Abstract
Decision-making processes rarely occur in isolation. Rather, representations are updated constantly based on feedback to past decisions and actions. However, previous research has focused on the reaction to feedback receipt itself, instead of examining how feedback information is integrated into future decisions. In the current study, we examined differential neural sensitivity during risk decisions following positive versus negative feedback in a risk-taking context, and how this differential sensitivity is linked to adolescent risk behavior. Fifty-eight adolescents (ages 13–17 years) completed the Balloon Analogue Risk Task (BART) during an fMRI session and reported on their levels of risk-taking behavior. Results show that reduced medial PFC (mPFC) response following negative versus positive feedback is associated with fewer reductions in task-based risky decisions following negative feedback, as well as increased self-reported risk-taking behavior. These results suggest that reduced neural integration of negative feedback into during future decisions supports risky behavior, perhaps by discounting negative relative to positive feedback information when making subsequent risky decisions.
Introduction
1.1
Decision-making processes almost never occur in isolation; rather individuals’ representations are constantly being updated based on internal and external information. Feedback, either positive or negative, can be used to update decision-making in real time, affecting subsequent behavior in complex ways (Gold & Shadlen, 2007). Successful monitoring of decision-making performance involves both the ability to extract relevant information from feedback stimuli and then adjust behavioral strategies in appropriate ways. However, these abilities are not uniform across development. Rather, different phases of development show differential susceptibility to the wide array of feedback available in the environment. Adolescence is a time when sensitivity to positive and negative feedback undergoes significant changes, which have important implications for adolescents’ real-world risk behavior (Somerville et al., 2010).
Theoretical and empirical work has explored how sensitivity to positive and negative feedback changes during the adolescent phase of development. Some models of adolescence have focused primarily on adolescent sensitivity to positive feedback (i.e., rewards), suggesting that increased approach motivation prompts adolescents to seek out risky situations and behavior (Casey et al., 2008, Steinberg, 2010). The rewarding nature of engaging in these behaviors can serve as an intrinsic form of positive feedback, supporting further risk behavior. A large body of empirical work has supported these suggestions, with adolescents demonstrating greater sensitivity than adults or children to a wide range of rewarding stimuli (e.g., Steinberg et al., 2008; Cauffman et al., 2010; Spear, 2011). On the neural level, adolescent reward circuitry (e.g., ventral striatum) shows hypersensitivity to rewards relative to both children and adults (e.g., Galván, 2013; see Telzer, 2016), and this neural reward sensitivity has been linked with increased engagement in risk-taking behaviors (Galván et al., 2007; Telzer et al., 2013; Braams et al., 2015; Qu et al., 2015). These findings support the idea that adolescence is a period of sensitivity to positive feedback, and that this sensitivity is an important contributor to increased risk behavior
Other models have proposed that adolescent sensitivity to negative feedback also contributes to increases in risk taking (Ernst et al., 2006). Specifically, decreased sensitivity to negative feedback results in low avoidance motivation, such that adolescents are less likely to subjectively experience negative outcomes related to their risky behavior, and are less likely to weigh potentially negative consequences into their decision-making representations compared with adults and children (Ernst et al., 2006). Empirical evidence for decreased sensitivity to negative feedback in adolescence has been sparse. On the behavioral level, there does appear to be a developmental decrease in sensitivity to negative feedback. However, this decrease continues through adolescence and into adulthood; that is, it is not unique to adolescents (van Duijvenvoorde et al., 2008; Cauffman et al., 2010; Humphreys et al., 2016). Neural evidence for a unique adolescent reduction in sensitivity to negative feedback is considerably more sparse (but see Ernst et al., 2005).
While there is little evidence for a decrease in sensitivity to negative feedback in adolescence, there is some accumulating evidence for adolescence as a transitional period in how positive and negative feedback are used in relation to one another during decision-making. During reversal learning, for instance, children show greater reward learning and adults show greater punishment learning, while adolescents show fewer distinctions in how feedback type relates to behavioral performance (van der Schaaf et al., 2011; van den Bos et al., 2012). Additionally, during risk taking, adolescents’ risk behavior is more likely to be unchanged after receiving negative feedback, while children tend to increase and adults tend to decrease their risk behavior (Humphreys et al., 2016). Moreover, decreases in sensitivity to negative feedback interact with increased rates of learning during adolescence to promote behavioral advantages over adults and children during high, but not low or medium, risk situations (Humphreys et al., 2016). These patterns of results may reflect changes in how positive and negative information are integrated together and differentially weighted.
Although subcortical regions are most often implicated in sensitivity to positive and negative feedback (the ventral striatum and amygdala, respectively), integration of multiple forms of feedback likely relies on higher-order regulatory regions, such as the prefrontal cortex (PFC). Initial evidence suggests that transitions in PFC activity and connectivity might play a role in changing sensitivity to feedback observed during adolescence. For instance, regulatory (e.g., DLPFC and superior parietal lobule) activity during feedback learning mimics a transition in how positive versus negative feedback affects participant learning (van Duijvenvoorde et al., 2008). However, other regions such as the pre-SMA/ACC and caudate do not show these adolescent transitions (van Duijvenvoorde et al., 2008), suggesting that neural systems may exhibit differential trajectories in their responses to positive and negative feedback. Additionally, medial PFC (mPFC) connectivity between both the amygdala (Gee et al., 2013; Gabard-Durman et al., 2014) and ventral striatum (van den Bos et al., 2012) show transitions (albeit in opposite directions) during adolescence, with neural coupling flipping in the direction of association during this period. The mPFC distinguishes between positive and negative feedback, and mPFC sensitivity to reward versus loss receipt relates to adolescents’ frequency of risk engagement (van Duijvenvoorde et al., 2014). This is consistent with adult work showing that the mPFC is involved in action-outcome evaluation (van Noordt & Segalowitz, 2012) and indexing of the relative risk of actions (Xue et al., 2009; van Leijenhorst, Moor, et al., 2010). These data suggest that the mPFC may play an important role in feedback integration and action evaluation, which impacts an individual’s propensity to both engage in risk initially, but also to modulate on-going behavioral strategies as feedback is received.
The majority of studies examining adolescent sensitivity to feedback focus on the actual receipt of reward or punishment (e.g. van Duijvenvoorde et al., 2014; Hauser et al., 2014). While an important first step, further examination of how adolescents continue to process feedback information and integrate it into future decision-making requires greater attention. Examining down-stream processes of feedback integration and neural adaptation offers additional insight into risky decision-making above looking solely at momentary reactivity to feedback. While certainly related, feedback reactivity and integration likely also involve different neural systems, which may be sensitive to unique individual differences. An understanding of how feedback information is integrated into decision making processes over time can give us new insights into how feedback processing relates to adolescents’ risky behavior. To address this question, we examined adolescents’ differential neural reactivity to positive and negative feedback in the context of risk and reward. During an fMRI session, adolescents completed the Balloon Analog Risk Task (BART; Lejuez et al., 2002), a well-established paradigm that gives both positive and negative feedback following participants’ risk behavior. In contrast to previous examinations of feedback processing, we focused on subsequent decisions adolescents made following positive (e.g., receipt of reward) or negative (loss of potential reward) feedback. We first examined differences in decision-making made in these separate contexts. Because these events (i.e., pump decisions) are otherwise identical, any observed differences could be linked to adolescents’ sensitivity to the differential contexts created by the previous trials’ feedback. Secondly, we characterized the consequences of individual differences in feedback processing during risk taking by examining associations between neural signatures of feedback integration and risky behavior, both from the task and adolescents’ self-reported real-world behavior. Importantly, examining these brain-behavior relationships not only allows us to explore how adolescents incorporate feedback information into future decisions, but also the impact of this differential neural processing on real-world behavior. We hypothesized that adolescents would show differential neural activation when making decisions made after negative relative to positive feedback. In particular, regions involved in feedback integration, such as the mPFC, should be sensitive to the context of different feedback conditions. Given previous work relating mPFC to risky behavior, we hypothesize that reduced mPFC integration of negative, relative to positive, feedback into decision-making processes would be associated with increases in risk-taking behavior during adolescence.
