Abstract
The placenta serves as the interface between the maternal and fetal circulations and regulates the transfer of oxygen, nutrients, and waste products. When exogenous substances are present in the maternal bloodstream—whether from environmental contact, occupational exposure, medication, or drug abuse—the extent to which this exposure affects the fetus is determined by transport and biotransformation processes in the placental barrier. Advances in drug delivery strategies are expected to improve the treatment of maternal and fetal diseases encountered during pregnancy.
Keywords: placenta, pregnancy, transporters, biotransformation
Graphical abstract
1. Introduction
Although most pregnant women use medications during pregnancy, the majority of drug trials to date have excluded the enrollment of pregnant women [1]. It is imperative that more studies be carried out in order to fully assess the risks of fetal exposure to these medications. A better understanding of the function of the placenta in controlling the maternal-fetal transfer of drugs will help scientists and clinicians to make informed decisions to improve the health of new moms and newborns.
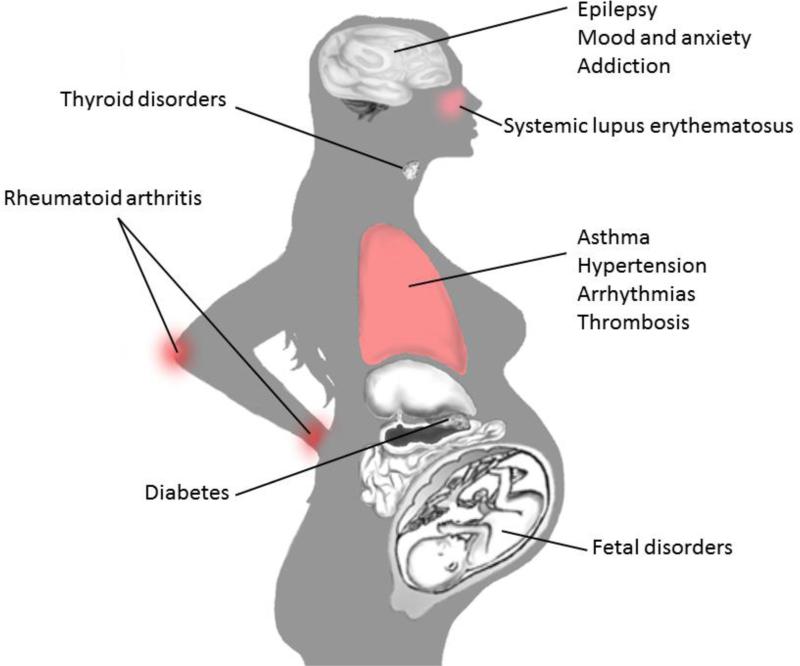
Given the risks of thalidomide and other teratogenic substances, some pregnant women may be reluctant to use any medications. However, for women with chronic conditions, or women who develop a temporary febrile illness, medications which maintain maternal health can improve neonatal outcomes [2,3]. In 2008, more than 93% of pregnant women took at least one over-the-counter or prescription medication at any time during pregnancy, and more than 50% took 4 or more medications [4]. Figure 1 highlights some disorders that may require medication during pregnancy. Between 2004–2008, the most common drugs prescribed to pregnant women included antibiotics, albuterol, progesterone, levothyroxine, and ondansetron [4]. From data collected between 1997-2004, the most common over-the-counter medications used during pregnancy were acetaminophen, ibuprofen, and pseudoephedrine [5]. Drugs of abuse are also of special concern, as data from 2013 report that 15.4% of women smoked, 9.4% drank alcohol, and 5.4% used illicit drugs during pregnancy [6].
Figure 1.
Selected disorders that may require pharmacotherapy during pregnancy.
The potential effects of drugs on fetal development are dependent on various factors, including gestational age, dose, dosing frequency, route of administration, and drug clearance [7,8]. Therefore, it is important to consider those physiological changes associated with the progression of pregnancy which can alter the pharmacokinetics of various drugs. Together with an increase in maternal blood volume during pregnancy, maternal serum albumin concentrations decrease. For drugs with high protein binding, the decreased albumin concentrations may result in a higher proportion of free drug, and hence, greater bioactivity [9,10]. The pH of maternal arterial blood increases slightly during pregnancy. This results in a shift of the oxy-hemoglobin dissociation curve, promoting the dissociation of oxygen and its transplacental transfer. This change in pH could also affect drug-protein binding. Pregnancy-associated increases in glomerular filtration can accelerate the renal clearance of many medications. Drug absorption during pregnancy could be reduced by progesterone-induced delays in gastric emptying, nausea and vomiting, or an increase in gastric pH [9]. The expression and function of drug metabolizing enzymes can also change significantly during pregnancy, some as the result of increased estrogen levels [11]. It is important to consider whether dose adjustments would be necessary to account for the pregnancy-associated changes affecting the pharmacokinetics of certain medications [12].
This review focuses on drug transport processes within the placenta which determine maternal-to-fetal transfer rates. Although the placenta is unlikely to prevent completely the transfer of typical small molecule compounds, the placenta may reduce the transfer of certain drugs based on properties such as size, lipophilicity, and affinity for transporter proteins [13]. Following a summary of human placental structure and function, mechanisms of drug transport across the placenta and drug metabolizing enzymes within the placenta will be discussed. Continued research and advances in these areas will lead us to a greater understanding and ability to control drug delivery across the placenta in order to improve the treatment of maternal and fetal diseases encountered during pregnancy.
2. Placental structure and function
2.1 Development of the maternal-fetal interface
The placenta is a unique organ of fetal origin that provides nutrients and oxygen to the developing fetus and also serves as the avenue for carbon dioxide and other fetal waste products to be eliminated via the maternal circulation [14]. The placenta starts to develop soon after blastocyst implantation. The outer blastocyst trophoblast cells facing the uterine epithelium fuse to form multinucleated syncytiotrophoblast. Proliferation of the syncytiotrophoblast comes about by fusion of precursor cytotrophoblast cells. Lacunae emerge within the interior of the trophoblastic complex and trophoblast invasion leads to the remodeling of maternal spiral arteries within the uterine wall. As the maternal endometrial vessel walls are eroded, maternal blood cells reach the lacunae. Arterial inlets into the lacunar system and venous outlets from the lacunae are established, and branching of trophoblast cells into the lacunar spaces result in the formation of villous trees. The lacunar spaces become the intervillous space where maternal blood flows among the villous trees. Fetal capillaries and larger vessels carry oxygenated blood from the villi to the umbilical vein, and deoxygenated fetal blood is returned from the fetus to the placental villi via the umbilical arteries. Maternal blood in the intervillous space and fetal blood in the villous capillaries are separated by a continuous layer of syncytiotrophoblast, a discontinuous layer of cytotrophoblast cells, basal lamina, connective tissue, and fetal endothelial cells [15,16].
The placenta undergoes several changes as pregnancy progresses. The ratio of cytotrophoblast to syncytiotrophoblast decreases with time; for example, in the second month of pregnancy, a complete layer of cytotrophoblast cells lines the syncytiotrophoblast. However, the previously cuboidal cytotrophoblast cells lose their thickness and the cytotrophoblast layer is less continuous at term. At that point, syncytiotrophoblasts account for 86% of the total villous trophoblast cell mass. Overall, the placental barrier becomes thinner over time. The distance separating the maternal circulation from the fetal circulation decreases from 50-100 μm in the second month to only 4-5 μm at term, and the total syncytiotrophoblast surface area increases from approximately 5 m2 at 28 weeks of gestation to 12 m2 at term [13,15–17]. The expression and cellular distribution of placental transporter proteins may also differ in early versus late pregnancy [18].
2.2 Experimental models and methods to study human placental drug transport
Maternal blood and cord blood samples collected at the time of delivery provide valuable information regarding the extent of fetal exposure to drugs present in the maternal circulation during pregnancy. Nevertheless, it is important to recognize certain pharmacokinetic variables when interpreting the fetal-to-maternal ratios obtained from these samples. First, the amount of time that had passed between the last dose of a drug and sampling of the maternal plasma is more likely to affect the maternal concentration than the effect of time on the cord blood concentration of the drug. Perhaps due to a reduced fetal capacity for biotransformation or processes effecting the transfer of drugs across the placenta in the fetal-to-maternal direction, the maternal clearance of some medications is substantially faster than fetal clearance, which means that the maternal levels of a drug would decline more rapidly than fetal levels [12,19]. If a maternal plasma sample is taken soon after the dose was administered, the maternal level would be high, whereas if the sample were taken several hours after the dose, the maternal level would be much lower. If concentrations of that same drug in the fetal circulation do not decline as rapidly, this would result in a lower fetal-to-maternal ratio when sampling closely followed the dose, but a higher fetal-to-maternal ratio if the sampling time is much later. A second variable important to consider when interpreting fetal-to-maternal ratios is whether a single dose of the medication was given or steady state had been reached due to repeated and regular dosing. Fetal concentrations of the drug could be higher at steady state than after a single dose due to the accumulation of a drug that cannot be as rapidly cleared from the fetal circulation. Therefore, fetal-to-maternal ratios could be higher at steady state than following a single dose [12].
Experimental models to gather more data on placental drug transfer kinetics than is possible with single time point sampling at delivery include ex vivo dual perfusion of human placental lobule, placental explants, membrane vesicles, primary cells, and cell lines. Although the focus of this review is upon human placenta, it should be noted that additional data can also be obtained by means of animal studies; in this case, care should be taken when extrapolating the findings to human data, due to differences in placental structure, blood flow, as well as any differences in the expression and function of transporters and enzymes [20,21]. For example, in guinea pig, rabbit, mouse, and rat placentae, where blood flow is countercurrent, the transfer rate of hydrophilic substances is permeability limited, but the transfer rate of hydrophobic substances is flow limited. However, the same pattern does not always apply to the diffusion of substances across the multivillous human placenta [20,22].
The ex vivo dually perfused human placental lobule system (DPPL) provides the most complete experimental model to predict placental transport and metabolism because it retains the anatomical and functional integrity of the tissue [23]. In the ex vivo placental perfusion model, tubing is cannulated into fetal vessels corresponding to a single lobule of a placenta obtained immediately following delivery. Tubing is likewise inserted in the space left by the maternal spiral arteries on the opposite side of the placenta. Maternal and fetal perfusate flow rates are controlled by pumps and gassed with O2, CO2, and N2 to maintain physiological levels. The system is maintained at 37°C, and oxygen transfer, glucose consumption, pH, and fetal volume loss are all monitored to ensure continuity of placental function. Antipyrine transport is often used as a control to confirm proper experimental setup and to normalize differences between placentas [24]. During the perfusion experiments, both maternal and fetal concentrations can be sampled at multiple time points. Studies have shown that functional characteristics of the placenta are retained for several hours after delivery [25,26].
Placental explants can be used to determine drug uptake and metabolism, as well as to evaluate the effects of exogenous substances on placental viability or endocrine function. Explants from term placental tissue can be maintained at 8% O2 and studied for up to 11 days. They can be cultured on the bottom of a well or on a supportive mesh. Out of concern for microbial contamination, the explants should be monitored closely for plasma membrane integrity and for any abnormalities in morphology. Tests to verify viability may include release of hCG, human placental lactogen, lactate dehydrogenase and alkaline phosphatase, lactate production, glucose consumption, trypan blue or inulin exclusion, and mitochondrial function assays such as JC-1 or MTT [27].
