Abstract
Phthalates are used in a large variety of products, such as building materials, medical devices, and personal care products. Most previous studies on the toxicity of phthalates have focused on single phthalates, but it is also important to study the effects of phthalate mixtures because humans are exposed to phthalate mixtures. Thus, we tested the hypothesis that prenatal exposure to an environmentally relevant phthalate mixture adversely affects female reproduction in mice. To test this hypothesis, pregnant CD-1 dams were orally dosed with vehicle (tocopherol-stripped corn oil) or a phthalate mixture (20 and 200 μg/kg/day, 200 and 500 mg/kg/day) daily from gestational day 10 to birth. The mixture was based on the composition of phthalates detected in urine samples from pregnant women in Illinois. The mixture included 35% diethyl phthalate, 21% di(2-ethylhexyl) phthalate, 15% dibutyl phthalate, 15% diisononyl phthalate, 8% diisobutyl phthalate, and 5% benzylbutyl phthalate. Female mice born to the exposed dams were subjected to tissue collections and fertility tests at different ages. Our results indicate that prenatal exposure to the phthalate mixture significantly increased uterine weight and decreased anogenital distance on postnatal days 8 and 60, induced cystic ovaries at 13 months, disrupted estrous cyclicity, reduced fertility-related indices, and caused some breeding complications at 3, 6, and 9 months of age. Collectively, our data suggest that prenatal exposure to an environmentally relevant phthalate mixture disrupts aspects of female reproduction in mice.
Keywords: environmentally relevant, phthalate mixture, female reproduction, prenatal exposure
Introduction
Phthalates are synthetic chemicals that are widely used in common consumer products, including various plastics, cosmetics, personal care products, polyvinyl chloride pipes, and vinyl flooring [1, 2]. Because of the ubiquitous characteristics of phthalates, humans at all ages are constantly exposed to phthalates through ingestion, inhalation, and dermal contact. Of particular concern, phthalates and their metabolites are present in pregnant women and can affect the health of the mothers as well as their offspring. Metabolites of many phthalates such as di(2-ethylhexyl) phthalate (DEHP), diethyl phthalate (DEP), dimethyl phthalate, di-n-butyl phthalate (DBP), benzyl butyl phthalate (BBzP), and diisobutyl phthalate (DiBP) have been frequently detected in urine samples from pregnant women [3–5]. Moreover, urinary levels of DEHP and its metabolite, mono-ethylhexyl phthalate (MEHP), have been detected in maternal plasma and urine samples, and have been associated with decreased gestational age [6, 7]. Phthalate metabolites are also commonly found in fetal samples such as amniotic fluid and cord blood samples [8–10]. Collectively, these studies suggest that humans are constantly exposed to phthalates and that this exposure starts as early as fetal life.
Fetal life is considered to be one of the most sensitive periods to toxicants because of the critical developmental events that occur during this time period. Prenatal phthalate exposure has been shown to induce “phthalate syndrome” in male rodents [11, 12]. This “phthalate syndrome” is characterized by malformations in the male reproductive organs, retention of nipples, and reduced anogenital distance (AGD) [11, 12]. Moreover, gestational exposures to different phthalates have been shown to reduce fertility in male rodents by decreasing steroidogenic capacity, sperm quality and quantity, and sexual behaviors [13–16]. However, the effects of prenatal phthalate exposure on female reproduction in the offspring are less extensively studied. Published studies have shown that gestational exposure to DEHP affects folliculogenesis [17], reduces oocyte quality [18], decreases steroidogenic capacity [18, 19], delays puberty [19], and decreases fertility [17, 18].
Although studies on prenatal exposure to a single phthalate provide important information on phthalate toxicity, it is important to study mixtures of phthalates because humans are exposed to mixtures. Prenatal exposure to a mixture of DBP, DEHP, and bisphenol A reduced ovarian follicular reserve and induced polycystic ovaries in female offspring [20]. Prenatal exposure to a phthalate mixture (BBzP, DBP, DEHP, DiBP, and dipentyl phthalate) induced uterine malformations in female offspring [21]. Prenatal exposure to a mixture of phthalates, pesticides, UV-filters, bisphenol A, butylparaben, and paracetamol significantly impaired female reproductive function in the offspring by reducing follicle numbers, disrupting estrous cycles, and decreasing ovarian weights [22]. Only one of these previous studies, however, used a mixture that was based on human exposure, but the lowest dose used in that study was 100 times greater than the estimates of human exposure [22]. Thus, there is a need for studies conducted using environmentally relevant phthalate mixtures at doses relevant to human exposure levels. Therefore, in our study, we developed a phthalate mixture based on estimates of phthalate exposure in pregnant women and we used this phthalate mixture at environmentally relevant doses to test the hypothesis that prenatal exposure to a phthalate mixture adversely affects reproductive outcomes in the F1 female offspring.
Materials and Methods
Chemicals
DEP, DEHP, DBP, DiBP, DiNP, and BBzP (>98% purity) were purchased from Sigma-Aldrich (St. Louis, MO). A pure phthalate mixture was made by calculating and combining the appropriate amount of each phthalate according to the following percentages: 21% DEHP, 35% DEP, 15% DBP, 8% DiBP, 5% BBzP, and 15% DiNP. The percentages were derived from levels of phthalate metabolites measured in urine samples from pregnant women in Illinois (unpublished data from the iKids study). Then, the phthalate mixture was mixed thoroughly before dilution in tocopherol-stripped corn oil (vehicle control).
The doses used for this study were 20 μg/kg/day, 200 μg/kg/day, 200 mg/kg/day, and 500 mg/kg/day. This is the first time this phthalate mixture has been tested in vivo. Thus, doses of phthalate mixture were chosen to cover a wide environmentally relevant range and to include some of the doses of individual phthalates that have been shown to adversely affect reproductive health during prenatal exposure [17–19, 23]. The estimated general population daily exposure level of DEHP is 3–30 μg/kg/day [24]. According to these estimates, the two lower doses used in our study mimic daily human exposure because they contain approximately 4 and 40 μg/kg/day of DEHP, respectively. We also included two high doses of mixture (200 and 500 mg/kg/day, which contain approximately 40 and 100 mg/kg/day of DEHP, respectively) to test the effects of this mixture at a level higher than human expose range and to compare our results with available information from single phthalate studies that used doses close to this level. Previous studies have shown that gestational exposure to DEHP decreased the thickness of thecal cell layers at 50 and 300 mg/kg/day [23], disrupted steroidogenic enzyme gene expression and increased estradiol levels at 100 mg/kg/day [19], increased preantral follicle numbers at 200 μg/kg/day and 500 mg/kg/day [17], increased ovarian weights at 0.05 and 5 mg/kg/day [18], and induced breeding complications at 200 μg/kg/day and 750 mg/kg/day [17].
Animals
Adult cycling female and adult male CD-1 mice were purchased from Jackson Laboratory (Bar Harbor, ME) and allowed to acclimate to the facility for at least two weeks before use. The mice were housed at the University of Illinois at Urbana-Champaign, Veterinary Medicine Animal Facility in polysulfone cages. Food (Harlan Teklad 8604) and water (reverse osmosis filtered) were provided for ad libitum consumption. Temperature was maintained at 22±1 °C, and animals were subjected to 12-h light-dark cycles. The Institutional Animal Care and Use Committee (IACUC) at the University of Illinois at Urbana-Champaign approved all procedures involving animal care, euthanasia, and tissue collection.
Study Design
At two months of age, 60 females were mated with untreated proven breeder male mice. Successful mating was confirmed by the presence of vaginal sperm plug. Gestational day (GD) 1 was set as the day of the presence of vaginal sperm plug. Once the vaginal sperm plug was observed, female mice were separated and singly housed. These dams were considered to be the F0 generation, and their body weights were monitored every three days to confirm pregnancy. On GD 10, F0 dams were assigned to five different treatment groups (12 F0 dams/treatment group) and then were dosed once a day until they give birth. F0 dams were orally dosed by pipetting tocopherol-stripped corn oil (vehicle control) or different doses of phthalate mixture (20 μg/kg/day, 200 μg/kg/day, 200 mg/kg/day, and 500 mg/kg/day) into the mouth from GD 10 to birth.
