Abstract
In the hippocampus, cyclic-adenosine monophosphate (cAMP) and cAMP-dependent protein kinase (PKA) form a critical signaling cascade required for long-lasting synaptic plasticity, learning and memory. Plasticity and memory are known to occur following pathway-specific changes in synaptic strength that are thought to result from spatially and temporally coordinated intracellular signaling events. To better understand how cAMP and PKA dynamically operate within the structural complexity of hippocampal neurons, we used live two-photon imaging and genetically-encoded fluorescent biosensors to monitor cAMP levels or PKA activity in CA1 neurons of acute hippocampal slices. Stimulation of β-adrenergic receptors (isoproterenol) or combined activation of adenylyl cyclase (forskolin) and inhibition of phosphodiesterase (IBMX) produced cAMP transients with greater amplitude and rapid on-rates in intermediate and distal dendrites compared to somata and proximal dendrites. In contrast, isoproterenol produced greater PKA activity in somata and proximal dendrites compared to intermediate and distal dendrites, and the on-rate of PKA activity did not differ between compartments. Computational models show that our observed compartmental difference in cAMP can be reproduced by a uniform distribution of PDE4 and a variable density of adenylyl cyclase that scales with compartment size to compensate for changes in surface to volume ratios. However, reproducing our observed compartmental difference in PKA activity required enrichment of protein phosphatase in small compartments; neither reduced PKA subunits nor increased PKA substrates were sufficient. Together, our imaging and computational results show that compartment diameter interacts with rate-limiting components like adenylyl cyclase, phosphodiesterase and protein phosphatase to shape the spatial and temporal components of cAMP and PKA signaling in CA1 neurons and suggests that small neuronal compartments are most sensitive to cAMP signals whereas large neuronal compartments accommodate a greater dynamic range in PKA activity.
Keywords: Computational modeling, cAMP, PKA, β-adrenergic receptors, FRET, Signaling dynamics
1. Introduction
In the hippocampus, long-term potentiation (LTP) and long-term memory (LTM) occur through changes in synaptic plasticity and neuronal excitability. Hippocampal dendrites integrate numerous excitatory and modulatory inputs through morphological and biophysical characteristics that influence excitability, current flow and posttranslational modifications (Spruston, 2008). Dendritic morphology also has a profound influence on kinase signaling that produces long-range signals from dendrites to the nucleus that alter epigenetic modifications (Li et al., 2015) or sustain structural plasticity (Zhai et al., 2013). Additionally, computational simulations and biosensor imaging studies find that dendritic geometry can also affect the spread of second-messenger signals within dendrites and influence downstream kinase signaling (Chay et al., 2016; Neves et al., 2008). Although much is known about how dendritic segments can serve as integrators of synaptic inputs, additional molecular studies are needed to determine how dynamic signaling pathways are coordinated with spatial and temporal precision.
Hippocampal LTP and LTM critically rely on production of the second-messenger, cyclic-adenosine 5’-3’-monophosphate (cAMP) and activity of cAMP-dependent protein kinase A (PKA). cAMP levels are tightly regulated through activation and inactivation mechanisms that are thought to operate in close proximity to produce “microdomains” of molecular activity and contribute to synapse-specific plasticity (Harvey et al., 2008; Zaccolo and Pozzan, 2002). Adenylyl cyclases (ACs) synthesize cAMP when stimulated by calcium influx or Gαs subunits released from neuromodulatory G-protein coupled receptors (GPCRs) while phosphodiesterase (PDE) proteins degrade cAMP to restore basal levels. During a cAMP transient, four molecules of cAMP bind to the regulatory subunits of PKA and release diffusible catalytic subunits that phosphorylate and regulate the activity of numerous intracellular targets that participate in plasticity and memory (Turnham and Scott, 2016; Woolfrey et al., 2015). Increasing hippocampal cAMP by application of either cell-permeable cAMP analogues, inhibition of PDE4 or direct activation of ACs can enhance several forms of LTM (Barad et al., 1998; Bernabeu et al., 1997; Bourtchouladze et al., 2003; Viola et al., 2000) or LTP (Duffy and Nguyen, 2003; Frey et al., 1993). Similarly, suppression of phosphatase activity by inducible expression of inhibitor-1 (I1) enhances spatial memory (Genoux et al., 2002), but surprisingly, bath application of phosphatase inhibitors do not enhance LTP (Woo et al., 2002).
During emotional experiences and heightened states of arousal, the noradrenergic system modulates neuronal activity and promotes LTM by secreting norepinephrine (NE) and increasing cAMP and PKA activity through activation of β-adrenergic receptors (β-ARs) (Cahill et al., 1994; Hu et al., 2007; Sara, 2009). Similarly, exogenous treatment with β-AR agonists can enhance consolidation of LTM in rodents (Izquierdo et al., 1998) or humans (Cahill and Alkire, 2003). However, in hippocampal slices, stimulation of β-ARs with NE or the β-AR agonist isoproterenol (ISO) requires weak synaptic stimulation and NMDA receptor activation to produce an “enhanced” form of pathway-specific LTP (Gelinas et al., 2008; Maity et al., 2015; Thomas et al., 1996).
To determine how the cAMP/PKA signaling pathway may operate in distinct dendritic compartments, we used live two-photon measurements of genetically encoded fluorescent biosensors to monitor cAMP or PKA activity in hippocampal slices during stimulation of β-ARs. Computational models were then developed to determine how dendritic morphology interacts with activation and inactivation mechanisms to produce molecular constraints that shape dynamic changes in cAMP and PKA activity and compartmentalize signal transduction pathways in hippocampal neurons.
2. Materials and Methods
2.1. Preparation of brain slices and viral transduction
Recombinant Sindbis virus encoding AKAR3 (Allen and Zhang, 2006) or EPAC-sh150 (Polito et al., 2013) were prepared as previously described (Castro et al., 2010). Brains were rapidly isolated from P10-P14, C57BL/6 mice (Janvier; Le Genest Saint Isle, France) and placed in ice-cold “cutting” artificial cerebrospinal fluid (aCSF) containing 110 mM choline Cl, 25 mM NaHCO3, 1.25 mM NaH2PO4, 2.5 mM KCl, 0.5 mM CaCl2, 7 mM MgCl2, 25 mM glucose, 11.6 mM ascorbic acid, 3.1 mM pyruvic acid and saturated with 95% O2/5% CO2. Coronal sections (250 μm) were made using a vibrating microtome (Thermo Scientific). Slices were recovered at 30°C for 20 minutes in “standard” aCSF containing 125 mM NaCl, 2.5 mM KCl, 1.25 mM NaH2PO4, 25 mM NaHCO3, 25 mM glucose, 2 mM CaCl2, 1 mM MgCl2 (~290 ± 5-10 mOsm) saturated with 95% O2/5% CO2 followed by an additional 30 minutes at ~21°C (room temperature). Slices were then transferred to Millicell-CM membranes (Millipore) in culture medium (50% MEM, 50% HBSS, 6.5 g/L glucose, penicillin-streptomycin; Invitrogen), equilibrated for 10 minutes at 35°C with 5% CO2, and 1 μL Sindbis virus (~5 × 105 particles / slice) was added directly over the hippocampus before overnight incubation at 35°C with 5% CO2. The following day, slices were transferred to “standard” aCSF saturated with 95% O2/5% CO2 at room temperature until used for imaging (<20 hours after viral infection).
2.2. Two-photon slice imaging and drug delivery
On the microscope stage, a nylon/platinum harp stabilized the slice while suspended on nylon mesh to facilitate continuous perfusion over the whole slice at 3 mL/min with “standard” aCSF at 32°C. Two-photon imaging was performed using an upright Leica TCS MP5 microscope with resonant scanning (8 kHz), a Leica 25X/0.95 HCX IRAPO immersion objective and a tunable Ti:sapphire laser (Coherent Chameleon Vision II) with dispersion correction set to 860 nm for CFP excitation. The emission path consisted of an initial 700 nm low-pass filter to remove excess excitation light (E700 SP, Chroma Technologies), 506 nm dichroic mirror for orthogonal separation of emitted signal, 479/40 CFP emission filter, 542/50 YFP emission filter (FF506-Di01-25×36; FF01-479/40; FF01-542/50; Brightline Filters; Semrock) and two-channel Leica HyD detector for simultaneous acquisition. Due to the high quantum efficiency and low dark noise of the HyD photodetectors, detector gain was typically set at 10-15% with laser power at 1-5%. Z-stack images (16-bit; 512 × 512) were typically acquired every 15 seconds, but in some cases the “default” minimal frame interval was used. The z-step size was 1-2 μm and total stack size was typically 40-90 sections depending on the slice (~40-180 μm).
