Abstract
Recent studies indicate that spontaneous low-frequency fluctuations (LFFs) of resting-state functional magnetic resonance imaging (rs-fMRI) blood oxygen level-dependent (BOLD) signals are driven by the slow (<0.1 Hz) modulation of ongoing neuronal activity synchronized locally and across remote brain regions. How regional LFFs of the BOLD fMRI signal are altered during anesthetic-induced alteration of consciousness is not well understood. Using rs-fMRI in 15 healthy participants, we show that during administration of propofol to achieve loss of behavioral responsiveness indexing unconsciousness, the fractional amplitude of LFF (fALFF index) was reduced in comparison to wakeful baseline in the anterior frontal regions, temporal pole, hippocampus, parahippocampal gyrus, and amygdala. Such changes were absent in large areas of the motor, parietal, and sensory cortices. During light sedation characterized by the preservation of overt responsiveness and therefore consciousness, fALFF was reduced in the subcortical areas, temporal pole, medial orbital frontal cortex, cingulate cortex, and cerebellum. Between light sedation and deep sedation, fALFF was reduced primarily in the medial and dorsolateral frontal areas. The preferential reduction of LFFs in the anterior frontal regions is consistent with frontal to sensory-motor cortical disconnection and may contribute to the suppression of consciousness during general anesthesia.
Keywords: Resting-state fMRI, Spontaneous neural activity, Fractional amplitude of low-frequency fluctuation (fALFF), Propofol sedation, Loss of consciousness
1. Introduction
Resting-state functional magnetic resonance imaging (rs-fMRI) is a powerful and unique tool for the noninvasive mapping of spontaneous neuronal activity and regional functional interactions in the brain in the absence of exogenous stimulation or task (Biswal 2012; Buckner et al. 2013). Accumulating experimental evidence indicates that the low-frequency (< 0.1 Hz) fluctuations (LFFs) of blood oxygen-dependent (BOLD) fMRI signals in the resting state are correlated with spontaneous neuronal activity synchronized locally or over long distances in the brain (Cabral et al. 2014; Foster et al. 2016). Rapid neuronal dynamics like spiking and gamma-band activity are modulated by neuronal processes occurring at a slower time scale, and it is this slow modulation that gives rise to the observed BOLD fMRI dynamics (Fox et al. 2005; Raichle 2011; Foster et al. 2016). Moreover, the long-range synchrony of network activity as revealed by rs-fMRI has been specifically linked to the slow modulation (< 0.1 Hz) of local field potentials in the gamma-band (Nir et al. 2008).
General anesthesia alters ongoing brain activity, regional cerebral metabolism and hemodynamics, with characteristic patterns depending on the anesthetic agent and dose (Alkire et al. 2008). In the past decade, numerous neuroimaging studies sought a better understanding of functional connectivity changes in anesthetic-induced altered states of consciousness (Hudetz 2012). However, with few exceptions, little attention has been given to the local or regional modulation of low-frequency fluctuations of BOLD fMRI signals by anesthesia (Guldenmund et al. 2016). This is important because functional connectivity is generally derived from the temporal correlation of BOLD signals between a pair of spatially remote brain regions, indexing the degree of their temporal coactivation. This derivation of functional connectivity does not reveal which of the involved brain regions are altered during any intervention. For example, an anesthetic may alter functional connectivity via the suppression or augmentation of local neuronal-hemodynamic fluctuations in either or both regions and may influence those in a similar or opposing manner. This argument motivated us to examine the effect of anesthesia on low-frequency BOLD fluctuations across various regions of the brain.
To quantify the intensity of regional spontaneous brain activity, a suitable index, the fractional amplitude of low-frequency fluctuations, or fALFF, was previously introduced (Zou et al. 2008) based on the similar index ALFF (Zang et al. 2007). The fALFF measures the ratio of power within the low-frequency range (e.g., 0.01–0.1 Hz) of BOLD signal fluctuations relative to total power in the entire measurable frequency range. It has been applied to detect abnormal spontaneous brain activity in various cognitive and neuropsychiatric disorders such as mild cognitive impairment (Han et al. 2012), schizophrenia (Hoptman et al. 2010), aging (La et al. 2016), and depression (Tadayonnejad et al. 2015). To our knowledge, fALFF has not been used to characterize the effect of general anesthesia on brain activity.
In this study, we used the fALFF index to quantify region-specific changes of spontaneous LFFs of the BOLD signal during propofol-induced alterations of consciousness. To delineate the important changes in fALFF associated with the transition between conscious and unconscious states, we used two levels of sedation, as called here, light and deep sedation. Memory and consciousness are two expressions of brain functioning that are suppressed by anesthetics (Hudetz and Pearce 2010). Memory is diminished at a light sedative dose of anesthetic at which responsiveness and consciousness are still present (Sanders et al. 2012). Overt behavioral responsiveness and, by inference, consciousness are abolished at a deep sedative dose.
