Abstract
Soluble oligomers of amyloid-beta (Aβo) are implicated by biochemical and genetic evidence as a trigger for Alzheimer’s disease (AD) pathophysiology. A key step is Aβo interaction with the neuronal surface to initiate a cascade of altered signal transduction leading to synaptic dysfunction and damage. This review discusses neuronal cell surface molecules with high affinity selectively for oligomeric disease-associated states of Aβ, with a particular focus on the role of cellular prion protein (PrPC) in this process. Additional receptors may contribute to mediation of Aβo action, but PrPC appears to play a primary role in a number of systems. The specificity of binding, the genetic necessity in mouse models of disease and downstream signaling pathways are considered. Signal transduction downstream of Aβo complexes with PrPC involves metabotropic glutamate receptor 5 (mGluR5), Fyn kinase and Pyk2 kinase, with deleterious effects on synaptic transmission and maintenance. Current data support the hypothesis that a substantial portion of Aβo toxicity in AD is mediated after initial interaction with PrPC on the neuronal surface. As such, the interaction of Aβo with PrPC is a potential therapeutic intervention site for AD.
Keywords: Alzheimer’s disease, cellular prion protein, Aβ = amyloid-beta, Aβo = amyloid-beta oligomers, PrPC = cellular prion protein, Prnp = prion gene name
Alzheimer’s disease (AD) is a highly prevalent and devastating dementia, with more than 5 million Americans afflicted by the disease [1]. AD is characterized pathologically by amyloid-β (Aβ) plaques composed of Aβ peptide and neurofibrillary tangles composed of hyperphosphorylated tau [2, 3]. The clinical presentation of AD includes early deposition of Aβ plaque followed by progressive cognitive decline, marked synapse loss and eventually brain atrophy, and death. After early Aβ pathology, tau pathology, and microglial inflammation become manifest as the disease progresses over a decade or more.
Amyloid-β peptide can misfold in a range of conformations, from monomer to various oligomers to insoluble fibrils of plaque [4]. Soluble Aβ oligomers (Aβo) are purported to be the initial insult in AD and are thought to mediate much of the synaptic dysfunction observed in AD [5, 6]. These synaptotoxic Aβo are have been detected in brain, in CSF, and in interstitial fluid. The focus of the current review is the molecular mechanism whereby Aβo interact with the neuronal surface to trigger synaptic dysfunction. The potential role of cellular Prion protein (PrPC) as a cell surface receptor for Aβo is discussed in detail.
Cellular Prion Protein (PrPC) as a receptor for Aβo
The emerging role of PrPC in neurodegeneration and especially AD is exciting and complex. The discovery of PrPC as a high-affinity binding partner of Aβo [7] is a key finding in determining the early trigger in disease pathophysiology. A genome wide screen identified PrPC as a selective and high-affinity binding partner of Aβo [7]. Specific binding of Aβo and PrPC was observed in human COS cells and in primary neurons. A mutational analysis revealed the selectivity of this binding by elucidating the 95-111 amino acid residues as the major residues responsible for binding of Aβo. Furthermore, constitutive deletion of PrPC was able to functionally restore synaptic deficits in long-term potentiation (LTP) induced by Aβo in acute brain slices. Further investigation revealed the importance of PrPC in mediating learning and memory deficits in a mouse model of familial AD [8]. Specifically, learning and memory deficits in a mouse model of familial AD (mice with APPSwe/PSEN1ΔE9 transgene) are dependent on PrPC. Furthermore, deletion of PrPC rescued the early death phenotype associated with this mouse model. Analysis of APP metabolism showed that this Aβo-PrPC interaction is downstream of Aβ plaque formation. Additionally, the absence of PrPC did not alter the gliosis present in these mice, distinguishing gliosis from the PrPC-dependent learning and memory deficits in this mouse model. These were the first studies to describe the role of PrPC in AD.