Concentrated stocks were diluted in standard aCSF saturated with 95% O2/5% CO2 and continuously bubbled during perfusion. Isoproterenol (10 mM; Tocris) was freshly prepared in Milli-Q water. Forskolin (10 mM; Sigma) and IBMX (100 mM; 3,7-Dihydro-1-methyl-3-(2-methylpropyl)-1H-purine-2,6-dione; Tocris) were prepared in 100% DMSO.
2.3. Image analysis and post-acquisition processing
Images were processed in ImageJ by using maximum z-projections followed by translation registration correction to reduce x/y movement. If Sindbis transduction and expression was especially efficient throughout the slice, a z-projection was made using a subset of the whole image stack to reduce mixing signal from multiple cells along the z-axis. However, z-projections were occasionally complicated by movement in the z-axis and were therefore corrected with a custom Matlab script before measurement in ImageJ. After correcting movement in the x/y/z directions, regions of interest (ROIs) were selected for measurement if they could only be measured for the whole experimental time course. ROIs were placed around the periphery of the soma to exclude the nuclear compartment and only somata that were reasonably considered to be in stratum pyramidale of hippocampal area CA1 were measured. Proximal dendrites were the region of apical dendrite between the soma and initial dendritic branch, usually <35 μm from the soma. Intermediate dendrites were between the proximal dendrite and distal dendrites, typically 35-90 μm. Distal dendrites were the most distal dendritic compartment that could be observed and were believed to be connected to neurons in the pyramidal cell layer with a distance 90-150 μm (see Fig. 1A).
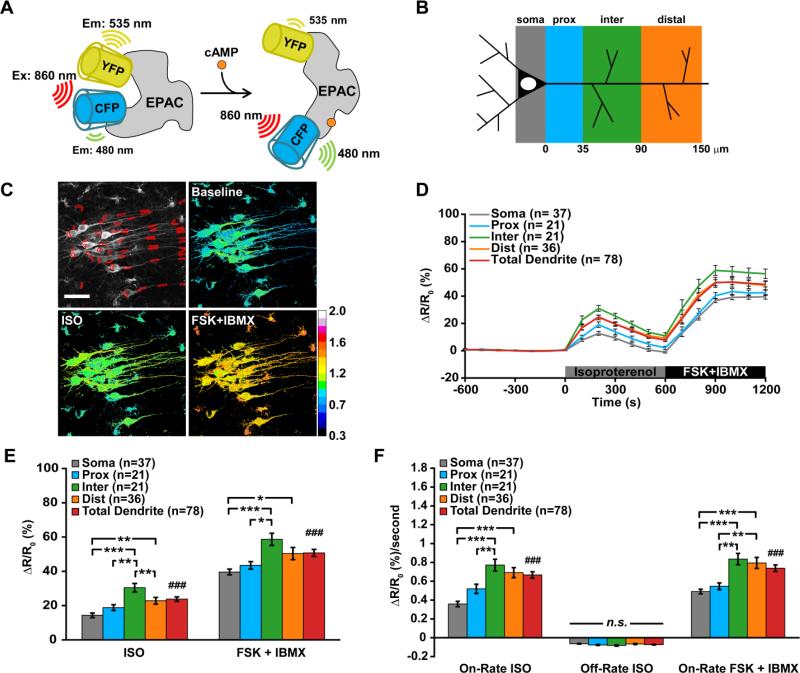
Figure 1. Cyclic-AMP transients in CA1 neurons have a greater rate and amplitude in dendrites compared to somata during β-adrenergic receptor activation.
(A) Cartoon of how EPAC-SH150 reports cAMP levels by decreasing the FRET response between a CFP and YFP fluorescent protein. (B) Classification of neuronal compartments by distance from soma. (C) Representative example of cAMP responses in CA1 neurons of brain slices. (D-F) Combined data from experiments using 100 nM or 1 μM isoproterenol. (D) Timecourse, (E) peak amplitudes during isoproterenol (F(3,111) = 12.62, p < 0.0001), (###p < 0.001) or FSK + IBMX application (F(3,111) = 7.613, p < 0.0001), (###p = 0.008), and (F) average rate of cAMP dynamics during isoproterenol (F(3,111) = 15.99, p < 0.0001), (###p < 0.001) or FSK + IBMX application (F(3,111) = 13.46, p < 0.0001), (###p < 0.001). Data are mean ± SEM. Soma vs dendritic compartments were analyzed by one-way ANOVA with Tukey's Test (*p < 0.05, **p < 0.01, ***p < 0.001). Soma vs total dendrites were analyzed by unpaired, two-tailed t-test with p-value as indicated. Scale bars are 40 μm.
After ROI placement, raw CFP and YFP intensity measurements for the entire time course were imported into Microsoft Excel. A fluorescence ratio was calculated for each time point in each ROI series and was normalized to the average baseline ratio for each respective ROI (average of 20 frames before first stimulus). For each ROI, peak amplitudes were determined by finding the maximum point during the stimulus interval (e.g. isoproterenol treatment), and taking the average of 2 points on either side of the max (average of 5 points total). For each ROI, the peak on-rate was determined by measuring the maximum slope over 4-5 points of the rising phase and peak off-rate was determined by measuring the most negative slope over 10-15 points of the falling phase starting after the peak isoproterenol response. Statistical analysis was performed in GraphPad Prism. One-way ANOVA followed by Tukey's multiple comparison test was used to identify significant differences in the means of each neuronal compartment. Two-tailed, unpaired t-test was used to compare the mean response in all dendrites to the mean response in somata.
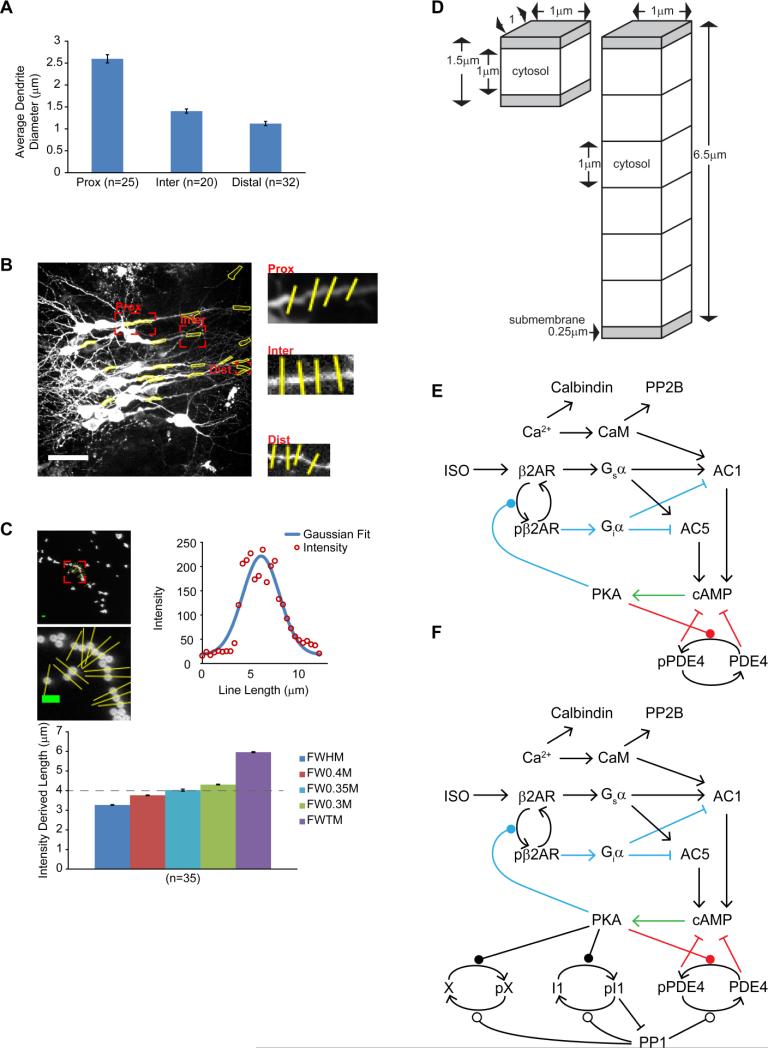
CA1 dendrite diameter measurements were determined with Image J. Line scans were placed across the dendrite and each intensity profile was plotted and fit with a Gaussian curve. The full-width of the Gaussian fit was measured at 35% of the maximum intensity and the average of four line scans was used to determine the diameter of each ROI for three slices.