Prior findings suggested that general anesthetics at a deep sedative dose preferentially reduce brain activation in higher-order information-processing regions but leave the reactivity of primary sensory cortices to stimuli unchanged (Hudetz 2006; Alkire et al. 2008; Hudetz and Mashour 2016). Accordingly, we hypothesized that during light sedation with propofol, fALFF would be reduced in memory-related brain areas, and while in deep sedation consistent with loss of consciousness, it would show preferential reduction in the frontal association regions involved in higher-order executive functions.
2. Materials and methods
2.1. Data acquisition
Fifteen healthy volunteers aged 19 – 35 years old (nine males and six females, mean age 26.7 years, standard deviation 4.8, body mass index < 25) participated in this study. The anesthetic agent propofol was administered with a bolus dose followed by a target-controlled continuous infusion (STANPUMP (Shafer 1996)). We targeted a plasma concentration of 0.98±0.18 μg ml−1 for light sedation and 1.88±0.24 μg ml−1 for deep sedation (the targeted plasma concentration varied slightly in individual participants in order to achieve a desired state of sedation). The lower dose for light sedation was intended to induce lethargic response to questions in participants (OAAS [observer’s assessment of alertness/sedation] score: 4; (Chernik et al. 1990)). The higher dose for deep sedation was chosen to achieve the desired endpoint, at which the participant showed no response when his/her name was called loudly at the scanner bedside and did not respond to mild prodding and shaking (OAAS score: 2–1). The order of administering light and deep sedation was counterbalanced in study participants. Standard American Society of Anesthesiologists monitoring was conducted during the experiment, including electrocardiogram, noninvasive blood pressure cuff, pulse oximetry, and end-tidal carbon dioxide gas monitoring. Supplemental oxygen was administered prophylactically via nasal cannula.
Resting-state structural and functional MRI data were acquired using a whole-body 3T Signa GE 750 scanner (GE Healthcare, Waukesha, Wisconsin, USA) with a standard 32-channel transmit-receive head coil. Functional imaging data were acquired during each of four 15-minute scans in wakefulness, light sedation, deep sedation, and recovery, respectively, with repetition time, 2 s; echo time, 25 ms; slice thickness, 3.5 mm; in-plane resolution, 3.5 × 3.5 mm; number of slices, 41; flip angle, 77°; field of view, 22.4 cm; matrix size, 64 × 64. High-resolution spoiled gradient-recalled echo (SPGR) anatomical images were acquired before functional scans with TE/TR/TI, 8.2/3.2/450 ms; slice thickness, 1 mm; number of slices, 150; flip angle, 12°; field of view, 24 cm; matrix size, 256 × 256.
2.2 Data preprocessing
Imaging data were preprocessed using a collection of Analysis of Functional NeuroImages (AFNI, http://afni.nimh.nih.gov/afni), FSL (http://www.fmrib.ox.ac.uk/fsl), FreeSurfer (http://freesurfer.net), and Matlab (The MathWorks, Natick, MA) software. Raw functional images were first retrospectively corrected for cardiac and respiratory motion (3dretroicor in AFNI). The first five data points were discarded to reduce the initial transient effects in data acquisition. Subsequent data preprocessing included despiking, detrending, and motion correction. No significant differences of head motion ranges were found between the four functional scan conditions. Physiological noise was estimated using the average BOLD time series from the regions of white matter (WM) and cerebrospinal fluid (CSF), which were determined in each individual’s anatomical images. The voxelwise BOLD time series from each run was then analyzed with a general linear regression model (3dDeconvolve in AFNI) using the eight regressors representing noise artifacts from the motion parameters, WM, and CSF, respectively. Each participant’s high-resolution anatomical images were spatially transformed to the standard MNI (Montreal Neuroimaging Institute) space (MNI152) (flirt in FSL); then, the functional data were coregistered into the MNI space with a sampling to a 3-mm cubic voxel size. In the MNI space, functional data were further cleaned by regressing out artifacts originating from subregions of the WM, CSF, and major vein (e.g., superior sagittal sinus) areas.
2.3 Computation of fALFF and statistical analysis
Computation of fALFF was performed in voxel-wise manner using the volumetric fMRI data. The BOLD time series of all brain voxels were converted to the frequency domain via the Fast Fourier Transform (FFT, Matlab), and normalized power spectrums were subsequently obtained. The fALFF index was computed in a voxel-wise manner as the sum of power in the frequency band of 0.01–0.1 Hz divided by the sum of power of the entire frequency range (0.01–0.25 Hz). To investigate the effect of propofol-induced differences in fALFF at the group level, two-sided paired t-tests were performed on the individual fALFF maps between different states of consciousness. The threshold of significance was set at a P = 0.01 combined with correction for multiple comparisons using the AlphaSim method in AFNI (a minimum cluster threshold of 153 voxels of 3-mm cubic in the MNI space).