Phenotypic studies in vitro and in vivo have also demonstrated a necessity for PrPC in synaptic loss, a hallmark of AD pathophysiology. Dendritic spine loss triggered by Aβo and observed by time lapse imaging in cultured hippocampal neurons, does not occur in neurons lacking PrPC [9-11]. Prnp null mice carrying an AD transgene do not exhibit the progressive synapse loss in the hippocampus observed in PrPC-intact AD transgenic mice [8, 12]. In fact, by repeated in vivo imaging, dendritic spine dynamics are normalized in the AD transgenic mice when PrPC is absent [11]. Thus, in these models PrPC is essential for the deleterious effects of Aβo and AD transgenes on synaptic anatomy as well as physiology.
Independent evidence from other groups has also made the case for PrPC as the cognate receptor for Aβo. Using surface plasmon resonance (SPR), Chen et al describe a dose dependent binding of Aβo to human PrPC and a lack of binding to monomeric and fibril forms of Aβ [13]. In agreement with these biophysical studies, Kohler et al [14] describe an Aβo-PrPC interaction from human AD autopsy brain. They were able to co-immunoprecipitate Aβ using a PrPC specific antibody. Furthermore, this study revealed that the PrPC interacting Aβo species are greater than 150 kD. Combined, these data demonstrate the ability of PrPC to bind Aβo with high affinity, and specificity whose binding leads to deleterious consequences in synaptic function.
Nevertheless, PrPC is not essential for all mouse phenotypes driven by familial AD transgenes. A follow up study on the initial discovery of PrPC as a receptor for Aβo, used organotypic slices infected with APPct100 as a model of familial AD in vitro [15]. This study observed Aβ42 dependent suppression of LTP and loss of dendritic spines independent of PrPC expression. This study underscored the heterogeneity of AD models, and hinted at the idea that PrPC is not the sole receptor that mediates Aβo induced pathology. In addition to this work, another study observed an Aβo dependent impairment of memory, by testing novel object recognition (NOR), that does not require expression of PrPC [16]. In this model they used an acute cerebral injection of Aβo as opposed to a chronic Aβo model of AD as in transgenic mice to recapitulate disease. The acute insult of Aβo may account for the lack of PrPC dependence on NOR test. An additional study observed a PrPC independent suppression of LTP in a mouse model of familial AD [17]. While early studies on the Aβo-PrPC interaction yielded some conflicting evidence, it has been clear that Aβo could interact with PrPC in certain contexts, and that his interaction could elicit synaptic deficits in certain scenarios.
The majority of work in elucidating the role of PrPC in Aβo-induced synaptic dysfunction is less controversial. Recent evidence continues to support for the hypothesis that PrPC is the major receptor responsible for Aβo induced pathology, conceding that it is not the sole receptor for Aβo (see below). For example, a study using Aβ-containing TBS soluble extract from human autopsy brain induced LTP deficits in rats in vivo, and observed that this deficit was PrPC dependent [18]. A separate study using Aβ-containing extracts of human AD brain displayed suppression of LTP in acute brain slices compared to non-demented control human brain extract [19]. This study found that robust LTP was induced in slices derived from PrPC null mice in the presence of AD human brain extract. Furthermore, anti-PrPC antibodies targeting a variety of regions in PrPC were able to abolish LTP deficits induced by human AD brain extract [19] and were effective when administered peripherally [20]. This effect was observed in acute brain slices and during in vivo recordings in rats. Importantly these studies utilized Aβo from human AD brain, reducing the possibility of an Aβo preparation artifact.
A more recent study has surveyed a wide range of mouse models for AD. Kostylev and colleagues used a PrPC-ELISA (PLISA) to quantify the Aβo-PrPC interaction in brain tissue from several mouse models of AD and also healthy and AD human brain [9, 21]. This study found that PrPC interacting Aβo are highly correlated with learning and memory deficits in multiple mouse models of AD as defined by PLISA activity [21]. Furthermore, PLISA activity disappeared with PrPC mediated depletion of high-molecular weight Aβo from mouse of the varying mouse models. These data suggest a high-molecular weight Aβo species is responsible for the learning and memory deficits seen in multiple mouse models of AD. While there exists some conflicting evidence, a large body of genetic and biochemical evidence suggest PrPC is the cognate receptor for Aβo-dependent synaptic deficits.
