Abstract
Background
Frontal QRS‐T angle reflects changes in regional action potential duration and the direction of repolarization. Although it has been suggested that abnormal ventricular repolarization predisposes to atrial arrhythmias, it is unknown whether abnormal frontal QRS‐T angle is associated with an increased risk of atrial fibrillation (AF).
Methods
We examined the association between frontal QRS‐T angle and AF in 4282 participants (95% white; 41% male) from the Cardiovascular Health Study (CHS). QRS‐T angle was computed from baseline electrocardiogram data. Abnormal QRS‐T angle was defined as values greater than the sex‐specific 95th percentile (men >131°; women: >104°). AF cases were identified from study electrocardiograms and from hospitalization discharge data through December 31, 2010. Cox regression was used to compute hazard ratios (HR) and 95% confidence intervals (CI) for the association between abnormal QRS‐T angle and AF.
Results
Over a median follow‐up of 12.1 years, a total of 1276 (30%) participants developed AF. In a Cox regression model, adjusted for socio‐demographics and known AF risk factors, abnormal QRS‐T angle was associated with a 55% increased risk of AF (HR = 1.55, 95%CI = 1.23, 1.97). When QRS‐T angle was examined as a continuous variable, each 10° increase was associated with a 3% increased risk of AF (HR = 1.03, 95%CI = 1.01, 1.05). This finding was consistent in subgroups stratified by age, sex, and race.
Conclusion
Our findings suggest that an abnormal frontal QRS‐T angle on the electrocardiogram provides important prognostic information regarding AF risk in the elderly, and further implicate ventricular repolarization abnormalities in the pathogenesis of AF.
Keywords: electrocardiogram, atrial fibrillation, risk
The frontal QRS‐T axis angle is the difference in orientation between ventricular repolarization and depolarization. It reflects changes in regional action potential duration and the direction of repolarization sequence. Abnormalities in this measure indicate altered ventricular repolarization, possibly related to underlying structural and functional myocardial changes.1 Accordingly, abnormal QRS‐T angle predicts future cardiovascular disease events and all‐cause mortality.2, 3, 4, 5
Abnormalities in ventricular structure and function that accompany repolarization abnormalities are possibly associated with atrial pathology. Several reports have suggested that ventricular repolarization abnormalities, as indicated by QT interval prolongation, are independently associated with an increased risk for atrial fibrillation (AF).6, 7, 8 However, there have been no studies to determine the association between abnormal frontal QRS‐T angle, a “purer” marker of repolarization, and AF.9 Therefore, we sought to determine if abnormal frontal QRS‐T angle predicts AF in the Cardiovascular Health Study (CHS), a well‐characterized, population‐based cohort study of community‐dwelling older adults.
Methods
Study Population
Details of CHS have been previously described.10 Briefly, CHS is a prospective population‐based cohort study of risk factors for coronary heart disease and stroke in individuals 65 years and older. A total of 5888 participants with Medicare eligibility in the United States were recruited from four field centers located in the following locations: Forsyth County, NC; Sacramento County, CA; Washington County, MD; and Pittsburgh, PA. Subjects were followed with semiannual contacts, alternating between telephone calls and surveillance clinic visits. CHS clinic exams ended in June of 1999 and since that time 2 yearly phone calls to participants were used to identify events and collect data. The institutional review boards at each site approved the study and written informed consent was obtained from participants at enrollment.
A longitudinal cohort study design was used to examine the association between abnormal QRS‐T angle and incident AF. Participants were excluded if they had baseline AF, major intraventricular conduction delays were present (including complete bundle branch blocks and/or QRS duration ≥120 ms), baseline covariate data were missing, or follow‐up data were missing.
Frontal QRS‐T Angle
Identical electrocardiographs (MAC PC, Marquette Electronics Inc., Milwaukee, WI, USA) were used at all clinic sites, and resting, 10‐second standard simultaneous 12‐lead electrocardiograms (ECG) were recorded in all participants. All ECGs were processed in a central laboratory (initially at the University of Alberta, Edmonton, Alberta, Canada, and later at the Epidemiological Cardiology Research Center, Wake Forest School of Medicine, Winston‐Salem, NC, USA). The methodology and prevalence of ECG abnormalities in CHS have been previously reported.11 QRS‐axis was measured by examining the areas of positive and negative deflections. T axis was determined by analyzing in which frontal leads the highest T waves were observed. The frontal QRS‐T angle was calculated as the absolute difference between QRS‐axis and T axis which yielded values between 0° and 180°. Abnormal QRS‐T angle was defined as values greater than the sex‐specific 95th percentile (men >131°; women: >104°).