Placental membrane vesicles represent another experimental model which can be especially valuable in determining the activity of efflux and uptake transporters localized on apical and basal membranes of syncytiotrophoblast cells. Apical and basal membrane vesicles can be prepared by differential centrifugation according to established protocols [28–30]. Some of the vesicles will be oriented inside-out, as identified by acetylcholinesterase activity [31]. The activity of uptake transporters can be characterized by means of substrate entrapment into right-side out vesicles. On the other hand, entrapment of efflux transporter substrates into the core of inside-out vesicles represents a unique method to characterize efflux transporter activity. While the activity of efflux transporters is determined in the presence of ATP, the activity of transport via some organic anion transporters can be assessed in the presence of α-ketoglutarate [28,32,33].
Primary cytotrophoblast cells can be isolated from a placenta shortly after delivery. Villous tissue is trypsinized and then layered onto a Percoll gradient to obtain the density fraction containing the cytotrophoblast cells. Further purification can be achieved by means of immunomagnetic separation. Villous cytotrophoblast cells do not express HLA class Ia molecules, so other cells resulting from the tissue digestion and centrifugation steps can be removed using magnetic beads bound to the HLA class-I-positive cells. As the cell mixture passes through a magnetic column, the cytotrophoblast cells pass through while the non-cytotrophoblast cells remain in the column. Although primary isolated cytotrophoblast cells do not proliferate in culture, they may syncytialize spontaneously. They can be used in uptake studies and toxicological assays [34–36].
Immortalized human placental trophoblast cell lines include JEG-3 cells, JAr cells, and BeWo cells, each derived from choriocarcinoma cells. Similar to primary trophoblast cells isolated from term placentas, JEG-3 cells, JAr cells, and BeWo cells all express the efflux transporters MDR1, MDR3, MRP1, MRP2, MRP3, MRP4, and BCRP [37,38]. Likewise, the facilitative glucose transporters GLUT1 and GLUT3 are expressed in the JEG-3, JAr, and Bewo cell lines [39–41]. BeWo cells secrete hCG, human placental lactogen, progesterone, and estradiol, and cell fusion can be induced by forskolin or cyclic adenosine monophosphate [42]. Unlike the parent BeWo cell line, the b24 and b30 clones form confluent monolayers which can be utilized for drug transport studies [43,44]. Culturing JEG-3 cells with medium containing acidic fibroblast growth factor can reduce paracellular transport and increase transepithelial electrical resistance as compared to normal JEG-3 culture protocols [45].
3. Mechanisms of drug transport across the placenta
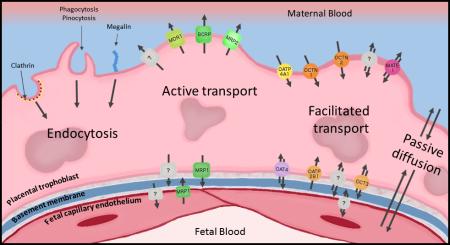
Any drugs, xenobiotics, or endogenous compounds that cross the placenta and enter the fetal circulation must cross the syncytiotrophoblast, basement membrane, and the fetal capillary endothelium. The route that these compounds can take to cross the placenta can be energy dependent or energy independent, and depend largely on their physical and chemical properties [46–48]. Furthermore, interindividual differences in transport can be influenced by epigenetic changes, genetic differences, differences in protein expression, and maternal and fetal health [47]. Figure 2 provides a visual overview of some of the major transport processes in the placental barrier.
Figure 2.
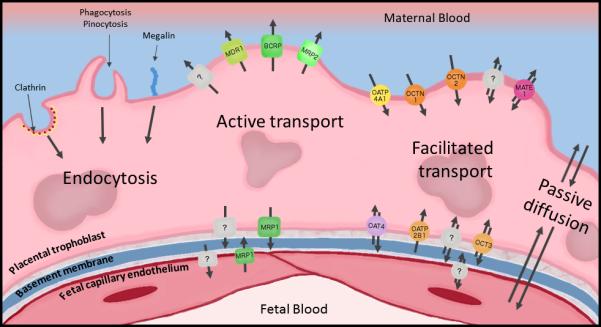
Highlighted transport processes within the human placental barrier. It should be noted that this does not represent an exhaustive list of transporters present in the placental trophoblast or fetal capillary endothelium. Trophoblast cells represent the rate-limiting barrier separating maternal and fetal circulations within the placenta. MDR1, multidrug resistance protein 1 (P-glycoprotein); BCRP, breast cancer resistance protein; MRP2, multidrug resistance-associated protein 2; OATP4A1, organic anion transporting polypeptide 4A1; OCTN1, novel organic cation transporter 1; OCTN2, novel organic cation transporter 2; MATE1, multidrug and toxin extruding protein 1; MRP1, multidrug resistance-associated protein 1; OAT4, organic anion transporter 4; OATP2B1, organic anion transporting polypeptide 2B1; OCT1, organic cation transporter 1; OCT3, organic cation transporter 3. Question marks indicate transport processes requiring further characterization.
3.1 Passive diffusion
Passive diffusion of a drug across the placenta is favored for a drug that is lipophilic, allowing it to cross the phospholipid bilayer. Lipophilicity of the compound, typically measured by the octanol/water partition coefficient, affects the partitioning of the drug between the extracellular aqueous environment and the phospholipid bilayer [49]. Smaller compounds tend to cross the placenta more readily, with compounds having a molecular weight less than 500 Da crossing the most [46]. Being that this process is not ATP-dependent and not dependent on the presence of a membrane protein facilitator, compounds are more likely to move down a concentration gradient [46]. An example of this type of movement is the transplacental transfer of antipyrine, a widely used internal standard and marker in ex vivo placental perfusions [50]. It freely diffuses across the placenta and has little tissue accumulation, allowing the equilibration of antipyrine concentrations between maternal and fetal compartments to be considered an indicator of experimental validity [50]. Many other drugs have been shown, at least partially, to passively diffuse across the placenta, including lidocaine, azidothymidine, warfarin, and others [51–53]. In some of these cases, it is still unclear the extent of the role that placental transporters play in their passage across the placenta [52].
Ionizable compounds may have pH-dependent accumulation in the fetal compartment. In their ionized state, compounds are much less lipophilic and are therefore less likely to cross the lipid bilayer membrane. Generally, the pH of the fetal circulation is somewhat lower than the pH of the maternal circulation, which can change the proportion of ionized to non-ionized species, effectively trapping or excluding them from one compartment or another [46].
Endogenous compounds may also enter the fetal circulation by this route. It has been shown in the placental perfusion model that that gangliosides GM3 and GD3 are rapidly taken up by the placenta [54], though the authors speculated that GD3 was metabolized during the perfusion and that more studies needed to be conducted to elucidate the precise mechanisms of uptake.
It was widely accepted that high plasma protein binding of some drugs preclude their diffusion across the placenta, and that drugs must dissociate from plasma proteins before they can enter the apical syncytiotrophoblast membrane [55,56]. Nevertheless, it has been shown that albumin and other proteins may enter the syncytiotrophoblast through endocytosis mechanisms [57].
3.2 Facilitated diffusion
Membrane proteins may allow passage of certain compounds across the placenta without directly hydrolyzing ATP, and for the purposes of this review they will be categorized as transporters that allow facilitated diffusion of compounds. These transporters, though not directly able to hydrolyze adenosine triphosphate (ATP), may function based on energy dependent concentration or ionic gradients [48,58], though the precise mechanisms for many of them are unknown. The largest and most studied groups of these transporters in the placenta include members of the Solute Carrier Family, including organic anion transporters (OATs), organic anion transporting polypeptides (OATPs), organic cation transporters (OCTs and OCTNs), multidrug and toxin extruding protein 1 (MATE1), and nucleoside transporters (NTs) [48].
OAT4 is expressed on the basolateral membrane of the syncytiotrophoblast [58]. OAT4 is capable of bidirectional transport and has broad specificity, as evidenced by its involvement in the transport of endogenous and exogenous compounds. It has been shown that OAT4 is involved in the transport of 16-α-hydroxydehydroepiandrosterone sulfate, a metabolite of dehydroepiandrosterone sulfate, into the syncytiotrophoblast for estriol synthesis [32], as well as the transport of estrone-3-sulfate. OAT4 is responsible for the transport of exogenous compounds including olmesartan [33], perfluoroalkyl acids [59], and others [48]. Interestingly, OAT4 transporter function is enhanced in the presence of ions or ionic gradients, with an inwardly directed chloride gradient increasing substrate transport out of the syncytiotrophoblast, and transport function generally being reduced in the absence of sodium [32,33]. The mechanism by which the presence of ions affects the function of the transporter has yet to be elucidated.
The two members of the OATP family observed in placental trophoblast that have been most studied to date are OATP2B1 and OATP4A1 [48,58]. Readers are referred to [60] for translating between former and current OATP nomenclature. OATP2B1 is expressed on the basolateral surface of the syncytiotrophoblast and is responsible for the uptake of anions from the fetal circulation into the trophoblast cells [61]. Like OAT4, this transporter is involved in the transport of estrone sulfate and dehydroepiandrosterone sulfate, and this process may be sodium dependent [62]. It has also been proposed that the transport of glutamate may also be coupled to anion transport by both OATP2B1 and OAT4, though this may need to be studied with more substrates [63]. OATP4A1, on the other hand, is expressed on the apical membrane of the syncytiotrophoblast [58,61]. To date, little is known regarding the substrate profile or specificity of this transporter. It was reported that OATP4A1 may be involved in hormone transport, but studies are required to determine substrates, dependence on ionic gradients or exchanger activity, and if it works in conjunction with any other transporters [48,64,65].
Organic cation transporter 3 (OCT3) is expressed on the basolateral membrane of the syncytiotrophoblast throughout pregnancy, though its expression may be variable at different gestational ages [48,61,66]. OCT3 has been shown to be involved in acetylcholine release from the placenta [67]. OCT3 is also involved in catecholamine transport, though this happens to a larger extent by monoamine transporters [68]. Interestingly, the expression of OCT3 was found to be significantly lower in patients with preeclampsia. It was postulated that these changes during preeclampsia may be related to alterations in placental blood flow [69]. In terms of xenobiotics in the placenta, OCT3 is involved in transport of metformin in the kidney and is implicated in the transplacental transfer of metformin, working together with the multidrug and toxin extrusion 1 protein (MATE1). Another compound that is commonly used experimentally as an OCT3 substrate is 1-methyl-4-phenylpyridinium (MPP+). This cationic compound is frequently used to inhibit the MATE1/OCT3 transport pathway [66,70,71].
MATE1 has been known to work in conjunction with OCT3 in both the placenta and in renal tissues. This transporter is heavily affected by pH, leading to the theory that transport by this protein works by proton exchange [72]. A similar mechanism of organic cation transport has been proposed to be at work in the placenta, with MATE1 on the apical membrane of the syncytiotrophoblast and OCT3 on the basolateral membrane, both pumping organic cations into the maternal circulation. More studies must be conducted to verify the validity of this transport scheme and its effect on maternal and fetal xenobiotic concentrations [66].
