Abstract
Aging is associated with reduced resources needed to perform difficult cognitive tasks, but the neural underpinnings are not well understood, especially as there is scant evidence linking functional brain differences to aging cognition. Therefore, the current study examined modulation of fMRI activation from easier to harder spatial distance judgments across a large lifespan sample (N=161; ages 20–94) to identify when in the lifespan modulation to difficulty begins to show deficits and if age-related modulation predicts cognition. Analyses revealed two sets of regions in which modulation increased with difficulty due to either more activation (positive modulation) or more deactivation (negative modulation) to difficulty. These two networks evidenced differential aging trajectories: a right-lateralized fronto-parietal network that decreased in modulation to difficulty between middle- and older-age, and a network of regions in ventromedial prefrontal cortex, posterior cingulate, left angular and middle frontal gyri that showed decreased modulation at the transition from younger to middle-age. Critically, older adults who maintained negative modulation to difficulty showed higher task accuracy. Further, individuals who showed greater coupling between positive and negative modulation performed better on a fluid reasoning task. Age-related preservation of coupled modulation in both cognitive control regions and regions typically associated with default network may be a salient marker of how the brain adapts to maintain cognitive function as we age.
Keywords: aging, fMRI, cognitive control, adult lifespan, reasoning
1. Introduction
Decline in cognition is a well-documented phenomenon of aging, even in the absence of significant disease (Salthouse 2000; Park et al. 2002; Schaie 1994). Specifically, older adults often experience greater deficits on tasks with high demands on regulatory processing compared to younger adults (Braver and Barch 2002; Reuter-Lorenz and Lustig 2005; Verhaegen et al. 2005). It has been proposed that this deficit occurs because aging is accompanied by a reduced availability of the cognitive and neural processing resources required to perform more difficult tasks (Craik and Byrd 1982; Reuter-Lorenz and Cappell 2008). Furthermore, aging studies often reveal large inter-individual differences in cognitive performance, where some older adults may perform as well as, or even better than some young adults (Christensen et al. 1994; Glisky 2007). To date, there are numerous studies examining the neural correlates of increased cognitive demands in young adults (Braver et al. 1997; Barch et al. 1997; Linden et al. 2003), and there is an increasing focus on group differences between younger and older adults (see Reuter-Lorenz and Cappell 2008 for a review). However, individual differences in neural processing resources with aging and their ability to predict cognitive performance (or lack thereof) are poorly understood.
Functional magnetic resonance imaging (fMRI) studies find that strategic cognitive processing (which is required during difficult cognitive operations), relies heavily on fronto-parietal cortices (Miller and Cohen 2001; Cabeza and Nyberg 2000). Specifically, dorsolateral prefrontal cortex, insula, posterior parietal cortex, and the precuneus constitute a “cognitive control network” which is responsive to changes in task demands (Braver et al. 1997; Linden et al. 2003; Cole et al. 2013). There is ample evidence that association cortices of the frontal and parietal lobes show age-related degradation in both gray (Raz et al. 2005) and white matter structure (Kennedy and Raz 2009), and these structural declines have been associated with reduced cognition (see Raz and Rodrigue 2006; Kennedy and Raz 2009 for review).
Additionally, fronto-parietal cognitive control regions are thought to function as a switch responsible for the flexible engagement and disengagement of other brain networks (Cole et al. 2013). In young adults, blood-oxygen-level-dependent (BOLD) activity in fronto-parietal cortices is actively coupled with suppression of activity in default regions of the brain (Spreng et al. 2010) which are generally more active during rest or “default” states (Greicius et al. 2003). Recent evidence suggests that the coupling between fronto-parietal activity and default mode suppression weakens in old age, especially in the face of more challenging cognitive operations (Turner and Spreng 2015). Therefore, BOLD response in fronto-parietal regions may be predictive of age differences in cognition due to structural degradation as well as the potential influence on functional activity in proximal and perhaps distal brain regions, including the default mode system.
Indeed, studies examining age differences in functional response during tasks with low and high cognitive demands frequently report that the greatest age differences are in cognitive control regions. Comparative studies of younger and older adults find that when task demands are low, functional activity in old age is characterized by increased activity in fronto-parietal regions compared to younger adults (Cappell et al. 2010; Spaniol and Grady 2012; Mattay et al. 2006; Schneider-Garces et al. 2010). This “over-recruitment” is thought to serve as compensatory activation that enables older adults to perform at similar levels as younger adults (Reuter-Lorenz and Cappell 2008). However, there remains conflicting evidence regarding the nature of prefrontal over-activation, as it has been associated with both better (Davis et al. 2012; Reuter-Lorenz and Lustig 2005) and worse cognitive performance (de Chastelaine et al. 2011) in older adults. Therefore, caution is advised when interpreting relative age-group differences in functional activity, particularly in the absence of clear behavioral associations (Grady 2012).
As task difficulty increases, younger adults increase activity in fronto-parietal regions (Schneider-Garces et al. 2010; Turner and Spreng 2015; Kennedy et al. 2015) and decrease activity in default regions (Persson et al. 2007), suggesting that young adults are able to flexibly modulate neural response in both cognitive control and default mode cortex to account for increased task demands. In contrast, older adults may under-recruit prefrontal regions in the context of increased cognitive demands (Cappell 2010; Schneider-Garces et al. 2010), and this “under-recruitment” co-occurs with reduced suppression of default mode regions (Turner and Spreng 2015; Persson et al. 2007; Sombarto et al. 2008). When processing capacity is reached, neural resources hit a ceiling in older adults, thereby limiting the ability to further engage the brain regions necessary to meet the increased task demands (Reuter-Lorenz and Cappell 2008). This failure to appropriately modulate fronto-parietal control regions may also account for age-related reductions in cognition due to the importance of the cognitive control network in engaging appropriate networks (i.e., task-facilitative) and disengaging other networks (i.e., default mode regions; Turner and Spreng 2015). Putatively, older adults may also recruit default mode regions to support task performance when neural resource capacity is challenged or exceeded (Turner and Spreng 2015).
Although the majority of studies in neurocognitive aging have focused on understanding group differences between younger and older adults, there is an increasing focus on the importance of including the entire adult lifespan to better understand the trajectory of functional brain aging. The inclusion of a continuous, adult lifespan sample is paramount to address these issues, as there is growing evidence that middle-age may be an important period in which age-differences in functional activity may first emerge (Kwon et al. 2015; Kennedy et al. 2015; Park et al. 2013; Chan et al. 2014; Grady et al. 2006; Ankudowich et al. 2016). Furthermore, different functional brain networks (like the fronto-parietal control and default mode networks) may age at different rates; thus inclusion of a full lifespan sample is essential to capture these differential effects. For example, we recently reported that during difficult semantic judgments, age differences in modulation to difficulty in fronto-parietal regions may emerge earlier in the lifespan (i.e., middle age to older age) than in subcortical structures of the dopaminergic pathway (i.e., old age to very old age; Kennedy et al. 2015). Finally, the inclusion of a lifespan sample allows for the examination of individual differences, rather than averaging across participant age groups, which squanders sensitivity to the large individual differences inherent within young and older adult age groups (Christensen et al. 1994; Glisky 2007).
In the current study, we use a nonverbal visuo-spatial distance judgment task with three levels of difficulty to examine how age affects the ability to modulate activation in different regions of the brain to difficulty, in a large, adult lifespan sample (ages 20 – 94). Given the nature of the task, which incorporates aspects of working memory and visuo-spatial ability, we expect that conducting distance judgments will engage large portions of right-lateralized fronto-parietal cortex and these regions will show modulation to difficulty at least in young adults (Baciu et al. 1999; Cabeza and Nyberg 2000). We predict that increased age will be associated with reduced modulation to difficulty both in regions that up-modulate or exhibit a “positive modulation effect” (i.e., right fronto-parietal) and down-modulate or exhibit a “negative modulation effect” (i.e., regions typically included within the default mode network; D.C. Park et al., 2010; Persson et al., 2007). The goals of the current study are two-fold: (1) characterize the lifespan trajectory of the modulation of activation from easier to more difficult levels of a cognitive task to potentially identify when in the adult lifespan functional processing resources (positive modulation and negative modulation to difficulty) may begin to show deficits and (2) evaluate whether and how individual differences in modulation to difficulty with aging are related to (i) task accuracy in the scanner and (ii) to cognition measured outside the scanner (fluid reasoning). Critically, this will help elucidate the unclear nature of associations between functional modulation of activation and cognition, by testing if these functional differences in the aging brain are compensatory, deleterious or unrelated to cognitive performance.
2. Methods
2.1. Participants
Participants included 161 healthy adults, ages 20–94 (mean age = 51.93 ± 18.9 years; 95 women; 66 men) who were recruited from the Dallas-Fort Worth metroplex. Participants with complete neuropsychological testing and fMRI were drawn from a larger sample N=181; twenty participants were excluded from the current study for the following reasons: excessive in-scanner head movement (n = 4); poor functional image acquisition (n = 2); poor structural image acquisition (n = 1); no response on > 15% of trials (n = 2); and low accuracy (< 70% correct) on the in-scanner control task (n = 11). Although age was used as a continuous variable in all analyses, some results were visualized by splitting participants into four age groups of roughly equal size. Demographics for these age groups and the entire sample can be found in Table 1.
