsay R, Shumaker M, Bochart R, Brim B, Zamzow D, Magnusson KR. Reducing expression of GluN1(0XX) subunit splice variants of the NMDA receptor interferes with spatial reference memory. Behavioural brain research. 2012;230:317–324. doi: 10.1016/j.bbr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SR, Magnusson KR. Relationship between mRNA expression of splice forms of the zeta1 subunit of the N-methyl-D-aspartate receptor and spatial memory in aged mice. Brain Res. 2008;1207:142–154. doi: 10.1016/j.brainres.2008.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SR, Magnusson KR. Changes in expression of splice cassettes of NMDA receptor GluN1 subunits within the frontal lobe and memory in mice during aging. Behavioural brain research. 2011;222:122–133. doi: 10.1016/j.bbr.2011.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies NM, Watson MS. Clinical pharmacokinetics of sulindac. A dynamic old drug. Clinical pharmacokinetics. 1997;32:437–459. doi: 10.2165/00003088-199732060-00002. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. The biological properties of interleukin-1. European cytokine network. 1994;5:517–531. [PubMed] [Google Scholar]
- Division UNDoEaSAP . World Population Prospects: The 2015 Revision, Key Findings and Advance Tables. United Nations; New York: 2015. [Google Scholar]
- Dore S, Otsuka T, Mito T, Sugo N, Hand T, Wu L, Hurn PD, Traystman RJ, Andreasson K. Neuronal overexpression of cyclooxygenase-2 increases cerebral infarction. Annals of neurology. 2003;54:155–162. doi: 10.1002/ana.10612. [DOI] [PubMed] [Google Scholar]
- Dunah AW, Standaert DG. Dopamine D1 receptor-dependent trafficking of striatal NMDA glutamate receptors to the postsynaptic membrane. J Neurosci. 2001;21:5546–5558. doi: 10.1523/JNEUROSCI.21-15-05546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eakin TJ, Baskin DG, Breininger JF, Stahl WL. Calibration of 14C-plastic standards for quantitative autoradiography with 33P. J Histochem Cytochem. 1994;42:1295–1298. doi: 10.1177/42.9.8064137. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Gage F, Dunnett S, Bjorklund A. Spatial learning and motor deficits in aged rats. Neurobiol Aging. 1984;5:43–48. doi: 10.1016/0197-4580(84)90084-8. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: Development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993;107:618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Nicolle MM. Animal models of normal aging: Relationship between cognitive decline and markers in hippocampal circuitry. Behav Brain Res. 1993;57:155–162. doi: 10.1016/0166-4328(93)90131-9. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Rapp PR. The use of animal models to study the effects of aging on cognition. Ann Rev Psychol. 1997;48:339–370. doi: 10.1146/annurev.psych.48.1.339. [DOI] [PubMed] [Google Scholar]
- Gilsky RL. Changes in cognitive function in human aging. In: Riddle DR, editor. Brain Aging: Models, Methods, and Mechanisms. CRC Press; Boca Raton: 2007. pp. 3–20. [Google Scholar]
- Goss JR, Finch CE, Morgan DG. Age-related changes in glial fibrillary acidic protein mRNA in the mouse brain. Neurobiol Aging. 1991;12:165–170. doi: 10.1016/0197-4580(91)90056-p. [DOI] [PubMed] [Google Scholar]
- Hascup ER, Wang F, Kopchick JJ, Bartke A. Inflammatory and Glutamatergic Homeostasis Are Involved in Successful Aging. J Gerontol A Biol Sci Med Sci. 2016;71:281–289. doi: 10.1093/gerona/glv010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head E, Mehta R, Hartley J, Kameka M, Cummings BJ, Cotman CW, Ruehl WW, Milgram NW. Spatial learning and memory as a function of age in the dog. Behav Neurosci. 1995;109:851–858. doi: 10.1037//0735-7044.109.5.851. [DOI] [PubMed] [Google Scholar]
- Ishii T, Moriyoshi K, Sugihara H, Sakurada K, Kadotani H, Yokoi M, Akazawa C, Shigemoto R, Mizuno N, Masu M, Nakanishi S. Molecular characterization of the family of the N-methyl-D-aspartate receptor subunits. J Biol Chem. 1993;268:2836–2843. [PubMed] [Google Scholar]