2.4 Computational Modeling
We created a computational model of the βAR and calcium activated signaling pathways in hippocampal CA1 pyramidal neurons using NeuroRD version 3.0 (Jedrzejewski-Szmek and Blackwell, 2016), which simulates reaction-diffusion systems stochastically. The morphology of the model represents a thin neuronal cross-section with reflective boundary conditions: either a 6.5 μm diameter compartment representing proximal dendrite or soma; or a thinner 1.5 μm diameter intermediate or distal dendrite. The morphology is subdivided into two 0.25 μm submembrane voxels and additional 1 μm cytosolic voxels, to allow for 1 dimensional diffusion from the membrane toward the core. In the model, βAR stimulation activates the Gs subtype of GTP binding protein, which activates adenylyl cyclase (AC) types 1 and 5, to produce cAMP. AC1 additionally requires calcium-bound calmodulin binding for activation. cAMP activates PKA, which triggers several feedback mechanisms controlling cAMP dynamics. PKA phosphorylates both phosphodiesterase type 4, which enhances its activity, and βAR, which inactivates the receptor. Calbindin and calcineurin are included in the model to maintain physiological levels of calcium and calmodulin. In the model, βAR, G protein, AC and PKA holoenzyme are localized in the submembrane domain and do not diffuse. Cyclic-AMP, the catalytic subunit of PKA, PDE4 and calmodulin diffuse. For one set of simulations, one additional set of molecules was included: inhibitor-1, which is phosphorylated by active PKA and dephosphorylated by calcineurin; protein phosphatase 1, which is inhibited by phosphorylated inhibitor-1; and a non-specific PKA phosphoprotein called molecule X. In simulations using this additional set of molecules, protein phosphatase 1 dephosphorylates PDE4 and molecule X. In all cases, phosphorylated PDE4 is used as a read-out of the PKA activity, comparable to the AKAR fluorescence measured in the experiments. All model files needed to run the simulations (or to examine molecule quantities, diffusion constants and reaction rate constants) are available on ModelDB (https://senselab.med.yale.edu/ModelDB/showModel.cshtml?model=187608).
3. Results
3.1. β-AR-induced cAMP transients are stronger in intermediate/dendrites than in cell bodies
In the first series of experiments, we explored how cAMP dynamics operate in hippocampal neurons during global stimulation of β-ARs. Young hippocampal slices were infected with Sindbis virus encoding the cAMP FRET-based biosensor EPAC-SH150, which consists of a truncated and catalytically inactive version of EPAC1 (Klarenbeek et al., 2011) sandwiched between mTurquoise2 and cp174Citrine (Polito et al., 2013). Cyclic-AMP transients were monitored in CA1 pyramidal neurons in real time using two-photon excitation, ratiometric (CFP/YFP) microscopy. Up to four compartments were measured in CA1 neurons that included proximal, intermediate, and distal segments of apical dendrites, and regions of the cell body excluding the nucleus (see Fig. 1C). The general design of these experiments included a 10 minute baseline before a 10 minute treatment with the β-AR agonist isoproterenol (100 nM or 1 μM) and finally, a 10 minute combined treatment with the adenylyl cyclase activator, forskolin (10 μM) and the broad-spectrum phosphodiesterase (PDE) inhibitor, IBMX (100 μM) (Fig. 1C-D). We initially tested two different concentrations of isoproterenol (100 nM or 1 μM) and observed a similar trend in cAMP levels with a greater amplitude and rate in dendrites compared to somata and therefore combined the data from these experiments. Overall, we found that isoproterenol produced the largest amplitude cAMP transients in intermediate (ΔRatio = 30.4 ± 2.5%, n = 21) and distal dendrites (ΔRatio = 22.8 ± 2.0%, n = 36) that were significantly greater than in proximal dendrites (ΔRatio = 18.8 ± 1.7%, n = 21) and somata (ΔRatio = 14.3 ± 1.3%, n = 37) (one-way ANOVA, F(3,111) = 12.62, p < 0.0001) (Fig. 1E). Additionally, the average amplitude of cAMP transients in all dendrites was significantly greater than all somatic compartments during isoproterenol treatment (soma: ΔRatio = 14.3 ± 1.3%, n = 37 vs. total dendrites: ΔRatio = 23.8 ± 1.3%, n = 78; t-test, p < 0.001) (Fig. 1E). During treatment with FSK+IBMX, cAMP amplitudes in intermediate (ΔRatio = 58.6 ± 3.6%, n = 21) and distal dendrites (ΔRatio = 50.4 ± 3.5%, n = 36) were significantly greater than proximal dendrites (ΔRatio = 43.4 ± 2.2%, n = 21) and somata (ΔRatio = 39.6 ± 1.7%, n = 37) (F(3,111) = 7.613, p < 0.0001), and the average amplitude of cAMP transients in all dendrites was significantly greater than all somatic compartments (soma: ΔRatio = 39.6 ± 1.7%, n = 37 vs. total dendrites: ΔRatio = 50.7 ± 2.1%, n = 78; t-test, p = 0.008) (Fig. 1E). Calculating the average on-rate of cAMP produced with isoproterenol (expressed as a the change in fluorescence ratio, ΔRatio, as a function of time) revealed that intermediate (ΔRatio = 0.77 ± 0.06%/second, n = 21) and distal dendrites (ΔRatio = 0.70 ± 0.05%/second, n = 36) were significantly greater than somatic compartments (ΔRatio = 0.36 ± 0.03%/second, n = 37), but only intermediate dendrites were significantly different compared to proximal dendrites (ΔRatio = 0.52 ± 0.05%/second, n = 21) (one-way ANOVA, F(3,111) = 15.99, p < 0.0001) (Fig. 1F). Overall, the average on-rate of cAMP transients in dendrites was nearly twice the observed rate in somata (soma: ΔRatio = 0.36 ± 0.03%/second, n = 37 vs. total dendrites: ΔRatio = 0.67 ± 0.03%/second, n = 78; t-test, ###p < 0.001) (Fig. 1F). The average on-rate of cAMP during endpoint treatment with FSK+IBMX followed the same pattern for the average on-rate with isoproterenol: intermediate (ΔRatio = 0.83 ± 0.06%/second, n = 21) and distal dendrites (ΔRatio = 0.79 ± 0.06%/second, n = 36) were greater than proximal dendrites (ΔRatio = 0.55 ± 0.04%/second, n = 21) and somatic compartments (ΔRatio = 0.49 ± 0.02%/second, n = 37) (one-way ANOVA, F(3,111) = 13.46, p < 0.0001) and the average on-rate of cAMP in all dendrites was significantly faster compared to somata (soma: ΔRatio = 0.49 ± 0.02%/second, n = 37 vs. total dendrites: ΔRatio = 0.74 ± 0.03%/second, n = 78; t-test, ###p < 0.001) (Fig. 1F).
