Abstract
In the latter half of the 20th century, among participants of the Framingham Heart Study, incidence of heart failure (HF) has declined by about a third in women but not in men and survival after the onset of HF has improved in both sexes; however, HF remains highly lethal with over 50% dying within 5 years after onset of HF. Overall, the 8-year relative risk of HF is 24% lower in women compared with men. The 8-year incidence rates of HF with preserved ejection fraction (HFPEF; EF >45%) and HF with reduced EF (HFREF; EF ≤45%) in women and HFPEF in men are similar; however, men have a 2-fold higher cumulative incidence of HFREF than HFPEF. The lifetime risk of HF is about 20% in both women and men at 40, 50, 60, 70, and 80 years of age. Contribution of hypertension and diabetes mellitus to the risk of HF was more prominent in women than in men. Serum levels of several biomarkers were distinctly different in women compared with men and had differential effects on left ventricular structure and function; however, the strength and direction of the association between biomarkers levels and HF risk were generally similar in women and men. In individuals with HF, about two-thirds of the underlying cause of death and about one-half of the immediate cause of death were due to cardiovascular causes. Non-cardiovascular underlying and immediate causes of death were more evident in HFPEF.
Keywords: Biomarkers, Epidemiology, Framingham heart study, Gender, Heart failure, Incidence, Men, Mortality, Risk factors, Sex-differences, Survival, Women
Introduction
Framingham Heart Study (FHS) is a large population-based study that started in the late 1940s. Over the last 65 years, three generations of participants have been enrolled and followed in the FHS clinic at every 2, 4, or 8-year intervals. Women and men are almost equally represented. Participants are under continuous surveillance for the incidence of clinical endpoints of interest, including heart failure (HF). Over the years at FHS, the same set of clinical criteria has been used for the diagnosis of HF [1]. Hence, FHS provides an optimal milieu to examine the incidence, survival, relative risk, and lifetime risk of HF; evaluate the risk factors and markers of HF; and describe the modes of death after the onset of HF. In the present report, we have reviewed key articles from the FHS to glean insights into sex-based differences in the epidemiology of HF.
Incidence of HF
In 1950–69, the HF incidence rate/10,000 person years of follow-up was 42 (95% confidence interval [CI] 34–50) in women and 63 (95% CI 48–78) in men [2]. Over the next 30 years, the incidence rate declined by about one-third (95% CI 31 to 40%) in women, primarily between time periods 1950–69 and 1970–79, but remained almost unchanged in men [2]. The reasons for the disparity in HF incidence trends between sexes are unclear.
Among FHS participants who developed acute myocardial infarction (n = 676, 34% women), the 30-day and 5-year incidence of HF increased from 10 and 27.6% in the decade of the 1970s to 23.1 and 31.9%, respectively, in the decade of the 1990s [3]. The increase in HF incidence rates was commensurate with the decline in mortality after myocardial infarction – the 30-day and 5-year mortality rate declined from 12.2 and 41.1% in the 1970s to 4.1 and 17.3%, respectively, in the 1990s [3]. However, among those who survived 30 days after acute myocardial infarction without HF, the incidence of HF remained unchanged. Sex-specific analyses were not performed.
Survival After Onset of HF
Between 1948 and 1988, median survival after the onset of HF was better in women than in men (3.2 years versus 1.7 years) [4]. One-, 2-, 5-, and 10-year survival rates were 64, 56, 38, and 21%, respectively, in women and the corresponding rates were 57, 46, 25, and 11%, respectively, in men [4]. Mortality increased with advancing age in both sexes (hazard ratio [HR] for women, 1.61 per decade of age; 95% CI, 1.37–1.90; HR for men, 1.27 per decade of age; 95% CI, 1.09–1.47). After adjusting for age, survival after HF onset was better in women than in men (HR 0.64; 95% CI, 0.54– 0.77).
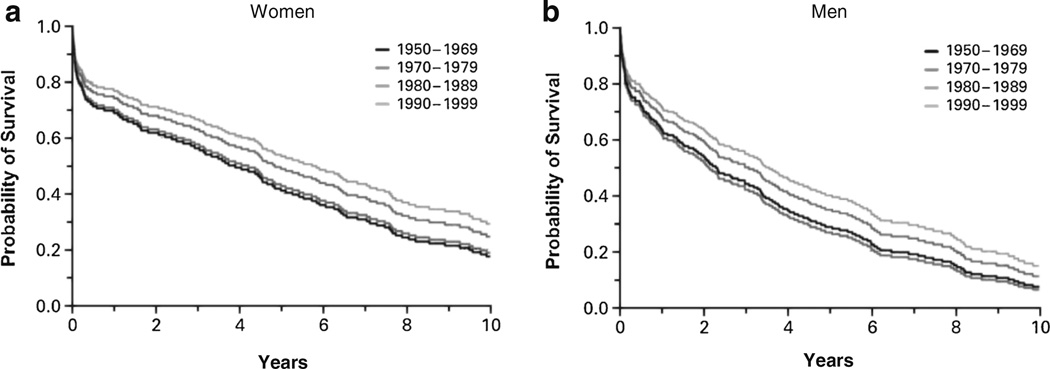
In subsequent analyses of data from the first 5 decades of follow-up, 1950s through 1990s, the age-adjusted probability of survival improved in both women and men (Fig. 1) [2]. The 30-day, 1-year, and 5-year age-adjusted mortality rates among women declined from 18, 28, and 57%, respectively, in 1950–69 to 10, 24, and 45%, respectively, in 1990–99. The corresponding rates among men declined from 12, 30, and 70%, respectively, in 1950–69 to 11, 28, and 59%, respectively, in 1990–99. Despite the improvement in overall survival after the onset of HF by 12% per decade (P = 0.02 for women and P = 0.01 for men), HF remains highly fatal; 5-year mortality was more than 50% among those diagnosed with HF in the 1990s.
Fig. 1.
Temporal trends in age-adjusted survival after the onset of heart failure among women (a) and men (b). Values were adjusted for age (<55, 55 to 64, 65 to 74, 75 to 84, and ≥85 years). Estimates are shown for participants who were 65 to 74 years of age. Source: Levy D et al. New Engl J Med. 2002;347:1397–1402
Relative Risk of HF
Using data on 9 commonly ascertained clinical factors, a profile for estimating the 4-year probability of HF in individuals aged 45 to 94 years has been published [5]. Participants in the top quintile of this multivariable risk accounted for 73% of HF events in women and 60% in men. In recent analyses of data between 1981 and 2008, women had a 24% (95% CI 7 to 38%) lower 8-year risk of new-onset HF than men [6]. Similar risk assessment models are available for prediction of HF among individuals with atrial fibrillation [7].
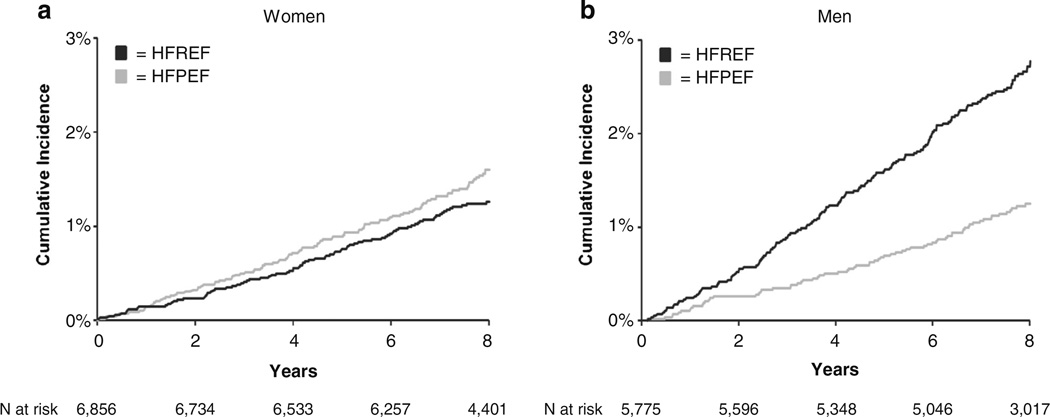
In FHS participants with new-onset HF, the multivariable odds of HF with preserved ejection fraction (HFPEF, EF >45%) are 2.8-fold higher in women than in men [8]. Overall, among individuals free of HF at baseline, women are 65% less likely to have HF with reduced ejection fraction (HFREF, EF ≤45%) [6]. However, the cumulative incidence of HFPEF and HFREF are similar in women and approximate that of HFPEF in men, whereas the cumulative incidence of HFREF in men is markedly high (Fig. 2) [6]. This disparity in the incidence of HFPEF and HFREF between women and men may partially be explained by differential left ventricular (LV) adaptation response to stress; e.g., isolated systolic hypertension is associated with concentric LV hypertrophy in women and eccentric hypertrophy in men [9]. A more detailed discussion on HFPEF is reported elsewhere [10,11].
Fig. 2.
