Abstract
In principle, proton therapy offers a substantial clinical advantage over the conventional photon therapy. This is because of the unique depth-dose characteristics of protons, which can be exploited to achieve significant reductions in normal tissue doses proximal and distal to the target volume. These may, in turn, allow escalation of tumor doses, greater sparing of normal tissues, thus potentially improving local control and survival while at the same time reducing toxicity and improving quality of life.
Protons, accelerated to therapeutic energies ranging from 70 to 250 MeV, typically with a cyclotron or a synchrotron, are transported to the treatment room where they enter the treatment head mounted on a rotating gantry. The initial thin beams of protons are spread laterally and longitudinally and shaped appropriately to deliver treatments. Spreading and shaping can be achieved by electro-mechanical means to treat the patients with “passively-scattered proton therapy (PSPT); or using magnetic scanning of thin “beamlets” of protons of a sequence of initial energies. The latter technique can be used to treat patients with optimized intensity modulated proton therapy (IMPT), the most powerful proton modality.
Despite the high potential of proton therapy, the clinical evidence supporting the broad use of protons is mixed. It is generally acknowledged that proton therapy is safe, effective and recommended for many types of pediatric cancers, ocular melanomas, chordomas and chondrosarcomas. Although promising results have been and continue to be reported for many other types of cancers, they are based on small studies. Considering the high cost or establishing and operating proton therapy centers, questions have been raised about their cost effectiveness. General consensus is that there is a need to conduct randomized trials and/or collect outcomes data in multi-institutional registries to unequivocally demonstrate the advantage of protons.
Treatment planning and plan evaluation of PSPT and IMPT requires special considerations compared to the processes used for photon treatment planning. The differences in techniques arise from the unique physical properties of protons but are also necessary because of the greater vulnerability of protons to uncertainties, especially from inter- and intra-fractional variations in anatomy. These factors must be considered in designing as well as evaluating treatment plans. In addition to anatomy variations, other sources of uncertainty in dose delivered to the patient include the approximations and assumptions of models used for computing dose distributions for planning of treatments. Furthermore, the relative biological effectiveness (RBE) of protons is simplistically assumed to have a constant value of 1.1. In reality, the RBE is variable and a complex function of energy of protons, dose per fraction, tissue and cell type, end point, etc.
These uncertainties, approximations and current technological limitations of proton therapy may limit the achievement of the true potential of proton therapy. Ongoing research is aimed at better understanding the consequences of the various uncertainties on proton therapy, and reducing the uncertainties image-guidance, adaptive radiotherapy, further study of biological properties of protons, and the development of novel dose computation and optimization methods. However, residual uncertainties will remain in spite of the best efforts. To increase the resilience of dose distributions in the face of uncertainties and improve our confidence in dose distributions seen on treatment plans, robust optimization techniques are being developed and implemented. We assert that, with such research, proton therapy will be a commonly applied radiotherapy modality for most types of solid cancers in the near future.
Graphical Abstract
1 Introduction
1.1 Conventional Radiotherapy of Cancers
Most of the current practice of clinical radiotherapy utilizes photon beams of energies ranging from 4 to 18 megavolt (MV). Less than 1% of the patients world-wide are treated with protons and heavier ions, though the number is increasing as new facilities are established. As illustrated in Figure 1, photon radiation dose as a function depth in the patient rises initially as the electrons ejected by photons build up to a maximum, and then declines exponentially as photons are absorbed. Thus, a photon beam deposits dose from entrance all the way to where it exits from the body. A crossfire arrangement of multiple beams is used to deliver high and curative dose to the tumor target while maintaining the normal tissue doses to below tolerance limits.
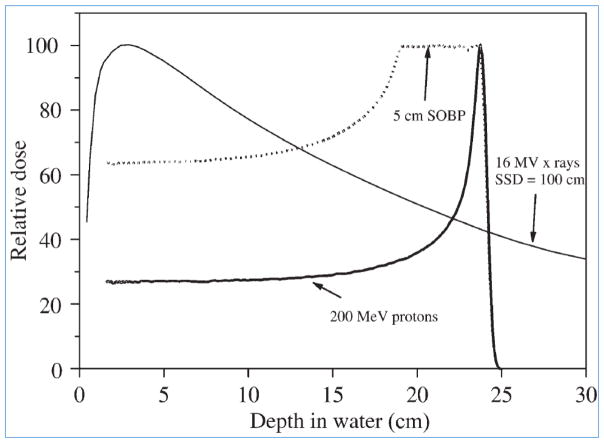
Figure 1.
Depth-dose curves for a 200 MeV proton beam: both unmodulated and with a 5 cm spread-out Bragg peak (SOBP), compared with a 16 MV x-ray beam (for 10 ×10 cm2 fields). The curves are normalized in each case to 100 at maximum dose. (Adapted from Jones, reproduced with permission).[1]
In the mid-1990 s, radiotherapy with photons took a giant leap forward when Intensity modulated photon radiotherapy (IMRT) was introduced. With IMRT, each of a group of broad beams of photons is subdivided into narrow beamlets of cross-sections of the order of ½ cm x ½ cm and delivered using dynamic multi-leaf collimators. Following its introduction over 20 years ago, IMRT has continued to steadily evolve and is now considered both state-of-the-art and standard of care for many malignancies. In IMRT, intensities of the beamlets are adjusted using optimization techniques to appropriately balance the target and normal tissue dose distributions. IMRT allows considerable control to tailor dose distributions to achieve desired clinical objectives. However, given the physical properties of photons, normal tissues surrounding the target volume still receive a substantial amount of unwanted dose, which often limits our ability to deliver curative dose to the tumor without unacceptable normal tissue toxicities.
1.2 Rationale for Proton Therapy
In contrast to photons, when protons of a given energy (typically in the range of 70 to 250 MeV) penetrate matter, they slow down continuously as a function of depth. The rate of their energy loss (called “linear energy transfer” or LET) increases with decreasing velocity. This continues until their entire energy is depleted and then they come to an abrupt stop. This process of dose (energy deposited per unit mass) deposition produces a characteristic depth-dose curve (“Bragg curve”) for a broad monoenergetic beam of protons as illustrated in Figure 1. The point of highest dose is called the Bragg peak. The depth of the peak, i.e., the range of protons, is a function of the initial energy. Dose deposited beyond the range is negligible. As protons traverse a medium, they also scatter laterally but the dose outside the boundary of a beam of protons falls rapidly.
Narrow, monoenergetic beams of protons for therapeutic use can be produced using cyclotrons or synchrotrons as discussed in Section 3. For clinical use, the beams are spread longitudinally (to create a “spread-out Bragg peak” or SOBP, Figure 1) and laterally and then shaped appropriately to conform the high dose regions to the target volume.
1.3 Brief History
The therapeutic potential of the depth-dose characteristics of protons was first recognized in a report by Wilson in 1946.[2] He theorized how proton beams could be used for treating localized cancers. In less than 10 years, the first patient was treated with protons in 1954 employing the synchrocyclotron at the University of California, Berkley.[3] Since then, and until about 1990, a number research accelerators at physics laboratories around the world were adapted for treating cancer patients with protons and, to a small extent, with heavier particles. Most prominent among these laboratories was the Harvard Cyclotron Laboratory (HCL) in Cambridge, Massachusetts, which was originally built for nuclear physics experiments. Under the leadership of Suit and Goitein, a program of proton therapy for several cancer sites was instituted at HCL in 1973.[4] In addition to UC Berkeley and HCL, substantial numbers of patients were treated at Uppsala University, Sweden; Dubna, Russia; and Chiba, Japan.
Physics laboratory-based particle therapy facilities have numerous limitations including beam orientations (typically horizontal beams only), competition for beam-on time, inadequate medical logistics, etc. The first hospital-based proton therapy facility was established in 1990 at the Loma Linda University Medical Center, CA. It included the capability to point proton beams from any direction using isocentric gantries. [5] Approximately 10 years later, Massachusetts General Hospital (MGH)-Harvard University opened the second hospital-based proton therapy center with gantries. It was followed in 2006 by proton therapy centers at MD Anderson Cancer Center (MDACC) in Houston and the University of Florida in Jacksonville. The MDACC Proton Therapy Center is the first one in the US to have scanning beam capability and first in the world to have a two-dimensional scanning beam.[6–9] Soon thereafter, there was a spate of new proton therapy facilities in the US and around the world. According to the PTCOG website (http://www.ptcog.ch), as of December 2014, there were approximately 15 active proton therapy facilities in the US and 15 more under construction or planned. There are many more around the world. As of March 2014, over 110,000 patients worldwide have been treated with protons.
Since the original proposal by Wilson in 1946, accelerator technology has evolved greatly. Currently, most of the proton accelerators in use are cyclotrons and a smaller number are synchrotrons. Each type has advantages and disadvantages discussed in Section 3. The technology of accelerators and ancillary systems, such as gantries and treatment delivery control systems, continues to be further developed to reduce their cost and to make them more compact, efficient and clinically effective.
In addition to the delivery devices, software systems to plan proton treatments and compute and optimize proton dose distributions are also required. Goitein, et al were the first to develop a three-dimensional conformal radiotherapy planning system for protons.[10, 11] For nearly two decades, the state-of-the-art of such systems remained relatively static. Only during the last decade or so, has there been a recognition of the need for further development.
In order to relate our clinical experience with photon treatments to the clinical application of protons and heavier ions, it is necessary to understand the biological effects of the latter. The first studies of the biological effects of particle beams were conducted at the University of California, Berkeley.[12] Subsequently, extensive in-vitro and in-vivo studies to determine the biological effectiveness of protons and other particles relative to photon irradiation (i.e., “relative biological effectiveness” or RBE) have been reported. Results of these studies have been summarized in two review articles by Paganetti et al.[13, 14] In the current practice of proton therapy, an average RBE of 1.1 is used, implying that, across the board, protons are 10% more effective biologically than photons. It is being recognized increasingly that this approximation is not appropriate and its continued use could limit the effectiveness of proton therapy. Biological effect issues of proton therapy are further discussed in Sections 2.2 and 6.2.
More historical details can be found in ICRU Report 78.[15]
2 Principles of Proton Therapy
In order to appreciate the observed characteristics of dose distributions, their therapeutic potential and limitations, and the uniqueness of the methods required for the planning and delivery of proton treatments, it is instructive to understand the fundamental processes underlying the transport of protons through matter.
2.1 Interactions of Protons with Matter
Protons interact with matter primarily through (1) Coulomb interactions with atomic electrons; (2) Coulomb interactions with nuclei; and (3) nuclear interactions. They lose most of their energy through interactions with electrons. Secondary electrons (called “delta rays”) travel very short distances from the path of the proton while ionizing and depositing energy. The energy deposited by a proton per unit distance traveled (the LET) increases inversely as the square of the proton velocity. In a uniform medium, monoenergetic protons will, therefore, travel a well-defined distance, losing energy at an increasing rate as they slow down, before coming to a stop. This leads to the formation of the characteristic Bragg curve shown in Figure 1. Because a proton is much heavier than an electron, its interactions with electrons do not result in an appreciable deviation from its original direction.
When a proton passes close to a nucleus, and if the distance of approach is not too small, it is deflected by Coulomb repulsion, but does not lose any energy. Each deflection may be small, but the accumulation of such deflections, called “multiple Coulomb scattering,” can lead to substantial lateral spreading of protons.
If the distance of approach is small, protons may also undergo scattering with nuclei. The probability of nuclear interactions is small relative to Coulomb interactions. However, it increases with the atomic number of the target nucleus and with the energy of protons. It is estimated that as many as 20% of protons of the highest energies in the therapeutic range undergo nuclear interactions along their path. In nuclear interactions, the primary proton imparts a large fraction of its energy to the nucleus and may scatter through a large angle. Nuclear interactions may be further classified as elastic and non-elastic. In elastic scattering, the nucleus only recoils and the total kinetic energy is conserved. In non-elastic scattering, on the other hand, the target nucleus absorbs some of the energy and may undergo several different types of secondary events such as disintegration into smaller fragments, emission of prompt gamma rays, becoming radioactive, etc. Recoil nuclei and the heavier fragments are absorbed essentially at the point of interaction. However, scattered protons and, especially, the secondary neutrons may travel relatively large distances and produce a “halo” of low dose.
2.2 Biological Effectiveness of Protons
As mentioned above, it is assumed that, biologically, protons are similar to photons. Based on numerous in-vitro and animal experiments, protons have been assumed to have a 10% higher biological effectiveness relative to photons (i.e., RBE of 1.1). In clinical practice, the physical dose, in units of Gy, delivered by protons is multiplied by 1.1 to obtain the biologically effective dose in units of Gy (RBE). However, past experiments were conducted under broad range of inconsistent conditions and have large variability in results. It is increasingly recognized that the current practice of using the average RBE of 1.1 may affect the quality of proton treatments. Further discussion of the biological effectiveness of protons is in Section 6.2.
3 Current Proton Therapy Delivery Mechanisms and Systems
As mentioned above, protons are accelerated to therapeutic energies, typically from 70 to 250 MeV, with cyclotrons or synchrotrons. The higher end of this range is required to reach the maximum depth of tumors encountered in clinical practice. An accelerated proton beam entering the treatment delivery head (the “nozzle”) is very thin and has depth dose characteristics shown as Bragg curve in Figure 1. As such, it is not suitable for treating three-dimensional, arbitrarily-shaped tumor targets. It must be broadened longitudinally and laterally and sculpted to conform to the target shape. There are two main approaches for achieving this: (1) passive scattering to deliver passively-scattered proton therapy (PSPT), and (2) magnetic-scanning of narrow “beamlets” of protons of a sequence of initial energies to deliver intensity-modulated proton therapy (IMPT). There are variations of each mode.
