Abstract
Alzheimer’s disease (AD) is the disease of lost memories. Synaptic loss is a major reason for memory defects in AD. Signaling pathways involved in memory loss in AD are under intense investigation. The role of deranged neuronal calcium (Ca2+) signaling in synaptic loss in AD is described in this review. Familial AD (FAD) mutations in presenilins are linked directly with synaptic Ca2+ signaling abnormalities, most likely by affecting endoplasmic reticulum (ER) Ca2+ leak function of presenilins. Excessive ER Ca2+ release via type 2 ryanodine receptors (RyanR2) is observed in AD spines due to increase in expression and function of RyanR2. Store-operated Ca2+ entry (nSOC) pathway is disrupted in AD spines due to downregulation of STIM2 protein. Because of these Ca2+ signaling abnormalities, a balance in activities of Ca2+-calmodulin-dependent kinase II (CaMKII) and Ca2+-dependent phosphatase calcineurin (CaN) is shifted at the synapse, tilting a balance between long-term potentiation (LTP) and long-term depression (LTD) synaptic mechanisms. As a result, synapses are weakened and eliminated in AD brains by LTD mechanism, causing memory loss. Targeting synaptic calcium signaling pathways offers opportunity for development of AD therapeutic agents.
Keywords: Alzheimer disease, Ca2+ signaling, ryanodine receptors, neuronal store-operated Ca2+ channels, mushroom spines, synapse, Ca2+-calmodulin-dependent kinase II (CaMKII), calcineurin
Introduction
Alzheimer’s disease (AD) is neurodegenerative disorder, which is characterized by alterations in memory formation and storage. Most cases of AD are sporadic and occur in the aging population (>60 years of age), but approximately 1%–2% of cases refer to early onset familial form of AD (40–50 years of age) [1]. Familial form of AD (FAD) is caused by mutations in genes encoding Presenilin 1 (PS1), Presenilin 2 (PS2) proteins and amyloid-precursor protein (APP) [1–4]. Several hypothesis about AD development have been proposed, but so-called «amyloid cascade hypothesis» is still considered to be the dominant. The amyloid cascade hypothesis states that overproduction and deposition of amyloid β-peptide (Aβ) in brain tissue driving AD pathogenesis [5]. Therefore, much effort was spent to develop agents which will reduce Aβ production or eliminate Aβ from the brain. Most drugs developed based on this approach failed to show any potential benefit for patients in clinical trials [6], although some promising trends have been recently reported in the trial of anti-Aβ antibody aducanumab [7]. These failures suggest that we urgently needed new approaches to AD treatment in addition to targeting Aβ. We and others previously proposed that neuronal Ca2+ dyshomeostasis has an important role in AD [8–12]. In this review, we will discuss Ca2+ signaling alterations observed in AD and point new ways of disease modifying drug development based on it.
Loss of synapses in Alzheimer disease
Several pathological hallmarks such as shrinkage of hippocampal and cortex brain regions, amyloid plaque deposition, intracellular neurofibrillary tangles and activated microglia are observed in AD patients. Another AD marker is a loss of synapses and changes in the shape and size of dendritic spines [13, 14]. Synaptic alterations observed at early stages of disease [15] and correlate strongly with cognitive decline in AD patients [16]. Synapse is formed by presynaptic axon ending and by postsynaptic dendritic spine. According to morphological structure, dendritic spines are classified into three groups: mushroom, thin and stubby spines [17, 18]. Mushroom spines, which are large and form strong synaptic contacts, were proposed to represent stable “memory spine”, while thin spines are “learning spines” which are responsible for formation of new memories [19]. We and others previously proposed that mushroom spines are strongly eliminated in AD and that loss of mushroom spines may underlie cognitive decline during the progression of the disease [20–22]. Consistent with this hypothesis, reduction in hippocampal mushroom spines was observed in different experimental mice models of AD, including PS1-M146V-KI mice [23], newly generated APP-KI mice [24, 25], in conditions of amyloid synaptotoxicity in vivo and in vitro [26], and ex vivo in hippocampal slice cultures from APPSDL transgenic mice [27, 28]. We suggest that mushroom spines loss is an early event, which precedes more severe neurodegenerative changes. Thus, we reasoned that stabilization of mushroom spines may help to prevent eventual loss of memories of AD patients. What cell biological mechanism is responsible for dendritic spine alterations in AD? In this review we discuss the hypothesis that dysregulation of neuronal Ca2+ signaling plays an important role in destabilization of synaptic spines in aging and AD brains.
Presenilins and neuronal calcium homeostasis
Genetic mutations in presenilins is one of the major causes of familial AD (FAD) [1–4]. Presenilins together with nicastrin, APH-1 and PEN-2 form the γ-secretase complex that cleaves several substrates including amyloid precursor protein (APP) [29]. Sequential cleavage of APP by β- and γ-secretases constitutes amyloidogenic pathway that leads to production of toxic Aβ peptides. FAD-associated mutations in presenilins disrupt the function of γ-secretase. There is a debate on whether FAD mutations in presenilins upregulate or downregulate γ-secretase activity [30–32]. Presenilins also exert a number of γ-secretase- independent functions [33]. One of these functions is related to Ca2+ signaling. The connection between presenilins and Ca2+ signaling was initially uncovered when it was reported that fibroblasts from FAD patients release supranormal amounts of Ca2+ in response to InsP3 [34]. Similar results were obtained in experiments with cells expressing FAD mutant presenilins [35] and in cortical neurons from FAD presenilin mutant knock-in mice [36, 37]. To explain these results it has been suggested that mutant presenilins affect store-operated Ca2+ influx [38, 39], increase activity and/or expression of intracellular Ca2+ release channels such as RyanR [37, 40–42] and InsP3R [43–45] or modulate function of SERCA ER Ca2+ pump [46]. We proposed that presenilins form passive ER Ca2+ leak channel, the function that is disrupted by many but not all FAD mutations [47–50] (Fig 1). This idea created some controversy and it was challenged [51–53]. However, independent experimental support for leak function of presenilin began to accumulate [54, 55]. Importantly, recent unbiased screen for modulators of intracellular Ca2+ homeostasis revealed key role of presenilins in mediating passive Ca2+ influx from ER, in agreement with the “leak channel” hypothesis [56, 57]. A large hole that traverses through the entire protein was observed in the recent high resolution crystal structure of archaeal presenilin homologue PSH1 [58]. The authors noted that this hole is large enough to allow passage of small ions [58]. Interestingly, PSH1 monomer in this crystal structure adopts a fold similar to the seven-helix fold of the ClC chloride channel family [59]. These latest evidence provide additional support to the “ER Ca2+ leak channel hypothesis”.