Cumulative incidence of heart failure with preserved ejection fraction (HFPEF) versus reduced ejection fraction (HFREF) in women (a) and men (b). Source: Ho JE et al. Circulation. Heart failure. 2013;6:279–286
Lifetime Risk of HF
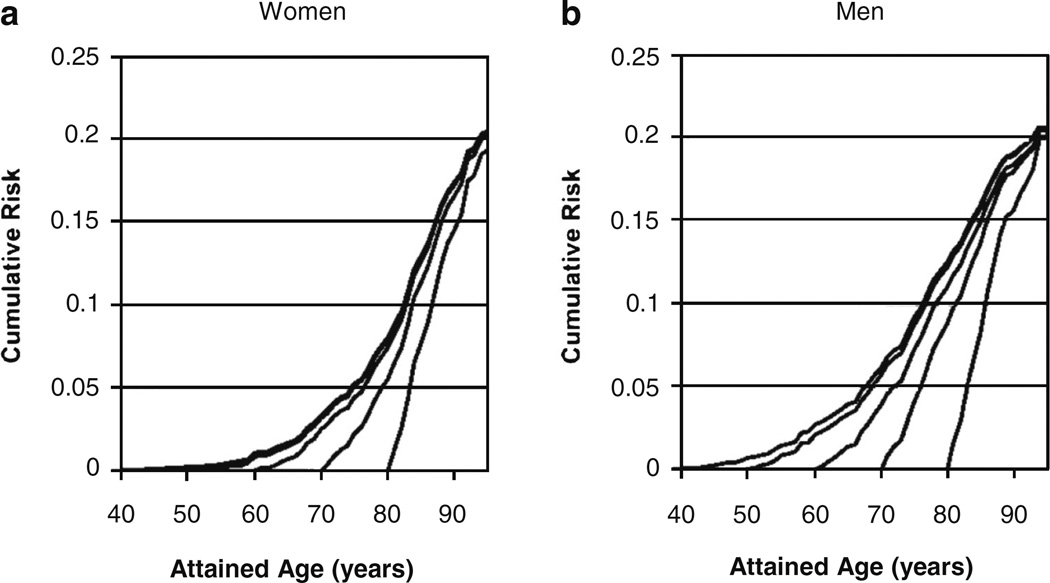
The lifetime risk statistic is a better indicator of the population burden of a disease than cumulative incidence because it quantifies the absolute cumulative risk of a disease during the remainder of one’s life and accounts for the competing causes of death. Among both women and men, the lifetime risk of developing HF at 40, 50, 60, 70, and 80 years of age continually remains high at about 20% (1 in 5) [12]. With increasing age, the slope of cumulative risk of HF becomes progressively steeper because the short-term risk of HF rapidly increases with advancing age (Fig. 3) [12]. For instance, among women, the 5-year risk of HF increases from 0.1% at age 40 to 8.3% at 80 years of age (Table 1) [12]. The persistently high lifetime risk of HF among the elderly, despite shorter life expectancy, is likely due to greater prevalence of hypertension (a major risk factor for HF) and improved survival after acute myocardial infarction (a potent risk factor for HF). Among those without prior myocardial infarction, the lifetime risk of HF at 40 years of age is approximately 15% for women and 11% for men [12].
Fig. 3.
Cumulative risk for heart failure at selected index ages for women (a) and men (b). Lifetime risk for heart failure for given index age is cumulative risk through age 94 years. Source: Lloyd-Jones DM et al. Circulation. 2002;106:3068–307
Table 1.
Short-term vs. lifetime cumulative risks of heart failure in women and men at selected index agesa
| Index age, yr | Women |
Men |
||
|---|---|---|---|---|
| 5-year risk,% |
Lifetime risk,% |
5-year risk,% |
Lifetime risk,% |
|
| 40 | 0.1 | 20.3 | 0.2 | 21.0 |
| 50 | 0.1 | 20.5 | 0.8 | 21.0 |
| 60 | 0.7 | 20.5 | 1.3 | 20.5 |
| 70 | 2.2 | 20.2 | 4.0 | 20.6 |
| 80 | 7.8 | 19.3 | 8.3 | 20.2 |
Source: Lloyd-Jones DM et al. Circulation. 2002;106:3068–3072
Clinical Risk Factors for HF
Hypertension and HF Risk
Role of hypertension in the pathogenesis of HF is well described [13,14]. In models adjusting for diabetes mellitus, angina, myocardial infarction, electrocardiographic LV hypertrophy (ECG-LVH), and valvular heart disease, presence of hypertension is associated with a 3.4-fold increase in the risk of HF in women and 2-fold increase in risk of HF in men (Table 2) [13]. Consequently, even though the prevalence of hypertension is similar in both sexes (about 60%), the population attributable fraction is much higher in women than in men (59% vs. 39%) [13]. In stepwise regression analyses considering 14 established HF risk factors, hypertension was significantly associated with HFREF but not with HFPEF [6].
Table 2.
Results of multivariable analyses examining the effect of clinical factors, electrocardiographic findings, and laboratory values and on the risk of heart failure in the Framingham heart studya
| Authors Year of Publication |
Number (women) Age, mean±SD Baseline exam |
Follow-up (yrs) Events (women) |
Variable | Prevalence,% | Multivariable HR(95% CI) | Interaction by Sex |
||
|---|---|---|---|---|---|---|---|---|
| Women | Men | Women | Men | |||||
| Levy D et al. [13] 1996 |
5143 (55% women) 56 (mean) 1970s |
14 (mean) 20 (max) 392 events |
Hypertension | 62 | 60 | 3.35(1.67–6.73) | 2.07(1.34–3.20) | Possible |
| Diabetes mellitus | 5 | 8 | 3.73(2.71–5.15) | 1.82(1.28–2.58) | Present | |||
| Valve disease | 8 | 5 | 2.13(1.54–2.94) | 2.47(1.70–3.60) | Not apparent | |||
| Angina | 9 | 11 | 1.68(1.23–2.30) | 1.43(1.03–1.98) | Not apparent | |||
| Myocardial infarction | 3 | 10 | 6.01 (4.37–8.28) | 6.34 (4.61–8.72) | Not apparent | |||
| ECG-LVH | 3 | 4 | 2.85(1.97–4.12) | 2.19(1.49–3.21) | Not apparent | |||
| Kenchaiah S et al. [15] 2002 |
5881 (54% women) 55 yrs (mean) 1976–79 (Original) 1979–83 (Offspring) |
14 (mean) 22 (max) 496 (258 women) |
Body-mass Index (kg/m2) per 1 kg/m2 increment |
1.07(1.04–1.10) | 1.05(1.02–1.09) | NS(P>0.10) | ||
| 18.5 to 24.9 | 54 | 32 | 1.00 (referent) | 1.00 (referent) | ||||
| 25 to 29.9 | 30 | 51 | 1.50(1.12–2.02) | 1.20 (0.87–1.64) | ||||
| ≥30 | 16 | 17 | 2.12(1.51–2.97) | 1.90(1.30–2.79) | ||||
| Walsh CR et al. [16] 2002 |
6289 (56% women) -- Pooled repeated data 1971–94 (Original) 1979–95 (Offspring) |
-- 219 (120 women) |
Alcohol intakeb | Present | ||||
| Nondrinkers | NA | NA | 1.00 (referent) | 1.00 (referent) | ||||
| Former drinkers | NA | NA | 1.15(0.72–1.86) | 0.64 (0.34–1.22) | ||||
| Mild drinkers | NA | NA | 0.82(0.48–1.39) | 0.46 (0.27–0.81) | ||||
| Moderate drinkers | NA | NA | 0.60(0.31–1.18) | 0.47 (0.24–0.94) | ||||
| Heavy drinkers | 17 | 23 | 1.04 (0.56–1.92) | 0.63(0.34–1.19) | ||||
| Lee DS et al. [17] 2006 |
2214 (52% women) 44 yrs (mean) 1978–98 |
20 (mean) 90 events |
Parental heart failure | Sex-pooled analyses | NS | |||
| No | 68 | 69 | 1.00 (referent) | |||||
| Yes | 32 | 31 | 1.70(1.11–2.60) | |||||
| Dhingra R et al. [18] 2006 |
1759 (63% women) 69 yrs (mean) 1979–84 |
13 (mean) 324 (205 women) |
QRS duration, ms | NS | ||||
| Per log SD increase | 1.23(1.08–1.38) | |||||||
| Normal (<100) | 85 | 62 | 1.00 (referent) | |||||
| Incomplete BBB (100–119) | 11 | 28 | 1.40(1.05–1.96) | |||||
| Complete BBB (≥120) | 4 | 10 | 1.70(1.28–2.35) | |||||
| Velagaleti RS et al. [19] 2009 |
6860 (54% women) 44 yrs (mean) 1968–74 (Original) 1971–74 (Offspring) |
26 (mean) 680 (333 women) |
Non-HDL-C, mg/dL | 156+45 | 1.19(1.11–1.27) | NS | ||
| Per 1SD increase | ||||||||
| HDL-C, mg/dL | 52±16 | 0.82 (0.75–0.90) | NS | |||||
| Per 1SD increase | ||||||||
| Kraigher-Krainer E et al. [20] 2013 |
1142 (65% women) 76 yrs (mean) 1986–90 |
10 (mean) 19 (max) 250 events |
Physical Activity Index | Not assessed | ||||
| Tertile 1 | 33 | 32 | 1.00 (referent) | |||||
| Tertile 2 | 33 | 33 | 0.84(0.60–1.17) | |||||
| Tertile 3 | 34 | 35 | 0.65 (0.46–0.91) | |||||
HR denotes hazard ratio. CI denotes confidence interval. Plus-minus values are means ± standard deviation. SD denotes standard deviation. NA denotes not available. NS denotes not significant. ECG-LVH denotes electrocardiographic left ventricular hypertrophy. BBB denotes bundle branch block. HDL-C denotes high density lipoprotein-cholesterol.