- Kamat PK, Kalani A, Rai S, Swarnkar S, Tota S, Nath C, Tyagi N. Mechanism of Oxidative Stress and Synapse Dysfunction in the Pathogenesis of Alzheimer's Disease: Understanding the Therapeutics Strategies. Molecular neurobiology. 2016;53:648–661. doi: 10.1007/s12035-014-9053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlamangla AS, Miller-Martinez D, Lachman ME, Tun PA, Koretz BK, Seeman TE. Biological correlates of adult cognition: midlife in the United States (MIDUS) Neurobiol Aging. 2014;35:387–394. doi: 10.1016/j.neurobiolaging.2013.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohama SG, Goss JR, Finch CE, McNeill TH. Increases of glial fibrillary acidic protein in the aging female mouse brain. Neurobiol Aging. 1995;16:59–67. doi: 10.1016/0197-4580(95)80008-f. [DOI] [PubMed] [Google Scholar]
- Kotilinek LA, Westerman MA, Wang Q, Panizzon K, Lim GP, Simonyi A, Lesne S, Falinska A, Younkin LH, Younkin SG, Rowan M, Cleary J, Wallis RA, Sun GY, Cole G, Frautschy S, Anwyl R, Ashe KH. Cyclooxygenase-2 inhibition improves amyloid-beta-mediated suppression of memory and synaptic plasticity. Brain. 2008;131:651–664. doi: 10.1093/brain/awn008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsuwada T, Kashiwabuchi N, Mori H, Sakimura K, Kushiya E, Araki K, Meguro H, Masaki H, Kumanishi T, Arakawa M, Mishina M. Molecular diversity of the NMDA receptor channel. Nature. 1992;358:36–41. doi: 10.1038/358036a0. [DOI] [PubMed] [Google Scholar]
- Larrabee GJ, Crook TH., 3rd Estimated prevalence of age-associated memory impairment derived from standardized tests of memory function. Int Psychogeriatr. 1994;6:95–104. doi: 10.1017/s1041610294001663. [DOI] [PubMed] [Google Scholar]
- Laurie DJ, Seeburg PH. Regional and developmental heterogeneity in splicing of the rat brain NMDAR1 mRNA. J Neurosci. 1994;14:3180–3194. doi: 10.1523/JNEUROSCI.14-05-03180.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim G, Yang F, Chu T, Chen P, Beech W, Teter B, Tran T, Ubeda O, Ashe K, Frautschy S, Cole G. Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer's disease. J Neurosci. 2000;20:5709–5714. doi: 10.1523/JNEUROSCI.20-15-05709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim GP, Yang F, Chu T, Gahtan E, Ubeda O, Beech W, Overmier JB, Hsiao-Ashec K, Frautschy SA, Cole GM. Ibuprofen effects on Alzheimer pathology and open field activity in APPsw transgenic mice. Neurobiol Aging. 2001;22:983–991. doi: 10.1016/s0197-4580(01)00299-8. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Fellous JM, Wang XJ. A role for NMDA-receptor channels in working memory. Nat Neurosci. 1998;1:273–275. doi: 10.1038/1086. [DOI] [PubMed] [Google Scholar]
- Magnusson KR. The effects of age and dietary restriction on metabotropic glutamate receptors. J Gerontol. 1997;52A:B291–B299. doi: 10.1093/gerona/52a.6.b291. [DOI] [PubMed] [Google Scholar]
- Magnusson KR. Aging of glutamate receptors: Correlations between receptor binding and spatial memory performance in C57Bl mice. Mech Ageing Dev. 1998;104:227–248. doi: 10.1016/s0047-6374(98)00076-1. [DOI] [PubMed] [Google Scholar]
- Magnusson KR. Declines in mRNA expression of different subunits may account for differential effects of aging on agonist and antagonist binding to the NMDA receptor. J Neurosci. 2000;20:1666–1674. doi: 10.1523/JNEUROSCI.20-05-01666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson KR. Influence of diet restriction on NMDA receptor subunits and learning during aging. Neurobiol Aging. 2001;22:613–627. doi: 10.1016/s0197-4580(00)00258-x. [DOI] [PubMed] [Google Scholar]
- Magnusson KR, Bai L, Zhao X. The effects of aging on different C-terminal splice forms of the zeta1(NR1) subunit of the N-methyl-d-aspartate receptor in mice. Brain Res Mol Brain Res. 2005;135:141–149. doi: 10.1016/j.molbrainres.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Magnusson KR, Brim BL, Das SR. Selective vulnerabilities of N-methyl-D-aspartate (NMDA) receptors during brain aging. Front Aging Neurosci. 2010;2 doi: 10.3389/fnagi.2010.00011. Article 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson KR, Cotman CW. Age-related changes in excitatory amino acid receptors in two mouse strains. Neurobiol Aging. 1993;14:197–206. doi: 10.1016/0197-4580(93)90001-r. [DOI] [PubMed] [Google Scholar]