Atrial Fibrillation
Incident AF cases were identified during the study ECGs that were performed annually until 1999. In addition, hospitalization discharge data were used to identify AF cases using International Classification of Diseases codes 427.31 and 427.32. Hospital diagnosis codes for AF ascertainment have been shown to have a positive predictive value of 98.6%.12
Covariates
Participant characteristics were collected during the initial CHS interview and questionnaire. Age, sex, race, income, and education were self‐reported. Annual income was dichotomized at $25,000 and education was dichotomized at “high school or less.” Smoking was defined as ever (e.g., current or former) or never smoker. Blood samples were obtained after a 12‐hour fast at a local field center and measurements of total cholesterol, high‐density lipoprotein cholesterol, and plasma glucose were used in this analysis. Diabetes was defined as a self‐reported history of a physician diagnosis, a fasting glucose value ≥126 mg/dL, or by the current use of insulin or oral hypoglycemic medications. Blood pressure was measured for each participant in the seated position and systolic measurements were used in this analysis. The use of aspirin and antihypertensive medications was self‐reported. Body mass index was computed as the weight in kilograms divided by the square of the height in meters. Baseline coronary heart disease was determined by self‐reported history or by medical record adjudication of the following diagnoses: myocardial infarction, angina pectoris without myocardial infarction, and coronary revascularization procedures (angioplasty and coronary artery bypass graft surgery).13 Baseline cases of stroke and heart failure were identified by self‐reported history of a physician diagnosis followed by medical record review.
Statistical Analysis
Categorical variables were reported as frequency and percentage, whereas continuous variables were recorded as mean ± standard deviation. Statistical significance for categorical variables was tested using the chi‐square method and the student's t‐test procedure for continuous variables. Follow‐up time was defined as the time between the initial study visit until one of the following: AF development, death, loss to follow‐up, or end of follow‐up (December 31, 2010). Kaplan–Meier estimates were used to compute cumulative incidence of AF by abnormal QRS‐T angle and the difference in estimates was compared using the log‐rank procedure.14 Cox regression was used to compute hazard ratios (HR) and 95% confidence intervals (CI) for the association between abnormal QRS‐T angle and incident AF. We also examined the association between QRS‐T angle and AF as a continuous variable per 10° increase in the QRS‐T angle. Multivariable models were constructed as follows: Model 1 adjusted for age, sex, race, education, and income; Model 2 adjusted for Model 1 covariates plus smoking, heart rate, systolic blood pressure, diabetes, body mass index, total cholesterol, high‐density lipoprotein cholesterol, aspirin, antihypertensive medications, coronary heart disease, stroke, and heart failure. We tested for interactions between our main effect variable and age (dichotomized at 75 years), sex, and race (white vs black). We also constructed a restricted cubic spline model to examine the graphical dose–response relationship between QRS‐T angle and AF at the 5th, 50th, and 95th percentiles.15 We examined the association between abnormal QRS‐T angle using the previously defined value of ≥100° to define abnormal QRS‐T angle in a sensitivity analysis.4 Due to the fact that men in our population had a value 6° (95%CI = 3.5, 7.8) higher than females, we defined abnormal QRS‐T angle as >100° for men and >94° for women. A sensitivity analysis also was performed with further adjustment for the QT interval to determine if the frontal QRS‐T angle provides prognostic information regarding AF risk independent of this common repolarization measure. Statistical significance for our main effect model and interaction terms was defined as P < 0.05. SAS Version 9.4 (Cary, NC, USA) was used for all analyses.
Results
A total of 4282 participants (95% white; 41% male) with complete data were used in this analysis. The mean QRS‐T angle was 40.1° ± 35.6° (median = 30°; 25th–75th percentiles = 13–56°). Abnormal QRS‐T angle values were identified in 206 (4.8%) participants. Baseline characteristics stratified by abnormal QRS‐T angle are shown in Table 1.