Table 1.
Participant Demographics
| Age Group |
Age range |
N (% female) |
Mean Age (sd) |
Mean Education (sd) |
Mean MMSE (sd) |
|---|---|---|---|---|---|
| Younger | 20 – 35 | 42 (54.8%) | 27.45 (4.4) | 15.57 (2.1) | 29.14 (.95) |
| Middle-Aged | 36 – 54 | 41 (56.1%) | 45.24 (5.4) | 15.12 (2.5) | 29.32 (.79) |
| Older | 55 – 69 | 40 (62.5%) | 61.05 (3.7) | 15.65 (2.4) | 28.95 (.72) |
| Oldest | 70 – 94 | 38 (63.2%) | 76.61 (6.1) | 15.71 (2.7) | 28.87 (.84) |
| Whole Sample | 20 – 94 | 161 (59.0%) | 51.93 (18.9) | 15.51 (2.4) | 29.07 (.84) |
Note. Abbreviations: sd – standard deviation; MMSE – Mini Mental Status Examination
All participants were screened to be right-handed and fluent English speakers with normal or corrected-to-normal vision (at least 20/40), and if necessary, vision was corrected using MRI-compatible corrective lenses during the fMRI session. Participants were additionally screened to be cognitively intact (Mini Mental Status Exam ≥ 26; Folstein et al. 1975) with no history of neurological or psychiatric conditions, head trauma, drug or alcohol problems, or significant cardiovascular disease (however, n = 32 reported diagnosis of hypertension). Participants’ informed consent was obtained in accordance with protocol approved by the University of Texas at Dallas and the University of Texas Southwestern Medical Center.
2.2. Neuropsychological Testing
Participants completed a battery of neuropsychological tests to measure performance across a variety of cognitive domains. For the current study, the Culture Fair Intelligence Test (CFIT) was the primary task of interest. CFIT is a fluid ability test consisting of matrix reasoning problems designed to evaluate the ability to recognize novel patterns and use these patterns to solve similar problems. We used Scale 3, Form B consisting of 50 items across four subtests (Cattell and Cattell 1963): 1) Series (3 minutes; 13 items) in which participants saw a series of three progressive matrices and had to identify the next matrix in the series, 2) Classification (4 minutes; 14 items) in which participants saw five matrices and had to identify the two matrices different from the other three, 3) Matrices (3 minutes; 13 items) where participants saw an array of 4 matrices (with one missing) and had to identify the matrix that would complete the array, 4) Topology (2.5 minutes; 10 items) where participants saw a series of abstract shapes with a dot and had to identify the matrix with a dot in an analogous location. After completing two to three practice items for each subtest, participants worked on the remaining problems, and scores were calculated as the number of items correct within the specified time limit for that subtest. Total number of items completed within the time limit across the four subtests was averaged to create a composite fluid reasoning score (CFIT).
2.3. fMRI Protocol
2.3.1. fMRI Acquisition
Participants were scanned on a single Philips Achieva 3T whole body scanner equipped with a 32-channel head coil. BOLD fMRI data were acquired using a T2*-weighted echo planar imaging sequence in 29 interleaved axial slices parallel to AC-PC line (64 × 64 × 29 matrix, 3.4 × 3.4 × 5 mm3, FOV = 220 mm, TE = 30 ms, TR = 1500 ms). In addition to functional volumes, high-resolution anatomical images were collected with a T1-weighted MP-RAGE sequence with the following parameters: 160 sagittal slices, 1×1×1 mm3 voxels; 256 × 204 × 160 matrix, FOV = 256 mm, TE = 3.8 ms, TR = 8.3 ms, FA = 12°.
2.3.2. fMRI Distance Judgment Task
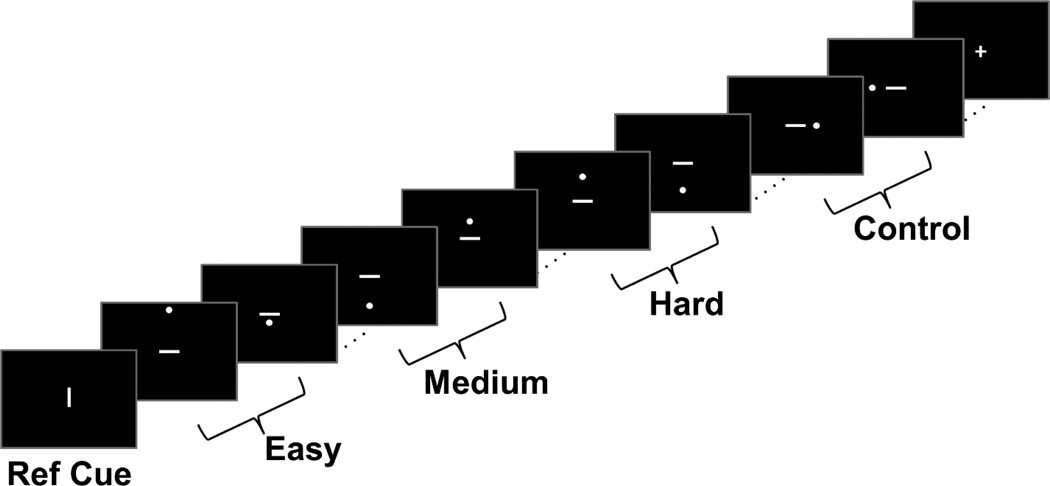
Participants performed a spatial distance judgment task in which they conducted two types of judgments (modeled after Baciu et al. 1999, and D.C. Park et al., 2010). The first judgment was a categorical (LEFT/RIGHT) judgment in which they saw a dot on the left or right side of a horizontal bar. Using their index and middle finger on the right hand, participants indicated whether the dot was on the left or right side of the bar. This task was used as a control condition. The second judgment was a coordinate (NEARER/FARTHER) judgment in which participants briefly saw a vertical reference line, followed by a dot either above or below a horizontal bar at some distance. Participants indicated whether the dot was “nearer to” or “farther from” the horizontal bar, using the previously viewed vertical line as a reference for the distance (see Figure 1 for a schematic). The coordinate judgment task included three levels of difficulty: easy, medium, and hard. In easy blocks, the dot was either very close to or very far from the horizontal bar. In medium conditions, the distance between the dot and horizontal bar was slightly closer to the length of the vertical reference line. In the hard condition, the distance between the dot and horizontal bar was very close to the length of the vertical reference line, making it more difficult to judge the “nearness” or “farness”. The range of distances used in each difficulty level was determined by behavioral piloting of the task in 11 younger and older adults using response time cost to determine which stimuli were used to create the three difficulty conditions. Pilot participants also made subjective ratings of the stimuli as “easy” or “difficult”.
Figure 1. Schematic of fMRI task.
While in scanner, participants made coordinate nearer/farther judgments of the dot to the bar relative to the length of the reference cue at varying levels of difficulty (easy, medium, and hard). They also made categorical left/right judgments as a control task.
Prior to entering the scanner, participants completed a short practice session of the task to ensure they understood the instructions. In scanner, each condition of the task (control, easy, medium, or hard) was presented in block design with five trials of a particular condition per block. Stimuli were presented for 2500 ms with a 500 ms interstimulus interval using PsychoPy v1.77.02 (Peirce 2007, Peirce 2009) and responses were recorded via Current Designs 4-button fiber response pad (Current Designs INC, Philadelphia, PA). Each run of data consisted of 20 blocks split across the four conditions presented in pseudo-random order. Each run also included two 15 sec blocks of fixation interspersed between the task blocks. There were three runs total yielding a total functional scan time of approximately 15 minutes.
2.3.3. fMRI Processing
Individual participant’s time series data were preprocessed with Statistical Parametric Mapping 8 (SPM8; Wellcome Department of Cognitive Neurology, London, UK) according to a standard pipeline of procedures. First, images were corrected for differences in slice time acquisition. Second, individual volumes were corrected for within-run participant movement. Finally, images were normalized to a common MNI space and smoothed with an isotropic 8 mm3 full-width-half-maximum Gaussian kernel. Additionally, ArtRepair (Mazaika et al. 2007) was used to identify potential outlier volumes for each participant. For each participant, runs that had more than 15% outlier volumes (~30 volumes) with greater than 3% deviation from the mean in global intensity spikes or greater than 2 mm of motion displacement were flagged. Five participants had one run of data (out of three runs) flagged for excessive outlier volumes and the noisy run was subsequently removed from further analysis. Participants with more than one run flagged for excessive outlier volumes were excluded from the study entirely (n = 4).
At the individual subject level, BOLD response to each condition (control, easy, medium, hard) was modeled in SPM as a block convolved with a canonical hemodynamic response function; six directions of motion-estimates for each volume generated from ArtRepair were also included as nuisance covariates. Several first-level contrasts were computed: Hard vs. Easy (to examine individual differences in modulation to difficulty) as well as Easy vs. Control, Medium vs. Control and Hard vs. Control for use in post-hoc examination of our second-level results (described below).