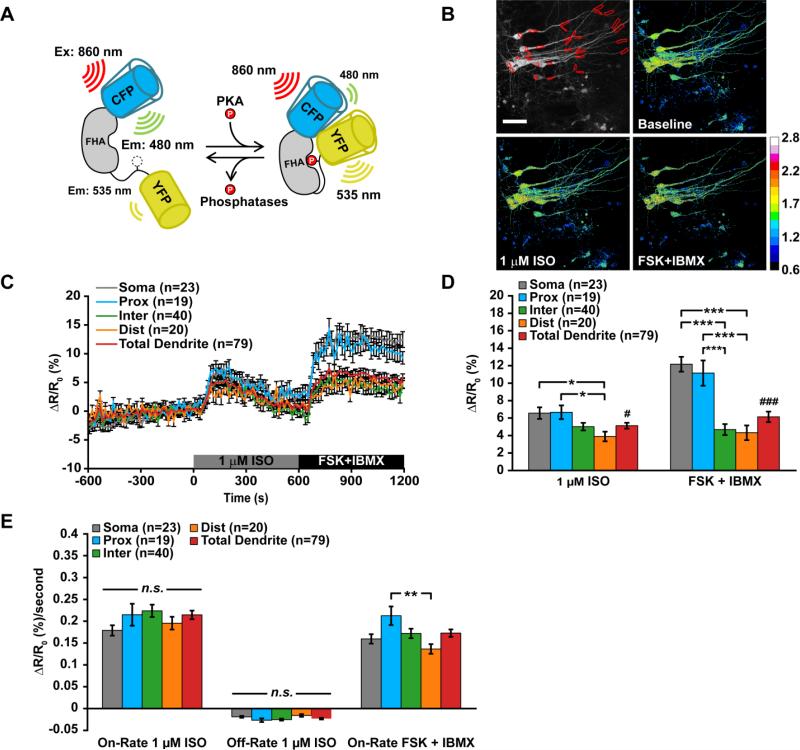
3.2. β-AR-induced PKA activation is stronger in cell bodies than in intermediate/distal dendrites
PKA is a major target of cAMP that plays an important role in regulating synaptic plasticity and memory. To determine how PKA activity is dynamically regulated during β-AR activation, we used Sindbis virus to express the PKA FRET sensor, AKAR3, which contains a PKA consensus phosphorylation motif and a phospho-binding domain sandwiched between ECFP and cpVenus (Allen and Zhang, 2006) (Fig. 2A). PKA activity was monitored in CA1 neurons of young slices and the FRET ratio was calculated over time (YFP/CFP) during stimulation with 1 μM isoproterenol before endpoint treatment with FSK+IBMX (Fig. 2B-C). With isoproterenol treatment, the amplitude of PKA activity was significantly larger in somata (ΔRatio = 6.6 ± 0.7%, n = 23) and proximal dendrites (ΔRatio = 6.7 ± 0.8%, n = 19) than in distal dendrites (ΔRatio = 3.9 ± 0.6%, n = 20), although it was not significantly different from intermediate (ΔRatio = 5.0 ± 0.4%, n = 40) (one-way ANOVA, F(3,98) = 3.837, p = 0.0121) or total dendrites (ΔRatio = 5.1 ± 0.3%, n = 79; t-test, #p = 0.0491) (Fig. 2D). Treatment with FSK+IBMX produced PKA activity in somata (ΔRatio = 12.2 ± 0.8%, n = 23) and proximal dendrites (ΔRatio = 11.2 ± 1.4%, n = 19) that was nearly twice the amplitude produced by isoproterenol treatment, and both were significantly greater than peak PKA activity in intermediate (ΔRatio = 4.7 ± 0.5%, n = 40) and distal dendrites (ΔRatio = 4.3 ± 0.8%, n = 20) (one-way ANOVA, F(3,98) = 5.373, p < 0.0001) (Fig. 2D). Additionally, PKA activity in somatic compartments was significantly greater than the average dendritic response produced with FSK+IBMX (soma: ΔRatio = 12.2 ± 0.8%, n = 23 vs. total dendrites: ΔRatio = 6.2 ± 0.6%, n = 79; t-test, ###p < 0.0001) (Fig. 2D).
Figure 2. PKA activity in CA1 neurons is greater in somata and proximal dendrites during β-adrenergic receptor activation.
(A) Left: Cartoon of how AKAR3 functions as a substrate and reporter of PKA activity. Right: Representative example of PKA activity in CA1 neurons of brain slices. (B, C and D) (B) Average timecourse, (C) average peak amplitude of PKA activity during application of 1 μM isoproterenol (F(3,98) = 3.837, p = 0.0121) ), (#p = 0.0491) or FSK + IBMX (F(3,98) = 5.373, p < 0.0001), (###p < 0.0001) and (D) average rates of PKA activity during application of 1 μM isoproterenol or FSK + IBMX (F(3,98) = 4.371, p = 0.0062). Data are mean ± SEM. Neuronal compartments were analyzed by one-way ANOVA and Tukey's Test (*p < 0.05, **p < 0.01, ***p < 0.001). Soma vs total dendrites were analyzed by unpaired, two-tailed t-test with p-value as indicated. Image scale bars are 40 μm.
During isoproterenol treatment, the apparent rates of PKA activation or deactivation (i.e. AKAR3 dephosphorylation) were not significantly different across neuronal compartments for either the on-rate or off-rate (Fig. 2E). However, stimulation with FSK+IBMX significantly increased the on-rate of PKA activity in proximal dendrites (ΔRatio = 0.21 ± 0.02%/second, n = 19) compared to distal dendritic segments (ΔRatio = 0.14 ± 0.01%/second, n = 20) (one-way ANOVA, F(3,98) = 4.371, p = 0.0062) (Fig. 2E).
3.3. Computational models show that an asymmetric density of adenylyl cyclase underlies compartmental differences in cAMP transients
To better understand the molecular signaling events producing the compartmental differences in cAMP concentration that we observed during live-imaging experiments, we developed a spatial, stochastic computational model. The signaling network and feedback loops are shown in Figure 3E. Simulated compartment sizes were approximated from the diameter of CA1 dendrites present in our imaging experiments. Line scan intensity distributions were calibrated to 4 μm fluorescent beads (Fig. 3C) and the average of 4 line scans were placed across each dendritic compartment (Fig. 3B). CA1 dendrite diameter progressively decreased from somata (~15 μm, not shown) to proximal dendrites (2.6 ± 0.1 μm), intermediate dendrites (1.4 ± 0.1 μm) and distal dendrites (1.1 ± 0.1 μm) (Fig. 3A-B). Therefore, a 1.5 μm width compartment served as a small intermediate or distal dendritic compartment and a 6.5 μm width compartment was used to approximate a large proximal dendrite or somatic compartment.
Figure 3. Spatial compartmental model of CA1 neuronal compartments.
(A) Compartment diameter of proximal dendrites (2.6 ± 0.1 μm), intermediate dendrites (1.4 ± 0.1 μm) and distal dendrites (1.1 ± 0.1 μm) on CA1 neurons. Values are mean ± standard error of dendrites measured from three slices. (B) Four line scans were taken across the same regions used for measuring cAMP transients to calculate the average diameter at that region. White scale bar is 40 μm. (C) Line scans intensity plots were calibrated to 4 μm fluorescent beads and the full-width of the intensity plot was calculated at 35% of the max was used to for dendrite measurements. Other measures along the intensity plot are full-width half-max (FWHM), 40% max (FW0.4M), 35% (FW0.35M), 30% (FW0.3M) and full-width tenth max (FWTM). Green scale bar is 8 μm. (D) Compartment dimensions used in computational simulations. (E-F) Major components of the signaling pathway used to simulate cAMP transients and PKA activity without (E), and with (F) the addition of protein phosphatases and molecule X. Feedback loop 1 is in blue, feedback loop 2 is in red and the green arrow is common to both pathways.
