Abstract
Young and older adults experience benefits in attention and memory for emotional compared to neutral information, but this memory benefit is greatly diminished in Alzheimer’s disease (AD). Little is known about whether this impairment arises early or late in the time course between healthy aging and AD. This study compared memory for positive, negative, and neutral items with neutral backgrounds between patients with mild cognitive impairment (MCI) and healthy older adults. We also used a divided attention condition in older adults as a possible model for the deficits observed in MCI patients. Results showed a similar pattern of selective memory for emotional items while forgetting their backgrounds in older adults and MCI patients, but MCI patients had poorer memory overall. Dividing attention during encoding disproportionately reduced memory for backgrounds (versus items) relative to a full attention condition. Participants performing in the lower half on the divided attention task qualitatively and quantitatively mirrored the results in MCI patients. Exploratory analyses comparing lower- and higher-performing MCI patients showed that only higher-performing MCI patients had the characteristic scene memory pattern observed in healthy older adults. Together, these results suggest that the effects of emotion on memory are relatively well preserved for patients with MCI, although emotional memory patterns may start to be altered once memory deficits become more pronounced.
Keywords: Aging, Mild cognitive impairment, Selective memory, Emotion, Divided attention
1. Introduction
While the typical belief is that memory declines with advancing age, memory for emotional information often remains relatively well preserved across the lifespan (Hess, 2005; Mather and Carstensen, 2005; Park et al., 2002). A wealth of evidence has shown that younger and older adults preferentially attend to emotional information and also subsequently remember it better than neutral information (Denburg et al., 2003; Leclerc and Kensinger, 2008; Otani et al., 2007; reviewed by Murphy and Isaacowitz (2008)). In contrast, emotion does not convey the same benefits upon memory in those with moderate to severe stages of Alzheimer’s disease (AD); patients with AD retain very little neutral (reviewed by McKhann et al. (2011)) or emotional information (reviews by Klein-Koerkamp et al. (2012) and Waring and Kensinger (2010)). However, the trajectory of this decline is unclear; it raises questions about the point in the disease course when the emotional enhancement in memory dissipates.
There are still many questions remaining about the characterization of Mild Cognitive Impairment (MCI), preceding a diagnosis of AD. MCI as a clinical condition has not been researched to nearly the same extent as AD. The limited research about emotional memory in MCI has provided disparate findings and left a number of questions remaining. The extent to which the enhancing effect of emotional enhancement on memory is preserved in MCI is unclear—does it dissipate early in the disease progression at the same rate as overall memory decline, or is emotional enhancement in memory relatively well preserved? Some studies of working memory for emotional images have revealed a negativity bias in the memories of MCI patients (but the negative items were also more arousing than positive items, making it impossible to attribute these differences to valence (how positive or negative) alone (Dohnel et al., 2008, 2007)). Another investigation of MCI patients’ recognition memory for emotional and neutral IAPS images showed higher true recognition and false alarms for negative compared to positive or neutral images, and also that dividing attention did not impact discrimination ability (Sava et al., 2016). In other studies MCI patients have shown a memory benefit for positive or negative words (Brueckner and Moritz, 2009; Callahan et al., 2016), but impaired memory for faces studied with emotional expressions (Wang et al., 2013; Werheid et al., 2010). A recent study examined recall and recognition memory for a short series of emotional pictures (8 of each positive, negative, neutral) in healthy older adults, MCI, and patients with unspecified diagnosis of ‘dementia’(Gorenc-Mahmutaj et al., 2015). The authors reported comparable delayed recognition for positive and negative pictures in healthy older adults and MCI, but better memory for positive than negative pictures in patients with dementia. Given the small number of studies and differences in diagnostic testing measures reported, it is difficult to draw conclusions about the extent of impairment or preservation in emotional memory ability in MCI from the existing literature. The disparate findings among the few studies of emotional memory in MCI may be due to differences between task design, stimuli, or degree of impairment among participants (Klein-Koerkamp et al., 2012). Further investigation of the extent of impairment and preservation of selective emotional memory enhancement in MCI can increase our understanding of the course and extent of functional deficits resulting from early AD pathophysiological changes.
The effects of attention and encoding-related phenomena upon subsequent memory are a particularly important area to investigate in patients with MCI because the attention deficits observed in MCI parallel those of healthy older adults: namely, difficulty ignoring or suppressing irrelevant information (Gazzaley and D’Esposito, 2007; Hasher and Zacks, 1988). Previous studies have shown that dividing attention resources during encoding of emotional information is one factor that can quantitatively and qualitatively affect contents of memory in young adults (Clark-Foos and Marsh, 2008; Kensinger and Corkin, 2004; Kern et al., 2005), and older adults (Mather and Knight, 2005: expt 3). Stimuli with emotional arousal increase the difficulty of working memory tasks because they bias attention away from less arousing information also competing for processing resources (Mather and Sutherland, 2011). There is strong evidence for progressive decline in attention processing abilities from healthy aging into MCI; patients with MCI have more difficulties in tasks requiring divided attention than healthy older adults (Okonkwo et al., 2008). These studies provide further evidence that initial attention and encoding phase processes play an important role in subsequent memory in healthy and pathological aging. Notably, older adults with normal cognition often have AD neuropathology while remaining pre-symptomatic of AD for years (Morris et al., 1996), so examining the breakdown in attention and encoding processes with the onset of MCI may provide information about the earliest behavioral changes in older adults that signal advancing underlying pathology.
The first goal of this study was to examine the early effects of the AD pathophysiological process compared to healthy aging on the type of information retained from emotional scenes. To address this goal, healthy older adults and patients with a mild degree of AD pathophysiological change (psychometrically fitting clinical criteria for MCI; Sperling et al., 2011) viewed photographic visual scenes containing positive, negative, or neutral items within a neutral background context. It seemed likely that although early AD pathophysiological changes cause mild memory loss, the relative preservation of brain regions vital for emotion processing (Apostolova and Thompson, 2008; Whitwell et al., 2007) would allow emotional information to retain a higher degree of salience in memory than neutral information. Consequently, we expected that MCI patients would demonstrate selective memory for emotional items in scenes similar to that of healthy older adults.
The second goal of this study was to examine whether dividing the attention of older adults could serve as a model for the memory patterns present in MCI patients granting full attention to encoding. To address this goal, an additional group of older adults encoded emotional and neutral scenes under divided attention. We hypothesized that dividing attention while encoding emotional scenes may not impair encoding of emotionally salient items, but would particularly inhibit older adults’ ability to successfully encode background scene information. The disruption of early attention processes in older adults may provide a model of the pattern of results naturally developing in MCI patients, leading to similar memory patterns between older adults with divided attention and MCI patients. There is a growing body of research about MCI patients’ memory for neutral information, but still little examining emotional memory in MCI. This research advances knowledge about the earliest effects of the AD pathophysiological process on memory for positive and negative information. Exploratory analyses also examined whether there were differences between higher- and lower-performing MCI patients’ memory patterns, to more fully explicate the trajectory of emotional memory changes within the spectrum from healthy aging to the cusp of AD.
2. Methods
2.1. Participants
Participants included 22 patients with a mild degree of AD pathophysiological change, as determined by a neurologist (AEB) or clinical neuropsychologists. 46 healthy older adults approximately matched to the patient sample in age and years of education, (age M=74.4 years, SD=7.0; education M=16.2 years, SD=2.4; 21 men, 25 women). Individuals in the patient group fit criteria for diagnosis of MCI due to AD pathology including 1) concern of a change in cognition over time, 2) impairment in one or more cognitive domains including memory, executive function, attention, language, and visuospatial skills; most commonly presenting with impairment in episodic memory, 3) preservation of independent functional abilities, and 4) not demented (Albert et al., 2011). Data from four individuals were not included in analyses due to elevated scores on a self-reported measure of depression (GDS > 6), and an additional participant repeatedly fell asleep during the protocol, so the final patient sample included 17 participants (age M =79.5 years, SD=5.7; education M =15.6 years, SD=2.5; 12 men, 5 women).