Mild drinkers (1–2 drinks/week in women, 1–7 drinks/week in men), moderate drinkers (3–7 drinks/wk in women, 8–14 drinks/week in men), and heavy drinkers (≥8 drinks/wk in women, ≥15 drinks/week in men).
Diabetes Mellitus and HF Risk
Diabetes mellitus is an established risk factor for HF [5,6,14,21,22]. Analyses based on initial 18-years of follow-up revealed that diabetes mellitus conferred a 5-fold increased risk of HF in women and a 2-fold increased risk of HF in men [21]. Among individuals with diabetes mellitus, regardless of coronary artery disease status, HF occurred approximately 2-times more commonly in women than in men [21]. These findings imply that women are particularly susceptible to the deleterious impact of diabetes mellitus on the heart. In recent sex-pooled multivariable analyses, diabetes mellitus was associated with a similar, nearly 3-fold, increase in the risk of HFPEF as well as HFREF [6].
Individuals with diabetes mellitus had higher heart rates than those without diabetes mellitus (73 vs 68 beats/min, P = 0.004, in women; 68 vs 64 beats/min, P = 0.002, in men) [23]. Women with diabetes mellitus had greater evidence of LV remodeling characterized by increased LV wall thickness, relative wall thickness, LV end-diastolic dimension, and LV mass indexed to height [23]. In multivariable analyses, diabetes mellitus remained significantly associated with greater LV mass and wall thickness in women (all P <0.01) but not in men [23]. Of note, worsening glucose tolerance was associated with higher LV mass and wall thickness; this association was stronger in women compared with men [24]. Insulin resistance was associated with increased LV mass in women alone, but this relation was attenuated by obesity [24]. Other mechanisms for diabetic cardiomyopathy have been previously described in a separate review [22].
Obesity and HF Risk
Among FHS participants, every 1 kg/m2 increment in body mass index (BMI) was associated with a 7% increase in HF risk in women and a 5% increase in HF risk in men (Table 2) [15]. As compared with normal BMI (defined as BMI between 18.5 and 24.9 kg/m2), Obesity (defined as BMI ≥30 kg/m2) was associated with a 2.1-fold increase in the risk of HF in women (P <0.001) and 90% increase in the risk of HF in men (P <0.001). The influence of elevated BMI on the risk of HF was not altered by sex (P for interaction >0.10). In addition to current BMI, evidence of elevated BMI in the past 10 years or past 10 to 20 years were each associated with increased HF risk [25]. This finding suggests that excess weight in early life increases the risk of HF in later life. Potential mechanisms by which obesity may result in the development of HF include promotion of atherogenic risk traits, alteration of neuroendocrine pathways, predisposition to sleep-disordered breathing, potentiation of cardiac remodeling, and induction of proteinuria and renal dysfunction [26].
Physical Inactivity and HF Risk
In 1142 elderly FHS participants (mean age 76 years) without prior myocardial infarction, levels of physical activity were similar in women and men [20]. In age and sex-adjusted analyses, higher levels of physical activity (middle and upper tertile compared with lower tertile) were associated with a 15–56% lower risk of HF. In multivariable models adjusting for established clinical risk factors for HF, this association was evident for risk of any HF and HFPEF (EF >45%) but attenuated for HFREF (EF ≤45%). Effect modification of these associations by sex was not assessed in this investigation.
Dyslipidemia and HF Risk
Every 1SD increment in non-HDL-C was associated with a 19% increase in the risk of HF (Table 2) [19]. To the contrary, every 1SD increase in HDL-C conferred an 18% reduction (HR 0.82, 95% CI 0.75–0.90) in HF risk. These results remained significant even after adjustment for myocardial infarction as a time-dependent covariate. All analyses were conducted on data for pooled sexes; there was no significant effect modification by sex of the association of lipid levels with the risk of HF.
Cigarette Smoking and HF Risk
In recent multivariable analyses accounting for established HF risk factors, current cigarette smoking was associated with a 1.6-fold increase in the risk of HF (HR 1.64, 95% CI 1.28– 2.01, P <0.001) [6]. In stepwise regression analyses including 14 potential HF risk factors, current cigarette smoking was associated with a 2-fold increase in the risk of HFPEF (HR 2.04, 95% CI 1.39–2.99, P <0.001) but was not a significant contributor to the risk of HFREF [6]. Interactions of these associations by sex were not evaluated in this report.
Alcohol Intake and HF Risk
In both women and men, alcohol intake was not associated with increased risk for HF even among heavy drinkers (defined as ≥15 drinks/week in men and ≥8 drinks/week in women) (Table 2) [16]. Among women, age-adjusted risk for HF was lower among women who consumed 3 to 7 drinks/week (HR 0.49, 95% CI 0.25–0.96) than in nondrinkers. This association remained marginally statistically significant after adjustment for multiple predictors of HF. The risk for HF was lower among men at all levels of alcohol consumption compared with nondrinkers, before and after adjustment for multiple predictors of HF. The lowest risk for HF was seen in men who consumed 8 to 14 drinks/week (HR 0.41, 95% CI 0.25– 0.96). The observed association between alcohol consumption and risk for HF in men was not affected by adjustment for hypertension, but it was mildly attenuated by adjustment for HDL cholesterol. This suggests that part of the protective effect of alcohol against HF may be possibly related to an alcohol-mediated increase in serum HDL cholesterol levels.
Parental HF and HF Risk
In cross-sectional analyses, history of HF in at least one parent (parental HF) was associated with increased LV mass [17] and LV internal dimensions [17] and reduced LV systolic function [17] and circumferential strain [27] in the offspring. Further, abnormal LV geometric patterns aggregate in families and parental HF is associated with eccentric LV geometry in offspring [28]. In prospective multivariable analyses, parental HF was associated with a 70% increase (95% CI 11 to 160%) in the risk of HF in the offspring (Table 2) [17]. This association was similar in women and men (no effect modification by sex was observed).
Myocardial Infarction and HF Risk
Myocardial infarction is a potent risk factor for HF. In prior analyses adjusted for angina pectoris, diabetes mellitus, ECG-LVH, and valvular heart disease, presence of myocardial infarction conferred a 6-fold increase in the risk of HF (Table 2) [13]. Effect modification of this association by sex was not apparent. In recent analyses on a contemporary FHS cohort where 14 covariates were considered in stepwise regression models, previous myocardial infarction and previous coronary heart disease were associated with a 3.5-fold and 1.7-fold increase respectively in the risk of HFREF but was not significantly associated with the risk of HFPEF [6].
Valvular Heart Disease and HF Risk
In FHS, valvular heart disease is defined as the presence of Grade 3/6 or greater intensity systolic murmur, or any diastolic murmur. In models adjusting for age, hypertension, diabetes mellitus, angina, myocardial infarction, ECG-LVH, presence of valvular heart disease was associated with a 2.1-fold and 2.5-fold increase in HF risk in women and men, respectively (Table 2) [13]. There was no apparent effect modification of this association by sex. In recent sex-pooled analyses examining 14 clinical risk factors, presence of valvular heart disease conferred a 3.2-fold (95% CI 2.3 to 4.4-fold) increase in HF risk and was associated with both HFPEF and HFREF [6].
Heart Rate and HF Risk
In earlier analyses, every 10 bpm increase in heart rate was associated with 10–15% greater odds of HF [5]. In more contemporary analyses, based on data from 1988 to 2008, every 12 bpm increment in heart rate resulted in a 28% increase (95% CI 19 to 38%) in the 8-year risk of HF [6]. Effect modification of this association by sex was not assessed in this specific investigation.
Noncardiac Dysfunction and HF Risk
In a small group of FHS participants (n = 676; mean age 75 years; 58% women) with data on noncardiac risk factors of interest, after adjustment for known clinical risk factors and cardiac systolic and diastolic dysfunction, higher serum creat-inine (>1.05 mg/dL), lower FEV1:FVC ratios (<91% predicted), and lower hemoglobin levels (<13 g/dL) were associated with increased HF risk (all P <0.05); however, serum albumin and white blood cell count were not associated with HF risk (P >0.30) [29]. Interaction by sex was not examined in this report.
Electrocardiographic Findings and HF Risk
Electrocardiographic LV Hypertrophy and HF Risk
In multivariable analyses, presence of LV hypertrophy by ECG criteria was associated with an increased risk of HF in both women and men (Table 2) [13]. Overlapping 95% CIs suggests lack of effect modification by sex; however, formal assessment by including interaction term for sex in regression models was not performed [13]. In stepwise multivariable regression models consisting of 14 established HF risk factors, ECG-LVH was associated with a 2.7-fold increase in the risk of HFREF (HR 2.73, 95% CI 2.04–3.65, P<0.001) but was not significantly associated with the risk of HFPEF [6].
Electrocardiographic QRS Duration and HF Risk
Delay in ventricular depolarization was associated with an increased risk of HF [18]. Each SD increment in log-QRS duration was associated with a multivariable-adjusted 23% increase in HF risk (Table 2). In time- dependent models with QRS category and risk factors updated every 2 years, incomplete bundle branch block was associated with a 1.4-fold and complete bundle branch block with a 1.7-fold increase in risk of HF. These associations were maintained on adjustment for baseline LV mass. There was no effect modification of the association between QRS duration and HF risk by sex.