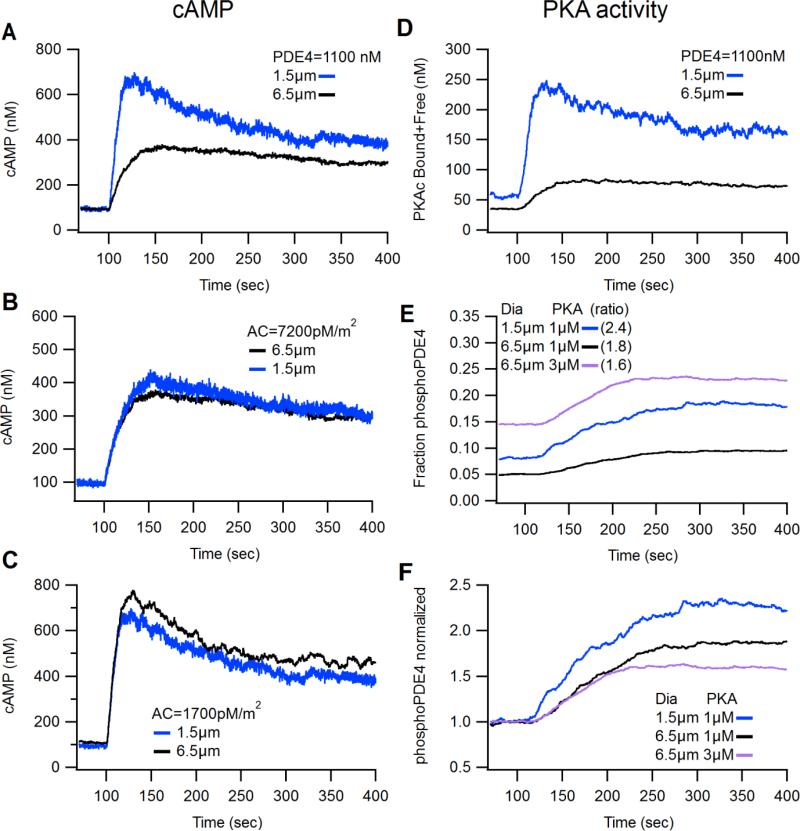
The resting level of cAMP was determined by a balanced, steady-state reaction between cAMP production by adenylyl cyclase (AC) and cAMP degradation by phosphodiesterase (modeled here with kinetics of PDE4), and thus initial simulations used basal cAMP concentration as the constraint on the relative quantities of AC and PDE4. Two different sets of AC/PDE4 concentrations were able to achieve a resting level of ~100 nM cAMP in both size compartments. One approach was to keep PDE4 concentration identical in both compartment sizes. With this constraint, the density of AC needed to be higher in the 6.5 μm compartment compared to the 1.5 μm compartment. Specifically, to achieve a resting level of ~100 nM cAMP, AC density was adjusted so that the 1.5 μm compartment contained 1,700 pM/m2 and the 6.5 μm compartment contained 7,200 pM/m2 (Fig. 4A). The second approach was to keep AC density the same in both compartment sizes. With this latter approach and a high, uniform density of AC (7,200 pM/m2) in both compartment sizes, the small compartment required 4 fold more PDE4 (4,420 nM) compared to the large compartment (1,100 nM) to maintain a 100 nM resting level of cAMP (Fig. 4B). Conversely, with a low, uniform density of AC (1,700 pM/m2) in both small and large compartments, the large compartment required 4 fold less PDE4 (250 nM) compared to the small compartment (1,100 nM) to achieve stable baseline cAMP levels (Fig. 4C). It is apparent that basal cAMP levels are controlled by the ratio between production and degradation pathways. Because AC is a “surface” (membrane) molecule regulating cAMP production and PDE4 is a “volume” (cytosolic) molecule regulating degradation, this ratio implies that basal cAMP is proportional to the AC:PDE4 ratio. Thus, if AC is uniformly distributed between both compartments, PDE4 needs to be reduced in large compartment to maintain the same AC:PDE4 ratio.
Figure 4. Computational modeling determines compartment size and adenylyl cyclase density shape cAMP dynamics.
(A) Simulations of a small (1.5 μm) and large (6.5 μm) dendritic compartment with a uniform concentration of PDE4 (1,100 nM) in both compartments and an asymmetric density of adenylyl cyclase (small=1,700 pmol/m2; large= 7,200 pmol/m2) produced compartmental differences in cAMP transients that resembled cAMP dynamics measured in live-imaging experiments. (B) A “high” uniform density of AC (7,200 pmol/m2) requires a greater concentration of PDE4 in the small compartment (4,420 nM) compared to large compartment (1,100 nM) to maintain basal cAMP levels. Induced cAMP transients are similar in both compartments. (C) A “low” uniform density of AC (1,700 pmol/m2) requires a lower concentration of PDE4 in the large compartment (250 nM) compared to the small compartment (1,100 nM) to maintain basal cAMP levels. Induced cAMP transients are similar in both compartments. (D-F) Simulations using the conditions for AC and PDE4 Fig. 3A. (D) cAMP-activated PKA is greater in the small compartment compared to the large compartment. “PKAc released” is the total PKA catalytic subunits released from PKA regulatory subunits whether interacting with a phospho-substrate or freely diffusing. (E and F) Phosphorylation status of PDE4 as a fraction of total pPDE4 (panel E) or normalized fraction (panel F). The small compartment has more initial and stimulated pPDE4 compared to large compartments and thus the relative increase in PKA activity did not resemble PKA activity measured in live-imaging experiments. Increasing the concentration of PKA subunits increased the basal fraction of pPDE4 (panel E), but reduced the peak fractional change (panel F).
When the models with the uniform PDE4 concentration were stimulated with 1 μM isoproterenol, the rate and amplitude of cAMP transients were greater in the small compartment compared to the large compartment (Fig. 4A) suggesting that an asymmetric distribution of AC density may underlie our observed experimental result (Fig. 1). In contrast, Figures 4B and 4C show that when AC density was the same in both compartment sizes, stimulated cAMP transients were similar in both compartments and did not reproduce the experimental observations of larger cAMP transient in the small dendrites. Because the conditions used in Fig. 4A produced cAMP transients that resembled the pattern we observed in imaging experiments, we concluded that a uniform distribution of PDE4 and an asymmetric distribution of adenylyl cyclase may be representative of how cAMP signaling is coordinated in vivo and these conditions were used to explore the mechanisms regulating PKA dynamics.
3.4 Computational models show that increased phosphatase concentrations can limit the normalized change in phosphorylated PKA substrates
Protein kinase-A (PKA) is one of the major effectors regulated by cAMP; thus a greater cAMP signal is predicted to produce a larger PKA signal. Because our live imaging experiments revealed a larger PKA signal in dendritic regions with smaller cAMP signals, we used computational modeling to evaluate several mechanisms that might account for these results. When active, PKA releases diffusible catalytic subunits (PKAc) that interact with and phosphorylate substrate proteins that carry the consensus phosphorylation sequence specific for PKA. Protein phosphatases, such as PP1, PP2A or PP2B/calcineurin dephosphorylate substrates phosphorylated by PKA, which serves to maintain steady-state levels of phosphorylated substrates and reset the signaling pathway after receiving a stimulus. We hypothesized three potential molecular mechanisms that may account for our observed measures of greater PKA activity in large neuronal compartments compared to small compartments: 1. small dendritic compartments have a lower concentration of PKA, 2. small dendritic compartments have a higher concentration of phosphatases that dephosphorylate the AKAR sensor, or 3. small compartments have a greater concentration of substrate proteins which compete with AKAR for PKAc. We evaluated PKA activity using two measures: 1. “PKAc released” represents all PKAc subunits that are released from PKA regulatory subunits (PKAreg). This measure does not take into account phosphatase activity, but varies with the amount of substrate proteins. 2. Because the AKAR sensor has not been biochemically characterized, we evaluated the phosphorylation of PDE4 to represent PKA activity. This measure is sensitive to both phosphatase activity and the amount of competing substrate proteins.
Using our conditions determined by modeling cAMP signaling, which included a uniform distribution of PDE4 and an asymmetric distribution of adenylyl cyclase (Fig. 4A; Section 3.3), we evaluated whether PKA activity would be greater in the large compartment, as observed experimentally. When PKA enzyme concentration, PKA substrate concentration and phosphatase concentration were the same in both the small and large compartment, the model showed that activation of the GPCR produced a greater concentration of PKAc released (Fig. 4D) and phosphorylated PDE4 (Fig. 4E-F) in small compartments compared to large compartments, similar to the difference observed with the cAMP response (Fig. 4A). These simulations did not match the experimental results, suggesting that other signaling molecules or parameters contributed to the observations.
We then tested which of the three mechanisms suggested above (i.e., different concentrations of PKA, of phosphatases, or of competing endogenous substrates) could account for the experimental results. First, we increased the concentration of PKA in the large compartment by three times and found the resting fraction of phosphoPDE4 was greater in the large compartment than in the small compartment (Fig. 4E, pink trace). However, since live imaging experiments measured PKA activity as the ratio of stimulated to basal AKAR fluorescence, we expressed PKA activity in the model as the normalized ratio of stimulated phosphoPDE4 to basal phosphoPDE4. Using the normalized phosphorylation status of uniformly distributed PDE4, our model showed that increasing the amount of PKA in large compartments did not increase phosphorylation of PDE4 (Fig. 4F, pink trace). Together these findings suggested that the concentration of PKA cannot account for the greater apparent PKA activity observed in somata as compared to dendrites.
Next, to evaluate the role of phospho-substrate competition or dephosphorylation via phosphatase activity, we added additional components to the model. These components included: an additional PKA substrate, molecule X, which served as an unidentified PKA substrate that competes with PDE4; dephosphorylation of PDE4 by protein phosphatase 1 (PP1); and inhibitor-1 (I-1) which binds to and inhibits PP1 when phosphorylated by PKA to establish a positive-feedback mechanism that prolongs the PKA-dependent phosphorylation of substrates (Fig. 3F). We used affinities of PKA and PP1 for molecule X that were similar to those for PDE4.