Methods
2.1 Participants
Sixty adolescent participants completed an fMRI scan. Subjects were recruited from the community through a variety of methods, including flyers, recruiting from a pool of subjects, and through local schools. One participant was excluded for excessive head motion (> 2.0 mm inter-slice movement on ≥10% of slices), another for lacking sufficient trial types for modeling functional events, and two participants were excluded for being on medication for ADD/ADHD; leaving a final sample of fifty-eight adolescents (30 female; Mage=15.62 years, SD=1.38, range=12.3–17.7 years; 40 European-American, 14 African-American, 2 Latin-American, 1 Asian-American, and 3 mixed/multiple ethnicity). Adolescents provided written consent and assent in accordance with the University of Illinois’ Institutional Review Board.
2.2 Self-Reported Risk Taking
In order to examine real-world behaviors associated with neural sensitivity to feedback, adolescents reported on their risk-taking behavior using a modified version of the Adolescent Risk-Taking Scale (Alexander et al., 1990; Telzer, Fuligni, Lieberman, & Galván 2013). Participants completed 12 questions indicating how frequently (1= Never to 4= Many Times) they engaged in a variety of risky behaviors (e.g., “I have gotten high or drunk at a party,” and “I have slipped out at night while my parents thought I was asleep.”). The scale had excellent reliability (α = .91).
2.3 Pubertal Development
Adolescents completed the self-report Peterson Pubertal Development Scale (PDS; Peterson et al., 1988) which is used to assess the development of secondary sexual characteristics. The PDS is composed of 5 general questions which capture information related to physical growth, changes in body hair, and changes in the skin. Adolescents also completed additional sex-specific questions; 2 for boys related to changes in the voice and facial hair, and 3 for girls related to breast development and menstruation. For each question, adolescents indicated on a 4-point scale whether that aspect of development: (1) had not yet started, (2) had barely started, (3) had definitely started, or (4) seemed completed. Participants’ responses were averaged to calculate a pubertal development score which was used in analyses.
2.4 Behavioral Paradigm
Adolescents completed a variant of the Balloon Analog Risk Task (BART) during an fMRI scan. The BART is a widely-used paradigm to measure risk taking (Lejuez et al., 2002; Telzer et al., 2015) and provides positive and negative feedback following participants’ risk decisions. Prior to the scan, participants were shown a box filled with age-appropriate prizes and were told they could choose prizes from the box based on how many points they earned during the scan; however, in reality all participants were able to choose 3 prizes at the end of the scan session regardless of how many points they earned.
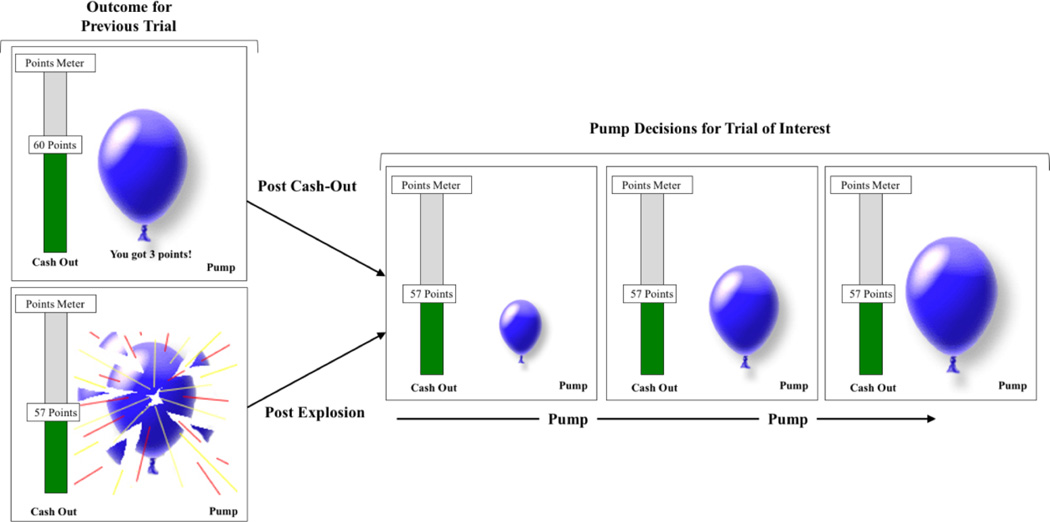
During the scan session, adolescents were shown a series of 24 balloons, which they could choose to pump up in order to earn points. Each pump increased the likelihood that the balloon would explode, which resulted in the participant losing all the points they had earned on that balloon. Participants could choose to cash-out their points at any point after the first pump, which saved the points they earned on that balloon to their total score. Participants’ running total of points were presented in a points meter on the left side of the screen, and participants were instructed to try to earn as many points as possible during the task. Depending on their choices during each balloon, participants received either negative (i.e., explosion) or positive (i.e., point receipt) feedback. Cash-out events (i.e., positive feedback) involved the display of the points that the participant earned on that balloon, accompanied by a positive, cash tone which was played through speakers in the scan bore. Explosion events (i.e., negative feedback) were accompanied by an aversive, loud tone. Participants’ decisions on the different balloons were categorized by the type of feedback that was received on the previous trial (Figure 1). In particular, balloons were categorized as either (1) post-explosion trials (i.e., following negative feedback) or (2) post-cash-out trials (i.e., following positive feedback). Each decision and outcome were separated by a jitter (500–4000 ms), and the task only advanced when participants made the decision to either pump or save their points. Participants were informed that after each decision, there would be a brief delay where further responses would not advance the task. During the delay, the on-screen decisions “pump” and “cash-out” disappeared. These periods corresponded to the jittered interval between pump decisions and/or outcomes. By not allowing the task to advance during the jitter period, we ensured that the hemodynamic function of each decision could be modeled successfully. Balloons were presented in a fixed order (pseudo-randomly selected), such that each participant was shown the same sequence of balloons, and each balloon exploded at a set level (range=3–10 pumps). The probability of explosion was equally distributed across explosion levels (i.e., each explosion level appeared 3 times). Participants were not made aware at any point of the rules of the task, or the pump levels at which point balloons could explode. Rather, they were only instructed to earn as many points as they could, and were only able to learn about task parameters through completing the task.
Figure 1.
The outcome of the previous trial (i.e. cash-out or explosion) was used to group subsequent pump decisions into conditions of interest. The first trial was excluded from analyses but was used to group pump decisions in trial 2.
After the scan, adolescents reported on a 4-point scale (1=Not at All, 4=Definitely) their level of motivation to earn the available prizes. Adolescents reported being moderately motivated by the prizes, although there was individual variation (M=2.74, SD=.99, range=1–4). Adolescents’ motivation to earn prizes was not related to any of our behavioral or neural variables of interest. Furthermore, controlling for motivation in our analyses does not alter the effects.