Patients were recruited from the Bedford, MA Veterans Administration Hospital, Boston University Alzheimer’s Disease Center, and a local adult day health program. Older adults were recruited using fliers posted in the community. Individuals were excluded if they reported a history of psychiatric or neurological disorder (other than MCI) at any point over the past 3 years or if they had participated in a study in this research laboratory that used the same stimulus set or task instructions. All participants were native English speakers with normal or corrected to normal vision.
At the time of the study, participants scored within the normal range on measures of depression and anxiety (see Table 1). Participants were paid at the rate of $10 per hour, and took 2–2.5 h to complete the study protocol. Written informed consent was obtained from all participants prior to beginning the study, in accordance with the protocols approved by the Institutional Review Boards of Boston College (healthy older adults) or the Boston University Medical Center (MCI patients), and the study was performed in accordance with the standards of the 1964 Declaration of Helsinki.
Table 1.
Characterization of participants.
| Test | Older adults | MCI | Older Adults Full: Divided attn | All Older Adults: MCI | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Full attention | Divided attention | |||||||||
|
|
|
|
|
|
||||||
| M (SE) | Range | M (SE) | Range | M (SE) | Range | F (1,45) | p | F (1,62) | p | |
| Mini-Mental State Examination Folstein et al. (2010) |
29.48 (.17) | 27–30 | 29.00 (.22) | 27–30 | 28.18 (.38) | 24–30 | 3.07 | n/s | 10.85 | < .01 |
| Digit Symbols Substitution Wechsler (1997) |
33.61 (1.54) | 20–49 | 35.00 (1.28) | 25–54 | 19.88 (1.70) | 2–29 | .49 | n/s | 55.74 | < .0005 |
| Verbal Fluency to letters Spreen and Benton (1977) |
48.57 (1.92) | 30–73 | 51.78 (2.85) | 28–81 | 36.41 (3.65) | 10–67 | .88 | n/s | 14.42 | < .0005 |
| Digits Recall, backward Wechsler (1997) |
8.61 (.48) | 4–13 | 8.30 (.54) | 5–14 | 6.41 (.68) | 2–14 | .18 | n/s | 8.21 | < .01 |
| Shipley Vocabulary Test Shipley (1946) |
36.61 (.52) | 31–40 | 36.96 (.58) | 30–40 | 31.88 (1.76) | 9–39 | .20 | n/s | 15.76 | < .0005 |
| Trails B Adjutant General’s Office (1944) |
95.13 (6.72) | 51–171 | 94.36 (8.35) | 45–226 | 140.06 (21.93) | 50–300 | .01 | n/s | 8.01 | < .01 |
| Dysexecutive Questionnaire Wilson et al. (1996) |
15.74 (1.95) | 2–42 | 15.65 (1.41) | 3–28 | 15.59 (2.14) | 2–39 | .001 | n/s | < .01 | n/s |
| Beck Anxiety Inventory Beck et al. (1988) |
4.87 (1.06) | 0–18 | 5.04 (1.23) | 0–24 | 3.47 (.84) | 0–11 | .01 | n/s | 1.10 | n/s |
| Geriatric Depression Scale Sheikh and Yesavage (1986) |
.83 (.21) | 0–3 | .39 (.15) | 0–3 | .94 (.30) | 0–5 | 2.92 | n/s | 1.40 | n/s |
| CERAD (recall/delayed recall/recognition) Morris et al. (1989) |
– | – | – | – | 16.53/3.18/8.71 | – | – | – | – | – |
Notes: Verbal Fluency to letters represents the total number of words produced in 60 s each for letters F, A, and S. Trails B represents time to complete. CERAD values represent means for 3 task sections, respectively. M=mean, SE=standard error, n/s=not significant at level of p < .05.
2.2. Materials
The stimulus set included 50 positive, 50 negative, and 50 neutral items and 150 backgrounds. Scenes were created by placing an emotional or neutral item on a background (e.g., a snake by a river). The stimulus set is a subset of the items and backgrounds used in prior studies (Waring and Kensinger, 2009, 2011). Prior ratings from healthy younger and older adults (using a 7-pt scale) indicated that positive (arousal M =3.12; range 2.57–4.47; SD=.58) and negative items (arousal M =3.29; range 2.00–3.75; SD=.42), were significantly more arousing than neutral items (arousal M =2.25; range 1.07–3.40; SD=.62; F(1,149) > 102.02, p < .0005). An important methodological improvement over our earlier studies investigating emotional scene memory (Waring, Addis, and Kensinger, 2013; Waring and Kensinger, 2009, 2011) is that the sets of positive and negative items were matched for arousal level (F(1,99)=2.27, p=.10), allowing for more direct comparison of the effects of emotion on scene memory.1 (Stimuli described in further detail in Waring et al. (2013) and Waring and Kensinger (2009, 2011)).
2.3. Procedure
Each participant saw 75 composite scenes (e.g., a chipmunk by a river. See Fig. 1A), with each scene containing an item (25 positive, 25 negative, 25 neutral) in the context of a neutral background). Backgrounds were paired with items of each emotion type (positive, negative, neutral) across participants (e.g. one person saw a snake [negative item] by the river [neutral background], and one person saw a kitten [positive item] by the river [neutral background]) to isolate effects attributed to emotionality of items and to avoid any confounds related to the backgrounds themselves. Participants viewed each item and background only once across studied scenes.
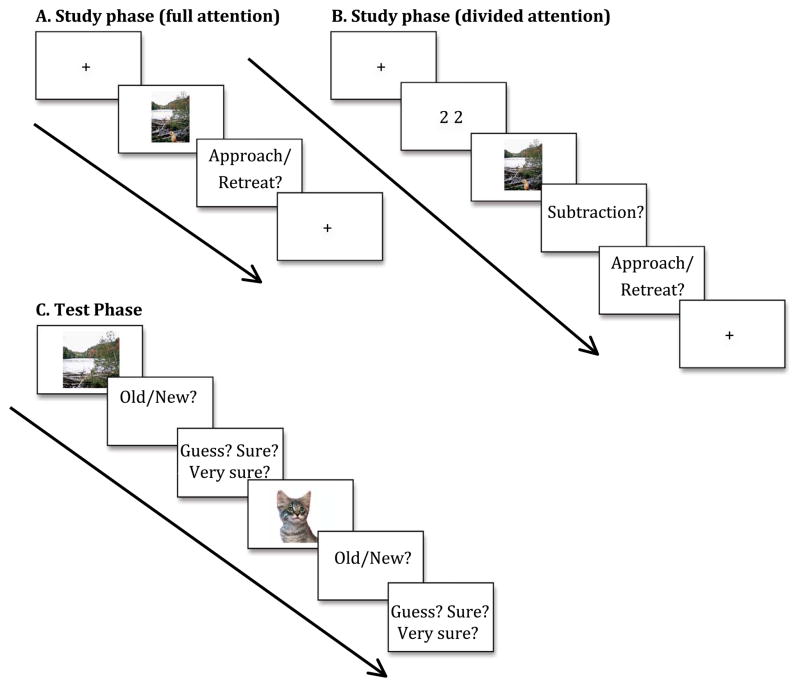
Fig. 1. Depiction of experimental design.
Schematic of study tasks for full attention (A) and divided attention (B) conditions (between subjects design), and the recognition memory test (C) common to both study conditions. MCI patients only experienced the full attention condition.
Healthy older adult participants were divided into two experimental groups. Half of the older adults completed the scene-encoding task while simultaneously performing a second task that served to divide their attention (“divided attention”), and the other half were only asked to perform the scene-encoding task (“full attention”). All MCI participants completed the encoding task in the full attention condition. Healthy older adults entered their responses on a computer keyboard independently, and the experimenter entered responses for the MCI patients to avoid the possibility of impaired performance attributable to difficulty with computer use.