- Magnusson KR, Nelson SE, Young AB. Age-related changes in the protein expression of subunits of the NMDA receptor. Mol Brain Res. 2002;99:40–45. doi: 10.1016/s0169-328x(01)00344-8. [DOI] [PubMed] [Google Scholar]
- Magnusson KR, Scruggs B, Aniya J, Wright KC, Ontl T, Xing Y, Bai L. Age-related deficits in mice preforming working memory tasks in a water maze. Behav Neurosci. 2003;117:485–495. doi: 10.1037/0735-7044.117.3.485. [DOI] [PubMed] [Google Scholar]
- Magnusson KR, Scruggs B, Zhao X, Hammersmark R. Age-related declines in a two-day reference memory task are associated with changes in NMDA receptor subunits in mice. BMC Neurosci. 2007;8:43. doi: 10.1186/1471-2202-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura K, Otani M, Takano M, Kadoyama K, Matsuyama S. The influence of chronic ibuprofen treatment on proteins expressed in the mouse hippocampus. Eur J Pharmacol. 2015;752:61–68. doi: 10.1016/j.ejphar.2015.01.047. [DOI] [PubMed] [Google Scholar]
- McKee AC, Carreras I, Hossain L, Ryu H, Klein WL, Oddo S, LaFerla FM, Jenkins BG, Kowall NW, Dedeoglu A. Ibuprofen reduces Abeta, hyperphosphorylated tau and memory deficits in Alzheimer mice. Brain Res. 2008;1207:225–236. doi: 10.1016/j.brainres.2008.01.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meguro H, Mori H, Araki K, Kushiya E, Kutsuwada T, Yamazaki M, Kumnainshi T, Arakawa M, Sakimura K, Mishina M. Functional characterization of a heteromeric NMDA receptor channel expressed from cloned cDNAs. Nature. 1992;357:70–74. doi: 10.1038/357070a0. [DOI] [PubMed] [Google Scholar]
- Mesches MH, Gemma C, Veng LM, Allgeier C, Young DA, Browning MD, Bickford PC. Sulindac improves memory and increases NMDA receptor subunits in aged Fischer 344 rats. Neurobiol Aging. 2004;25:315–324. doi: 10.1016/S0197-4580(03)00116-7. [DOI] [PubMed] [Google Scholar]
- Mondadori C, Weiskrantz L, Buerki H, Petschke F, Fagg GE. NMDA receptor antagonists can enhance or impair learning performance in animals. Exp Brain Res. 1989;75:449–456. doi: 10.1007/BF00249896. [DOI] [PubMed] [Google Scholar]
- Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg PH. Heteromeric NMDA receptors: Molecular and functional distinction of subtypes. Science. 1992;256:1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Morris RGM. Synaptic plasticity and learning: Selective impairment of learning in rats and blockade of long-term potentiation in vivo by the N-methyl-D-aspartate receptor antagonist AP5. J Neurosci. 1989;9:3040–3057. doi: 10.1523/JNEUROSCI.09-09-03040.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RGM, Davis M. The role of NMDA receptors in learning and memory. In: Collingridge GL, Watkins JC, editors. The NMDA Receptor. Oxford University Press; Oxford: 1994. pp. 340–375. [Google Scholar]
- Nair A, Bonneau RH. Stress-induced elevation of glucocorticoids increases microglia proliferation through NMDA receptor activation. Journal of neuroimmunology. 2006;171:72–85. doi: 10.1016/j.jneuroim.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Ojo JO, Rezaie P, Gabbott PL, Stewart MG. Impact of age-related neuroglial cell responses on hippocampal deterioration. Front Aging Neurosci. 2015;7:57. doi: 10.3389/fnagi.2015.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okura T, Langa KM. Caregiver burden and neuropsychiatric symptoms in older adults with cognitive impairment: The Aging, Demographics, and Memory Study (ADAMS) Alzheimer Dis Assoc Disord. 2011;25:116–121. doi: 10.1097/WAD.0b013e318203f208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychol Aging. 2002;17:299–320. [PubMed] [Google Scholar]
- Pelleymounter MA, Beatty G, Gallagher M. Hippocampal 3H-CPP binding and spatial learning deficits in aged rats. Psychobiology. 1990;18:298–304. [Google Scholar]
- Prolla TA. DNA microarray analysis of the aging brain. Chemical senses. 2002;27:299–306. doi: 10.1093/chemse/27.3.299. [DOI] [PubMed] [Google Scholar]
- Rao JS, Keleshian VL, Klein S, Rapoport SI. Epigenetic modifications in frontal cortex from Alzheimer's disease and bipolar disorder patients. Translational psychiatry. 2012;2:e132. doi: 10.1038/tp.2012.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp P, Rosenberg R, Gallagher M. An evaluation of spatial information processing in aged rats. Behav Neurosci. 1987;101:3–12. doi: 10.1037//0735-7044.101.1.3. [DOI] [PubMed] [Google Scholar]