3.1 Proton Accelerators
Most commonly, protons for therapeutic applications are accelerated using a cyclotron (Figure 2) or a synchrotron (Figure 3); each has its advantages and disadvantages. Cyclotrons produce a continuous stream of protons. In theory, they are more compact and have higher beam intensity. Protons are accelerated to the maximum of the energy of the cyclotron (e.g., 230 MeV), and the required lower energies are achieved by electromechanically inserting energy degraders in the path of protons between the accelerator and the treatment room.
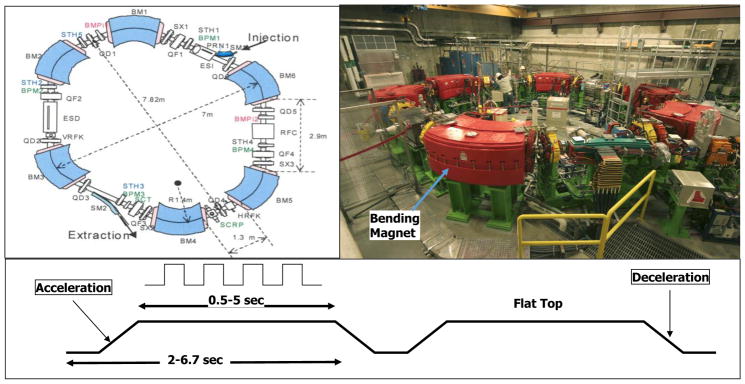
Figure 2.
Acceleration of protons in a cyclotron. A fixed magnetic field bends the path of protons, and they are accelerated by a square wave electric field applied between gaps of two D-shaped regions (known as “Dees”). As energy increases, the radius of the proton path increases until the designated maximum is reached and protons are extracted. Panel on the right shows key components of the cyclotrons. (Adapted from [16].)
Figure 3.
The synchrotron at MD Anderson Proton Therapy Center. A batch of protons is initially accelerated by a linear accelerator to a low energy (7 MeV) and injected into the synchrotron. Protons, as they are accelerated by the successive application of an alternating electric field, are constrained to move in a fixed circular path by increasing the magnetic field. When the batch of protons has reached the specified energy, it is extracted and transmitted to one of the treatment rooms. (Adapted from [16].)
Synchrotrons, on the other hand, accelerate batches (pulses) of protons to the desired energy. Once a batch has reached the required energy, it is extracted and transmitted via the “beam line” (see Figure 5) to the treatment room. The extraction may occur over a variable period of time from 0.5 to 4.5 seconds, depending on the application (Figure 3). The duration of the pulse, i.e., the cycle time, is one to two seconds longer to allow for resetting of the acceleration system between pulses. Each cycle can produce protons of a different energy. Generally, the advantage of synchrotrons is that they have greater energy flexibility, smaller energy spread, and lower power consumption.
Figure 5.
Layout of the treatment floor of MD Anderson s Proton Therapy Center.
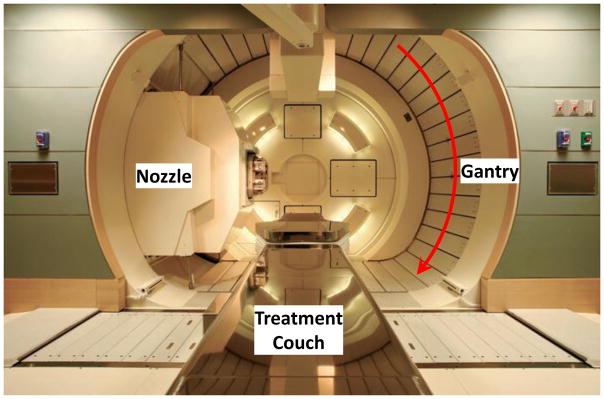
Regardless of the type of accelerator, the extracted narrow monoenergetic beam is magnetically guided through the beam line to the nozzle mounted, in most cases, on a rotating gantry in the treatment room (Figure 4). The gantry is used to aim the beam at the target in the patient lying on a treatment couch. The couch can also be rotated and shifted to achieve optimum beam directions to avoid as much normal tissue as possible.
Figure 4.
Nozzle (treatment head) mounted on a rotating gantry to direct the beam to the tumor in the patient lying on the treatment couch.
A typical proton accelerator serves multiple rooms. The beam is switched automatically from one room to the next based on the order of request and priority. Figure 5 shows the treatment floor configuration at MD Anderson Proton Therapy Center. Considering the high cost of establishing and operating multi-room proton therapy centers, single room systems are now being produced. Such systems were initially introduced by Mevion based on their unique gantry-mounted design. Currently, other vendors also offer single room designs.
3.2 Passively Scattered Proton Therapy
For PSPT, the lateral and longitudinal spreading of the thin beam entering the nozzle is achieved with a combination of a rotating modulation wheel (RMW) and one or two scatterers. Figure 6 shows the schematic of the passive scattering nozzle of the Hitachi proton synchrotron at MDACC and describes its components. Protons of only a small number of discrete initial energies, spanning the therapeutic range, enter the nozzle. The MDACC PSPT system, for instance, uses 8 initial energies from 100 to 250 MeV. An initially monoenergetic beam of the required maximum penetration is modulated using an RMW.
Figure 6.
(a) A passive scattering nozzle. The beam entering the nozzle is spread longitudinally by the range modulator wheel (RMW) and spread laterally by two scatterers; one of them is built into the RMW. The inset (b) illustrates how the RMW interposes steps of different thickness to produce Bragg peaks of different ranges and intensities, which combine to create an SOBP shown in panel (c). The proton beam is turned on at the thinnest step. The width of the Bragg peak can be changed by gating the beam off at an appropriate step. Panels (d) and (e) show a brass aperture and a compensator respectively.
An RMW is like a propeller that interposes steps of different thicknesses of material in the path of protons as it rotates. It has multiple banks of steps of a range of thicknesses. Figure 6(b) shows the design of a Hitachi RMW. It has six banks of steps and rotates at 400 revolutions per minute. The step thicknesses and widths are designed so that the sum of the resulting individual Bragg peaks results in a flat, homogeneous depth dose distributions, the SOBP. The thinnest step corresponds to the deepest penetration and the thickest one corresponds to the most proximal Bragg peak desired. SOBPs of in-between widths may be achieved by turning the incident beam off and on repeatedly at predetermined angles during the wheel s rotation.
In addition to spreading the Bragg peak longitudinally, thin pencils of protons must be spread laterally. This is achieved typically using two scatterers made of high Z materials to produce a broad beam that is flat within the region of interest. Thus, a combination of an RMW and a pair of scatterers produce a uniform cylindrically-shaped dose distribution with relatively low dose proximally and rapidly falling dose distally.
3.2.1 Field Shaping for Passively Scattered Proton Beams
To conform the dose distribution laterally to the shape of the target volume (plus appropriate margins), an aperture, typically made from blocks of brass of sufficient thickness (2 cm to 8 cm) to absorb incident protons of the highest energy, is used (see Figure 6(d)). To create a dose distribution that conforms to the distal shape of the target, the spread-out Bragg peak of the passively scattered beam is shaped further by using a range compensator (Figure 6(e)). A compensator is usually made of a nearly water-equivalent material such as Lucite. It is designed to degrade the beam energy ray-by-ray by variable amounts so that the distal edge of the beam conforms to the shape of the target plus a suitable margin. In computing the compensator thickness at each point, the water-equivalent pathlength to the distal edge of the target along the ray from the source of protons through the compensator point to the distal edge is determined. The surface irregularities and tissue inhomogeneities are taken into consideration in this computation. The compensator is the final element in the nozzle. The air gap between the patient and the compensator is minimized to reduce the penumbra by moving the end of the treatment head (“snout”, Figure 6(a)) close to the patient. The aperture and compensator for each beam are designed by the planning system, and the design information is used to fabricate these devices using computer-controlled milling machines.
Since the SOBP width for a passively scattered beam is designed to be constant across the entire field, passive scattering provides no control over dose distribution proximal to the target. For a target with a highly irregular distal edge, this may lead to a substantial excess volume of high dose proximal to the target.
3.3 Scanning Beams
A more efficient and potentially clinically more effective alternative to the use of RMWs, apertures and compensators to shape the beams is magnetic scanning of thin beamlets of protons. Multiple beams incident from different directions, each comprising the scanning beamlets of a sequence of energies, are used to produce the desired pattern of dose. For each scanned beam, the treatment is delivered in “layers,” one layer per energy. Upon completion of one layer, the energy is changed to the next in the sequence. Compared to passive scattering, beamlets of a much larger number of closely-spaced incident energies are required.
Protons in a beamlet incident on a patient or a phantom are very nearly monoenergetic and are distributed essentially as a narrow Gaussian function of position relative to the beamlet s central axis. The lateral dimension of a beamlet is expressed in terms of the full-width-at-half-maximum (FWHM) of the Gaussian, or its σ. A smaller FWHM is desirable since it allows for a sharper penumbra and a greater control over dose distributions. In air, higher energy proton pencil beams have a smaller FWHM than the lower energy ones. Typically, the smallest achievable FWHMs in air for the highest energies (220 to 250 MeV) range from 7 to 12 mm (or σ of 3 to 5 mm) depending on the vendor and the machine model. Once the pencil beam enters a medium, such as a phantom or a patient, the FWHM increases substantially, especially near the end of the range of protons.
Magnetic scanning of beamlets provides greater flexibility and control for creating the optimum conformal proton dose distribution. In addition, the elimination of mechanical shaping devices (such as apertures and compensators) saves the cost of fabricating them and the time required for the insertion of these devices, obviates the need to enter the treatment room between fields and makes the treatments more efficient. Most importantly, scanned techniques allow the delivery of intensity-modulated proton therapy (IMPT), potentially the most effective form of proton therapy. The positions and intensities (in terms of monitor units) for a matrix of spots within the target volume for each scanned beam are determined by the treatment planning system to achieve acceptable or the best possible approximation of the desired dose distribution. (See Section 4.2.)
Figure 7 shows the schematic of the scanning beam nozzle of the Hitachi proton synchrotron at MDACC and describes its components. Field sizes of up to 30 cm x 30 cm can be achieved with it. There are 94 energies from 72 to 222 MeV in the MDACC Hitachi system.
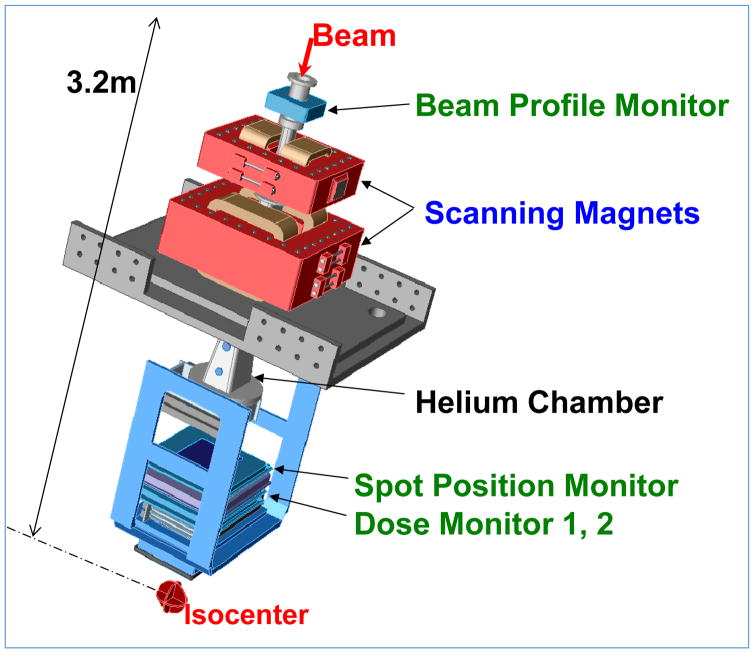
Figure 7.
A Scanning beam nozzle (Hitachi at MDACC). The thin beamlets of a sequence of energies entering the nozzle are spread laterally by a pair of x- and y-magnets to create a three-dimensional pattern of dose distribution. Magnet strengths are adjusted to confine the Bragg peaks of beamlets (“spots”) to within the target volume. Intensities of beamlets, computed using a treatment planning system, are optimized in order to conform the high and uniform dose pattern to the target volume and appropriately spare critical normal tissues. Various monitoring systems ensure that the characteristics of the proton beam are within specifications and that the requisite dose is accurately delivered. Part of the path from the beamlet entry position to the isocenter is replaced with a helium chamber to reduce lateral dispersion of the scanning beamlet in air.
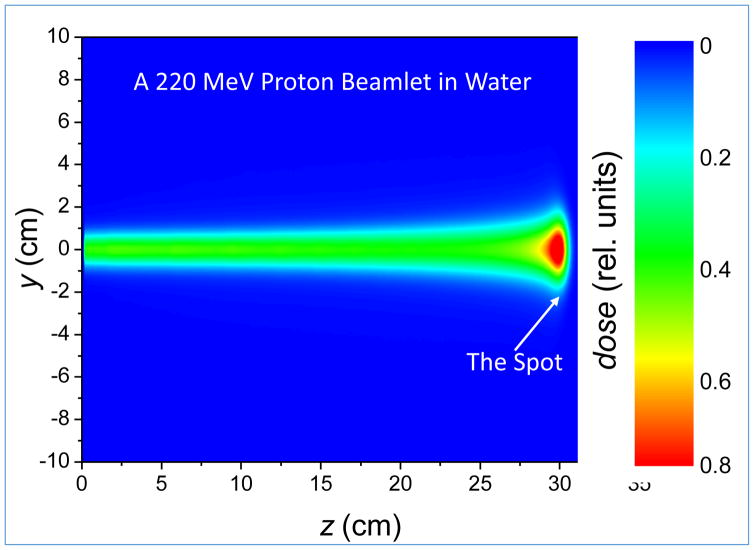
Figure 8 shows the dose distribution of a single beamlet in water for a proton beam of range 30.5 cm (corresponding to an energy of 222 MeV) for the Hitachi proton therapy system at MDACC. The FWHM of the pencil beam at the end of its range in water as a function of energy varies from approximately 18 mm (σ~8 mm) for 222 MeV to 30 mm (σ~13 mm) for 72 MeV. The high dose region at the end of the range of a beamlet is often referred to as a “spot.” The spot size is of special interest for scanned proton beam therapy. It affects the width of the penumbra and limits the fineness of the adjustment of the dose possible to achieve optimum IMPT dose distributions.