2.5 fMRI Data Acquisition and Analysis
Imaging data were collected using a 3 Tesla Siemens Trio MRI scanner. Structural scans consisted of a T1* magnetization-prepared rapid-acquisition gradient echo (MPRAGE; TR=1.9sec; TE=2.3msec; FOV=230; matrix=256×256; sagittal plane; slice thickness=1mm; 192 slices) and a T2*weighted, matched-bandwidth (MBW), high-resolution, anatomical scan (TR=4sec; TE=64msec; FOV=230; matrix=192×192; slice thickness=3mm; 38 slices). During the BART, T2*-weighted echoplanar images (EPI) (slice thickness=3 mm; 38 slices; TR=2sec; TE=25msec; matrix=92×92; FOV=230 mm; voxel size 2.5×2.5×3mm3) were acquired. MBW and EPI scans were obtained using an oblique axial orientation in order to maximize brain coverage.
fMRI data were analyzed using Statistical Parametric Mapping software package (SPM8; Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK). Preprocessing involved spatial realignment to correct for head motion (adolescents included in the final sample had no motion in excess of 1.5mm inter-slice motion; average framewise displacement per TR = 0.15 mm), coregistration of all images to the high-resolution T1* MPRAGE structural scan, and segmentation into grey matter, white matter, and cerebrospinal fluid. MBW and EPI images were warped into the standard stereotactic space defined by the Montreal Neurological Institute (MNI) and the International Consortium for Brain Mapping by applying the transformation matrices used in MPRAGE segmentation. An 8mm Gaussian kernel, full-width-at-half maximum was used to smooth EPI images to increase the signal-to-noise ratio. Each trial was convolved with a canonical hemodynamic response function. A high-pass temporal filter with a 128s cutoff was applied to remove low-frequency drift in the time series, and a restricted maximum likelihood algorithm with an autoregressive model order of 1 was used to estimate serial autocorrelations.
In each person’s fixed effects model, a general linear model (GLM) was created with seven regressors of interest, modeled as events. Our primary conditions of interest included pumps on balloons following explosions (i.e., risk taking post negative feedback) and pumps on balloons following successful cash-outs (i.e., risk taking post positive feedback). The first balloon of the task was modeled separately and was excluded from analysis because decisions during this trial lacked any previous feedback information. Similarly, outcome events (i.e., cash-outs and explosions) were modeled separately from both types of risk decisions and from one another, but were not included in the current analyses, as our focus was not on sensitivity to the feedback event itself but instead to decisions adolescents made following positive (e.g., receipt of reward) or negative (loss of potential reward) feedback. Thus, risk decisions were categorized by the feedback adolescents’ received immediately prior and modeled separately in order to compare differential neural sensitivity to positive and negative feedback on future risk-taking decisions. To help ensure lower levels of noise in our estimation of the BOLD signal during the task, we excluded participants who had fewer than five balloons for either condition (following positive or negative feedback). Because each pump decision was modeled for each balloon, all participants had over 15 individual decision events following both positive and negative feedback (M=27.61, SD=11.43, range=15–67). The jittered inter-trial periods between decision events were not modeled explicitly and therefore served as the implicit baseline. Within a balloon trial, each pump decision was modeled separately such that the number of events per balloon was equal to the number of times participants pumped that balloon. A parametric modulator (PM) was included for the two conditions of interest and corresponded to the number of pumps at each pump decision. The PM was included as a control to ensure that the observed effects were not simply due to differences in neural processing of the progressively-larger balloons within a trial. Individual contrasts were then computed for each condition of interest.
Random effects, group-level analyses were run on all individual subject contrasts using GLMFlex, which removes outliers and sudden activation changes in the brain, corrects for variance-covariance inequality, partitions error terms, and analyzes all voxels containing data (http://mrtools.mgh.harvard.edu/index.php/GLM_Flex). Our group level fMRI analyses contrasted risk-taking decisions (i.e., pumping behavior) on post-explosion versus post-cash-out trials. To examine the consequences of differential sensitivity to negative versus positive feedback, we examined links between neural activation and both task behavior and adolescent self-report risk taking. For our task-based behavioral measure of sensitivity to negative feedback, we computed a difference score representing the average number of pumps on balloons following negative feedback (i.e., an explosion) and the average number of pumps on balloons following positive feedback (i.e., cash-out). The resultant score (negative – positive) reflects adolescents’ differential risk behavior following feedback, with greater negative values reflecting greater reductions in pumping following an explosion relative to a cash-out. We hypothesized that adolescents who showed reduced neural reactivity to negative versus positive feedback would show less sensitivity to negative feedback behaviorally (i.e., would show less reduction in pumping behavior after receiving negative feedback) as well as report greater frequency of engaging in risk-taking behaviors. To help control for differences in maturation between our subjects, we ran whole-brain regressions with age, pubertal development, and gender, as well as the interactions between age/puberty and gender. Furthermore, we included these variables as covariates in our analyses of interest, both in whole-brain regressions and mediation analyses. Age, gender, and their interaction were not associated with neural activation during decisions following positive relative to negative feedback. Moreover, including age, gender, and pubertal development as covariates did not change the effects in any of our regressions of interest. As such, we removed these controls for parsimony.
Correction for multiple comparisons was run using a Monte Carlo simulation through the 3dFWHMx and 3dClustSim packages from the AFNI software package (Ward, 2000; updated April 2016) using the group-level brain mask generated through GLMFlex. Group-level brain masks included all voxels in functional space containing functional information that were included in our analyses. Separate simulations were run for each analysis using the analysis-specific group-level mask. To determine the overall cluster threshold, we selected the largest threshold from each individual simulation and applied it for all analyses. Simulations resulted in a voxel-wise threshold of p<.005 and a minimum cluster size of 51 contiguous voxels for the whole brain, corresponding to p<.05, Family-Wise Error (FWE) corrected. All results are available on Neurovault (http://neurovault.org/collections/1985/; Gorgolewski et al., 2015).
Results
3.1 Behavioral Results
Consistent with prior research (Rao et al., 2008;Telzer et al., 2015) and adolescents’ goals for the task, adolescents were more likely to cash-out than pump until balloons exploded (Cash-outs: M=18.05, SD=1.19; Explosions: M=5.95, SD=1.19; t(57)=21.25, p<.001). Furthermore, adolescents showed differential pumping behavior on balloons after positive versus negative feedback, such that they pumped more on average following a cashed-balloon (M=7.25, SD=0.86) than an exploded balloon (M=5.83, SD=0.83; t(57)=14.22, p<.001). Furthermore, when examining behavior on a trial-by-trial basis, adolescents tend to reduce their pumping following negative feedback and increase their pumping following positive feedback (B= −1.25, SE = .08, t(55) = −14.91, p< .001), controlling for both trial number and how much they pumped on the previous trial. Differences in pumping behavior after negative versus positive feedback was not significantly related to adolescents’ self-reported risk-taking behavior (r=.087, p=.524) or to the total number of points earned during the task (r=−.192, p=.157).
3.2 fMRI Main Effects
3.2.1.Risk-related neural responses following positive feedback
We first examined neural activity during risk decisions after participants received positive feedback (i.e., following cash-out trials). In other words, what neural patterns of activation do adolescents show when making risky choices on a balloon after receiving a reward? Adolescents demonstrated heightened activation in the dorsal anterior cingulate cortex (dACC), bilateral insula, bilateral ventral striatum (VS), bilateral dorsolateral PFC (DLPFC), and bilateral amygdala. We also found decreased activation in the bilateral inferior frontal gyrus (IFG) and bilateral superior temporal gyrus (STG; Table 1).
Table 1.