Atrial Fibrillation and HF Risk
Atrial fibrillation and HF are inter-related; one begets the other [30]. Its presence confers a 1.9-fold increase in the 8-year risk of HF [6]. Among 725 FHS participants (mean age 73 years, 45% women) with atrial fibrillation followed over 10 years, the incidence of HF was similar in women (4.3 per 100 person-years) and men (3.3 per 100 person-years) [7].
Echocardiographic Measures and HF Risk
Left Ventricular Enlargement and HF Risk
Echocardiographic LV end-diastolic and end-systolic dimensions were highly correlated (r=0.86 in both men and women). Every 1SD increment in LVend-diastolic and end-systolic dimension was associated with a 47 and 43% increase in HF risk respectively (Table 3) [31]. Sex had no significant interaction with LV end-diastolic or end-systolic dimensions and the risk of HF (P for interaction >0.39).
Table 3.
Results of multivariable analyses examining the effect of echocardiographic measures on the risk of heart failure in the Framingham heart studya
| Authors Year of Publication |
Number (women) Age, mean±SD Baseline exam |
Follow-up, yrs Events (women) |
Variable | Variable value |
Exposure units | Multivariable HR (95% CI) |
Interaction by Sex |
|
|---|---|---|---|---|---|---|---|---|
| Women | Men | |||||||
| Vasan RS et al. [31] 1997 |
4744 (56% women) 50±0.3 yrs 1979–83 |
7.7 (mean) 11 (max) 74 events |
LV internal dimension, mm | |||||
| End-diastole (LVIDd) | 45.8±0.08 | 50.9±0.09 | 1SD increase in LVIDd/height | 1.47(1.25–1.73) | NS (P>0.39) | |||
| End-systole (LVIDs) | 28.3±0.07 | 32.6±0.08 | 1SD increase in LVIDs/height | 1.43 (1.24–1.65) | NS (P>0.39) | |||
| Wang TJ et al. [32] 2003 |
4257 (56% women) 61 yrs |
12 (mean) 175 events |
ALVD (LVEF ≤50%) | 0.8% | 6.0% | ALVD (yes/no) | 4.70(2.72–8.14) | NS |
| No ALVD (EF >50%) | NA | NA | No ALVD (EF >50%) | 1.00 (referent) | ||||
| Mild ALVD (EF 40–50%) | NA | NA | Mild ALVD (EF 40–50%) | 3.32 (1.65–6.64) | ||||
| Mod-Sev ALVD (EF <40%) | NA | NA | Mod-Sev ALVD (EF <40%) | 7.77 (3.86–15.63) | ||||
| Lam CSP et al. [29] 2011 |
1038 (61% women) 76±5 yrs 1986–90 |
11 (mean) 248 (146 women) |
Sex-pooled data | Not assessed | ||||
|
LV systolic dysfunction (EF<45%) |
5% | Yes/No | 2.33(1.43–3.78) | |||||
| LV diastolic dysfunctionb | 36% | Yes/No | 1.32(1.01–1.71) | |||||
| Lam CSP et al. [33] 2013 |
6493 (54% women) 56±14 yrs Pooled repeated data |
-- 415 events |
Aortic root diameter | Sex-pooled data | NS (P=0.99) | |||
| Baseline dimension | 32±4 | 1SD increase | 1.19(1.07–1.33) | |||||
| 4523 (54% women) 58±12 yrs Pooled repeated data |
-- 228 events |
Aortic root diameter | NS | |||||
| Change over 8 years | 1.1±3.2 | 1SD increase | 1.20(1.04–1.38) | |||||
| Velagaleti RS et al. [34] 2014 |
4768 (56% women) 50 yrs 1978–80 (Original) 1979–82 (Offspring) |
21 (mean) 458 (250 women) |
LV geometric patternsc | NS (P=0.53) | ||||
| Normal LV | 72% | 69% | LV geometric patterns were | 1.00 (referent) | ||||
| Concentric remodeling | 13% | 16% | evaluated as a categorical | 1.09(0.85–1.40) | ||||
| Concentric hypertrophy | 8% | 8% | variable. | 1.40(1.04–1.87) | ||||
| Eccentric hypertrophy | 7% | 7% | 1.89(1.41–2.54) | |||||
HR denotes hazard ratio. CI denotes confidence interval. Plus-minus values are means ± standard deviation. SD denotes standard deviation. NS denotes not significant. NA denotes not available. LV denotes left ventricle or left ventricular. BBB denotes bundle branch block. ALVD denotes asymptomatic left ventricular systolic dysfunction. EF denotes ejection fraction.
LV diastolic dysfunction was defined on the basis of LV filling pattern as any abnormal relaxation, pseudonormal filling, or restrictive filling. Abnormal relaxation (mitral E/A <0.5, deceleration time >280 milliseconds) or restrictive filling (mitral E/A >2.0, deceleration time <120 milliseconds) was classified on the basis of mitral inflow patterns. In the absence of tissue Doppler imaging, pseudonormal LV filling was distinguished from normal LV diastolic function by the presence of left atrial size at or above the sex-specific 80th percentile, LV mass at or above the sex-specific 80th percentile, or any atrial fibrillation.
Normal LV group (normal LV mass and relative wall thickness), concentric remodeling (normal LV mass with increased relative wall thickness), concentric hypertrophy (increased LV mass and relative wall thickness), and eccentric hypertrophy (increased LV mass with normal relative wall thickness).
Left Ventricular Dysfunction and HF Risk
Among FHS participants without HF, the prevalence of asymptomatic LV systolic dysfunction (ALVD, defined as echocardiographic LV ejection fraction ≤0.50) was lower in women than in men (0.8% in women versus 6.0% in men) [32]. Presence of ALVD conferred a 4.7-fold increase in HF risk (Table 3). This increased risk did not vary by sex (p for interaction was not statistically significant).
In subsequent analyses accounting for clinical HF risk factors, LV systolic dysfunction (defined as LV ejection fraction ≤0.45) was associated with 130% increase in HF risk and LV diastolic dysfunction was associated with a 32% increase in HF risk [29]. Effect modification of these associations by sex was not assessed in this report.
Aortic Root Remodeling and HF Risk
Among FHS participants, the average aortic root measurement in women was 2.4 mm smaller than that of men of comparable age, height, and weight [35]. After accounting for clinical risk factors, HF risk increased by 19% for every 1 SD increase in aortic root dimension at baseline and by 20% for every 1SD increase in aortic root size over 8 years (Table 3) [33]. This increased risk was attenuated on additional adjustment for LV mass suggesting that arterial and ventricular remodeling may be occurring parallel to each other (correlation co-efficient between aortic root dimension and LV mass was 0.50, P<0.001). The association between baseline aortic root dimension and HF risk was similar in both women and men (p for interaction 0.99).
LV Hypertrophy Patterns and HF Risk
Over a mean follow-up of 21 years, the age- and sex-adjusted incidence of HF increased from 7.0% in the normal LV group (normal LV mass and relative wall thickness) to 8.7, 13.4, and 15.3% in the concentric remodeling (normal LV mass with increased relative wall thickness), concentric hypertrophy (increased LV mass and relative wall thickness), and eccentric hypertrophy (increased LV mass with normal relative wall thickness) groups, respectively [34]. In multivariable analyses adjusting for known risk factors for HF, compared with normal LV group, concentric remodeling was not associated with greater HF risk; however, significant increase in HF risk was noted among those with concentric hypertrophy (1.4-fold increase) and eccentric hypertrophy (1.9-fold increase) (Table 3) [34]. There was no statistically significant interaction by sex (p for interaction = 0.53) [34]. Of note, concentric hypertrophy was associated with a greater risk of HFPEF and eccentric hypertrophy was more likely to be associated with HFREF [34].
Other Sex-Related Disparity in Cardiac Structure and Function
Women have lower LV mass and LV wall thickness than men but experience greater age-associated increase in LV wall thickness [36]. Women also have better LV mechanical function (higher longitudinal, transverse, circumferential, and radial strain by speckle tracking echocardiography) than men (P<0.001 in multivariable regression analyses) and history of having at least one parent with HF (parental history of HF) was associated with worse circumferential strain in offspring free of HF (β 0.23, P=0.01) [27]. Studies to examine these implications on subsequent development of HF are awaited.
Biomarkers and HF
Homocysteine and HF Risk
Plasma homocysteine was directly related to LV mass and wall thickness in women (P =0.004 to 0.04) but not in men (P =0.28–0.68) [37]. Plasma homocysteine was not related to left atrial size or LV fractional shortening in either sex. In prospective analyses, plasma homocysteine levels higher than sex-specific median value were associated with a 93% increase in HF risk among women (Table 4) [38]. Sex-related differences in the associations of plasma homocysteine with LV mass and HF has been detailed in a prior report [49].
Table 4.