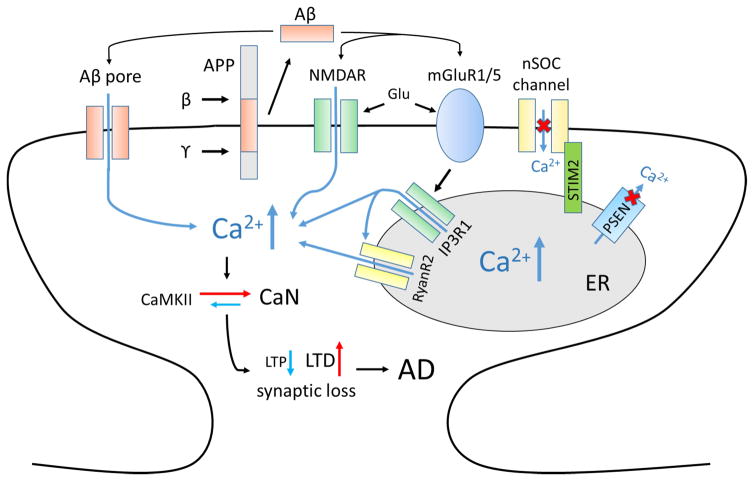
Figure 1. Ca2+ dysregulation in AD and synaptic loss.
Amyloid β-peptide (Aβ) is generated by sequential cleavages of amyloid-precursor protein (APP) by β-secretase (β) and γ-secretase (γ). Aβ is able to form Ca2+-permeable pore in cell membrane. Aβ affects activity of synaptic NMDAR and mGluR5. Glutamate stimulates activation of mGluR1/5 receptors, production of IP3 and IP3R1-mediated Ca2+ release from the ER. Presenilins (PSEN) acts as Ca2+-leak pore. Many familial AD mutations disrupt this function of presenilins, which leads to ER Ca2+ overload and subsequent downregulation of neuronal store-operated calcium entry (nSOC) gated by STIM2. Increased ER Ca2+ levels result in enhanced Ca2+ release through IP3R1 and RyanR2. Dysregulated spine Ca2+ signals lead to reduction in CaMKII activity and enhanced CaN activity, subsequent facilitation of LTD and inhibition of LTP and loss of synapses. Abbreviations used in figure: AD - Alzheimer’s disease, NMDAR - N-methyl-D-aspartate receptor, mGluR1/5 - metabotropic glutamate receptor type 1 or 5, IP3 - inositol trisphosphate, IP3R1- inositol trisphosphate receptor, ER - endoplasmic reticulum, RyanR2 - ryanodine receptor type 2, CaMKII - Ca2+/calmodulin-dependent protein kinase II, CaN – calcineurin, LTD - long-term depression, LTP - long-term potentiation, Glu - glutamate.
Ryanodine receptors in AD
There are several lines of evidence supporting dysregulation on RyanR-mediated Ca2+ signaling in AD. Post-mortem hippocampal brain specimens from early-stage AD patients display increased [H]3ryanodine binding, indicative of increased RyanR protein levels hippocampal regions (subiculum, CA2 and CA1) compared to non-demented controls [60]. These results were recently supported in a study where post-mortem analysis of brain from individuals with mild cognitive impairment (MCI) at high risk for developing AD revealed the up-regulation of RyanR2 [61]. Recent data suggest that RyanRs are increased in expression and function in FAD models, particularly in the hippocampus and cortex of PS1-M146V knock-in (KI) mice [37, 40, 50] and in transgenic CRND8 (APP695(KM670/671NL + V717F) mice [62]. The up-regulation of RyanRs may be a part of AD pathology, but it may also be a protective and/or compensatory response to neuronal Ca2+ dysregulation (reviewed in [63]).
One approach used to dissect this issue is to obstruct the effects of RyanRs using pharmacological inhibitors or functional blockers. In our previous studies, we observed that long-term feeding of the RyanR inhibitor dantrolene exacerbated amyloid plaque formation and resulted in the loss of hippocampal synaptic markers and neuronal deterioration in 8 month old APPPS1 mice [50]. In contrast, studies from other groups showed that short term treatment with dantrolene was able to stabilize Ca2+ signals, ameliorate cognitive decline and reduce neuropathology, amyloid load and memory impairments in various AD mouse models [64–66], suggesting that blocking RyanR activity may actually be beneficial in the context of AD. One potential problem with interpreting these results is that specific RyanR inhibitors do not exist and the drug dantrolene, used in most studies, has additional targets such as store-operated Ca2+ channels [67]. Moreover, dantrolene is specific for skeletal muscle isoform RyanR1 [68], and does not block neuronal RyanR2 and RyanR3 effectively.
To resolve this issue, in the recent studies we took a genetic approach and generated APPPS1x RyanR3−/− mice [69]. We compared the phenotype of APPPS1x RyanR3−/− to the phenotype of WT, RyanR3−/− and APPPS1 mice. In this analysis we discovered that RyanR3 appears to play a dual role in the context of AD pathology, rather than an invariable positive or negative effect. In the young APPPS1 mice (≤3 month old) deletion of RyanR3 was detrimental and enhanced AD pathology [69]. We concluded that RyanR3 plays an important protective role in early stages of AD by helping to reduce neuronal excitability and activity-dependent Aβ production. These data support the hypothesis that blockade of RyanRs in the early stages of AD progression would produce a more aggressive AD phenotype compared to the placebo group, as we previously suggested [50, 63, 70]. In contrast, in older APPPS1 mice (≥6 month old) deletion of RyanR3 resulted in beneficial effects and reduced AD pathology [69]. These results consistent with reports that dantrolene exerted beneficial effects in several AD mouse models [64–66] and suggested that blocking RyanR is a viable therapeutic strategy. This idea is reviewed in depth by Dr. Grace Stutzmann and her colleagues in the accompanying review article [71].
Disruption of nSOC signaling in mouse models of AD
Another Ca2+ signaling defect in AD neurons is related to dysfunction of neuronal store-operated Ca2+ entry (SOC) pathway. The neuronal function of SOC pathway is poorly understood, however key molecular components of SOC are expressed in the brain and enriched in hippocampus. SOC activation is mediated by STIM proteins, which act as ER Ca2+ sensors. STIM2 protein is highly enriched in hippocampus and STIM1 is enriched in cerebellum. Recent report demonstrated impaired spatial memory in forebrain-specific double knockout of STIM1 and STIM2 proteins [72]. Differential role of STIM1 and STIM2 proteins in control of neuronal SOC was demonstrated [73]. In our studies we discovered that expression of STIM2 is downregulated in hippocampal neurons PS1-M146V-KI and APP-KI neurons and in post-mortem samples from AD patients [23, 24]. We reasoned that reduction in STIM2 expression level is a compensatory response to ER Ca2+ overload in these models. We further proposed that downregulation of STIM2 and synaptic SOC is responsible for loss of mushroom spines in PS1-M146V-KI and APP-KI in hippocampal neurons [23, 24] (Fig 1). Indeed, expression of STIM2 resulted in rescue of mushroom spine loss in PS1-M146V-KI and APP-KI hippocampal neurons [23, 24] and in neurons exposed to Aβ oligomers [26].