Neural Regions Showing Significant Activation During Risky Decisions after Positive and Negative Feedback.
| Anatomical Region | +/− | BA | x | y | z | t | k |
|---|---|---|---|---|---|---|---|
| Pumps After Positive Feedback | |||||||
| dACC | + | 24/32 | −6 | 20 | 31 | 7.26 | 1074 |
| Motor Cortex | + | 4 | −6 | −4 | 73 | 5.88 | |
| L Postcentral Gyrus | + | 1/2 | −66 | −22 | 22 | 6.34 | 1797 |
| L Insula | + | −45 | 11 | −5 | 5.98 | ||
| L Amygdala | + | −21 | −4 | −11 | 5.72 | ||
| L VS | + | −21 | 14 | −5 | 3.83 | ||
| R Insula | + | 54 | 11 | −2 | 5.71 | 838 | |
| R VS | + | 21 | 11 | −2 | 4.67 | ||
| L DLPFC | + | 9/46 | −36 | 41 | 28 | 5.26 | 315 |
| R DLPFC | + | 9/46 | 30 | 50 | 31 | 5.40 | 222 |
| R SupraMarginal Gyrus | + | 40 | 66 | −22 | 28 | 4.52 | 121 |
| Calcarine Gyrus | + | 17 | −3 | −99 | 7 | 5.76 | 517 |
| R Cerebellumd | + | 36 | −52 | −32 | 7.09 | 2698 | |
| L Cerebellumd | + | −42 | −52 | −29 | 4.95 | ||
| L STG | − | 22 | −48 | −25 | 4 | −4.61 | 104 |
| R STG | − | 22 | 54 | −16 | 4 | −5.52 | 169 |
| L IFG | − | 45 | −39 | 14 | 28 | −4.62 | 120 |
| R IFG | − | 45 | 45 | 29 | 22 | −3.71 | 58 |
| Pumps After Negative Feedback | |||||||
| dACC | + | 24/32 | 3 | 29 | 28 | 6.12 | 742 |
| L DLPFC | + | 9/46 | −30 | 56 | 19 | 5.57 | 420 |
| R DLPFC | + | 9/46 | 30 | 53 | 25 | 5.02 | 442 |
| L Insula | + | −45 | 14 | 5 | 4.88 | 537 | |
| L Caudate | + | −15 | −1 | 19 | 4.56 | ||
| L VS | + | −27 | 2 | −8 | 4.16 | ||
| R Insula | + | 51 | 17 | −5 | 5.23 | 556 | |
| R Caudate | + | 18 | 2 | 16 | 4.94 | ||
| R VS | + | 24 | 2 | −8 | 4.84 | ||
| L SupraMarginal Gyrus | + | 40 | −63 | −40 | 34 | 4.18 | 85 |
| Rectal Gyrus | − | 12 | 6 | 8 | −14 | −4.38 | 167 |
| Medial OFC | − | 11 | −9 | 26 | −23 | −3.99 | |
| L STG | − | 22 | −51 | −22 | 4 | −6.24 | 265 |
| R STG | − | 22 | 57 | −16 | 4 | −6.32 | 359 |
| L Occipital Lobe | − | 18/19 | −30 | −73 | 22 | −5.48 | 1148 |
| R Occipital Lobe | − | 18/19 | 39 | 085 | 25 | −4.69 | |
|
Pumps After Negative Feedback– Pumps After Positive Feedback |
|||||||
| MPFC | + | 8/9 | 0 | 41 | 49 | 5.02 | 205 |
| R SFG | + | 46 | 39 | 62 | 10 | 3.76 | 84 |
| R MTG | + | 21 | 69 | −31 | −8 | 4.10 | 105 |
| R VS | − | 9 | 11 | −11 | −5.38 | 352 | |
| Medial OFC | − | 11 | 6 | 38 | −17 | −3.53 | |
| L Postcentral Gyrus | − | 2/3 | −45 | −13 | 55 | −5.08 | 1294 |
| R MTG | − | 21 | 57 | 073 | 7 | −4.87 | 215 |
| R ITG | − | 20 | −54 | −58 | −11 | −3.66 | 142 |
| R Calcarine Gyrus | − | 17 | 24 | −64 | 19 | −5.59 | 2059 |
Note: L and R refer to left and right hemispheres; + and − refer to positive or negative activation; BA refers to Brodmann Area of peak voxel; k refers to the number of voxels in each significant cluster; t refers to peak activation level in each cluster; x, y, and z refer to MNI coordinates.
Superscripts (e.g. a, b, etc.) indicate that peak voxels are part of a contiguous cluster.
mPFC = Medial Prefrontal Cortex; ACC = Anterior Cingulate Cortex, dACC = dorsal ACC, DLPFC = Dorsolateral Prefrontal Cortex, STG = Superior Temporal sulcus, VS = Ventral Striatum, IFG = Inferior Frontal Gyrus, OFC = Orbitofrontal Cortex, ITG = Inferior Temporal Gyrus, SFG = Superior Frontal Gyrus, MTG = Middle Temporal Gyrus.
3.2.2 Risk-related neural responses following negative feedback
We then examined neural activity during risk decisions following negative feedback (i.e., explosion). In other words, what neural patterns of activation do adolescents show when making risky choices on a balloon after receiving a punishment? Adolescents demonstrated heightened activation in the dACC, bilateral anterior insula, bilateral VS, bilateral caudate, and bilateral DLPFC as well as decreased activation in regions of the subgenual ACC, orbitofrontal cortex (OFC), bilateral STG, bilateral occipital cortex, and left motor cortex (Table 1).
3.2.3 Differential responses following negative and positive feedback
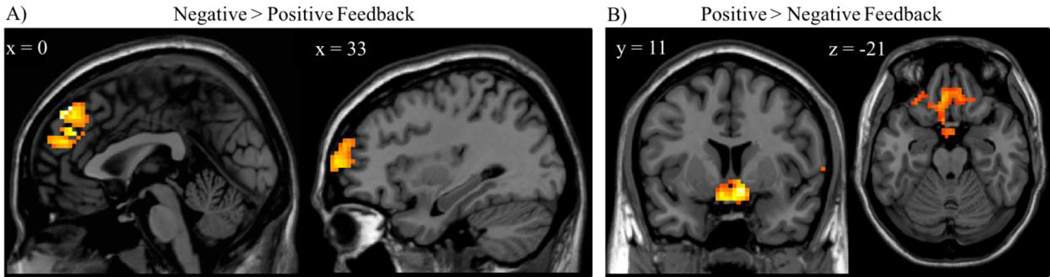
Next, we examined differences in neural activation during risk decisions after receiving negative relative to positive feedback. Adolescents demonstrated heightened activation to negative feedback in the mPFC, right superior frontal gyrus (SFG), and right middle temporal gyrus (MTG). Regions showing greater activation after positive feedback included the VS, OFC, bilateral inferior temporal gyrus (ITG), bilateral occipital cortex, and left motor cortex (Figure2; Table 1).
Figure 2.
Adolescents showed A) greater mPFC and R SFG activation to negative feedback and B) greater VS and OFC activation to positive feedback.