Results of multivariable analyses examining the effect of biomarkers and laboratory values on the risk of heart failure in the Framingham heart studya
| Authors Year of Publication |
Number (women) Age, mean±SD Baseline exam |
Follow-up, yrs Events (women) |
Variable | Variable value |
Exposure units | Multivariable HR(95%CI) P-value |
Interaction by Sex |
|
|---|---|---|---|---|---|---|---|---|
| Women | Men | |||||||
| Vasan RS et al. [38] 2003 |
2491 (62% women) 72±7 yrs 1979–82 and 1986–90 |
8 (mean) 156 (88 women) |
Homocysteine, µmol/L | 12.0±5.4 | 13.0±8.8 | Sex-specific median value | NS (albeit more continuous in women) |
|
| Women | 1.93(1.19–3.14) | |||||||
| Men | 1.84(1.06–3.17) | |||||||
| Vasan RS et al. [39] 2003 |
732 (67% women) 78 yrs 1992–1994 |
5.2 (mean) 56 (35 women) |
IL-6, pg/mL | 6.69±15.90 | 7.68±17.22 | 1SD increase in log value | 1.36(1.06–1.74) | NS |
| CRP, mg/L | 1.60±3.93 | 2.66±8.47 | ≥5 mg/L | 2.81 (1.22–6.50) | ||||
| TNFα, ng/mL | 4.68±3.66 | 5.33±4.34 | 1SD increase in log value | 1.46 (1.00–2.15) | ||||
| Vasan RS et al. [40] 2003 |
717 (67% women) 78.4 yrs 1992–94 |
5.2 (mean) 56 (35 women) |
IGF-I, µg/L | 138.4±54.2 | 154.7±64.0 | 1SD increase in log value | 0.73 (0.56–0.95) | NS |
| ≥140 µg/L (median) | 0.46 (0.23–0.91) | |||||||
| Wang TJ et al. [41] 2004 |
3346 (53% women) 58.5±10yrs 1995–98 |
5.2 (mean) 41 events |
Median | |||||
| BNP, pg/mL | 10.0 | 6.2 | 1SD increase in log value | 1.77(1.31–2.41) | NS | |||
| NT-proBNP, pmol/L | 351 | 256 | 1SD increase in log value | 1.94 (1.37–2.75) | NS | |||
| Frankel DS et al. [42] 2009 |
2739 (53% women) 61 yrs 1999–2001 |
6 (mean) 58 (25 women) |
Intertertile ranae (Sex-pooled data) | NS | ||||
| Resistin, ng/mL | (11, 15) | 1SD increase in log value | 1.26(1.01–1.60) | |||||
| Adiponectin, µg/mL | (6.5, 11) | 1SD increase in log value | NS | |||||
| Lieb W et al. [43] 2009 |
818 (62% women) 79 yrs 1992–93 |
8.0 (mean) 129 (73 women) |
Leptin, ng/mL | Median (Intequartile ranae) | 1SD increase in sex- standardized log value |
1.26(1.03–1.55) | NS (P>0.75) | |
| 17.4 (10.6, 28.7) | 7.2(4.5, 11.4) | |||||||
| Velagaleti RS et al. [44] 2010 |
2754 (54% women) 58±10 yrs 1995–98 |
9.4 (mean) 95 (41 women) |
Median (Interquartile range) | |||||
| CRP, mg/L | 2.40 (0.99, 5.63) | 1.81 (0.90,3.91) | Not retained in regression models using backward elimination approach |
|||||
| PAI-1, ng/mL | 20.2(12.1,31.8) | 25.6 (17.1, 36.0) | ||||||
| Homocysteine, mmol/L | 8.30 (6.97, 10.13) | 9.81 (8.26, 11.92) | ||||||
| ARR | 1.00 (0.55, 1.67) | 0.65(0.38, 1.14) | ||||||
| BNP, pg/mL | 9.70 (4.00, 19.65) | 6.10(4.00, 15.9) | 1SD increase in log value | 1.52 (1.24–1.87) | NS | |||
| UACR, mg/g | 8.55 (3.57, 17.24) | 4.88 (2.15, 10.93) | 1SD increase in log value | 1.35(1.11–1.66) | NS | |||
| Dhingra R et al. [45] 2010 |
3300 (51% women) 44 yrs 1979–82 |
17.4 (mean) 157 (63 women) |
Phosphorus, mg/dL, | Median (range) | 1 mg/dL increase | 1.74(1.17–2.59) | NS (P>0.05) | |
| Quartile 1 | 2.7 (1.8–2.9) | 2.5(1.6–2.7) | ||||||
| Quartile 2 | 3.1 (3.0–3.2) | 2.9 (2.8–3.0) | ||||||
| Quartile 3 | 3.4 (3.3–3.5) | 3.2(3.1–3.3) | ||||||
| Quartile 4 | 3.8 (3.6–6.2) | 3.6 (3.4–5.0) | ||||||
| Dhingra R et al. [46] 2010 |
3544 (52% women) 24 yrs 1978–82 |
23.6 (mean) 188 (77 women) |
GGT, U/L | Median (interquartile range) | 1SD increase in log value | 1.39(1.20–1.62) | NS (P>0.05) | |
| 9 (7, 14) | 16(11,25) | ≥median (yes/no) | 1.71 (1.21–2.42) | |||||
| Ho JE et al. [47] 2012 |
3353 (53% women) 59 yrs 1995–98 |
11.2 (mean) 166 events |
Galectin-3, ng/mL, median (interquartile range) |
14.3 (12.0, 16.8) | 13.1 (11.1, 15.4) | 1SD increase in log value | 1.23(1.04–1.47) | Not assessed |
| Coglianese EE et al. [48] 2012 |
3523 (59% women) 52 yrs (range 50–65) 1948–71 |
20 (max) 217 (100 women) |
Hematocrit,% | Range | Hematocrit,% | Not assessed |
||
| Low (Quintile 1) | 36.0 to <40.0 | 39.0 to <44.0 | Low (Quintile 1) | 1.00 (referent) | ||||
| Low-Normal (Quintile 2) | 40.1 to <42.0 | 44.1 to<45.0 | Low-Normal (Quintile 2) | 1.21 (0.78–1.87) | ||||
| Normal (Quintile 3 and 4) | 42.1 to <45.0 | 45.1 to<49.0 | Normal (Quintile 3 and 4) | 1.38 (0.95–2.01) | ||||
| High (Quintile 5) | ≥45.0 | ≥49.1 | High (Quintile 5) | 1.58 (1.02–2.44) | ||||
| P trend <0.0001 | ||||||||
HR denotes hazard ratio. CI denotes confidence interval. Plus-minus values are means ± standard deviation. SD denotes standard deviation. NS denotes not significant. IL-6 denotes interleukin-6. CRP denotes C-reactive protein, TNFα denotes tumor necrosis factor-alpha. IGF-I denotes insulin growth factor-I. BNP denotes B-type natriuretic peptide. NT-proBNP denotes N-terminal pro-B-type natriuretic peptide. PAI-1 denotes plasminogen activator inhibitor-1. ARR denotes aldosterone-to-renin ratio. UACR denotes urine albumin-to-creatinine ratio. GGT denotes gamma glutamyl transferase.
Inflammatory Cytokines and HF Risk
Elevated serum interleukin-6 (IL-6), C-reactive protein (CRP), and spontaneous production of tumor necrosis factor-α (TNFα) by peripheral blood mononuclear cell (PBMC) were associated with an increased risk of HF (Table 4) [39]. Participants with elevated levels of all 3 markers had a 3-fold increased risk of HF (HR 3.00, 95% CI 1.13 to 7.95, P =0.03] when adjusted for known HF risk factors at baseline and a 4-fold increase in risk when additionally adjusted for myocardial infarction as a time-dependent variable. In stepwise models directly comparing the prognostic utility of elevation of all 3 markers with that of an increase in serum IL-6 alone, elevated serum IL-6 entered the model first; elevation of the other two cytokines did not enter the model subsequently. The impact of inflammatory markers on HF risk did not vary by sex (p for interaction >0.10).
Serum Insulin-Like Growth Factor-I Level and HF Risk
Low serum IGF-I is a risk factor for new-onset HF in elderly individuals without previous myocardial infarction [40]. Among elderly FHS participants without myocardial infarction or HF at baseline, women had lower serum IFG-I level than men and, after adjustment for known HF risk factors, higher IFG-I level was associated with a lower risk of HF (Table 4). This association was alike in women and men (p for interaction >0.05).
Natriuretic Peptides Levels and HF Risk
The natriuretic peptides are counterregulatory hormones involved in volume homeostasis and cardiovascular remodeling [41]. Compared with men, women have a 1.6-fold higher plasma brain natriuretic peptide (BNP) levels and 1.3-fold higher N-terminal atrial natriuretic peptide (NT-ANP) levels [50]. Lower levels of circulating androgens and the potentiating effect of exogenous female hormone therapy contribute to the higher circulating NT-proBNP concentrations in women [51]. Both BNP and NT-ANP are positively associated with elevated LV mass and LV systolic dysfunction in both sexes [52]. However, presumably due to low prevalence of LV systolic dysfunction, ascertainment of natriuretic peptide levels is not a useful screening tool for the detection of increased LV mass or LV systolic dysfunction [52]. In multivariable analyses, every 1SD increase in log BNP and log NT-ANP level was associated with a 77 and 94% increase, respectively, in the risk of HF (Table 4) [41]. These associations were similar in the two sexes [41].