Based on these results we propose that positive modulators of nSOC activity may be considered as potential therapeutics agents for preventing synaptic and memory loss in AD [21, 23, 24, 26, 74]. However, in order to develop effective drug the knowledge of molecular identity of nSOC channels is necessary. The candidates for such role are members of Orai and/or TRPC families. Expression of Orai2 is enriched in hippocampus [75]. Association of neuronal STIM2 with Orai1 was demonstrated in biochemical experiments [76] and the functional role of Orai1 protein in control of synaptogenesis in hippocampal neurons has been recently described [77]. TRPC proteins have been demonstrated to play a role in neuronal Ca2+ signaling in multiple studies [78, 79]. Brain overexpression of TRPC6 channels resulted in spine proliferation and enhanced memory performance in transgenic mice [80], suggesting a potential role for TRPC6 in mediating nSOC. Interestingly, TRPC6 is activated by hyperforin [81] and it has been demonstrated in the previous studies that hyperforin and its derivatives were able to prevent beta-amyloid neurotoxicity and spatial memory impairments in AβPPSwe/PSEN1 E9 (AβPP/PS1) transgenic mice [82–84]. TRPC6 was also recently demonstarted to interact directly with APP and to affect APP cleavage by γ-secretase [85]. Future studies will determine the potential role of Orai and TRPC channels in supporting STIM2-gated nSOC and will help to establish if positive modulators of these channels exert beneficial effects in AD.
CaMKII and CaN “tug of war” and AD pathogenesis
Another possibility for the development of disease preventing therapies is to target signaling pathways downstream from synaptic Ca2+ dysregulation. Spine Ca2+ signaling sets up a balance between activities of Ca2+-calmodulin-dependent kinase II (CaMKII) and Ca2+-dependent phosphatase calcineurin (CaN). Both CaMKII and CaN are enriched in the brain and extremely abundant in synaptic locations [86, 87]. CaMKII is a holoenzyme of 12 subunits, each derived from one of four genes (α, β, γ and δ). In the brain αCaMKII and βCaMKII are the most abundant subunits, expressed at the ratio 3:1. The total concentration of CaMKII in the hippocampal spines was estimated to be on the order of 100 μM [88, 89]. CaMKII holoenzymes are activated by the binding of Ca2+/CaM. Following activation, CaMKII can undergo inter-subunit autophosphorylation at residue T286 (for αCaMKII), that results in “locking” CaMKII in an active state independently from Ca2+ levels. Presence of p(T286)-αCaMKII at synaptic locations is essential for LTP and it has been proposed to be critical for memory formation [90, 91], an idea supported by observation of LTP and memory defects in T286A αCaMKII mutant mice [92]. The role of αCaMKII T286 autophosphorylation in memory maintenance is less clear [91, 93]. CaN (PP2B) is a protein phosphatase composed of a large catalytic (CaNA) and a small regulatory subunit (CaNB). Increase in Ca2+ concentration leads to Ca2+ association with EF hand motifs in CaNB, partial activation of CaN and exposure of Ca2+-CaM binding site on CaNA. Association of Ca2+/CaM with CaNA then causes full activation of CaN [86, 94]. Activation of CaN in hippocampal neurons is essential for induction of LTD [95, 96]. Overexpression of CaN in the forebrain of mice impaired the transition from short-term to long-term memory as well as an intermediate form of LTP [97]. Conditional genetic knockout of CaN in mouse forebrain resulted in impairment of hippocampal-dependent tasks including working and episodic memory and blocked LTD [98].
As it is clear from this brief description, both CaMKII and CaN play key and opposing roles in synaptic plasticity and both enzymes are regulated by spine Ca2+ in a complex manner. Experimental evidence indicated that CaMKII functions as a high-frequency activity detector that stabilizes the spines (by LTP-like mechanism), whereas CaN is responsive to low frequency stimulation and destabilizes the spines (by LTD-like mechanism) [96, 99]. A proposed allosteric model suggests that CaM is more likely to activate either CaN or CaMKII depending on local Ca2+ concentration [100]. Intense and transient Ca2+ increase through NMDARs following tetanic stimulation result in the preferential activation of CaMKII within the dendritic spines. However, as Ca2+ decreases, but before it returns to baseline, CaM is more likely to bind and activate CaN [100]. CaN activates PP1, and PP1 in turn dephosphorylates CaMKII, consequently decreasing its kinase activity [95].
Because both CaMKII and CaN are Ca2+-dependent, subtle changes in synaptic Ca2+ signaling cause shift in the balance of CaMKII and CaN activities. The balance between activities of CaMKII and CaN appear to be shifted in AD synapses in favor of CaN (Fig 1) [11, 101]. Consistent with this hypothesis, a shift in the balance between LTP-like and LTD-like mechanisms has been recently reported based on the analysis of synaptic plasticity in mouse model of AD [102]. It has been proposed that deranged synaptic Ca2+ signaling causes aberrant metaplasticity in AD by shifting a balance in induction of LTP and LTD [103]. In support of these functional arguments, biochemical analysis revealed alterations in CaMKII localization and expression in AD brains. It was discovered that p(T286)-αCaMKII is reduced at synaptic locations in hippocampus of AD patients and in mouse hippocampal neurons treated with Aβ [104]. The degree of p(T286)-αCaMKII loss at synaptic locations correlated with severity of the disease [104]. These results suggested that reduction in synaptic CaMKII activity may play an important role in AD pathogenesis [105]. In our experiments we observed direct correlation between reduced nSOC, levels of synaptic p(T286)-αCaMKII and mushroom spine loss in mouse models of AD [23, 24, 26] (Figure 1). Importantly, STIM2 overexpression rescued p(T286)-αCaMKII levels [23, 24, 26]. The exact mechanism how nSOC influences synaptic CaMKII function is unclear. Dendritic spines have a poor intrinsic buffering capacity for Ca2+, and action potentials may increase Ca2+ only very briefly. It is possible that nSOC activity is necessary to support CaMKII autophosphorylation when high-frequency stimulation ceases. While activity of synaptic CaMKII is reduced in AD, activity of CaN appear to be enhanced. Superactivation of CaN in human AD samples was reported in biochemical experiments [106, 107]. Activation of CaN in AD human samples appears to occur due to calpain-mediated cleavage and hyperactivation. Importance of CaN in AD has been highlighted by previous studies in AD cellular and animal models. It has been shown that CaN mediates both the neurotoxic and cognitive effects of Aβ oligomers [108–114]. Beneficial effects of CaN inhibition have been demonstrated in several AD mouse models [108, 110, 111, 114–116]. It has been shown that CREB phosphorylation and LTP expression, which are disrupted by Aβ oligomers, are restored following FK506 treatment in hippocampal slice experiments [111]. These findings lead to proposal that CaN overactivation in one of the driving forces of AD pathology [117]. Very importantly, recent analysis revealed significantly reduced incidence of AD in transplant patients treated with calcineurin inhibitor FK506 [118]. These findings provide strong support to the hypothesis that excessive activation of CaN plays a key role in spine loss in AD.