Another set of organic cation transporters (OCTN1, OCTN2, and OCTN3), are expressed on the apical membrane of the syncytiotrophoblast [48,61]. These transporters most notably function as carnitine transporters. It was shown that OCTN2 is expressed in the apical membrane of the syncytiotrophoblast in much higher amounts than the basolateral membrane using membrane vesicles. OCTN2 is involved in the transport of carnitine from the maternal circulation to the fetal circulation. Transplacental carnitine transfer is significant because the fetus alone cannot synthesize sufficient amounts of this nutrient, which assists in the oxidation of fatty acids in the mitochondria. Carnitine deficiency may cause cardiomyopathy, muscle weakness, hypoglycemia, and sudden infant death [73]. The expression of OCTN2 starts early and is maintained throughout pregnancy [74]. Carnitine transport may be impaired in preeclampsia. It was shown in BeWo cells that OCTN2 transcription is increased under hypoxic conditions, but OCTN2 protein expression was unchanged and carnitine transport was impaired [75]. Another study showed a similar finding in placental samples from patients with preeclampsia, which complements the higher maternal carnitine levels found in pregnant patients [76]. It was also shown in BeWo cells that induction of syncytialization (which is impaired in preeclampsia) by forskolin treatment increased OCTN2 expression [77]. These data together support the involvement of OCTN2 in altered fetal carnitine delivery during preeclampsia.
OCTN1 is also involved in carnitine transport, and is found in a variety of tissues including the apical membrane of the placental syncytiotrophoblast [48,78]. This transporter has multiple cationic substrates, and its function is dependent on the presence of a proton gradient [79]. However, the functional relevance of this transporter in the human placenta is not yet fully understood.
3.3 Active transport
Energy-dependent drug and xenobiotic transport is largely governed ATP-binding cassette (ABC) proteins. This family of transporters contains 7 subfamilies and some of these transporters have been shown to be functionally expressed in the placenta, on both the apical and basolateral membranes. These transporters work by using the energy released by ATP hydrolysis to pump drugs and xenobiotics to one side of a membrane, often against a concentration gradient [48,61]. These transporters include P-glycoprotein (P-gp), breast cancer resistance protein (BCRP), and multidrug resistance-associated proteins (MRPs).
P-glycoprotein, also known as MDR1 (ABCB1), is perhaps the most well studied of all of the active placental transporters due to its diverse substrate profile [46]. It is expressed on the apical membrane of the syncytiotrophoblast [50,80]. P-gp is involved in the efflux of both endogenous and exogenous compounds. Cortisol, a well-known endogenous glucocorticoid, was shown to be effluxed by P-gp in a choriocarcinoma cell line [81]. P-gp is also known to prevent placental passage of dexamethasone, a synthetic glucocorticoid, as well as a number of other drugs including verapamil, paclitaxel, bupropion, zidovudine, tenofovir disoproxil fumarate, cyclosporine, and others [50,52,61,80–82]. P-gp is often inhibited in a competitive manner using many of these same substrates to test whether compounds are substrates of P-gp in certain models. Interestingly, P-gp can be inhibited in a non-competitive manner by some drug additives/excipients, such as Cremophor®-EL and polysorbate 80 [83]. The consequences for placental efflux by P-gp of compounds in the presence of these excipients are as of yet unclear. Nonetheless, P-gp undoubtedly plays a significant role in the placental barrier and fetal protection. Recently, it has been shown that there may be a significant risk of congenital anomalies when mothers are administered multiple drugs that are P-gp substrates or inhibitors [84].
Breast cancer resistance protein (ABCG2) is expressed in the apical membrane of the syncytiotrophoblast and is responsible for the efflux of a variety of compounds [50,80]. Endogenous substrates of this efflux transporter in the placenta include steroid sulfates (in conjunction with OATP2B1) and porphyrins, including heme [85,86]. An interesting modulator of BCRP expression is hypoxia [86]. Drugs that are known substrates of BCRP include zidovudine, tenofovir disoproxil fumarate, bupropion, nitrofurantoin, and glyburide, to name a few [52,80,82,87,88]. Many of the substrates of BCRP are also substrates of P-gp, demonstrating redundancy in placental efflux capacity. Efflux of glyburide by BCRP in the placenta is the likely cause of low fetal accumulation of glyburide, making it safe for use in gestational diabetes [87]. Genistein, an isoflavone and phytoestrogen, has been shown to both directly interfere with BCRP efflux and alter the transcription of BCRP, which may have clinical relevance for pregnant patients consuming soy products [87,89].
Multidrug resistance-associated protein 1, or MRP1 (ABCC1) is expressed on the basolateral membrane of the syncytiotrophoblast and on the abluminal side of the fetal capillary endothelium [61,90,91]. This protein is involved in the transport of both endogenous and exogenous compounds [48]. Cyclic adenosine monophosphate (cAMP) is a substrate of MRP1, and is involved in certain syncytiotrophoblast functions [92]. Interestingly, expression of MRP1 in immortalized placental trophoblast cells was shown to be modulated by interleukin-1β and estrone [92]. It was shown that MRP1 is also partially responsible for the transport of methyl mercury to the fetal circulation, which explains the higher levels of methyl mercury in umbilical cord blood in comparison to maternal blood [93]. The position of MRP1 in the syncytiotrophoblast suggests that MRP1 may not function as a component of the placental barrier, but may rather be involved in other physiological and developmental functions not yet fully understood [92,94]. Transport by MRP1 of estrogen sulfates does appear to be enhanced by reduced glutathione, but this functionality and its relevance in placental tissue is not yet known [95].
Unlike MRP1, MRP2 (ABCC2) is located on the apical membrane of the syncytiotrophoblast [48,61,80]. Transcription of MRP2 has been shown to occur in BeWo and JAr choriocarcinoma cells as well as in primary trophoblast cells. Protein expression is low in primary trophoblasts and not detectable in other cell lines, but the choriocarcinoma cell lines appear to retain MRP activity [38]. Its position in the membrane means the primary function of MRP2 is probably to protect the fetus against harmful xenobiotics, in addition to transporting endogenous compounds. The substrate specificity of MRP2 is similar to that of MRP1 [90]. MRP2 is known to transport some leukotrienes and bilirubin. In addition to this, it can transport a number of sulfate, glucuronide, and glutathione conjugates [96]. Xenobiotics that are transported by MRP2 include tenofovir disoproxil fumarate and talinolol, among others [48,61,80,97].
3.4 Endocytosis
The placental syncytiotrophoblast is capable of endocytosis through multiple mechanisms, which functions for both endogenous compound transport as well as xenobiotics such as drug-loaded nanoparticles. Albumin, for instance, has been shown to be taken up by the placental trophoblast through a clathrin-mediated process [57]. Megalin has recently been shown to be involved in receptor-mediated endocytosis in the syncytiotrophoblast, and is involved in the uptake of the aminoglycoside gentamicin [98]. Receptor-mediated endocytosis is involved in uptake of other materials from the maternal blood as well, including some essential nutrients [99].
It is already known that certain types of nanoparticles are capable of crossing the placental barrier, or causing indirect effects to placental or fetal development [53,100–102]. Endocytosis of nanoparticles by the placenta is important in that it can alter the transplacental passage of drugs. An example of this is the passage of nanoparticles made of the polymer poly(lactic-co-glycolic acid) (PLGA) or its PEGylated block copolymer, which may alter or prevent drug binding to efflux transporters and potentially alter their metabolism [102,103]. Other nanoparticles, such as poly(amidoamine) dendrimers and silica nanoparticles, however, may demonstrate reduced transfer across the placenta as compared to polystyrene or PLGA nanoparticles [100,101,103,104]. Another example is the use of cationic liposomes made of phospholipids and their ability to decrease the transfer of warfarin across the placenta [53]. There seems to be, as is the case with many tissues, a difference in nanoparticle transfer based on size, surface charge, and composition [105]. The clinical uses of diagnostic or therapeutic nanoparticles are expected to be extended to pregnant patients in the future.
4. Metabolizing enzymes in the placenta
The placenta is capable of both Phase I and Phase II biotransformation of many different types of drugs, xenobiotics, and endogenous compounds through the use of diverse enzymes that are capable of oxidation, reduction, and conjugation reactions. As such, the properties that these compounds possess may change, causing differences in receptor binding, off-target binding, solubility, or others. For this reason, it is essential to take metabolism into consideration when determining the transplacental passage of drugs.
4.1 Phase I biotransformation
The cytochrome P450 system is a major metabolic system largely studied in the liver, but it also plays important roles in placental metabolism. These enzymes are localized in the endoplasmic reticulum and mitochondria [46]. Interestingly, the expression of various cytochrome P450 enzymes can vary with gestational age (as measured by mRNA levels), which may have implications on the fetal exposure to different drugs and xenobiotics [106].
Endogenous compounds and vitamins may be metabolized by the placenta for normal physiological functions. Vitamin D3 can be hydroxylated at a number of sites by placental CYP11A1, yielding mono-, di-, and trihydroxylated forms. CYP27B1 is also involved in Vitamin D3 metabolism, causing 1α-hydroxylation of 20-hydroxyvitamin D3. CYP11A1 is responsible for the metabolism of plant sterols, cholesterol, and other steroids [107,108]. Aromatase, or CYP19A1, converts androgens to estrogens and has other steroidal substrates [109]. The protein levels of CYP19A1 (as well as non-cytochrome P450 enzymes, HSD17B1 and HSD3B1) were shown not to change in gestational diabetes or insulin resistance, though androgen levels were elevated in these patients [110]. Placental trophoblast cells (JEG-3 and primary human trophoblast cells) express CYP17, which catalyzes the conversion of progesterone to androstenedione, which is then converted to testosterone by HSD17B1. This process occurs in the placenta and it may be a source of estrogens, as aromatase converts androstenedione and testosterone to estrone and estradiol, respectively [111].
Many xenobiotics are metabolized by the placental cytochrome enzymes. CYP19A11 is capable of metabolizing glyburide, which exhibits limited transplacental passage. Glyburide can also be metabolized by CYP3A7, which is present in the placenta, but metabolism of glyburide by CYP3A7 has only been studied in the liver [112,113]. Another important xenobiotic, ethanol, is shown to be partially oxidized by CYP2E1 to make acetaldehyde [114].
Regulation of the cytochromes P450 may be dependent on a number of maternal factors. For instance, smoking is known in increase CYP1A1 expression (likely through the aryl hydrocarbon receptor), while CYP19A1 expression decreases. These types of changes probably have consequences for the metabolic profile of substrates entering the placenta and their overall effect on the fetus [109]. It was also shown that patients with higher maternal age have significantly lower CYP1A activity [114]. CYP19A1 has been shown to be downregulated (and consequently, less active) in patients exhibiting preeclampsia. This results in an overall decrease in circulating 17-β-estradiol and an increase in androgens, which may have a role in the pathophysiology of preeclampsia [115]. CYP27B1 and CYP24A1 are involved in vitamin D metabolism in the placenta and are upregulated in the presence of inflammatory cytokines in primary trophoblast cells [116].