Resistin, Adiponectin and HF Risk
In a fully-adjusted multivariable model, each SD increment in blood resistin concentrations (7.45 ng/ml) was associated with a 26% increase in HF risk (Table 4) even after accounting for prevalent coronary heart disease, obesity, and measures of insulin resistance and inflammation [42]. Adiponectin, however, was not associated with HF. Effect modification of these associations by sex was not examined in this report, likely due to the small sample size.
Leptin and HF Risk
Leptin levels were higher in women and strongly correlated with BMI (P <0.0001) [43,53]. Log leptin levels standardized to sex was inversely associated with LV mass, LV wall thickness, and left atrial size. Leptin levels were not correlated with fractional shortening, transmitral early/late diastolic filling velocities, and LV end-diastolic dimensions. Although, these cross-sectional associations suggest a cardioprotective effect [53], in multivariable Cox regression analyses adjusting for established risk factors, log-leptin was positively associated with the incidence of HF (Table 4) [43]. Additional adjustment for BMI nullified the association with HF (HR 0.97 [95% CI 0.75–1.24]) [43]. The association between serum leptin concentration and HF risk was similar in both sexes (P for interaction ≥0.75) [43].
Multibiomarker Panel and HF Risk
Biomarkers of inflammation (C-reactive protein), hemostasis (fibrinogen and plasminogen activator inhihibitor-1 [PAI-1), and activation of the renin-angiotensin-aldosterone system (al-dosterone-to-renin ratio [ARR]) were each associated with LV geometry in separate models [54]. However, only ARR was associated with eccentric as well as concentric LV hypertrophy when all biomarkers were included in the same model. Only the B-type natriuretic peptide (BNP) and the urinary albumin-to-creatinine ratio (UACR) emerged as significant predictors of non-ischemic HF risk in analyses that also included C-reactive protein, PAI-1, homocysteine, and ARR (Table 4) [44]. These associations were not modified by sex (p for interaction was not statistically significant).
Phosphorus and HF Risk
Cross-sectionally, serum phosphorus was related positively to LV mass, internal dimensions, and systolic dysfunction [45]. In prospective analyses adjusting for established risk factors as time-varying covariates, every 1 mg/dL increment in serum phosphorus was associated with a 1.7-fold increase in the risk of HF and individuals in the highest serum phosphorus quartile experienced a 2-fold increase in risk of HF compared with participants in the lowest quartile (Table 4) [45]. The association between phosphorus level and HF risk did not vary by sex (P for interaction >0.05).
Gamma Glutamyl Transferase and HF Risk
Each SD increase in log-GGT and a value at or greater than the median was associated with a 1.4-fold and 1.7-fold increase, respectively, in the risk of HF (Table 4) [46]. These associations were similar in both women and men (P for interaction was not statistically significant).
Galectin-3 and HF Risk
Elevated serum Galectin-3 level is a marker of myocardial fibrosis, which may potentiate myocardial dysfunction and subsequently result in overt HF. Among FHS participants, Galectin-3 levels were higher in women compared with men (Table 4, P<0.05) [47]. In multivariable models adjusting for established clinical risk factors for HF, every 1SD increment in sex-standardized log Galectin-3 levels was associated with a 28% increase in the risk of HF. This association remained statistically significant after additional adjustment for BNP levels. Effect modification of the association between Galectin-3 levels and HF risk by sex, if any, was not reported.
Hematocrit Level and HF Risk
In a large sample of FHS participants (N=3523), higher hematocrit levels, even within the normal range, were associated with a greater risk of HF (Table 4) [48]. Adjustment for interim occurrence of hypertension, diabetes mellitus, coronary heart disease or stroke and subgroup analyses of nonsmokers yielded similar results. However, in a smaller subset of FHS participants (N=676) with available data on noncardiac dysfunction, after accounting for clinical risk factors and cardiac systolic and diastolic dysfunction, lower hemoglobin level was positively associated with HF risk [29]. The reasons for these observations are unclear. Effect modification of these associations by sex was not examined in both these reports.
Other Biomarkers and LV Remodeling
Aldosterone and Cardiac Structure
Serum aldosterone, a marker of myocardial fibrosis and LV remodeling, were higher in women compared with men [55]. In multivariable regression models, serum aldosterone was positively associated with increased LV wall thickness and relative wall thickness but decreased internal dimensions in women (P <0.05 for all) but not in men (P >0.20 for all) [55]. In prospective analyses, aldosterone was associated with increase in systolic blood pressure and incident hypertension [56], which, in turn, might increase the risk of HF.
Matrix Metalloproteinase-9 and Cardiac Structure
Plasma levels of matrix metalloproteinase-9 (MMP-9), a key determinant of extracellular matrix degradation, was associated with increased LV end-diastolic dimensions and increased LV wall thickness in men but not in women [57]. Its effect on HF risk has not been assessed.
Leukocyte Telomere Length and LV Mass
Leukocyte telomere length shortens with increasing age, greater oxidative stress and inflammation, and exposure to atherosclerotic risk factors. Contrary to expectation, it was positively associated with echocardiographic LV mass and wall thickness [58]. Sex was not an effect-modifier of this association. Implications of these associations on development of overt HF are unknown.
Causes of Death After Onset of HF
Among participants who developed HF, about two-thirds of the underlying cause of death and about one-half of the immediate cause of death were due to cardiovascular causes such as coronary heart disease, stroke, progressive pump failure, and arrhythmias or sudden cardiac death [59]. Major underlying and/or immediate noncardiovascular causes of death were infectious/noninfectious respiratory disease, cancer and other systematic infections. Predominant noncardiovascular contributory causes of death were kidney disease (including genitourinary or electrolyte abnormalities), diabetes mellitus, and noninfectious respiratory conditions.
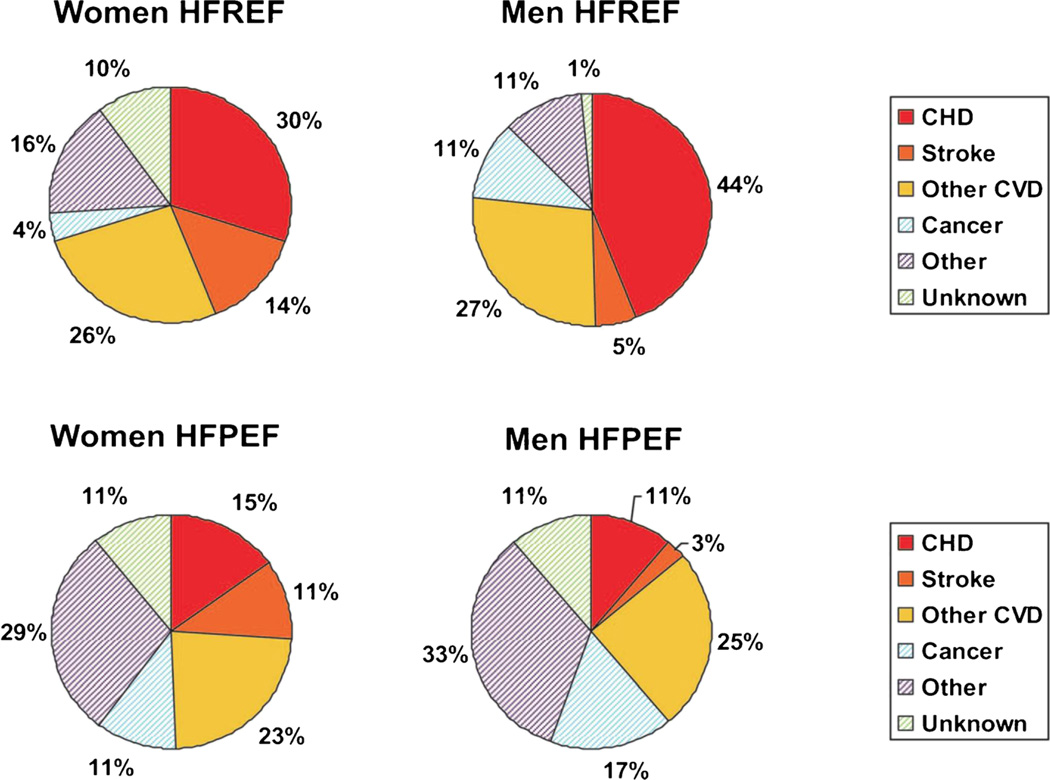
Older age at death was associated with greater odds of non-cardiovascular underlying cause of death in men (P=0.003) but did not reach statistical significance in women (P=0.14). Prior myocardial infarction was significantly associated with the increased odds of cardiovascular disease as the underlying cause of death in women (odds ratio [OR] 1.87, 95% CI 1.10 to 3.16) but not in men (OR 0.88, 95% CI 0.55–1.41). Cardiovascular underlying cause of death occured more frequently in the presence of HFREF (Fig. 4, 70% of deaths in women an 76% of deaths in men) than in the presence of HFPEF (49% of deaths in women and 39% of deaths in men). Among those with LV systolic dysfunction (HFREF), odds of cardiovascular death as the underlying cause was 2-fold higher in women (OR 2.39, 95% CI 1.39–4.08, P=0.002) and 3-fold higher in men (OR 3.16, 95% CI 1.73–5.78, P<0.001) and the odds of cardiovascular death as the immediate cause was 2-fold higher in women (OR 2.12, 95% CI 1.09–4.10, P=0.027) and 5-fold higher in men (OR 4.90, 95% CI 2.02–11.91, P<0.001). As a corollary, non-cardiovascular underlying and immediate causes of death were more common in HFPEF than in HFREF.