In the full attention condition, participants performed an incidental scene encoding task where they were asked to rate on a 1–7 scale whether they would prefer to approach or retreat from the scene if they were to encounter it in everyday life (1=move extremely close, 4= stay at present location, 7= move extremely far away). (Results and discussion of approach-retreat ratings available in Supplementary information.) The image was visible for 3 s regardless of response time. Scene-viewing procedures were identical in the full and divided attention conditions, and all participants had the opportunity to practice before beginning the task. (See Fig. 1 for schematic of experimental design).
In the divided attention condition (healthy older adults only), prior to the incidental scene-encoding task, participants viewed a numerical value between 17 and 100 for 750 ms. While the scene was visible, participants were asked to mentally subtract 16 from the value and enter their response after the scene was removed from view (See Fig. 1B). This task was chosen because it required participants to perform a mental operation the whole time the scene was in view, however it did not deliver additional visual or auditory stimulus input while the scene was visible. There were an additional 3 healthy older adult participants in the divided attention condition who were removed from all analyses because of poor performance (< 33% correct) on the math calculation (final sample older adult math calculation accuracy M =78%, N=23). Following the 3-second scene study phase, divided attention group participants were prompted to first type their numerical response to the mental calculation and then to enter their approach or retreat decision response (See Fig. 1B).
After a 10-min delay, composite scenes from the study phase were separated into their individual component items and backgrounds for an old/new recognition memory test (see Fig. 1C). The memory test included 75 old items (25 positive, 25 negative, 25 neutral), 75 old backgrounds (25 had been presented with a positive item, 25 with a negative item, and 25 with a neutral item), as well as 75 ’new’ (i.e., never studied) items (25 positive, 25 negative, and 25 neutral) and 75 new backgrounds (by definition, all neutral), for a combined total of 300 scene components tested individually. Scene components were counterbalanced across participants for studied versus novel status at test. The orientation (i.e. horizontal or vertical) of each ’old’ item presented at test was set to match its orientation in the studied scene. Order of stimulus presentation was varied across participants to minimize effects of fatigue and order.
At test, participants had up to 3 s to view each component and up to 7 s to indicate, via key press, whether it was from one of the scenes viewed during the study phase (’old’) or had not been previously viewed within a scene (’new’; see Fig. 1C). There was a short practice test given to ensure participants fully understood the meaning of “old” and “new” scene components. Additionally, for all test items and backgrounds participants were asked to rate their confidence in the accuracy of their memory (1= guessing, 2=sure, 3=very sure; results and discussion of confidence ratings available in Supplementary information).
3. Results
This investigation had two main goals. First, to examine how the emotional saliency of complex visual scenes affected memory in patients with MCI compared to healthy older adults. Secondly, to examine whether taxing older adults’ attention resources during scene encoding could serve as a model for the emotional memory patterns arising in MCI. Exploratory analyses also examined whether there were differences between higher- and lower-performing MCI patients’ memory patterns. After characterizing the groups’ performance on measure of neuropsychological function, we address the outcome of each of these goals.
3.1. Neuropsychological assessments
In order to characterize the sample, participants completed measures of cognition, as well as self-reports of anxiety, depression, and dysexecutive function. ANOVAs between groups (MCI, healthy older adults in full attention condition, healthy older adults in divided attention condition) revealed that MCI patients had significantly poorer performance on all measures of memory, processing speed, verbal fluency, and working memory than healthy older adults (Fs(1,61) > 8.23, ps < .01, all ). However, there were no significant differences between MCI and healthy older adults on self-reported measures of anxiety (Beck Anxiety Inventory; Beck et al., 1988), depression (Geriatric Depression Scale; Sheikh and Yesavage, 1986), or dysexecutive function (Dysexecutive Questionnaire; Wilson et al., 1996) (Fs(1,61) < 1.37, ps > .25 all η2ρ < .03; reported in Table 1). There were no differences on any measures between healthy older adults assigned to the full attention versus divided attention conditions (Fs(1,44) < 3.07, ps > .09, all η2ρ < .07).
3.2. Full attention: memory for emotional scenes
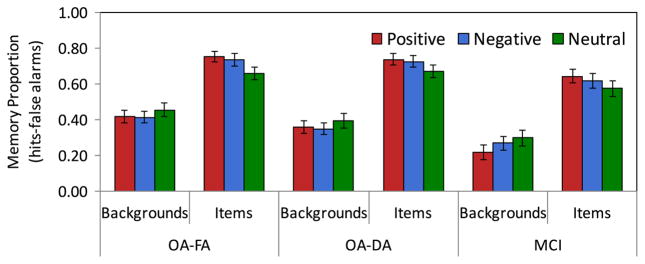
The primary goal of the study was to examine the effect of emotion on selective scene memory between MCI patients and older adults in the full attention condition. An ANOVA on corrected recognition values (proportion hits-false alarms) with factors of component (items, backgrounds), scene type (positive, negative, neutral), and group (older adults, MCI) revealed a main effect of component (F(1,38)=214.71, p < .0005, ) and an interaction between component and scene type (F(2,37)=6.66, p < .005, η2ρ =.27). This interaction emerged because emotional items were remembered better than neutral items (pos > neu t(39)=4.43, p < .0005, Cohen’s d=.66; neg > neu t(39)=2.74, p < .01, Cohen’s d=.41; pos M=.70, neg M=.68, neu M=.62), while there was no memory advantage for backgrounds that had been paired with emotional items; in fact, these backgrounds were remembered less well (numerically, though not significantly) than backgrounds that had been paired with neutral items (pos < neu t(39)=1.88, p=.07, Cohen’s d=.27; neg < neu t(39)=1.50, p=.14, Cohen’s d=.28; pos M=.32, neg M=.34, neu M=.38).
A key finding was that there was a main effect of group (F(1,38) =9.14, p < .005, ; older adults M=.57, MCI M=.44), but no interactions with group (Fs < 2.22, ps > .14, all η2ρ < .06). These results reflect that older adults had overall better memory for scenes than MCI patients, but the emotional scene memory pattern did not differ between groups. It established that there was enhanced memory for positive and negative emotional items compared to neutral items in MCI patients as well as healthy older adults (uncorrected raw recognition and false alarm counts presented in Table 2; results and discussion of response bias available in Supplementary information).
Table 2.
Count of hits and false alarms by attention condition and group.
| Items M (SE)
|
Backgrounds M (SE)
|
||||||
|---|---|---|---|---|---|---|---|
| Positive | Negative | Neutral | Positive | Negative | Neutral | ||
| Full Attention | |||||||
| Older Adults | Hits | 20.09 (.80) | 19.43 (.75) | 17.61 (.96) | 14.91 (1.12) | 14.74 (.97) | 15.83 (1.19) |
| FA | 3.52 (.59) | 3.17 (.43) | 3.26 (.56) | * | * | 13.17 (1.84) | |
| MCI | Hits | 18.12 (1.04) | 17.12 (1.27) | 16.06 (1.08) | 11.24 (.96) | 12.53 (.84) | 13.24 (1.07) |
| FA | 5.88 (.93) | 4.82 (.68) | 4.94 (.88) | * | * | 17.24 (2.28) | |
| Divided Attention | |||||||
| Older Adults | Hits | 20.13 (.62) | 19.61(.54) | 18.74(.73) | 14.48(.69) | 14.13(.74) | 15.09 (.68) |
| FA | 4.39 (.74) | 4.61 (.88) | 3.74 (.77) | * | * | 15.52(2.40) | |
Hits=number recognized, uncorrected; FA=number of false alarms, M=mean, SE= standard error. Item and background hits counts are out of 25 total, item FA counts are out of 25 total, and background FA counts are out of 75 total.
one FA value is applied to backgrounds of all scene types because novel backgrounds are inherently neutral.
3.3. Effects of reducing attention resources on memory for emotional scenes
The second goal of this study was to examine whether taxing older adults’ attention resources during scene encoding could serve as a model for the MCI patients’ general memory impairment and if dividing attention would have selective impact as a function of emotional scene type.