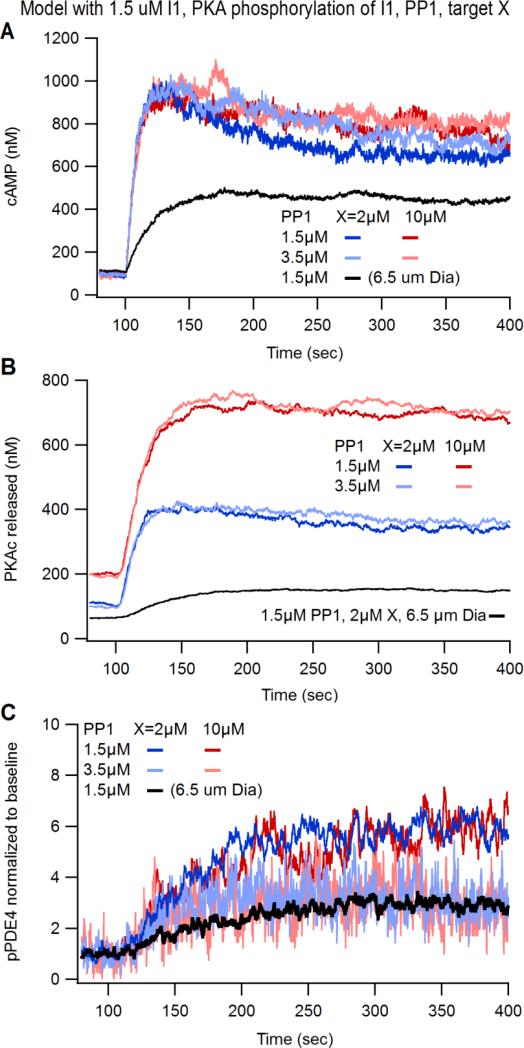
The quantity of PP1 and molecule X were varied to investigate whether a higher phosphatase activity in the dendrites or greater competition of substrates for PKA could explain the experimental results. Fig 5A shows that incorporating PP1, I-1, and X into our computational model did not change the cAMP responses significantly: the cAMP transient remained greater in the small compartment than in the large compartment. The addition of PP1, I-1 and X produced an increase in the total amount of PKAc released (Fig. 5B) relative to simulations without PP1, I-1 and X (Fig. 4D), but PKA activity remained greater in the small compartment compared to large compartment. Interestingly, simulations with 5 times more X showed a dramatic increase in the level of PKAc released (Fig. 5B; red traces) which is likely due to excess substrate X interacting with and delaying PKAc subunits from reuniting with PKAreg subunits to form the inactive PKA holoenzyme. Nonetheless, the greater quantity of molecule X did not lower the phosphorylation of PDE4 relative to baseline for either quantity of PP1, because molecule X equally affected both the basal and transient phosphorylation level. In contrast, if the amount of PP1 was increased from 1.5 to 3.5 μM in small compartments, the amount of pPDE4 was reduced (Fig. 5C). This result was observed for both high and low levels of molecule X. In summary, our simulation results suggest that protein phosphatase levels, and not PKA quantity or competition from other phosphoproteins, may differentially affect PKA signaling in small compartments and may explain the compartmental differences in PKA activity observed in our imaging experiments.
Figure 5. Computational modeling determines that protein phosphatase 1 is a potential mechanism suppressing PKA activity in small dendritic compartments and excess PKA substrate does not affect PKA signaling dynamics.
(A-C) The computational model defined in Fig. 3A was modified to include different concentrations of protein phosphatase 1 (PP1) and various concentrations of PKA substrate (molecule “X”). (A) cAMP transients were not affected by different concentrations of PP1 or X. (B) PKAc Released increases with greater concentrations of X but were unaffected by greater PP1. (C) The normalized phosphorylation status of PDE4 was not affected by different concentrations of X, but was reduced with greater concentrations of PP1.
4. Discussion
Here, we used live fluorescent imaging and computational modeling to investigate the molecular constraints shaping the compartmental dynamics of cAMP and PKA activity in hippocampal neurons. Our imaging experiments show that global stimulation of β-adrenergic receptors produced cAMP transients with a greater amplitude and faster on-rate in small dendrites compared to large compartments like somata. However, the same stimulation protocol produced greater PKA activity in large compartments compared to small dendrites. Computational modeling was used to investigate the mechanisms producing these spatial gradients. Simulations of cAMP found that as neuronal structures increase in size, the density of rate-limiting components such as adenylyl cyclase must also increase, scaling in a manner similar to cable theory (Zador and Koch, 1994). If AC density was uniformly distributed in our small and large compartmental model, we suspect that an asymmetric distribution of β-ARs could be an alternative mechanism to normalize resting cAMP levels and reproduce larger stimulated cAMP transients in the small compartment compared to the large compartment. Although β-ARs are clustered in CA1 somata, dendrites and Schaffer collateral synapses (Davare, 2001; Joiner et al., 2010), it is unclear if the receptors are enriched in dendrites relative to somata. However, the total amount of adenylyl cyclase appears to be enriched in stratum pyramidale of area CA1 (Mons et al., 1995) which supports our model's prediction that AC density increases with compartment size. Our computational model also relied on a uniform distribution of PDEs between small and large compartments to shape degradation of cAMP transients after GPCR activation. Interestingly, our experimental imaging results show that the off-rate of cAMP transients did not differ between somatic or dendritic compartments (Fig 1F), suggesting that PDE activity may be uniformly distributed as predicted in our simulation. Prior models have also found that dendritic spines can decrease axial diffusion of small molecules (Li et al., 2015; Mohapatra et al., 2016; Santamaria et al., 2006) and may serve as another alternative mechanism to produce spatial gradients of cAMP. However, our observed spatial gradients in cAMP and PKA signaling were produced by global stimulation of cAMP signaling. Therefore, we did not explore axial diffusion or the effect of spines in our model.
In the absence of protein phosphatases, our computational simulations found, as expected, that PKA activity was proportional to the size of the cAMP transient in a particular dendritic segment (i.e., a large cAMP transient produced a large amplitude PKA response). However when PP1 was included in the model, an increase in PP1 could reduce PKA activity as measured by phosphoprotein levels, more so for stimulated compared to basal levels. Although protein phosphatases are known to interact with A-Kinase Anchoring Proteins (AKAPs) (Skroblin et al., 2010), thereby positioning bidirectional regulatory mechanisms (i.e., kinases and phosphatases) in close spatial proximity, it is currently unknown if protein phosphatases are enriched in small dendritic compartments compared to large compartments. An important future direction would be to asses spatial distributions of protein phosphatases and if activity of any particular phosphatase dominates following activation of β-adrenergic signaling.
Our experimental results from brain slices and computational simulations agree with previous studies from cultured neurons that showed activation of β-ARs produced greater cAMP levels in smaller diameter processes (Kim et al., 2011; Neves et al., 2008). Because cultured neurons develop a dissociated network of synaptic connections and typically have few astrocytes, our results suggest that the observed spatial gradients are cell-autonomous and are not influenced by intercellular signaling following activation of β-ARs on hippocampal astrocytes or CA3 neurons. Similar to prior studies, we find that surface to volume ratio is important. Indeed, basal cAMP is controlled by surface to volume ratio because basal cAMP depends on the balance between production and degradation mechanisms. Since AC is a membrane-bound “surface” molecule and PDE4 is a cytosolic “volume” molecule, this ratio implies that basal cAMP is proportional to the AC:PDE4 ratio. Despite having the same AC:PDE4 ratio and basal cAMP for all models (Fig. 4 and 5), we find that the transient response still differs depending on the AC quantity. Specifically, simulations revealed that a uniform AC:PDE4 ratio across different compartment sizes normalizes basal cAMP levels and reproduces our experimentally observed spatial gradient in cAMP transients.