Figure 8.
Monoenergetic 222 MeV beamlet with FWHM of ~13 mm at the entrance to the water phantom and ~30 mm at the Bragg peak.
Proton scanning beams have been in use for patient treatments at the Paul Scherrer Institute since 1996, where a one-dimensional scanning of proton pencil beams of different energies in the patient s transverse plane is used. The other dimension is achieved by moving the couch along the patient s longitudinal axis. The first use of two-dimensional scanning occurred in May, 2008 at MDACC, where it is now used routinely. Recognizing the potential of scanning beams, most new proton therapy installations primarily or entirely employ scanning beams. Further research and development of this technology is continuing.
For scanning beams, proximal and lateral field shaping is achieved by limiting the positions of the spots to within the target regions only. Presumably, there is no need for an aperture. However, because of the substantial size of the pencil beam spots, consideration is now being given to the use of dynamic apertures that can change their shapes layer by layer.
4 Proton Treatment Planning and Treatment Plan Evaluation
The significant differences in dose deposition and scattering characteristics of protons and photons mean that many of the formalisms, algorithms and techniques used for photon treatment planning, optimization and plan evaluation are not extensible to protons. The finite range, sharp distal fall-off and scattering characteristics make proton dose distributions more sensitive to inter- and intra-fractional anatomy variations. The computed range of protons in patients is uncertain due to uncertainty in the transformation of Hounsfield Units (HUs) to stopping power ratios (SPRs). The conventional practice in photon therapy is to assign an adequate safety margin to the clinical target volume (CTV) to create a planning target volume (PTV) to ensure that the CTV will receive the prescribed dose in the face of treatment setup and anatomy variations over the course of radiotherapy. For protons, however, uncertainty in the range depends on the depth of point of interest and, therefore, on the direction of each proton beam. Furthermore, anatomic variations perturb the dose distribution within the target volume, not just near the boundaries. Consequently, the conventional practice of assigning CTV-to-PTV margins is not appropriate for the planning and evaluation of proton treatments. Similar margin issues exist for margins for organs at risk.
Another noteworthy difference between protons and photons is that an attenuator placed in the path of photons changes the intensity (number) of photons with only a small effect on the energy spectrum; whereas for protons, the attenuator changes their energy, and, therefore, their range, and has only a small effect on the number of protons.
For proton therapy in general, due to the lower dose proximally and distally to the target, the number of beams needed are typically much smaller than for photons. This is assumed to be an advantage for protons, though, in some aspects, e.g., robustness of dose distributions, it may be a detriment. Preferred beam directions for protons tend to be those that minimize passage through complex tissue heterogeneities and have shorter paths to the distal tumor edge. Furthermore, because of concern about higher biological effectiveness at the end of proton range and uncertainty in proton range, directions, which could potentially lead to higher biologically effective dose to a critical tissue, such as spinal cord, at or just beyond the distal edge of the target, are avoided.
For these reasons, alternative techniques have been and continue to be further developed. Techniques are different for passively-scattered protons and scanning beams and IMPT for reasons that may become apparent from the discussion below.
4.1 Treatment Planning and Plan Evaluation for Passively-Scattered Proton Therapy
For each beam in PSPT, incident energy and range modulation (i.e., SOBP) width are chosen so as to cover the target distally and proximally. Lateral safety margin for uncertainties is assigned to the target volume in the same manner as for photons, whereas proximal and distal margins are assigned to account for range uncertainty.
As described in Section 3.2.1, to conform proton dose distribution laterally and distally, an aperture and a compensator for each beam are designed to conform dose distribution to the lateral and distal shape of the target volume plus margins for uncertainties in setup, anatomy variations and range. The compensator is “smeared” [17] to reduce the sensitivity of proton dose distributions to day-to-day variations in positioning and anatomy (see description in Figure 9).
Figure 9.
Compensator smearing. Panel (a) shows a compensator designed assuming perfect alignment. Panel (b) shows a smeared (expanded) compensator to account for misalignment of the compensator with anatomy and anatomic structures relative to each other. The smearing process essentially reduces the width of the higher thickness regions of the compensator, which allows protons to penetrate more deeply even when adjacent higher density tissues move into their path. Smearing may necessitate an increase in the modulation width to ensure that dose to the proximal edge is not compromised. [17]
Dose distributions for each of the beams are computed by the treatment planning system (TPS) and summed, with appropriate weighting, to produce the composite optimum dose distribution expected to be delivered. In the current state-of-the-art, semi-empirical analytical formalisms and algorithms are used for such computations. The approximations and assumptions of these methods, and of the software systems based on them, contribute to the overall uncertainty in dose distributions delivered.
Computed PSPT dose distributions are evaluated, one beam at a time, by viewing them superimposed on CT image sections. The process involves making certain that, for each beam individually, the distances between the lateral, distal and proximal edges of the CTV and the prescription isodose surface are equal to or greater than the assigned lateral, distal and proximal margins, and at the same time, the distances are not so large as to unnecessarily expose large volumes of normal tissues. A similar process is used to ensure that normal critical structures are at sufficient distances from the high-dose isodose surfaces and are adequately spared.
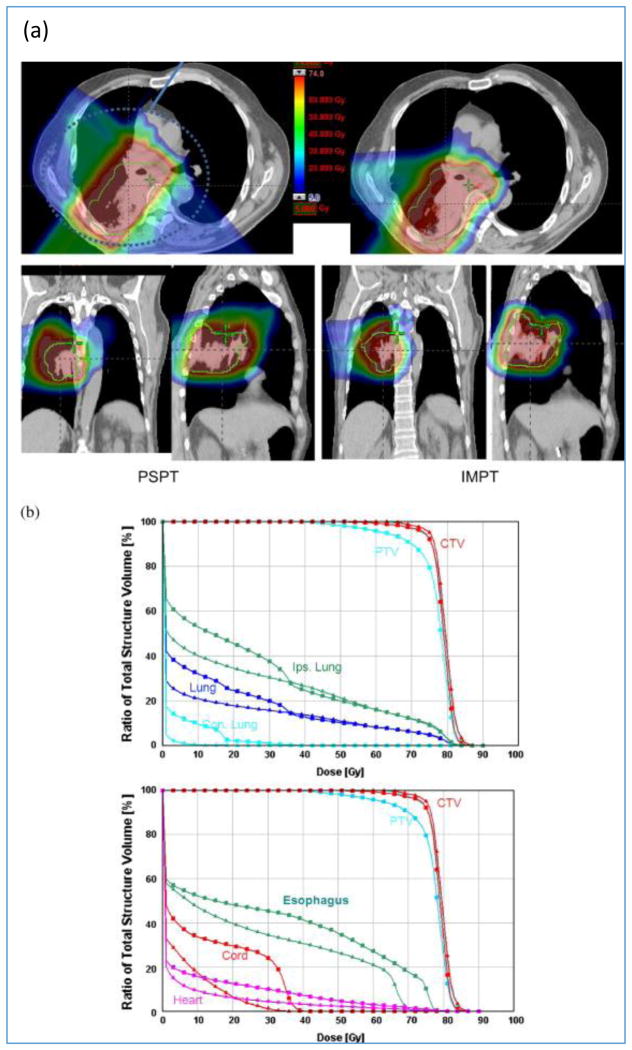
There is also a need to evaluate the composite dose distribution of a plan and to compare composite dose distributions of competing treatment plans. As implied earlier, the concepts of PTV (and the organs at risk volumes, i.e., ORVs) are not strictly valid for proton therapy. Nevertheless, in the current practice, dose-volume histograms (DVHs) of PTVs and ORVs are commonly used. Techniques that are more appropriate for protons are being developed and introduced clinically. Some of them are described in Section 6.4. Figures 10 compare PSPT vs. IMPT (discussed in Section 4.2) dose distributions and DVHs for a lung case.
Figure 10.
(a) PSPT vs. IMPT dose distributions. Due to the requirement of sparing of critical normal structures, adequate coverage of the target could be achieved with PSPT but was possible with IMPT. (b) DVHs for the PSPT (squares) and IMPT (triangles) plans are shown. [18]
For the PSPT of some complex targets, “patched fields” are sometimes employed to maximize sparing of normal tissues. For instance, for the treatment of an “L” shaped target, the distal edge of one field is patched with the lateral boundary of a “through field”. Imperfections in patching may lead to hot and cold spots in dose distributions. To achieve relatively uniform doses at the patch junctions, multiple patched pairs on different days are used to “feather” the junctions. Similarly, the long treatment fields required for craniospinal irradiations need to be divided into multiple fields with junctions. These junction must also be feathered by shifting them from fraction to fraction.
4.2 Treatment Planning and Plan Evaluation for Scanning Beams and IMPT
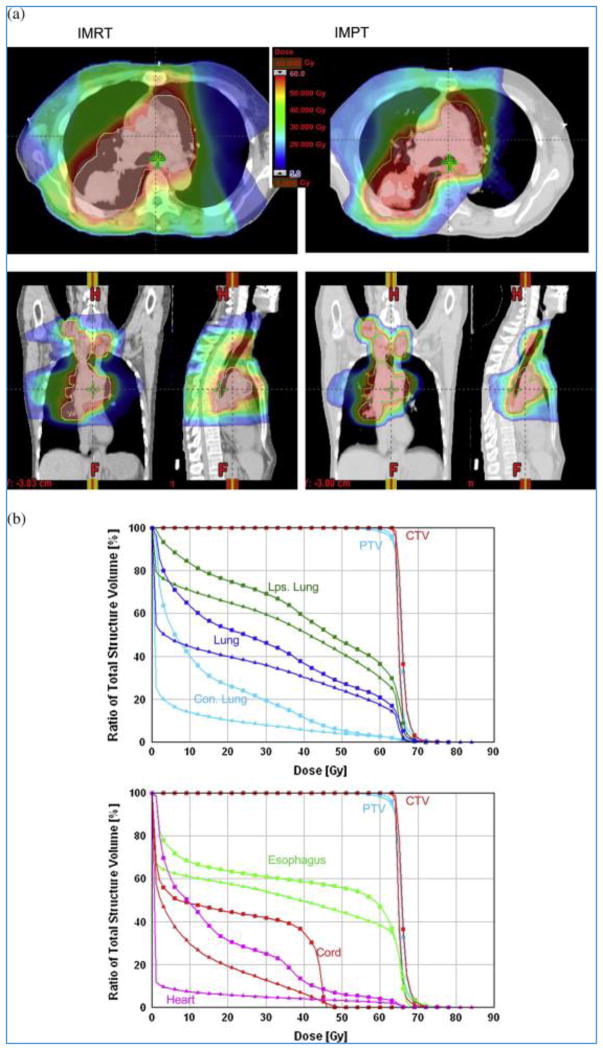
Compared to the scattering and range modulation of proton beams for PSPT, the use of magnetic scanning of beamlets of protons of a sequence of incident energies offers considerable additional flexibility to achieve optimal dose distributions. In particular, it allows the delivery of intensity-modulated proton therapy (IMPT) in which scanning beamlets of protons are used to “paint” radiation dose on the target.[19–23] The flexibility of IMPT can be exploited to safely bend beams around complex critical structures, allowing improved sparing of these structures without compromising target coverage. The energy of the beamlets is varied to paint the target layer-by-layer. The intensities of beamlets comprising multiple scanning beams, aimed at the tumor from different directions, are determined using computer-aided mathematical methods to optimally balance the tumor dose versus the limits of normal tissue exposures. Because of its ability to control proton energies and intensities, the process produces dose distributions that are, in general, vastly superior not only to the corresponding photon-based techniques (i.e., intensity-modulated [photon] radiotherapy or IMRT) but also to PSPT (Figures 10 and 11). While considerable improvement in IMPT technology is possible with ongoing research, thousands of patients have already been treated with it, mainly at Paul Scherrer Institute in Switzerland and MD Anderson Cancer Center in Houston, including prostate, head and neck, CNS, spine, retroperitoneal, pleural and lung. It is expected that IMPT will become the dominant mode of treatments in the near future.
Figure 11.
(a) IMRT vs. IMPT dose distributions. Large dose bath outside the target for IMRT is apparent. (b) DVHs for IMRT (squares) and IMPT (triangles) plans are shown.[18]
The relative dosimetric advantages of lower entrance dose and no dose beyond the range of protons over photons in PSPT are also true of IMPT over IMRT. In numerous treatment planning studies, it has been shown that protons deliver two to three times less energy (i.e., integral dose) outside the target volume than high-energy x-rays.[24] This dose advantage can be used either to increase the tumor dose and, hence, the probability of tumor control, or to reduce morbidity, or an intermediate combination of the two.