3.3.1. Divided versus full attention at encoding
An ANOVA of healthy older adults’ corrected recognition with factors of component (items, backgrounds), scene type (positive, negative, neutral), and condition (full attention, divided attention) was used to determine the effects of dividing attention on memory for emotional scenes. There was a main effect of component (F(1,44) =368.69, p < .0005, ), and an interaction between component and scene type (F(2,43)=5.05, p=.01, η2ρ =.19). This interaction reflects better memory for emotional items (pos M=.75, pos > neu t(45)=3.77, p < .0005, Cohen’s d=.69; neg M=.73, neg > neu t(45) =2.05, p < .05, Cohen’s d=.31) than neutral items (M=.68), and poorer memory for backgrounds that had been paired with negative than neutral items (neg M=.38, neu M=.42; neg < neu t(45)=1.98, p=.05, Cohen’s d=.30; pos M=.39, pos < neu t(45)=1.31, p=.20, Cohen’s d=.19). A key finding was the interaction between factors of component and condition with medium effect size (F(1,44)=4.11, p < .05, η2ρ =.09) reflecting poorer memory for backgrounds in the divided attention (M=.37) than full attention condition (M=.43), but comparable memory for items in both conditions (Ms=.72). There were no significant interactions between factors of scene type and condition (Fs < 1), indicating that although dividing attention at encoding reduced memory for backgrounds generally, the effects did not differ as a function of emotional scene type (See Fig. 2).
Fig. 2. Comparison of memory for emotional scenes between conditions and groups.
Comparison of memory for items and backgrounds by emotional valence between attention conditions and participant groups. Emotional valence of backgrounds refers to the valence of the item it was paired with during encoding phase. OA-FA = older adults, full attention encoding; OA-DA = older adults, divided attention encoding.
3.3.2. Healthy older adults with divided attention at encoding versus MCI patients
To determine whether dividing healthy older adults’ attention during encoding would produce a similar pattern of emotional scene memory as MCI patients, we performed an ANOVA on corrected recognition values with factors of component (item, background), scene type (positive, negative, neutral) and group (older adults divided attention, MCI full attention). As in prior analyses, the results showed a significant main effect of component (F(1,38)=310.26, p < .0005, ) qualified by an interaction between component and scene type (F(2,37)=6.91, p=.003, η2ρ =.27; see Fig. 2). As also observed in the analysis comparing MCI patients to older adults with full attention, there was a main effect of group (F(1,38)=6.45,p=.02, η2ρ =.15; older adults divided attention M=.54, MCI M=.44), indicating that MCI patients were significantly impaired in their memory accuracy, but memory impairment did not alter the pattern of emotional scene memory from that of healthy older adults (for all interactions with group Fs < 1).
To test whether the divided attention condition would more closely approximate MCI in the individuals who found the divided attention task most challenging, we conducted exploratory analyses comparing scene memory between healthy older adults whose math calculation accuracy was in the lower half of the sample (N=11, < 85% correct) versus the MCI patient sample. As in previous analyses, we computed an ANOVA on corrected recognition values with factors of component (item, background), scene type (positive, negative, neutral), and group (older adults divided attention, MCI full attention). In contrast to analyses employing the entire divided attention group, there was no main effect of group (F(1,26) < 1, p=.51, ). MCI patients’ scene memory performance did not differ from the subgroup of healthy older adults who had the most difficulty accurately completing in the divided attention condition (i.e., lowest math calculation scores). There were also no interactions with the factor of groups (Fs < .5). There was a main effect of component (F(1,26)=224.81, p < .0005, η2ρ =.90) and interaction between component and scene type (F(2,25)=4.73, p < .05, η2ρ =.27), as also described previously. Moreover, there was no correlation between math calculation accuracy and overall corrected recognition rate (hits-FAs) in the divided attention group (r(21)=.26, p=.23). It is also important to point out that there were no significant differences on any neuropsychological measures between the individuals who had higher versus lower accuracy on the divided attention math calculation (for measures see Table 1; all Fs(1,22) < 3.76, ps > .07, η2ρ < .15).
3.4. Comparison of higher and lower performing MCI patients
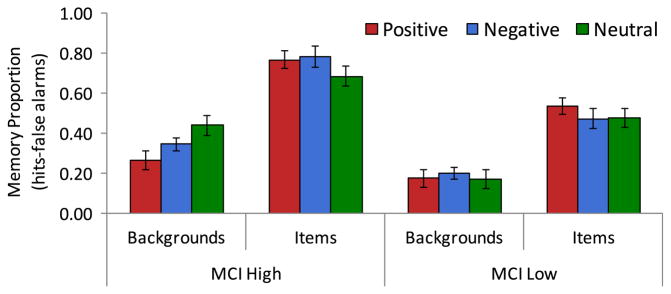
To understand whether there was a uniform pattern in emotional scene memory among the MCI patients or whether effects differed as a function of overall memory ability, we conducted exploratory analyses of differences between higher and lower performing MCI patients, as determined using a median split of corrected recognition scores (hits-false alarms; split at M=.40) for overall task performance, agnostic to the specific composition of memory.2 This created a subgroup of higher performers (n=8; 3 female, 5 male) and a subgroup of lower performers (n=9; 2 female, 7 male); age did not differ between these two subgroups (t < 1). An ANOVA of corrected recognition values with factors of component (item, background), valence (positive, negative, neutral), and group (higher, lower performers) revealed a main effect of component (F(1,15) =104.99, p < .0005, ), and interaction between component and valence (F(2,30) =4.32, p=.02, η2ρ =.22), reflecting that, as a group, MCI patients showed the normal pattern of better memory for positive and negative items relative to neutral items, and poorer memory for backgrounds that had been paired with positive and negative items relative to neutral (as described earlier in results). The 3-way interaction among these factors had a medium-large effect size (F(2,30) 2.91, p=.07, η2ρ =.16). Higher performing MCI patients remembered negative items (M=.79) and positive items (M=.77) better than neutral items (M=.69), as reflected in their large and medium effect sizes, respectively (neg > neu t(7)=2.29, p=.056, Cohen’s d=.79; pos > neu t(7)=1.57, p=.16, Cohen’s d=.63), yet low performing MCI patients remembered only positive items (M=.54) better than neutral items (M=.48; pos > neu t(8)=2.34, p < .05, Cohen’s d=.91; neg M=.47; neg v neu t(8) < 1, Cohen’s d=.10). Higher performing MCI patients also had poorer memory for backgrounds from positive (M=.27) and negative scenes (M=.35) than neutral scenes (M=.44; pos < neu t(7) =2.71, p=.03, Cohen’s d=.94; neg < neu t(7)=2.68, p=.03, Cohen’s d=.92), but low performing MCI patients did not show the same decrement in memory for backgrounds from emotional scenes (pos M=.18, neg M=.20, neu M=.17; ts < .5, Cohen’s d’s < .20. See Fig. 3). Plainly stated, the higher-performing MCI patients had enhancement in memory for positive and negative items relative to neutral and also poorer memory for backgrounds from positive and negative scenes than neutral scenes, while the lower-performing MCI patients had only better memory for positive than neutral items. The main effect of group (F(1,15) =31.67, p < .0005, η2ρ =.68) mirrors the split that assigned membership to higher versus lower performing MCI groups.
Fig. 3. Comparison of memory for emotional scenes between higher- and lower-performing MCI patients.
Comparison of memory for items and backgrounds by emotional valence between higher- performing (MCI High) and lower-performing MCI patients (MCI Low). Emotional valence of backgrounds refers to the valence of the item it was paired with during encoding phase.