We found that standardization of voxel BOLD time series (normalizing to zero mean and standard deviation, i.e., z-score) did not affect the fALFF index results. This is because fALFF represents the relative power of low-frequency fluctuations to that of a wider frequency range, whereas the ALFF index measures the absolute power of LFFs. For the purpose of comparison, we also computed the ALFF index and compared the changes in ALFF across the four states of consciousness (see Supplemental Information). However, standardizing the voxel BOLD time series became a critical factor that substantially influenced the results for ALFF. In our experiments, BOLD fMRI signals were acquired using a standard 32-channel transmit-receive head coil with a signal-detection sensitivity that is several times higher for tissues near the surface of the brain than for tissues located deep inside the brain (Supplemental Fig. 1). As a result, the baseline amplitude and the absolute oscillating amplitude of BOLD signals obtained near the surface of the brain were much higher than those that are deep inside, and the same was true for ALFF. To correct this imaging-acquisition-induced distortion to the ALFF index, the voxel BOLD time series had to be standardized. With the standardization (normalizing using z-scores), comparisons of ALFF between the four states of consciousness produced results similar to those obtained using the fALFF index (Supplemental Figs. 2 and 3). Conversely, without the standardization, comparisons of ALFF showed few significant effects which were inconsistent with the state of consciousness (Supplemental Figs. 4 and 5).
A common practice in computing subject-level maps of fALFF and ALFF has been to normalize the obtained values across the brain by dividing them by the global mean value or transforming them to z-scores (Zang et al. 2007; Zuo et al. 2010). However, this type of standardization did not seem to be appropriate for the type of comparisons investigated in this study. In our study, each participant underwent different states of consciousness, as modulated by propofol administration. The group statistical analyses were performed by subject-matched paired t-tests. Although useful for comparison between independent groups, the above-mentioned standardization would have removed the propofol-induced changes in fALFF and ALFF between the paired states within the same subject (e.g., global mean changes). Because fALFF represents the contribution of LFF relative to the total power of each voxel time series, it is more resistant to data-standardization. Therefore, we reported fALFF as the primary measure and included ALFF in the Supplemental Information.
To obtain information about detailed region-specific fALFF changes, a standard brain atlas was used to divide the brain into a parcellation of 116 anatomical structures (Tzourio-Mazoyer et al. 2002). The sum of fALFF over voxels included in six representative anatomical regions or groups was computed and compared among the four states of consciousness.
3. Results
3.1. Distribution of fALFF in wakeful baseline and altered states of consciousness
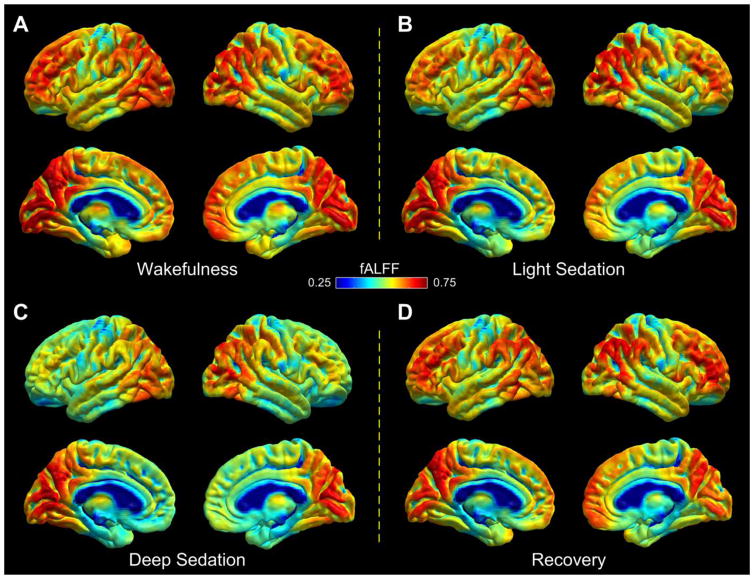
The regional distribution of mean fALFF across study participants in the resting wakeful baseline is depicted in Fig. 1A. Areas showing relatively high fALFF include the medial and dorsolateral frontal cortices, visual cortex, supramarginal and angular gyrus, precuneus, posterior cingulate cortex (PCC), and posterior portion of the inferior and middle temporal lobe. During light sedation, fALFF was reduced relative to wakeful baseline in subcortical areas and the anterior portion of the temporal lobe (Fig. 1B). During deep sedation, fALFF was markedly reduced in comparison to wakeful baseline in anterior brain regions and subcortical structures (Fig. 1C). The most prominent decreases were observed in the dorsolateral and medial frontal cortices, insular cortex, anterior portion of the temporal lobe, and subcortical areas. During recovery, decreases in fALFF as observed in deep sedation were reversed (Fig. 1D), yielding a distribution pattern of fALFF values similar to that observed in light sedation.
Fig. 1.
Group average of fALFF index presented on the brain surface (fsaverage in FreeSurfer) in the four states of consciousness in wakefulness (A), light sedation (B), deep sedation (C), and recovery (D), respectively.