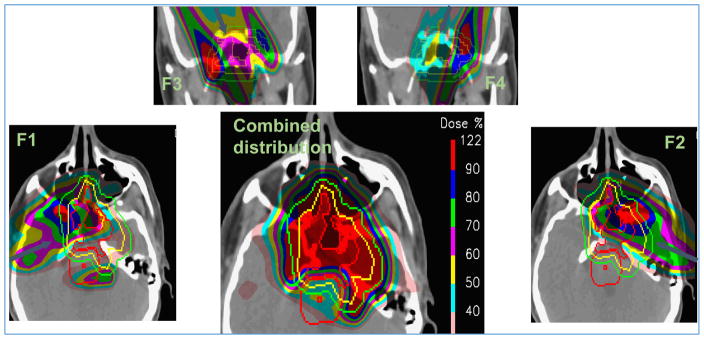
In IMPT, we often aim to achieve homogeneous dose to the target but not necessarily deliver it using SOBPs from each beam direction. The achievement of homogeneous target dose distribution with minimum and optimally balanced normal tissue doses would generally lead to inhomogeneous per-field target dose distributions as illustrated in Figure 12.
Figure 12.
Inhomogeneous individual field IMPT target dose distributions (F1, F2, F3, F4) and a homogenous combined dose distribution for a head and neck case. (Adapted from a figure provided by A. Lomax, PSI, private communication.)
Such highly complex per-beam dose distributions, when combined, fit somewhat like a jigsaw puzzle to create the desired pattern of homogeneous dose distribution in the target and sparing of normal tissues as illustrated in Figure 12. However, in the face of uncertainties (e.g., in range), the fit may be lost, creating hot and/or cold regions. Thus, in general, IMPT dose distributions are more sensitive to (i.e., less robust in the face of) uncertainties in positioning and motion than PSPT dose distributions. For the latter, the dose distribution due to each incident beam is designed independently of other beams to cover the target adequately. It is relatively uniform (in water) and is terminated beyond the distal edge of the target plus a margin. Therefore, the composite dose distribution due to all beams is relatively less sensitive to perturbations. It should also be noted that smearing implicitly improves the robustness of PSPT dose distributions.
To reduce such sensitivity of IMPT to uncertainties, “robust optimization” techniques are being actively investigated (see Section 6.5).
5 Clinical Outcomes
As noted in the introduction, the number of patients treated with photon therapy vastly outnumbers those treated with proton therapy. This is due to the historically low number of centers able to offer proton therapy to patients. Even given these limitations, there is substantial clinical evidence to support the clinical use and continued study of proton therapy. Moreover, as the cost of proton therapy systems falls and clinical interest grows, larger numbers of patients will have access to proton therapy. With this change, it is expected that the clinical data to support the routine use of proton therapy will emerge. To date the majority of clinical evidence supporting the use of proton therapy stems from early stage, non-randomized trials. However, increasingly, randomized trials have either been proposed or are already accruing. Given the increased cost of proton therapy in relation to photons, such evidence is greatly in demand.
Children are particularly susceptible to late adverse effects of radiation, including but not limited to secondary cancers, cardiac disease, endocrinopathies, cognitive dysfunction etc. Given the dramatic reductions in normal tissue exposure using proton therapy and, therefore, the potential for reduction in adverse effects, proton therapy is widely accepted for childhood cancers. While some have voiced concerns that the sharp distal falloff with proton therapy could lead to higher rates of disease recurrence, numerous publications now suggest that disease control and survival rates seen with proton therapy are comparable to those seen with photons. Disease sites studied include medulloblastoma, ependymoma, craniopharyngioma, and rhabdomyosarcoma among others.[25–29] Investigators from MGH, one of the pioneering institutions in proton therapy, have published intriguing results on the incidence of secondary malignancies comparing patients treated with protons vs. matched photon patients.[30, 31] Such studies, albeit non-randomized, suggests a potential reduction in the incidence of secondary malignancies. Given potential ethical as well as practical issues, randomized trials of protons vs. photons in pediatric patients will likely never be seen. However, increasingly within the pediatric radiation oncology community, participation in large multi-institutional registry trials is common. Such trials will further serve to document reductions in late adverse effects.
The second disease site where proton therapy has solidified its presence is for skull based or sino-nasal malignancies. For the treatment of these tumors, high radiation doses are required in order to achieve disease control. However, the close proximity of critical normal tissues, e.g., the brainstem or optic structures, frequently precludes the delivery of such high doses even using the most advanced photon techniques. The physical properties of particle therapy are uniquely suited for the treatment of these challenging cases. Here again, investigators from MGH have published the largest studies of proton therapy with high disease control and acceptable toxicity rates.[32, 33] As noted previously, the majority of patients treated with proton therapy have received passively scattered treatments. Investigators from the Paul Scherrer Institute, the first to clinically implement scanning beam proton therapy, have, however, published excellent outcomes using such techniques for patients with skull based lesions.[34] Early results have also been reported from MD Anderson.[35] For sinonasal tumors, Patel, et al compiled a multi-institutional dataset suggesting improved survival outcomes, a remarkable finding.[36]
Proton therapy also shows particular promise in the treatment of brain tumors. The potential benefits of proton therapy in the treatment of brain tumors center on the reduction of adverse effects, particularly cognitive dysfunction, as well as potential dose escalation for radiation resistant tumors such as glioblastoma. Wenkel, et al studied 46 patients with benign base of skull meningiomas treated with a combination of photons and protons and reported recurrence-free rates of 100% and 88% at 5 and 10 years, respectively.[37] Noel, et al also reported outcomes for 51 patients with base of skull meningiomas treated with a combination of photons and proton therapy. [38] Four-year local control and overall survival rates were 98% and 100% respectively. Weber, et al. from the Paul Scherrer Institute, studied 39 cases treated only with protons.[39] At least 10 patients had WHO Grade II/III meningiomas, and the average tumor volumes were larger than in other series. Five-year local control and overall survival rates were 84.8% and 81.8% for all histology types and 100% for benign histology. The 5-year Grade 3/4 toxicity-free survival was 84.5%. Patients who experienced late-grade toxicities were those with large tumor volumes and optic tract meningiomas. Thus, initial outcomes appear to support the use of particle therapy for meningiomas, especially for lesions in close proximity to critical structures. Proton therapy for low-grade gliomas has also been evaluated. Investigators from the MGH first utilized mixed photon/proton treatments for dose escalation studies including patients with grades II and III gliomas.[40] Investigators from the University of Heidelberg, which employs scanning beam proton delivery technology, have also reported on 19 patients treated for low-grade gliomas. Similar to photon-based treatments, their initial results suggest high rates of tumor control and acceptable toxicity rates.[41] Importantly, in a recent study Shih, et al. reported results of a prospective trial, which enrolled patients with grade II gliomas and assessed cognitive function and quality of life following proton therapy. Twenty patients, all with supratentorial tumors, were enrolled. With a median follow-up of 5.1 years, measures of cognitive function were stable to improved compared to the baseline.[42] For glioblastoma, MGH as well as investigators in Japan have assessed the role of proton therapy for dose escalation with promising results. Such results in combination with other factors have led to the introduction of a multi-institution dose escalation trial employing either IMRT or protons.
Within the United States, the high cost of proton therapy commonly attracts negative media attention when utilized for the treatment of prostate cancer. Numerous other, presumably equally effective, treatment options including brachytherapy, surgery, external beam radiation therapy or even observation exist for prostate cancer patients. While there is not yet a direct comparison of protons to modern photon techniques for prostate cancer, historical studies, including ones evaluating dose escalation, have utilized proton therapy and documented excellent outcomes.[43–46] On the other hand, more recently, SEER-based analyses of prostate data have called into question the utility of proton therapy.[47] However, in interpreting such studies as the Seer study it is important to understand their substantial limitations. In an attempt to provide a direct comparison, a new randomized trial comparing IMRT and protons has begun for patients with prostate cancer.
Perhaps one of the most technically challenging disease sites to treat, particularly for proton therapy, is lung cancer. Recently, a large trial comparing 60 to 74Gy, all done using photon therapy, was completed.[48] Surprisingly, dose escalation was associated with inferior outcomes. Potential reasons for this are many; however, inadvertent increases in lung and heart exposure and the longer duration of 74 Gy radiotherapy have emerged as likely culprits. This highlights the potential promise of proton therapy in reducing normal tissue exposure, allowing for dose and dose per fraction escalation to the target. Initial retrospective and single arm early phase trials suggest excellent toxicity profiles and disease control rates for protons.[49–52] In a first of its kind, a randomized study of IMRT vs. PSPT recently completed accrual. While the results are not yet published, a randomized phase III study is now underway through NRG comparing the two modalities. In the setting of lung cancer it is important to highlight that PSPT has been the modality used to date. Newer delivery modalities, in particular IMPT, may offer substantial dose sparing in comparison to PSPT. Other thoracic malignancies, including esophageal tumors, thymoma and mesothelioma may also benefit. For such lesions, excess dose to the heart and/or lung may also be associated with increase morbidity and, potentially, mortality. Retrospective studies suggest reduced toxicity rates combined with promising disease control rates and further prospective studies, including a randomized trial of protons vs. photons for esophageal cancer, have been initiated.[53]
In addition to esophageal cancer, proton therapy may benefit other gastrointestinal malignancies. For primary hepatocellular carcinoma (HCC), cholangiocarcinoma and isolated hepatic metastases, the normal tissue sparing and, therefore, the dose escalation proton therapy allows, shows great promise in numerous studies from Asia and the US.
Similar to lung, treatment of head and neck malignancies is challenging. In contrast to lung tumors, where issues such as respiratory motion are of concern, with malignancies of the head and neck often times highly conformal dose distributions are required, for example in treatment of the bilateral neck. Frequently, with PSPT, it is difficult to achieve high dose conformality on a par with IMRT. This is not the case with IMPT where both high and low dose conformality are easily achieved even with the most complex target volumes. Initial retrospective results suggest measurably lower toxicities in patients treated with IMPT, and a randomized trial comparing IMPT with IMRT is underway.[54, 55] As the incidence of HPV associated head and neck malignancies in young, never-smokers increases, a reduction in radiation associated toxicities is of paramount importance.
For breast cancer, one of the malignancies most commonly treated with radiation therapy, there are relatively few reports involving proton therapy. However, increasingly there is interest in utilizing proton therapy both for patients having undergone lumpectomy as well as those requiring adjuvant radiation following mastectomy. Initial results have suggested good outcomes.[56, 57] Moreover, hypo-fractionated radiation schedules, gaining increasing popularity using photon treatments, may be ideal for proton therapy and potentially be associated with cost reductions. Similar to other disease sites, here again, large prospective randomized trials are planned in order to formally document the potential benefits of protons, particularly the sparing of cardiac structures, thereby reducing radiation induced adverse effects.
It is notable that there are few randomized trials comparing any type of radiation modality, either photon or particle based. Regardless, as both the availability of proton therapy and the pressure for clinical evidence increases, so does the number of clinical studies. As of September 2014 the Particle Therapy Co-Operative Group (PTCOG) listed nearly 140 clinical protocols of particle therapy. These include randomized studies for cancers of the lung, head and neck, brain, prostate and breast. Finally, on a technical note, looking into the future, it is important to realize that the vast majority of clinical studies published to date have employed PSPT. As the utilization of scanning beam and IMPT increases, it is reasonable to believe that further improvements in clinical outcomes will be made. Indeed, in comparing clinical outcomes, it will be important to compare best photon therapy (IMRT) to best proton therapy (IMPT) in order to document the true clinical benefits of each modality.
6 Current Limitations and Challenges in Proton Therapy and Research and Development to Address Them
While proton therapy is not new, the high cost of establishing and operating proton therapy facilities has constrained the research and development necessary to maximize its clinical effectiveness. Technological advancement has been relatively slow. One of the most important issues, alluded to in Section 4, is the greater sensitivity of proton, in particular IMPT, dose distributions to setup variability, inter-fractional anatomy changes and intra-fractional motion. In contrast, photon, especially IMRT, dose distributions are much more resilient.
Protons are considered to have an advantage from the point of view of their physical characteristics in that they deposit lower dose outside the target volume, i.e., they have lower integral dose in the body as a whole. On the other hand, because of their scattering characteristics, they have a larger penumbra. While a large volume of tissue away from the target may receive considerably lower dose, the dose to the volume of normal tissue immediately surrounding the target volume may be higher for protons. It is possible to reduce the penumbra of beams incident on the patient using apertures and minimizing the spot size; however, there is little that can be done about lateral scattering in the patient.
Furthermore, variations in anatomy in the path of protons during a single fraction and over the course of proton therapy can impair the conformality of dose distributions. This is especially true for treatment sites, such as lung and head and neck, where complex heterogeneities, such as lung and head and neck, are encountered and where it may be difficult to prevent high doses from being delivered to significant volumes of normal tissues distal to the target.
Moreover, in the current practice, the proton RBE is assumed to have a constant value of 1.1 under all circumstances. Once the physical proton dose distribution is multiplied by 1.1, it is assumed to have the same biological and clinical effectiveness as the equivalent photon dose distribution. In reality, the RBE is, in fact, variable. It may be close to 1 (even lower values have been reported) in the entrance regions, and considerably higher than 1.1 at larger depth depending on the LET (which is a function of the residual range of protons), dose per fraction, tissue type, end point, etc. If the region of low RBE happens to be in the tumor volume or the region of high RBE is in normal tissue, the presumed advantage of protons may be lost or may even lead to unanticipated recurrences or toxicities. On the other hand, if a mechanism could be developed to incorporate the dependence of RBE on the multiple variables mentioned, the biological characteristic of protons would offer an additional advantage. This is achievable in theory using IMPT, where it is possible to use optimization techniques to preferentially deposit dose with higher RBE protons in the target and away from normal tissues. While the current detailed knowledge of RBE is limited, it should be sufficient to lead to safe and potentially more effective IMPT.