4. Discussion
To our knowledge, this is the first study examining selective memory for emotional scenes in patients with MCI (but see Gorenc-Mahmutaj et al. (2015) and Sava et al. (2016)) for memory for complete emotional images in patients with MCI or dementia). The primary goal of the study was to examine the effect of emotion on selective scene memory between MCI patients and healthy older adults. Results showed that there was a similar pattern of emotional scene memory between groups, despite poorer overall memory in MCI patients. Results of the second goal of this study showed that dividing attention during encoding disproportionately reduced memory for backgrounds (versus items), when compared to encoding with full attention. Dividing attention did not uniformly serve as a reliable model for the MCI patients’ broader memory impairment pattern. However, the older adults whose math calculation accuracy was in the lower half of the divided attention group showed no quantitative or qualitative difference in scene memory from the MCI patients, suggesting that sufficiently taxing attentional resources during encoding can model emotional scene memory in MCI patients. Exploratory analyses comparing lower- and higher-performing MCI patients revealed that scene memory differed as a function of overall memory ability; only the higher performing MCI patients demonstrated the characteristic pattern of selective emotional scene memory.
4.1. Selective memory for emotional items
Results showed that MCI patients, as well as healthy older adults, have enhanced memory for emotional items compared to neutral items. For MCI patients, like the healthy older adult participants, memory enhancement was selective to the emotional item within a scene and did not extend to a benefit in memory for the background paired with the emotional item. These results suggest that although overall memory performance is poorer in patients with MCI (as would be expected with this condition), their pattern of memory for visual scenes containing emotional components generally resembles that of healthy older adults.
In the present dataset, although there was a numerical detriment to memory for the backgrounds paired with emotional compared to neutral items, this difference did not always reach significance. This weaker effect of emotion on background memory may be related to the fact that, because the present study matched the arousal level of positive and negative items (see methods), this required removal of the most arousing negative items. Consequently, the set of positive items is similar to ’positive higher-arousal’ items in our past investigations, while the set of negative items is similar to ’negative lower-arousal’ items in our past investigations (Waring and Kensinger, 2009). The present results generally replicate those of Waring and Kensinger (2009) for comparable stimuli and study-test delay interval (Fig. 2B, comparing positive-high arousal and negative-low arousal bars). In a broader sense, these results contribute to the literature of preserved emotion processing in late life, and memory preferences for positive information in healthy older adults (reviewed by Reed, Chan, and Mikels, 2014), and individuals with MCI (Callahan et al., 2016; Gorenc-Mahmutaj et al., 2015; Leal et al., 2016).
4.2. Dividing attention during encoding as a model for MCI
Taxing the attention resources of older adults by dividing their attention during scene encoding did not mitigate the enhancement in memory for emotional items, nor did it significantly affect overall levels of item memory. Instead, dividing attention during encoding selectively reduced memory accuracy for backgrounds. These results may indicate that encoding of items is a relatively easier or more automatic process for older adults, while encoding background contexts is a more controlled process, requiring additional mental resources (Gazzaley, 2011). Thus, when attention is divided there is a general item-processing bias, with available resources prioritized toward item encoding, regardless of valence (Chee et al., 2006; Gutchess et al., 2007).
Importantly, because the scene-encoding task was constant between the two attention conditions, memory differences between the full and divided attention conditions cannot be attributed to instructions encouraging a shift in visual focus within the scene or competing sensory (e.g., auditory) information. Rather, the working memory resources occupied by the divided attention task (mental math calculation) may have taxed the limited capacity of attention resources (Baddeley, 2000) such that available resources could only be allocated toward encoding one portion of the scene. Alternatively, it is possible that items can be remembered with limited post-encoding elaborative processes whereas successfully remembering backgrounds requires greater elaboration. Performing the math calculations during the time interval after the scene was removed from view may have interrupted the elaborative processing necessary for successful encoding of backgrounds (Anderson et al., 2000; Ritchey et al., 2011).
The present study suggests that dividing the attention of older adults at encoding can provide a model for MCI in individuals whose attentional resources are sufficiently taxed by a simultaneous secondary task. When examining the divided attention group as a whole, results were essentially identical whether comparing MCI patients to older adults with full or divided attention during encoding. However, closer inspection of scene memory the older adults whose math calculation accuracy was in the lower half of the divided attention group showed no quantitative or qualitative difference in scene memory from the MCI patients. Evaluation of performance on neuropsychological measures between individuals with higher and lower math calculation accuracy on the divided attention task confirmed that calculation accuracy is not merely a proxy for dividing the sample by overall cognitive ability. There was also no correlation between calculation accuracy and emotional scene memory. One would predict a positive relationship between these factors if there were individuals with greater cognitive abilities as evidenced in both greater math accuracy and task performance. Moreover, if participants had merely compromised math accuracy in favor of the encoding task, one would predict a negative relationship between these factors; e.g., memory increases as math accuracy decreases. This was not observed in the current sample.
Results showed that completing a rapid math calculation concurrent with a scene encoding task was sufficiently taxing for many of the individuals in the divided attention condition (indeed, anecdotal reports during debriefing confirmed many participants found the task demanding). When completing a concurrent divided attention task at encoding, healthy older adults’ emotional scene memory can be attenuated to the level of MCI patients. This suggests that reduced attentional deployment at encoding may account, at least partially, for the recognition memory deficits in MCI. Others have shown that memory impairments can be explained by impairments in attention processing, which limits efficient memory encoding, and leads to downstream deficits in memory retrieval (Balota and Faust, 2001; Castel et al., 2009; Hedden et al., 2012). However, we cannot preclude the possibility that ineffective post-encoding elaboration or consolidation processes also play a role in reduced memory performance in MCI patients.
4.3. Relation between memory impairment and emotional enhancement of memory
The results of this study may better situate MCI on the spectrum between healthy aging and AD. AD patients experience severe impairments in overall memory accuracy and most laboratory evidence has shown they are also largely unable to experience recognition memory enhancement for emotional information (Abrisqueta-Gomez et al., 2002; Brueckner and Moritz, 2009; Budson et al., 2006; Chainay et al., 2014; Hamann et al., 2000; Kensinger et al., 2004, 2002; Landre et al., 2013; Perrin et al., 2012, but see Borg et al., 2011; Gallo et al., 2010). Studies that have demonstrated any degree of emotional memory enhancement in AD patients have required very deep and elaborative encoding or cued retrieval procedures (reviewed by Klein-Koerkamp et al., 2012; Sava et al., 2015). Past investigation of the neural mechanisms underlying loss of emotional enhancement in memory in patients with mild AD has elucidated that greater loss of emotional enhancement in memory correlates with amygdalar and hippocampal volume loss (Landre et al., 2013).
In the present study, we observed that despite poorer memory accuracy overall in the MCI patients, differences in the pattern of their emotional scene memory occurred only within the poorest-performing MCI patients. The characteristic enhancement of memory for emotional items, and simultaneous decrement in memory for backgrounds from scenes containing emotional items (compared to neutral), was apparent in the higher performing MCI patients. Thus, the higher performing MCI patients’ pattern of selective emotional scene memory is similar to that of healthy older adults, while lower performing MCI patients’ emotional memory patterns may resemble the more substantial emotional memory deficits of AD patients. These results suggest that changes in the pattern of emotional scene memory develop along with broader decline in memory. Volumetric measurement has shown that, after considering hippocampal atrophy, regional volume losses within fusiform and inferior and middle temporal gyri have the highest predictive value for transition from healthy aging to MCI (Apostolova and Thompson, 2008) so emotional memory deficits may not appear until there is more extensive involvement of frontal regions as well as more posterior perceptual processing regions (Perry and Hodges, 1999).
The literature demonstrating relatively better preservation of frontal than posterior brain regions in aging and AD pathology provides a possible mechanistic explanation for results of the present study. Prefrontal activation and network connectivity is best preserved and functions compensatorily for deficits in the aging brain (Davis, Dennis, Daselaar, Fleck, and Cabeza, 2008; Grady, 2008; Park and Reuter-Lorenz, 2009). Prefrontal activation also preferentially supports encoding of positive images, while posterior regions support encoding of negative images (Kensinger and Schacter, 2008; Mickley and Kensinger, 2008). Moreover, structural and functional evidence from past studies demonstrate that the earliest and heaviest AD pathological burden develops within temporal and parietal lobes, while the prefrontal cortex remains relatively preserved until later disease stages (Perry and Hodges, 1999). Taken together, previous findings suggest a likely mechanism for the pattern of results observed in the present study. Selective preservation of memory enhancement for positive items in the lower performing MCI patients follows from evidence that frontal regions are relatively more resistant to early MCI pathology and also support encoding of positive images.