Future directions
Synaptic Ca2+ dysregulation appears to play an important role in synaptic loss in AD. This knowledge provides a number of potential therapeutic targets for prevention of memory loss in AD (Fig 1). Potential approaches include modulation of RyanR2 activity and activity of nSOC channels. Molecular identity of nSOC channels needs to be established to facilitate these efforts. Pharmacological tools aimed at restoring the balance between CaMKII and CaN activities in synaptic spines may also provide a potential for therapeutic interference. It is necessary to establish if beneficial effects can be achieved following inhibition of CaN without immunosuppression side-effects. It is also important to identify downstream relevant targets of CaMKII and CaN at the synapse.
Highlights.
Synaptic loss is a basis for memory loss in Alzheimer’s disease (AD)
Dysregulation of synaptic calcium (Ca2+) signaling plays an important role in synaptic loss in AD
Function of ryanodine receptors and store-operated calcium channels is abnormal in AD neurons
There is a shift in the balance of Ca2+-calmodulin-dependent kinase II (CaMKII) and Ca2+-dependent phosphatase calcineurin (CaN) activities at the synapse
Balance between long-term potentiation (LTP) and long-term depression (LTD) synaptic mechanisms is tilted in AD spines, causing elimination of synapses by LTD-like mechanism
Acknowledgments
Ilya Bezprozvanny is a holder of the Carl J. and Hortense M. Thomsen Chair in Alzheimer’s Disease Research. This work was supported by the National Institutes of Health grant R01NS080152 (IB) (chapter: CaMKII and CaN “tug of war” and AD pathogenesis), Russian Science Foundation Grant 14-25-00024 (IB) (chapters: introduction, synaptic loss in AD), by the state grant 17.1360.2014/κ (IB) (chapters: Ryanodine receptors in AD, Disruption of nSOC signaling in mouse models of AD), and by the Dynasty foundation grant DP-B-49-15 (EP) (chapter: Presenilins and neuronal calcium homeostasis).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–6. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 2.Hardy J. The amyloid hypothesis for Alzheimer’s disease: a critical reappraisal. Journal of neurochemistry. 2009;110:1129–34. doi: 10.1111/j.1471-4159.2009.06181.x. [DOI] [PubMed] [Google Scholar]
- 3.Bergmans BA, De Strooper B. gamma-secretases: from cell biology to therapeutic strategies. Lancet Neurol. 2010;9:215–226. doi: 10.1016/S1474-4422(09)70332-1. [DOI] [PubMed] [Google Scholar]
- 4.Karch CM, Cruchaga C, Goate AM. Alzheimer’s disease genetics: from the bench to the clinic. Neuron. 2014;83:11–26. doi: 10.1016/j.neuron.2014.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–5. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 6.Karran E, Hardy J. A critique of the drug discovery and phase 3 clinical programs targeting the amyloid hypothesis for Alzheimer disease. Ann Neurol. 2014;76:185–205. doi: 10.1002/ana.24188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sevigny J, Chiao P, Bussiere T, Weinreb PH, Williams L, Maier M, Dunstan R, Salloway S, Chen T, Ling Y, O’Gorman J, Qian F, Arastu M, Li M, Chollate S, Brennan MS, Quintero-Monzon O, Scannevin RH, Arnold HM, Engber T, Rhodes K, Ferrero J, Hang Y, Mikulskis A, Grimm J, Hock C, Nitsch RM, Sandrock A. The antibody aducanumab reduces Abeta plaques in Alzheimer’s disease. Nature. 2016;537:50–6. doi: 10.1038/nature19323. [DOI] [PubMed] [Google Scholar]
- 8.Green KN, LaFerla FM. Linking calcium to Abeta and Alzheimer’s disease. Neuron. 2008;59:190–4. doi: 10.1016/j.neuron.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 9.Bezprozvanny I. Calcium signaling and neurodegenerative diseases. Trends Mol Med. 2009;15:89–100. doi: 10.1016/j.molmed.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bezprozvanny I, Mattson MP. Neuronal calcium mishandling and the pathogenesis of Alzheimer’s disease. Trends Neurosci. 2008;31:454–63. doi: 10.1016/j.tins.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berridge MJ. Calcium signalling and Alzheimer’s disease. Neurochem Res. 2011;36:1149–56. doi: 10.1007/s11064-010-0371-4. [DOI] [PubMed] [Google Scholar]
- 12.Chakroborty S, Stutzmann GE. Calcium channelopathies and Alzheimer’s disease: Insight into therapeutic success and failures. Eur J Pharmacol. 2013 doi: 10.1016/j.ejphar.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 13.Koffie RM, Hyman BT, Spires-Jones TL. Alzheimer’s disease: synapses gone cold. Mol Neurodegener. 2011;6:63. doi: 10.1186/1750-1326-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–91. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 15.Scheff SW, Price DA, Schmitt FA, DeKosky ST, Mufson EJ. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology. 2007;68:1501–1508. doi: 10.1212/01.wnl.0000260698.46517.8f. [DOI] [PubMed] [Google Scholar]
- 16.DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: Correlation with cognitive severity. Annals of Neurology. 1990;27:457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- 17.Bourne JN, Harris KM. Balancing structure and function at hippocampal dendritic spines. Annu Rev Neurosci. 2008;31:47–67. doi: 10.1146/annurev.neuro.31.060407.125646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H. Structure-stability-function relationships of dendritic spines. Trends Neurosci. 2003;26:360–8. doi: 10.1016/S0166-2236(03)00162-0. [DOI] [PubMed] [Google Scholar]
- 19.Bourne J, Harris KM. Do thin spines learn to be mushroom spines that remember? Current opinion in neurobiology. 2007;17:381–6. doi: 10.1016/j.conb.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Popugaeva E, Bezprozvanny I. Role of endoplasmic reticulum Ca2+ signaling in the pathogenesis of Alzheimer disease. Front Mol Neurosci. 2013;6:29. doi: 10.3389/fnmol.2013.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Popugaeva E, Supnet C, Bezprozvanny I. Presenilins, deranged calcium homeostasis, synaptic loss and dysfunction in Alzheimer’s disease. Messenger. 2012;1:53–62. [Google Scholar]
- 22.Tackenberg C, Ghori A, Brandt R. Thin, stubby or mushroom: spine pathology in Alzheimer’s disease. Curr Alzheimer Res. 2009;6:261–8. doi: 10.2174/156720509788486554. [DOI] [PubMed] [Google Scholar]