Aβo-PrPC dependent downstream signaling
A growing body of evidence suggests the Aβo-PrPC interaction is necessary for many downstream intracellular processes the mediate Aβo-dependent synaptotoxic effects. Several studies suggest that PrPC couples with metabotropic glutamate receptor 5 (mGluR5) [10, 22, 23]. The initial insight to PrPC and mGluR5 coupling was the identification of mGluR5 in a screen of 81 post-synaptic density transmembrane proteins [10]. This study showed that PrPC and mGluR5 together induced synaptic dysfunction. Furthermore, the mGluR5 antagonist MTEP was able to reverse learning and memory deficits seen a mouse model of familial AD. In addition, this study demonstrated that the decrease in synapse density observed in this mouse model of AD (APPSwe/PSEN1?E9) was reversed after treatment with MTEP. This evidence provided strong support for the dual relationship of PrPC and mGluR5 mediating the Aβo-dependent synaptotoxicity.
A follow up study utilized a transheterozygote model of PrPC and mGluR5 to genetically link PrPC and mGluR5 to AD [23]. Although evidence suggested a synergistic role of PrPC and mGluR5 in mediating Aβo-dependent synaptotoxicity it was unclear whether Aβo were binding PrPC and mGluR5 independently, or whether this signaling cascade was a sequential event. Haas et al found PrPC and mGluR5 to interact biochemically as evident by dual co-immunoprecipitation in acute and chronic models of AD, as well as co-immunoprecipitation of PrPC and mGluR5 in human AD brain tissue. Acute brain slices derived from PrPC and mGluR5 transheterozygote animals with one allele of each gene displayed a rescue of Aβo induced suppression of LTP. Furthermore, downstream activation of effector kinases required PrPC and mGluR5 transheterozygosity. This transheterozygous state also reversed a decrease in synapse density and increased animal survival in APPSwe/PSEN1?E9 mice, a mouse model of familial AD.
A separate study assessed the long-term depression (LTD) deficit seen in AD models and showed this deficit was dependent on mGluR5 and PrPC [16]. This study described the disruption of both LTD and LTP by either synthetic Aβo or human AD brain extract. Researchers observed an enhancement of LTD by synthetic Aβo or human AD brain extract, and this enhancement was reversed by either systemic administration of MTEP or treatment with anti-PrPC antibodies. Similarly, MTEP treatment blocked LTP suppression caused by synthetic Aβo or human AD brain extract. Together these data suggest a pivotal role of PrPC and mGluR5 complex in mediating the synaptic deficits seen in AD.
In addition to PrPC and mGluR5 coupling, this interaction leads to activation of intracellular kinases. One of these molecules is Fyn kinase. A member of the Src family of kinases, Fyn was identified to play a key role in mediating Aβo dependent phenotypes seen in acute and chronic models of AD [9, 24, 25]. Um and colleagues showed Fyn to be activated acutely by Aβo and that Aβo-driven Fyn activation is PrPC-dependent [16]. Activated Fyn in this disease context displayed a preference for activating NMDAR subunits in a PrPC dependent manner. Furthermore, Aβo-dependent activation of Fyn leads to a decrease in dendritic spines in primary neurons. Together these findings study suggest Fyn plays an important role in mediating the synaptotoxic effects induced by Aβo. Further evidence from a separate group supported this hypothesis and identified Fyn as a downstream effector of the Aβo-PrPC interaction and importantly implicated Fyn in linking Aßo with Tau pathology in AD [24]. This study identified an increase in membrane bound PrPC-Fyn interaction in human brain tissue from AD patients. Furthermore, they demonstrated that tau hyperphosphorylation in primary neurons was dependent on the Aβo-PrPC interaction induced by human derived Aβo. Importantly, they also found that phosphorylated tau at Tyr18 (p-tau Y18) was increased in a mouse model of familial AD, and that this increase was reversed by reduction of Prnp in an AD mouse model heterozygous for Prnp. Hyperphosphorylated tau at p-tau Y18 was increased further when APP/PS1 transgenic mice were crossed to mice over expressing PrPC. Overall, these studies suggest Fyn as a key molecule in connecting the two hallmark pathologies of AD.