4.4. Limitations and future directions
One limitation of this study was that neuroimaging evidence was not available to further elucidate the neural mechanisms underlying the observed effects of MCI and divided attention on emotional scene memory. Identifying the relationship between task performance and structural measures of grey matter volume or white matter path integrity would further clarify the relationship between MCI-related brain changes and memory performance. Moreover, functional neuroimaging of task performance would provide a more complete picture of how older adults with divided attention and MCI patients may be recruiting additional resources to fulfill task demands, relative to healthy older adults with full attention at encoding. These are intriguing possibilities for future research.
Another limitation of this study is the small patient sample size, which may have limited power to detect differences between groups. Because no prior study had examined selective emotional memory in an MCI or AD patient sample, we had limited basis for conducting an a priori power analysis to determine sample size. We considered prior research that observed main and interactive effects of group on emotional scene memory (Waring et al., 2013; Waring and Kensinger, 2009) and effects of AD on memory for emotional pictures (Chainay et al., 2014; Landre et al., 2013; Perrin et al., 2012), and chose a sample size that was comparable or slightly larger than in these prior studies. The patient sample size was limited due to diligence to obtain a well-characterized amnestic MCI sample (excluding frank Alzheimer’s disease, multi-domain MCI, or signs of clinically meaningful depression). Even with a small patient sample we observed the hypothesized main effect of memory differences between the full attention older adult and MCI patient groups. Nevertheless, null effects should be interpreted with caution because the sample may have been underpowered to detect such effects, e.g., interactions.3 Future studies that enroll larger patient samples may permit broader conclusions about how emotional scene memory presents in individuals across a spectrum of late life cognitive impairments.
Additional possible future directions are to modulate the study-test delay interval to interrogate the impact of emotion on memory consolidation in MCI patients (see Sava et al., 2015). As described above, we cannot exclude the possibility that ineffective post-encoding elaboration or consolidation processes play a role in reduced memory performance in MCI patients, so varying the amount of consolidation time could further clarify the impact of elaboration and consolidation on subsequent memory (Waring and Kensinger, 2009).
Lastly, our study was limited to testing recognition memory for details from emotional scenes, although testing memory for gist as well as detail memory may provide more information about how emotional information influences memory strength. Moreover, examining recall in addition to recognition could further probe the nuances of memory decline in healthy older adults and MCI patients. A recent study reported that individuals with below-average episodic memory preferentially recalled the gist of positive stories relative to individuals with above-average episodic memory, although this effect did not extend to memory for story details (Leal et al., 2016). This raises the question of whether the patterns of emotional scene memory in healthy older adults and MCI patients would appear to differ if recall or gist were assessed as well as recognition of specific scene items and backgrounds. There may be subtle variations in memory as a function of the methodology applied. This is an intriguing question for future investigations.
4.5. Conclusions
The results of the present study, taken together with the existing literature, suggest that emotional enhancement in memory is relatively well preserved for patients with mild MCI, but emotional memory patterns may become altered once memory deficits are more pronounced. Although patients with MCI had poorer accuracy overall than older adults –even those older adults with divided attention during scene encoding– higher performing MCI patients showed a similar pattern of memory performance as the healthy older adults; they selectively remembered emotional items from complex visual scenes. These results better situate the development of impaired memory for emotional materials on the spectrum from healthy aging to AD.
Supplementary Material
Acknowledgments
We thank the staff of the Boston University Alzheimer’s Disease Center, Marion Dunn at The Community Family of Everett, MA, and Erin Hussey for assistance with participant recruitment. We also thank Sara Samaha and Anna Petracca for assistance with data collection. We also thank an anonymous reviewer for encouraging us to more fully consider the effects of difficulty of the divided attention condition on memory.
Support for this research was provided by National Institutes of Health grants MH080833 (EAK) and P30 AG13846 (AEB), the Boston University Alzheimer’s Disease Center, and the American Psychological Association (JDW).
Appendix A. Supplementary information
Supplementary information associated with this article can be found in the online version at doi:10.1016/j.neuropsychologia.2017. 01.011.
Footnotes
Previous literature has shown that patients with AD have normal perception of emotional stimuli; ratings of stimulus arousal and valence are not significantly different between healthy older adults and patients with AD (Dohnel et al., 2008; Gallo et al., 2010).
All MCI patients were classified as members of the same higher- or lower-performing subgroup regardless of whether using hits-false alarms or d′ as the criteria for subdividing the sample. Values for d′ and C were calculated using the formulas from Snodgrass and Corwin (1988) because they are undefined when proportion of responses equals 0 or 1. H =(# hits +.5) /(# studied items +1); FA =(#false alarms +.5)/(# unstudied items +1).
Given the observed effect size of the 3-way interaction among factors of group (MCI, OA full attention), scene type, and component, future studies hypothesizing such interactive effects would be advised to recruit 60 or 75 participants per group to detect significant interaction between 2 groups or 3 groups, respectively (power =.80, alpha=.05).
References
- Abrisqueta-Gomez J, Bueno OFA, Oliveira MGM, Bertolucci PHF. Recognition memory for emotional pictures in Alzheimer’s patients. Acta Neurol Scand. 2002;105(1):51–54. doi: 10.1034/j.1600-0404.2002.00035.x. [DOI] [PubMed] [Google Scholar]
- Adjutant General’s Office. Army individual test battery: manual of directions and scoring. War Department; Washington, DC: 1944. [Google Scholar]
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. < https://doi.org/16/j.jalz.2011.03.008>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson ND, Iidaka T, Cabeza R, Kapur S, McIntosh AR, Craik FI. The effects of divided attention on encoding-and retrieval-related brain activity: a PET study of younger and older adults. J Cogn Neurosci. 2000;12(5):775–792. doi: 10.1162/089892900562598. [DOI] [PubMed] [Google Scholar]