In addition to cytochrome P450 enzymes, 3β-hydroxysteroid dehydrogenase (HSD3B1) is present in the placenta [117]. This enzyme has the function of metabolizing steroid hormones, namely 3β-hydroxy-5-ene-steroids such as pregnenolone and dehydroepiandrosterone. The products of these conversions (progesterone and androstenedione) are important in determining the activity or inactivity of the uterus in the context of labor [117,118]. In polycystic ovary syndrome, or PCOS, androstenedione and testosterone are significantly higher compared to patients without PCOS. This has been linked to elevated activity of placental HSD3B1 and reduced activity of CYP19A1 in pregnant PCOS patients, which may alter androgen concentrations [119]. Monoamine oxidase is involved in the oxidative deamination of neurotransmitters such as serotonin, and this enzyme has also been investigated as a target for prodrug activation [120]. Reduced activity of monoamine oxidase A in placentas from preeclamptic pregnancies may be responsible for increases in serotonin levels and blood pressure [121].
4.2 Phase II biotransformation
Glucuronide conjugation is conducted by uridine 5′-diphosphate glucuronosyltransferase enzymes, or UGTs. It has been shown that UGT1A1, UGT1A4, UGT1A6, UGT1A9, UGT2B4, UGT2B7, UGT2B10, UGT2B11, UGT2B15 and in some cases UGT2B17 transcription occurs in the placenta, while UGT1A1, UGT1A4, UGT1A6, UGT1A9, UGT2B4 and UGT2B7 have been observed at the protein level [122–124]. These enzymes are known for their protective role in fetal development by conjugating glucuronic acid to xenobiotics. The expression of UGT enzymes may be altered by polycyclic aromatic hydrocarbons or other xenobiotics, but the extent of this and the effect on pregnancy is not clear [124].
Azidothymidine has been shown to be glucuronidated by UGT2B7 in the placenta. The conversion of azidothymidine to its glucuronide conjugate is low, varying between in vitro models. However, it was shown that the glucuronide conjugate exhibits a faster fetal-to-maternal transfer than maternal-to-fetal transfer, perhaps meaning that glucuronidation may be protective of the fetus to xenobiotic exposure [125]. Ethanol can be partially oxidized to acetaldehyde by CYP2E1 and it can also be conjugated to glucuronide to make ethyl glucuronide. This compound can cross the placenta but it is not yet clear whether this is mediated by placental UGT2B7 [126]. UGT1A4 is responsible for lamotrigine glucuronidation. During pregnancy, lamotrigine levels fall faster than in non-pregnant patients, which may be due to the activity of placental UGT1A4 [122]. One compound that has gained significant attention due to its ubiquity and estrogenic activity is bisphenol A. It was shown that both bisphenol A and its glucuronide derivative are present in the fetal circulation. Bisphenol A can readily cross the placenta in both the fetal-to-maternal and maternal-to-fetal directions, but bisphenol A glucuronide has limited permeability across the placenta in either direction. Conjugation by the placenta or fetus may affect the levels of bisphenol A glucuronide in the fetal circulation [127].
Sulfotransferases (SULTs) mediate the conjugation of sulfate groups to certain compounds using a cofactor, 3′-phosphoadenosine 5′-phosphosulfate (PAPS). Evidence for SULT1A1, SULT1A3, SULT2B1a, SULT2B1b, and SULT1E1 expression in the placenta has been shown [128,129]. SULT2B1b is almost exclusively expressed in the nuclei of syncytiotrophoblast in human placenta, though it is found in other tissues as well. In terms of physiological functions, this enzyme may be involved in the sulfation of dehydroepiandrosterone in the placenta [128]. Cholesterol sulfate is also synthesized in the placenta [130]. Sulfation of hormones can occur by SULT1A1 and SULT1A3 activity, though SULT1E1 (also known as estrogen sulfotransferase) functions in the placenta as well [129]. Sulfated steroids are inactive at their receptors, unlike unconjugated steroids [131]. Other hormones that may be sulfated include iodothyronines [129]. It is, as of yet, unclear how much sulfate is generated in the placenta by sulfur-containing amino acids [132].
Sulfatase enzymes catalyze the removal of sulfate groups from compounds, including steroid hormones and others. Steroid sulfotransferase, or STS (ARSC1) removes sulfate from steroid sulfates, rendering them active at their respective receptors, e.g., estrone sulfate to estrone [131]. This enzyme is present in the placenta, where it removes sulfate from 3β-hydroxysteroids. This allows estrogen production to occur during pregnancy [133]. Arylsulfatase A is also active in the placenta, though its role in fetal health and development is unclear. Its function may be related to the triggering of labor [134]. A number of compounds are being developed as sulfatase inhibitors for use in some cancer patients, but they may affect placental sulfatase activity and could potentially be dangerous for use during pregnancy [131,135]. Sulfatase deficiency results in significantly reduced estriol with moderate reductions in estrone and estradiol. These reductions are not complete, which may be evidence that androgen and estrogen synthesis may occur in the placenta de novo [111].
Glutathione S-transferase-π (GST-π) is the major GST isoform present in the placenta [46]. It is responsible for the conjugation of glutathione to certain compounds to diminish their toxicity. It is not normally expressed in adult tissues, and it is usually associated with carcinogenesis if present in the adult [136]. GST-π in the placenta may be inhibited by fluoxetine, potentially causing fetal toxicity [137]. Other GST enzymes have been shown to be transcribed in the placenta, but their functional relevance is not yet fully understood [138]. Interestingly, the level of glutathione S-transferase expression in the human placenta was shown to be higher in pregnancies where women were experiencing unexplained recurrent pregnancy loss. This may be a protective response in placentas that are experiencing some type of stress associated with pregnancy loss [139]. There is also a correlation between the risk for recurrent pregnancy loss and a specific polymorphism of glutathione S-transferase, GSTM1 [140]. There may be some practical benefit to the development of inhibitors of glutathione S-transferase or specifically the placental isoform, but these will have to be examined closely in the context of pregnancy as they may be unsafe for fetal development [141]. Esterases in the placenta may also be responsible for the hydrolysis of several drugs, but since placental tissue is highly perfused, it may be difficult to distinguish placental esterase activity from that of blood esterases [142].
5. Conclusion
Understanding the structure and function of the various components of the placental barrier—together with an appreciation for the influence of a drug's physicochemical properties—will guide the selection of therapeutics and strategies appropriate for treating diseases during pregnancy. Although most focus is usually placed on the use of medication to treat maternal disease, fetal disorders can also be treated in utero, where concerns for maternal exposure to fetal medication supplant the typical concerns for fetal drug exposure [143]. Recent advances in new technologies are expected to improve drug delivery during pregnancy. These include the potential for elastin-like polypeptides to reduce the transplacental transfer of drugs intended for maternal therapy [144] and the development of nanoparticles targeted to the placenta to address fetal growth, placental insufficiency, and fetal diseases [145–147].
Acknowledgments
The authors are grateful for research support from Citizens United for Research in Epilepsy, the Saudi Arabian Cultural Mission, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01HD083003 and U54HD047891). The authors also wish to thank Dr. Tatiana Nanovskaya for helpful comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Endicott S, Haas DM. The current state of therapeutic drug trials in pregnancy. Clin. Pharmacol. Ther. 2012;92:149–150. doi: 10.1038/clpt.2012.81. doi:10.1038/clpt.2012.81. [DOI] [PubMed] [Google Scholar]
- 2.Patel SI, Pennell PB. Management of epilepsy during pregnancy: an update. Ther. Adv. Neurol. Disord. 2016;9:118–129. doi: 10.1177/1756285615623934. doi:10.1177/1756285615623934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feldkamp ML, Meyer RE, Krikov S, Botto LD. Acetaminophen use in pregnancy and risk of birth defects: findings from the National Birth Defects Prevention Study. Obstet. Gynecol. 2010;115:109–115. doi: 10.1097/AOG.0b013e3181c52616. doi:10.1097/AOG.0b013e3181c52616. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell AA, Gilboa SM, Werler MM, Kelley KE, Louik C, Hernández-Díaz S. Medication use during pregnancy, with particular focus on prescription drugs: 1976-2008. Am. J. Obstet. Gynecol. 2011;205:51.e1–51.e8. doi: 10.1016/j.ajog.2011.02.029. doi:10.1016/j.ajog.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Werler MM, Mitchell AA, Hernandez-Diaz S, Honein MA. Use of over-the-counter medications during pregnancy. Am. J. Obstet. Gynecol. 2005;193:771–777. doi: 10.1016/j.ajog.2005.02.100. doi:10.1016/j.ajog.2005.02.100. [DOI] [PubMed] [Google Scholar]
- 6.Substance Abuse and Mental Health Services Administration . Substance Abuse and Mental Health Services Administration (2014) Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-48, HHS Publication No.(SMA) 14-4863. Subst. Abus. Ment. Health Serv. Adm.; Rockville, MD.: 2014. [Google Scholar]
- 7.Buhimschi CS, Weiner CP. Medications in pregnancy and lactation: part 1. Teratology. Obstet. Gynecol. 2009;113:166–188. doi: 10.1097/AOG.0b013e31818d6788. doi:10.1097/AOG.0b013e31818d6788. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell AA. Adverse drug reactions in utero: perspectives on teratogens and strategies for the future. Clin. Pharmacol. Ther. 2011;89:781–783. doi: 10.1038/clpt.2011.52. doi:10.1038/clpt.2011.52. [DOI] [PubMed] [Google Scholar]