The route of exposure was selected to mimic the major route of exposure in humans [25]. The exposure window was chosen because it is a critical period for ovarian development in the mouse. All doses were given in 28–44 μl based on their body weights. Dams were allowed to give birth naturally and the number of total and live pups, body weights of live pups, and sex ratio of the F1 pups were recorded on postnatal day (PND) 0.
Tissue Collection, Body Weights, and Organ Weights
On PNDs 1, 4, 8, and 21, at least one female F1 pup per litter (n = 10–12 dams/treatment group) was randomly chosen for tissue collections. On PND 60 and 13 months of age, the remaining F1 female pups were euthanized and their tissues were collected during diestrus. At each time point, sera, ovaries, uteri, and livers were collected, organ weights and body weights were recorded, and anogenital distances (AGD) were measured. Organ weights were recorded as whole organ weights in grams, AGD was recorded in millimeters (mm), and ovaries from the same pup were measured together and recorded as one weight for PND 1–60. At 13 months of age, ovarian weights were based on one healthy ovary per mouse. The presence of cystic ovaries was recorded. We normalized AGD to cubic root of body weight to account for body size effects [26]. Sera were stored for measurement of hormone levels in future studies. Ovaries, uteri, and liver were snap-frozen or fixed for molecular or histological analyses in future studies.
Onset of Puberty
After weaning, one F1 female per litter (n = 11–12 litters/treatment group) was kept for analyses of onset of puberty, estrous cyclicity, and fertility. Vaginal opening was used as a sign of onset of puberty and was monitored every morning after weaning. Body weights were recorded on the day of weaning and on the day of vaginal opening. After vaginal opening, estrous cyclicity was monitored every morning by examining vaginal smears daily for 30 consecutive days.
Fertility Tests
One F1 female per litter (n = 7–12 litters/treatment group) was subjected to fertility tests at 3, 6, and 9 months of age. For each fertility test, estrous cyclicity was monitored every morning for 14 consecutive days prior to mating. Then, female mice were housed with untreated proven breeder male mice for maximum of 2 weeks to evaluate fertility of F1 females. Once a vaginal sperm plug was present, female mice were singly housed. Body weights were recorded twice a week starting from the first day of estrous cyclicity examination until the birth of pups. Maintenance or loss of pregnancy were monitored by body weight gain. For every female, the presence of a vaginal sperm plug, days to pregnancy, the ability to become and maintain pregnancy, and pregnancy lengths were recorded. The ability to deliver live pups, size of the litter, average live pup birth weight, and pup sex ratios were also recorded to evaluate the birth outcomes.
To examine fertility, we used several equations to calculate mating index, pregnancy rate, fertility index, and gestation index as previously described [27] and as follows:
Pregnancy rate = number of pregnant females/number of breeding pairs × 100
Mating index = number of females with vaginal sperm plugs/number of breeding pairs × 100
Fertility index = number of pregnant females/number of females with vaginal sperm plugs × 100
Gestational index = number of females who delivered/number of pregnant females × 100
Statistical Analyses
Data analyses were conducted using SPSS statistical software (SPSS Inc., Chicago, IL). One-way analysis of variance (ANOVA) was used to conduct multiple comparisons between normally distributed experimental groups, then followed by Dunnett post-hoc comparisons if equal variances were assumed, or Games-Howell post-hoc comparisons if equal variances were not assumed. Kruskal-Wallis H tests were used for comparison between groups if data were not normally distributed, followed by Mann-Whitney U two-independent sample tests. Statistical significance was assigned at p < 0.05.
Results
Effect of Phthalate Mixture Exposure on F0 Fertility Outcomes
The phthalate mixture did not affect the number of F0 females that gave birth to live litters (n = 10–12 dams/treatment group, p > 0.05, data not shown). The phthalate mixture also did not cause any effects on F0 body weight, ovary weight, uterine weight, liver weight, or relative organ weight compared to vehicle control (n = 11–12 dams/treatment group, p > 0.05, data not shown). Further, the phthalate mixture did not affect the number of total pups, number of live pups, average live pup body weights, and sex ratio of the F1 generation compared to the control groups (n = 10–12 dams/treatment group, p > 0.05, data not shown).
Effect of Prenatal Phthalate Mixture Exposure on F1 Female Pup Tissues
Prenatal exposure to phthalate mixture did not affect the body and liver weights of F1 females compared to controls on PND 1 and 4 (Table 1, n = 10–12 dams/treatment). Prenatal exposure to phthalate mixture at 500 mg/kg/day significantly decreased AGD in F1 females compared to controls on PND 8 (Table 1, n = 8–12 dams/treatment, p < 0.05). Moreover, prenatal exposure to phthalate mixture (20 μg/kg/day) increased uterine weight in the F1 females compared to controls on PND 8 (Table 1, n = 8–12 dams/treatment, p < 0.05). Prenatal exposure to phthalate mixture did not affect body weight, liver weight, ovary weight, uteri weight, or AGD in F1 females compared to controls on PND 21 or at 13 months of age (Table 1, n = 6–11 dams/treatment). However, prenatal exposure to phthalate mixture at 20 and 200 μg/kg/day significantly decreased AGD in F1 females and it (500 mg/kg/day) increased uterine weight in F1 females compared to controls on PND 60 (Table 1, n = 5–9 dams/treatment, p < 0.05).
Table 1.
The effects of prenatal exposure to phthalate mixture on body and organ weights and AGD in F1 females.