- Apostolova LG, Thompson PM. Mapping progressive brain structural changes in early Alzheimer’s disease and mild cognitive impairment. Neuropsychologia. 2008;46(6):1597–1612. doi: 10.1016/j.neuropsychologia.2007.10.026. http://dx.doi.org/10.1016/j.neuropsychologia.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A. The episodic buffer: a new component of working memory? Trends Cogn Sci. 2000;4(11):417–423. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- Balota DA, Faust ME. Attention in dementia of the Alzheimer’s type. In: Boller F, Cappa S, editors. Handbook of Neuropsychology. 2. Vol. 6. Elsevier; New York: 2001. pp. 51–80. [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56(6):893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Borg C, Leroy N, Favre E, Laurent B, Thomas-Antérion C. How emotional pictures influence visuospatial binding in short-term memory in ageing and Alzheimer’s disease. Brain Cogn. 2011;76(1):20–25. doi: 10.1016/j.bandc.2011.03.008. http://dx.doi.org/10.1016/j.bandc.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Brueckner K, Moritz S. Emotional valence and semantic relatedness differentially influence false recognition in mild cognitive impairment, Alzheimer’s disease, and healthy elderly. J Int Neuropsychol Soc. 2009;15:268–276. doi: 10.1017/S135561770909047X. [DOI] [PubMed] [Google Scholar]
- Budson A, Todman R, Chong H, Adams E, Kensinger E, Krangel T, Wright C. False recognition of emotional word lists in aging and Alzheimer disease. Cogn Behav Neurol: Off J Soc Behav Cogn Neurol. 2006;19(2):71–78. doi: 10.1097/01.wnn.0000213905.49525.d0. [DOI] [PubMed] [Google Scholar]
- Callahan BL, Simard M, Mouiha A, Rousseau F, Laforce R, Hudon C. Impact of depressive symptoms on memory for emotional words in mild cognitive impairment and late-life depression. J Alzheimer’s Dis. 2016;52(2):451–462. doi: 10.3233/JAD-150585. http://dx.doi.org/10.3233/JAD-150585. [DOI] [PubMed] [Google Scholar]
- Castel AD, Balota DA, McCabe DP. Memory efficiency and the strategic control of attention at encoding: impairments of value-directed remembering in Alzheimer’s disease. Neuropsychology. 2009;23(3):297–306. doi: 10.1037/a0014888. http://dx.doi.org/10.1037/a0014888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chainay H, Sava A, Michael GA, Landré L, Versace R, Krolak-Salmon P. Impaired emotional memory enhancement on recognition of pictorial stimuli in Alzheimer’s disease: No influence of the nature of encoding. Cortex. 2014;50:32–44. doi: 10.1016/j.cortex.2013.10.001. http://dx.doi.org/10.1016/j.cortex.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Chee MWL, Goh JOS, Venkatraman V, Tan JC, Gutchess AH, Sutton B, Park D. Age-related changes in object processing and contextual binding revealed using fMR adaptation. J Cogn Neurosci. 2006;18(4):495–507. doi: 10.1162/jocn.2006.18.4.495. http://dx.doi.org/10.1162/jocn.2006.18.4.495. [DOI] [PubMed] [Google Scholar]
- Clark-Foos A, Marsh RL. Recognition memory for valenced and arousing materials under conditions of divided attention. Memory. 2008;16(5):530–537. doi: 10.1080/09658210802007493. http://dx.doi.org/10.1080/09658210802007493. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. queue PASA? The posterior-anterior shift in aging. Cereb Cortex. 2008;18(5):1201–1209. doi: 10.1093/cercor/bhm155. http://dx.doi.org/10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denburg NL, Buchanan TW, Tranel D, Adolphs R. Evidence for preserved emotional memory in normal older persons. Emotion. 2003;3(3):239–253. doi: 10.1037/1528-3542.3.3.239. http://dx.doi.org/10.1037/1528-3542.3.3.239. [DOI] [PubMed] [Google Scholar]
- Dohnel K, Sommer M, Reindl G, Muller J, Hajak G, Ibach B. Information processing in patients with mild cognitive impairment (MCI) Psychiatr Prax. 2007;34(Supplement 1):S117–S118. [Google Scholar]
- Dohnel K, Sommer M, Lbach B, Rothmayr C, Meinhardt J, Hajak G. Neural correlates of emotional working memory in patients with mild cognitive impairment. Neuropsychologia. 2008;46(1):37–48. doi: 10.1016/j.neuropsychologia.2007.08.012. http://dx.doi.org/10.1016/j.neuropsychologia.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Folstein M, Folstein S, White T, Messer M. MMSE-2 Mini-Mental State Examination. 2. Vol. 12. Psychological Assessment Resources Inc; 2010. (User’s Manual) [Google Scholar]
- Gallo DA, Foster KT, Wong JT, Bennett DA. False recollection of emotional pictures in Alzheimer’s disease. Neuropsychologia. 2010 doi: 10.1016/j.neuropsychologia.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A. Influence of early attentional modulation on working memory. Neuropsychologia. 2011;49(6):1410–1424. doi: 10.1016/j.neuropsychologia.2010.12.022. < https://doi.org/16/j.neuropsychologia.2010.12.022>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, D’Esposito M. Top-down modulation and normal aging. Ann NY Acad Sci. 2007;1097(1):67–83. doi: 10.1196/annals.1379.010. http://dx.doi.org/10.1196/annals.1379.010. [DOI] [PubMed] [Google Scholar]
- Gorenc-Mahmutaj L, Degen C, Wetzel P, Urbanowitsch N, Funke J, Schröder J. The positivity effect on the intensity of experienced emotion and memory performance in mild cognitive impairment and dementia. Dement Geriatr Cogn Disord Extra. 2015;5(2):233–243. doi: 10.1159/000381537. http://dx.doi.org/10.1159/000381537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL. Cognitive neuroscience of aging. Ann NY Acad Sci. 2008;1124:127–144. doi: 10.1196/annals.1440.009. http://dx.doi.org/10.1196/annals.1440.009. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Hebrank A, Sutton BP, Leshikar E, Chee MW, Tan JC, Park DC. Contextual interference in recognition memory with age. NeuroImage. 2007;35(3):1338–1347. doi: 10.1016/j.neuroimage.2007.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann SB, Monarch E, Goldstein F. Memory enhancement for emotional stimuli is impaired in early Alzheimer’s disease. Neuropsychology. 2000;14(1):82–92. [PubMed] [Google Scholar]
- Hasher L, Zacks RT. Working memory, comprehension, and aging: a review and a new view. In: Bower G, editor. The Psychology of Learning and Motivation 22. Academic Press; New York: 1988. pp. 193–225. [Google Scholar]
- Hedden T, Van Dijk KRA, Shire EH, Sperling RA, Johnson KA, Buckner RL. Failure to modulate attentional control in advanced aging linked to white matter pathology. Cereb Cortex. 2012;22(5):1038–1051. doi: 10.1093/cercor/bhr172. http://dx.doi.org/10.1093/cercor/bhr172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess TM. Memory and aging in context. Psychol Bull. 2005;131(3):383–406. doi: 10.1037/0033-2909.131.3.383. http://dx.doi.org/10.1037/0033-2909.131.3.383. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Corkin S. Two routes to emotional memory: distinct neural processes for valence and arousal. Proc Natl Acad Sci. 2004;101(9):3310–3315. doi: 10.1073/pnas.0306408101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. Neural processes supporting young and older adults’ emotional memories. J Cogn Neurosci. 2008;20(7):1161–1173. doi: 10.1162/jocn.2008.20080. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Brierley B, Medford N, Growdon J, Corkin S. Effects of normal aging and Alzheimer’s disease on emotional memory. Emotion. 2002;2:118–134. doi: 10.1037/1528-3542.2.2.118. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Anderson A, Growdon J, Corkin S. Effects of Alzheimer disease on memory for verbal emotional information. Neuropsychologia. 2004;42(6):791–800. doi: 10.1016/j.neuropsychologia.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Kern R, Libkuman T, Otani H, Holmes K. Emotional stimuli, divided attention, and memory. Emotion. 2005;5(4):408–417. doi: 10.1037/1528-3542.5.4.408. http://dx.doi.org/10.1037/1528-3542.5.4.408. [DOI] [PubMed] [Google Scholar]
- Klein-Koerkamp Y, Baciu M, Hot P. Preserved and impaired emotional memory in Alzheimer’s disease. Front Psychol. 2012;3:1–12. doi: 10.3389/fpsyg.2012.00331. http://dx.doi.org/10.3389/fpsyg.2012.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landre L, Sava AA, Krainik A, Lamalle L, Krolak-Salmon P, Chainay H. Effects of emotionally-rated material on visual memory in Alzheimer’s disease in relation to medial temporal atrophy. J Alzheimers Dis. 2013;36(3):535–544. doi: 10.3233/JAD-130170. http://dx.doi.org/10.3233/JAD-130170. [DOI] [PubMed] [Google Scholar]