- 9.Costantine MM. Physiologic and pharmacokinetic changes in pregnancy. Front. Pharmacol. 2014;5 doi: 10.3389/fphar.2014.00065. doi:10.3389/fphar.2014.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pacheco LD, Costantine MM, Hankins GD. Physiologic changes during pregnancy. In: Mattison DR, editor. Clinical Pharmacology During Pregnancy. Elsevier; Amsterdam: 2013. pp. 5–16. [Google Scholar]
- 11.Koh KH, Jurkovic S, Yang K, Choi S-Y, Jung JW, Kim KP, et al. Estradiol induces cytochrome P450 2B6 expression at high concentrations: implication in estrogen-mediated gene regulation in pregnancy. Biochem. Pharmacol. 2012;84:93–103. doi: 10.1016/j.bcp.2012.03.016. doi:10.1016/j.bcp.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rytting E, Nanovskaya TN, Wang X, Vernikovskaya DI, Clark SM, Cochran M, et al. Pharmacokinetics of indomethacin in pregnancy. Clin. Pharmacokinet. 2014;53:545–551. doi: 10.1007/s40262-014-0133-6. doi:10.1007/s40262-014-0133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Audus KL. Controlling drug delivery across the placenta. Eur. J. Pharm. Sci. 1999;8:161–165. doi: 10.1016/s0928-0987(99)00031-7. [DOI] [PubMed] [Google Scholar]
- 14.Moore KL, Persaud TVN, Torchia MG. Before We Are Born: Essentials of Embryology and Birth Defects. Elsevier; Philadelphia: 2016. [Google Scholar]
- 15.Benirschke K, Kaufmann P, Baergen RN. Pathology of the Human Placenta. Springer; New York: 2006. [Google Scholar]
- 16.Wang Y. Vascular Biology of the Placenta. Colloq. Ser. Integr. Syst. Physiol. From Mol. to Funct. 2010;2:1–98. doi:10.4199/C00016ED1V01Y201008ISP009. [Google Scholar]
- 17.Mori M, Ishikawa G, Luo S-S, Mishima T, Goto T, Robinson JM, et al. The cytotrophoblast layer of human chorionic villi becomes thinner but maintains its structural integrity during gestation. Biol. Reprod. 2007;76:164–172. doi: 10.1095/biolreprod.106.056127. doi:10.1095/biolreprod.106.056127. [DOI] [PubMed] [Google Scholar]
- 18.Gude NM, Roberts CT, Kalionis B, King RG. Growth and function of the normal human placenta. Thromb. Res. 2004;114:397–407. doi: 10.1016/j.thromres.2004.06.038. doi:10.1016/j.thromres.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 19.Quinney SK, Mohamed AN, Hebert MF, Haas DM, Clark S, Umans JG, et al. A Semi-Mechanistic Metabolism Model of CYP3A Substrates in Pregnancy: Predicting Changes in Midazolam and Nifedipine Pharmacokinetics. CPT: Pharmacometrics & Syst. Pharmacol. 2012;1 doi: 10.1038/psp.2012.5. doi:10.1038/psp.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ala-Kokko TI, Myllynen P, Vähäkangas K. Ex vivo perfusion of the human placental cotyledon: implications for anesthetic pharmacology. Int. J. Obstet. Anesth. 2000;9:26–38. [Google Scholar]
- 21.Rytting E, Wang X, Vernikovskaya DI, Zhan Y, Bauer C, Abdel-Rahman SM, et al. Metabolism and disposition of bupropion in pregnant baboons (Papio cynocephalus). Drug Metab. Dispos. Biol. Fate Chem. 2014;42:1773–1779. doi: 10.1124/dmd.114.058255. doi:10.1124/dmd.114.058255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malassiné A, Frendo JL, Evain-Brion D. A comparison of placental development and endocrine functions between the human and mouse model. Hum. Reprod. Update. 2003;9:531–539. doi: 10.1093/humupd/dmg043. [DOI] [PubMed] [Google Scholar]
- 23.Hemauer SJ, Yan R, Patrikeeva SL, Mattison DR, Hankins GDV, Ahmed MS, et al. Transplacental transfer and metabolism of 17-alpha-hydroxyprogesterone caproate. Am. J. Obstet. Gynecol. 2008;199:169.e1–169.e5. doi: 10.1016/j.ajog.2007.11.065. doi:10.1016/j.ajog.2007.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathiesen L, Mørck TA, Zuri G, Andersen MH, Pehrson C, Frederiksen M, et al. Modelling of human transplacental transport as performed in Copenhagen, Denmark. Basic & Clin. Pharmacol. & Toxicol. 2014;115:93–100. doi: 10.1111/bcpt.12228. doi:10.1111/bcpt.12228. [DOI] [PubMed] [Google Scholar]
- 25.Di Santo S, Malek A, Sager R, Andres A-C, Schneider H. Trophoblast viability in perfused term placental tissue and explant cultures limited to 7-24 hours. Placenta. 2003;24:882–894. doi: 10.1016/s0143-4004(03)00142-5. [DOI] [PubMed] [Google Scholar]
- 26.Schneider H. Tolerance of human placental tissue to severe hypoxia and its relevance for dual ex vivo perfusion. Placenta. 2009;30(Suppl A):S71–S76. doi: 10.1016/j.placenta.2008.11.004. doi:10.1016/j.placenta.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Miller RK, Genbacev O, Turner MA, Aplin JD, Caniggia I, Huppertz B. Human placental explants in culture: approaches and assessments. Placenta. 2005;26:439–448. doi: 10.1016/j.placenta.2004.10.002. doi:10.1016/j.placenta.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Hemauer SJ, Patrikeeva SL, Nanovskaya TN, Hankins GDV, Ahmed MS. Opiates inhibit paclitaxel uptake by P-glycoprotein in preparations of human placental inside-out vesicles. Biochem. Pharmacol. 2009;78:1272–1278. doi: 10.1016/j.bcp.2009.07.002. doi:10.1016/j.bcp.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Illsley NP, Wang ZQ, Gray A, Sellers MC, Jacobs MM. Simultaneous preparation of paired, syncytial, microvillous and basal membranes from human placenta. Biochim. et Biophys. Acta. 1990;1029:218–226. doi: 10.1016/0005-2736(90)90157-j. [DOI] [PubMed] [Google Scholar]
- 30.Eaton BM, Oakey MP. Sequential preparation of highly purified microvillous and basal syncytiotrophoblast membranes in substantial yield from a single term human placenta: inhibition of microvillous alkaline phosphatase activity by EDTA. Biochim. et Biophys. Acta. 1994;1193:85–92. doi: 10.1016/0005-2736(94)90336-0. [DOI] [PubMed] [Google Scholar]
- 31.Steck TL, Kant JA. Preparation of impermeable ghosts and inside-out vesicles from human erythrocyte membranes. Methods Enzymol. 1974;31:172–180. doi: 10.1016/0076-6879(74)31019-1. [DOI] [PubMed] [Google Scholar]
- 32.Tomi M, Eguchi H, Ozaki M, Tawara T, Nishimura S, Higuchi K, et al. Role of OAT4 in Uptake of Estriol Precursor 16α-Hydroxydehydroepiandrosterone Sulfate Into Human Placental Syncytiotrophoblasts From Fetus. Endocrinology. 2015;156:2704–2712. doi: 10.1210/en.2015-1130. doi:10.1210/en.2015-1130. [DOI] [PubMed] [Google Scholar]
- 33.Noguchi S, Nishimura T, Fujibayashi A, Maruyama T, Tomi M, Nakashima E. Organic Anion Transporter 4-Mediated Transport of Olmesartan at Basal Plasma Membrane of Human Placental Barrier. J. Pharm. Sci. 2015;104:3128–3135. doi: 10.1002/jps.24434. doi:10.1002/jps.24434. [DOI] [PubMed] [Google Scholar]
- 34.Petroff MG, Phillips TA, Ka H, Pace JL, Hunt JS. Isolation and culture of term human trophoblast cells. Methods Mol. Med. 2006;121:203–217. doi: 10.1385/1-59259-983-4:201. [DOI] [PubMed] [Google Scholar]
- 35.Göhner C, Svensson-Arvelund J, Pfarrer C, Häger J-D, Faas M, Ernerudh J, et al. The placenta in toxicology. Part IV: Battery of toxicological test systems based on human placenta. Toxicol. Pathol. 2014;42:345–351. doi: 10.1177/0192623313482206. doi:10.1177/0192623313482206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaccioli F, Aye ILMH, Roos S, Lager S, Ramirez VI, Kanai Y, et al. Expression and functional characterisation of System L amino acid transporters in the human term placenta. Reprod. Biol. Endocrinol.: RB&E. 2015;13 doi: 10.1186/s12958-015-0054-8. doi:10.1186/s12958-015-0054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Serrano MA, Macias RIR, Briz O, Monte MJ, Blazquez AG, Williamson C, et al. Expression in human trophoblast and choriocarcinoma cell lines, BeWo, Jeg-3 and JAr of genes involved in the hepatobiliary-like excretory function of the placenta. Placenta. 2007;28:107–117. doi: 10.1016/j.placenta.2006.03.009. doi:10.1016/j.placenta.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 38.Evseenko DA, Paxton JW, Keelan JA. ABC drug transporter expression and functional activity in trophoblast-like cell lines and differentiating primary trophoblast. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:R1357–R1365. doi: 10.1152/ajpregu.00630.2005. doi:10.1152/ajpregu.00630.2005. [DOI] [PubMed] [Google Scholar]
- 39.Illsley NP, Sellers MC, Wright RL. Glycaemic regulation of glucose transporter expression and activity in the human placenta. Placenta. 1998;19:517–524. doi: 10.1016/s0143-4004(98)91045-1. [DOI] [PubMed] [Google Scholar]
- 40.Baumann MU, Zamudio S, Illsley NP. Hypoxic upregulation of glucose transporters in BeWo choriocarcinoma cells is mediated by hypoxia-inducible factor-1. Am. J. Physiol. Cell Physiol. 2007;293:C477–C485. doi: 10.1152/ajpcell.00075.2007. doi:10.1152/ajpcell.00075.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown K, Heller DS, Zamudio S, Illsley NP. Glucose transporter 3 (GLUT3) protein expression in human placenta across gestation. Placenta. 2011;32:1041–1049. doi: 10.1016/j.placenta.2011.09.014. doi:10.1016/j.placenta.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orendi K, Kivity V, Sammar M, Grimpel Y, Gonen R, Meiri H, et al. Placental and trophoblastic in vitro models to study preventive and therapeutic agents for preeclampsia. Placenta. 2011;32(Suppl):S49–S54. doi: 10.1016/j.placenta.2010.11.023. doi:10.1016/j.placenta.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 43.Poulsen MS, Rytting E, Mose T, Knudsen LE. Modeling placental transport: correlation of in vitro BeWo cell permeability and ex vivo human placental perfusion. Toxicol. Vitr.: Int. J. Publ. Assoc. with BIBRA. 2009;23:1380–1386. doi: 10.1016/j.tiv.2009.07.028. doi:10.1016/j.tiv.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 44.Bode CJ, Jin H, Rytting E, Silverstein PS, Young AM, Audus KL. In vitro models for studying trophoblast transcellular transport. Methods Mol. Med. 2006;122:225–239. doi: 10.1385/1-59259-989-3:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ikeda K, Yamasaki K, Homemoto M, Yamaue S, Ogawa M, Nakao E, et al. Efflux transporter mRNA expression profiles in differentiating JEG-3 human choriocarcinoma cells as a placental transport model. Die Pharm. 2012;67:86–90. [PubMed] [Google Scholar]