Table 1.
Baseline Characteristics (N = 4282)
| Characteristic | Abnormal QRS‐T Angle (n = 206) | Normal QRS‐T Angle (n = 4076) | P Valuea |
|---|---|---|---|
| Age, years | |||
| 65–70 (%) | 76 (37) | 1840 (45) | <0.001 |
| 71–74 (%) | 37 (18) | 961 (24) | |
| 75–80 (%) | 64 (31) | 899 (22) | |
| >80 (%) | 29 (14) | 376 (9) | |
| Male (%) | 80 (39) | 1655 (41) | 0.61 |
| White (%) | 190 (92) | 3883 (95) | 0.049 |
| High school or less (%) | 133 (65) | 2302 (56) | 0.022 |
| Annual income <$25,000 (%) | 140 (68) | 2524 (62) | 0.081 |
| Ever smoker (%) | 121 (59) | 2177 (53) | 0.13 |
| Diabetes (%) | 48 (23) | 556 (14) | <0.001 |
| Body mass index, mean (SD), kg/m2 | 26 (3.9) | 26 (4) | 0.98 |
| Systolic blood pressure, mean (SD), mm Hg | 145 (22) | 138 (20) | <0.001 |
| Total cholesterol, mean (SD), mg/dL | 210 (38) | 213 (39) | 0.30 |
| HDL cholesterol, mean (SD), mg/dL | 51 (14) | 55 (16) | <0.001 |
| Heart rate, mean (SD), bpm | 65 (12) | 65 (11) | 0.25 |
| Antihypertensive medication use (%) | 122 (59) | 1719 (42) | <0.001 |
| Aspirin use (%) | 78 (38) | 1336 (33) | 0.13 |
| Coronary heart disease (%) | 74 (36) | 656 (16) | <0.001 |
| Stroke (%) | 11 (5) | 122 (3) | 0.058 |
| Heart failure (%) | 18 (9) | 102 (3) | <0.001 |
Statistical significance, for continuous data was tested using the student's t‐test and, categorical data was tested using the chi‐square test.
AF = atrial fibrillation; bpm = beats per minute; HDL = high‐density lipoprotein; SD = standard deviation.
Over a median follow‐up of 12.1 years, a total of 1276 (30%) participants developed AF. The incidence rate of AF was higher for those with abnormal QRS‐T angle (42.6 cases per 1000 person‐years) than normal QRS‐T angle (23.7 cases per 1000 person‐years). The unadjusted cumulative incidence for AF by abnormal QRS‐T angle is shown in Figure 1.
Figure 1.
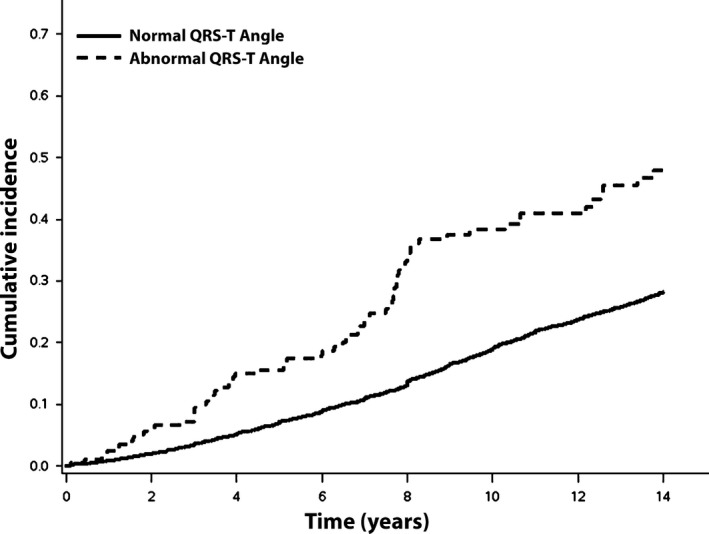
Unadjusted cumulative incidence of atrial fibrillation. Incidence curves are statistically different (log‐rank P < 0.001).
In an unadjusted Cox regression analysis, abnormal QRS‐T angle was associated with an increased risk for AF (HR = 1.97, 95%CI = 1.56, 2.49). After adjustment for socio‐demographics and known AF risk factors, the association between abnormal QRS‐T angle and AF remained statistically significant (Table 2). Interactions were not detected when the analysis was stratified by age, sex, or race (Table 3).