3.2. Significant changes of fALFF in propofol sedation
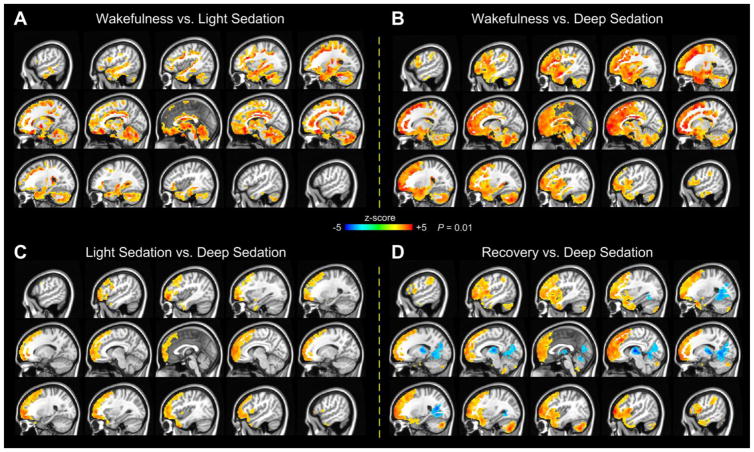
During light sedation relative to wakeful baseline, fALFF was reduced in areas of the bilateral temporal pole; ventromedial and orbitomedial PFC; PCC; cingulate cortex; cerebellum; and subcortical areas including the hippocampus, parahippocampal gyrus, and amygdala (Fig. 2A). No significant increases in fALFF were observed in light sedation at the reporting threshold.
Fig. 2.
Two-sided one-sample paired t-tests of fALFF between wakefulness and light sedation (A), between wakefulness and deep sedation (B), between light sedation and deep sedation (C), and between recovery and deep sedation (D). Warm colors indicate higher fALFF in the first state of each paired comparison. The significance of results is reported at P = 0.01, corrected for multiple comparisons.
During deep sedation compared with wakeful baseline, fALFF showed extensive reductions in large areas of the dorsolateral and medial frontal cortices; temporal pole; cingulate cortex; insular cortex; cerebellum; brain stem; and subcortical areas including the hippocampus, parahippocampal gyrus, and amygdala (Fig. 2B). Such changes, however, were absent in most middle and posterior portions of the brain, including the motor cortex; widespread parietal and temporal regions; and visual, auditory, and somatosensory cortices. No significant increases of fALFF were observed in deep sedation at the reporting threshold.
Contrasting light and deep sedation revealed a reduction of fALFF during deep sedation in large areas of the medial and dorsolateral frontal cortex and small sections of the bilateral temporal pole (Fig. 2C). We emphasize that during light sedation, participants maintained lethargic responses to verbal commands; whereas in deep sedation, they completely lost behavioral responsiveness, which we used to index the loss of consciousness. In comparison, the re-establishment of consciousness, relative to deep sedation, was accompanied by increased fALFF in large areas of the medial and dorsolateral frontal cortex, temporal pole, and the cerebellum (Fig. 2D). Unexpectedly, part of the thalamus, visual cortex, and cerebellum showed further reduction in fALFF during recovery compared with deep sedation. For a better visualization, comparisons of fALFF among the different states of consciousness are also presented on the brain surface (Supplemental Fig. 6).
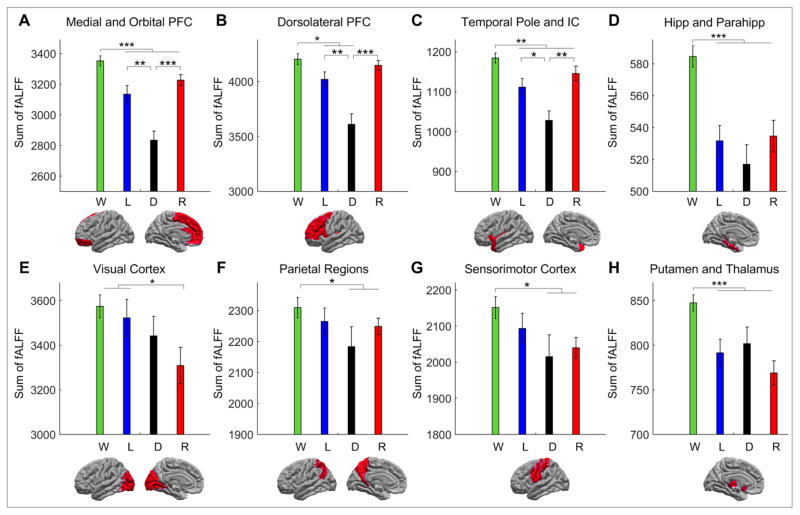
To quantify the regional changes in fALFF, we calculated the sum of fALFF over all voxels within eight major anatomical regions (Fig. 3). The first row of Fig. 3 shows the sum of fALFF in three anatomical groups including the medial and orbital prefrontal cortex (PFC) (Fig. 3A); dorsolateral PFC (Fig. 3B); and the temporal pole and insular cortex (Fig. 3C). The bar plots showed consistent anesthetic-dose-dependent changes of fALFF in all groups. Specifically, the sum of fALFF was significantly greater in wakeful baseline than in both light and deep sedation. In all three groups, fALFF was also greater in both light sedation and recovery than in deep sedation.