2.4. fMRI Data Analysis
Second-level modeling proceeded with two major analyses: First, we examined the effect of increasing task difficulty across all participants. For the entire sample, single-sample t-tests were conducted on the first-level contrasts of the linear effect of difficulty, which is equivalent to the contrast of Hard vs. Easy. This allowed us to determine which regions of cortex across all participants showed increased or decreased activity (positive modulation or negative modulation, respectively) in response to changing difficulty. Next, we examined the effect of age on modulation of difficulty from the easiest level of the task to the hardest level of the task. A voxel-wise regression was conducted using age (as a continuous variable) to predict increases or decreases in activity for Easy vs. Hard levels of distance judgment (positive modulation and negative modulation effects). All second-level analyses were whole-brain voxel-wise multiple regressions, cluster corrected at FWE p < .05 with a height threshold of p < .0001.
3. Results
3.1. Behavioral Results
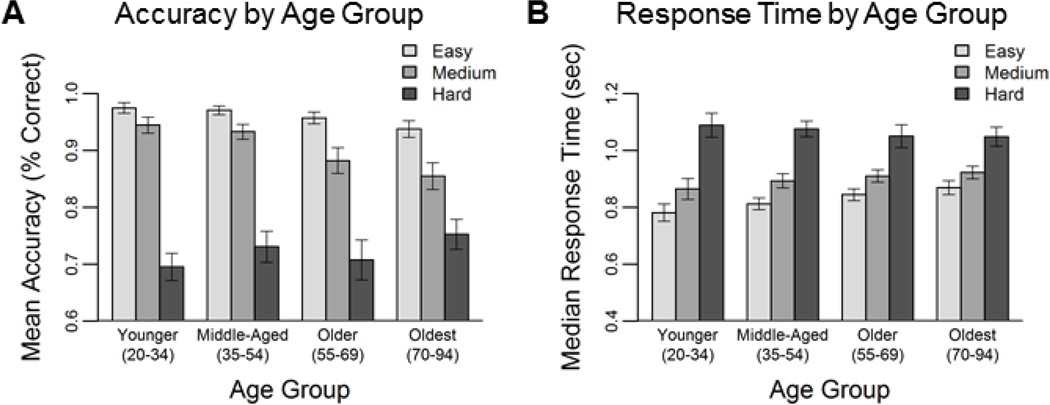
To examine the effects of age and difficulty manipulation on task performance two repeated-measures general linear models were conducted with difficulty as a three-level (Easy, Medium, and Hard) within-subjects measure and age as a continuous between-subjects measure to predict mean accuracy and median response time across trials. For task accuracy, we found significant main effects of age (F(1,159) = 4.12, p = .044), and difficulty (F(2,318) = 38.23, p <.001), as well as an age × difficulty interaction (F(2,318) = 5.72, p = .004). To decompose this interaction we conducted regressions with age on each level of difficulty1 (however for clarity of illustration, the results are plotted using binned age variables; Figure 2). With increasing age, participants were less accurate on easy (t(159) = −2.98, p = .003) and medium (t(159) = −3.73, p < .001), but not on hard (t < 1, p = .35) levels of difficulty (Figure 2A). For response time we found significant main effect of difficulty (F(2,318) = 62.06, p <.001) and an age × difficulty interaction (F(2,318) = 9.96, p <.001), which indicated that increasing age was associated with slower response times on easy t(159) = 3.23, p = .002) and medium (t(159) = 2.04, p =. 043), but not on hard (t < 1, p = .48) levels (Figure 2B).
Figure 2. Behavioral results from fMRI task.
Accuracy (A) decreased and response time (B) increased with increasing levels of difficulty. Error bars indicate standard error of the mean.
3.2. Imaging Results
3.2.1. Supplementary Contrast Effects
Details of contrasts of potential interest are included as supplementary reports in Kennedy and colleagues (in press). These include the main effect of Coordinate vs. Categorical Judgments (Figure 1 and Table 1 in Kennedy et al in press Data in Brief) which illustrates largely right lateralized regions of activity greater during coordinate than categorical judgments consisting of fronto-parietal regions including dorsolateral prefrontal cortex, anterior cingulate, and precuneus which are regions largely consistent with both spatial judgment (Baciu et al., 1999) and working memory (Barch et al. 1997; Cole et al., 2013) networks. Regions where categorical control task was greater than coordinate task included bilateral temporo-parietal junction, middle temporal, posterior parietal (including angular and supramarginal gyrus), posterior cingulate, and medial prefrontal cortex, regions largely consistent with a “default mode” network of activity (Greicius et al. 2003). For additional supplementary information on incrementing levels of difficulty from Easy to Medium and Medium to Hard see Figure 2A, B and Tables 2 and 3 in Kennedy et al., (in press) Data in Brief.
Table 2.
Cluster Peaks for whole sample effect of difficulty (Hard vs. Easy)
| A. Positive Modulation to Difficulty Effect [Hard > Easy] | |||||||
|---|---|---|---|---|---|---|---|
| Cluster Label | BA | k | X | Y | Z | t-value | p FWE |
| L/R anterior cingulate & R insula / inferior frontal |
8 | 3050 | 6 | 24 | 45 | 10.30 | <.001 |
| 13 | 30 | 24 | −3 | 10.06 | |||
| 46 | 45 | 36 | 18 | 9.94 | |||
| R posterior/inferior parietal & middle occipital |
7 | 2809 | 30 | −57 | 54 | 9.81 | <.001 |
| 18 | 27 | −87 | 18 | 9.67 | |||
| 40 | 48 | −39 | 48 | 9.65 | |||
| L Cerebellar Crus I & II | 483 | −33 | −69 | −45 | 8.49 | <.001 | |
| −30 | −66 | −33 | 8.43 | ||||
| −9 | −78 | −30 | 7.94 | ||||
| L insula | 13 | 153 | −30 | 21 | 0 | 8.18 | <.001 |
| L precentral & inferior frontal | 44 | 119 | −45 | 3 | 27 | 6.02 | .001 |
| 44 | −57 | 18 | 33 | 4.58 | |||
| L inferior orbital frontal | 47 | 64 | −45 | 45 | −6 | 5.61 | .009 |
| B. Negative Modulation to Difficulty Effect [Hard < Easy] | |||||||
| Cluster Label | BA | k | X | Y | Z | t-value | pFWE |
| L/R Precuneus | 31 | 13885 | 0 | −63 | 24 | 11.90 | <.001 |
| 31 | 9 | −54 | 33 | 11.51 | |||
| 23 | −6 | −54 | 27 | 11.42 | |||
| R inferior occipital | 18 | 163 | 27 | −96 | −3 | 10.70 | <.001 |
| L inferior occipital | 18 | 115 | −24 | −96 | −9 | 8.42 | .001 |
| R cerebellum | 49 | 9 | −54 | −45 | 6.98 | .017 | |
| R cerebellar crus II | 206 | 24 | −84 | −36 | 6.67 | .005 | |
| 45 | −75 | −39 | 5.36 | ||||
| L middle orbital frontal | 47 | 79 | −27 | 36 | −15 | 6.64 | <.001 |
| R middle frontal | 8 | 40 | 24 | 33 | 39 | 5.30 | .032 |
Note. p < .0001 uncorrected; Abbreviations: BA – Brodmann Area; L – left; R – Right
Table 3.