Table 2.
Risk of Atrial Fibrillation
| Events/No. at Risk | Incidence Rate Per 1000 Person‐years (95%CI) | Model 1a HR (95%CI) | P Value | Model 2b HR (95%CI) | P Value | |
|---|---|---|---|---|---|---|
| Normal QRS‐T angle | 1200/4076 | 23.7 (22.4, 25.1) | Ref | – | Ref | – |
| Abnormal QRS‐T angle | 76/206 | 42.6 (34.1, 53.4) | 1.86 (1.47, 2.34) | <0.001 | 1.55 (1.23, 1.97) | <0.001 |
| QRS‐T angle (per 10° increase) | 1276/4282 | 24.3 (23.0, 25.7) | 1.05 (1.03, 1.06) | <0.001 | 1.03 (1.01, 1.05) | <0.001 |
Adjusted for age, sex, race, education, and income.
Adjusted for Model 1 covariates plus smoking, heart rate, systolic blood pressure, diabetes, body mass index, total cholesterol, high‐density lipoprotein cholesterol, aspirin, antihypertensive medications, coronary heart disease, stroke, and heart failure.
CI = confidence interval; HR = hazard ratio.
Table 3.
Subgroup Analyses
| HR (95%CI)a | P Value | P Interaction | |
|---|---|---|---|
| Age <75 | 1.61 (1.14, 2.25) | 0.0062 | 0.99 |
| Age ≥75 | 1.59 (1.14, 2.22) | 0.0065 | |
| Male | 1.37 (0.93, 2.01) | 0.11 | 0.41 |
| Female | 1.68 (1.24, 2.27) | <0.001 | |
| White | 1.57 (1.23, 1.99) | <0.001 | 0.62 |
| Black | 2.28 (0.49, 10.7) | 0.30 |
Adjusted for age, sex, race, education, income, smoking, heart rate, systolic blood pressure, diabetes, body mass index, total cholesterol, high‐density lipoprotein cholesterol, aspirin, antihypertensive medications, coronary heart disease, stroke, and heart failure.
CI = confidence interval; HR = hazard ratio.
The risk of AF increased linearly when we examined QRS‐T angle as a continuous variable per 10° increase (Table 2). This dose–response relationship between QRS‐T angle and AF is graphically depicted in Figure 2. When we used prior definitions of abnormal QRS‐T angle (men >100°; women >94°), the relationship between QRS‐T angle and AF remained statistically significant (HR = 1.37, 95%CI = 1.13, 1.66). The association between QRS‐T angle and AF was not materially altered with further adjustment for the QT interval (HR = 1.54, 95%CI = 1.20, 1.98).
Figure 2.
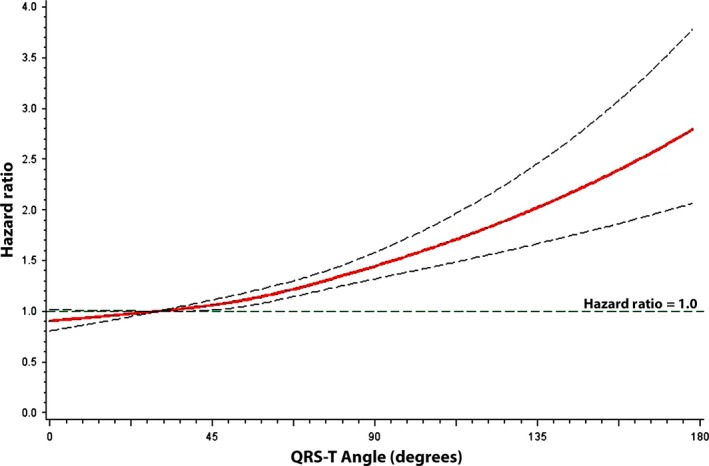
Risk of atrial fibrillation across QRS‐T angle values. Each hazard ratio was computed with the median QRS‐T angle value of 30° as the reference and was adjusted for age, sex, race, education, income, smoking, heart rate, systolic blood pressure, diabetes, body mass index, total cholesterol, high‐density lipoprotein cholesterol, aspirin, antihypertensive medications, coronary heart disease, stroke, and heart failure.