Fig. 3.
Region-specific changes in the sum of fALFF across the four states of consciousness in wakefulness (W), light sedation (L), deep sedation (D), and recovery (R). Paired t-test comparisons that show a main effect of difference are marked by an asterisk. Error bars represent the standard error of measurement. (* denotes p<=0.05; ** p<=0.01; and *** p<=0.001. PFC: prefrontal cortex; IC: insular cortex; Hipp: hippocampus; Parahipp: parahippocampal gyrus)
The sum of fALFF in the hippocampus and parahippocampal gyrus showed decreases in light sedation, deep sedation, and recovery compared with wakeful baseline (Fig. 3D). In deep sedation, the sum of fALFF exhibited a trend of decrease relative to light sedation and recovery, but no significant effects were demonstrated between these three states. The similarity of fALFF values in light sedation and recovery in the hippocampus and parahippocampal gyrus indicates that the recovery of fALFF in these regions was incomplete, presumably from a lingering effect of propofol.
The visual cortex showed no significant decrease of fALFF in deep sedation compared with wakeful baseline or light sedation. Moreover, a trend of further decrease in fALFF was observed in recovery (Fig. 3E). The parietal and sensorimotor cortices showed a decrease of fALFF in deep sedation and in recovery in comparison to wakeful baseline (Fig. 3F and 3G). There were no significant differences in these regions between wakeful baseline and light sedation. In addition, fALFF values in deep sedation were not significantly different from those in light sedation and recovery. Compared with wakeful baseline, the thalamus and putamen showed decreased fALFF in light sedation, deep sedation, and recovery, with no significant differences among the latter three states, although there was a trend for further decrease in recovery (Fig. 3H). A lack of reversal of the summated fALFF during recovery suggested the continued suppression of these regions after the termination of propofol infusion and a drop of predicted propofol plasma concentration (Ward et al. 2002).
4. Discussion
This study used the fALFF index to quantify changes in spontaneous BOLD LFFs during propofol-induced alterations of consciousness for the first time. Previously, fALFF was used to characterize various cognitive and neuropsychiatric disorders including mild cognitive impairment (Han et al. 2012), schizophrenia (Hoptman et al. 2010), aging (La et al. 2016), and depression (Tadayonnejad et al. 2015), but not anesthesia or sedation. In our study of healthy participants, the distribution of fALFF in wakeful baseline was consistent with previous findings (Zou et al. 2008), revealing relatively high fALFF in the medial default mode network (precuneus, PCC, and ventromedial PFC), bilateral inferior parietal lobule, dorsolateral PFC, and visual cortex.
Prior studies suggest that general anesthetics preferentially suppress the activity of higher-order information-processing regions (Hudetz 2006; Alkire et al. 2008; Hudetz and Mashour 2016), particularly those of the frontal lobes (Davis et al. 2007; Boveroux et al. 2010; Liu et al. 2012; Guldenmund et al. 2016). Such observations are consistent with the dependence of conscious sensory perception on frontal cortex activation and recurrent feedback from frontal association to sensory regions (Dehaene et al. 2006; Lamme 2006; Mashour 2014). Accordingly, we hypothesized that LFFs would be suppressed mainly in the frontal association regions involved in higher-order executive functions as consciousness was suppressed by propofol infusion.
One of the key findings of our study was that propofol reduced fALFF in partially disjoint brain regions during light versus deep sedation. Light sedation was accompanied by a reduction of fALFF mainly in memory-related brain regions including the temporal pole, orbitomedial PFC, inferior frontal gyrus, anterior cingulate cortex, and subcortical structures consisting of the hippocampus, parahippocampal gyrus, and amygdala (Fig. 2A). This effect of propofol during light sedation is consistent with diminished memory along with preserved responsiveness and consciousness (Sanders et al. 2012). During deep sedation, the reduction in fALFF extended, beyond those in light sedation, to widespread areas of the anterior medial and dorsolateral PFC and insular cortex.
The selective reduction of fALFF in medial and dorsolateral prefrontal cortex between light and deep sedation deserves special emphasis. Since responsiveness and therefore, consciousness, was present during light sedation in all study participants, the findings collectively support that loss of consciousness was associated with a preferential reduction of LFFs in the anterior frontal regions. The significance of frontal lobe suppression for loss of consciousness is profound. Through their roles in executive interpretation and top-down attentional control, the frontal lobes contribute to conscious perception as emphasized by the global workspace theory (GWT) of consciousness (Baars 2002; Baars 2005) and the global neuronal workspace (GNW) model (Dehaene et al. 2003). The frontal lobes enable conscious cognitive functions such as working memory, voluntary action, selective attention, planning, learning, report, cognitive flexibility, inhibition, and abstract reasoning (Miller and Cohen 2001; Baars 2002; Miller and Cummings 2007). The ventral and dorsal medial PFC perform conscious functions related to action and outcome monitoring, self-knowledge, person perception, and mentalizing (Amodio and Frith 2006). Neuroimaging studies have shown that in the unconscious state produced by anesthetics, integrative functions of higher-order information-processing regions, such as the anterior frontal areas but not the posterior sensory cortices, are preferentially reduced (Hudetz 2006; Alkire et al. 2008). Propofol exerts a prominent suppression on the activity and connectivity of the frontal lobe (Davis et al. 2007; Boveroux et al. 2010; Liu et al. 2012; Guldenmund et al. 2016), and sevoflurane has a similar effect (Deshpande et al. 2010; Palanca et al. 2015). Thus, the dominant reduction of fALFF upon loss of consciousness in widespread frontal regions, but not in sensory and motor cortices, suggests that a loss of frontal executive function may be the primary factor in propofol-induced unconsciousness.