Thus, as a consequence, the various factors mentioned above may lead to dose distributions used to make treatment decisions that are quite different from the biologically-effective dose distributions actually delivered to the patient over the course of radiotherapy. This may diminish the achievement of the true potential of proton therapy and may lead to a poor correlation of treatment response to planned dose distributions. Fortunately, with the establishment of many more proton therapy centers, research to address the known limitations of proton therapy is accelerating. The following subsections give some examples of uncertainties and their consequences and ongoing research to overcome them.
6.1 Physical Uncertainties
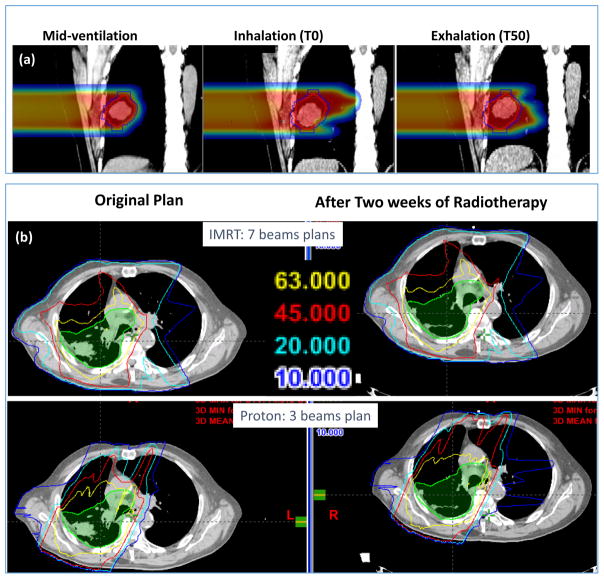
Figure 13(a) illustrates the effect of respiratory motion on dose distribution from end-inhale phase (middle panel) to end-exhale phase (right panel) compared to the mid-ventilation phase. The beam dimension needs to be wide enough to cover the moving tumor, but protons penetrate further depositing high dose into the lung distal to the tumor when it moves. In the current practice of proton therapy, a CT image, averaged over all phases of respiration, is used to compute dose distributions and to design and evaluate treatment plans. Thus, such perturbations do not become obvious.
Figure 13.
(a) shows the impact of the respiratory motion of a lung tumor on the penetration of a proton beam. Figure 13(b) shows that, for a 7-beam IMRT plan, the effect of significant tumor shrinkage after two weeks to treatments is negligible, but it is considerable for a 3-beam passively scattered proton plan (lower panel).
It is also important to note that, for protons, it is not just the motion of the tumor but also the anatomy in the path of the proton beam that can have a significant effect on dose distributions.[25] In contrast, for photons, if the respiration-induced tumor motion is small (e.g., ≤ 5 mm end-to-end), it can safely be assumed that the impact of such motion on dose distribution can be neglected.
Figure 13(b) illustrates the effect of tumor shrinkage during the course of radiotherapy. After two weeks of proton therapy, the tumor has shrunk significantly. Such shrinkage has minimal impact on 7-field IMRT dose distribution. However, protons can penetrate deeper and significantly perturb the dose distribution.
In spite of our best efforts to minimize uncertainties through such techniques as image-guidance, respiratory gating, adaptive replanning to accommodate anatomy changes from fraction-to-fraction, etc., residual uncertainties will remain. It is important to account for them in treatment planning so that there is high confidence that, in the face of uncertainties, the target remains covered by the prescribed dose and normal tissues are adequately spared. This is discussed further in Sections 6.4 and 6.5.
6.2 Dose Computation Algorithms
As stated earlier, computed dose distributions are used for the planning of treatments. Dose distribution displays, dose-volume histograms (DVHs) and dose-volume indices derived from dose distributions are used to make treatment decisions. It is commonly assumed that the dose distribution seen on a treatment plan is actually delivered. Furthermore, dose distributions for cohorts of patients are also used to determine associations between dose and dose-volume indices and dose-response models vs. observed tumor and normal tissue responses. However, in addition to the perturbation of dose distributions by anatomy changes as mentioned in the previous section, approximations and assumptions in algorithms and formalisms used for computing dose distributions introduce additional uncertainty. Some contributing factors include the process of converting CT numbers to proton stopping power ratios (SPRs), assumptions and approximations in dose computation algorithms necessitated for practical reasons, such as achieving adequate speed for the planning of treatments on affordable computers, etc. In particular, when a proton beam passes through a complex heterogeneity, the consequent variations in lateral dispersion can lead to substantial dose heterogeneity. Inadequate management of the lateral dispersion is one of the important weaknesses of the semi-empirical methods of dose computation used in the current treatment planning systems. Ultimately, Monte Carlo techniques (or their abridged or accelerated variations) will be necessary to overcome the limitations of the current models. These are being developed and implemented for clinical use; though obstacles, such as complexity of development, requirements of computing resources, etc. remain to their broad clinical use.
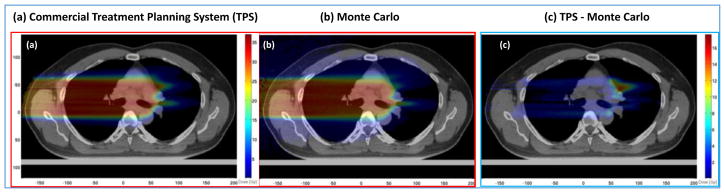
Figure 14 is an illustrative example of the difference between a widely used dose computation model implemented on a commercial treatment planning system and a Monte Carlo system. A carefully commissioned and calibrated Monte Carlo system can be assumed to be the “gold standard” since it simulates the transport of particles (tens of millions of them) based on basic principles of physics.
Figure 14.
Passively scattered proton therapy dose distributions for a non-small cell lung cancer (NSCLC) patient computed with (a) Monte Carlo simulations and (b) a conventional commercial treatment planning system (TPS). Panel (c) shows the TPS – MC difference dose distribution, illustrating the under-dosing in conventional model predictions. Similarly the difference MC – TPS (not shown) would illustrate regions of over-dosing. (Mirkovic, et al. Unpublished, private communication)
6.3 Biological Uncertainties
As mentioned above, the biologic effectiveness of protons relative to photons (i.e., “relative biologic effectiveness” or RBE) is simplistically assumed to have a generic fixed value of 1.1.[14, 58] This value of RBE is based on an average of the results of numerous in vitro and in vivo experiments conducted under varied conditions. Most commonly, these experiments were conducted at high doses per fraction (e.g., 6–8 Gy) and in the middle of the spread-out Bragg peak where the RBE is relatively constant and close to the average value of 1.1. Furthermore, the RBE data acquired so far are for only a limited number of cell lines, tissues, and endpoints. The resulting data also have large error bars.[14] To justify the assumption of the use of the RBE of 1.1, it is argued that, clinically, no adverse responses have been demonstrated with the use of this value. On the other hand, the large uncertainties in the treatment processes may have obscured the effect of not using a variable RBE to calculate biologically effective dose distributions. Furthermore, as mentioned above, a case can be made that an improved knowledge of RBE could lead to improvements in treatment planning, which in turn may enhance the effectiveness of proton therapy. In reality, the biological interactions of protons are considerably more complex than what has been assumed so far in clinical practice. They are a complex function of LET, dose per fraction, tissue and cell type, end point, etc. Thus RBE is variable and increases as a function of depth and is highest at the distal edge. Such variations in RBE are ignored in the current practice. For a passively scattered beam, this might mean that the biologically effective dose near the end of the range is uncertain and higher than what is seen on a treatment plan. For this reason, and because of uncertainties in range, aiming the beams toward a normal critical structure is avoided and special precautions are taken to avoid situations where the distal edge of a proton beam is in or close to a critical normal tissue.
In IMPT dose distributions, the distal edge of each beam does not necessarily conform to the distal edge of the target. Instead, beamlets of individual beams meet inside the target volume. Therefore, the LET and the RBE may be quite different from the values for the PSPT for the same physical dose distribution. In the current state-of-the-art of IMPT optimization, the biological effect would be uncertain to a degree depending on the heterogeneity of the spot intensities.
Models to predict RBE have been published. However, they are simplistic and based on limited measured data. Research is ongoing to improve our understanding of RBE using experiments and computer simulations. Research is also taking place to develop novel models and to improve the accuracy of the current models to predict RBE as a function of tissue type, dose and LET. With the aid of such models, it would be possible to more reliably estimate the impact of variable biological effectiveness on dose distributions. It would also be possible to use optimization methodology to confine protons with higher biological effectiveness to within the tumor target and away from critical normal tissues near the boundary.
Figure 15 is an illustrative example of IMPT optimized based on variable RBE.
Figure 15.
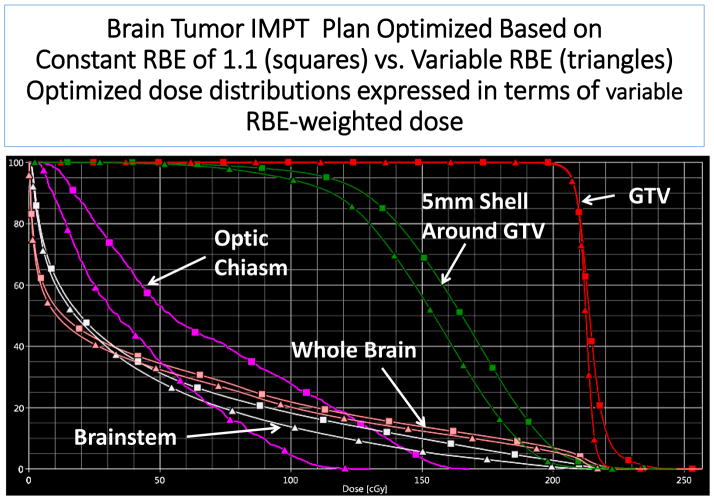
A two-beam IMPT plan for brain tumor optimized based on criteria defined in terms of constant RBE of 1.1 (squares) and in terms of variable RBE computed using a model published by Wilkens, et al. (triangles). After optimization, both dose distributions were converted to variable RBE-weighted dose for comparison. The dose to the gross tumor volume (GTV) is more homogeneous for variable RBE-weighted optimization but lower to normal tissues to varying degrees. Notably, the dose in a 5 mm shell surrounding the GTV is reduced substantially. (Unpublished, Courtesy of Cao, University of Houston.)
6.4 Evaluation of Planned Dose Distributions and their Robustness
While beam-specific margins and smearing are appropriate for designing individual beams for passively-scattered and uniform scanning proton therapy planning, they cannot be integrated in the evaluation and comparison of combined dose distributions from all beams. Nevertheless, for lack of alternatives, displays of combined dose distributions, and the DVHs, TCPs, NTCPs, EUDs, etc. derived from them, are used for both PSPT and IMPT plan evaluation.
A simple strategy for evaluation of proton dose distributions, which is applicable to both PSPT and IMPT, is to examine individual dose distributions and derived indices for each of a set of uncertainty scenarios.[59–63] These scenarios may, for instance, include shifts along the orthogonal axes, range uncertainty, end-inhale and end-exhale phases, etc. The magnitudes of shifts may be chosen to be the same as the margins for CTV to PTV and the magnitudes for range uncertainty may the same as those used for designing PSPT beams. Such a review should reveal deficiencies in a dose distribution in one or more scenarios and steps may be taken to rectify them.
A composite worst case dose distribution may also be obtained, for instance, by assigning to each voxel inside the CTV the minimum dose on any of the scenarios and to each voxel outside the CTV the maximum dose on any of the scenarios. The resulting combined dose distributions and DVHs, representing the worst-case scenario, may then be used to evaluate the dose distribution. Although the worst case scenario may be considered to be overly conservative, it is analogous to the way photon dose distributions are evaluated using dose distributions and DVHs of PTVs and ORVs; the point being that, for instance, the DVH of a PTV represents the worst case dose distribution for the CTV.
It is also useful to assess the resilience, i.e., robustness, of a dose distribution in the face of uncertainties. A simple strategy is to compute dose distributions for each of a sufficiently large set of plausible uncertainty scenarios and plot families of DVHs for each anatomic structure of interest. The band of DVHs for a given structure represents the range of possible dose distributions. Band width at the critical dose-volume points on the DVH (e.g., at volume receiving 20 Gy (RBE) or higher for lung) may be used as a quantitative measure of robustness. (See Figure 16.)
Figure 16.