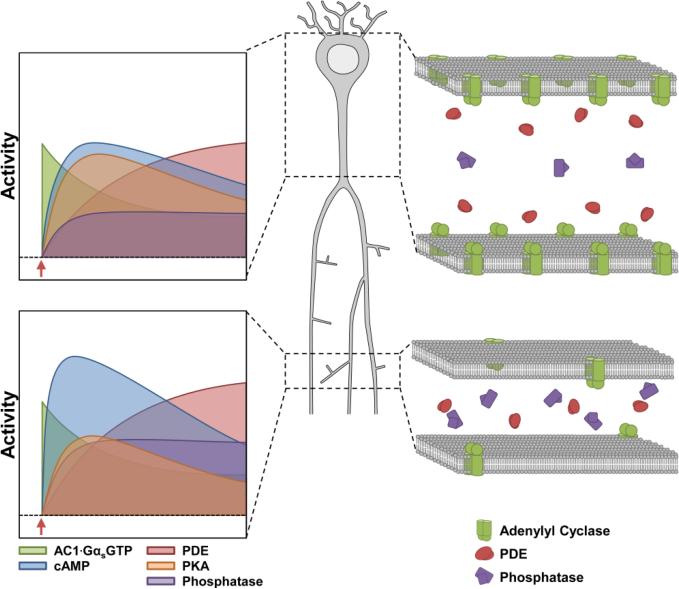
The shape of a cAMP transient is caused by inactivation mechanisms that follow the activation phase. These inactivation mechanisms include inactivation of β-AR and enhancement of PDE4 activity by PKA phosphorylation (Fig. 3E-F). The latter forms a negative feedback loop controlling cAMP; thus the amplitude of the observed cAMP transient is dependent on the rate of cAMP production as well as the onset and rate of inactivation mechanisms (Novák and Tyson, 2008; Yang and Ferrell, 2013). This suggests that the observed bias toward a greater cAMP transient in the small compartment results from generating cAMP at a rate that greatly exceeds inactivation mechanisms. In contrast, the large compartment exhibits a gradual rise in cAMP, which cannot escape inactivation mechanisms (Fig. 6). Our model also suggests that activated pPDE4 can compensate for changes in upstream signaling dynamics (Fig. 4E) and surprisingly, a compensatory increase in pPDE4 activity was observed in transgenic mice that expressed a conditionally active Gαs* subunit (Kelly et al., 2007). Similarly, the greater PKA activity observed in large compartments would enhance inactivation mechanisms relative to the rate of cAMP production to attenuate cAMP transients relative to small compartments (Fig. 6).
Figure 6. Summary of compartmental differences influencing cAMP and PKA dynamics.
(Left graphs) Interactions between “activation mechanisms” (ACs) and “inactivation mechanisms” (include PDEs and PPs) shape compartmental differences in cAMP and PKA signaling dynamics. (Right panels) Illustrations showing that AC density increases with compartment size and PDE concentrations are relatively the same in both small and large compartments. Phosphatase enrichment in small compartments may be a mechanism limiting bulk PKA activity in small dendritic compartments.
Overall, our findings help clarify how signaling dynamics may operate during induction of hippocampal LTP. Previous studies have shown that isoproterenol treatment alone does not alter synaptic plasticity, but long-lasting LTP can be produced when isoproterenol is paired with weak stimulation of Schaffer collateral synapses (O'Dell et al., 2015). However, bath application of forskolin without synaptic stimulation can produce a form of long-lasting synaptic facilitation that requires cAMP, PKA activity and PKA anchoring to AKAPs (Chavez-Noriega and Stevens, 1992; Kim et al., 2011; Woo et al., 2002). Our imaging results show that isoproterenol produces a transient response in cAMP and PKA activity, whereas stimulation with forskolin + IBMX produces a sustained, large-amplitude response, suggesting that the cAMP/PKA activity observed with isoproterenol treatment is subthreshold for LTP. Although it is unclear whether the threshold for cAMP/PKA-dependent LTP relies on the amplitude, duration or total amount of activity, future experiments may be able to explore this question by combining fluorescent biosensor imaging with electrophysiological recordings.
Highlights.
Neuronal β-AR activity produces spatially patterns of cAMP different than PKA
Non-uniform AC normalizes basal cAMP and reproduces spatially-distinct transients
Enrichment of PP1 is proposed mechanism shaping spatially-distinct PKA activity
Acknowledgements
This work was supported by NIMH grant R01 MH086415 (TA), NIAAA grant R01 AA018060 (TA and KB), NSF grant 1515458 (TA), NSF grant 1515686 (KB), ERC-2009-AdG-250349 (JAG), and Chateaubriand Fellowship (VL). We wish to thank Pierre Vincent for providing the Sindbis EPAC-SH150, Ludovic Tricoire (UPMC, Paris) for the Matlab z-registration script, and members of the Abel lab for helpful discussions in preparing the manuscript. TA is the Brush Family Professor of Biology at the University of Pennsylvania.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen MD, Zhang J. Subcellular dynamics of protein kinase A activity visualized by FRET-based reporters. Biochem. Biophys. Res. Commun. 2006;348:716–721. doi: 10.1016/j.bbrc.2006.07.136. [DOI] [PubMed] [Google Scholar]
- Barad M, Bourtchouladze R, Winder DG, Golan H, Kandel E. Rolipram, a type IV-specific phosphodiesterase inhibitor, facilitates the establishment of long-lasting long- term potentiation and improves memory. Proc. Natl. Acad. Sci. U. S. A. 1998;95:15020–15025. doi: 10.1073/pnas.95.25.15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernabeu R, Bevilaqua L, Ardenghi P, Bromberg E, Schmitz P, Bianchin M, Izquierdo I, Medina JH. Involvement of hippocampal cAMP/cAMP-dependent protein kinase signaling pathways in a late memory consolidation phase of aversively motivated learning in rats. Proc. Natl. Acad. Sci. U. S. A. 1997;94:7041–7046. doi: 10.1073/pnas.94.13.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourtchouladze R, Lidge R, Catapano R, Stanley J, Gossweiler S, Romashko D, Scott R, Tully T. A mouse model of Rubinstein-Taybi syndrome: defective long-term memory is ameliorated by inhibitors of phosphodiesterase 4. Proc. Natl. Acad. Sci. U. S. A. 2003;100:10518–10522. doi: 10.1073/pnas.1834280100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, Alkire MT. Epinephrine enhancement of human memory consolidation: Interaction with arousal at encoding. Neurobiol. Learn. Mem. 2003;79:194–198. doi: 10.1016/s1074-7427(02)00036-9. [DOI] [PubMed] [Google Scholar]
- Cahill L, Prins B, Weber M, McGaugh JL. Beta-adrenergic activation and memory for emotional events. Nature. 1994;371:702–704. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- Castro LRV, Gervasi N, Guiot E, Cavellini L, Nikolaev VO, Paupardin-Tritsch D, Vincent P. Type 4 phosphodiesterase plays different integrating roles in different cellular domains in pyramidal cortical neurons. J. Neurosci. 2010;30:6143–6151. doi: 10.1523/JNEUROSCI.5851-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez-Noriega LE, Stevens CF. Modulation of synaptic efficacy in field CA1 of the rat hippocampus by forskolin. Brain Res. 1992;574:85–92. doi: 10.1016/0006-8993(92)90803-h. [DOI] [PubMed] [Google Scholar]
- Chay A, Zamparo I, Koschinski A, Zaccolo M, Blackwell KT, Thomas M, Moody T, Makhinson M, O'Dell T, Winder D, et al. Control of βAR- and N-methyl-D-aspartate (NMDA) Receptor-Dependent cAMP Dynamics in Hippocampal Neurons. PLOS Comput. Biol. 2016;12:e1004735. doi: 10.1371/journal.pcbi.1004735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davare MA. A beta 2 Adrenergic Receptor Signaling Complex Assembled with the Ca2+ Channel Cav1.2. Science (80−. ) 2001;293:98–101. doi: 10.1126/science.293.5527.98. [DOI] [PubMed] [Google Scholar]
- Duffy SN, Nguyen PV. Postsynaptic application of a peptide inhibitor of cAMP-dependent protein kinase blocks expression of long-lasting synaptic potentiation in hippocampal neurons. J. Neurosci. 2003;23:1142–1150. doi: 10.1523/JNEUROSCI.23-04-01142.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey U, Huang YY, Kandel ER. Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science. 1993;260:1661–1664. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- Gelinas JN, Tenorio G, Lemon N, Abel T, Nguyen PV. Beta-adrenergic receptor activation during distinct patterns of stimulation critically modulates the PKA-dependence of LTP in the mouse hippocampus. Learn. Mem. 2008;15:281–289. doi: 10.1101/lm.829208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genoux D, Haditsch U, Knobloch M, Michalon A, Storm D, Mansuy IM, M K, a M, D S, I M. Protein phosphatase 1 is a molecular constraint on learning and memory. Nature. 2002;418:970–975. doi: 10.1038/nature00928. [DOI] [PubMed] [Google Scholar]