Interestingly, although the summated fALFF showed a decrease in the middle and posterior portions of the brain including the motor cortex, parietal regions, and sensory cortices upon loss of consciousness (Fig. 3), the decrease did not reverse in recovery and there were no significant differences in fALFF between light and deep sedation in these areas. This finding is contrary to the recent emphasis of a posterior “hot zone” of consciousness (Koch et al. 2016). However, it is possible that fALFF (and ALFF) may be reduced in more posterior brain regions at a higher dose of propofol. A recent study reported a decrease in standard deviation of band-pass filtered (0.01–0.08 Hz) BOLD fMRI signals, reflecting a change in the power of LFF, in widespread frontal and parietal regions when propofol was infused to an effect-site concentration of 4.0 μg/ml (Huang et al. 2016).
The changes in fALFF during light sedation involved a reduction in the temporal pole, inferior frontal gyrus, anterior cingulate cortex, and subcortical structures including the hippocampus, parahippocampal gyrus, and amygdala, consistent with propofol’s amnesic effect. The temporal pole is an association cortex involved in multimodal analysis, especially in social and emotional processing, with the left temporal pole associated with semantic memory and the right temporal pole with personal and episodic memory (Bonner and Price 2013). The anterior cingulate cortex is involved in formation and consolidation of recent and remote memories (Einarsson and Nader 2012) and the inferior frontal gyrus is involved in semantic processing and working memory (Curtis and D’Esposito 2003; Binder et al. 2009). The subcortical structures generally affected by propofol including the hippocampus, parahippocampal gyrus, and amygdala are part of the limbic system critically involved in memory, spatial orientation, and emotional reaction (Baars and Gage 2013). When sedation was deepened, additional reduction of fALFF also occurred in the insula, which plays an important role in diverse functions such as emotion or salience processing and is probably involved in consciousness, especially in interoceptive awareness (Craig 2009).
The thalamus and putamen were previously identified as important sites of anesthetic modulation (White and Alkire 2003; Mhuircheartaigh et al. 2010; Liu et al. 2013). In our study, both regions showed a decrease in fALFF during deep sedation, but fALFF failed to recover after propofol infusion was terminated and the participants regained responsiveness. Indeed, fALFF in the thalamus and putamen showed a trend to decrease further during recovery. Moreover, there were no significant differences in fALFF in the thalamus and putamen between light and deep sedation that would have accompanied the transition between consciousness and unconsciousness. We conclude that the fALFF changes in the thalamus and putamen are inconsistent with the depth of propofol sedation, casting doubt about the causal role of these regions in the state of consciousness as indexed by behavioral responsiveness, sometimes referred to as “external awareness” (Boly et al. 2013; Hudetz and Mashour 2016). This conclusion, however, does not preclude the possibility that “internal awareness” (i.e., the subjective experience of internally generated thoughts, images, dreams, and daydreams) may depend on the functionality of thalamic and other subcortical circuits. Participation of the nonspecific (intralaminar) thalamus, although not investigated here, may be particularly important in this regard (Liu et al. 2013).
A potential caveat to the analysis of fALFF is that it is derived from BOLD signal fluctuations that directly depend on cerebral hemodynamics. Even if the latter is driven by the slow modulation of ongoing neuronal activity (Fox et al. 2005; Nir et al. 2008; Raichle 2011; Foster et al. 2016), anesthetic agents may influence the cerebral vasculature directly, leading to a change in cerebral hemodynamics. However, this limitation applies to all BOLD signal-based neuroimaging studies, including those of functional connectivity, and would not make fALFF any less reliable than those. Moreover, propofol at sedative doses has small to negligible effects on cerebral blood flow and flow autoregulation (Fiset et al. 1999; Veselis et al. 2005), suggesting that the observed changes in fALFF are mainly of neuronal origin.
In summary, we found that during sedation with propofol, fALFF is reduced in multiple cortical and subcortical areas in a dose-dependent manner. In light sedation, fALFF was reduced in memory-related regions. Deepening the level of sedation to loss of behavioral responsiveness indexing loss of external consciousness, fALFF was markedly reduced in the anterior medial and dorsolateral PFC. Imaging the spatiotemporal patterns of spontaneous low-frequency activity of the brain may yield further insight into the system-level mechanisms of general anesthesia and the state of consciousness.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by grants of the National Institute of General Medical Sciences of the National Institutes of Health under Award Number R01-GM103894 and T32 GM89586. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank Ms. Lydia Washechek, BA, for editorial assistance.