Cluster peaks for the regression of age on modulation to difficulty
| A. Age-Related Decreases in Positive Modulation to Difficulty | |||||||
|---|---|---|---|---|---|---|---|
| Cluster Label | BA | k | X | Y | Z | t-value | p FWE |
| R intraparietal sulcus, inferior & superior parietal lobules |
40 | 603 | 45 | −39 | 54 | 5.70 | < .001 |
| 7 | 27 | −63 | 54 | 5.36 | |||
| 40 | 36 | −45 | 42 | 5.22 | |||
| R insula | 13 | 176 | 42 | 21 | −6 | 5.35 | < .001 |
| 47 | 30 | 30 | −6 | 4.96 | |||
| 44 | 33 | 30 | 12 | 4.91 | |||
| R middle frontal | 6 | 86 | 33 | 0 | 57 | 5.13 | .003 |
| R inferior frontal | 44 | 94 | 51 | 9 | 24 | 5.03 | .002 |
| 6 | 57 | 12 | 42 | 4.49 | |||
| 44 | 48 | 12 | 12 | 4.12 | |||
| R anterior cingulate | 32 | 65 | 6 | 18 | 48 | 4.64 | .007 |
| 32 | 3 | 33 | 33 | 4.55 | |||
| B. Age-Related Decreases in Negative Modulation to Difficulty | |||||||
| Cluster Label | BA | k | X | Y | Z | t-value | pFWE |
| L middle frontal | 9 | 69 | −27 | 33 | 42 | 5.41 | .006 |
| L angular gyrus | 39 | 91 | −45 | −75 | 33 | 5.35 | .002 |
| 39 | −42 | −72 | 48 | 4.26 | |||
| L/R cuneus, precuneus & posterior cingulate |
18 | 241 | −3 | −87 | 24 | 5.20 | <. 001 |
| 23 | −9 | −60 | 15 | 5.14 | |||
| 31 | 9 | −60 | 18 | 5.05 | |||
| L/R ventromedial prefrontal | 10 | 135 | 0 | 51 | −6 | 4.76 | < .001 |
| 10 | −6 | 51 | 6 | 4.22 | |||
Note. p < .0001 uncorrected; Abbreviations: BA – Brodmann Area; L – left; R – Right
3.2.2. Effect of Increasing Difficulty from Easier to Harder Judgments
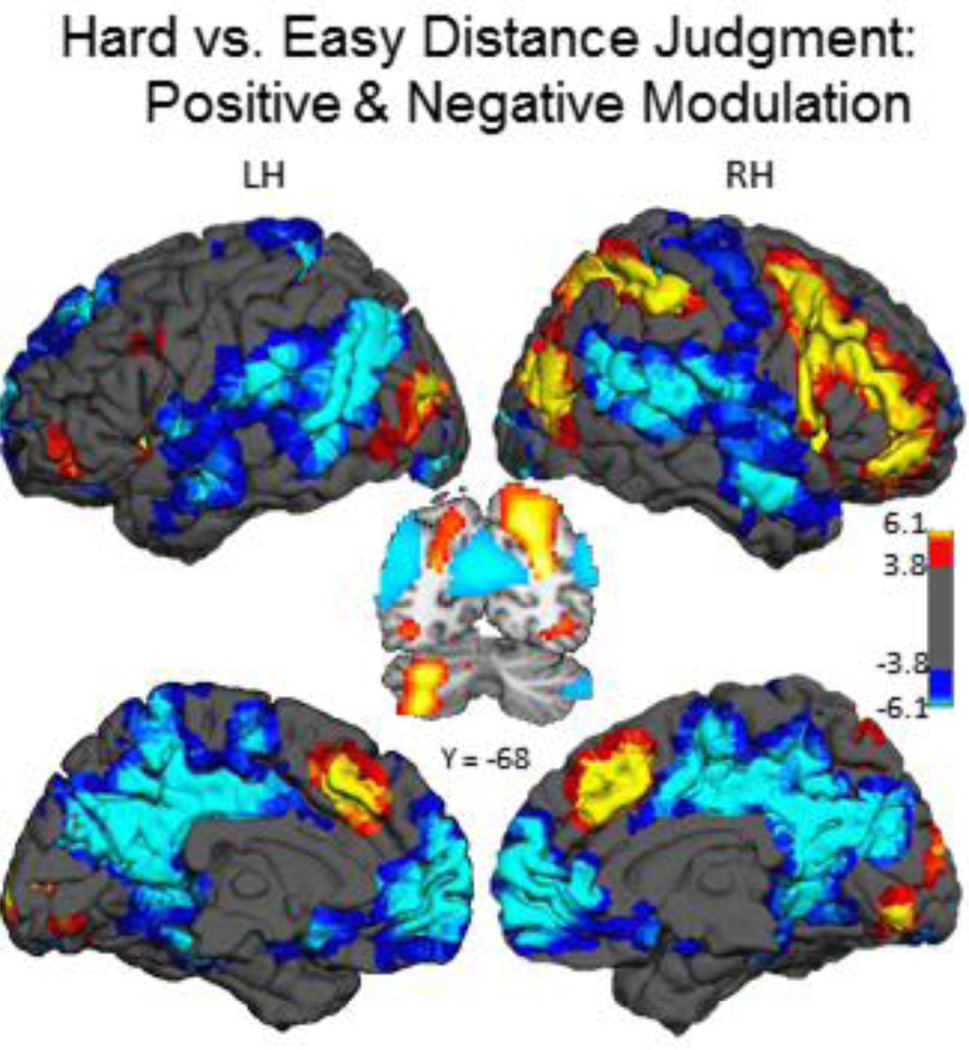
Our primary contrast of interest to examine activation for the difficulty manipulation was a linear contrast across the three levels of difficulty, which is equivalent to a contrast of Hard vs. Easy conditions. We found regions with positive modulation and regions with negative modulation effects. The positive modulation effect was characterized by large and widespread increases in activation to Hard compared to Easy in largely right-lateralized fronto-parietal regions including: middle and inferior frontal gyrus, inferior parietal and anterior cingulate (Figure 3 hot clusters; coordinates in Table 2A). In the negative modulation effect, activity was greater for Easy compared to Hard levels in lateral temporo-parietal, medial frontal, precuneus, and posterior cingulate cortices (Figure 3; cool clusters; Table 2B).
Figure 3. Main effect of difficulty manipulation.
On the coordinate distance judgment task, increasing difficulty from easier to harder judgments was associated with increased modulation of activation (positive modulation effect) in largely right-lateralized frontal and parietal association cortex, left-lateralized cerebellum, and bilateral anterior cingulate (hot clusters) and increased negative modulation (negative modulation effect) in posterior cingulate, precuneus and medial frontal cortex (cool clusters). Color-scale indicates t-values. Abbreviations: LH – Left Hemisphere; RH – Right Hemisphere.
3.2.3. Effect of Age on Positive and Negative Modulation to Increasing Difficulty
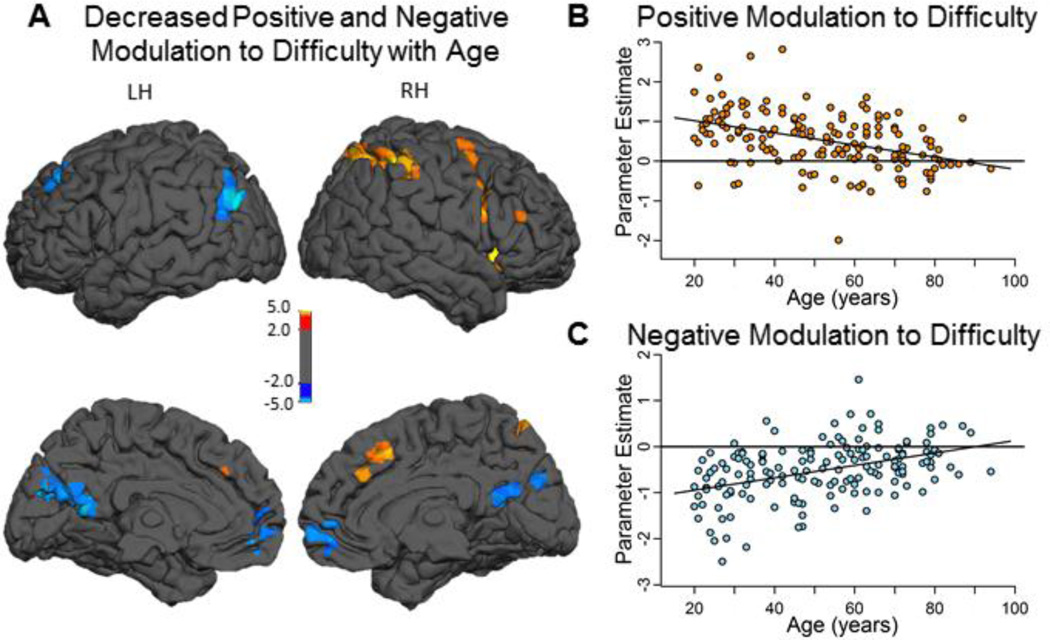
To investigate differences in ability to modulate activity to increased difficulty across the adult lifespan we tested an age × difficulty [Hard, Easy] interaction with Age (continuous) and difficulty (2-level) as factors2. This voxelwise regression revealed that BOLD modulation to difficulty weakens with aging in both positive and negative modulated regions (see Figure 4A; coordinates in Table 3). Specifically, increasing age was associated with decreasing positive modulation of activation to difficulty in right-lateralized fronto-parietal regions including middle and inferior frontal gyri (BA 6 and 44/47), insula (BA 13), intraparietal sulcus (including both superior [BA 7] and inferior [BA 40] parietal lobules), and dorsal anterior cingulate (BA 32) (see hot colors in Figure 4A, B).
Figure 4. Effects of aging on positive and negative modulation of activation to difficulty.
Increasing age was associated with decreased modulation to difficulty in both regions of positive modulation (hot colors in panel A) and regions of negative modulation (cool colors in panel A). The regions of age-reduced modulation included right lateralized fronto-parietal and anterior cingulate regions (A; hot clusters) and ventromedial prefrontal, posterior cingulate, left angular and left middle frontal cortices (A; cool clusters). Panel B illustrates age-related decreases in mean positive modulation regions to Hard relative to Easy averaged across all hot clusters. Panel C illustrates age-related decreases in mean negative modulation averaged across all cool clusters. Color-scale indicates t-values. Abbreviations: LH – Left Hemisphere; RH – Right Hemisphere.
Increased age was also associated with significantly decreased negative modulation in two midline regions -- ventromedial prefrontal cortex (vmPFC) (BA 10) and ventral posterior cingulate/precuneus (BA 23 and 31), in addition to left angular (BA 39) and left middle frontal (BA 9) gyri (see cool colors Figure 4A, C).
3.2.4. Decomposing the Age × Difficulty Interaction
To understand the nature of the age × difficulty interaction (i.e., to determine when in the lifespan these difficulty modulation differences arise for the two networks), we decomposed the effects as follows. Using the MarsBar (Brett et al. 2002) toolbox in SPM8, mean parameter estimates from each cluster from the age-regression analysis (Table 3) were extracted for each participant for first-level Easy vs. Control, Medium vs. Control and Hard vs. Control contrasts. Parameter estimates from the five positive modulation effect clusters (i.e., right middle frontal, right inferior frontal, right insula, right posterior parietal and anterior cingulate) were averaged to create a summary estimate of age-related decreases in modulation in these regions. Parameter estimates extracted from the four negative modulation effect clusters (left angular, left middle frontal, precuneus/posterior cingulate, ventromedial prefrontal) were averaged to create an estimate of age-related decreases in modulation in these regions.