- 46.Syme MR, Paxton JW, Keelan JA. Drug transfer and metabolism by the human placenta. Clin. Pharmacokinet. 2004;43:487–514. doi: 10.2165/00003088-200443080-00001. doi:10.2165/00003088-200443080-00001. [DOI] [PubMed] [Google Scholar]
- 47.Eshkoli T, Sheiner E, Ben-Zvi Z, Holcberg G. Drug transport across the placenta. Curr. Pharm. Biotechnol. 2011;12:707–714. doi: 10.2174/138920111795470877. [DOI] [PubMed] [Google Scholar]
- 48.Staud F, Cerveny L, Ceckova M. Pharmacotherapy in pregnancy; effect of ABC and SLC transporters on drug transport across the placenta and fetal drug exposure. J. Drug Target. 2012;20:736–763. doi: 10.3109/1061186X.2012.716847. doi:10.3109/1061186X.2012.716847. [DOI] [PubMed] [Google Scholar]
- 49.Sharom FJ. Complex Interplay between the P-Glycoprotein Multidrug Efflux Pump and the Membrane: Its Role in Modulating Protein Function. Front. Oncol. 2014;4 doi: 10.3389/fonc.2014.00041. doi:10.3389/fonc.2014.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nanovskaya TN, Patrikeeva SL, Paul J, Costantine MM, Hankins GDV, Ahmed MS. Transplacental transfer and distribution of pravastatin. Am. J. Obstet. Gynecol. 2013;209:373.e1–373.e5. doi: 10.1016/j.ajog.2013.05.038. doi:10.1016/j.ajog.2013.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson RF, Herman N, Arney TL, Olenick M, Paschall RL, Johnson HV, et al. Transfer of lidocaine across the dual perfused human placental cotyledon. Int. J. Obstet. Anesth. 1999;8:17–23. doi: 10.1016/s0959-289x(99)80147-2. [DOI] [PubMed] [Google Scholar]
- 52.Neumanova Z, Cerveny L, Greenwood SL, Ceckova M, Staud F. Effect of drug efflux transporters on placental transport of antiretroviral agent abacavir. Reprod. Toxicol. 2015;57:176–182. doi: 10.1016/j.reprotox.2015.07.070. doi:10.1016/j.reprotox.2015.07.070. [DOI] [PubMed] [Google Scholar]
- 53.Bajoria R, Sooranna S, Chatterjee R. Effect of lipid composition of cationic SUV liposomes on materno-fetal transfer of warfarin across the perfused human term placenta. Placenta. 2013;34:1216–1222. doi: 10.1016/j.placenta.2013.10.005. doi:10.1016/j.placenta.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 54.Mitchell MD, Henare K, Balakrishnan B, Lowe E, Fong BY, McJarrow P. Transfer of gangliosides across the human placenta. Placenta. 2012;33:312–316. doi: 10.1016/j.placenta.2011.12.018. doi:10.1016/j.placenta.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 55.Nekhayeva IA, Nanovskaya TN, Pentel PR, Keyler DE, Hankins GDV, Ahmed MS. Effects of nicotine-specific antibodies, Nic311 and Nic-IgG, on the transfer of nicotine across the human placenta. Biochem. Pharmacol. 2005;70:1664–1672. doi: 10.1016/j.bcp.2005.08.013. doi:10.1016/j.bcp.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 56.Berveiller P, Vinot C, Mir O, Broutin S, Deroussent A, Seck A, et al. Comparative transplacental transfer of taxanes using the human perfused cotyledon placental model. Am. J. Obstet. Gynecol. 2012;207:514.e1–514.e7. doi: 10.1016/j.ajog.2012.10.007. doi:10.1016/j.ajog.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 57.Lambot N, Lybaert P, Boom A, Delogne-Desnoeck J, Vanbellinghen AM, Graff G, et al. Evidence for a clathrin-mediated recycling of albumin in human term placenta. Biol. Reprod. 2006;75:90–97. doi: 10.1095/biolreprod.105.050021. doi:10.1095/biolreprod.105.050021. [DOI] [PubMed] [Google Scholar]
- 58.Roth M, Obaidat A, Hagenbuch B. OATPs, OATs and OCTs: the organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br. J. Pharmacol. 2012;165:1260–1287. doi: 10.1111/j.1476-5381.2011.01724.x. doi:10.1111/j.1476-5381.2011.01724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kummu M, Sieppi E, Koponen J, Laatio L, Vähäkangas K, Kiviranta H, et al. Organic anion transporter 4 (OAT 4) modifies placental transfer of perfluorinated alkyl acids PFOS and PFOA in human placental ex vivo perfusion system. Placenta. 2015;36:1185–1191. doi: 10.1016/j.placenta.2015.07.119. doi:10.1016/j.placenta.2015.07.119. [DOI] [PubMed] [Google Scholar]
- 60.Hagenbuch B, Meier PJ. Organic anion transporting polypeptides of the OATP/ SLC21 family: phylogenetic classification as OATP/ SLCO superfamily, new nomenclature and molecular/functional properties. Pflügers Arch.: Eur. J. Physiol. 2004;447:653–665. doi: 10.1007/s00424-003-1168-y. doi:10.1007/s00424-003-1168-y. [DOI] [PubMed] [Google Scholar]
- 61.Vähäkangas K, Myllynen P. Drug transporters in the human blood-placental barrier. Br. J. Pharmacol. 2009;158:665–678. doi: 10.1111/j.1476-5381.2009.00336.x. doi:10.1111/j.1476-5381.2009.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ugele B, Bahn A, Rex-Haffner M. Functional differences in steroid sulfate uptake of organic anion transporter 4 (OAT4) and organic anion transporting polypeptide 2B1 (OATP2B1) in human placenta. J. Steroid Biochem. Mol. Biol. 2008;111:1–6. doi: 10.1016/j.jsbmb.2008.04.001. doi:10.1016/j.jsbmb.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 63.Lofthouse EM, Brooks S, Cleal JK, Hanson MA, Poore KR, O'Kelly IM, et al. Glutamate cycling may drive organic anion transport on the basal membrane of human placental syncytiotrophoblast. J. Physiol. 2015;593:4549–4559. doi: 10.1113/JP270743. doi:10.1113/JP270743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sato K, Sugawara J, Sato T, Mizutamari H, Suzuki T, Ito A, et al. Expression of organic anion transporting polypeptide E (OATP-E) in human placenta. Placenta. 2003;24:144–148. doi: 10.1053/plac.2002.0907. [DOI] [PubMed] [Google Scholar]
- 65.Nishikawa M, Iwano H, Yanagisawa R, Koike N, Inoue H, Yokota H. Placental transfer of conjugated bisphenol A and subsequent reactivation in the rat fetus. Environ. Health Perspect. 2010;118:1196–1203. doi: 10.1289/ehp.0901575. doi:10.1289/ehp.0901575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ahmadimoghaddam D, Zemankova L, Nachtigal P, Dolezelova E, Neumanova Z, Cerveny L, et al. Organic cation transporter 3 (OCT3/SLC22A3) and multidrug and toxin extrusion 1 (MATE1/SLC47A1) transporter in the placenta and fetal tissues: expression profile and fetus protective role at different stages of gestation. Biol. Reprod. 2013;88 doi: 10.1095/biolreprod.112.105064. doi:10.1095/biolreprod.112.105064. [DOI] [PubMed] [Google Scholar]
- 67.Wessler I, Roth E, Deutsch C, Brockerhoff P, Bittinger F, Kirkpatrick CJ, et al. Release of non-neuronal acetylcholine from the isolated human placenta is mediated by organic cation transporters. Br. J. Pharmacol. 2001;134:951–956. doi: 10.1038/sj.bjp.0704335. doi:10.1038/sj.bjp.0704335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eisenhofer G. The role of neuronal and extraneuronal plasma membrane transporters in the inactivation of peripheral catecholamines. Pharmacol. & Ther. 2001;91:35–62. doi: 10.1016/s0163-7258(01)00144-9. [DOI] [PubMed] [Google Scholar]
- 69.Bottalico B, Larsson I, Brodszki J, Hernandez-Andrade E, Casslén B, Marsál K, et al. Norepinephrine transporter (NET), serotonin transporter (SERT), vesicular monoamine transporter (VMAT2) and organic cation transporters (OCT1, 2 and EMT) in human placenta from pre-eclamptic and normotensive pregnancies. Placenta. 2004;25:518–529. doi: 10.1016/j.placenta.2003.10.017. doi:10.1016/j.placenta.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 70.Lee N, Hebert MF, Prasad B, Easterling TR, Kelly EJ, Unadkat JD, et al. Effect of gestational age on mRNA and protein expression of polyspecific organic cation transporters during pregnancy. Drug Metab. Dispos. Biol. Fate Chem. 2013;41:2225–2232. doi: 10.1124/dmd.113.054072. doi:10.1124/dmd.113.054072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ahmadimoghaddam D, Staud F. Transfer of metformin across the rat placenta is mediated by organic cation transporter 3 (OCT3/SLC22A3) and multidrug and toxin extrusion 1 (MATE1/SLC47A1) protein. Reprod. Toxicol. 2013;39:17–22. doi: 10.1016/j.reprotox.2013.03.001. doi:10.1016/j.reprotox.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 72.Terada T, Masuda S, Asaka J-I, Tsuda M, Katsura T, Inui K. Molecular cloning, functional characterization and tissue distribution of rat H+/organic cation antiporter MATE1. Pharm. Res. 2006;23:1696–1701. doi: 10.1007/s11095-006-9016-3. doi:10.1007/s11095-006-9016-3. [DOI] [PubMed] [Google Scholar]
- 73.Rytting E, Audus KL. Novel organic cation transporter 2-mediated carnitine uptake in placental choriocarcinoma (BeWo) cells. J. Pharmacol. Exp. Ther. 2005;312:192–198. doi: 10.1124/jpet.104.072363. doi:10.1124/jpet.104.072363. [DOI] [PubMed] [Google Scholar]
- 74.Grube M, Meyer Zu Schwabedissen H, Draber K, Präger D, Möritz K-U, Linnemann K, et al. Expression, localization, and function of the carnitine transporter octn2 (slc22a5) in human placenta. Drug Metab. Dispos. Biol. Fate Chem. 2005;33:31–37. doi: 10.1124/dmd.104.001560. doi:10.1124/dmd.104.001560. [DOI] [PubMed] [Google Scholar]
- 75.Rytting E, Audus KL. Effects of low oxygen levels on the expression and function of transporter OCTN2 in BeWo cells. J. Pharm. Pharmacol. 2007;59:1095–1102. doi: 10.1211/jpp.59.8.0006. doi:10.1211/jpp.59.8.0006. [DOI] [PubMed] [Google Scholar]
- 76.Chang T-T, Shyu M-K, Huang M-C, Hsu C-C, Yeh S-Y, Chen M-R, et al. Hypoxia-mediated down-regulation of OCTN2 and PPARα expression in human placentas and in BeWo cells. Mol. Pharm. 2011;8:117–125. doi: 10.1021/mp100137q. doi:10.1021/mp100137q. [DOI] [PubMed] [Google Scholar]
- 77.Huang F-D, Kung F-L, Tseng Y-C, Chen M-R, Chan H-S, Lin C-J. Regulation of protein expression and function of octn2 in forskolin-induced syncytialization in BeWo Cells. Placenta. 2009;30:187–194. doi: 10.1016/j.placenta.2008.11.016. doi:10.1016/j.placenta.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 78.Wu X, George RL, Huang W, Wang H, Conway SJ, Leibach FH, et al. Structural and functional characteristics and tissue distribution pattern of rat OCTN1, an organic cation transporter, cloned from placenta. [NaN, 2000];Biochim. et Biophys. Acta. 2000 1466:315–327. doi: 10.1016/s0005-2736(00)00189-9. http://www.ncbi.nlm.nih.gov/pubmed/10825452. [DOI] [PubMed] [Google Scholar]