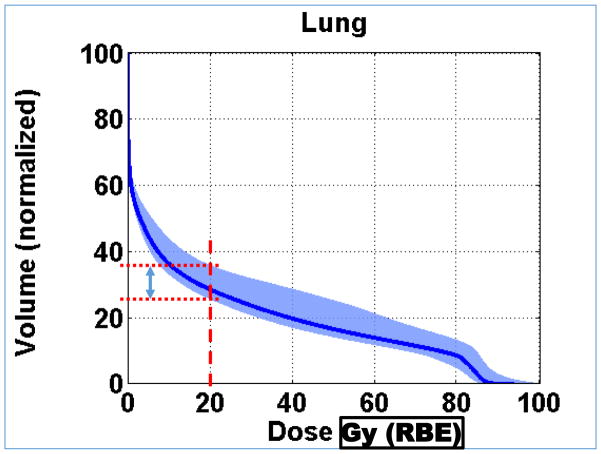
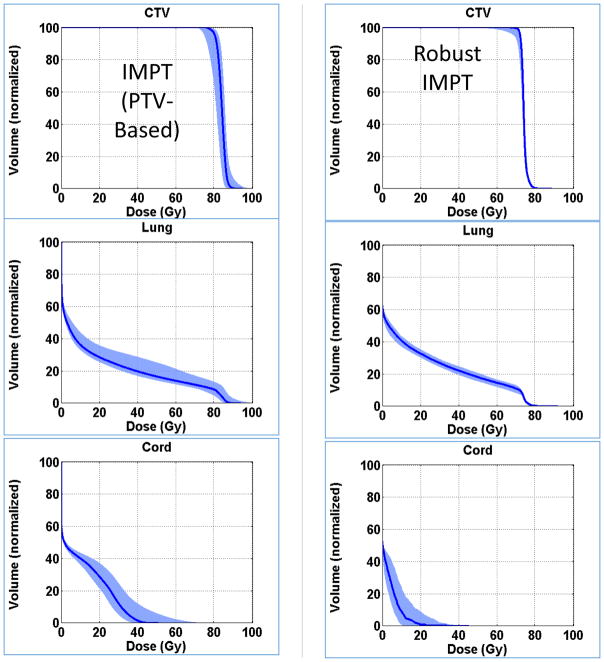
Normal lung (i.e., Lung – CTV) dose volume histograms for a NSCLC IMPT plan optimized based on conventional (i.e., PTV-based) criteria. The blue band represents nine different dose distributions. Six of them were obtained by shifting the patient (i.e., the CT image) along +/− x, y and z directions by margins typically assigned for positioning uncertainty. Two more were obtained by scaling the CT numbers so that the range of protons is increased or decreased by the estimated uncertainty in range. The ninth dose distribution is the nominal dose distribution without considering any uncertainty. Its corresponding lung DVH is represented by the dark blue line. During optimization, the volume receiving 20 Gy (RBE) is constrained to within 37% of the total lung volume. The width of the band at 20 Gy (RBE) along the volume axis is a measure of the robustness of this critical dose-volume index for lung. The worst case DVH will be the upper bound of the band. (Adapted from Liu, et al.[64])
6.5 Robustness Improvement and Robust Optimization
The robustness of proton dose distributions depends on many factors. Plans with larger numbers of beams tend to be more robust. The passage of beams through highly heterogeneous anatomy increases uncertainty and reduces robustness. For dose distributions affected by respiratory motion, there magnitude of the effect often depends on the beam direction. For PSPT, careful selection of treatment parameters can improve robustness.
As mentioned previously, in general, IMPT dose distributions are less robust than PSPT dose distributions, which are less robust than IMRT dose distributions. To overcome the high vulnerability of IMPT to motion and positioning uncertainties, additional measures are required. To this end, IMPT “robust optimization” techniques are being developed and their potential is being investigated.
A typical robust optimization process would simultaneously consider multiple uncertainty scenarios and optimize intensities in the face of all scenarios considered. As an example, it may consider the nominal dose distribution, six dose distributions obtained by shifting the patient image by, for instance, ±5 mm (equal to the CTV-to-PTV margin) along three orthogonal directions, and two additional dose distributions incorporating uncertainty in the range of, for instance, ±3%. The optimization algorithm computes the score (i.e., the value of the objective function to be minimized) in each iteration by selecting the worst dose in each voxel from among the nine scenarios. For the target volume, the worst would be the minimum value and for normal tissues, it would be the maximum value. This is the so-called “voxel-by-voxel” worst-case approach [64–71]. Figure 17 shows an example. Alternate approaches have been proposed and have different strengths [72–74].
Figure 17.
An illustrative example comparing DVHs of NSCLC IMPT plans optimized using two different approaches. The conventional approach used the criteria defined based on PTV dose distributions; whereas the robust optimization used the approach described in the text. Afterwards, the robustness of both dose distributions was evaluated using the approach described in section 6.4. The narrower DVH bands resulting from robust optimization demonstrate its benefit. An interesting byproduct of robust optimization is that, in general, it leads to improved homogeneity of target dose and greater sparing of normal tissues. (Adapted from Liu, et al.[64])
6.6 Technological Limitations
Other challenges and obstacles in the path maximizing the effectiveness of proton therapy are related to the slow pace of development of the technology to-date and the very high cost of establishing and operating proton therapy facilities. The following are some examples of such challenges. Fortunately, commercial vendors (IBA, Hitachi, Varian, others) are now making strong efforts to overcome them.
Spot size: For scanning beams, smaller spot size would allow greater control over deliverable dose distributions. Up until recently, commercial proton therapy systems had incident beamlet spot sizes (σ s) that ranged from about 5 mm for the highest energies (~220 MeV) to about 14 mm for the lowest energies (~70 MeV). Such spot sizes are considered too large to allow effective control over deliverable dose distributions.
Energy switching: Regardless of the accelerator type, it takes of the order of a couple of seconds to change from one energy to another. This is particularly important for IMPT where up to 50 or 60 layers of energy may be required for each beam. This means for a three or four beam IMPT plan, five to six minutes are used just to switch energy in addition to the actual beam on to deliver dose. This is inefficient and prolongs the time the patient needs to stay still on the treatment table.
In-Room Volumetric Image-Guidance: Volumetric imaging, using cone-beam CT or CT-on-Rails, is commonplace in the photon domain but has been rare so far in the proton facilities. It does affect the throughput of patients, thus impacting the facility economically. On the other hand, accurate image-guided setup is much more important for high quality of treatments with protons than with photons.
Respiratory Gating: In spite of the fact that tumor and anatomy motion can affect proton dose distribution to a significantly greater extent, respiratory gating techniques are rarely employed in proton therapy. In general, respiratory gating increases the treatment delivery time by a factor of two to three, which would be a concern at expensive proton facilities.
Dynamic Collimation: As mentioned, the large spots sizes of proton beams can broaden the penumbra of IMPT dose distributions and lead to higher than necessary dose outside the target volume. A solution would be the use of an aperture. However, since the goal of IMPT is to conform the dose three-dimensionally to the target, the collimation would need to be changed, energy layer-by-energy layer, as protons beamlets of different energies paint the target. Hyer, et al have designed an innovative dynamic collimation system exactly for such purposes.[75]
High Cost: Current proton therapy facilities with three to four treatment rooms cost well over a $100 million. A single room facility costs of the order of $30 million. These costs are an order of magnitude higher than the cost of a high-end photon treatment unit. Numerous efforts are underway to develop novel, lower cost compact accelerators and gantries based on innovative designs and super-conducting magnets.
Particles other than Protons: Of the various particles that can potentially be used for radiotherapy, protons are by far the most prevalent. However, as explained earlier, protons scatter substantially limiting the control over dose distributions and producing a relatively wide penumbra. Heavier charged particles such as helium and carbon scatter much less laterally. They, carbon in particular, also have higher RBE. Heavier particles also means more costly accelerators and gantries. Carbon and heavier ions also have a significant fragmentation tail beyond the distal fall-off. At this point it is not clear which particle would be optimum overall from the point of view of physical and biological characteristics and cost. Further research is needed.
7 Summary
Dose deposition characteristics of protons imply that, in principle, it is possible to achieve higher tumoricidal dose for the same or lower normal tissue doses. Furthermore, with IMPT, the additional degree of freedom, that of energy, offers a crucial ability to optimally balance tumor and normal tissue doses. However, despite the high promise of proton therapy, and despite the fact that well in excess of 100,000 patients have been treated with protons, the clinical evidence for protons has been mixed.
In the recent past, there have been concerns expressed about the high cost of proton therapy considering the limited convincing favorable clinical evidence. For instance, Brada, et al performed a systematic review and analysis of published clinical results of proton therapy in 2007 and “found no convincing evidence that protons are superior to photons.”[76] Five years later, De Ruysscher, et al updated the findings of Brada, et al and confirmed that Brada s conclusions still stand and that “except for rare indications such as childhood cancer, the gain from introducing proton therapies into clinical practice remains controversial.”[77]
However, ASTRO s Emerging Technology Committee report about the evidence on proton therapy, published in 2012, [78] concluded that there is evidence of benefit of proton therapy over photon therapy in large ocular melanomas, chordomas and chondrosarcomas. Furthermore, in pediatric CNS malignancies there is “a suggestion” that proton therapy is superior to photon therapy, but there is insufficient supporting data. In HCC and prostate cancer, there is evidence of efficacy but no suggestion of superiority. The report also concluded that the current data do not provide sufficient evidence of benefit for lung, head and neck cancer, GI (except for HCC) and pediatric non-CNS malignancies. It recommended clinical trials for these and other disease sites to ascertain the potential of protons.
More recently, a 2014 ASTRO Model Policy document on proton beam therapy states Proton Beam Therapy (PBT) “is considered reasonable in instances where sparing the surrounding normal tissue cannot be adequately achieved with photon-based radiotherapy and is of added clinical benefit to the patient.”[79] Based on the medical necessity requirements, stated in the document, and published data, the policy supports the use of PBT for several tumors such as ocular tumors, including ocular melanomas; base-of-skull chordomas and chondrosarcomas; tumors of the spine where spinal cord tolerance may be exceeded with conventional radiotherapy; primary HCC treated with hypofractionation; etc. The policy also supports the use of PBT for a number of other sites, e.g., head and neck, thoracic, abdominal and some pelvic malignancies, for the generation of clinical evidence in IRB-approved clinical trials or in multi-institutional patient registries adhering to Medicare requirements.
As the number of institutions practicing proton therapy increases, more positive results, though based on small studies, are being reported as detailed in Section 5. However, considerable additional and higher level evidence is needed. An obvious question is “Why has the clear advantage of protons in principle not translated into broad unequivocal clinical evidence?” The answer may lie in the multiple issues discussed in Section 6. These include the still maturing technology and limited experience with proton therapy up to now; the greater uncertainty in delivered biologically-effective dose distributions due to such factors as inter-fractional changes, intra-fractional motion and setup variability; the approximations and assumptions of dose computation methods; the assumption of constant RBE of 1.1; etc. Many of the limitations of proton therapy and concerns about them have been known for several decades.[17, 80–82] However, even in the face of such limitations, the gap between protons and photons at that time was sufficiently large that protons could be assumed to be superior. That is no longer the case. Over the last three decades, photon therapy has advanced considerably. In particular, IMRT was introduced in mid-1990s and has continued to evolve steadily. In addition, there has been continued enhancement in ancillary imaging, treatment planning and delivery technologies. In contrast proton therapy state-of-the-art had not advance significantly from the 1980 s through the middle of the last decade. Thus, the gap between photon therapy and proton therapy had essentially vanished.
In order to demonstrate the true potential of proton therapy, there is a need for further research and development. Over the last decade or so, this effort has been accelerating. With the availability of advanced and sophisticated imaging and treatment planning tools, research has started to quantify the consequences of the impact of uncertainties on proton therapy. Furthermore, although PSPT typically has an advantage in low-dose sparing due to the reduced integral dose compared to IMRT, PSPT shows poor high dose conformality. Only IMPT can effectively compete with IMRT. IMPT is still in its early stages of development, but many technical and biological issues are being addressed. In addition, clinical trials directly comparing IMRT and IMPT, rather than PSPT vs. IMRT, are needed and, as stated in Section 5, are being initiated.
Moreover, as alluded to in Section 6, the use of MC techniques to improve the accuracy, reduction in uncertainties through improved image guidance, incorporation of residual uncertainties in robust optimization to improve confidence in delivered dose distributions should lead considerable enhancement in IMPT. Efforts are also underway to experimentally acquire large amounts of biological response data for protons and to develop reliable models for predicting RBE. It is anticipated that ongoing clinical trials, especially randomized IMRT vs. IMPT ones, will provide data to correlate treatment responses with dose distributions and lead to improved understanding of various issues related to proton therapy and, therefore, to its further enhancement. Thus, in spite of the current concerns, the future of proton therapy is promising.
Acknowledgments
Supported in part by NCI U19 CA021239
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Radhe Mohan, Department of Radiation Physics.
David Grosshans, Department of Radiation Oncology, MD Anderson Cancer Center, Houston, TX 77030.