| Age of pups (F1) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | PND 1 | PND 4 | PND 8 | PND 21 | PND 60 | 13 months | ||
| Body weight (g) | Control | 1.8279 ± 0.0456 | 3.3657 ± 0.1362 | 6.1867 ± 0.3138 | 14.9986 ± 1.2510 | 30.4500 ± 0.9917 | 44.3971 ± 2.2984 | |
| 20 μg/kg/day | 1.7677 ± 0.0404 | 3.3603 ± 0.1277 | 6.0878 ± 0.1271 | 15.1017 ± 0.4504 | 28.6614 ± 0.8763 | 46.6067 ± 2.6242 | ||
| 200 μg/kg/day | 1.8442 ± 0.0487 | 3.3253 ± 0.0883 | 6.5079 ± 0.1839 | 15.7880 ± 0.2801 | 29.1700 ± 1.5292 | 46.8811 ± 2.2391 | ||
| 200 mg/kg/day | 1.7742 ± 0.0490 | 3.2851 ± 0.1237 | 5.9298 ± 0.1863 | 14.8082 ± 0.5059 | 29.2856 ± 1.0320 | 48.4640 ± 1.9372 | ||
| 500 mg/kg/day | 1.7737 ± 0.0395 | 3.1211 ± 0.1512 | 5.9311 ± 0.1121 | 15.4883 ± 0.3072 | 31.1767 ± 1.3363 | 44.8367 ± 3.7498 | ||
| Liver weight (g) | Control | 0.0776 ± 0.0024 | 0.1220 ± 0.0063 | 0.2031 ± 0.0149 | 0.8298 ± 0.0922 | 1.9251 ± 0.1499 | 2.6497 ± 0.1789 | |
| 20 μg/kg/day | 0.0705 ± 0.0013 | 0.1186 ± 0.0075 | 0.1941 ± 0.0115 | 0.8415 ± 0.0393 | 1.6657 ± 0.1016 | 2.6943 ± 0.1463 | ||
| 200 μg/kg/day | 0.0766 ± 0.0027 | 0.1195 ± 0.0042 | 0.2131 ± 0.0063 | 0.9101 ± 0.0221 | 1.8083 ± 0.0918 | 2.5070 ± 0.1086 | ||
| 200 mg/kg/day | 0.0737 ± 0.0019 | 0.1137 ± 0.0038 | 0.1875 ± 0.0090 | 0.8169 ± 0.0403 | 1.8529± 0.0986 | 2.6211 ± 0.1209 | ||
| 500 mg/kg/day | 0.0760 ± 0.0020 | 0.1128 ± 0.0067 | 0.1918 ± 0.0039 | 0.8800 ± 0.0405 | 1.8505 ± 0.1512 | 2.5688 ± 0.1192 | ||
|
|
Control | 0.7162 ± 0.0669 | 1.7418 ± 0.0822 | 2.1497 ± 0.1105 | 1.6733 ± 0.0545 | |||
| 20 μg/kg/day | 0.6714 ± 0.0365 | 1.5916 ± 0.0501 | 1.7781 ± 0.0769* | 1.7680 ± 0.0671 | ||||
| 200 μg/kg/day | 0.6711 ± 0.0414 | 1.6945 ± 0.0592 | 1.8112 ± 0.0772* | 1.7481 ± 0.0533 | ||||
| 200 mg/kg/day | 0.7343 ± 0.0432 | 1.6683 ± 0.0567 | 1.9686 ± 0.0641 | 1.8108 ± 0.0450 | ||||
| 500 mg/kg/day | 0.5899 ± 0.0253* | 1.6103 ± 0.0120 | 1.9055 ± 0.0620 | 1.6997 ± 0.0843 | ||||
| Uterine weight (g) | Control | 0.0065 ± 0.0006 | 0.0409 ± 0.0066 | 0.0902 ± 0.0119 | 0.2365 ± 0.0316 | |||
| 20 μg/kg/day | 0.0071 ± 0.0002* | 0.0384 ± 0.0042 | 0.1119 ± 0.0093 | 0.2419 ± 0.0271 | ||||
| 200 μg/kg/day | 0.0067 ± 0.0002 | 0.0448 ± 0.0035 | 0.0902 ± 0.0093 | 0.2201 ± 0.0221 | ||||
| 200 mg/kg/day | 0.0064 ± 0.0003 | 0.0392 ± 0.0031 | 0.1120 ± 0.0096 | 0.2779 ± 0.0597 | ||||
| 500 mg/kg/day | 0.0064 ± 0.0002 | 0.0391 ± 0.0019 | 0.1411 ± 0.0201* | 0.3144 ± 0.0742 | ||||
| Ovary weight (g) | Control | 0.0048 ± 0.0004 | 0.0169 ± 0.0015 | 0.0096 ± 0.0012 | ||||
| 20 μg/kg/day | 0.0045 ± 0.0002 | 0.0135 ± 0.0014 | 0.0096 ± 0.0008 | |||||
| 200 μg/kg/day | 0.0044 ± 0.0004 | 0.0138 ± 0.0011 | 0.0118 ± 0.0018 | |||||
| 200 mg/kg/day | 0.0045 ± 0.0003 | 0.0157 ± 0.0020 | 0.0094 ± 0.0011 | |||||
| 500 mg/kg/day | 0.0051 ± 0.0004 | 0.0151 ± 0.0014 | 0.0102 ± 0.0014 | |||||
Interestingly, prenatal exposure to the phthalate mixture caused cystic ovaries in F1 females at 13 months of age (Figure 1). In controls, none of the F1 females had cystic ovaries, but females in all phthalate treated groups had cystic ovaries with enlarged fluid or blood filled cysts (Table 2).
Figure 1.
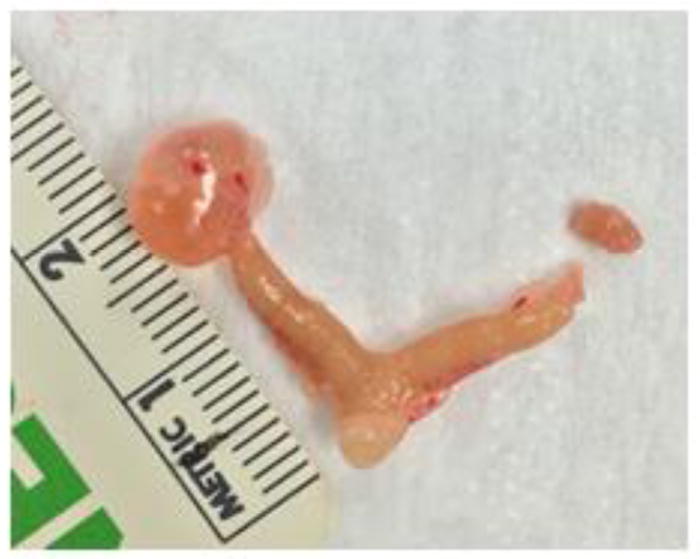
A representative photograph of a cystic ovary in the phthalate mixture exposed F1 mice at 13 months of age. The cystic ovary is shown on the left and a normal ovary is shown on the right. A scale (in centimeters) was photographed along with the left uterine horn and ovary to indicate the sizes.
Table 2.
The effects of prenatal exposure to phthalate mixture on the occurrence of cystic ovaries in F1 females.
| Treatment | Total number of females | Number of females with cystic ovaries | Number of females with 1 cystic ovary | Number of females with 2 cystic ovaries | Number of females with blood filled cysts | Number of females with fluid filled cysts |
|---|---|---|---|---|---|---|
| Control | 7 | 0 | 0 | 0 | 0 | 0 |
| 20 μg/kg/day | 9 | 5 | 5 | 0 | 0 | 5 |
| 200 μg/kg/day | 9 | 7 | 4 | 3 | 3 | 4 |
| 200 mg/kg/day | 10 | 5 | 4 | 1 | 1 | 4 |
| 500 mg/kg/day | 9 | 2 | 1 | 1 | 2 | 0 |
Effect of Prenatal Phthalate Mixture Exposure on F1 Pubertal Outcomes
Prenatal exposure to phthalate mixture at 20 μg/kg/day decreased the F1 body weights at vaginal opening compared to controls (Figure 2A, n = 10–12, p < 0.05), but it did not significantly affect the age at vaginal opening in F1 females compared to controls (Figure 2B, n = 10–12). However, prenatal exposure to phthalate mixture at 200 μg/kg/day borderline significantly reduced the days between vaginal opening and the first estrus in F1 females compared to controls (Figure 2C, n = 10–12, p = 0.061).
Figure 2.