Discussion
In this analysis from CHS, we have demonstrated that an abnormal frontal QRS‐T angle is associated with an increased risk for AF development, independent of several AF risk factors. The results were consistent when the analysis was stratified by advanced age (>75 years), sex, and race. Overall, our findings suggest that important prognostic information is obtained from the frontal QRS‐T angle on the 12‐lead ECG regarding AF risk. In addition, these findings further implicate ventricular repolarization abnormalities in the pathogenesis of AF.
An abnormal QRS‐T angle indicates underlying alterations in myocardial ion channels which result in aberrant ventricular repolarization.5 It has been proposed that an abnormal QRS‐T angle is the result of increased dispersion of repolarization that increases one's risk for malignant arrhythmias. Accordingly, abnormalities in this measure are associated with several adverse cardiovascular outcomes, including sudden cardiac death.2, 3, 4, 5 The findings of this study extend the adverse events associated with an abnormal QRS‐T angle to include atrial arrhythmias, such as AF.
The QRS‐T angle is a more sensitive and reproducible marker of abnormal ventricular repolarization. Imperfections in the ability of the QT interval to assess ventricular repolarization are well‐known, as the QT interval includes abnormalities in depolarization (e.g., QRS duration). In addition, inconsistencies among clinicians in the measurement and interpretation of the QT interval are documented.16 Due to these reported shortcomings, the QRS‐T angle has emerged as a repolarization measure of interest, as it is thought to represent a “purer” marker of repolarization. This is related to the fact that abnormalities in the QRS‐T angle detect repolarization abnormalities before other common markers are apparent (e.g., T wave inversion or ST depression).9 Therefore, the QRS‐T angle is likely to signify a more robust and reproducible measurement of repolarization than the QT interval. Furthermore, our findings demonstrate that important prognostic information is obtained from the QRS‐T angle regarding the risk of AF.
Abnormal ventricular repolarization is an established mechanism of arrhythmiogenesis.17 Abnormalities in ventricular repolarization have been linked to AF by showing that prolongation of the QT interval results in altered atrial conduction. For example, patients with long QT syndrome have demonstrated prolonged atrial action potential durations which result in polymorphic atrial tachyarrhythmias.18 These polymorphic atrial arrhythmias have been shown to degenerate into AF.19 Alternative explanations are related to enhancement in the activity of the late sodium current that possibly results in the initiation of atrial ectopic activity.20 In addition, it is possible that the relationship between abnormal ventricular repolarization and AF is driven by common comorbid conditions (e.g., hypertension, diabetes) that were unable to be fully accounted for in the statistical analysis. Although we provide several explanations to link abnormal QRS‐T angle with AF, studies are needed to elucidate the underlying mechanisms.
There are some limitations in this study that should be considered. CHS included participants aged 65 years and older who were predominately Caucasian. Therefore, the generalizability of our findings to younger, racially diverse populations is uncertain. AF cases were identified from study‐scheduled ECGs and hospital discharge data. Accordingly, AF cases possibly were missed. Although we included several covariates in our multivariable models, we cannot exclude the possibility of residual confounding. Despite these limitations, this is the first study to report abnormal frontal QRS‐T angle is an important marker to predict AF.
Acknowledgements
This manuscript was prepared using Cardiovascular Health Study Research Materials obtained from the National Heart, Lung, and Blood Institute Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of the Cardiovascular Health Study or the National Heart, Lung, and Blood Institute.
Ann Noninvasive Electrocardiol 2017;22(2):e12388, 10.1111/anec.12388
Funding: Dr. Shah is sponsored by the American Heart Association (SDG‐20593449) and the National Institutes of Health (UL1‐TR‐000454, KL2‐TR‐00045, K23‐HL 127251). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
Disclosures: The authors declare no conflicts of interest.