- 23.Sun S, Zhang H, Liu J, Popugaeva E, Xu NJ, Feske S, White CL, 3rd, Bezprozvanny I. Reduced synaptic STIM2 expression and impaired store-operated calcium entry cause destabilization of mature spines in mutant presenilin mice. Neuron. 2014;82:79–93. doi: 10.1016/j.neuron.2014.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang H, Wu L, Pchitskaya E, Zakharova O, Saito T, Saido T, Bezprozvanny I. Neuronal Store-Operated Calcium Entry and Mushroom Spine Loss in Amyloid Precursor Protein Knock-In Mouse Model of Alzheimer’s Disease. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:13275–86. doi: 10.1523/JNEUROSCI.1034-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saito T, Matsuba Y, Mihira N, Takano J, Nilsson P, Itohara S, Iwata N, Saido TC. Single App knock-in mouse models of Alzheimer’s disease. Nature neuroscience. 2014;17:661–3. doi: 10.1038/nn.3697. [DOI] [PubMed] [Google Scholar]
- 26.Popugaeva E, Pchitskaya E, Speshilova A, Alexandrov S, Zhang H, Vlasova O, Bezprozvanny I. STIM2 protects hippocampal mushroom spines from amyloid synaptotoxicity. Mol Neurodegener. 2015;10:37. doi: 10.1186/s13024-015-0034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Penazzi L, Tackenberg C, Ghori A, Golovyashkina N, Niewidok B, Selle K, Ballatore C, Smith AB, III, Bakota L, Brandt R. Aβ-mediated spine changes in the hippocampus are microtubule-dependent and can be reversed by a subnanomolar concentration of the microtubule-stabilizing agent epothilone D. Neuropharmacology. 2016;105:84–95. doi: 10.1016/j.neuropharm.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tackenberg C, Brandt R. Divergent pathways mediate spine alterations and cell death induced by amyloid-beta, wild-type tau, and R406W tau. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:14439–50. doi: 10.1523/JNEUROSCI.3590-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Strooper B. Aph-1, Pen-2, and Nicastrin with Presenilin generate an active gamma-Secretase complex. Neuron. 2003;38:9–12. doi: 10.1016/s0896-6273(03)00205-8. [DOI] [PubMed] [Google Scholar]
- 30.Xia D, Kelleher RJ, 3rd, Shen J. Loss of Abeta43 Production Caused by Presenilin-1 Mutations in the Knockin Mouse Brain. Neuron. 2016;90:417–22. doi: 10.1016/j.neuron.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xia D, Watanabe H, Wu B, Lee SH, Li Y, Tsvetkov E, Bolshakov VY, Shen J, Kelleher RJ., 3rd Presenilin-1 knockin mice reveal loss-of-function mechanism for familial Alzheimer’s disease. Neuron. 2015;85:967–81. doi: 10.1016/j.neuron.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veugelen S, Saito T, Saido TC, Chavez-Gutierrez L, De Strooper B. Familial Alzheimer’s Disease Mutations in Presenilin Generate Amyloidogenic Abeta Peptide Seeds. Neuron. 2016;90:410–6. doi: 10.1016/j.neuron.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 33.Duggan SP, McCarthy JV. Beyond gamma-secretase activity: The multifunctional nature of presenilins in cell signalling pathways. Cell Signal. 2016;28:1–11. doi: 10.1016/j.cellsig.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 34.Ito E, Oka K, Etcheberrigaray R, Nelson TJ, McPhie DL, Tofel-Grehl B, Gibson GE, Alkon DL. Internal Ca2+ mobilization is altered in fibroblasts from patients with Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:534–8. doi: 10.1073/pnas.91.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leissring MA, Paul BA, Parker I, Cotman CW, LaFerla FM. Alzheimer’s presenilin-1 mutation potentiates inositol 1,4,5-trisphosphate-mediated calcium signaling in Xenopus oocytes. Journal of neurochemistry. 1999;72:1061–8. doi: 10.1046/j.1471-4159.1999.0721061.x. [DOI] [PubMed] [Google Scholar]
- 36.Stutzmann GE, Caccamo A, LaFerla FM, Parker I. Dysregulated IP3 signaling in cortical neurons of knock-in mice expressing an Alzheimer’s-linked mutation in presenilin1 results in exaggerated Ca2+ signals and altered membrane excitability. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:508–13. doi: 10.1523/JNEUROSCI.4386-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stutzmann GE, Smith I, Caccamo A, Oddo S, Laferla FM, Parker I. Enhanced ryanodine receptor recruitment contributes to Ca2+ disruptions in young, adult, and aged Alzheimer’s disease mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:5180–9. doi: 10.1523/JNEUROSCI.0739-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leissring MA, Akbari Y, Fanger CM, Cahalan MD, Mattson MP, LaFerla FM. Capacitative calcium entry deficits and elevated luminal calcium content in mutant presenilin-1 knockin mice. The Journal of cell biology. 2000;149:793–8. doi: 10.1083/jcb.149.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoo AS, Cheng I, Chung S, Grenfell TZ, Lee H, Pack-Chung E, Handler M, Shen J, Xia W, Tesco G, Saunders AJ, Ding K, Frosch MP, Tanzi RE, Kim TW. Presenilin-mediated modulation of capacitative calcium entry. Neuron. 2000;27:561–72. doi: 10.1016/s0896-6273(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 40.Chan SL, Mayne M, Holden CP, Geiger JD, Mattson MP. Presenilin-1 mutations increase levels of ryanodine receptors and calcium release in PC12 cells and cortical neurons. The Journal of biological chemistry. 2000;275:18195–200. doi: 10.1074/jbc.M000040200. [DOI] [PubMed] [Google Scholar]
- 41.Rybalchenko V, Hwang SY, Rybalchenko N, Koulen P. The cytosolic N-terminus of presenilin-1 potentiates mouse ryanodine receptor single channel activity. Int J Biochem Cell Biol. 2008;40:84–97. doi: 10.1016/j.biocel.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 42.Hayrapetyan V, Rybalchenko V, Rybalchenko N, Koulen P. The N-terminus of presenilin-2 increases single channel activity of brain ryanodine receptors through direct protein-protein interaction. Cell calcium. 2008;44:507–18. doi: 10.1016/j.ceca.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 43.Cai C, Lin P, Cheung KH, Li N, Levchook C, Pan Z, Ferrante C, Boulianne GL, Foskett JK, Danielpour D, Ma J. The presenilin-2 loop peptide perturbs intracellular Ca2+ homeostasis and accelerates apoptosis. The Journal of biological chemistry. 2006;281:16649–55. doi: 10.1074/jbc.M512026200. [DOI] [PubMed] [Google Scholar]