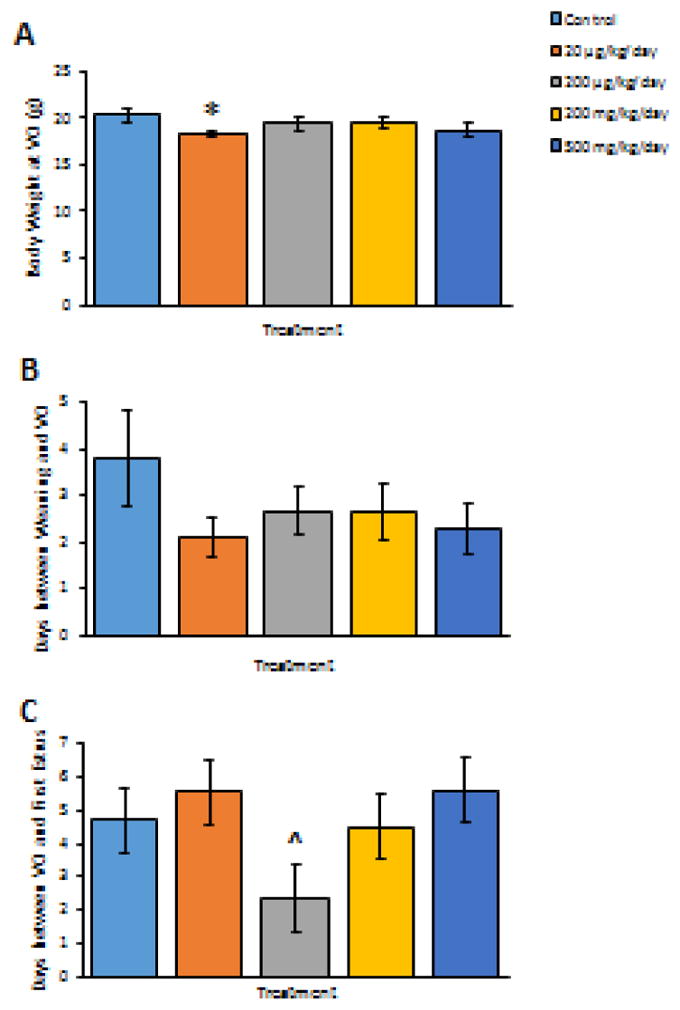
The effects of prenatal exposure to phthalate mixture on pubertal outcomes in F1 females. The effects of prenatal exposure to phthalate mixture on body weights at vaginal opening in F1 females are shown in panel A. The effects of prenatal exposure to phthalate mixture on days between weaning and vaginal opening in F1 females are shown in panel B. The effects of prenatal exposure to phthalate mixture on days between vaginal opening and the presence of first estrus in F1 females are shown in panel C. Graphs represent means ± SEM from 10–12 dams per treatment group. Asterisks (*) indicate significant differences from the control (p < 0.05). ^ indicates borderline significance compared to control, p = 0.061. VO, vaginal opening
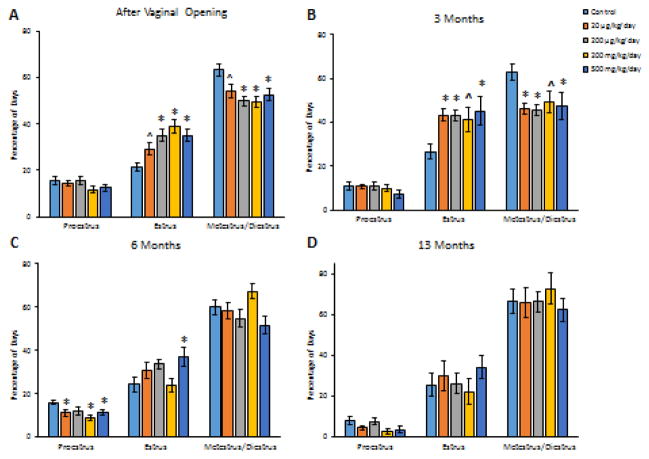
Prenatal phthalate mixture exposure did not affect the time mice spent in proestrus in the 30 days estrous cyclicity after vaginal opening in F1 females compared to control (Figure 3A, n = 10–12). However, at all doses, prenatal phthalate mixture exposure increased the time F1 mice spent in estrus and decreased the time they spent in metestrus and diestrus combined in the 30 days estrous cyclicity after vaginal opening compared to control (Figure 3A, n = 10–12, *p < 0.05, ^p = 0.057 for estrus, ^p = 0.063 for metestrus/diestrus).
Figure 3.
The effects of prenatal exposure to phthalate mixture on estrous cyclicity after vaginal opening, and at 3, 6, and 13 months of age in F1 females. Estrous cyclicity after vaginal opening in F1 females is shown in panel A. Estrous cyclicity at 3 months in F1 females is shown in panel B. Estrous cyclicity at 6 months in F1 females is shown in panel C. Estrous cyclicity at 13 months in F1 females is shown in panel D. Graphs represent means ± SEM from 7–12 dams per treatment group. Asterisks (*) indicate significant differences from the control (p < 0.05). ^ indicates borderline significance compared to control (panel A, p = 0.057 for estrus, p = 0.063 for metestrus/diestrus. Panel B, p = 0.077 for estrous, p = 0.059 for metestrus/diestrus).
Effect of Prenatal Phthalate Mixture Exposure on F1 Body Weights and Cyclicity over Time
Prenatal phthalate mixture exposure did not affect F1 body weights at 3, 6, 9, and 13 months of age compared to controls (Table 3, n = 7–12). At 3 months, prenatal exposure to phthalate mixture did not affect the time F1 mice spent in proestrus (Figure 3B, n = 11–12), but it increased the time spent in estrus and decreased the time spent in metestrus/diestrus combined at all doses in F1 females compared to controls (Figure 3B, n = 11–12, ^p = 0.077 for estrous, ^p = 0.059 for metestrus/diestrus). At 6 months, prenatal exposure to phthalate mixture at 20 μg/kg/day, 200 mg/kg/day, and 500 mg/kg/day significantly decreased the time F1 mice spent in proestrus, but at 500 mg/kg/day, it increased the time F1 mice spent in estrus compared to control (Figure 3C, n = 10–12, p < 0.05). At 13 months, prenatal exposure to phthalate mixture did not affect F1 estrous cyclicity compared to control (Figure 3D, n = 7–9).
Table 3.
The effects of prenatal exposure to phthalate mixture on body weight in F1 females.
| Treatments | Age of pups (F1) | |||
|---|---|---|---|---|
| 3 months (g) | 6 months (g) | 9 months (g) | 13 months (g) | |
| Control | 32.34 ± 1.23 | 39.37 ± 1.08 | 44.86 ± 1.50 | 46.07 ± 2.42 |
| 20 μg/kg/day | 32.23 ± 0.78 | 39.14 ± 1.99 | 43.66 ± 1.28 | 48.00 ± 2.56 |
| 200 μg/kg/day | 32.71 ± 0.85 | 40.16 ± 0.97 | 44.84 ± 1.60 | 49.95 ± 2.13 |
| 200 mg/kg/day | 33.95 ± 0.79 | 41.39 ± 0.93 | 46.68 ± 1.54 | 50.29 ± 2.33 |
| 500 mg/kg/day | 32.78 ± 1.08 | 39.97 ± 1.12 | 46.27 ± 2.36 | 49.56 ± 3.06 |
Effect of Prenatal Phthalate Mixture Exposure on F1 Fertility over Time
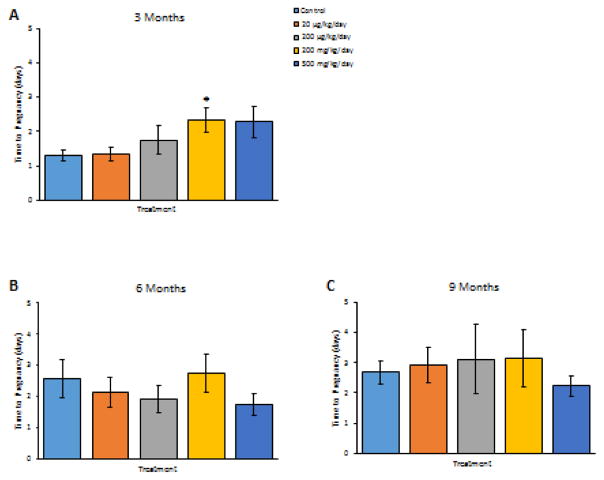
At 3 months, prenatal exposure to phthalate mixture at 200 mg/kg/day significantly increased the number of days the F1 mice needed to become pregnant compared to controls (Figure 4A, n = 11–12, p < 0.05), but it did not affect pregnancy length in F1 females compared to controls (n = 10–12, data not shown). At this time point, prenatal exposure to phthalate mixture also caused some breeding complications in the F1 females. Specifically, one F1 female in the control group lost her pregnancy. One F1 female in the 200 mg/kg/day group successfully mated, but did not become pregnant and one F1 female in the same treatment group lost her pregnancy. One F1 female in the 500 mg/kg/day group successfully mated, but did not become pregnant. This resulted in a reduced pregnancy rate and fertility index in the 200 and 500 mg/kg/day treatment groups compared to control (Table 4, n = 10–12). As far as birth outcomes, the phthalate mixture did not affect live and total F2 pup numbers, average F2 pup birth weight, or percent F2 females compared to control (n = 9–11, data not shown).
Figure 4.