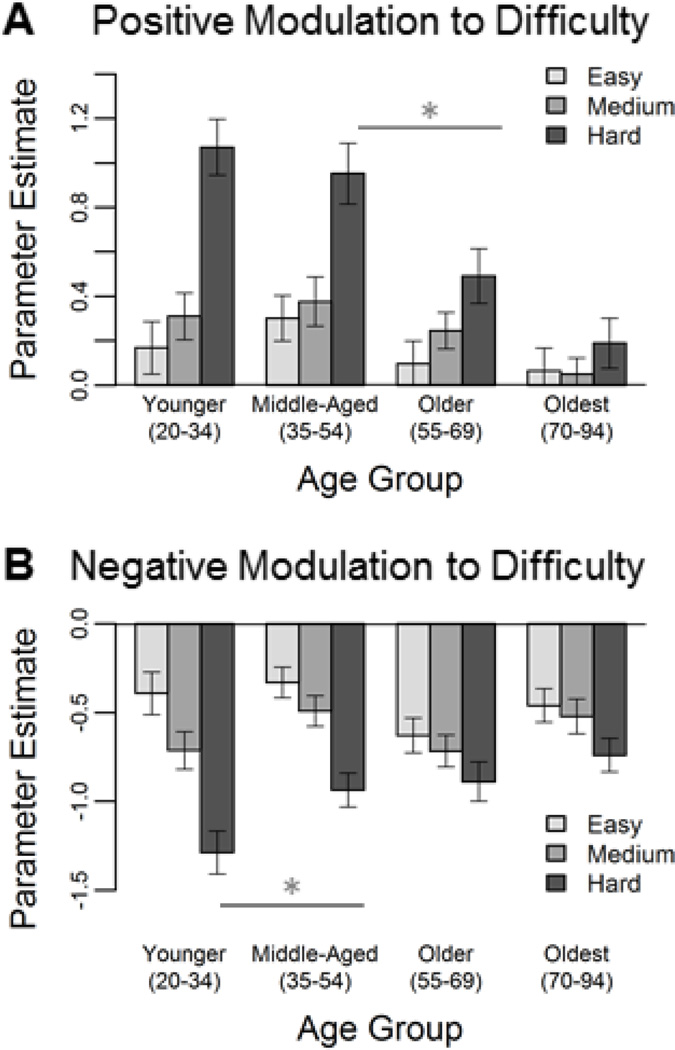
To determine at what point in the lifespan there were shifts in modulation, participants were split into four age groups and mean parameter estimates for each level of difficulty relative to control were examined (Figure 5). For the positive modulation network, younger and middle-aged adults showed increased activation from easier to harder judgments (Figure 5A), with the biggest age difference occurring with a decrease in activity to hard judgments from middle-age to old age (t(79) = 2.51, p = .014). Interestingly, the oldest group no longer showed increased activation to more difficult items. For the negative modulation effect it is clear that these regions deactivate more to harder judgments, and this modulation difference across difficulty levels is lost with increasing age (Figure 5B), with the largest age difference from young to middle-age (t(81) = −2.26, p = .027).
Figure 5. Differential aging of positive modulation and negative modulation regions.
Age-related decreases in difficulty modulation in the positive modulation network (i.e., right fronto-parietal cortices) were not evident until between middle and older age (Panel A). Age-related differences in the negative modulation network (i.e., ventromedial prefrontal, precuneus/posterior cingulate, left angular and middle frontal gyri) occurred earlier in the lifespan, between younger and middle-age (Panel B). Error bars indicate standard error of the mean.
3.3. Relationship between positively modulated and negatively modulated networks
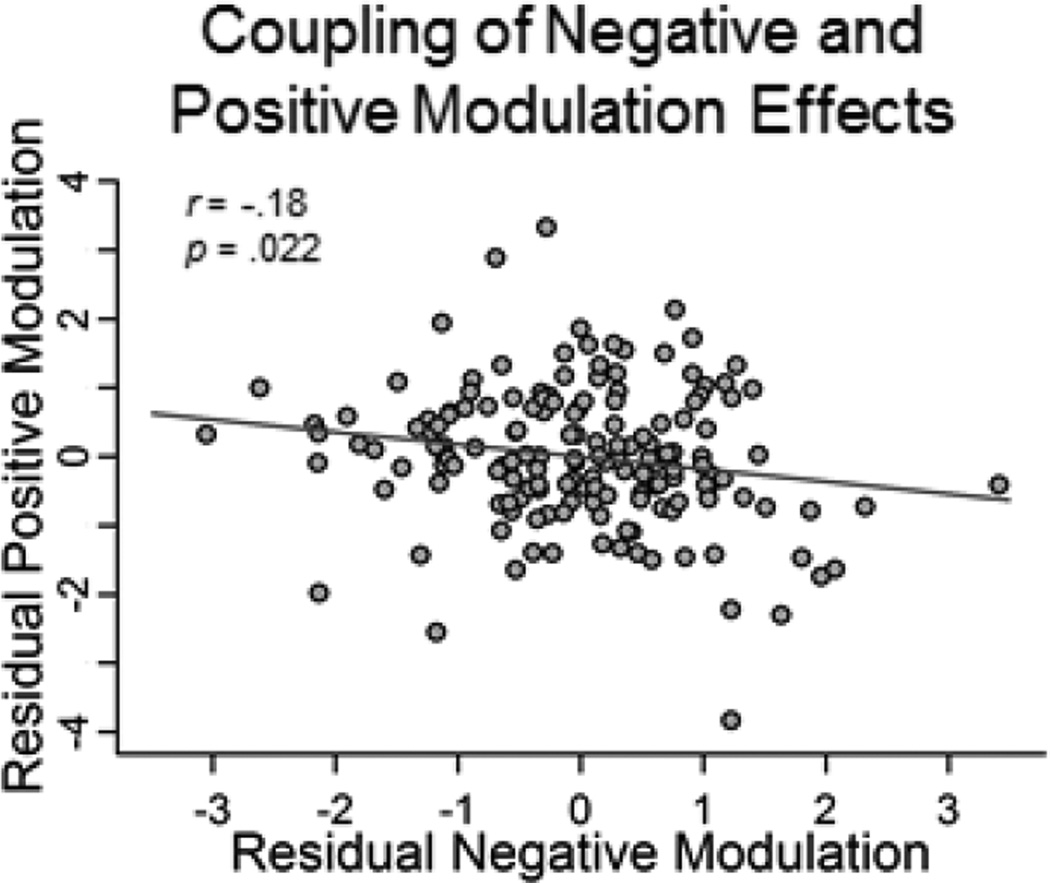
We were next interested in whether ability to modulate BOLD response (i.e., exhibit greater differences in activity between Hard and Easy judgments) was related between the two networks. To investigate whether there was coupling of these networks (beyond their shared age-variance) we computed a partial correlation (controlling for age) between modulation (Hard > Easy parameter estimates) in positive modulation regions and negative modulation regions and found that there was small but significant coupling (r = −.18, p = .022) between the two functional measures. In other words, regardless of age, those participants that exhibited increased modulation in positive regions (e.g., right fronto-parietal cortices) also showed increased modulation in negative regions (i.e., greater deactivation in ventromedial prefrontal, precuneus/posterior cingulate), suggesting that the ability to flexibly modulate activation to difficulty is somewhat related between activated and deactivated networks (Figure 6).
Figure 6. Coupling between positive modulation and negative modulation to difficulty.
A significant association between BOLD modulation to difficulty in positive modulation and negative modulation regions was observed, regardless of age, indicating that greater activation in positive modulation regions was associated with greater deactivation of negative modulation regions.
3.4. Linking Age-Related Modulation to Difficulty with Behavior
Our second major goal was to link age-related differences in modulation to difficulty with measures of behavior (both on the in-scanner task and to a relevant measure of cognitive ability outside of the scanner, fluid reasoning) to determine if these age-related alterations in modulation were beneficial or detrimental. As in the prior analysis, modulation to difficulty was calculated as the difference in BOLD response to Hard and Easy judgments in the two networks resulting from the age regression (Table 3).