3.3 Associations between Neural Activation and Behavior
3.3.1 Behavioral sensitivity to negative versus positive feedback
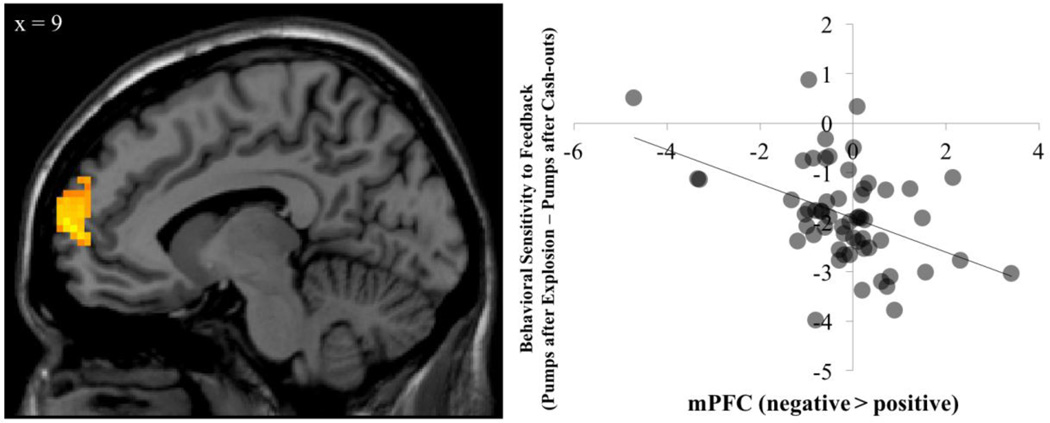
To examine how behavioral sensitivity to feedback is related to neural sensitivity to feedback, we entered our metric of differential behavioral sensitivity to negative versus positive feedback (i.e., pump behavior post-explosions minus pump behavior post-cash-outs) as a regressor in a whole-brain regression analysis on the contrast of interest (risk taking following negative feedback > positive feedback). We found that adolescents who showed fewer reductions in pump behavior post-explosions relative to post-cash-outs showed reduced sensitivity to negative (vs. positive) feedback in the mPFC, left DLPFC, bilateral posterior insula, and bilateral caudate (Figure 3; Table 2). This suggests that reduced neural sensitivity to negative feedback is associated with sustained risk taking (i.e., greater pumping behavior) after negative feedback.
Figure 3.
Adolescents who showed reduced mPFC sensitivity to negative versus positive feedback, also showed blunted behavioral sensitivity to negative feedback. (i.e., smaller reductions in pumping following an explosions compared to pumping after a cash-out).
Table 2.
Neural Regions Activated During Risky Decisions Following Negative-Positive Feedback that are Associated with Behavioral Measures
| Anatomical Region | +/− | BA | x | y | z | t | k |
|---|---|---|---|---|---|---|---|
|
Behavioral Sensitivity to Feedback |
|||||||
| R MPFC | + | 8/9 | 6 | 65 | 10 | 3.65 | 121 |
| R DLPFC | + | 9/46 | −45 | 47 | 19 | 4.05 | 101 |
| L Insula | + | −36 | −1 | 13 | 4.01 | 172 | |
| R Insula | + | 36 | −13 | 10 | 4.21 | 377 | |
| R Caudate | + | 12 | 11 | 22 | 3.75 | ||
| L Caudate | + | −6 | 17 | 16 | 3.32 | 55 | |
| Self-reported Risk-taking | |||||||
| R MPFC | + | 8/9 | 9 | 53 | 25 | 4.10 | 663 |
| R ACC | + | 24/32 | 3 | 35 | 13 | 3.63 | |
| R pre-SMA | + | 6/32 | 9 | 11 | 49 | 3.88 | 104 |
| R Caudate | + | 18 | 17 | 13 | 3.92 | 109 |
Note: L and R refer to left and right hemispheres; + and − refer to positive or negative activation; BA refers to Brodmann Area of peak voxel; k refers to the number of voxels in each significant cluster; t refers to peak activation level in each cluster; x, y, and z refer to MNI coordinates.
Superscripts (e.g. a, b, etc.) indicate that peak voxels are part of a contiguous cluster.
MPFC = Medial Prefrontal Cortex; DLPFC = Dorsolateral Prefrontal Cortex, SMA = Supplementary Motor Area.
To unpack which components of our contrasts were driving this effect, we decomposed our findings in two ways. First we examined risk taking following positive and negative feedback separately. We found that behavioral sensitivity to negative versus positive feedback is associated with reduced mPFC processing following negative but not positive feedback (see Table 3 for full results). Secondly, we split our behavioral metric of differential sensitivity into its two component parts (i.e. average pumps following positive feedback and average pumps following negative feedback). We then correlated each of these indices with neural activation during risk taking following positive and negative feedback respectively. Results indicate that our effects are being driven by the association between the average number of pumps following negative feedback and reduced mPFC activation following negative feedback (see Table 3 for full results). Average pumps following positive feedback is not associated with neural activation in the mPFC during risk taking following positive feedback These results indicate that reduced mPFC integration of negative feedback into future behavior has an important role in the maintenance of risky behavior following negative feedback.
Table 3.
Decomposition of Neural Contrasts Showing Significant Associations with Behavioral Measures
| Behavioral Measure |
Neural Contrast |
Anatomical Region | +/− | BA | x | y | z | t | k |
|---|---|---|---|---|---|---|---|---|---|
|
Behavioral Sensitivity to Feedback |
|||||||||
|
Pumps After Positive Feedback |
|||||||||
| R Insula | + | 42 | 17 | 1 | 3.98 | 88 | |||
| R DLPFC | + | 9/46 | 39 | 56 | 22 | 3.71 | 91 | ||
|
Pumps After Negative Feedback |
|||||||||
| L MPFC | − | 8/9 | −9 | 62 | 13 | 3.33 | 61 | ||
| R Posterior Insula | − | 36 | −13 | 10 | 4.12 | 129 | |||
|
Average Number of Pumps After Positive Feedback |
|||||||||
|
Pumps After Negative Feedback –Pumps After Positive Feedback |
|||||||||
| L Pons | − | −12 | −28 | −44 | 4.52 | 74 | |||
|
Average Number of Pumps After Negative Feedback |
|||||||||
|
Pumps After Negative Feedback –Pumps After Positive Feedback |
|||||||||
| MPFC | − | 8/9 | −3 | 62 | 4 | 4.37 | 819 | ||
| Medial OFC | − | 11 | 3 | 38 | −17 | 4.54 | |||
| R VS | − | 9 | 2 | −11 | 3.89 | ||||
| L Lateral OFC | − | 11 | −24 | 32 | −17 | 5.14 | 855 | ||
| L IFG | − | 45 | −54 | 17 | 4 | 3.24 | |||
| L Posterior Insula | − | −60 | −19 | 7 | 3.62 | ||||
| L Temporal Pole | − | 38 | −36 | 20 | −26 | 3.78 | |||
|
Self- reported Risk- taking |
|||||||||
|
Pumps After Positive Feedback |
|||||||||
| Medial OFC | + | 11 | −9 | 56 | −20 | 4.06 | 191 | ||
| Motor Cortex | + | 4 | −3 | 2 | 61 | 4.03 | 163 | ||
| R IFG | + | 45 | 54 | 26 | 31 | 3.85 | 144 | ||
| R Lingual Gyrus | + | 19 | 24 | −79 | 4 | 3.76 | 87 | ||
|
Pumps After Negative Feedback |
|||||||||
| R MPFC | − | 8/9 | 15 | 53 | 22 | 3.67 | 258 | ||
| R dACC | − | 24/32 | 9 | 35 | 16 | 3.97 | |||
| R Insula | − | 27 | 20 | 7 | 4.51 | 75 |
Note: Text in bold indicate behavioral regressors of interest; text in italics indicate neural contrasts. L and R refer to left and right hemispheres; + and − refer to positive or negative activation; BA refers to Brodmann Area of peak voxel; k refers to the number of voxels in each significant cluster; t refers to peak activation level in each cluster; x, y, and z refer to MNI coordinates.