Footnotes
Conflict of Interest Statement
All authors declare no conflict of Interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alkire MT, Hudetz AG, et al. Consciousness and anesthesia. Science. 2008;322(5903):876–880. doi: 10.1126/science.1149213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7(4):268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Baars BJ. The conscious access hypothesis: origins and recent evidence. Trends Cogn Sci. 2002;6(1):47–52. doi: 10.1016/s1364-6613(00)01819-2. [DOI] [PubMed] [Google Scholar]
- Baars BJ. Global workspace theory of consciousness: toward a cognitive neuroscience of human experience. Prog Brain Res. 2005;150:45–53. doi: 10.1016/S0079-6123(05)50004-9. [DOI] [PubMed] [Google Scholar]
- Baars BJ, Gage NM. Cognition, brain, and consciousness: introduction to cognitive neuroscience. Burlington, MA: Academic Press/Elsevier; 2013. [Google Scholar]
- Binder JR, Desai RH, et al. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19(12):2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal BB. Resting state fMRI: a personal history. Neuroimage. 2012;62(2):938–944. doi: 10.1016/j.neuroimage.2012.01.090. [DOI] [PubMed] [Google Scholar]
- Boly M, Seth AK, et al. Consciousness in humans and non-human animals: recent advances and future directions. Front Psychol. 2013;4:625. doi: 10.3389/fpsyg.2013.00625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner MF, Price AR. Where Is the Anterior Temporal Lobe and What Does It Do? Journal of Neuroscience. 2013;33(10):4213–4215. doi: 10.1523/JNEUROSCI.0041-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveroux P, Vanhaudenhuyse A, et al. Breakdown of within- and between-network resting state functional magnetic resonance imaging connectivity during propofol-induced loss of consciousness. Anesthesiology. 2010;113(5):1038–1053. doi: 10.1097/ALN.0b013e3181f697f5. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, et al. Opportunities and limitations of intrinsic functional connectivity MRI. Nat Neurosci. 2013;16(7):832–837. doi: 10.1038/nn.3423. [DOI] [PubMed] [Google Scholar]
- Cabral J, Kringelbach ML, et al. Exploring the network dynamics underlying brain activity during rest. Prog Neurobiol. 2014;114:102–131. doi: 10.1016/j.pneurobio.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Chernik DA, Gillings D, et al. Validity and reliability of the Observer’s Assessment of Alertness/Sedation Scale: study with intravenous midazolam. J Clin Psychopharmacol. 1990;10(4):244–251. [PubMed] [Google Scholar]
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D’Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci. 2003;7(9):415–423. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- Davis MH, Coleman MR, et al. Dissociating speech perception and comprehension at reduced levels of awareness. Proc Natl Acad Sci U S A. 2007;104(41):16032–16037. doi: 10.1073/pnas.0701309104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Changeux JP, et al. Conscious, preconscious, and subliminal processing: a testable taxonomy. Trends Cogn Sci. 2006;10(5):204–211. doi: 10.1016/j.tics.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Sergent C, et al. A neuronal network model linking subjective reports and objective physiological data during conscious perception. Proc Natl Acad Sci U S A. 2003;100(14):8520–8525. doi: 10.1073/pnas.1332574100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande G, Kerssens C, et al. Altered local coherence in the default mode network due to sevoflurane anesthesia. Brain Res. 2010;1318:110–121. doi: 10.1016/j.brainres.2009.12.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einarsson EO, Nader K. Involvement of the anterior cingulate cortex in formation, consolidation, and reconsolidation of recent and remote contextual fear memory. Learn Mem. 2012;19(10):449–452. doi: 10.1101/lm.027227.112. [DOI] [PubMed] [Google Scholar]
- Fiset P, Paus T, et al. Brain mechanisms of propofol-induced loss of consciousness in humans: a positron emission tomographic study. J Neurosci. 1999;19(13):5506–5513. doi: 10.1523/JNEUROSCI.19-13-05506.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster BL, He BJ, et al. Spontaneous Neural Dynamics and Multi-scale Network Organization. Front Syst Neurosci. 2016;10:7. doi: 10.3389/fnsys.2016.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guldenmund P, Gantner IS, et al. Propofol-Induced Frontal Cortex Disconnection: A Study of Resting-State Networks, Total Brain Connectivity, and Mean BOLD Signal Oscillation Frequencies. Brain Connect. 2016;6(3):225–237. doi: 10.1089/brain.2015.0369. [DOI] [PubMed] [Google Scholar]
- Han Y, Lui S, et al. Anatomical and functional deficits in patients with amnestic mild cognitive impairment. PLoS One. 2012;7(2):e28664. doi: 10.1371/journal.pone.0028664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoptman MJ, Zuo XN, et al. Amplitude of low-frequency oscillations in schizophrenia: a resting state fMRI study. Schizophr Res. 2010;117(1):13–20. doi: 10.1016/j.schres.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Zhang J, et al. Decoupled temporal variability and signal synchronization of spontaneous brain activity in loss of consciousness: An fMRI study in anesthesia. Neuroimage. 2016;124(Pt A):693–703. doi: 10.1016/j.neuroimage.2015.08.062. [DOI] [PubMed] [Google Scholar]