References
- 1. Rautaharju PM, Kooperberg C, Larson JC, et al. Electrocardiographic predictors of incident congestive heart failure and all‐cause mortality in postmenopausal women: The Women's Health Initiative. Circulation 2006;113:481–489. [DOI] [PubMed] [Google Scholar]
- 2. Kardys I, Kors JA, van der Meer IM, et al. Spatial QRS‐T angle predicts cardiac death in a general population. Eur Heart J 2003;24:1357–1364. [DOI] [PubMed] [Google Scholar]
- 3. Yamazaki T, Froelicher VF, Myers J, et al. Spatial QRS‐T angle predicts cardiac death in a clinical population. Heart Rhythm 2005;2:73–78. [DOI] [PubMed] [Google Scholar]
- 4. Aro AL, Huikuri HV, Tikkanen JT, et al. QRS‐T angle as a predictor of sudden cardiac death in a middle‐aged general population. Europace 2012;14:872–876. [DOI] [PubMed] [Google Scholar]
- 5. Whang W, Shimbo D, Levitan EB, et al. Relations between QRS|T angle, cardiac risk factors, and mortality in the third National Health and Nutrition Examination Survey (NHANES III). Am J Cardiol 2012;109:981–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mandyam MC, Soliman EZ, Alonso A, et al. The QT interval and risk of incident atrial fibrillation. Heart Rhythm 2013;10:1562–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nielsen JB, Graff C, Pietersen A, et al. J‐shaped association between QTc interval duration and the risk of atrial fibrillation: Results from the Copenhagen ECG study. J Am Coll Cardiol 2013;61:2557–2564. [DOI] [PubMed] [Google Scholar]
- 8. O'Neal WT, Efird JT, Kamel H, et al. The association of the QT interval with atrial fibrillation and stroke: The Multi‐Ethnic Study of Atherosclerosis. Clin Res Cardiol 2015;104:743–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang ZM, Prineas RJ, Case D, et al. Comparison of the prognostic significance of the electrocardiographic QRS/T angles in predicting incident coronary heart disease and total mortality (from the atherosclerosis risk in communities study). Am J Cardiol 2007;100:844–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: Design and rationale. Ann Epidemiol 1991;1:263–276. [DOI] [PubMed] [Google Scholar]
- 11. Furberg CD, Manolio TA, Psaty BM, et al. Major electrocardiographic abnormalities in persons aged 65 years and older (the Cardiovascular Health Study). Cardiovascular Health Study Collaborative Research Group. Am J Cardiol 1992;69:1329–1335. [DOI] [PubMed] [Google Scholar]
- 12. Psaty BM, Manolio TA, Kuller LH, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation 1997;96:2455–2461. [DOI] [PubMed] [Google Scholar]
- 13. Psaty BM, Kuller LH, Bild D, et al. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol 1995;5:270–277. [DOI] [PubMed] [Google Scholar]
- 14. Gray RJ, Tsiatis AA. A linear rank test for use when the main interest is in differences in cure rates. Biometrics 1989;45:899–904. [PubMed] [Google Scholar]
- 15. Marrie RA, Dawson NV, Garland A. Quantile regression and restricted cubic splines are useful for exploring relationships between continuous variables. J Clin Epidemiol 2009;62:511–517. [DOI] [PubMed] [Google Scholar]
- 16. Al‐Khatib SM, LaPointe NM, Kramer JM, et al. What clinicians should know about the QT interval. JAMA 2003;289:2120–2127. [DOI] [PubMed] [Google Scholar]
- 17. Kuo CS, Reddy CP, Munakata K, et al. Mechanism of ventricular arrhythmias caused by increased dispersion of repolarization. Eur Heart J 1985;6(Suppl D):63–70. [DOI] [PubMed] [Google Scholar]
- 18. Kirchhof P, Eckardt L, Franz MR, et al. Prolonged atrial action potential durations and polymorphic atrial tachyarrhythmias in patients with long QT syndrome. J Cardiovasc Electrophysiol 2003;14:1027–1033. [DOI] [PubMed] [Google Scholar]
- 19. Satoh T, Zipes DP. Cesium‐induced atrial tachycardia degenerating into atrial fibrillation in dogs: Atrial torsades de pointes? J Cardiovasc Electrophysiol 1998;9:970–975. [DOI] [PubMed] [Google Scholar]
- 20. Song Y, Shryock JC, Belardinelli L. An increase of late sodium current induces delayed afterdepolarizations and sustained triggered activity in atrial myocytes. Am J Physiol Heart Circ Physiol 2008;294:H2031–2039. [DOI] [PubMed] [Google Scholar]