Superscripts (e.g. a, b, etc.) indicate that peak voxels are part of a contiguous cluster.
MPFC = Medial Prefrontal Cortex; DLPFC = Dorsolateral Prefrontal Cortex; OFC = Orbitofrontal Cortex; IFG = Inferior Frontal Gyrus; dACC = Dorsal Anterior Cingulate Cortex.
3.3.2 Real-world risk taking
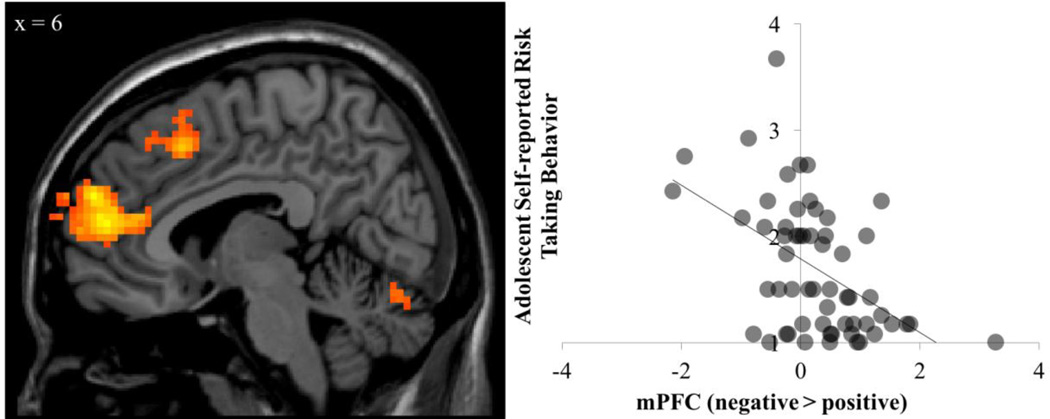
We next investigated how differential neural responding following negative versus positive feedback is associated with real-world risk-taking behavior in adolescents. To do this, we entered self-reported risk-taking behavior as a regressor in a whole-brain regression analysis on the contrast of interest (risk taking following negative feedback > positive feedback). Adolescents who engaged in greater risk-taking behavior showed less activation to negative versus positive feedback in the mPFC, right caudate, and pre-SMA (Figure 4; Table 2), suggesting that reduced neural sensitivity to negative feedback is related greater risk-taking behavior. Furthermore, when we decompose our contrast of interest (risk taking following positive and negative feedback separately), we find that these effects are driven by the association between self-reported risk-taking behavior and a decrease in mPFC activation following negative feedback but not positive feedback (see Table 3 for full results). Importantly, reduced mPFC sensitivity to negative feedback was linked to both task-based behavior and real-world risk-taking, suggesting that these processes depend on similar underlying neural processes.1
Figure 4.
Adolescents who showed reduced mPFC sensitivity to negative versus positive feedback, reported greater frequency of real-world risk-taking behaviors.
3.3.3 Links between task-based sensitivity to negative feedback and risk taking
Because of the overlapping regions found in the mPFC for both task-based sensitivity to negative feedback and real-world risk taking, we wanted to see if differential mPFC feedback sensitivity might link task and real-world behavior. We extracted parameter estimates of signal intensity from the mPFC from the voxels that showed overlap in the two sets of independent analyses. We then standardized our behavioral measures of interest in order to test for the indirect effect, using the methods outlined by Hayes (Hayes, 2013). We used 1000 sample bootstrapping to calculate the significance and magnitude of the indirect effect, in order to construct a bias-corrected confidence interval (CI). We tested whether our behavioral measure of sensitivity to negative feedback was associated with adolescents’ self-reported risk-taking behavior through a shared effect of dampened mPFC activation during risk taking following negative feedback relative to positive feedback. Results indicated a significant indirect effect (B=.09 SE=.05; 95% CI = [.02, .21]), relating reduced behavioral sensitivity to negative feedback and risk-taking behavior through reduced mPFC sensitivity to negative feedback, suggesting that adolescent’s blunted sensitivity to negative feedback contributes positively to increased risky behavior via blunted mPFC sensitivity to negative feedback.
3.3.4. Associations between neural sensitivity to feedback and adaptive task performance
Finally, we wanted to examine whether differential mPFC activation following negative versus positive feedback was related to adolescents’ earnings during the task. We extracted parameter estimates of signal intensity from the mPFC region showing differential sensitivity to negative > positive feedback, and correlated it with the total number of points adolescents earned during the task. We found a significant association between differential mPFC activation and total points earned (r=−.293, p=.03), such that adolescents who showed reduced mPFC activation following negative feedback were more likely to earn a higher number of points in the task. Thus, differential sensitivity to negative feedback contributes to both risk taking but also adaptive outcomes in the form of point acquisition.
Discussion
4.1
A majority of research on adolescent neurodevelopment and risk-taking behavior has focused on adolescent-specific increases in sensitivity to positive feedback (e.g., rewards; see Telzer, 2016). However, possible links between changes in sensitivity to negative feedback during adolescence and risk taking have received comparatively little attention. Moreover, previous research examining adolescent feedback sensitivity and risk taking has focused on neural responsivity to feedback receipt (e.g., van Duijvenvoorde et al., 2014). We took a novel approach by modeling risk decisions made after positive or negative feedback in order to examine how feedback information is integrated into on-going decision-making representations. We present the first evidence that blunted mPFC sensitivity to negative feedback supports risk-taking behavior during adolescence. This suggests that blunted sensitivity to negative feedback is an important component supporting increases in risk-taking seen during adolescence.
Our findings indicate that adolescents display neural sensitivity to differential forms of feedback. Adolescents showed robust neural differences when making decisions following negative versus positive feedback in regions involved in performance monitoring, reward evaluation, and cognitive regulation, suggesting that on-going risky decision-making representations take into account an individual’s history of reward and punishment. In particular, regions that showed greater sensitivity to positive feedback during subsequent decisions included the OFC and ventral striatum, which have been implicated in reward sensitivity and learning (Galvan et al., 2005; see Telzer, 2016; O’Doherty et al., 2001; Schoenbuam & Roesch, 2005; McCormick & Telzer, in press). This suggests that receiving positive feedback can potentiate future risky decisions by activating reward-related regions, perhaps increasing approach behaviors. In contrast, regions showing greater sensitivity to negative feedback during risky decision-making included the mPFC and SFG, which are involved in regulating decision-making processes. The mPFC is involved in integrating feedback information (van Noordt & Segalowitz, 2012; van Duijvenvoorde et al., 2014) and the SFG is involved in higher-level regulatory processes, including goal maintenance and behavioral regulation (Ridderinkhof et al., 2004; Braver et al., 2009). This suggests that decisions made after receiving negative feedback recruit greater neural regulation than those made after receiving positive feedback. Negative feedback may be signaling to the adolescents that a change in behavioral strategy is necessary to avoid future negative feedback, engaging regions important for implementing new behavioral patterns.