- Rodriguez Gonzalez F, Jimenez Romero C, Rodriguez Romano D, Loinaz Segurola C, Marques Medina E, Perez Saborido B, Garcia Garcia I, Rodriguez Canete A, Moreno Gonzalez E. Orthotopic liver transplantation with 100 hepatic allografts from donors over 60 years old. Transplantation proceedings. 2002;34:233–234. doi: 10.1016/s0041-1345(01)02738-5. [DOI] [PubMed] [Google Scholar]
- Royer GL, Seckman CE, Welshman IR. Safety profile: fifteen years of clinical experience with ibuprofen. The American journal of medicine. 1984;77:25–34. doi: 10.1016/s0002-9343(84)80015-7. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlieb P, Robinson RM. Stevens-Johnson syndrome plus toxic hepatitis due to ibuprofen. New York state journal of medicine. 1978;78:1239–1243. [PubMed] [Google Scholar]
- Tang Y-P, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- Van Hecken A, Schwartz JI, Depre M, De Lepeleire I, Dallob A, Tanaka W, Wynants K, Buntinx A, Arnout J, Wong PH, Ebel DL, Gertz BJ, De Schepper PJ. Comparative inhibitory activity of rofecoxib, meloxicam, diclofenac, ibuprofen, and naproxen on COX-2 versus COX-1 in healthy volunteers. Journal of clinical pharmacology. 2000;40:1109–1120. [PubMed] [Google Scholar]
- Watanabe M, Inoue Y, Sakimura K, Mishina M. Distinct distributions of five N-methyl-D-aspartate receptor channel subunit mRNAs in the forebrain. J Comp Neurol. 1993;338:377–390. doi: 10.1002/cne.903380305. [DOI] [PubMed] [Google Scholar]
- Weggen S, Eriksen JL, Das P, Sagi SA, Wang R, Pietrzik CU, Findlay KA, Smith TE, Murphy MP, Bulter T, Kang DE, Marquez-Sterling N, Golde TE, Koo EH. A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature. 2001;414:212–216. doi: 10.1038/35102591. [DOI] [PubMed] [Google Scholar]
- Wilkinson BL, Cramer PE, Varvel NH, Reed-Geaghan E, Jiang Q, Szabo A, Herrup K, Lamb BT, Landreth GE. Ibuprofen attenuates oxidative damage through NOX2 inhibition in Alzheimer's disease. Neurobiol Aging. 2012;33:197, e121–132. doi: 10.1016/j.neurobiolaging.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki M, Mori H, Araki K, Mori KJ, Mishina M. Cloning, expression and modulation of a mouse NMDA receptor subunit. FEBS Lett. 1992;300:39–45. doi: 10.1016/0014-5793(92)80160-i. [DOI] [PubMed] [Google Scholar]
- Yan Q, Zhang J, Liu H, Babu-Khan S, Vassar R, Biere A, Citron M, Landreth G. Anti-inflammatory drug therapy alters beta-amyloid processing and deposition in an animal model of Alzheimer's disease. J Neurosci. 2003;23:7504–7509. doi: 10.1523/JNEUROSCI.23-20-07504.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong VW, Moumdjian R, Yong FP, Ruijs TC, Freedman MS, Cashman N, Antel JP. Gamma-interferon promotes proliferation of adult human astrocytes in vitro and reactive gliosis in the adult mouse brain in vivo. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:7016–7020. doi: 10.1073/pnas.88.16.7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamzow DR, Elias V, Shumaker M, Larson C, Magnusson KR. An increase in the association of GluN2B containing NMDA receptors with membrane scaffolding proteins was related to memory declines during aging. J Neurosci. 2013;33:12300–12305. doi: 10.1523/JNEUROSCI.0312-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Rosenke R, Kronemann D, Brim B, Das SR, Dunah AW, Magnusson KR. The effects of aging on N-methyl-D-aspartate receptor subunits in the synaptic membrane and relationships to long-term spatial memory. Neuroscience. 2009;162:933–945. doi: 10.1016/j.neuroscience.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Su Y, Li B, Liu F, Ryder JW, Wu X, Gonzalez-DeWhitt PA, Gelfanova V, Hale JE, May PC, Paul SM, Ni B. Nonsteroidal anti-inflammatory drugs can lower amyloidogenic Abeta42 by inhibiting Rho. Science. 2003;302:1215–1217. doi: 10.1126/science.1090154. [DOI] [PubMed] [Google Scholar]
- Zukin RS, Bennett MVL. Alternatively spliced isoforms of the NMDAR1 receptor subunit. Trends in Neurosciences. 1995;18:306–311. doi: 10.1016/0166-2236(95)93920-s. [DOI] [PubMed] [Google Scholar]