- 44.Cheung KH, Shineman D, Muller M, Cardenas C, Mei L, Yang J, Tomita T, Iwatsubo T, Lee VM, Foskett JK. Mechanism of Ca2+ disruption in Alzheimer’s disease by presenilin regulation of InsP(3) receptor channel gating. Neuron. 2008;58:871–83. doi: 10.1016/j.neuron.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheung KH, Mei L, Mak DO, Hayashi I, Iwatsubo T, Kang DE, Foskett JK. Gain-of-function enhancement of IP3 receptor modal gating by familial Alzheimer’s disease-linked presenilin mutants in human cells and mouse neurons. Sci Signal. 2010;3:ra22. doi: 10.1126/scisignal.2000818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Green KN, Demuro A, Akbari Y, Hitt BD, Smith IF, Parker I, LaFerla FM. SERCA pump activity is physiologically regulated by presenilin and regulates amyloid beta production. The Journal of cell biology. 2008;181:1107–16. doi: 10.1083/jcb.200706171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nelson O, Supnet C, Liu H, Bezprozvanny I. Familial Alzheimer’s disease mutations in presenilins: effects on endoplasmic reticulum calcium homeostasis and correlation with clinical phenotypes. J Alzheimers Dis. 2010;21:781–93. doi: 10.3233/JAD-2010-100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tu H, Nelson O, Bezprozvanny A, Wang Z, Lee S-F, Hao YH, Serneels L, De Strooper B, Yu G, Bezprozvanny I. Presenilins form ER calcium leak channels, a function disrupted by mutations linked to familial Alzheimer’s disease. Cell. 2006;126:981–993. doi: 10.1016/j.cell.2006.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nelson O, Supnet C, Tolia A, Horre K, De Strooper B, Bezprozvanny I. Mutagenesis mapping of the presenilin 1 calcium leak conductance pore. The Journal of biological chemistry. 2011;286:22339–47. doi: 10.1074/jbc.M111.243063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang H, Sun S, Herreman A, De Strooper B, Bezprozvanny I. Role of presenilins in neuronal calcium homeostasis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:8566–80. doi: 10.1523/JNEUROSCI.1554-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shilling D, Mak DO, Kang DE, Foskett JK. Lack of evidence for presenilins as endoplasmic reticulum Ca2+ leak channels. The Journal of biological chemistry. 2012;287:10933–10944. doi: 10.1074/jbc.M111.300491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu B, Yamaguchi H, Lai FA, Shen J. Presenilins regulate calcium homeostasis and presynaptic function via ryanodine receptors in hippocampal neurons. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:15091–6. doi: 10.1073/pnas.1304171110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bezprozvanny I, Supnet C, Sun S, Zhang H, De Strooper B. Response to Shilling et al. (10.1074/jbc.M111.300491) The Journal of biological chemistry. 2012;287:20469. doi: 10.1074/jbc.L112.356790. author reply 20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Das HK, Tchedre K, Mueller B. Repression of transcription of presenilin-1 inhibits gamma-secretase independent ER Ca(2)(+) leak that is impaired by FAD mutations. Journal of neurochemistry. 2012;122:487–500. doi: 10.1111/j.1471-4159.2012.07794.x. [DOI] [PubMed] [Google Scholar]
- 55.Kuo IY, Hu J, Ha Y, Ehrlich BE. Presenilin-like GxGD Membrane Proteases Have Dual Roles as Proteolytic Enzymes and Ion Channels. The Journal of biological chemistry. 2015;290:6419–27. doi: 10.1074/jbc.M114.629584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bandara S, Malmersjo S, Meyer T. Regulators of Calcium Homeostasis Identified by Inference of Kinetic Model Parameters from Live Single Cells Perturbed by siRNA. Science signaling. 2013;6:ra56. doi: 10.1126/scisignal.2003649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bezprozvanny I. Presenilins and calcium signaling--systems biology to the rescue. Sci Signal. 2013;6:pe24. doi: 10.1126/scisignal.2004296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li X, Dang S, Yan C, Gong X, Wang J, Shi Y. Structure of a presenilin family intramembrane aspartate protease. Nature. 2013;493:56–61. doi: 10.1038/nature11801. [DOI] [PubMed] [Google Scholar]
- 59.Theobald DL. Presenilin adopts the ClC channel fold. Protein Sci. 2016;25:1363–5. doi: 10.1002/pro.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kelliher M, Fastbom J, Cowburn RF, Bonkale W, Ohm TG, Ravid R, Sorrentino V, O’Neill C. Alterations in the ryanodine receptor calcium release channel correlate with Alzheimer’s disease neurofibrillary and beta-amyloid pathologies. Neuroscience. 1999;92:499–513. doi: 10.1016/s0306-4522(99)00042-1. [DOI] [PubMed] [Google Scholar]
- 61.Bruno AM, Huang JY, Bennett DA, Marr RA, Hastings ML, Stutzmann GE. Altered ryanodine receptor expression in mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging. 2012;33:1001 e1–6. doi: 10.1016/j.neurobiolaging.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Supnet C, Grant J, Kong H, Westaway D, Mayne M. Amyloid-beta-(1–42) increases ryanodine receptor-3 expression and function in neurons of TgCRND8 mice. The Journal of biological chemistry. 2006;281:38440–7. doi: 10.1074/jbc.M606736200. [DOI] [PubMed] [Google Scholar]
- 63.Supnet C, Bezprozvanny I. The dysregulation of intracellular calcium in Alzheimer disease. Cell calcium. 2010;47:183–189. doi: 10.1016/j.ceca.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oules B, Del Prete D, Greco B, Zhang X, Lauritzen I, Sevalle J, Moreno S, Paterlini-Brechot P, Trebak M, Checler F, Benfenati F, Chami M. Ryanodine Receptor Blockade Reduces Amyloid-beta Load and Memory Impairments in Tg2576 Mouse Model of Alzheimer Disease. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:11820–11834. doi: 10.1523/JNEUROSCI.0875-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peng J, Liang G, Inan S, Wu Z, Joseph DJ, Meng Q, Peng Y, Eckenhoff MF, Wei H. Dantrolene ameliorates cognitive decline and neuropathology in Alzheimer triple transgenic mice. Neurosci Lett. 2012;516:274–9. doi: 10.1016/j.neulet.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chakroborty S, Briggs C, Miller MB, Goussakov I, Schneider C, Kim J, Wicks J, Richardson JC, Conklin V, Cameransi BG, Stutzmann GE. Stabilizing ER Ca2+ channel function as an early preventative strategy for Alzheimer’s disease. PloS one. 2012;7:e52056. doi: 10.1371/journal.pone.0052056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao X, Weisleder N, Han X, Pan Z, Parness J, Brotto M, Ma J. Azumolene inhibits a component of store-operated calcium entry coupled to the skeletal muscle ryanodine receptor. The Journal of biological chemistry. 2006;281:33477–86. doi: 10.1074/jbc.M602306200. [DOI] [PubMed] [Google Scholar]
- 68.Krause T, Gerbershagen MU, Fiege M, Weisshorn R, Wappler F. Dantrolene--a review of its pharmacology, therapeutic use and new developments. Anaesthesia. 2004;59:364–73. doi: 10.1111/j.1365-2044.2004.03658.x. [DOI] [PubMed] [Google Scholar]