The effects of prenatal exposure to phthalate mixture on time to pregnancy at 3, 6, and 9 months of age in F1 females. The effects of prenatal exposure to phthalate mixture on time to pregnancy at 3 months of age in F1 females are shown in panel A. The effects of prenatal exposure to phthalate mixture on time to pregnancy at 6 months of age in F1 females are shown in panel B. The effects of prenatal exposure to phthalate mixture on time to pregnancy at 9 months of age in F1 females are shown in panel C. Graphs represent means ± SEM from 7–12 dams per treatment group. Asterisk (*) indicates significant differences from the control (p < 0.05).
Table 4.
The effects of prenatal exposure to phthalate mixture on fertility at 3, 6, and 9 months of age.
| Age | Treatment | Total female | Plugged female | Pregnant females | Delivered female | Mating index | Pregnancy rate | Fertility index | Gestational index |
|---|---|---|---|---|---|---|---|---|---|
| 3 Months | Control | 11 | 11 | 11 | 10 | 100 | 100 | 100 | 91 |
| 20 μg/kg/day | 12 | 12 | 12 | 12 | 100 | 100 | 100 | 100 | |
| 200 μg/kg/day | 11 | 11 | 11 | 11 | 100 | 100 | 100 | 100 | |
| 200 mg/kg/day | 12 | 12 | 11 | 10 | 100 | 92 | 92 | 91 | |
| 500 mg/kg/day | 11 | 11 | 10 | 10 | 100 | 91 | 91 | 100 | |
| 6 Months | Control | 10 | 10 | 10 | 9 | 100 | 100 | 100 | 90 |
| 20 μg/kg/day | 11 | 11 | 10 | 10 | 100 | 91 | 91 | 100 | |
| 200 μg/kg/day | 11 | 11 | 9 | 8 | 100 | 82 | 82 | 89 | |
| 200 mg/kg/day | 12 | 12 | 10 | 8 | 100 | 83 | 83 | 80 | |
| 500 mg/kg/day | 11 | 11 | 10 | 9 | 100 | 91 | 91 | 90 | |
| 9 Months | Control | 7 | 7 | 6 | 6 | 100 | 86 | 86 | 100 |
| 20 μg/kg/day | 11 | 11 | 11 | 8 | 100 | 100 | 100 | 73 | |
| 200 μg/kg/day | 10 | 10 | 10 | 7 | 100 | 100 | 100 | 70 | |
| 200 mg/kg/day | 10 | 9 | 9 | 8 | 90 | 90 | 100 | 89 | |
| 500 mg/kg/day | 9 | 9 | 9 | 8 | 100 | 100 | 100 | 89 |
At 6 months of age, prenatal exposure to the phthalate mixture did not affect the time F1 mice needed to become pregnant (Figure 4B, n = 10–12). Phthalate mixture exposure also did not affect pregnancy length compared to controls (n = 10–12, data not shown). At this time point, prenatal exposure to phthalate mixture caused some breeding complications in F1 females. Specifically, one F1 female in the 20 μg/kg/day group successfully mated, but did not become pregnant, and two F1 females in the 200 μg/kg/day group successfully mated, but did not become pregnant and one F1 female in this group lost her pregnancy. Further, two F1 females in the 200 mg/kg/day group successfully mated, but did not become pregnant and two F1 females in the same group lost their pregnancy. In addition, one F1 female in the 500 mg/kg/day group successfully mated, but did not become pregnant and one F1 female in the same group experienced dystocia. In contrast, only one F1 female in the control group lost her pregnancy. Taken together, this resulted in a reduced pregnancy rate and fertility index in the phthalate mixture treated groups compared to controls (Table 4, n = 10–12). As far as birth outcomes, the phthalate mixture at 200 μg/kg/day significantly decreased the number of live and total F2 pups born to the F1 females compared to control animals (Figure 5A, n = 7–10, p < 0.05), but it did not affect average F2 pup birth weight or percent of F2 females compared to control (Figure 5B and 5C, n = 7–10).
Figure 5.
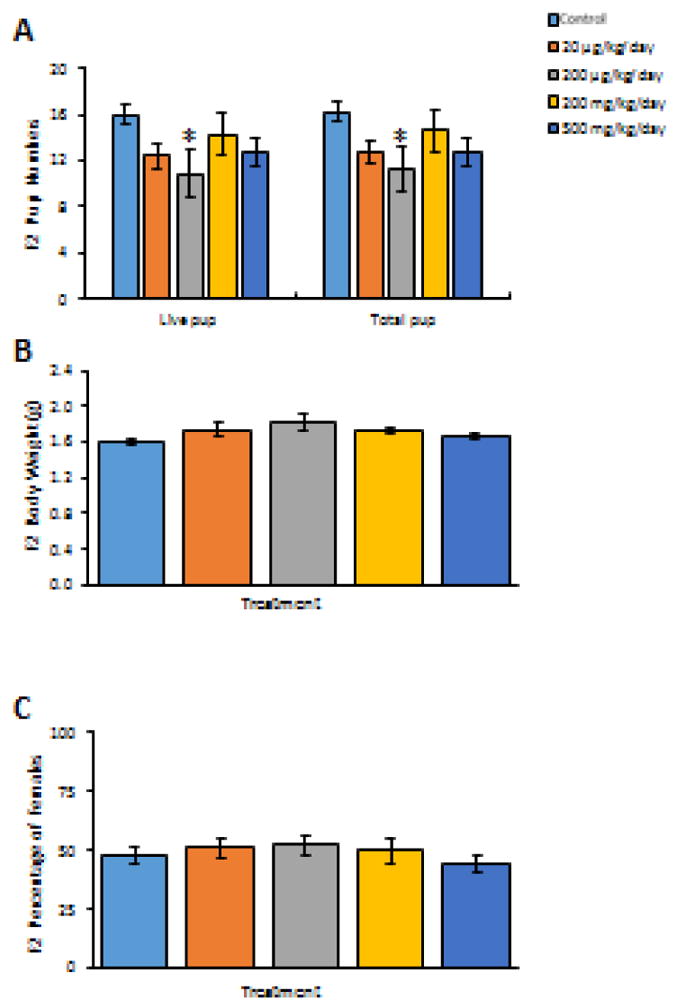
The effects of prenatal exposure to phthalate mixture on F2 birth outcomes at the 6 months F1 female fertility test. Litter size (including live and total F2 pup numbers) is shown in panel A. Average pup birth weights for F2 pups are shown in panel B. Percentages of female of F2 pups are shown in panel C. Graphs represent means ± SEM from 7–10 dams per treatment group. Asterisks (*) indicate significant differences from the control (p < 0.05).
At 9 months of age, the phthalate mixture did not affect the time F1 mice needed to become pregnant (Figure 4C, n = 7–11). Phthalate mixture exposure also did not affect pregnancy length compared to controls (n = 7–11, data not shown). However, prenatal exposure to phthalate mixture caused breeding complications in F1 females. Specifically, one F1 female in the 20 μg/kg/day lost her pregnancy and two F1 females in the same group experienced dystocia. Further, three F1 females in the 200 μg/kg/day group lost their pregnancies. In addition, one F1 female in the 200 mg/kg/day group lost her pregnancy and one female in the same group never mated. One F1 female in the 500 mg/kg/day group lost her pregnancy and two F1 females in the same group experienced dystocia. In contrast, only one F1 control female did not become pregnant after a successful mating. Taken together, this resulted in a reduced mating index and gestational index in the phthalate mixture treated groups compared to controls (Table 4, n = 7–11). As far as birth outcomes, the phthalate mixture did not affect F2 live and total pup numbers, average F2 pup birth weight, or percent F2 females compared to control (n = 7–11, data not shown).