Given the relationship of Fyn between Aβo and tau pathology, Fyn emerges as an exciting therapeutic target. To underscore the therapeutic potential of targeting Fyn for the treatment of AD, Kaufman et al utilized a previously identified small molecule competitive inhibitor of Fyn (AZD0530, Saracatinib) to examine its efficacy in a mouse model of familial AD [25]. Importantly, this study showed that AZD0530 was able to successfully cross the blood-brain barrier as demonstrated by observing AZD0530 levels in mouse brain and mouse cerebrospinal fluid (CSF), with equivalent levels in human patient CSF. This study used the APPSwe/PSEN1?E9 mouse model of familial AD and treated these mice for a month at 5 mg/kg/d. Treatment for one month but not two weeks was able to reverse synaptic, learning, and memory deficits. Additionally, the same treatment paradigm was able to significantly reduce the amount of hyperphosphorylated tau in 3xTg mice, a mouse model of AD containing a human tau transgene. Furthermore, treatment with AZD0530 did not affect gliosis or APP metabolism, and did not have obvious toxicity. This study underscores the therapeutic potential of AZD0530 for the treatment of Alzheimer’s disease.
Additional downstream effectors in Aβo-PrPC signaling include Homer, eEF2, CamKII, and Pyk2 [16]. The aforementioned transgenic mouse model study with PrPC and mGluR5 transheterozygotes [16] identified, as have others [26-29], that Homer, eEF2, CamKII, and Pyk2 are differentially regulated in an AD context. In the transheterozygote study [16], Homer and Pyk2 co-immunoprecipitation was significantly decreased in mouse models of AD and in human AD brain. This suggests a release or disassembly of Homer and Pyk2 from the PrPC-mGluR5 complex in disease. Furthermore, Pyk2 and CamKII activation was increased in acute brain slices incubated with synthetic Aβo, and Pyk2 along with p-eEF2 (T56) also displayed an increase in APPSwe/PSEN1?E9 mice while p-CamKII (T286) displayed a decrease. These differences reveal a distinction between acute models of AD using synthetic Aβo and chronic models AD using APPSwe/PSEN1?E9 transgenic mice. Overall, these studies identify key intracellular effectors that are potential candidates in mediating AD pathophysiology.
Additional non-PrPC receptors for Aβo
As alluded to previously, PrPC is not the only receptor for Aβo. A variety of conflicting evidence suggests a plethora of potential Aβo receptors interacting with a diverse collection of Aβo preparations. To provide a sense of this literature, a few putative receptor examples are discussed. Leukocyte immunoglobulin (Ig)-like receptor B 2 (LilrB2) and its murine homolog paired immunoglobulin-like receptor B (PirB) have been reported to regulate synaptic plasticity in AD [16]. Kim et al were able to observe a high-affinity interaction of their Aβo preparation with HEK cell expressed PirB. Furthermore, this interaction displayed isoform specificity as other isoforms of PirB did not bind Aβo with the same affinity. PirB deficient primary neurons showed a 50% decrease in Aβo binding suggesting, as described above, that additional Aβo receptors contribute to Aβo binding. A mutational analysis of PirB and LilrB2 (PirB human homolog) revealed a high affinity for Aβo of the D1D2 domain. Functional analysis in PirB deficient mice was able to rescue Aβo-dependent deficits seen in LTP and NOR. Given the ~50% reduction in Aβo binding to neurons after Prnp deletion [16], these findings suggest PrPC and PirB comprise a majority of Aβo binding sites on primary neurons.