- 69.Liu J, Supnet C, Sun S, Zhang H, Good L, Popugaeva E, Bezprozvanny I. The role of ryanodine receptor type 3 in a mouse model of Alzheimer disease. Channels. 2014;8:230–42. doi: 10.4161/chan.27471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Supnet C, Noonan C, Richard K, Bradley J, Mayne M. Up-regulation of the type 3 ryanodine receptor is neuroprotective in the TgCRND8 mouse model of Alzheimer’s disease. Journal of neurochemistry. 2010;112:356–65. doi: 10.1111/j.1471-4159.2009.06487.x. [DOI] [PubMed] [Google Scholar]
- 71.Briggs CA, Chakroborty S, Stutzmann GE. Emerging pathways driving early synaptic pathology in Alzheimer’s disease. BBRC. 2016 doi: 10.1016/j.bbrc.2016.09.088. in revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Garcia-Alvarez G, Shetty MS, Lu B, Yap KA, Oh-Hora M, Sajikumar S, Bichler Z, Fivaz M. Impaired spatial memory and enhanced long-term potentiation in mice with forebrain-specific ablation of the Stim genes. Front Behav Neurosci. 2015;9:180. doi: 10.3389/fnbeh.2015.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gruszczynska-Biegala J, Pomorski P, Wisniewska MB, Kuznicki J. Differential Roles for STIM1 and STIM2 in Store-Operated Calcium Entry in Rat Neurons. PloS one. 2011;6:e19285. doi: 10.1371/journal.pone.0019285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Popugaeva E, Bezprozvanny I. Can the calcium hypothesis explain synaptic loss in Alzheimer’s disease? Neurodegener Dis. 2014;13:139–41. doi: 10.1159/000354778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hoth M, Niemeyer BA. The neglected CRAC proteins: Orai2, Orai3, and STIM2. Curr Top Membr. 2013;71:237–71. doi: 10.1016/B978-0-12-407870-3.00010-X. [DOI] [PubMed] [Google Scholar]
- 76.Gruszczynska-Biegala J, Kuznicki J. Native STIM2 and ORAI1 proteins form a calcium-sensitive and thapsigargin-insensitive complex in cortical neurons. Journal of neurochemistry. 2013;126:727–38. doi: 10.1111/jnc.12320. [DOI] [PubMed] [Google Scholar]
- 77.Korkotian E, Oni-Biton E, Segal M. The role of the store-operated calcium entry channel Orai1 in cultured rat hippocampal synapse formation and plasticity. The Journal of physiology. 2016 doi: 10.1113/JP272645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sun Y, Sukumaran P, Bandyopadhyay BC, Singh BB. Physiological Function and Characterization of TRPCs in Neurons. Cells. 2014;3:455–75. doi: 10.3390/cells3020455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Prakriya M, Lewis RS. Store-Operated Calcium Channels. Physiol Rev. 2015;95:1383–436. doi: 10.1152/physrev.00020.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou J, Du W, Zhou K, Tai Y, Yao H, Jia Y, Ding Y, Wang Y. Critical role of TRPC6 channels in the formation of excitatory synapses. Nature neuroscience. 2008;11:741–3. doi: 10.1038/nn.2127. [DOI] [PubMed] [Google Scholar]
- 81.Leuner K, Kazanski V, Muller M, Essin K, Henke B, Gollasch M, Harteneck C, Muller WE. Hyperforin--a key constituent of St. John’s wort specifically activates TRPC6 channels. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2007;21:4101–11. doi: 10.1096/fj.07-8110com. [DOI] [PubMed] [Google Scholar]
- 82.Inestrosa NC, Tapia-Rojas C, Griffith TN, Carvajal FJ, Benito MJ, Rivera-Dictter A, Alvarez AR, Serrano FG, Hancke JL, Burgos PV, Parodi J, Varela-Nallar L. Tetrahydrohyperforin prevents cognitive deficit, Abeta deposition, tau phosphorylation and synaptotoxicity in the APPswe/PSEN1DeltaE9 model of Alzheimer’s disease: a possible effect on APP processing. Transl Psychiatry. 2011;1:e20. doi: 10.1038/tp.2011.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cerpa W, Hancke JL, Morazzoni P, Bombardelli E, Riva A, Marin PP, Inestrosa NC. The hyperforin derivative IDN5706 occludes spatial memory impairments and neuropathological changes in a double transgenic Alzheimer’s mouse model. Curr Alzheimer Res. 2010;7:126–33. doi: 10.2174/156720510790691218. [DOI] [PubMed] [Google Scholar]
- 84.Dinamarca MC, Cerpa W, Garrido J, Hancke JL, Inestrosa NC. Hyperforin prevents beta-amyloid neurotoxicity and spatial memory impairments by disaggregation of Alzheimer’s amyloid-beta-deposits. Molecular psychiatry. 2006;11:1032–48. doi: 10.1038/sj.mp.4001866. [DOI] [PubMed] [Google Scholar]
- 85.Wang J, Lu R, Yang J, Li H, He Z, Jing N, Wang X, Wang Y. TRPC6 specifically interacts with APP to inhibit its cleavage by gamma-secretase and reduce Abeta production. Nat Commun. 2015;6:8876. doi: 10.1038/ncomms9876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baumgartel K, Mansuy IM. Neural functions of calcineurin in synaptic plasticity and memory. Learn Mem. 2012;19:375–84. doi: 10.1101/lm.027201.112. [DOI] [PubMed] [Google Scholar]
- 87.Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–90. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- 88.Otmakhov N, Lisman J. Measuring CaMKII concentration in dendritic spines. J Neurosci Methods. 2012;203:106–14. doi: 10.1016/j.jneumeth.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee SJ, Escobedo-Lozoya Y, Szatmari EM, Yasuda R. Activation of CaMKII in single dendritic spines during long-term potentiation. Nature. 2009;458:299–304. doi: 10.1038/nature07842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lisman J, Yasuda R, Raghavachari S. Mechanisms of CaMKII action in long-term potentiation. Nat Rev Neurosci. 2012;13:169–82. doi: 10.1038/nrn3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Irvine EE, von Hertzen LS, Plattner F, Giese KP. alphaCaMKII autophosphorylation: a fast track to memory. Trends Neurosci. 2006;29:459–65. doi: 10.1016/j.tins.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 92.Giese KP, Fedorov NB, Filipkowski RK, Silva AJ. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science. 1998;279:870–3. doi: 10.1126/science.279.5352.870. [DOI] [PubMed] [Google Scholar]
- 93.Buard I, Coultrap SJ, Freund RK, Lee YS, Dell’Acqua ML, Silva AJ, Bayer KU. CaMKII “autonomy” is required for initiating but not for maintaining neuronal long-term information storage. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:8214–20. doi: 10.1523/JNEUROSCI.1469-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li H, Rao A, Hogan PG. Interaction of calcineurin with substrates and targeting proteins. Trends Cell Biol. 2011;21:91–103. doi: 10.1016/j.tcb.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mulkey RM, Endo S, Shenolikar S, Malenka RC. Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature. 1994;369:486–8. doi: 10.1038/369486a0. [DOI] [PubMed] [Google Scholar]