References
- 1.Jones DTL. Present status and future trends of heavy particle radiotherapy. In: Baron E, Lieuvin M, editors. Cyclotrons and Their Applications 1998. Institute of Physics Publishing; Bristol, UK: 1999. pp. 13–20. [Google Scholar]
- 2.Wilson RR. Radiological use of fast protons. Radiology. 1946;47:487–491. doi: 10.1148/47.5.487. [DOI] [PubMed] [Google Scholar]
- 3.Lawrence JH, Tobias CA, Born JL, Mc CR, Roberts JE, Anger HO, Low-Beer BV, Huggins CB. Pituitary irradiation with high-energy proton beams: a preliminary report. Cancer research. 1958;18:121–134. [PubMed] [Google Scholar]
- 4.Suit HD, Goitein M, Tepper J, Koehler AM, Schmidt RA, Schneider R. Explorotory study of proton radiation therapy using large field techniques and fractionated dose schedules. Cancer. 1975;35:1646–1657. doi: 10.1002/1097-0142(197506)35:6<1646::aid-cncr2820350626>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 5.Coutrakon G, Hubbard J, Johanning J, Maudsley G, Slaton T, Morton P. A performance study of the Loma Linda proton medical accelerator. Med Phys. 1994;21:1691–1701. doi: 10.1118/1.597270. [DOI] [PubMed] [Google Scholar]
- 6.Gillin MT, Sahoo N, Bues M, Ciangaru G, Sawakuchi G, Poenisch F, Arjomandy B, Martin C, Titt U, Suzuki K, Smith AR, Zhu XR. Commissioning of the discrete spot scanning proton beam delivery system at the University of Texas M.D. Anderson Cancer Center, Proton Therapy Center, Houston. Med Phys. 2010;37:154–163. doi: 10.1118/1.3259742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith AR. Vision 20/20: proton therapy. Med Phys. 2009;36:556–568. doi: 10.1118/1.3058485. [DOI] [PubMed] [Google Scholar]
- 8.Smith A, Gillin M, Bues M, Zhu XR, Suzuki K, Mohan R, Woo S, Lee A, Komaki R, Cox J, Hiramoto K, Akiyama H, Ishida T, Sasaki T, Matsuda K. The M. D. Anderson proton therapy system. Med Phys. 2009;36:4068–4083. doi: 10.1118/1.3187229. [DOI] [PubMed] [Google Scholar]
- 9.Smith AR. Proton therapy. Phys Med Biol. 2006;51:R491–504. doi: 10.1088/0031-9155/51/13/R26. [DOI] [PubMed] [Google Scholar]
- 10.Goitein M, Abrams M. Multi-Dimensional Treatment Planning: I. Delineation of Anatomy. Int J Radiat Oncol Biol Phys. 1983;9:777–787. doi: 10.1016/0360-3016(83)90002-0. [DOI] [PubMed] [Google Scholar]
- 11.Goitein M, Abrams M, Rowell D, Pollari H, Wiles J. Multi-Dimensional Treatment Planning: II. Beam's Eye-View, Back Projection, and Projection through CT Sections. Int J Radiat Oncol Biol Phys. 1983;9:789–797. doi: 10.1016/0360-3016(83)90003-2. [DOI] [PubMed] [Google Scholar]
- 12.Tobias CA, Anger HO, Lawrence JH. Radiological use of high energy deuterons and alpha particles, The American journal of roentgenology, radium therapy. and nuclear medicine. 1952;67:1–27. [PubMed] [Google Scholar]
- 13.Paganetti H. Nuclear interactions in proton therapy: dose and relative biological effect distributions originating from primary and secondary particles. Phys Med Biol. 2002;47:747–764. doi: 10.1088/0031-9155/47/5/305. [DOI] [PubMed] [Google Scholar]
- 14.Paganetti H. Relative biological effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose, and linear energy transfer. Phys Med Biol. 2014;59:R419–472. doi: 10.1088/0031-9155/59/22/R419. [DOI] [PubMed] [Google Scholar]
- 15.ICRU-78-Report-Committee. ICRU Report 78, Prescribing, Recording, and Reporting Proton Beam Therapy. Journal of the ICRU. 2007;7 [Google Scholar]
- 16.Mohan R, Bortfeld T. Proton therapy: clinical gains through current and future treatment programs. Frontiers of radiation therapy and oncology. 2011:440–464. doi: 10.1159/000322509. [DOI] [PubMed] [Google Scholar]
- 17.Urie M, Goitein M, Wagner M. Compensating for heterogeneities in proton radiation therapy. Phys Med Biol. 1984;29:553–566. doi: 10.1088/0031-9155/29/5/008. [DOI] [PubMed] [Google Scholar]
- 18.Chang JY, Komaki R, Lu C, Wen HY, Allen PK, Tsao A, Gillin M, Mohan R, Cox JD. Phase 2 study of high-dose proton therapy with concurrent chemotherapy for unresectable stage III nonsmall cell lung cancer. Cancer. 2011;117:4707–4713. doi: 10.1002/cncr.26080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lomax A. Intensity modulation methods for proton radiotherapy. Phys Med Biol. 1999;44:185–205. doi: 10.1088/0031-9155/44/1/014. [DOI] [PubMed] [Google Scholar]
- 20.Lomax AJ, Boehringer T, Coray A, Egger E, Goitein G, Grossmann M, Juelke P, Lin S, Pedroni E, Rohrer B, Roser W, Rossi B, Siegenthaler B, Stadelmann O, Stauble H, Vetter C, Wisser L. Intensity modulated proton therapy: a clinical example. Med Phys. 2001;28:317–324. doi: 10.1118/1.1350587. [DOI] [PubMed] [Google Scholar]
- 21.Pedroni E, Bohringer T, Coray A, Egger E, Grossmann M, Lin S, Lomax A, Goitein G, Roser W, Schaffner B. Initial experience of using an active beam delivery technique at PSI. Strahlenther Onkol. 1999;175(Suppl 2):18–20. doi: 10.1007/BF03038879. [DOI] [PubMed] [Google Scholar]
- 22.Cella L, Lomax A, Miralbell R. New techniques in hadrontherapy: intensity modulated proton beams. Phys Med. 2001;17(Suppl 1):100–102. [PubMed] [Google Scholar]
- 23.Lomax AJ, Pedroni E, Rutz H, Goitein G. The clinical potential of intensity modulated proton therapy. Z Med Phys. 2004;14:147–152. doi: 10.1078/0939-3889-00217. [DOI] [PubMed] [Google Scholar]
- 24.Lomax AJ, Bortfeld T, Goitein G, Debus J, Dykstra C, Tercier PA, Coucke PA, Mirimanoff RO. A treatment planning inter-comparison of proton and intensity modulated photon radiotherapy. Radiother Oncol. 1999;51:257–271. doi: 10.1016/s0167-8140(99)00036-5. [DOI] [PubMed] [Google Scholar]
- 25.Jimenez RB, Sethi R, Depauw N, Pulsifer MB, Adams J, McBride SM, Ebb D, Fullerton BC, Tarbell NJ, Yock TI, Macdonald SM. Proton Radiation Therapy for Pediatric Medulloblastoma and Supratentorial Primitive Neuroectodermal Tumors: Outcomes for Very Young Children Treated With Upfront Chemotherapy. Int J Radiat Oncol Biol Phys. 2013;21:017. doi: 10.1016/j.ijrobp.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 26.Bishop AJ, Greenfield B, Mahajan A, Paulino AC, Okcu MF, Allen PK, Chintagumpala M, Kahalley LS, McAleer MF, McGovern SL, Whitehead WE, Grosshans DR. Proton beam therapy versus conformal photon radiation therapy for childhood craniopharyngioma: multi-institutional analysis of outcomes, cyst dynamics, and toxicity. Int J Radiat Oncol Biol Phys. 2014;90:354–361. doi: 10.1016/j.ijrobp.2014.05.051. doi:310.1016/j.ijrobp.2014.1005.1051. Epub 2014 Jul 1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ladra MM, Szymonifka JD, Mahajan A, Friedmann AM, Yong Yeap B, Goebel CP, MacDonald SM, Grosshans DR, Rodriguez-Galindo C, Marcus KJ, Tarbell NJ, Yock TI. Preliminary results of a phase II trial of proton radiotherapy for pediatric rhabdomyosarcoma. J Clin Oncol. 2014;32:3762–3770. doi: 10.1200/JCO.2014.56.1548. doi:3710.1200/JCO.2014.3756.1548. Epub 2014 Oct 3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGovern SL, Okcu MF, Munsell MF, Kumbalasseriyil N, Grosshans DR, McAleer MF, Chintagumpala M, Khatua S, Mahajan A. Outcomes and acute toxicities of proton therapy for pediatric atypical teratoid/rhabdoid tumor of the central nervous system. Int J Radiat Oncol Biol Phys. 2014;90:1143–1152. doi: 10.1016/j.ijrobp.2014.08.354. doi:1110.1016/j.ijrobp.2014.1108.1354. Epub 2014 Oct 1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sethi RV, Giantsoudi D, Raiford M, Malhi I, Niemierko A, Rapalino O, Caruso P, Yock TI, Tarbell NJ, Paganetti H, Macdonald SM. Patterns of failure after proton therapy in medulloblastoma; linear energy transfer distributions and relative biological effectiveness associations for relapses. Int J Radiat Oncol Biol Phys. 2014;88:655–663. doi: 10.1016/j.ijrobp.2013.11.239. [DOI] [PubMed] [Google Scholar]
- 30.Chung CS, Yock TI, Nelson K, Xu Y, Keating NL, Tarbell NJ. Incidence of second malignancies among patients treated with proton versus photon radiation. Int J Radiat Oncol Biol Phys. 2013;87:46–52. doi: 10.1016/j.ijrobp.2013.04.030. [DOI] [PubMed] [Google Scholar]
- 31.Sethi RV, Shih HA, Yeap BY, Mouw KW, Petersen R, Kim DY, Munzenrider JE, Grabowski E, Rodriguez-Galindo C, Yock TI, Tarbell NJ, Marcus KJ, Mukai S, Macdonald SM. Second nonocular tumors among survivors of retinoblastoma treated with contemporary photon and proton radiotherapy. Cancer. 2014;120:126–133. doi: 10.1002/cncr.28387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Debus J, Hug EB, Liebsch NJ, O'Farrel D, Finkelstein D, Efird J, Munzenrider JE. Brainstem tolerance to conformal radiotherapy of skull base tumors. Int J Radiat Oncol Biol Phys. 1997;39:967–975. doi: 10.1016/s0360-3016(97)00364-7. [DOI] [PubMed] [Google Scholar]
- 33.Fagundes MA, Hug EB, Liebsch NJ, Daly W, Efird J, Munzenrider JE. Radiation therapy for chordomas of the base of skull and cervical spine: patterns of failure and outcome after relapse. Int J Radiat Oncol Biol Phys. 1995;33:579–584. doi: 10.1016/0360-3016(95)02014-3. [DOI] [PubMed] [Google Scholar]
- 34.Ares C, Hug EB, Lomax AJ, Bolsi A, Timmermann B, Rutz HP, Schuller JC, Pedroni E, Goitein G. Effectiveness and safety of spot scanning proton radiation therapy for chordomas and chondrosarcomas of the skull base: first long-term report. Int J Radiat Oncol Biol Phys. 2009;75:1111–1118. doi: 10.1016/j.ijrobp.2008.12.055. doi:1110.1016/j.ijrobp.2008.1112.1055. Epub 2009 Apr 1120. [DOI] [PubMed] [Google Scholar]
- 35.Grosshans DR, Zhu XR, Melancon A, Allen PK, Poenisch F, Palmer M, McAleer MF, McGovern SL, Gillin M, DeMonte F, Chang EL, Brown PD, Mahajan A. Spot scanning proton therapy for malignancies of the base of skull: treatment planning, acute toxicities, and preliminary clinical outcomes. Int J Radiat Oncol Biol Phys. 2014;90:540–546. doi: 10.1016/j.ijrobp.2014.07.005. doi:510.1016/j.ijrobp.2014.1007.1005. Epub 2014 Sep 1026. [DOI] [PubMed] [Google Scholar]
- 36.Patel SH, Wang Z, Wong WW, Murad MH, Buckey CR, Mohammed K, Alahdab F, Altayar O, Nabhan M, Schild SE, Foote RL. Charged particle therapy versus photon therapy for paranasal sinus and nasal cavity malignant diseases: a systematic review and meta-analysis. Lancet Oncol. 2014;15:1027–1038. doi: 10.1016/S1470-2045(14)70268-2. doi:1010.1016/S1470-2045(1014)70268-70262. Epub 72014 Jun 70226. [DOI] [PubMed] [Google Scholar]
- 37.Wenkel E, Thornton AF, Finkelstein D, Adams J, Lyons S, De La Monte S, Ojeman RG, Munzenrider JE. Benign meningioma: partially resected, biopsied, and recurrent intracranial tumors treated with combined proton and photon radiotherapy. Int J Radiat Oncol Biol Phys. 2000;48:1363–1370. doi: 10.1016/s0360-3016(00)01411-5. [DOI] [PubMed] [Google Scholar]
- 38.Noel G, Feuvret L, Calugaru V, Dhermain F, Mammar H, Haie-Meder C, Ponvert D, Hasboun D, Ferrand R, Nauraye C, Boisserie G, Beaudre A, Gaboriaud G, Mazal A, Habrand JL, Mazeron JJ. Chordomas of the base of the skull and upper cervical spine. One hundred patients irradiated by a 3D conformal technique combining photon and proton beams. Acta Oncol. 2005;44:700–708. doi: 10.1080/02841860500326257. [DOI] [PubMed] [Google Scholar]
- 39.Weber DC, Schneider R, Goitein G, Koch T, Ares C, Geismar JH, Schertler A, Bolsi A, Hug EB. Spot scanning-based proton therapy for intracranial meningioma: long-term results from the Paul Scherrer Institute. Int J Radiat Oncol Biol Phys. 2012;83:865–871. doi: 10.1016/j.ijrobp.2011.08.027. doi:810.1016/j.ijrobp.2011.1008.1027. Epub 2011 Dec 1012. [DOI] [PubMed] [Google Scholar]
- 40.Fitzek MM, Thornton AF, Harsh Gt, Rabinov JD, Munzenrider JE, Lev M, Ancukiewicz M, Bussiere M, Hedley-Whyte ET, Hochberg FH, Pardo FS. Dose-escalation with proton/photon irradiation for Daumas-Duport lower-grade glioma: results of an institutional phase I/II trial. Int J Radiat Oncol Biol Phys. 2001;51:131–137. doi: 10.1016/s0360-3016(01)01589-9. [DOI] [PubMed] [Google Scholar]