Task Accuracy
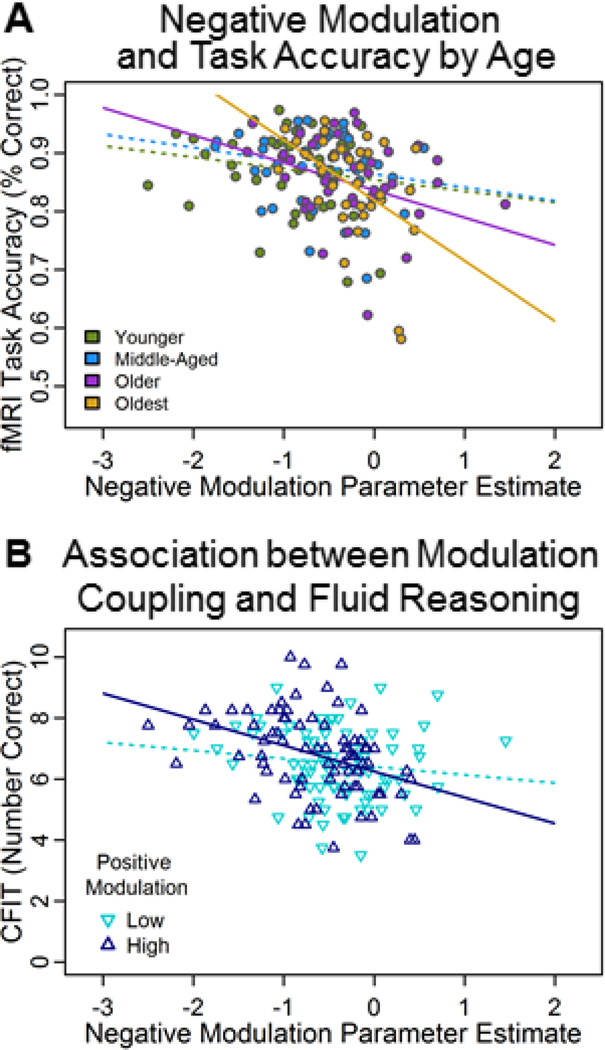
To examine whether age-related reductions in modulation of the BOLD response were related to task performance we conducted a multiple regression with age (continuous), mean positive modulation, mean negative modulation, and their interaction terms to predict accuracy on the functional task (averaged across easy, medium, and hard trials). Results revealed a significant age × negative modulation interaction (F(6,154) = 6.86, p = .009). To decompose this interaction, we performed univariate regressions for each of the four age-groups to predict accuracy from negative modulation regions (Figure 7A). These post-hoc analyses revealed that the effect was selective to the old adults, specifically for the two oldest age groups (ages 55–69: t(38) = −2.01, p = .051 and 70–94: t(36) = −3.24, p = .002), more negative modulation predicted higher task accuracy, suggesting that greater deactivation of this network is beneficial to performance.
Figure 7. Predicting cognitive performance from modulation to difficulty.
A). More negative modulation to difficulty was associated with greater mean judgment accuracy selectively in old adults (ages 55–69 and 70–94). B). Significant positive modulation by negative modulation interaction indicated an interdependency of these networks, such that those participants showing both high positive modulation in right fronto-parietal regions and greater negative modulation in deactivated regions exhibited higher fluid reasoning (CFIT) scores, regardless of age. Significant regression lines (p ≤ .05) are indicated by a solid line trend; nonsignificant regression lines are indicated by dotted lines.
3.4.1. Fluid Reasoning
Finally, to examine whether modulation to difficulty also predicted a related task completed outside the scanner, a second analysis was run using age, mean positive modulation, mean negative modulation, and their interactions to predict performance on CFIT, a test of general reasoning ability and fluid intelligence. We found a significant positive modulation × negative modulation interaction (F(6,154) = 4.16, p = .047). To examine the nature of the significant interaction between two continuous variables on reasoning, a median split was performed on the positive modulation variable (after regressing out age) to identify those participants with “high” and “low” modulation and was plotted against negative modulation as a continuous variable. Post-hoc analyses revealed that those participants who demonstrated both higher positive modulation and greater negative modulation had better fluid reasoning scores (t(78) = −3.70, p < .001; Figure 7B), suggesting that individuals with better fluid reasoning ability were able to better modulate both regions of activation and regions of deactivation.
4. Discussion
In the current study we examined the effect of increased cognitive difficulty on brain activation during a visuo-spatial distance judgment task across a large, adult lifespan sample. Across all participants, engaging in more difficult spatial distance judgments was associated with increased brain activity in largely right-lateralized fronto-parietal regions (positive modulation effect) and greater deactivation of regions typically associated with the default mode network (negative modulation effect). Next, we report that older age was characterized by reduced modulation to difficulty in right-lateralized posterior parietal, inferior and middle frontal gyri, and anterior cingulate (positive modulation) and in ventromedial prefrontal, precuneus/posterior cingulate and left lateralized angular and middle frontal gyri (negative modulation). Importantly, greater modulation was associated with better performance on both the in-scanner task and an out-of-scanner measure of fluid reasoning. These results support theories that when faced with increased difficulty, older adults may have fewer neural resources available for cognitive control, coupled with a reduced ability to dampen activity which may impede cognitive performance.
4.1. How does the brain respond to increased spatial judgment difficulty?
In our study, participants completed a visuo-spatial distance judgment task in which they estimated the distance between a dot and horizontal bar for easy, medium, and hard difficulty trials. This distance judgment task engaged both regions involved in visuo-spatial and working memory processes (see Kennedy et al in press Data in Brief). When examining the BOLD response to difficulty across all participants (i.e., regardless of age), we found increased activity in right-lateralized fronto-parietal regions and anterior cingulate for Hard distance judgments, and increased activity in large portions of medial prefrontal, posterior cingulate, and temporo-parietal cortex for Easy judgments (consistent with typical default mode regions; Greicius et al. 2003). The observation that default regions were more active during easier levels of the task is in accord with previous work (Persson et al. 2007). The right-lateralization of increased difficulty modulation at Hard levels may indicate a greater reliance on right posterior parietal regions responsible for making coordinate (i.e., “nearer” vs. “farther”) distinctions when cognitive demands are increased. This finding supports early work suggesting that right posterior parietal cortex has an advantage to processing coordinate compared to categorical information (Baciu et al. 1999) and suggests that as coordinate judgments become more difficult, participants may engage task-relevant regions of right parietal cortex to meet task demands.
4.2. How does modulation to difficulty differ across the lifespan?
When examining the lifespan effect of age on ability to modulate functional activity to difficulty (Easy vs. Hard), we found that increasing age was associated with decreased modulation in right-lateralized fronto-parietal regions. Unlike prior work suggesting that older adults “over-activate” frontal regions (compared to young adults) during difficult cognitive operations (Reuter-Lorenz and Cappell 2008; Schneider-Garces et al. 2010; see Grady 2012 for a review), in the current study we did not find any regions in the positive modulation network in which modulation increased with age. Furthermore, as seen in Figure 6, the decreased right fronto-parietal modulation in old age was not due to a higher level of activity at easy levels of the task; rather, with increasing age, the ability to increase activation as task demands became harder was diminished, suggesting that older adults showed similar levels of activity to young at easy levels of the task, but as difficulty increased, they were unable to bring relevant neural resources online. Indeed, prior studies finding age-related over-recruitment have utilized extreme age-group comparisons between young and older adults; therefore, findings of age-related “over- or under-recruitment” are, by definition, based on relative age-group differences. Here we utilized a continuous lifespan age analysis approach, which allowed us to model incrementing age as a biological construct rather than examining how old are different from young, as the extant literature has done.
Our findings are partially consistent with a recent hypothesis suggesting that age-related decreases in modulation to difficulty in frontal cortex will co-occur with decreased modulation in default mode regions (Turner and Spreng 2015). We observed that increased age was associated with decreased modulation in typically default regions (ventromedial prefrontal, posterior cingulate and left angular gyrus). This decreased modulation appeared to be driven by less deactivation at Hard levels of the task in the middle-aged through older adults, relative to younger adults. Our finding of increased deactivation with greater difficulty also supports previous work in which difficulty levels were parametrically manipulated across a variety of cognitive tasks (Turner and Spreng 2015; Persson et al. 2007) suggesting that the ability to suppress activity is imperative as cognitive demands increase.
Our findings are also consistent with earlier work using a similar experimental paradigm. D.C. Park and colleagues (2010) investigated age-group differences associated with categorical (i.e., “left” / “right”) compared to coordinate (i.e., “nearer” / “farther”) judgments which they operationalized as “easy” and “hard” judgments, respectively and found that compared to young, older adults failed to suppress activity in default regions during more difficult coordinate compared to the easier categorical spatial judgments. The current work extends this prior study by utilizing the categorical judgment as a control task, including a parametric modulation of difficulty in coordinate judgments (i.e., easy, medium, hard), and testing a large lifespan sample. Here we add to this knowledge showing robust age reductions in modulation in fronto-parietal regions.
4.3. When in the lifespan are differences in modulation to difficulty apparent?
In the current study, age differences in modulation to difficulty were observed in both positive modulation and negative modulation networks, but at different points of the lifespan. Specifically, during difficult distance judgments, regions including right-lateralized middle and inferior frontal, posterior parietal, and anterior cingulate, showed decreased BOLD response at the transition between middle age (35–54 years) and older age (55–69 years). On the other hand, for regions in which activity was deactivated during distance judgments (ventromedial PFC, precuneus/posterior cingulate, left angular and middle frontal gyri), the greatest age difference in modulation was already apparent from young (20–34 years) to middle-age (35–54 years) and was characterized by less deactivation in middle-aged compared to young adults. These cross-sectional results hint at a differential time-course in the effect of age on BOLD response in typically default regions compared to fronto-parietal cognitive control regions.