- 79.Tamai I, Nakanishi T, Kobayashi D, China K, Kosugi Y, Nezu J, et al. Involvement of OCTN1 (SLC22A4) in pH-dependent transport of organic cations. Mol. Pharm. 2004;1:57–66. doi: 10.1021/mp0340082. [DOI] [PubMed] [Google Scholar]
- 80.Neumanova Z, Cerveny L, Ceckova M, Staud F. Interactions of tenofovir and tenofovir disoproxil fumarate with drug efflux transporters ABCB1, ABCG2, and ABCC2; role in transport across the placenta. AIDS. 2014;28:9–17. doi: 10.1097/QAD.0000000000000112. doi:10.1097/QAD.0000000000000112. [DOI] [PubMed] [Google Scholar]
- 81.Mark PJ, Waddell BJ. P-glycoprotein restricts access of cortisol and dexamethasone to the glucocorticoid receptor in placental BeWo cells. Endocrinology. 2006;147:5147–5152. doi: 10.1210/en.2006-0633. doi:10.1210/en.2006-0633. [DOI] [PubMed] [Google Scholar]
- 82.Hemauer SJ, Patrikeeva SL, Wang X, Abdelrahman DR, Hankins GDV, Ahmed MS, et al. Role of transporter-mediated efflux in the placental biodisposition of bupropion and its metabolite, OH-bupropion. Biochem. Pharmacol. 2010;80:1080–1086. doi: 10.1016/j.bcp.2010.06.025. doi:10.1016/j.bcp.2010.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Netsomboon K, Laffleur F, Suchaoin W, Bernkop-Schnürch A. Novel in vitro transport method for screening the reversibility of P-glycoprotein inhibitors. Eur. J. Pharm. Biopharm.: Off. J. Arbeitsgemeinschaft Für Pharm. Verfahrenstechnik e.V. 2016;100:9–14. doi: 10.1016/j.ejpb.2015.11.019. doi:10.1016/j.ejpb.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 84.Daud ANA, Bergman JEH, Bakker MK, Wang H, Kerstjens-Frederikse WS, de Walle HEK, et al. P-Glycoprotein-Mediated Drug Interactions in Pregnancy and Changes in the Risk of Congenital Anomalies: A Case-Reference Study. Drug Saf. 2015;38:651–659. doi: 10.1007/s40264-015-0299-3. doi:10.1007/s40264-015-0299-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grube M, Reuther S, Meyer Zu Schwabedissen H, Köck K, Draber K, Ritter CA, et al. Organic anion transporting polypeptide 2B1 and breast cancer resistance protein interact in the transepithelial transport of steroid sulfates in human placenta. Drug Metab. Dispos. Biol. Fate Chem. 2007;35:30–35. doi: 10.1124/dmd.106.011411. doi:10.1124/dmd.106.011411. [DOI] [PubMed] [Google Scholar]
- 86.Krishnamurthy P, Ross DD, Nakanishi T, Bailey-Dell K, Zhou S, Mercer KE, et al. The stem cell marker Bcrp/ABCG2 enhances hypoxic cell survival through interactions with heme. J. Biol. Chem. 2004;279:24218–24225. doi: 10.1074/jbc.M313599200. doi:10.1074/jbc.M313599200. [DOI] [PubMed] [Google Scholar]
- 87.Bircsak KM, Gupta V, Yuen PYS, Gorczyca L, Weinberger BI, Vetrano AM, et al. Genetic and Dietary Regulation of Glyburide Efflux by the Human Placental Breast Cancer Resistance Protein Transporter. J. Pharmacol. Exp. Ther. 2016;357:103–113. doi: 10.1124/jpet.115.230185. doi:10.1124/jpet.115.230185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Feinshtein V, Holcberg G, Amash A, Erez N, Rubin M, Sheiner E, et al. Nitrofurantoin transport by placental choriocarcinoma JAr cells: involvement of BCRP, OATP2B1 and other MDR transporters. Arch. Gynecol. Obstet. 2010;281:1037–1044. doi: 10.1007/s00404-009-1286-7. doi:10.1007/s00404-009-1286-7. [DOI] [PubMed] [Google Scholar]
- 89.Bircsak KM, Aleksunes LM. Interaction of Isoflavones with the BCRP/ABCG2 Drug Transporter. Curr. Drug Metab. 2015;16:124–140. doi: 10.2174/138920021602150713114921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang J-S, Newport DJ, Stowe ZN, Donovan JL, Pennell PB, DeVane CL. The emerging importance of transporter proteins in the psychopharmacological treatment of the pregnant patient. Drug Metab. Rev. 2007;39:723–746. doi: 10.1080/03602530701690390. doi:10.1080/03602530701690390. [DOI] [PubMed] [Google Scholar]
- 91.Nagashige M, Ushigome F, Koyabu N, Hirata K, Kawabuchi M, Hirakawa T, et al. Basal membrane localization of MRP1 in human placental trophoblast. Placenta. 2003;24:951–958. doi: 10.1016/s0143-4004(03)00170-x. [DOI] [PubMed] [Google Scholar]
- 92.Biondi C, Ferretti ME, Lunghi L, Medici S, Cervellati F, Pavan B, et al. cAMP efflux from human trophoblast cell lines: a role for multidrug resistance protein (MRP)1 transporter. Mol. Hum. Reprod. 2010;16:481–491. doi: 10.1093/molehr/gaq023. doi:10.1093/molehr/gaq023. [DOI] [PubMed] [Google Scholar]
- 93.Straka E, Ellinger I, Balthasar C, Scheinast M, Schatz J, Szattler T, et al. Mercury toxicokinetics of the healthy human term placenta involve amino acid transporters and ABC transporters. Toxicology. 2016;340:34–42. doi: 10.1016/j.tox.2015.12.005. doi:10.1016/j.tox.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 94.Aye ILMH, Paxton JW, Evseenko DA, Keelan JA. Expression, localisation and activity of ATP binding cassette (ABC) family of drug transporters in human amnion membranes. Placenta. 2007;28:868–877. doi: 10.1016/j.placenta.2007.03.001. doi:10.1016/j.placenta.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 95.Qian YM, Song WC, Cui H, Cole SP, Deeley RG. Glutathione stimulates sulfated estrogen transport by multidrug resistance protein 1. J. Biol. Chem. 2001;276:6404–6411. doi: 10.1074/jbc.M008251200. doi:10.1074/jbc.M008251200. [DOI] [PubMed] [Google Scholar]
- 96.Gerk PM, Vore M. Regulation of expression of the multidrug resistance-associated protein 2 (MRP2) and its role in drug disposition. J. Pharmacol. Exp. Ther. 2002;302:407–415. doi: 10.1124/jpet.102.035014. doi:10.1124/jpet.102.035014. [DOI] [PubMed] [Google Scholar]
- 97.May K, Minarikova V, Linnemann K, Zygmunt M, Kroemer HK, Fusch C, et al. Role of the multidrug transporter proteins ABCB1 and ABCC2 in the diaplacental transport of talinolol in the term human placenta. Drug Metab. Dispos. Biol. Fate Chem. 2008;36:740–744. doi: 10.1124/dmd.107.019448. doi:10.1124/dmd.107.019448. [DOI] [PubMed] [Google Scholar]
- 98.Akour AA, Gerk P, Kennedy MJ. Megalin expression in human term and preterm placental villous tissues: effect of gestational age and sample processing and storage time. J. Pharmacol. Toxicol. Methods. 2015;71:147–154. doi: 10.1016/j.vascn.2014.10.001. doi:10.1016/j.vascn.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 99.Akour AA, Kennedy MJ, Gerk P. Receptor-mediated endocytosis across human placenta: emphasis on megalin. Mol. Pharm. 2013;10:1269–1278. doi: 10.1021/mp300609c. doi:10.1021/mp300609c. [DOI] [PubMed] [Google Scholar]
- 100.Menjoge AR, Rinderknecht AL, Navath RS, Faridnia M, Kim CJ, Romero R, et al. Transfer of PAMAM dendrimers across human placenta: prospects of its use as drug carrier during pregnancy. J. Control. Release. 2011;150:326–338. doi: 10.1016/j.jconrel.2010.11.023. doi:10.1016/j.jconrel.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Grafmueller S, Manser P, Diener L, Diener P-A, Maeder-Althaus X, Maurizi L, et al. Bidirectional Transfer Study of Polystyrene Nanoparticles across the Placental Barrier in an ex Vivo Human Placental Perfusion Model. Environ. Health Perspect. 2015;123:1280–1286. doi: 10.1289/ehp.1409271. doi:10.1289/ehp.1409271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Albekairi NA, Al-Enazy S, Ali S, Rytting E. Transport of digoxin-loaded polymeric nanoparticles across BeWo cells, an in vitro model of human placental trophoblast. Ther. Deliv. 2015;6:1325–1334. doi: 10.4155/tde.15.79. doi:10.4155/tde.15.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ali H, Kalashnikova I, White MA, Sherman M, Rytting E. Preparation, characterization, and transport of dexamethasone-loaded polymeric nanoparticles across a human placental in vitro model. Int. J. Pharm. 2013;454:149–157. doi: 10.1016/j.ijpharm.2013.07.010. doi:10.1016/j.ijpharm.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sønnegaard Poulsen M, Mose T, Leth Maroun L, Mathiesen L, Ehlert Knudsen L, Rytting E. Kinetics of silica nanoparticles in the human placenta. Nanotoxicology. 2013 doi: 10.3109/17435390.2013.812259. doi:10.3109/17435390.2013.812259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Albanese A, Tang PS, Chan WCW. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012;14:1–16. doi: 10.1146/annurev-bioeng-071811-150124. doi:10.1146/annurev-bioeng-071811-150124. [DOI] [PubMed] [Google Scholar]
- 106.Pavek P, Smutny T. Nuclear receptors in regulation of biotransformation enzymes and drug transporters in the placental barrier. Drug Metab. Rev. 2014;46:19–32. doi: 10.3109/03602532.2013.835819. doi:10.3109/03602532.2013.835819. [DOI] [PubMed] [Google Scholar]
- 107.Slominski AT, Kim T-K, Li W, Yi A-K, Postlethwaite A, Tuckey RC. The role of CYP11A1 in the production of vitamin D metabolites and their role in the regulation of epidermal functions. J. Steroid Biochem. Mol. Biol. 2014;144(Pt A):28–39. doi: 10.1016/j.jsbmb.2013.10.012. doi:10.1016/j.jsbmb.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Slominski AT, Li W, Kim T-K, Semak I, Wang J, Zjawiony JK, et al. Novel activities of CYP11A1 and their potential physiological significance. J. Steroid Biochem. Mol. Biol. 2015;151:25–37. doi: 10.1016/j.jsbmb.2014.11.010. doi:10.1016/j.jsbmb.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Storvik M, Huuskonen P, Pehkonen P, Pasanen M. The unique characteristics of the placental transcriptome and the hormonal metabolism enzymes in placenta. Reprod. Toxicol. 2014;47:9–14. doi: 10.1016/j.reprotox.2014.04.010. doi:10.1016/j.reprotox.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 110.Morisset A-S, Dubé M-C, Drolet R, Pelletier M, Labrie F, Luu-The V, et al. Androgens in the maternal and fetal circulation: association with insulin resistance. J. Matern. & Neonatal Med.: Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet. 2013;26:513–519. doi: 10.3109/14767058.2012.735725. doi:10.3109/14767058.2012.735725. [DOI] [PubMed] [Google Scholar]
- 111.Escobar JC, Patel SS, Beshay VE, Suzuki T, Carr BR. The human placenta expresses CYP17 and generates androgens de novo. J. Clin. Endocrinol. Metab. 2011;96:1385–1392. doi: 10.1210/jc.2010-2504. doi:10.1210/jc.2010-2504. [DOI] [PubMed] [Google Scholar]