A separate putative receptor for Aβo is the Sigma-2/PGRMC1 (Progesterone receptor membrane component 1). Recent work implicated Sigma-2/PGRMC1 function with an Aβo displacement assay [16]. This assay measured the displacement of a Sigma-2/PGRMC1 radioligand with molecules that reverse Aβo mediated vesicle trafficking deficits and cognitive dysfunction in mouse models of AD [16]. Aβo binding increased Sigma-2/PGRMC1 expression as a function of time in primary neurons, and Aβo binding decreased with Sigma-2/PGRMC1 depletion. Sigma-2/PGRMC1 may contribute by indirect or direct mechanisms to Aßo surface binding, highlighting the complexity of Aβo mediated synaptotoxicity.
Conclusions
Overall, many groups have identified several putative receptors for Aβo. A detailed description of these various receptors is provided elsewhere [30, 31], nevertheless these studies suggest a complex network of signaling that is induced by Aβo. It is evident that PrPC plays a critical role in mediating the synaptic deficits induced by Aβo. Future studies will more fully define the connections between Aβo-PrPC effectors and their linkage to tau pathology. The Aβo-PrPC network of molecules provides an exciting avenue for further research and lends to the possibility of novel therapeutics for the treatment of Alzheimer’s disease.
Supplementary Material
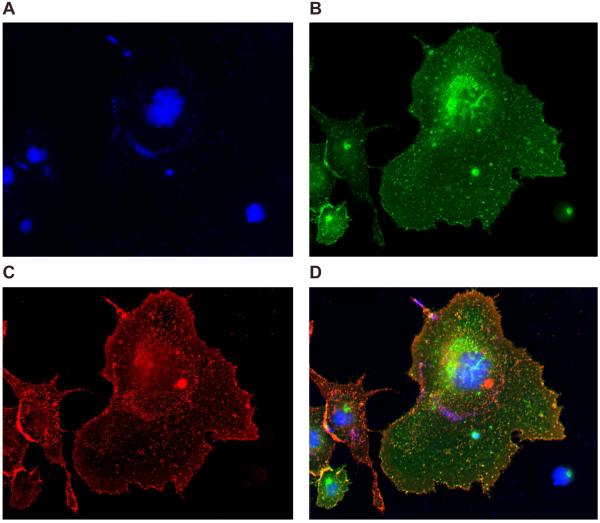
Figure 1.
Aβo-PrPC binding on COS cells. COS cells were transiently transfected with myc-hPrPC and incubated with biotin-Aβo (1 µM). A. Immunofluorescence images of DAPI staining in COS cells. B. Immunofluorescence image of myc-hPrPC immunoreactivity using an anti-myc antibody. C. Immunofluorescence image of 568-conjugated streptavidin fluorescence in COS cells incubated with biotin-Aβo (1 µM). D. Merge image of DAPI, myc-hPrPC immunoreactivity, and 568-conjugated streptavidin fluorescence. All images are at 20x magnification on a Zeiss epifluorescence microscope.
Aß oligomers trigger synaptic dysfunction in Alzheimer via neuronal receptors.
PrPC is a high affinity binding site for Aßo mediating deleterious effects in mice.
Signal transduction downstream of Aßo/PrPC involves mGluR5, Fyn and Pyk2.
ACKNOWLEDGMENTS
This work was supported by grants from NIH, Alzheimer’s Association and Falk Medical Research Trust to S.M.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
S.M.S. is a co-founder of Axerion Therapeutics seeking to develop PrP-based therapeutics for Alzheimer’s disease.