Discussion
Previous animal studies have shown that exposure to phthalates during different exposure windows causes various reproductive and developmental defects in both male and female animals (reviewed in [28, 29]). Most of these previous studies were conducted using single phthalates and the doses used in these studies were usually much higher than human exposure levels (reviewed in [28, 29]). Given that humans and animals are exposed to a mixture of different phthalates on daily basis, it is important to study the effects of phthalate mixtures on reproductive outcomes. Thus, this study was designed to determine if exposure to an environmentally relevant phthalate mixture at doses comparable to human exposure levels during a critical ovarian developmental window has adverse effects on female reproduction throughout reproductive life. To our knowledge, this is the first study to provide information on the effects of an environmentally relevant phthalate mixture on female reproduction. In our study, we found that prenatal exposure to the phthalate mixture decreased AGD, increased uterine weight, disrupted estrous cyclicity, reduced fertility-related indices, caused some breeding complications, and induced cystic ovaries in the F1 female mice (Table 5).
Table 5.
Summary of the effects of prenatal exposure to phthalate mixture in F1 females.
| Effects | Treatments | |||
|---|---|---|---|---|
| 20 μg/kg/day | 200 μg/kg/day | 200 mg/kg/day | 500 mg/kg/day | |
| Reduced AGD | √ (PND 60) | √ (PND 60) | √ (PND 8) | |
| Increased Uterine Weight | √ (PND 8) | √ (PND 60) | ||
| Induced Enlarged Cystic Ovaries | √ (13 months) | √ (13 months) | √ (13 months) | √ (13 months) |
| Reduced Mating Index | √ (9 months) | |||
| Reduced Pregnancy Rate | √ (6 months) | √ (6 months) | √ (3, 6, and 9 months) | √ (3 and 6 months) |
| Reduced Fertility Index | √ (6 months) | √ (6 months) | √ (3 and 6 months) | √ (3 and 6 months) |
| Reduced Gestational Index | √ (9 months) | √ (6 and 9 months) | √ (3, 6, and 9 months) | √ (6 and 9 months) |
| Disrupted Estrous Cyclicity | √ (3 and 6 months) | √ (after vaginal opening and at 3 months) | √ (after vaginal opening and at 6 months) | √ (after vaginal opening, and at 3 and 6 months) |
| Increased Time to Pregnancy | √ (3 months) | |||
| Decreased Litter Size | √ (6 months) | |||
Our data indicate that phthalate mixture exposure did not cause gestational complications in F0 females. F0 female mice in all treatment groups successfully gave birth to live litters, and the birth outcomes in the phthalate mixture exposed animals were comparable to controls. This indicates that phthalate mixture exposure did not cause fetal toxicity or overt maternal toxicity, which could lead to reduced litter size and birth defects in the pups. These findings differ from previous studies on gestational exposure to BBzP, DBP, and DEHP, which showed that these individual phthalates induced pregnancy loss and reduced litter size in rats and mice [18, 30–32]. The reasons for differences in our mixture study versus previous studies using single phthalates are probably due to the lower doses used in our study compared to previous studies. The dose range we included in our study was from 20 μg/kg/day to 500 mg/kg/day, however, the lowest doses that caused adverse effects used in the aforementioned studies was 500 mg/kg/day (doses used in previous studies ranged from 0.05 to 1500 mg/kg/day for a single phthalate) [18, 30–32]. In addition, differences in our results from previous studies could be due to the differences in exposure window. In our study, we exposed pregnant dams during the second half of gestation, however, in the studies in which pregnancy loss and reduced litter size were observed, the exposure windows were either only during the first half of the gestation [30, 31] or throughout the whole gestation period [18, 32].
Our data showed that prenatal exposure to the phthalate mixture increased uterine weight on PND 8 and 60. Uterine weight is affected by sex steroid hormone levels. Thus, it is possible that in the phthalate mixture exposed F1 females, steroidogenesis was disrupted by the mixture exposure. On PND 8, the pups still depend on the dams and are sexually immature. The hypothalamus-pituitary-ovary axis is not functioning on PND 8, and as a result, the hormone levels in F1 females are heavily influenced by the hormone levels in the dams. Studies have shown that exposure to single phthalates affects steroidogenesis in female mice [18, 33, 34]. Thus, it is possible that the phthalate mixture exposure adversely affected sex steroid hormone levels in the dams and in turn induced differences in uterine weights in the PND 8 pups. Moreover, our data showed that prenatal exposure to phthalate mixture also increased uterine weight on PND 60 in F1 females. At this age, mice have a functional hypothalamus-pituitary-ovary axis and the uteri are responsive to hormone actions. Thus, it is possible that at this age, the phthalate mixture induced disruption in steroid hormone levels in the exposed F1 females and this led to an increase in uterine weight in the F1 pups. Increased uterine weight has been observed in F1 females prenatally exposed to DEHP and altered steroidogenesis has been observed in follicles exposed to DEHP [17, 35]. Thus, future studies should examine the effects of the phthalate mixture on sex steroid hormone levels and determine if the phthalate mixture induced increase in uterine weight is due to altered sex steroid hormone levels.
Prenatal exposure to the phthalate mixture reduced AGD on PNDs 8 and 60. Phthalates have been shown to reduce AGD in male offspring after prenatal exposure in both singular and mixture forms [11, 36, 37]. Similar to results obtained from prenatal phthalate exposure studies in males, our results show that the phthalate mixture reduces AGD in F1 females. This outcome is likely due to the mixture reducing maternal androgen levels in the F1 females because AGD is determined by maternal androgen levels. Future studies are needed to measure sex steroid hormone levels of pregnant F0 dams to further confirm our hypotheses.
In phthalate mixture exposed F1 females, we frequently observed enlarged ovaries with fluid or blood filled cysts, whereas enlarged cystic ovaries were not observed in any control animals. The presence of ovarian cysts is often a sign of reproductive aging [38]. Similar phenotypes have been observed in aged mice, transgenic mice, and ovarian diseases mouse models [39–41]. Specifically, one study showed that CD-1 mice develop ovarian inclusion cysts starting at 6 months of age, but enlarged ovarian cysts were observed in a 9 month old animal [39]. In a transgenic mouse model with conditionally activated Notch1, enlarged ovarian cysts developed in 8 month old animals [40]. In a transgenic mouse model for the study of ovarian endosalpingiosis, enlarged ovarian cysts, similar to the cysts observed in our study, were observed in animals at 8 months of age or older [41]. Because we only observed the ovarian cysts in phthalate mixture treated F1 females, it is likely that phthalate mixture advanced reproductive senescence, or the mixture triggered other pathological changes indicated by the transgenic or induced ovarian diseases mouse models. Based on the origin, ovarian cysts can be divided into follicular cysts, rete ovarii cysts, paraovarian cysts, luteal cysts, luteinized follicle cysts, and unspecified cysts [42]. Based on appearance, the cysts that we observed in the phthalate mixture treated F1 females are likely to be epithelial cysts or rete ovarii cysts, but it is difficult to distinguish epithelial ovarian cysts and rete ovarii cysts based on appearance alone [42]. Thus, further histology evaluations are needed to determine the origin of the cysts in the phthalate mixture treated F1 mice.
In our study, prenatal exposure to phthalate mixture did not affect age at vaginal opening and age of first estrus. This is similar to previous study results on prenatal exposure to DEHP, in which no effects of DEHP were observed on the age at vaginal opening, weight at vaginal opening, or age at first estrus at any doses (20 μg/kg/day to 750 mg/kg/day) [17]. In contrast, prenatal exposure to BBzP or MEHP have been shown to delay puberty onset [19, 43]; however, the doses that were reported to delay puberty onset (≥ 500 mg/kg/day) were much higher than any of the individual phthalates used in the mixture in our study.