- 112.Shuster DL, Risler LJ, Prasad B, Calamia JC, Voellinger JL, Kelly EJ, et al. Identification of CYP3A7 for glyburide metabolism in human fetal livers. Biochem. Pharmacol. 2014;92:690–700. doi: 10.1016/j.bcp.2014.09.025. doi:10.1016/j.bcp.2014.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Maezawa K, Matsunaga T, Takezawa T, Kanai M, Ohira S, Ohmori S. Cytochrome P450 3As gene expression and testosterone 6 beta-hydroxylase activity in human fetal membranes and placenta at full term. Biol. & Pharm. Bull. 2010;33:249–254. doi: 10.1248/bpb.33.249. [DOI] [PubMed] [Google Scholar]
- 114.Collier AC, Tingle MD, Paxton JW, Mitchell MD, Keelan JA. Metabolizing enzyme localization and activities in the first trimester human placenta: the effect of maternal and gestational age, smoking and alcohol consumption. Hum. Reprod. 2002;17:2564–2572. doi: 10.1093/humrep/17.10.2564. [DOI] [PubMed] [Google Scholar]
- 115.Perez-Sepulveda A, Monteiro LJ, Dobierzewska A, España-Perrot PP, Venegas-Araneda P, Guzmán-Rojas AM, et al. Placental Aromatase Is Deficient in Placental Ischemia and Preeclampsia. PloS One. 2015;10 doi: 10.1371/journal.pone.0139682. doi:10.1371/journal.pone.0139682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Noyola-Martínez N, Díaz L, Zaga-Clavellina V, Avila E, Halhali A, Larrea F, et al. Regulation of CYP27B1 and CYP24A1 gene expression by recombinant pro-inflammatory cytokines in cultured human trophoblasts. J. Steroid Biochem. Mol. Biol. 2014;144(Pt A):106–109. doi: 10.1016/j.jsbmb.2013.12.007. doi:10.1016/j.jsbmb.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 117.Simard J, Moisan AM, Morel Y. Congenital adrenal hyperplasia due to 3beta-hydroxysteroid dehydrogenase/Delta(5)-Delta(4) isomerase deficiency. Semin. Reprod. Med. 2002;20:255–276. doi: 10.1055/s-2002-35373. doi:10.1055/s-2002-35373. [DOI] [PubMed] [Google Scholar]
- 118.Nguyen PTT, Conley AJ, Sneyd J, Lee RSF, Soboleva TK, Shorten PR. The role of enzyme compartmentalization on the regulation of steroid synthesis. J. Theor. Biol. 2013;332:52–64. doi: 10.1016/j.jtbi.2013.04.021. doi:10.1016/j.jtbi.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 119.Maliqueo M, Lara HE, Sánchez F, Echiburú B, Crisosto N, Sir-Petermann T. Placental steroidogenesis in pregnant women with polycystic ovary syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013;166:151–155. doi: 10.1016/j.ejogrb.2012.10.015. doi:10.1016/j.ejogrb.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 120.Stella V, Borchardt R, Hageman M, Oliyai R, Maag H, Tilley J. Prodrugs: challenges and rewards. Springer; New York: 2007. [Google Scholar]
- 121.Sivasubramaniam SD, Finch CC, Billett MA, Baker PN, Billett EE. Monoamine oxidase expression and activity in human placentae from pre-eclamptic and normotensive pregnancies. Placenta. 2002;23:163–171. doi: 10.1053/plac.2001.0770. doi:10.1053/plac.2001.0770. [DOI] [PubMed] [Google Scholar]
- 122.Reimers A, Østby L, Stuen I, Sundby E. Expression of UDP-glucuronosyltransferase 1A4 in human placenta at term. Eur. J. Drug Metab. Pharmacokinet. 2011;35:79–82. doi: 10.1007/s13318-010-0021-x. doi:10.1007/s13318-010-0021-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Collier AC, Thévenon AD, Goh W, Hiraoka M, Kendal-Wright CE. Placental profiling of UGT1A enzyme expression and activity and interactions with preeclampsia at term. Eur. J. Drug Metab. Pharmacokinet. 2015;40:471–480. doi: 10.1007/s13318-014-0243-4. doi:10.1007/s13318-014-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Collier AC, Ganley NA, Tingle MD, Blumenstein M, Marvin KW, Paxton JW, et al. UDP-glucuronosyltransferase activity, expression and cellular localization in human placenta at term. Biochem. Pharmacol. 2002;63:409–419. doi: 10.1016/s0006-2952(01)00890-5. [DOI] [PubMed] [Google Scholar]
- 125.Collier AC, Keelan JA, Van Zijl PE, Paxton JW, Mitchell MD, Tingle MD. Human placental glucuronidation and transport of 3’azido-3’-deoxythymidine and uridine diphosphate glucuronic acid. Drug Metab. Dispos. Biol. Fate Chem. 2004;32:813–820. doi: 10.1124/dmd.32.8.813. [DOI] [PubMed] [Google Scholar]
- 126.Matlow JN, Lubetsky A, Aleksa K, Berger H, Koren G. The transfer of ethyl glucuronide across the dually perfused human placenta. Placenta. 2013;34:369–373. doi: 10.1016/j.placenta.2012.12.016. doi:10.1016/j.placenta.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 127.Corbel T, Gayrard V, Puel S, Lacroix MZ, Berrebi A, Gil S, et al. Bidirectional placental transfer of Bisphenol A and its main metabolite, Bisphenol A-Glucuronide, in the isolated perfused human placenta. Reprod. Toxicol. 2014;47:51–58. doi: 10.1016/j.reprotox.2014.06.001. doi:10.1016/j.reprotox.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 128.Falany CN, He D, Dumas N, Frost AR, Falany JL. Human cytosolic sulfotransferase 2B1: isoform expression, tissue specificity and subcellular localization. J. Steroid Biochem. Mol. Biol. 2006;102:214–221. doi: 10.1016/j.jsbmb.2006.09.011. doi:10.1016/j.jsbmb.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pacifici GM. Sulfation of drugs and hormones in mid-gestation human fetus. Early Hum. Dev. 2005;81:573–581. doi: 10.1016/j.earlhumdev.2004.10.021. doi:10.1016/j.earlhumdev.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 130.Dawson PA. Role of sulphate in development. Reproduction. 2013;146:R81–R89. doi: 10.1530/REP-13-0056. doi:10.1530/REP-13-0056. [DOI] [PubMed] [Google Scholar]
- 131.Phan C-M, Liu Y, Kim B, Mostafa Y, Taylor SD. Inhibition of steroid sulfatase with 4-substituted estrone and estradiol derivatives. Bioorganic & Med. Chem. 2011;19:5999–6005. doi: 10.1016/j.bmc.2011.08.046. doi:10.1016/j.bmc.2011.08.046. [DOI] [PubMed] [Google Scholar]
- 132.Rakoczy J, Lee S, Weerasekera SJ, Simmons DG, Dawson PA. Placental and fetal cysteine dioxygenase gene expression in mouse gestation. Placenta. 2015;36:956–959. doi: 10.1016/j.placenta.2015.06.003. doi:10.1016/j.placenta.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 133.Selcer KW, Difrancesca HM, Chandra AB, Li P-K. Immunohistochemical analysis of steroid sulfatase in human tissues. J. Steroid Biochem. Mol. Biol. 2007;105:115–123. doi: 10.1016/j.jsbmb.2006.12.105. doi:10.1016/j.jsbmb.2006.12.105. [DOI] [PubMed] [Google Scholar]
- 134.Indraccolo U, Traini E, Baldoni E, Indraccolo SR, Vitaioli L. Arylsulphatase A activity and sulphatide concentration in placenta, membranes and cord after delivery. J. Perinat. Med. 2009;37:497–502. doi: 10.1515/JPM.2009.092. doi:10.1515/JPM.2009.092. [DOI] [PubMed] [Google Scholar]
- 135.Kozak W, Daśko M, Masłyk M, Kubiński K, Rachon J, Demkowicz S. Steroid Sulfatase Inhibitors Based on Phosphate and Thiophosphate Flavone Analogs. Drug Dev. Res. 2015;76:450–462. doi: 10.1002/ddr.21281. doi:10.1002/ddr.21281. [DOI] [PubMed] [Google Scholar]
- 136.Noguti J, Barbisan LF, Cesar A, Dias Seabra C, Choueri RB, Ribeiro DA. Review: In vivo models for measuring placental glutatione-S-transferase (GST-P 7-7) levels: a suitable biomarker for understanding cancer pathogenesis. Vivo. 2012;26:647–650. [PubMed] [Google Scholar]
- 137.Dalmizrak O, Kulaksiz-Erkmen G, Ozer N. Fluoxetine-induced toxicity results in human placental glutathione S-transferase-π (GST-π) dysfunction. Drug Chem. Toxicol. 2016:1–6. doi: 10.3109/01480545.2016.1141422. doi:10.3109/01480545.2016.1141422. [DOI] [PubMed] [Google Scholar]
- 138.Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human phase I metabolizing enzymes except for cytochrome P450 and phase II metabolizing enzymes. Drug Metab. Pharmacokinet. 2006;21:357–374. doi: 10.2133/dmpk.21.357. [DOI] [PubMed] [Google Scholar]
- 139.Gharesi-Fard B, Zolghadri J, Kamali-Sarvestani E. Alteration in the expression of proteins in unexplained recurrent pregnancy loss compared with in the normal placenta. J. Reprod. Dev. 2014;60:261–267. doi: 10.1262/jrd.2013-096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sata F, Yamada H, Kondo T, Gong Y, Tozaki S, Kobashi G, et al. Glutathione S-transferase M1 and T1 polymorphisms and the risk of recurrent pregnancy loss. Mol. Hum. Reprod. 2003;9:165–169. doi: 10.1093/molehr/gag021. [DOI] [PubMed] [Google Scholar]
- 141.Alparslan MM, Danış Ö. In Vitro Inhibition of Human Placental Glutathione STransferase by 3-Arylcoumarin Derivatives. Arch. Der Pharm. 2015;348:635–642. doi: 10.1002/ardp.201500151. doi:10.1002/ardp.201500151. [DOI] [PubMed] [Google Scholar]
- 142.Sastry BR. Placental toxicology. CRC Press; 1995. [Google Scholar]
- 143.Rytting E, Ahmed MS. Fetal drug therapy. In: Mattison DR, editor. Clinical Pharmacology During Pregnancy. Elsevier; Amsterdam: 2013. pp. 55–72. [Google Scholar]
- 144.George EM, Liu H, Robinson GG, Bidwell GL. A polypeptide drug carrier for maternal delivery and prevention of fetal exposure. J. Drug Target. 2014;22:935–947. doi: 10.3109/1061186X.2014.950666. doi:10.3109/1061186X.2014.950666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Abd Ellah N, Taylor L, Troja W, Owens K, Ayres N, Pauletti G, et al. Development of Non-Viral, Trophoblast-Specific Gene Delivery for Placental Therapy. PloS One. 2015;10 doi: 10.1371/journal.pone.0140879. doi:10.1371/journal.pone.0140879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Albrecht C, Caniggia I, Clifton V, Göhner C, Harris L, Hemmings D, et al. IFPA meeting 2015 workshop report IV: Nanomedicine applications and exosome biology, xenobiotics and endocrine disruptors and pregnancy, and lipid mediators and placental function. Placenta. 2016 doi: 10.1016/j.placenta.2016.01.003. doi:10.1016/j.placenta.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 147.Ilekis JV, Tsilou E, Fisher S, Abrahams VM, Soares MJ, Cross JC, et al. Placental origins of adverse pregnancy outcomes: potential molecular targets: an Executive Workshop Summary of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Am. J. Obstet. Gynecol. 2016 doi: 10.1016/j.ajog.2016.03.001. doi:10.1016/j.ajog.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]