- Hudetz AG. Suppressing Consciousness: mechanisms of general anesthesia. Seminars in Anesthesia, Perioperative Medicine and Pain. 2006;25:196–204. [Google Scholar]
- Hudetz AG. General anesthesia and human brain connectivity. Brain Connect. 2012;2(6):291–302. doi: 10.1089/brain.2012.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudetz AG, Mashour GA. Disconnecting Consciousness: Is There a Common Anesthetic End Point? Anesth Analg. 2016 doi: 10.1213/ANE.0000000000001353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudetz AG, Pearce RA. Supressing the mind: anesthetic modulation of memory and consciousness. New York: Springer; 2010. [Google Scholar]
- Koch C, Massimini M, et al. Neural correlates of consciousness: progress and problems. Nat Rev Neurosci. 2016;17(5):307–321. doi: 10.1038/nrn.2016.22. [DOI] [PubMed] [Google Scholar]
- La C, Mossahebi P, et al. Differing Patterns of Altered Slow-5 Oscillations in Healthy Aging and Ischemic Stroke. Front Hum Neurosci. 2016;10:156. doi: 10.3389/fnhum.2016.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamme VA. Towards a true neural stance on consciousness. Trends Cogn Sci. 2006;10(11):494–501. doi: 10.1016/j.tics.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Liu X, Lauer KK, et al. Differential effects of deep sedation with propofol on the specific and nonspecific thalamocortical systems: a functional magnetic resonance imaging study. Anesthesiology. 2013;118(1):59–69. doi: 10.1097/ALN.0b013e318277a801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Lauer KK, et al. Propofol disrupts functional interactions between sensory and high-order processing of auditory verbal memory. Hum Brain Mapp. 2012;33(10):2487–2498. doi: 10.1002/hbm.21385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashour GA. Top-down mechanisms of anesthetic-induced unconsciousness. Front Syst Neurosci. 2014;8:115. doi: 10.3389/fnsys.2014.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhuircheartaigh RN, Rosenorn-Lanng D, et al. Cortical and subcortical connectivity changes during decreasing levels of consciousness in humans: a functional magnetic resonance imaging study using propofol. J Neurosci. 2010;30(27):9095–9102. doi: 10.1523/JNEUROSCI.5516-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BL, Cummings JL. The human frontal lobes: functions and disorders. New York, NY: Guilford Press; 2007. [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Nir Y, Mukamel R, et al. Interhemispheric correlations of slow spontaneous neuronal fluctuations revealed in human sensory cortex. Nat Neurosci. 2008;11(9):1100–1108. doi: 10.1038/nn.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanca BJ, Mitra A, et al. Resting-state Functional Magnetic Resonance Imaging Correlates of Sevoflurane-induced Unconsciousness. Anesthesiology. 2015;123(2):346–356. doi: 10.1097/ALN.0000000000000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME. The restless brain. Brain connectivity. 2011;1(1):3–12. doi: 10.1089/brain.2011.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders RD, Tononi G, et al. Unresponsiveness not equal unconsciousness. Anesthesiology. 2012;116(4):946–959. doi: 10.1097/ALN.0b013e318249d0a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer SL. STANPUMP User’s Manual. Stanford, CA: Stanford University; 1996. [Google Scholar]
- Tadayonnejad R, Yang S, et al. Clinical, cognitive, and functional connectivity correlations of resting-state intrinsic brain activity alterations in unmedicated depression. J Affect Disord. 2015;172:241–250. doi: 10.1016/j.jad.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Veselis RA, Feshchenko VA, et al. Propofol and thiopental do not interfere with regional cerebral blood flow response at sedative concentrations. Anesthesiology. 2005;102(1):26–34. doi: 10.1097/00000542-200501000-00008. [DOI] [PubMed] [Google Scholar]
- Ward DS, Norton JR, et al. Pharmacodynamics and pharmacokinetics of propofol in a medium-chain triglyceride emulsion. Anesthesiology. 2002;97(6):1401–1408. doi: 10.1097/00000542-200212000-00011. [DOI] [PubMed] [Google Scholar]
- White NS, Alkire MT. Impaired thalamocortical connectivity in humans during general-anesthetic-induced unconsciousness. Neuroimage. 2003;19(2 Pt 1):402–411. doi: 10.1016/s1053-8119(03)00103-4. [DOI] [PubMed] [Google Scholar]
- Zang YF, He Y, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29(2):83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Zou QH, Zhu CZ, et al. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods. 2008;172(1):137–141. doi: 10.1016/j.jneumeth.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo XN, Di Martino A, et al. The oscillating brain: complex and reliable. Neuroimage. 2010;49(2):1432–1445. doi: 10.1016/j.neuroimage.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.