- 41.Hauswald H, Rieken S, Ecker S, Kessel KA, Herfarth K, Debus J, Combs SE. First experiences in treatment of low-grade glioma grade I and II with proton therapy. Radiat Oncol. 2012;7:189. doi: 10.1186/1748-1717X-1187-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shih HA, Sherman JC, Nachtigall LB, Colvin MK, Fullerton BC, Daartz J, Winrich BK, Batchelor TT, Thornton LT, Mancuso SM, Saums MK, Oh KS, Curry WT, Loeffler JS, Yeap BY. Proton therapy for low-grade gliomas: Results from a prospective trial. Cancer. 2015:29237. doi: 10.1002/cncr.29237. [DOI] [PubMed] [Google Scholar]
- 43.Rossi CJ. Conformal proton beam therapy of prostate cancer--update on the Loma Linda University medical center experience. Strahlenther Onkol. 1999;175:82–84. doi: 10.1007/BF03038897. [DOI] [PubMed] [Google Scholar]
- 44.Shipley WU, Verhey LJ, Munzenrider JE, Suit HD, Urie MM, McManus PL, Young RH, Shipley JW, Zietman AL, Biggs PJ, et al. Advanced prostate cancer: the results of a randomized comparative trial of high dose irradiation boosting with conformal protons compared with conventional dose irradiation using photons alone. Int J Radiat Oncol Biol Phys. 1995;32:3–12. doi: 10.1016/0360-3016(95)00063-5. [DOI] [PubMed] [Google Scholar]
- 45.Slater JD, Rossi CJ, Jr, Yonemoto LT, Bush DA, Jabola BR, Levy RP, Grove RI, Preston W, Slater JM. Proton therapy for prostate cancer: the initial Loma Linda University experience. Int J Radiat Oncol Biol Phys. 2004;59:348–352. doi: 10.1016/j.ijrobp.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 46.Zietman AL, Bae K, Slater JD, Shipley WU, Efstathiou JA, Coen JJ, Bush DA, Lunt M, Spiegel DY, Skowronski R, Jabola BR, Rossi CJ. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: long-term results from proton radiation oncology group/american college of radiology 95–09. J Clin Oncol. 2010;28:1106–1111. doi: 10.1200/JCO.2009.25.8475. doi:1110.1200/JCO.2009.1125.8475. Epub 2010 Feb 1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sheets NC, Goldin GH, Meyer AM, Wu Y, Chang Y, Sturmer T, Holmes JA, Reeve BB, Godley PA, Carpenter WR, Chen RC. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA. 2012;307:1611–1620. doi: 10.1001/jama.2012.460. doi:1610.1001/jama.2012.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bradley JD, Paulus R, Komaki R, Masters G, Blumenschein G, Schild S, Bogart J, Hu C, Forster K, Magliocco A, Kavadi V, Garces YI, Narayan S, Iyengar P, Robinson C, Wynn RB, Koprowski C, Meng J, Beitler J, Gaur R, Curran W, Jr, Choy H. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16:187–199. doi: 10.1016/S1470-2045(14)71207-0. doi:110.1016/S1470-2045(1014)71207-71200. Epub 72015 Jan 71216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bush DA, Slater JD, Shin BB, Cheek G, Miller DW, Slater JM. Hypofractionated proton beam radiotherapy for stage I lung cancer. Chest. 2004;126:1198–1203. doi: 10.1378/chest.126.4.1198. [DOI] [PubMed] [Google Scholar]
- 50.Chang JY, Komaki R, Lu C, Wen HY, Allen PK, Tsao A, Gillin M, Mohan R, Cox JD. Phase 2 study of high-dose proton therapy with concurrent chemotherapy for unresectable stage III nonsmall cell lung cancer. Cancer. 2011;117:4707–4713. doi: 10.1002/cncr.26080. doi:4710.1002/cncr.26080. Epub 22011 Mar 26022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koay EJ, Lege D, Mohan R, Komaki R, Cox JD, Chang JY. Adaptive/nonadaptive proton radiation planning and outcomes in a phase II trial for locally advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2012;84:1093–1100. doi: 10.1016/j.ijrobp.2012.02.041. doi:1010.1016/j.ijrobp.2012.1002.1041. Epub 2012 Apr 1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakayama H, Sugahara S, Tokita M, Satoh H, Tsuboi K, Ishikawa S, Tokuuye K. Proton beam therapy for patients with medically inoperable stage I non-small-cell lung cancer at the university of tsukuba. Int J Radiat Oncol Biol Phys. 2010;78:467–471. doi: 10.1016/j.ijrobp.2009.07.1707. doi:410.1016/j.ijrobp.2009.1007.1707. Epub 2010 Jan 1017. [DOI] [PubMed] [Google Scholar]
- 53.Lin SH, Komaki R, Liao Z, Wei C, Myles B, Guo X, Palmer M, Mohan R, Swisher SG, Hofstetter WL, Ajani JA, Cox JD. Proton beam therapy and concurrent chemotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys. 2012;83:e345–351. doi: 10.1016/j.ijrobp.2012.01.003. doi:310.1016/j.ijrobp.2012.1001.1003. Epub 2012 Mar 1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Frank SJ, Cox JD, Gillin M, Mohan R, Garden AS, Rosenthal DI, Gunn GB, Weber RS, Kies MS, Lewin JS, Munsell MF, Palmer MB, Sahoo N, Zhang X, Liu W, Zhu XR. Multifield optimization intensity modulated proton therapy for head and neck tumors: a translation to practice. Int J Radiat Oncol Biol Phys. 2014;89:846–853. doi: 10.1016/j.ijrobp.2014.04.019. doi:810.1016/j.ijrobp.2014.1004.1019. Epub 2014 May 1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holliday EB, Frank SJ. Proton radiation therapy for head and neck cancer: a review of the clinical experience to date. Int J Radiat Oncol Biol Phys. 2014;89:292–302. doi: 10.1016/j.ijrobp.2014.02.029. doi:210.1016/j.ijrobp.2014.1002.1029. Epub 2014 May 1015. [DOI] [PubMed] [Google Scholar]
- 56.MacDonald SM, Patel SA, Hickey S, Specht M, Isakoff SJ, Gadd M, Smith BL, Yeap BY, Adams J, Delaney TF, Kooy H, Lu HM, Taghian AG. Proton therapy for breast cancer after mastectomy: early outcomes of a prospective clinical trial. Int J Radiat Oncol Biol Phys. 2013;86:484–490. doi: 10.1016/j.ijrobp.2013.01.038. doi:410.1016/j.ijrobp.2013.1001.1038. Epub 2013 Mar 1021. [DOI] [PubMed] [Google Scholar]
- 57.Strom EA, Ovalle V. Initial clinical experience using protons for accelerated partial-breast irradiation: longer-term results. Int J Radiat Oncol Biol Phys. 2014;90:506–508. doi: 10.1016/j.ijrobp.2014.06.039. doi:510.1016/j.ijrobp.2014.1006.1039. Epub 2014 Sep 1026. [DOI] [PubMed] [Google Scholar]
- 58.Paganetti H, Niemierko A, Ancukiewicz M, Gerweck LE, Goitein M, Loeffler JS, Suit HD. Relative biological effectiveness (RBE) values for proton beam therapy. Int J Radiat Oncol Biol Phys. 2002;53:407–421. doi: 10.1016/s0360-3016(02)02754-2. [DOI] [PubMed] [Google Scholar]
- 59.Lomax AJ. Intensity modulated proton therapy and its sensitivity to treatment uncertainties 2: the potential effects of inter-fraction and inter-field motions. Phys Med Biol. 2008;53:1043–1056. doi: 10.1088/0031-9155/53/4/015. [DOI] [PubMed] [Google Scholar]
- 60.Lomax AJ. Intensity modulated proton therapy and its sensitivity to treatment uncertainties 1: the potential effects of calculational uncertainties. Phys Med Biol. 2008;53:1027–1042. doi: 10.1088/0031-9155/53/4/014. [DOI] [PubMed] [Google Scholar]
- 61.Park PC, Cheung JP, Zhu XR, Lee AK, Sahoo N, Tucker SL, Liu W, Li H, Mohan R, Court LE, Dong L. Statistical assessment of proton treatment plans under setup and range uncertainties. Int J Radiat Oncol Biol Phys. 2013;86:1007–1013. doi: 10.1016/j.ijrobp.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pflugfelder D, Wilkens JJ, Oelfke U. Worst case optimization: a method to account for uncertainties in the optimization of intensity modulated proton therapy. Phys Med Biol. 2008;53:1689–1700. doi: 10.1088/0031-9155/53/6/013. [DOI] [PubMed] [Google Scholar]
- 63.Unkelbach J, Chan TC, Bortfeld T. Accounting for range uncertainties in the optimization of intensity modulated proton therapy. Phys Med Biol. 2007;52:2755–2773. doi: 10.1088/0031-9155/52/10/009. [DOI] [PubMed] [Google Scholar]
- 64.Liu W, Liao Z, Schild SE, Liu Z, Li H, Li Y, Park PC, Li X, Stoker J, Shen J, Keole S, Anand A, Fatyga M, Dong L, Sahoo N, Vora S, Wong W, Zhu XR, Bues M, Mohan R. Impact of respiratory motion on worst-case scenario optimized intensity modulated proton therapy for lung cancers. Practical radiation oncology. 2015;5:e77–86. doi: 10.1016/j.prro.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu W, Zhang X, Li Y, Mohan R. Robust optimization in intensity-modulated proton therapy. Med Phys. 2012;39:1079–1091. doi: 10.1118/1.3679340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu W, Frank SJ, Li X, Li Y, Zhu RX, Mohan R. PTV-based IMPT optimization incorporating planning risk volumes vs robust optimization. Med Phys. 2013;40:021709. doi: 10.1118/1.4774363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu W, Frank SJ, Li X, Li Y, Park PC, Dong L, Ronald Zhu X, Mohan R. Effectiveness of robust optimization in intensity-modulated proton therapy planning for head and neck cancers. Med Phys. 2013;40:051711. doi: 10.1118/1.4801899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu W, Li Y, Li X, Cao W, Zhang X. Influence of robust optimization in intensity-modulated proton therapy with different dose delivery techniques. Med Phys. 2012;39:3089–3101. doi: 10.1118/1.4711909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu W, Liao Z, Schild SE, Liu Z, Li H, Li Y, Park PC, Li X, Stoker J, Shen J, Keole S, Anand A, Fatyga M, Dong L, Sahoo N, Vora S, Wong W, Zhu XR, Bues M, Mohan R. Impact of respiratory motion on worst-case scenario optimized intensity modulated proton therapy for lung cancers. Practical radiation oncology. 2014 doi: 10.1016/j.prro.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu W, Mohan R, Park P, Liu Z, Li H, Li X, Li Y, Wu R, Sahoo N, Dong L, Zhu XR, Grosshans DR. Dosimetric benefits of robust treatment planning for intensity modulated proton therapy for base-of-skull cancers. Practical radiation oncology. 2014;4:384–391. doi: 10.1016/j.prro.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu W, Zhang X, Li Y, Mohan R. Robust optimization of intensity modulated proton therapy. Med Phys. 2012;39:1079–1091. doi: 10.1118/1.3679340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fredriksson A, Forsgren A, Hardemark B. Minimax optimization for handling range and setup uncertainties in proton therapy. Med Phys. 2011;38:1672–1684. doi: 10.1118/1.3556559. [DOI] [PubMed] [Google Scholar]
- 73.Fredriksson A. A characterization of robust radiation therapy treatment planning methods-from expected value to worst case optimization. Med Phys. 2012;39:5169–5181. doi: 10.1118/1.4737113. [DOI] [PubMed] [Google Scholar]
- 74.Fredriksson A, Bokrantz R. A critical evaluation of worst case optimization methods for robust intensity-modulated proton therapy planning. Med Phys. 2014;41:081701. doi: 10.1118/1.4883837. [DOI] [PubMed] [Google Scholar]
- 75.Hyer DE, Hill PM, Wang D, Smith BR, Flynn RT. A dynamic collimation system for penumbra reduction in spot-scanning proton therapy: proof of concept. Med Phys. 2014;41:091701. doi: 10.1118/1.4837155. [DOI] [PubMed] [Google Scholar]
- 76.Brada M, Pijls-Johannesma M, De Ruysscher D. Proton therapy in clinical practice: current clinical evidence. J Clin Oncol. 2007;25:965–970. doi: 10.1200/JCO.2006.10.0131. [DOI] [PubMed] [Google Scholar]
- 77.De Ruysscher D, Mark Lodge M, Jones B, Brada M, Munro A, Jefferson T, Pijls-Johannesma M. Charged particles in radiotherapy: a 5-year update of a systematic review. Radiother Oncol. 2012;103:5–7. doi: 10.1016/j.radonc.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 78.Allen AM, Pawlicki T, Dong L, Fourkal E, Buyyounouski M, Cengel K, Plastaras J, Bucci MK, Yock TI, Bonilla L, Price R, Harris EE, Konski AA. An evidence based review of proton beam therapy: the report of ASTRO's emerging technology committee. Radiother Oncol. 2012;103:8–11. doi: 10.1016/j.radonc.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 79.ASTRO Model Policies. ASTRO; 2014. Proton Beam Therapy. [Google Scholar]
- 80.Goitein M. Calculation of the uncertainty in the dose delivered during radiation therapy. Med Phys. 1985;12:608–612. doi: 10.1118/1.595762. [DOI] [PubMed] [Google Scholar]
- 81.Urie M, Goitein M, Holley WR, Chen GT. Degradation of the Bragg peak due to inhomogeneities. Phys Med Biol. 1986;31:1–15. doi: 10.1088/0031-9155/31/1/001. [DOI] [PubMed] [Google Scholar]
- 82.Urie MM, Goitein M, Doppke K, Kutcher JG, LoSasso T, Mohan R, Munzenrider JE, Sontag M, Wong JW. The role of uncertainty analysis in treatment planning. Int J Radiat Oncol Biol Phys. 1991;21:91–107. doi: 10.1016/0360-3016(91)90170-9. [DOI] [PubMed] [Google Scholar]