Our estrous cyclicity data indicated that prenatal exposure to the phthalate mixture significantly disrupted estrous cyclicity in F1 females. We observed that prenatal phthalate mixture exposure at all doses significantly prolonged the stage of estrus at peripuberty and at 3 months of age. This differs from previous studies which showed that prenatal exposure to DEHP did not affect estrous cyclicity at the peripubertal stage [17]. However, our results are similar to a study that showed that adult exposure to DEHP increased the time mice spent in estrus compared to controls [44]. Further, a prepubertal DEHP exposure study has shown that DEHP exposure affects ovarian steroidogenesis [45], which could lead to disrupted estrous cyclicity. In addition, we speculate that the phthalate mixture could affect the establishment of hypothalamic-pituitary-ovarian axis, possibly by affecting hypothalamic kisspeptin levels to disrupt estrous cyclicity. This speculation is supported by both animal and epidemiology studies, which showed that phthalates exposure interferes with kisspeptin levels [45–47]. Disrupted estrous cyclicity often indicates disrupted ovarian function and sex steroid hormone levels and it may also indicate early reproductive senescence [38]. Our results on estrous cyclicity are in accordance with our observation of cystic ovaries at 13 months of age in the phthalate mixture exposed females, in which the presence of the cysts may indicate disrupted ovarian function and ovarian aging.
Prenatal exposure to the phthalate mixture affected fertility-related indices in F1 females at 3, 6, and 9 months of age. At 3 months of age, our results showed that F1 females in the 200 mg/kg/day group took longer to become pregnant compared to control. This is likely due to the disruption of estrous cyclicity by the phthalate mixture at 3 months of age. Many phthalates have been shown to induce breeding complications and pregnancy loss [17, 18, 30–32, 48]. In our study, phthalate mixture exposed F1 females experienced some breeding complications, including failure to mate, failure to become pregnant after successful mating, loss of pregnancy, and dystocia. Several of these complications became more apparent as the animals aged. Specifically, we observed more pregnancy loss and dystocia at 6 and 9 months of age than at 3 months of age, even at the lower doses. It is possible that prenatal exposure to phthalate mixture disrupted the development and functionality of the uterine tissues, and as a result, accelerated uterine aging. It could also due to the disrupted production of hormones that are required to maintain pregnancy. Further investigations on the uterine tissue and pregnancy related hormone levels are needed to elucidate the causes of pregnancy loss. It is difficult to distinguish early pregnancy loss and implantation failure based on the body weight alone. In our study, we defined pregnancy loss as more than 4 grams of weight loss during gestation. As a result, the rate of pregnancy loss might be higher than what we reported.
In conclusion, our data indicate that prenatal exposure to an environmentally relevant phthalate mixture disrupts female reproduction in the F1 offspring in mice. Specifically, prenatal exposure to the phthalate mixture affects organ weights, induces pathological changes in ovaries, disrupts estrous cyclicity, reduces fertility-related indices, and causes some breeding complications in F1 female mice. To our knowledge, this is the first study that used a mixture mimicking human exposure and examined the effects on female reproduction. However, our study did not provide mechanistic evaluation of the phthalate mixture induced effects. Thus, future analyses are needed to determine the underlying mechanisms of action of this phthalate mixture. Additionally, many endocrine disrupting chemicals have been shown to have transgenerational effects [27–29]; thus, it is of great interest to determine if a mixture of phthalates could induce transgenerational effects on female reproduction.
Highlights.
Prenatal exposure to a phthalate mixture disrupts F1 estrous cyclicity
Prenatal exposure to a phthalate mixture induces F1 ovarian cysts
Prenatal exposure to a phthalate mixture decreases F1 female fertility-related indices
Prenatal exposure to a phthalate mixture induces F1 breeding complications
Acknowledgments
This work was supported by NIH P01 ES022848 (JAF), EPA RD-83459301 (JAF), and an Environmental Toxicology Fellowship (CZ). The authors also wish to thank all the members of the Flaws’ lab for their assistance and constructive input.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Koniecki D, et al. Phthalates in cosmetic and personal care products: concentrations and possible dermal exposure. Environ Res. 2011;111(3):329–36. doi: 10.1016/j.envres.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 2.Xu Y, Cohen Hubal EA, Little JC. Predicting residential exposure to phthalate plasticizer emitted from vinyl flooring: sensitivity, uncertainty, and implications for biomonitoring. Environ Health Perspect. 2010;118(2):253–8. doi: 10.1289/ehp.0900559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meeker JD, Sathyanarayana S, Swan SH. Phthalates and other additives in plastics: human exposure and associated health outcomes. Philos Trans R Soc Lond B Biol Sci. 2009;364(1526):2097–113. doi: 10.1098/rstb.2008.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adibi JJ, et al. Prenatal exposures to phthalates among women in New York City and Krakow, Poland. Environ Health Perspect. 2003;111(14):1719–22. doi: 10.1289/ehp.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adibi JJ, et al. Characterization of phthalate exposure among pregnant women assessed by repeat air and urine samples. Environ Health Perspect. 2008;116(4):467–73. doi: 10.1289/ehp.10749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Latini G, et al. Exposure to Di(2-ethylhexyl)phthalate in humans during pregnancy. A preliminary report. Biol Neonate. 2003;83(1):22–4. doi: 10.1159/000067012. [DOI] [PubMed] [Google Scholar]
- 7.Weinberger B, et al. Effects of maternal exposure to phthalates and bisphenol A during pregnancy on gestational age. J Matern Fetal Neonatal Med. 2014;27(4):323–7. doi: 10.3109/14767058.2013.815718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Latini G, et al. In utero exposure to di-(2-ethylhexyl)phthalate and duration of human pregnancy. Environ Health Perspect. 2003;111(14):1783–5. doi: 10.1289/ehp.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen MS, et al. Amniotic fluid phthalate levels and male fetal gonad function. Epidemiology. 2015;26(1):91–9. doi: 10.1097/EDE.0000000000000198. [DOI] [PubMed] [Google Scholar]
- 10.Huang Y, et al. Phthalate levels in cord blood are associated with preterm delivery and fetal growth parameters in Chinese women. PLoS One. 2014;9(2):e87430. doi: 10.1371/journal.pone.0087430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foster PM. Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. Int J Androl. 2006;29(1):140–7. doi: 10.1111/j.1365-2605.2005.00563.x. discussion 181–5. [DOI] [PubMed] [Google Scholar]
- 12.Gray LE, Jr, et al. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol Sci. 2000;58(2):350–65. doi: 10.1093/toxsci/58.2.350. [DOI] [PubMed] [Google Scholar]
- 13.Giribabu N, Sainath SB, Sreenivasula Reddy P. Prenatal di-n-butyl phthalate exposure alters reproductive functions at adulthood in male rats. Environ Toxicol. 2014;29(5):534–44. doi: 10.1002/tox.21779. [DOI] [PubMed] [Google Scholar]
- 14.Saffarini CM, et al. Induction and persistence of abnormal testicular germ cells following gestational exposure to di-(n-butyl) phthalate in p53-null mice. J Androl. 2012;33(3):505–13. doi: 10.2164/jandrol.111.013706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrade AJ, et al. A dose response study following in utero and lactational exposure to di-(2-ethylhexyl) phthalate (DEHP): reproductive effects on adult male offspring rats. Toxicology. 2006;228(1):85–97. doi: 10.1016/j.tox.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 16.Dalsenter PR, et al. Phthalate affect the reproductive function and sexual behavior of male Wistar rats. Hum Exp Toxicol. 2006;25(6):297–303. doi: 10.1191/0960327105ht624oa. [DOI] [PubMed] [Google Scholar]