References
- [1].Alzheimer's A. 2012 Alzheimer's disease facts and figures. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2012;8:131–168. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]
- [2].Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta neuropathologica. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- [3].Selkoe DJ. Alzheimer's disease. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Benilova I, Karran E, De Strooper B. The toxic Abeta oligomer and Alzheimer's disease: an emperor in need of clothes. Nature neuroscience. 2012;15:349–357. doi: 10.1038/nn.3028. [DOI] [PubMed] [Google Scholar]
- [5].Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- [6].Sheng M, Sabatini BL, Sudhof TC. Synapses and Alzheimer's disease. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lauren J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457:1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gimbel DA, Nygaard HB, Coffey EE, Gunther EC, Lauren J, Gimbel ZA, Strittmatter SM. Memory impairment in transgenic Alzheimer mice requires cellular prion protein. J Neurosci. 2010;30:6367–6374. doi: 10.1523/JNEUROSCI.0395-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Um JW, Nygaard HB, Heiss JK, Kostylev MA, Stagi M, Vortmeyer A, Wisniewski T, Gunther EC, Strittmatter SM. Alzheimer amyloid-beta oligomer bound to postsynaptic prion protein activates Fyn to impair neurons. Nature neuroscience. 2012;15:1227–1235. doi: 10.1038/nn.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Um JW, Kaufman AC, Kostylev M, Heiss JK, Stagi M, Takahashi H, Kerrisk ME, Vortmeyer A, Wisniewski T, Koleske AJ, Gunther EC, Nygaard HB, Strittmatter SM. Metabotropic glutamate receptor 5 is a coreceptor for Alzheimer abeta oligomer bound to cellular prion protein. Neuron. 2013;79:887–902. doi: 10.1016/j.neuron.2013.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Heiss JK, Barrett J, Yu Z, Haas LT, Kostylev MA, Strittmatter SM. Early Activation of Experience-Independent Dendritic Spine Turnover in a Mouse Model of Alzheimer's Disease. Cerebral cortex. 2016 doi: 10.1093/cercor/bhw188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Haas LT, Salazar SV, Kostylev MA, Um JW, Kaufman AC, Strittmatter SM. Metabotropic glutamate receptor 5 couples cellular prion protein to intracellular signalling in Alzheimer's disease. Brain : a journal of neurology. 2016;139:526–546. doi: 10.1093/brain/awv356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chen S, Yadav SP, Surewicz WK. Interaction between human prion protein and amyloid-beta (Abeta) oligomers: role OF N-terminal residues. The Journal of biological chemistry. 2010;285:26377–26383. doi: 10.1074/jbc.M110.145516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Dohler F, Sepulveda-Falla D, Krasemann S, Altmeppen H, Schluter H, Hildebrand D, Zerr I, Matschke J, Glatzel M. High molecular mass assemblies of amyloid-beta oligomers bind prion protein in patients with Alzheimer's disease. Brain : a journal of neurology. 2014;137:873–886. doi: 10.1093/brain/awt375. [DOI] [PubMed] [Google Scholar]
- [15].Kessels HW, Nguyen LN, Nabavi S, Malinow R. The prion protein as a receptor for amyloid-beta. Nature. 2010;466:E3–4. doi: 10.1038/nature09217. discussion E4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. (!!! INVALID CITATION !!! )
- [17].Calella AM, Farinelli M, Nuvolone M, Mirante O, Moos R, Falsig J, Mansuy IM, Aguzzi A. Prion protein and Abeta-related synaptic toxicity impairment. EMBO Mol Med. 2010;2:306–314. doi: 10.1002/emmm.201000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Barry AE, Klyubin I, Mc Donald JM, Mably AJ, Farrell MA, Scott M, Walsh DM, Rowan MJ. Alzheimer's disease brain-derived amyloid-beta-mediated inhibition of LTP in vivo is prevented by immunotargeting cellular prion protein. J Neurosci. 2011;31:7259–7263. doi: 10.1523/JNEUROSCI.6500-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Freir DB, Nicoll AJ, Klyubin I, Panico S, Mc Donald JM, Risse E, Asante EA, Farrow MA, Sessions RB, Saibil HR, Clarke AR, Rowan MJ, Walsh DM, Collinge J. Interaction between prion protein and toxic amyloid beta assemblies can be therapeutically targeted at multiple sites. Nature communications. 