Fig. 4.
Underlying causes of death in women and men with heart failure according to status of left ventricular ejection fraction. HFREF denotes heart failure with reduced ejection fraction. HFPEF denotes heart failure with preserved ejection fraction. CHD denotes coronary heart disease. CVD denotes cardiovascular disease. Source: Lee DS et al. Circulation. Heart failure. 2011;4:36–43
Overall, these observations emphasize the need to prevent infections, control diabetes mellitus, correct fluid-electrolyte imbalance, maintain renal function, and treat other comorbidities to achieve further reductions in mortality among HF patients on optimal cardiovascular therapy.
Conclusions – Salient Features
Over a 50-year period, from 1950s to 1990s, the incidence of HF has declined by about one-third in women but has remained almost unchanged in men. The survival after the onset of HF has improved in both women and men; however, the HF mortality rate remains high – over 50% die within 5 years after onset of HF.
Between 1970s and 1990s, improved survival after myocardial infarction was accompanied by concomitant increase in occurrence of new cases of HF.
Overall, the 8-year relative risk of HF is 24% lower in women compared with men.
In those with new-onset HF, women have 2.8-fold higher odds of HFPEF (LVEF >45%) than HFREF (LVEF ≤45%). This disparity is primarily because men have a 2-fold higher cumulative incidence of HFREF than HFPEF. Of note, 8-year incidence rates of HFPEF and HFREF in women and HFPEF in men are similar.
The lifetime risk of HF is about 20% in both women and men at 40, 50, 60, 70, and 80 years of age. Persistently high lifetime risk despite shorter life expectancy with advancing age is intriguing. Higher short-term HF risk in elderly due to greater prevalence of hypertension (a major risk factor for HF) and improved survival after acute myocardial infarction (a potent risk factor for HF) are potential explanations.
Hypertension was associated with higher hazard for HF in women than men (3.4-fold vs 2-fold higher) and the sex-related difference in population attributable fraction was even more prominent (59% vs. 39% in women vs. men).
Women are particularly susceptible to the deleterious impact of diabetes mellitus on the heart. Diabetes mellitus was associated with a 5-fold higher relative risk for HF in women and 2-fold higher risk in men.
Alcohol intake was not associated with increased risk for HF even among heavy drinkers (≥15 drinks/week in men and ≥8 drinks/week in women). The inverse association between alcohol intake and risk for HF was less pronounced in women than men.
All other clinical, electrocardiographic, and echocardiographic risk factors, many of which are differentially distributed in women and men, posed a similar overall HF risk in both sexes.
Several circulating biomarker concentrations were distinctly different in women compared with men and had differential cross-sectional associations with LV structure and function (e.g., homocysteine, B-type natriuretic peptide, and leptin); however, the strength or direction of the association between biomarkers levels and HF risk were generally similar in women and men.
In individuals who developed HF, about two-thirds of the underlying cause of death and about one-half of the immediate cause of death were due to cardiovascular causes. Prior myocardial infarction was significantly associated with the increased odds of cardiovascular disease as the underlying cause of death in women but not in men. Non-cardiovascular underlying and immediate causes of death were more commonly noted among individuals with HFPEF.
Acknowledgments
Dr. Kenchaiah was partly supported by the Translational Research Institute (TRI), grants UL1TR000039 and KL2TR000063 through the National Institutes of Health (NIH) National Center for Research Resources and the National Center for Advancing Translational Sciences. This work was also partly supported by grant No. N01-HC-25195 (Dr. Vasan) from the National Heart, Lung, and Blood Institute, Bethesda, Maryland. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 2.Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KK, et al. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–1402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 3.Velagaleti RS, Pencina MJ, Murabito JM, Wang TJ, Parikh NI, D’Agostino RB, et al. Long-term trends in the incidence of heart failure after myocardial infarction. Circulation. 2008;118:2057–2062. doi: 10.1161/CIRCULATIONAHA.108.784215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham heart study subjects. Circulation. 1993;88:107–115. doi: 10.1161/01.cir.88.1.107. [DOI] [PubMed] [Google Scholar]
- 5.Kannel WB, D’Agostino RB, Silbershatz H, Belanger AJ, Wilson PW, Levy D. Profile for estimating risk of heart failure. Arch Intern Med. 1999;159:1197–1204. doi: 10.1001/archinte.159.11.1197. [DOI] [PubMed] [Google Scholar]
- 6.Ho JE, Lyass A, Lee DS, Vasan RS, Kannel WB, Larson MG, et al. Predictors of new-onset heart failure: differences in preserved versus reduced ejection fraction. Circ Heart Fail. 2013;6:279–286. doi: 10.1161/CIRCHEARTFAILURE.112.972828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schnabel RB, Rienstra M, Sullivan LM, Sun JX, Moser CB, Levy D, et al. Risk assessment for incident heart failure in individuals with atrial fibrillation. Eur J Heart Fail. 2013;15:843–849. doi: 10.1093/eurjhf/hft041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho JE, Gona P, Pencina MJ, Tu JV, Austin PC, Vasan RS, et al. Discriminating clinical features of heart failure with preserved vs. Reduced ejection fraction in the community. Eur Heart J. 2012;33:1734–1741. doi: 10.1093/eurheartj/ehs070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krumholz HM, Larson M, Levy D. Sex differences in cardiac adaptation to isolated systolic hypertension. Am J Cardiol. 1993;72:310–313. doi: 10.1016/0002-9149(93)90678-6. [DOI] [PubMed] [Google Scholar]
- 10.Lam CS, Donal E, Kraigher-Krainer E, Vasan RS. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:18–28. doi: 10.1093/eurjhf/hfq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersson C, Vasan RS. Epidemiology of heart failure with preserved ejection fraction. Heart Fail Clin. 2014;10:377–388. doi: 10.1016/j.hfc.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D’Agostino RB, Kannel WB, et al. Lifetime risk for developing congestive heart failure: the Framingham heart study. Circulation. 2002;106:3068–3072. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 13.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA. 1996;275:1557–1562. [PubMed] [Google Scholar]
- 14.Vasan RS, Levy D. The role of hypertension in the pathogenesis of heart failure. A clinical mechanistic overview. Arch Intern Med. 1996;156:1789–1796. [PubMed] [Google Scholar]
- 15.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 16.Walsh CR, Larson MG, Evans JC, Djousse L, Ellison RC, Vasan RS, et al. Alcohol consumption and risk for congestive heart failure in the Framingham heart study. Ann Intern Med. 2002;136:181–191. doi: 10.7326/0003-4819-136-3-200202050-00005. [DOI] [PubMed] [Google Scholar]
- 17.Lee DS, Pencina MJ, Benjamin EJ, Wang TJ, Levy D, O’Donnell CJ, et al. Association of parental heart failure with risk of heart failure in offspring. N Engl J Med. 2006;355:138–147. doi: 10.1056/NEJMoa052948. [DOI] [PubMed] [Google Scholar]
- 18.Dhingra R, Pencina MJ, Wang TJ, Nam BH, Benjamin EJ, Levy D, et al. Electrocardiographic qrs duration and the risk of congestive heart failure: the Framingham heart study. Hypertension. 2006;47:861–867. doi: 10.1161/01.HYP.0000217141.20163.23. [DOI] [PubMed] [Google Scholar]
- 19.Velagaleti RS, Massaro J, Vasan RS, Robins SJ, Kannel WB, Levy D. Relations of lipid concentrations to heart failure incidence: the Framingham heart study. Circulation. 2009;120:2345–2351. doi: 10.1161/CIRCULATIONAHA.109.830984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kraigher-Krainer E, Lyass A, Massaro JM, Lee DS, Ho JE, Levy D, et al. Association of physical activity and heart failure with preserved vs. reduced ejection fraction in the elderly: the Framingham heart study. Eur J Heart Fail. 2013;15:742–746. doi: 10.1093/eurjhf/hft025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34:29–34. doi: 10.1016/0002-9149(74)90089-7. [DOI] [PubMed] [Google Scholar]
- 22.Dhingra R, Vasan RS. Diabetes and the risk of heart failure. Heart Fail Clin. 2012;8:125–133. doi: 10.1016/j.hfc.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galderisi M, Anderson KM, Wilson PW, Levy D. Echocardiographic evidence for the existence of a distinct diabetic cardiomyopathy (the framingham heart study) Am J Cardiol. 1991;68:85–89. doi: 10.1016/0002-9149(91)90716-x. [DOI] [PubMed] [Google Scholar]
- 24.Rutter MK, Parise H, Benjamin EJ, Levy D, Larson MG, Meigs JB, et al. Impact of glucose intolerance and insulin resistance on cardiac structure and function: sex-related differences in the Framingham heart study. Circulation. 2003;107:448–454. doi: 10.1161/01.cir.0000045671.62860.98. [DOI] [PubMed] [Google Scholar]