- 96.Luscher C, Malenka RC. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD) Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a005710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mansuy IM, Mayford M, Jacob B, Kandel ER, Bach ME. Restricted and regulated overexpression reveals calcineurin as a key component in the transition from short-term to long-term memory. Cell. 1998;92:39–49. doi: 10.1016/s0092-8674(00)80897-1. [DOI] [PubMed] [Google Scholar]
- 98.Zeng H, Chattarji S, Barbarosie M, Rondi-Reig L, Philpot BD, Miyakawa T, Bear MF, Tonegawa S. Forebrain-specific calcineurin knockout selectively impairs bidirectional synaptic plasticity and working/episodic-like memory. Cell. 2001;107:617–29. doi: 10.1016/s0092-8674(01)00585-2. [DOI] [PubMed] [Google Scholar]
- 99.Woolfrey KM, Dell’Acqua ML. Coordination of Protein Phosphorylation and Dephosphorylation in Synaptic Plasticity. The Journal of biological chemistry. 2015;290:28604–12. doi: 10.1074/jbc.R115.657262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stefan MI, Edelstein SJ, Le Novere N. An allosteric model of calmodulin explains differential activation of PP2B and CaMKII. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:10768–73. doi: 10.1073/pnas.0804672105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bezprozvanny I, Hiesinger PR. The synaptic maintenance problem: membrane recycling, Ca2+ homeostasis and late onset degeneration. Mol Neurodegener. 2013;8:23. doi: 10.1186/1750-1326-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Megill A, Tran T, Eldred K, Lee NJ, Wong PC, Hoe HS, Kirkwood A, Lee HK. Defective Age-Dependent Metaplasticity in a Mouse Model of Alzheimer’s Disease. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:11346–57. doi: 10.1523/JNEUROSCI.5289-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jang SS, Chung HJ. Emerging Link between Alzheimer’s Disease and Homeostatic Synaptic Plasticity. Neural Plast. 2016;2016:7969272. doi: 10.1155/2016/7969272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Reese LC, Laezza F, Woltjer R, Taglialatela G. Dysregulated phosphorylation of Ca(2+) /calmodulin-dependent protein kinase II-alpha in the hippocampus of subjects with mild cognitive impairment and Alzheimer’s disease. Journal of neurochemistry. 2011;119:791–804. doi: 10.1111/j.1471-4159.2011.07447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ghosh A, Giese KP. Calcium/calmodulin-dependent kinase II and Alzheimer’s disease. Mol Brain. 2015;8:78. doi: 10.1186/s13041-015-0166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Qian W, Yin X, Hu W, Shi J, Gu J, Grundke-Iqbal I, Iqbal K, Gong CX, Liu F. Activation of protein phosphatase 2B and hyperphosphorylation of Tau in Alzheimer’s disease. J Alzheimers Dis. 2011;23:617–27. doi: 10.3233/JAD-2010-100987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mohmmad Abdul H, Baig I, Levine H, 3rd, Guttmann RP, Norris CM. Proteolysis of calcineurin is increased in human hippocampus during mild cognitive impairment and is stimulated by oligomeric Abeta in primary cell culture. Aging Cell. 2011;10:103–13. doi: 10.1111/j.1474-9726.2010.00645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Taglialatela G, Hogan D, Zhang WR, Dineley KT. Intermediate- and long-term recognition memory deficits in Tg2576 mice are reversed with acute calcineurin inhibition. Behav Brain Res. 2009;200:95–9. doi: 10.1016/j.bbr.2008.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Reese LC, Zhang W, Dineley KT, Kayed R, Taglialatela G. Selective induction of calcineurin activity and signaling by oligomeric amyloid beta. Aging Cell. 2008;7:824–35. doi: 10.1111/j.1474-9726.2008.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dineley KT, Hogan D, Zhang WR, Taglialatela G. Acute inhibition of calcineurin restores associative learning and memory in Tg2576 APP transgenic mice. Neurobiol Learn Mem. 2007;88:217–24. doi: 10.1016/j.nlm.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dineley KT, Kayed R, Neugebauer V, Fu Y, Zhang W, Reese LC, Taglialatela G. Amyloid-beta oligomers impair fear conditioned memory in a calcineurin-dependent fashion in mice. J Neurosci Res. 2010;88:2923–32. doi: 10.1002/jnr.22445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yao W, Zou HJ, Sun D, Ren SQ. Abeta induces acute depression of excitatory glutamatergic synaptic transmission through distinct phosphatase-dependent mechanisms in rat CA1 pyramidal neurons. Brain Res. 2013;1515:88–97. doi: 10.1016/j.brainres.2013.03.049. [DOI] [PubMed] [Google Scholar]
- 113.Cavallucci V, Berretta N, Nobili A, Nistico R, Mercuri NB, D’Amelio M. Calcineurin inhibition rescues early synaptic plasticity deficits in a mouse model of Alzheimer’s disease. Neuromolecular Med. 2013;15:541–8. doi: 10.1007/s12017-013-8241-2. [DOI] [PubMed] [Google Scholar]
- 114.Rozkalne A, Hyman BT, Spires-Jones TL. Calcineurin inhibition with FK506 ameliorates dendritic spine density deficits in plaque-bearing Alzheimer model mice. Neurobiol Dis. 2011;41:650–4. doi: 10.1016/j.nbd.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wu HY, Hudry E, Hashimoto T, Kuchibhotla K, Rozkalne A, Fan Z, Spires-Jones T, Xie H, Arbel-Ornath M, Grosskreutz CL, Bacskai BJ, Hyman BT. Amyloid beta induces the morphological neurodegenerative triad of spine loss, dendritic simplification, and neuritic dystrophies through calcineurin activation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:2636–49. doi: 10.1523/JNEUROSCI.4456-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.D’Amelio M, Cavallucci V, Middei S, Marchetti C, Pacioni S, Ferri A, Diamantini A, De Zio D, Carrara P, Battistini L, Moreno S, Bacci A, Ammassari-Teule M, Marie H, Cecconi F. Caspase-3 triggers early synaptic dysfunction in a mouse model of Alzheimer’s disease. Nature neuroscience. 2011;14:69–76. doi: 10.1038/nn.2709. [DOI] [PubMed] [Google Scholar]
- 117.Reese LC, Taglialatela G. A role for calcineurin in Alzheimer’s disease. Curr Neuropharmacol. 2011;9:685–92. doi: 10.2174/157015911798376316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Taglialatela G, Rastellini C, Cicalese L. Reduced Incidence of Dementia in Solid Organ Transplant Patients Treated with Calcineurin Inhibitors. J Alzheimers Dis. 2015;47:329–33. doi: 10.3233/JAD-150065. [DOI] [PMC free article] [PubMed] [Google Scholar]