Age group differences in default mode suppression during difficult cognitive tasks are well studied (D.C. Park et al. 2010; Persson et al. 2007). However, it is only with recent lifespan approaches that examine middle-age that it appears that these age-related changes may emerge much earlier in the lifespan than previously thought. For example, a study of functional network connectivity in a sample of 17–62 year olds found connectivity between fronto-insular cortex and default mode regions showed degradation as early as middle age (He et al. 2013). It is important to note that longitudinal studies of age-related changes in default mode activity (specifically in medial default regions) have not found substantial age-related longitudinal change (Persson et al. 2014; Beason-Held et al. 2009); however, the samples included in those studies were age-restricted (i.e., the youngest participants included were 55 years old). Thus our finding between young and middle age in medial and left angular default regions in the current study would have preceded the entry of the subjects to those studies and required a wide age-span sample to capture. Future work including longitudinal measures across the entire adult lifespan will help to better understand how default mode activity changes with age.
Although the number of functional neuroimaging studies with full lifespan samples is limited, there is evidence that other brain systems may also show different trajectories of aging. For example, recent work examining modulation of activity during difficult semantic judgments found that fronto-parietal regions exhibited age differences earlier in the lifespan (i.e., from middle to old age), whereas subcortical dopaminergic regions showed age differences much later (from old to very old age; Kennedy et al. 2015). Likewise, a lifespan study of neural correlates of successful memory encoding reports that engagement of “positive subsequent memory” regions (i.e., temporal, parietal and occipital regions) may begin to decline from young to middle age, whereas suppression of activity in “negative subsequent memory” regions (i.e., regions typical of the default mode network) may not differ until the transition from middle to old age (Park et al. 2013). Taken together with the current results, it is possible that some neural systems may age more rapidly than others. Further work examining interactions between functional systems as well as longitudinal research examining individual change in functional activity is needed to more precisely characterize the trajectory of functional brain aging.
4.4. What are the cognitive consequences of reduced modulation to difficulty?
One of the greatest challenges in the cognitive neuroscience of aging is linking age-differences in functional activity under task conditions to meaningful differences in behavior. Yet, a clear interpretation of age-related increases or decreases in activity as either compensatory or maladaptive necessitates demonstrating a link between those neural patterns to differences in cognitive performance. For example, prior work examining frontal activation across a variety of cognitive domains reports that in older adults, over-recruitment of prefrontal cortex (compared to young adults) has been associated both with better task performance (suggesting a prefrontal activation is providing a compensatory role; Cabeza 2002; Davis et al. 2008; Rossi et al. 2004) and worse task performance (suggesting that prefrontal activation may be indicative of inefficient processing; de Chastelaine et al. 2011). Similarly, less selective activation in specialized visual regions has been associated with poorer fluid processing (Park J. et al. 2010; Rieck et al. 2015) and poorer memory performance (Burianova et al. 2013).
The current study revealed a significant age-dependent brain-behavior relationship selective to our old participants (ages 55–69 and 70–94). Specifically, greater negative modulation predicted better performance during the in-scanner distance judgment task. These results support the idea that engagement of regions that are normally suppressed during the task is not necessarily compensatory, as it is not linked to better performance. Rather, the ability to modulate negative activity is an important component of maintained cognitive ability in old age. Prior work in different cognitive domains finds similar results—greater suppression of default regions has been linked to better executive function (Damoiseaux et al. 2008) and better subsequent memory performance (Otten and Rugg 2001), and this relationship may be moderated by age such that it is strongest in old age (Miller et al. 2008; Damoiseaux et al. 2008) or apparent even as early as the transition from middle to older age (Park et al. 2013). Furthermore, our findings suggest that even though functional differences in negative modulation were evident as early as middle-age, the behavioral correlates of inadequate modulation of activation to difficulty might not be observed until much later in the lifespan.
We also report a novel brain-brain relationship predicting performance on our out-of-scanner task; we found an interactive effect between right fronto-parietal network modulation (positive modulation) and negative modulation, such that better fluid ability was predicted by increased modulation in positive regions and increased modulation in negative regions, even after controlling for age. Importantly, these findings suggest evidence for an interdependent relationship between functional activity in two different brain systems. Prior work finds that fronto-parietal control regions may be responsible for the engagement and disengagement of other brain networks like default mode regions (Cole et al. 2013). In the current study, it is possible that more robust right fronto-parietal modulation might aid in the modulation of negative activity, thereby enabling greater fluid reasoning ability (or this ability to flexibly modulate activation is enjoyed by those with higher reasoning ability). However, the exact direction of the relationship between these two functional systems cannot be determined from the current cross-sectional analysis. Regardless, our findings suggest a potentially strong relationship between activating fronto-parietal control and deactivating “default-like” systems in predicting cognitive performance, and this work provides further impetus to examine interactive relationships between different brain systems across the lifespan.
4.5. Conclusions
In sum, we provide novel evidence for widespread differences in ability to modulate functional resources to increased difficulty across the lifespan. We found that during a visuo-spatial distance judgment task, increasing age was associated with reduced modulation of both positive modulation in right-lateralized fronto-parietal regions and of negative modulation in regions showing deactivation to difficulty (ventromedial prefrontal, precuneus/posterior cingulate, left angular and middle frontal gyri). We also provide important evidence that the reduced ability to modulate activity to difficulty was predictive of poorer performance on the in-scanner task and of poorer fluid intelligence. Interestingly, the trajectory of neurocognitive aging may differ across functional brain systems—we found evidence that these deactivated regions may show greater age differences earlier in the lifespan than fronto-parietal cognitive control regions. Even though functional differences were evident as early as middle-age (i.e, starting around 35 years old), the behavioral correlates of inadequate negative modulation might not be observed until much later in the lifespan (i.e., after the age of 55). The precise mechanisms underlying these functional processes and influencing the time-course in which these changes appear motivates further research into the (longitudinal) time course of neurocognitive aging.
Highlights.
BOLD activity is up-modulated and down-modulated in response to cognitive difficulty
Both positive modulation and negative modulation weaken with increasing age
Negative modulation weakens earlier in the lifespan than positive modulation
Greater coupling of positive and negative modulation predicts higher fluid reasoning
Greater negative modulation in older adults predicts better task accuracy
Acknowledgments
This work was supported in part by the National Institutes of Health (AG-036818, AG-036848). We thank Andy Hebrank for assistance with the functional task programming and Asha Unni for help with behavioral piloting and data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
To test for non-linear effects of age across the lifespan, additional regression models were conducted testing the quadratic effect of age on each behavioral measure. In no analysis did the quadratic age term explain significantly more variance than the linear term.
To test for non-linear effects of age on modulation to difficulty an additional whole-brain analysis adding a quadratic term was conducted. There were no regions in which a quadratic age term explained more variance than the linear term.
conflict of interest
The authors (JRR, KMR, MAB, KMK) of this manuscript (Age-related Reduction of BOLD Modulation to Cognitive Difficulty Predicts Poorer Task Accuracy and Poorer Fluid Reasoning Ability) have no conflicts of interest to report.