2011;2:336. doi: 10.1038/ncomms1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Klyubin I, Nicoll AJ, Khalili-Shirazi A, Farmer M, Canning S, Mably A, Linehan J, Brown A, Wakeling M, Brandner S, Walsh DM, Rowan MJ, Collinge J. Peripheral administration of a humanized anti-PrP antibody blocks Alzheimer's disease Abeta synaptotoxicity. J Neurosci. 2014;34:6140–6145. doi: 10.1523/JNEUROSCI.3526-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kostylev MA, Kaufman AC, Nygaard HB, Patel P, Haas LT, Gunther EC, Vortmeyer A, Strittmatter SM. Prion-Protein-interacting Amyloid-beta Oligomers of High Molecular Weight Are Tightly Correlated with Memory Impairment in Multiple Alzheimer Mouse Models. The Journal of biological chemistry. 2015;290:17415–17438. doi: 10.1074/jbc.M115.643577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hu NW, Nicoll AJ, Zhang D, Mably AJ, O'Malley T, Purro SA, Terry C, Collinge J, Walsh DM, Rowan MJ. mGlu5 receptors and cellular prion protein mediate amyloid-beta-facilitated synaptic long-term depression in vivo. Nature communications. 2014;5:3374. doi: 10.1038/ncomms4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Haas LT, Salazar SV, Kostylev MA, Um JW, Kaufman AC, Strittmatter SM. Metabotropic glutamate receptor 5 couples cellular prion protein to intracellular signalling in Alzheimer's disease. Brain : a journal of neurology. 2015 doi: 10.1093/brain/awv356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Larson M, Sherman MA, Amar F, Nuvolone M, Schneider JA, Bennett DA, Aguzzi A, Lesne SE. The complex PrP(c)-Fyn couples human oligomeric Abeta with pathological tau changes in Alzheimer's disease. J Neurosci. 2012;32:16857–16871a. doi: 10.1523/JNEUROSCI.1858-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kaufman AC, Salazar SV, Haas LT, Yang J, Kostylev MA, Jeng AT, Robinson SA, Gunther EC, van Dyck CH, Nygaard HB, Strittmatter SM. Fyn inhibition rescues established memory and synapse loss in Alzheimer mice. Annals of neurology. 2015;77:953–971. doi: 10.1002/ana.24394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Huang Y, Lu W, Ali DW, Pelkey KA, Pitcher GM, Lu YM, Aoto H, Roder JC, Sasaki T, Salter MW, MacDonald JF. CAKbeta/Pyk2 kinase is a signaling link for induction of long-term potentiation in CA1 hippocampus. Neuron. 2001;29:485–496. doi: 10.1016/s0896-6273(01)00220-3. [DOI] [PubMed] [Google Scholar]
- [27].Roselli F, Hutzler P, Wegerich Y, Livrea P, Almeida OF. Disassembly of shank and homer synaptic clusters is driven by soluble beta-amyloid(1-40) through divergent NMDAR-dependent signalling pathways. PloS one. 2009;4:e6011. doi: 10.1371/journal.pone.0006011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ma T, Chen Y, Vingtdeux V, Zhao H, Viollet B, Marambaud P, Klann E. Inhibition of AMP-activated protein kinase signaling alleviates impairments in hippocampal synaptic plasticity induced by amyloid beta. J Neurosci. 2014;34:12230–12238. doi: 10.1523/JNEUROSCI.1694-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Raka F, Di Sebastiano AR, Kulhawy SC, Ribeiro FM, Godin CM, Caetano FA, Angers S, Ferguson SS. Ca(2+)/Calmodulin-dependent protein Kinase II interacts with group I Metabotropic Glutamate and facilitates Receptor Endocytosis and ERK1/2 signaling: role of beta-Amyloid. Molecular brain. 2015;8:21. doi: 10.1186/s13041-015-0111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Haas LT, Strittmatter SM. Targeting Aβ Receptors to Modify Alzheimer's Disease Progression. In: Wolfe MS, editor. Developing Therapeutics For Alzheimer's Disease Progress and Challenges. Academic Press; 2016. pp. 227–250. [Google Scholar]
- [31].Smith LM, Strittmatter SM. Binding Site for Amyloid-β Oligomers and Synaptic Toxicity. Cold Spring Harb Perspect Med. doi: 10.1101/cshperspect.a024075. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.