- 17.Niermann S, et al. Prenatal exposure to di-(2-ethylhexyl) phthalate (DEHP) affects reproductive outcomes in female mice. Reprod Toxicol. 2015;53:23–32. doi: 10.1016/j.reprotox.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pocar P, et al. Exposure to di(2-ethyl-hexyl) phthalate (DEHP) in utero and during lactation causes long-term pituitary-gonadal axis disruption in male and female mouse offspring. Endocrinology. 2012;153(2):937–48. doi: 10.1210/en.2011-1450. [DOI] [PubMed] [Google Scholar]
- 19.Moyer B, Hixon ML. Reproductive effects in F1 adult females exposed in utero to moderate to high doses of mono-2-ethylhexylphthalate (MEHP) Reprod Toxicol. 2012;34(1):43–50. doi: 10.1016/j.reprotox.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manikkam M, et al. Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PLoS One. 2013;8(1):e55387. doi: 10.1371/journal.pone.0055387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hannas BR, et al. In utero phthalate effects in the female rat: a model for MRKH syndrome. Toxicol Lett. 2013;223(3):315–21. doi: 10.1016/j.toxlet.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johansson HK, et al. Perinatal exposure to mixtures of endocrine disrupting chemicals reduces female rat follicle reserves and accelerates reproductive aging. Reprod Toxicol. 2016;61:186–94. doi: 10.1016/j.reprotox.2016.03.045. [DOI] [PubMed] [Google Scholar]
- 23.Meltzer D, et al. In utero exposure to the endocrine disruptor di(2-ethylhexyl) phthalate targets ovarian theca cells and steroidogenesis in the adult female rat. Reprod Toxicol. 2015;51:47–56. doi: 10.1016/j.reprotox.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 24.Doull J, et al. A cancer risk assessment of di(2-ethylhexyl)phthalate: application of the new U.S. EPA Risk Assessment Guidelines. Regul Toxicol Pharmacol. 1999;29(3):327–57. doi: 10.1006/rtph.1999.1296. [DOI] [PubMed] [Google Scholar]
- 25.Latini G. Monitoring phthalate exposure in humans. Clin Chim Acta. 2005;361(1–2):20–9. doi: 10.1016/j.cccn.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Gallavan RH, Jr, et al. Interpreting the toxicologic significance of alterations in anogenital distance: potential for confounding effects of progeny body weights. Reprod Toxicol. 1999;13(5):383–90. doi: 10.1016/s0890-6238(99)00036-2. [DOI] [PubMed] [Google Scholar]
- 27.Ziv-Gal A, et al. The effects of in utero bisphenol A exposure on reproductive capacity in several generations of mice. Toxicol Appl Pharmacol. 2015 doi: 10.1016/j.taap.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kay VR, Bloom MS, Foster WG. Reproductive and developmental effects of phthalate diesters in males. Crit Rev Toxicol. 2014;44(6):467–98. doi: 10.3109/10408444.2013.875983. [DOI] [PubMed] [Google Scholar]
- 29.Kay VR, Chambers C, Foster WG. Reproductive and developmental effects of phthalate diesters in females. Crit Rev Toxicol. 2013;43(3):200–19. doi: 10.3109/10408444.2013.766149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ema M, Miyawaki E, Kawashima K. Effects of dibutyl phthalate on reproductive function in pregnant and pseudopregnant rats. Reprod Toxicol. 2000;14(1):13–9. doi: 10.1016/s0890-6238(99)00066-0. [DOI] [PubMed] [Google Scholar]
- 31.Ema M, Miyawaki E, Kawashima K. Reproductive effects of butyl benzyl phthalate in pregnant and pseudopregnant rats. Reprod Toxicol. 1998;12(2):127–32. doi: 10.1016/s0890-6238(97)00127-5. [DOI] [PubMed] [Google Scholar]
- 32.Gray LE, Jr, Laskey J, Ostby J. Chronic di-n-butyl phthalate exposure in rats reduces fertility and alters ovarian function during pregnancy in female Long Evans hooded rats. Toxicol Sci. 2006;93(1):189–95. doi: 10.1093/toxsci/kfl035. [DOI] [PubMed] [Google Scholar]
- 33.Svechnikova I, Svechnikov K, Soder O. The influence of di-(2-ethylhexyl) phthalate on steroidogenesis by the ovarian granulosa cells of immature female rats. J Endocrinol. 2007;194(3):603–9. doi: 10.1677/JOE-07-0238. [DOI] [PubMed] [Google Scholar]
- 34.Ma M, et al. Exposure of prepubertal female rats to inhaled di(2-ethylhexyl)phthalate affects the onset of puberty and postpubertal reproductive functions. Toxicol Sci. 2006;93(1):164–71. doi: 10.1093/toxsci/kfl036. [DOI] [PubMed] [Google Scholar]
- 35.Hannon PR, et al. Di(2-ethylhexyl) phthalate inhibits antral follicle growth, induces atresia, and inhibits steroid hormone production in cultured mouse antral follicles. Toxicol Appl Pharmacol. 2015;284(1):42–53. doi: 10.1016/j.taap.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howdeshell KL, et al. Dose addition models based on biologically-relevant reductions in fetal testosterone accurately predict postnatal reproductive tract alterations by a phthalate mixture in rats. Toxicol Sci. 2015 doi: 10.1093/toxsci/kfv196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Howdeshell KL, et al. Cumulative effects of dibutyl phthalate and diethylhexyl phthalate on male rat reproductive tract development: altered fetal steroid hormones and genes. Toxicol Sci. 2007;99(1):190–202. doi: 10.1093/toxsci/kfm069. [DOI] [PubMed] [Google Scholar]
- 38.Creasy D. Chapter 9 - Reproduction of the rat, mouse, dog, non-human primate and minipig A2 - McInnes, Elizabeth F. In: Mann P, editor. Background Lesions in Laboratory Animals. W.B. Saunders; Saint Louis: 2012. pp. 101–122. [Google Scholar]
- 39.Fleming JS, et al. E-cadherin expression and bromodeoxyuridine incorporation during development of ovarian inclusion cysts in age-matched breeder and incessantly ovulated CD-1 mice. Reprod Biol Endocrinol. 2007;5:14. doi: 10.1186/1477-7827-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferguson L, et al. Constitutive Notch Signaling Causes Abnormal Development of the Oviducts, Abnormal Angiogenesis, and Cyst Formation in Mouse Female Reproductive Tract. Biol Reprod. 2016;94(3):67. doi: 10.1095/biolreprod.115.134569. [DOI] [PubMed] [Google Scholar]
- 41.Bristol-Gould SK, et al. The development of a mouse model of ovarian endosalpingiosis. Endocrinology. 2005;146(12):5228–36. doi: 10.1210/en.2005-0697. [DOI] [PubMed] [Google Scholar]
- 42.Dixon D, et al. Nonproliferative and proliferative lesions of the rat and mouse female reproductive system. J Toxicol Pathol. 2014;27(3–4 Suppl):1s–107s. doi: 10.1293/tox.27.1S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moral R, et al. In utero exposure to butyl benzyl phthalate induces modifications in the morphology and the gene expression profile of the mammary gland: an experimental study in rats. Environ Health. 2011;10(1):5. doi: 10.1186/1476-069X-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hannon PR, Peretz J, Flaws JA. Daily exposure to Di(2-ethylhexyl) phthalate alters estrous cyclicity and accelerates primordial follicle recruitment potentially via dysregulation of the phosphatidylinositol 3-kinase signaling pathway in adult mice. Biol Reprod. 2014;90(6):136. doi: 10.1095/biolreprod.114.119032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lai FN, et al. Di (2-ethylhexyl) phthalate impairs steroidogenesis in ovarian follicular cells of prepuberal mice. Arch Toxicol. 2016 doi: 10.1007/s00204-016-1790-z. [DOI] [PubMed] [Google Scholar]
- 46.Hu J, et al. Short-term neonatal/prepubertal exposure of dibutyl phthalate (DBP) advanced pubertal timing and affected hypothalamic kisspeptin/GPR54 expression differently in female rats. Toxicology. 2013;314(1):65–75. doi: 10.1016/j.tox.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 47.Chen CY, et al. Phthalates may promote female puberty by increasing kisspeptin activity. Hum Reprod. 2013;28(10):2765–73. doi: 10.1093/humrep/det325. [DOI] [PubMed] [Google Scholar]
- 48.Schmidt JS, et al. Effects of di(2-ethylhexyl) phthalate (DEHP) on female fertility and adipogenesis in C3H/N mice. Environ Health Perspect. 2012;120(8):1123–9. doi: 10.1289/ehp.1104016. [DOI] [PMC free article] [PubMed] [Google Scholar]