Our results reveal that adolescents who choose to reduce their pumping less after receiving negative feedback also show reduced neural sensitivity to negative feedback in a region of the mPFC that has been previously implicated in aspects of feedback integration and risk monitoring (Xu et al., 2009; van Noordt & Segalowitz, 2012; van Duijvenvoorde et al., 2014). This suggests that adolescents who show blunted mPFC and concomitant blunted behavioral sensitivity to negative feedback in subsequent decisions do so because they do not integrate the negative feedback into decision-making representations to the same degree. While adolescents who show more mPFC sensitivity to negative feedback during risky decision-making reliably reduce their pump behavior after explosion events, adolescents who show more-blunted mPFC sensitivity to negative feedback show relatively smaller reductions in their pump behaviors. This link between mPFC sensitivity and reductions in pumping behavior following negative feedback might represent an unconscious reduced sensitivity, whereby individual differences in intrinsic sensitivity to negative feedback drive differences in adolescents’ risky behavior. Adolescents showing reduced mPFC activation following negative feedback may also be less able to detect and adapt to error messages signaled by the negative feedback. Alternatively, adolescents may be consciously suppressing their sensitivity to negative feedback in order to pursue goal-directed behavior, namely pumping in order to earn more points. Future research should explore potential differences between adolescents’ intrinsic and goal-directed suppression of reaction to feedback.
We also found that reduced sensitivity to negative feedback in the mPFC was related to adolescents’ self-reported risk-taking behavior. Adolescents who showed reduced mPFC sensitivity to negative feedback reported increased engagement in real-world risk-taking behaviors. While considerable research has explored the role of positive feedback sensitivity in adolescent risky behavior (e.g., van Leijenhorst, Zanolie, et al., 2010; van Duijvenvoorde et al., 2014, see Telzer, 2016), we report the first data showing that reduced mPFC sensitivity to negative feedback also promotes risk taking. These data complement and extend previous research relating increased mPFC sensitivity to reward (versus loss) receipt with increased engagement in risk (van Duijvenvoorde et al., 2014). While prior work showed that greater mPFC reactivity to reward receipt promotes risk engagement (van Duijvenvoorde et al., 2014), we found that reduced mPFC sensitivity during risk decisions following negative feedback promotes risk taking behaviors. One difference may be in the negative stimuli used in the two studies; explosions in the current study were especially aversive, with loss of points being accompanied by a loud and sudden blast of noise. Additionally, van Duijvenvoorde and colleagues examined neural sensitivity to feedback events (i.e., reward and loss) themselves, while the current study examined neural sensitivity during risky decisions following different forms of feedback events, where adolescents could apply information they gleaned from the different forms of feedback. Despite these differences, both studies point to the fact that individuals who show reduced sensitivity to negative feedback, either in the moment or in subsequent decisions, are more likely to engage in risk-taking behaviors. Importantly, this discounting of negative feedback information likely interacts with increases in sensitivity to positive feedback adolescents may receive (e.g., peer-approval, positive sensations) to promote risk behavior. Increased sensitivity to positive feedback likely prompts increased adolescent approach motivation while decreased sensitivity to negative feedback then reduces motivation to avoid potential negative consequences. As such, changes in sensitivity to both types of feedback may create a feedback loop that works to escalate risk-taking and sensation-seeking behaviors during adolescence.
Finally, we found that reduced behavioral sensitivity to negative feedback is related to adolescent’s self-reported risk taking through reduced neural sensitivity to negative feedback in the mPFC. This association between risk-taking and reduced sensitivity to negative feedback implies that adolescents who engage in more risky behavior may be insensitive to potential negative outcomes they experience as a result of their risky decisions. Thus, when these adolescents make subsequent risky decisions, they may weigh negative feedback (e.g., parent/authority disapproval, health consequences) less in their decision-making representations (Ernst et al., 2006). While previous research focused on amygdala reactivity to negative feedback in the form of reward omission (Ernst et al., 2005), we found no differences in amygdala activation in decisions made after adolescents received negative feedback relative to after they received positive feedback. Instead, we found that differential mPFC sensitivity to negative versus positive feedback during decision-making predicted adolescent risk-taking behavior. This difference may be on account of the role of feedback for informing future behavior. While participants in Ernst and colleagues (2005) made choices and received feedback on a series of independent trials, adolescents in the current study made sequential decisions, where feedback on a given balloon transmitted important information for future risk behavior.
Our results offer a new insight into how adolescents integrate feedback information into future decisions. Adolescence is a developmental window during which feedback processing can have important implications for health and achievement outcomes. Our results offer a new insight into how adolescents integrate feedback information into future decisions. However, additional work should investigate whether sensitivity to negative feedback is limited to adolescence by examining feedback-related changes in risky decision-making and links to real-work risk behavior in both children and adults. This extension of the current study will help to clarify the specificity of these feedback-related processes to adolescence. Additionally, while the current study focused on feedback sensitivity during risk-taking behavior, we did not have information on some important individual difference measures (e.g., IQ, SES, clinical psychopathology, etc.) that may interact with these feedback-related processes in risky decision-making. Furthermore, given the important role of social context (i.e., peers, parents) in adolescent risk taking (e.g. Chein et al., 2011; Telzer et al., 2015), future work should build on the current study by examining the effect of social information on these feedback-related processes. Finally, due to the nature of participants’ behavior on the BART, the number of trials in each condition (i.e., post-positive and post-negative feedback) are not balanced. While controlling for each participants’ number of post-negative feedback trials does not change the reported findings, future research examining these processes should take steps to make these conditions more-equal.
In summary, we took a novel approach by examining how adolescents integrate feedback to inform their risky decisions in real time, and how differential neural sensitivity following negative versus positive feedback promotes risky behavior. We found that blunted sensitivity to negative feedback in a region of mPFC previously implicated in outcome evaluation (van Duijvenvoorde et al., 2014) and risk indexing (van Leijenhorst, Moor, et al., 2010) was related to greater risky behavior in the face of negative feedback and engagement in more real-world risk taking. While reward (i.e., positive feedback) sensitivity has received much attention in the study of adolescent risk taking, results from the current study highlight the fact that the emergence and maintenance of risky behavior likely involves complex changes in how adolescents react to both positive and negative feedback. Additionally, the current study points to the importance of not simply examining neural reactivity to the delivery of positive or negative feedback, but how individuals integrate and implement those neural representations into future decision-making processes.
Acknowledgments
We greatly appreciate the assistance of the Biomedical Imaging Center at the University of Illinois. This research was supported by a grant from the National Institutes of Health (R01DA039923) and generous funds from the Department of Psychology at the University of Illinois.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: E.H.T designed research; E.H.T performed research; E.M.M. and E.H.T. analyzed data; E.M.M. and E.H.T. wrote the paper.
The authors declare no competing financial interests.
To control for differences between the number of post-positive and post-negative feedback, main effect and regression analyses were also run including each participants’ number of post-negative feedback trials as a covariate. Results were unchanged with this addition, so we removed the covariate for parsimony.