References
- Ankudowich E, Pasvanis S, Rajah MN. Changes in the modulation of brain activity during context encoding vs. context retrieval across the adult lifespan. NeuroImage. 2016;139:103–113. doi: 10.1016/j.neuroimage.2016.06.022. [DOI] [PubMed] [Google Scholar]
- Baciu M, Koenig O, Vernier MP, Bedoin N, Rubin C, Segebarth C. Categorical and coordinate spatial relations: fMRI evidence for hemispheric specialization. Neuroreport. 1999;10:1373–1378. doi: 10.1097/00001756-199904260-00040. [DOI] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Nystrom LE, Forman SD, Noll DC, Cohen JD. Dissociating working memory from task difficulty in human prefrontal cortex. Neuropsychologia. 1997;35:1373–1380. doi: 10.1016/s0028-3932(97)00072-9. [DOI] [PubMed] [Google Scholar]
- Beason-Held LL, Kraut MA, Resnick SM. Stability of default-mode network activity in the aging brain. Brain Imaging Behav. 2009;3:123–131. doi: 10.1007/s11682-008-9054-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Barch DM. A theory of cognitive control, aging cognition, and neuromodulation. Neurosci Biobehav Rev. 2002;26:809–817. doi: 10.1016/s0149-7634(02)00067-2. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. NeuroImage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using the MarsBar toolbox for SPM 99; Poster session presented at: the Functional Mapping of the Human Brain; Japan. 2002. [Google Scholar]
- Burianová H, Lee Y, Grady CL, Moscovitch M. Age-related dedifferentiation and compensatory changes in the functional network underlying face processing. Neurobiol Aging. 2013;34:2759–2767. doi: 10.1016/j.neurobiolaging.2013.06.016. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychol Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Cappell KA, Gmeindl L, Reuter-Lorenz PA. Age differences in prefrontal recruitment during verbal working memory maintenance depend on memory load. Cortex. 2010;46:462–473. doi: 10.1016/j.cortex.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattell RB, Cattell AKS. Test of” g”: Culture fair. Institute for Personality and Ability Testing. 1963 [Google Scholar]
- Chan MY, Park DC, Savalia NK, Petersen SE, Wig GS. Decreased segregation of brain systems across the healthy adult lifespan. Proc Natl Acad Sci U S A. 2014;111:E4997–E5006. doi: 10.1073/pnas.1415122111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen H, Mackinnon A, Jorm AF, Henderson AS, Scott LR, Korten AE. Age differences and interindividual variation in cognition in community-dwelling elderly. Psychol Aging. 1994;9:381–390. doi: 10.1037//0882-7974.9.3.381. [DOI] [PubMed] [Google Scholar]
- Cole MW, Reynolds JR, Power JD, Repovs G, Anticevic A, Braver TS. Multi-task connectivity reveals flexible hubs for adaptive task control. Nat Neurosci. 2013;16:1348–1355. doi: 10.1038/nn.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik FIM, Byrd M. Aging and cognitive deficits. In: Craik FIM, Trehub S, editors. Aging and Cognitive Processes. Springer US; 1982. pp. 191–211. [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJS, Barkhof F, Scheltens P, Stam CJ, Smith SM, Rombouts SA. Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex. 2008;18:1856–1864. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Qué PASA? The posterior-anterior shift in aging. Cereb Cortex. 2008;18:1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SW, Kragel JE, Madden DJ, Cabeza R. The architecture of cross-hemispheric communication in the aging brain: linking behavior to functional and structural connectivity. Cereb Cortex. 2012;22:232–242. doi: 10.1093/cercor/bhr123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Chastelaine M, Wang TH, Minton B, Muftuler LT, Rugg MD. The effects of age, memory performance, and callosal integrity on the neural correlates of successful associative encoding. Cereb Cortex. 2011;21:2166–2176. doi: 10.1093/cercor/bhq294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Glisky EL. Changes in cognitive function in human aging. In: Riddle DR, editor. Brain aging: models, methods, and mechanisms. Florida: CRC Press; 2007. [PubMed] [Google Scholar]
- Grady CL. The cognitive neuroscience of ageing. Nat Rev Neurosci. 2012;13:491–505. doi: 10.1038/nrn3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Springer MV, Hongwanishkul D, McIntosh AR, Winocur G. Age-related changes in brain activity across the adult lifespan. J Cogn Neurosci. 2006;18:227–241. doi: 10.1162/089892906775783705. [DOI] [PubMed] [Google Scholar]
- Grahn JA, Parkinson JA, Owen AM. The cognitive functions of the caudate nucleus. Prog Neurobiol. 2008;86:141–155. doi: 10.1016/j.pneurobio.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Qin W, Liu Y, Zhang X, Duan Y, Song J, Li K, Jiang T, Yu C. Age-related decrease in functional connectivity of the right fronto-insular cortex with the central executive and default-mode networks in adults from young to middle age. Neurosci Let. 2013;544:74–79. doi: 10.1016/j.neulet.2013.03.044. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Raz N. Aging white matter and cognition: differential effects of regional variations in diffusion properties on memory, executive functions, and speed. Neuropsychologia. 2009;47:916–927. doi: 10.1016/j.neuropsychologia.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KM, Rieck JR, Boylan MA, Rodrigue KM. Functional magnetic resonance imaging data of incremental increases in visuo-spatial difficulty in a large adult lifespan sample. Data in Brief, submitted. doi: 10.1016/j.dib.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KM, Rodrigue KM, Bischof GN, Hebrank AC, Reuter-Lorenz PA, Park DC. Age trajectories of functional activation under conditions of low and high processing demands: an adult lifespan fMRI study of the aging brain. NeuroImage. 2015;104:21–34. doi: 10.1016/j.neuroimage.2014.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon D, Maillet D, Pasvanis S, Ankudowich E, Grady CL, Rajah MN. Context memory decline in middle aged adults is related to changes in prefrontal cortex function. Cereb Cortex. 2015;26:2440–2460. doi: 10.1093/cercor/bhv068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DEJ, Bittner RA, Muckli L, Waltz JA, Kriegeskorte N, Goebel R, Singer W, Munk MHJ. Cortical capacity constraints for visual working memory: dissociation of fMRI load effects in a fronto-parietal network. NeuroImage. 2003;20:1518–1530. doi: 10.1016/j.neuroimage.2003.07.021. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hariri AR, Berman KF, Das S, Meyer-Lindenberg A, Goldberg TE, Callicott JH, Weinberger DR. Neurophysiological correlates of age-related changes in working memory capacity. Neurosci Lett. 2006;392:32–37. doi: 10.1016/j.neulet.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Mazaika P, Whitfield-Gabrieli S, Reiss A. Artifact repair for fMRI data from high motion clinical subjects. Chicago, IL: Poster session presented at Human Brain Mapping; 2007. [Google Scholar]
- Miller SL, Celone K, DePeau K, Diamond E, Dickerson BC, Rentz D, Pihlajamäki M, Sperling RA. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc Natl Acad Sci U S A. 2008;105:2181–2186. doi: 10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Rugg MD. When more means less: neural activity related to unsuccessful memory encoding. Curr Biol. 2001;11:1528–1530. doi: 10.1016/s0960-9822(01)00454-7. [DOI] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuo-spatial and verbal memory across the adult life span. Psychol Aging. 2002;17:299–320. [PubMed] [Google Scholar]
- Park DC, Polk TA, Hebrank AC, Jenkins LJ. Age differences in default mode activity on easy and difficult spatial judgment tasks. Front Hum Neurosci. 2010;3:75. doi: 10.3389/neuro.09.075.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Kennedy KM, Rodrigue KM, Hebrank AC, Park DC. An fMRI study of episodic encoding across the lifespan: Changes in subsequent memory effects are evident by middle-age. Neuropsychologia. 2013;51:448–456. doi: 10.1016/j.neuropsychologia.2012.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Carp J, Hebrank A, Park DC, Polk TA. Neural specificity predicts fluid processing ability in older adults. J Neurosci. 2010;30:9253–9259. doi: 10.1523/JNEUROSCI.0853-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce JW. PsychoPy--Psychophysics software in Python. J Neurosci Methods. 2007;162:8–13. doi: 10.1016/j.jneumeth.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce JW. Generating stimuli for neuroscience using PsychoPy. Front Neuroinform. 2009;2:10. doi: 10.3389/neuro.11.010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Lustig C, Nelson JK, Reuter-Lorenz PA. Age differences in deactivation: A link to cognitive control? J Cogn Neurosci. 2007;19:1021–1032. doi: 10.1162/jocn.2007.19.6.1021. [DOI] [PubMed] [Google Scholar]
- Persson J, Pudas S, Nilsson LG, Nyberg L. Longitudinal assessment of default-mode brain function in aging. Neurobiol Aging. 2014;35:2107–2117. doi: 10.1016/j.neurobiolaging.2014.03.012. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Cappell KA. Neurocognitive aging and the compensation hypothesis. Curr Dir Psychol Sci. 2008;17:177–182. [Google Scholar]
- Reuter-Lorenz PA, Lustig C. Brain aging: reorganizing discoveries about the aging mind. Curr Opin Neurobiol. 2005;15:245–251. doi: 10.1016/j.conb.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Rieck JR, Rodrigue KM, Kennedy KM, Devous MD, Park DC. The effect of beta-amyloid on face processing in young and old adults: A multivariate analysis of the BOLD signal. Hum Brain Mapp. 2015;36:2514–2526. doi: 10.1002/hbm.22788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Miniussi C, Pasqualetti P, Babiloni C, Rossini PM, Cappa SF. Age-related functional changes of prefrontal cortex in long-term memory: a repetitive transcranial magnetic stimulation study. J Neurosci. 2004;24:7939–7944. doi: 10.1523/JNEUROSCI.0703-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Methodological assumptions in cognitive aging research. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. 2nd. Mahwah (NJ): Lawrence Erlbaum Associates Publishers; 2000. pp. 467–498. [Google Scholar]
- Sambataro F, Murty VP, Callicott JH, Tan HY, Das S, Weinberger DR, Mattay VS. Age-related alterations in default mode network: Impact on working memory performance. Neurobiol Aging. 2010;31:839–852. doi: 10.1016/j.neurobiolaging.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaie KW. The course of adult intellectual development. Am Psychol. 1994;49:304–313. doi: 10.1037//0003-066x.49.4.304. [DOI] [PubMed] [Google Scholar]
- Schneider-Garces NJ, Gordon BA, Brumback-Peltz CR, Shin E, Lee Y, Sutton BP, Maclin EL, Gratton G, Fabiani M. Span, CRUNCH, and Beyond: Working memory capacity and the aging brain. J Cogn Neurosci. 2010;22:655–669. doi: 10.1162/jocn.2009.21230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaniol J, Grady CL. Aging and the neural correlates of source memory: over-recruitment and functional reorganization. Neurobiol Aging. 2012;33:425.e3–425.e18. doi: 10.1016/j.neurobiolaging.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. NeuroImage. 2010;53:303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner GR, Spreng RN. Prefrontal engagement and reduced default network suppression co-occur and are dynamically coupled in older adults: The default-executive coupling hypothesis of aging. J Cogn Neurosci. 2015;27:2462–2476. doi: 10.1162/jocn_a_00869. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Cerella J, Bopp KL, Basak C. Aging and varieties of cognitive control: A review of meta-analyses on resistance to interference, coordination, and task switching, and an experimental exploration of age-sensitivity in the newly identified process of focus switching. In: Engle RW, Sedek G, von Hecker U, McIntosh DN, editors. Cognitive limitations in aging and psychopathology. Cambridge: Cambridge University Press; 2005. [Google Scholar]