- Leal SL, Noche JA, Murray EA, Yassa MA. Positivity effect specific to older adults with subclinical memory impairment. Learn Memory. 2016;23:415–421. doi: 10.1101/lm.042010.116. http://dx.doi.org/10.1101/lm.042010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc CM, Kensinger EA. Effects of age on detection of emotional information. Psychol Aging. 2008;23(1):209–215. doi: 10.1037/0882-7974.23.1.209. http://dx.doi.org/10.1037/0882-7974.23.1.209. [DOI] [PubMed] [Google Scholar]
- Mather M, Knight M. Goal-directed memory: the role of cognitive control in older adults’ emotional memory. Psychol Aging. 2005;20(4):554–570. doi: 10.1037/0882-7974.20.4.554. http://dx.doi.org/10.1037/0882-7974.20.4.554. [DOI] [PubMed] [Google Scholar]
- Mather M, Carstensen LL. Aging and motivated cognition: the positivity effect in attention and memory. Trends Cogn Sci. 2005;9(10):496–502. doi: 10.1016/j.tics.2005.08.005. http://dx.doi.org/10.1016/j.tics.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Mather M, Sutherland MR. Arousal-Biased Competition in Perception and Memory. Perspect Psychol Sci. 2011;6(2):114–133. doi: 10.1177/1745691611400234. http://dx.doi.org/10.1177/1745691611400234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, Phelps CH. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. < https://doi.org/16/j.jalz.2011.03.005>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickley KR, Kensinger EA. Emotional valence influences the neural correlates associated with remembering and knowing. Cogn Affect Behav Neurosci. 2008;8:143–152. doi: 10.3758/cabn.8.2.143. [DOI] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Clark C. The consortium to establish a registry for Alzheimer’s disease (CERAD): Part I. clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39(9):1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Morris JC, Storandt M, McKeel D, Rubin E, Price J, Grant E, Berg L. Cerebral amyloid deposition and diffuse plaques in normal” aging: evidence for presymptomatic and very mild Alzheimer’s disease. Neurology. 1996;46(3):707–719. doi: 10.1212/wnl.46.3.707. [DOI] [PubMed] [Google Scholar]
- Murphy NA, Isaacowitz DM. Preferences for emotional information in older and younger adults: a meta-analysis of memory and attention tasks. Psychol Aging. 2008;23(2):263–286. doi: 10.1037/0882-7974.23.2.263. http://dx.doi.org/10.1037/0882-7974.23.2.263. [DOI] [PubMed] [Google Scholar]
- Okonkwo O, Wadley V, Ball K, Vance D, Crowe M. Dissociations in visual attention deficits among persons with mild cognitive impairment. Aging Neuropsychol Cogn. 2008;15(4):492–505. doi: 10.1080/13825580701844414. http://dx.doi.org/10.1080/13825580701844414. [DOI] [PubMed] [Google Scholar]
- Otani H, Libkuman TM, Widner RL, Graves EI. Memory for emotionally arousing stimuli: a comparison of younger and older adults. J Gen Psychol. 2007;134(1):23–42. doi: 10.3200/GENP.134.1.23-42. [DOI] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. http://dx.doi.org/10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychol Aging. 2002;17(2):299–320. http://dx.doi.org/10.1037//0882-7974.17.2.299. [PubMed] [Google Scholar]
- Perrin M, Henaff MA, Padovan C, Faillenot I, Merville A, Krolak-Salmon P. Influence of emotional content and context on memory in mild Alzheimer’s disease. J Alzheimers Dis. 2012;29(4):817–826. doi: 10.3233/JAD-2012-111490. http://dx.doi.org/10.3233/JAD-2012-111490. [DOI] [PubMed] [Google Scholar]
- Perry R, Hodges J. Attention and executive deficits in Alzheimer’s disease - a critical review. Brain. 1999;122:383–404. doi: 10.1093/brain/122.3.383. [DOI] [PubMed] [Google Scholar]
- Reed AE, Chan L, Mikels JA. Meta-analysis of the age-related positivity effect: age differences in preferences for positive over negative information. Psychol Aging. 2014;29(1):1–15. doi: 10.1037/a0035194. http://dx.doi.org/10.1037/a0035194. [DOI] [PubMed] [Google Scholar]
- Ritchey M, Bessette-Symons B, Hayes SM, Cabeza R. Emotion processing in the aging brain is modulated by semantic elaboration. Neuropsychologia. 2011;49(4):640–650. doi: 10.1016/j.neuropsychologia.2010.09.009. http://dx.doi.org/10.1016/j.neuropsychologia.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sava AA, Paquet C, Krolak-Salmon P, Dumurgier J, Hugon J, Chainay H. Emotional memory enhancement in respect of positive visual stimuli in Alzheimer’s disease emerges after rich and deep encoding. Cortex. 2015;65:89–101. doi: 10.1016/j.cortex.2015.01.002. http://dx.doi.org/10.1016/j.cortex.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Sava AA, Paquet C, Dumurgier J, Hugon J, Chainay H. The role of attention in emotional memory enhancement in pathological and healthy aging. J Clin Exp Neuropsychol. 2016;38(4):434–454. doi: 10.1080/13803395.2015.1123225. http://dx.doi.org/10.1080/13803395.2015.1123225. [DOI] [PubMed] [Google Scholar]
- Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. In: Brink TL, editor. Clinical gerontology: A Guide to Assessment and Intervention. The Haworth Press, Inc; NY: 1986. pp. 165–173. [Google Scholar]
- Shipley W. Shipley Institute of Living Scale. Western Psychological Services; Los Angeles: 1946. [Google Scholar]
- Snodgrass J, Corwin J. Pragmatics of measuring recognition memory-Applications to dementia and amnesia. J Exp Psychol: Gen. 1988;117(1):34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Phelps CH. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’S Dement. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. < https://doi.org/16/j.jalz.2011.03.003>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreen O, Benton A. Neurosensory Center Comprehensive Examination for Aphasia: Manual of instructions (NCCEA) University of Victoria; Victoria, BC: 1977. Rev. [Google Scholar]
- Wang P, Li J, Li H, Li B, Jiang Y, Bao F, Zhang S. Is emotional memory enhancement preserved in amnestic mild cognitive impairment? Evidence from separating recollection and familiarity. Neuropsychology. 2013;27(6):691–701. doi: 10.1037/a0033973. http://dx.doi.org/10.1037/a0033973. [DOI] [PubMed] [Google Scholar]
- Waring JD, Kensinger EA. Effects of emotional valence and arousal upon memory trade-offs with aging. Psychol Aging. 2009;24(2):412–422. doi: 10.1037/a0015526. http://dx.doi.org/10.1037/a0015526. [DOI] [PubMed] [Google Scholar]
- Waring JD, Kensinger EA. Emotional memory in Alzheimer’s Disease. In: Sun M-K, editor. Research Progress in Alzheimer’s Disease and Dementia. Vol. 4. Nova Science Publisher; New York: 2010. pp. 9–36. [Google Scholar]
- Waring JD, Kensinger EA. How emotion leads to selective memory: Neuroimaging evidence. Neuropsychologia. 2011;49(7):1831–1842. doi: 10.1016/j.neuropsychologia.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring JD, Addis DR, Kensinger EA. Effects of aging on neural connectivity underlying selective memory for emotional scenes. Neurobiol Aging. 2013;34(2):451–467. doi: 10.1016/j.neurobiolaging.2012.03.011. http://dx.doi.org/10.1016/j.neurobiolaging.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Technical Manual for the Wechsler Adult Intelligence Scale and Wechsler Memory Scale. 3. The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Werheid K, Gruno M, Kathmann N, Fischer H, Almkvist O, Winblad B. Biased recognition of positive faces in aging and amnestic mild cognitive impairment. Psychol Aging. 2010;25(1):1–15. doi: 10.1037/a0018358. http://dx.doi.org/10.1037/a0018358. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Przybelski SA, Weigand SD, Knopman DS, Boeve BF, Petersen RC, Jack CR. 3D maps from multiple MRI illustrate changing atrophy patterns as subjects progress from mild cognitive impairment to Alzheimer’s disease. Brain. 2007;130(7):1777–1786. doi: 10.1093/brain/awm112. http://dx.doi.org/10.1093/brain/awm112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson B, Alderman N, Burgess P, Emslie H, Evans J. The Behavioural Assessment of the Dysexecutive Syndrome. Thames Valley Test Company; Flempton, Bury St Edmunds, England: 1996. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.