References
- Alexander CS, Kim YJ, Ensminger M, Johnson KE, Smith BJ, Dolan LJ. A measure of risk taking for young adolescents: Reliability and validity assessments. Journal of Youth and Adolescence. 1990;19(6):559–569. doi: 10.1007/BF01537176. [DOI] [PubMed] [Google Scholar]
- Braams BR, van Duijvenvoorde AC, Peper JS, Crone EA. Longitudinal changes in adolescent risk-taking: a comprehensive study of neural responses to rewards, pubertal development, and risk-taking behavior. The Journal of Neuroscience. 2015;35(18):7226–7238. doi: 10.1523/JNEUROSCI.4764-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Paxton JL, Locke HS, Barch DM. Flexible neural mechanisms of cognitive control within human prefrontal cortex. Proceedings of the National Academy of Sciences. 2009;106(18):7351–7356. doi: 10.1073/pnas.0808187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA. The adolescent brain. Annals of the New York Academy of Sciences. 2008;1124(1):111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauffman E, Shulman EP, Steinberg L, Claus E, Banich MT, Graham S, Woolard J. Age differences in affective decision making as indexed by performance on the Iowa Gambling Task. Developmental Psychology. 2010;46(1):193. doi: 10.1037/a0016128. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, Pine DS. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25(4):1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine. 2006;36(03):299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabard-Durnam LJ, Flannery J, Goff B, Gee DG, Humphreys KL, Telzer E, Tottenham N. The development of human amygdala functional connectivity at rest from 4 to 23years: A cross-sectional study. NeuroImage. 2014;95:193–207. doi: 10.1016/j.neuroimage.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A. The Teenage Brain Sensitivity to Rewards. Current Directions in Psychological Science. 2013;22(2):88–93. doi: 10.1177/0963721413476512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Davidson M, Spicer J, Glover G, Casey BJ. The role of ventral frontostriatal circuitry in reward-based learning in humans. The Journal of Neuroscience. 2005;25(38):8650–8656. doi: 10.1523/JNEUROSCI.2431-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare T, Voss H, Glover G, Casey BJ. Risk-taking and the adolescent brain: who is at risk? Developmental science. 2007;10(2):F8–F14. doi: 10.1111/j.1467-7687.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, Tottenham N. A developmental shift from positive to negative connectivity in human amygdala–prefrontal circuitry. The Journal of Neuroscience. 2013;33(10):4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Rapoport JL. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN. The neural basis of decision making. Annual Review of Neuroscience. 2007;30:535–574. doi: 10.1146/annurev.neuro.29.051605.113038. [DOI] [PubMed] [Google Scholar]
- Gorgolewski KJ, Varoquaux G, Rivera G, Schwarz Y, Ghosh SS, Maumet C, Yarkoni T. NeuroVault.org: A web-based repository for collecting and sharing unthresholded statistical maps of the human brain. Frontiers in Neuroinformatics. 2015;9:8. doi: 10.3389/fninf.2015.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser TU, Iannaccone R, Ball J, Mathys C, Brandeis D, Walitza S, Brem S. Role of the medial prefrontal cortex in impaired decision making in juvenile attention-deficit/hyperactivity disorder. JAMA Psychiatry. 2014;71(10):1165–1173. doi: 10.1001/jamapsychiatry.2014.1093. [DOI] [PubMed] [Google Scholar]
- Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. Guilford Press; 2013. [Google Scholar]
- Humphreys KL, Telzer EH, Flannery J, Goff B, Gabard-Durnam L, Gee DG, Tottenham N. Risky decision making from childhood through adulthood: contributions of learning and sensitivity to negative feedback. Emotion. 2016;16(1):101–109. doi: 10.1037/emo0000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, Brown RA. Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task (BART) Journal of Experimental Psychology: Applied. 2002;8(2):75. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- McCormick EM, Telzer EH. Adaptive adolescent flexibility: Neurodevelopment of decision-making and learning in a risky context. Journal of Cognitive Neuroscience. doi: 10.1162/jocn_a_01061. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neuroscience. 2001;4(1):95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Petersen A, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17(2):117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Qu Y, Galvan A, Fuligni AJ, Lieberman MD, Telzer EH. Longitudinal changes in prefrontal cortex activation underlie declines in adolescent risk taking. The Journal of Neuroscience. 2015;35(32):11308–11314. doi: 10.1523/JNEUROSCI.1553-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao H, Korczykowski M, Pluta J, Hoang A, Detre JA. Neural correlates of voluntary and involuntary risk taking in the human brain: an fMRI Study of the Balloon Analog Risk Task (BART) NeuroImage. 2008;42(2):902–910. doi: 10.1016/j.neuroimage.2008.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, Van Den Wildenberg WP, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain and Cognition. 2004;56(2):129–140. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch M. Orbitofrontal cortex, associative learning, and expectancies. Neuron. 2005;47(5):633–636. doi: 10.1016/j.neuron.2005.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Casey BJ. A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain and Cognition. 2010;72(1):124–133. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. Rewards, aversions and affect in adolescence: emerging convergences across laboratory animal and human data. Developmental Cognitive Neuroscience. 2011;1(4):390–403. doi: 10.1016/j.dcn.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Albert D, Cauffman E, Banich M, Graham S, Woolard J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: evidence for a dual systems model. Developmental Psychology. 2008;44(6):1764. doi: 10.1037/a0012955. [DOI] [PubMed] [Google Scholar]
- Telzer EH. Dopaminergic reward sensitivity can promote adolescent health: A new perspective on the mechanism of ventral striatum activation. Developmental Cognitive Neuroscience. 2016;17:57–67. doi: 10.1016/j.dcn.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Fuligni AJ, Lieberman MD, Galván A. Meaningful family relationships: neurocognitive buffers of adolescent risk taking. Journal of Cognitive Neuroscience. 2013;25(3):374–387. doi: 10.1162/jocn_a_00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Fuligni AJ, Lieberman MD, Miernicki ME, Galván A. The quality of adolescents’ peer relationships modulates neural sensitivity to risk taking. Social Cognitive and Affective Neuroscience. 2015;10(3):389–398. doi: 10.1093/scan/nsu064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bos W, Cohen MX, Kahnt T, Crone EA. Striatum–medial prefrontal cortex connectivity predicts developmental changes in reinforcement learning. Cerebral Cortex. 2012;22(6):1247–1255. doi: 10.1093/cercor/bhr198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Schaaf ME, Warmerdam E, Crone EA, Cools R. Distinct linear and non-linear trajectories of reward and punishment reversal learning during development: Relevance for dopamine's role in adolescent decision making. Developmental cognitive neuroscience. 2011;1(4):578–590. doi: 10.1016/j.dcn.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Duijvenvoorde AC, de Macks ZAO, Overgaauw S, Moor BG, Dahl RE, Crone EA. A cross-sectional and longitudinal analysis of reward-related brain activation: effects of age, pubertal stage, and reward sensitivity. Brain and Cognition. 2014;89:3–14. doi: 10.1016/j.bandc.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Van Duijvenvoorde AC, Zanolie K, Rombouts SA, Raijmakers ME, Crone EA. Evaluating the negative or valuing the positive? Neural mechanisms supporting feedback-based learning across development. The Journal of Neuroscience. 2008;28(38):9495–9503. doi: 10.1523/JNEUROSCI.1485-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leijenhorst L, Moor BG, de Macks ZAO, Rombouts SA, Westenberg PM, Crone EA. Adolescent risky decision-making: neurocognitive development of reward and control regions. NeuroImage. 2010;51(1):345–355. doi: 10.1016/j.neuroimage.2010.02.038. [DOI] [PubMed] [Google Scholar]
- Van Leijenhorst L, Zanolie K, Van Meel CS, Westenberg PM, Rombouts SA, Crone EA. What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cerebral Cortex. 2010;20(1):61–69. doi: 10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- Van Noordt SJ, Segalowitz SJ. Performance monitoring and the medial prefrontal cortex: a review of individual differences and context effects as a window on self-regulation. Frontiers in Human Neuroscience. 2012;6:197. doi: 10.3389/fnhum.2012.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue G, Lu Z, Levin IP, Weller JA, Li X, Bechara A. Functional dissociations of risk and reward processing in the medial prefrontal cortex. Cerebral Cortex. 2009;19(5):1019–1027. doi: 10.1093/cercor/bhn147. [DOI] [PMC free article] [PubMed] [Google Scholar]