- Gervasi N, Hepp R, Tricoire L, Zhang J, Lambolez B, Paupardin-Tritsch D, Vincent P. Dynamics of protein kinase A signaling at the membrane, in the cytosol, and in the nucleus of neurons in mouse brain slices. J. Neurosci. 2007;27:2744–2750. doi: 10.1523/JNEUROSCI.5352-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey CD, Yasuda R, Zhong H, Svoboda K. The spread of Ras activity triggered by activation of a single dendritic spine. Science. 2008;321:136–140. doi: 10.1126/science.1159675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Real E, Takamiya K, Kang M-G, Ledoux J, Huganir RL, Malinow R. Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell. 2007;131:160–173. doi: 10.1016/j.cell.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Medina JH, Izquierdo LA, Barros DM, de Souza MM, Mello e Souza T. Short- and long-term memory are differentially regulated by monoaminergic systems in the rat brain. Neurobiol. Learn. Mem. 1998;69:219–224. doi: 10.1006/nlme.1998.3825. [DOI] [PubMed] [Google Scholar]
- Jędrzejewski-Szmek Z, Blackwell KT. Asynchronous τ-leaping. The Journal of Chemical Physics. 2016;144(12):125104. doi: 10.1063/1.4944575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner MA, Lisé M-F, Yuen EY, Kam AYF, Zhang M, Hall DD, Malik ZA, Qian H, Chen Y, Ulrich JD, et al. Assembly of a β2-adrenergic receptor—GluR1 signalling complex for localized cAMP signalling. EMBO J. 2010;29:482–495. doi: 10.1038/emboj.2009.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MP, Isiegas C, Cheung Y-F, Tokarczyk J, Yang X, Esposito MF, Rapoport DA, Fabian SA, Siegel SJ, Wand G, et al. Constitutive activation of Galphas within forebrain neurons causes deficits in sensorimotor gating because of PKA-dependent decreases in cAMP. Neuropsychopharmacology. 2007;32:577–588. doi: 10.1038/sj.npp.1301099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Park AJ, Havekes R, Chay A, Guercio LA, Oliveira RF, Abel T, Blackwell KT. Colocalization of protein kinase A with adenylyl cyclase enhances protein kinase A activity during induction of long-lasting long-term-potentiation. PLoS Comput. Biol. 2011;7:e1002084. doi: 10.1371/journal.pcbi.1002084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarenbeek JB, Goedhart J, Hink MA, Gadella TWJ, Jalink K. A mTurquoise-based cAMP sensor for both FLIM and ratiometric read-out has improved dynamic range. PLoS One. 2011;6:e19170. doi: 10.1371/journal.pone.0019170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Gervasi N, Girault J-A. Dendritic geometry shapes neuronal cAMP signalling to the nucleus. Nat. Commun. 2015;6:6319. doi: 10.1038/ncomms7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maity S, Jarome TJ, Blair J, Lubin FD, Nguyen PV. Norepinephrine goes nuclear: Epigenetic modifications during long-lasting synaptic potentiation triggered by activation of beta-adrenergic receptors. J. Physiol. 2015 doi: 10.1113/JP271432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra N, Tønnesen J, Vlachos A, Kuner T, Deller T, Nägerl UV, Santamaria F, Jedlicka P, Koninck Y. De, Fiumelli H, et al. Spines slow down dendritic chloride diffusion and affect short-term ionic plasticity of GABAergic inhibition. Sci. Rep. 2016;6:23196. doi: 10.1038/srep23196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mons N, Harry A, Dubourg P, Premont RT, Iyengar R, Cooper DM. Immunohistochemical localization of adenylyl cyclase in rat brain indicates a highly selective concentration at synapses. Proc. Natl. Acad. Sci. U. S. A. 1995;92:8473–8477. doi: 10.1073/pnas.92.18.8473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves SR, Tsokas P, Sarkar A, Grace EA, Rangamani P, Taubenfeld SM, Alberini CM, Schaff JC, Blitzer RD, Moraru II, et al. Cell shape and negative links in regulatory motifs together control spatial information flow in signaling networks. Cell. 2008;133:666–680. doi: 10.1016/j.cell.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novák B, Tyson JJ. Design principles of biochemical oscillators. Nat. Rev. Mol. Cell Biol. 2008;9:981–991. doi: 10.1038/nrm2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell TJ, Connor SA, Guglietta R, Nguyen PV, Dell TJO, Connor SA, Guglietta R, Nguyen PV, O'Dell TJ, Connor SA, et al. β-Adrenergic receptor signaling and modulation of long-term potentiation in the mammalian hippocampus. Learn. Mem. 2015;22:461–471. doi: 10.1101/lm.031088.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polito M, Klarenbeek J, Jalink K, Paupardin-Tritsch Daniele, Vincent P, Castro LRVV, Paupardin-Tritsch D, Vincent P, Castro LRVV. The NO/cGMP pathway inhibits transient cAMP signals through the activation of PDE2 in striatal neurons. Front. Cell. Neurosci. 2013;7:211. doi: 10.3389/fncel.2013.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamaria F, Wils S, De Schutter E, Augustine GJ. Anomalous diffusion in Purkinje cell dendrites caused by spines. Neuron. 2006;52:635–648. doi: 10.1016/j.neuron.2006.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat. Rev. Neurosci. 2009;10:211–223. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- Skroblin P, Grossmann S, Schäfer G, Rosenthal W, Klussmann E. Mechanisms of protein kinase A anchoring. Int. Rev. Cell Mol. Biol. 2010;283:235–330. doi: 10.1016/S1937-6448(10)83005-9. [DOI] [PubMed] [Google Scholar]
- Spruston N. Pyramidal neurons: dendritic structure and synaptic integration. Nat. Rev. Neurosci. 2008;9:206–221. doi: 10.1038/nrn2286. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Moody TD, Makhinson M, O'Dell TJ. Activity-dependent beta-adrenergic modulation of low frequency stimulation induced LTP in the hippocampal CA1 region. Neuron. 1996;17:475–482. doi: 10.1016/s0896-6273(00)80179-8. [DOI] [PubMed] [Google Scholar]
- Turnham RE, Scott JD. Protein kinase A catalytic subunit isoform PRKACA; History, function and physiology. Gene. 2016;577:101–108. doi: 10.1016/j.gene.2015.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola H, Furman M, Izquierdo LAI, Alonso M, Barros DM, de Souza MM, Izquierdo I, Medina JH, Souza M.M. De, Medina JH, et al. Phosphorylated cAMP response element-binding protein as a molecular marker of memory processing in rat hippocampus: effect of novelty. J. Neurosci. 2000;20:RC112. doi: 10.1523/JNEUROSCI.20-23-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo NH, Abel T, Nguyen PV. Genetic and pharmacological demonstration of a role for cyclic AMP-dependent protein kinase-mediated suppression of protein phosphatases in gating the expression of late LTP. Eur. J. Neurosci. 2002;16:1871–1876. doi: 10.1046/j.1460-9568.2002.02260.x. [DOI] [PubMed] [Google Scholar]
- Woolfrey KM, Dell'Acqua ML, Acqua XMLD, Dell'Acqua ML. Coordination of Protein Phosphorylation and Dephosphorylation in Synaptic Plasticity. J. Biol. Chem. 2015;290:jbc.R115.657262. doi: 10.1074/jbc.R115.657262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Ferrell JE. The Cdk1-APC/C cell cycle oscillator circuit functions as a time-delayed, ultrasensitive switch. Nat. Cell Biol. 2013;15:519–525. doi: 10.1038/ncb2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccolo M, Pozzan T. Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science. 2002;295:1711–1715. doi: 10.1126/science.1069982. [DOI] [PubMed] [Google Scholar]
- Zador A, Koch C. Linearized models of calcium dynamics: formal equivalence to the cable equation. J. Neurosci. 1994;14:4705–4715. doi: 10.1523/JNEUROSCI.14-08-04705.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai S, Ark ED, Parra-Bueno P, Yasuda R. Long-distance integration of nuclear ERK signaling triggered by activation of a few dendritic spines. Science. 2013;342:1107–1111. doi: 10.1126/science.1245622. [DOI] [PMC free article] [PubMed] [Google Scholar]