- 25.Lee DS, Massaro JM, Wang TJ, Kannel WB, Benjamin EJ, Kenchaiah S, et al. Antecedent blood pressure, body mass index, and the risk of incident heart failure in later life. Hypertension. 2007;50:869–876. doi: 10.1161/HYPERTENSIONAHA.107.095380. [DOI] [PubMed] [Google Scholar]
- 26.Kenchaiah S, Gaziano JM, Vasan RS. Impact of obesity on the risk of heart failure and survival after the onset of heart failure. Med Clin N Am. 2004;88:1273–1294. doi: 10.1016/j.mcna.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 27.Cheng S, McCabe EL, Larson MG, Chen MH, Osypiuk E, Lehman BT, et al. Left ventricular mechanical function: clinical correlates, heritability, and association with parental heart failure. Eur J Heart Fail. 2015;17:44–50. doi: 10.1002/ejhf.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lam CS, Liu X, Yang Q, Larson MG, Pencina MJ, Aragam J, et al. Familial aggregation of left ventricular geometry and association with parental heart failure: the Framingham heart study. Circ Cardiovasc Genet. 2010;3:492–498. doi: 10.1161/CIRCGENETICS.110.941088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lam CS, Lyass A, Kraigher-Krainer E, Massaro JM, Lee DS, Ho JE, et al. Cardiac dysfunction and noncardiac dysfunction as precursors of heart failure with reduced and preserved ejection fraction in the community. Circulation. 2011;124:24–30. doi: 10.1161/CIRCULATIONAHA.110.979203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham heart study. Circulation. 2003;107:2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 31.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Levy D. Left ventricular dilatation and the risk of congestive heart failure in people without myocardial infarction. N Engl J Med. 1997;336:1350–1355. doi: 10.1056/NEJM199705083361903. [DOI] [PubMed] [Google Scholar]
- 32.Wang TJ, Evans JC, Benjamin EJ, Levy D, LeRoy EC, Vasan RS. Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circulation. 2003;108:977–982. doi: 10.1161/01.CIR.0000085166.44904.79. [DOI] [PubMed] [Google Scholar]
- 33.Lam CS, Gona P, Larson MG, Aragam J, Lee DS, Mitchell GF, et al. Aortic root remodeling and risk of heart failure in the framing-ham heart study. JACC Heart Fail. 2013;1:79–83. doi: 10.1016/j.jchf.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Velagaleti RS, Gona P, Pencina MJ, Aragam J, Wang TJ, Levy D, et al. Left ventricular hypertrophy patterns and incidence of heart failure with preserved versus reduced ejection fraction. Am J Cardiol. 2014;113:117–122. doi: 10.1016/j.amjcard.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vasan RS, Larson MG, Levy D. Determinants of echocardiographic aortic root size. The Framingham heart study. Circulation. 1995;91:734–740. doi: 10.1161/01.cir.91.3.734. [DOI] [PubMed] [Google Scholar]
- 36.Cheng S, Xanthakis V, Sullivan LM, Lieb W, Massaro J, Aragam J, et al. Correlates of echocardiographic indices of cardiac remodeling over the adult life course: longitudinal observations from the Framingham heart study. Circulation. 2010;122:570–578. doi: 10.1161/CIRCULATIONAHA.110.937821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sundstrom J, Sullivan L, Selhub J, Benjamin EJ, D’Agostino RB, Jacques PF, et al. Relations of plasma homocysteine to left ventricular structure and function: the Framingham heart study. Eur Heart J. 2004;25:523–530. doi: 10.1016/j.ehj.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 38.Vasan RS, Beiser A, D’Agostino RB, Levy D, Selhub J, Jacques PF, et al. Plasma homocysteine and risk for congestive heart failure in adults without prior myocardial infarction. JAMA. 2003;289:1251–1257. doi: 10.1001/jama.289.10.1251. [DOI] [PubMed] [Google Scholar]
- 39.Vasan RS, Sullivan LM, Roubenoff R, Dinarello CA, Harris T, Benjamin EJ, et al. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: the Framingham heart study. Circulation. 2003;107:1486–1491. doi: 10.1161/01.cir.0000057810.48709.f6. [DOI] [PubMed] [Google Scholar]
- 40.Vasan RS, Sullivan LM, D’Agostino RB, Roubenoff R, Harris T, Sawyer DB, et al. Serum insulin-like growth factor i and risk for heart failure in elderly individuals without a previous myocardial infarction: the Framingham heart study. Ann Intern Med. 2003;139:642–648. doi: 10.7326/0003-4819-139-8-200310210-00007. [DOI] [PubMed] [Google Scholar]
- 41.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655–663. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 42.Frankel DS, Vasan RS, D’Agostino Sr RB, Benjamin EJ, Levy D, Wang TJ, et al. Resistin, adiponectin, and risk of heart failure the framingham offspring study. J Am Coll Cardiol. 2009;53:754–762. doi: 10.1016/j.jacc.2008.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lieb W, Sullivan LM, Harris TB, Roubenoff R, Benjamin EJ, Levy D, et al. Plasma leptin levels and incidence of heart failure, cardiovascular disease, and total mortality in elderly individuals. Diabetes Care. 2009;32:612–616. doi: 10.2337/dc08-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Velagaleti RS, Gona P, Larson MG, Wang TJ, Levy D, Benjamin EJ, et al. Multimarker approach for the prediction of heart failure incidence in the community. Circulation. 2010;122:1700–1706. doi: 10.1161/CIRCULATIONAHA.109.929661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dhingra R, Gona P, Benjamin EJ, Wang TJ, Aragam J, D’Agostino Sr RB, et al. Relations of serum phosphorus levels to echocardio-graphic left ventricular mass and incidence of heart failure in the community. Eur J Heart Fail. 2010;12:812–818. doi: 10.1093/eurjhf/hfq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dhingra R, Gona P, Wang TJ, Fox CS, D’Agostino Sr RB, Vasan RS. Serum gamma-glutamyl transferase and risk of heart failure in the community. Arterioscler Thromb Vasc Biol. 2010;30:1855–1860. doi: 10.1161/ATVBAHA.110.207340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ho JE, Liu C, Lyass A, Courchesne P, Pencina MJ, Vasan RS, et al. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J Am Coll Cardiol. 2012;60:1249–1256. doi: 10.1016/j.jacc.2012.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coglianese EE, Qureshi MM, Vasan RS, Wang TJ, Moore LL. Usefulness of the blood hematocrit level to predict development of heart failure in a community. Am J Cardiol. 2012;109:241–245. doi: 10.1016/j.amjcard.2011.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sundstrom J, Vasan RS. Homocysteine and heart failure: a review of investigations from the framingham heart study. Clin Chem Lab Med. 2005;43:987–992. doi: 10.1515/CCLM.2005.173. [DOI] [PubMed] [Google Scholar]
- 50.Wang TJ, Larson MG, Levy D, Leip EP, Benjamin EJ, Wilson PW, et al. Impact of age and sex on plasma natriuretic peptide levels in healthy adults. Am J Cardiol. 2002;90:254–258. doi: 10.1016/s0002-9149(02)02464-5. [DOI] [PubMed] [Google Scholar]
- 51.Lam CS, Cheng S, Choong K, Larson MG, Murabito JM, Newton-Cheh C, et al. Influence of sex and hormone status on circulating natriuretic peptides. J Am Coll Cardiol. 2011;58:618–626. doi: 10.1016/j.jacc.2011.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vasan RS, Benjamin EJ, Larson MG, Leip EP, Wang TJ, Wilson PW, et al. Plasma natriuretic peptides for community screening for left ventricular hypertrophy and systolic dysfunction: the Framingham heart study. JAMA. 2002;288:1252–1259. doi: 10.1001/jama.288.10.1252. [DOI] [PubMed] [Google Scholar]
- 53.Lieb W, Sullivan LM, Aragam J, Harris TB, Roubenoff R, Benjamin EJ, et al. Relation of serum leptin with cardiac mass and left atrial dimension in individuals >70 years of age. Am J Cardiol. 2009;104:602–605. doi: 10.1016/j.amjcard.2009.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Velagaleti RS, Gona P, Levy D, Aragam J, Larson MG, Tofler GH, et al. Relations of biomarkers representing distinct biological pathways to left ventricular geometry. Circulation. 2008;118:2252–2258. doi: 10.1161/CIRCULATIONAHA.108.817411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vasan RS, Evans JC, Benjamin EJ, Levy D, Larson MG, Sundstrom J, et al. Relations of serum aldosterone to cardiac structure: gender-related differences in the framingham heart study. Hypertension. 2004;43:957–962. doi: 10.1161/01.HYP.0000124251.06056.8e. [DOI] [PubMed] [Google Scholar]
- 56.Vasan RS, Evans JC, Larson MG, Wilson PW, Meigs JB, Rifai N, et al. Serum aldosterone and the incidence of hypertension in nonhypertensive persons. N Engl J Med. 2004;351:33–41. doi: 10.1056/NEJMoa033263. [DOI] [PubMed] [Google Scholar]
- 57.Sundstrom J, Evans JC, Benjamin EJ, Levy D, Larson MG, Sawyer DB, et al. Relations of plasma matrix metalloproteinase-9 to clinical cardiovascular risk factors and echocardiographic left ventricular measures: the Framingham heart study. Circulation. 2004;109:2850–2856. doi: 10.1161/01.CIR.0000129318.79570.84. [DOI] [PubMed] [Google Scholar]
- 58.Vasan RS, Demissie S, Kimura M, Cupples LA, White C, Gardner JP, et al. Association of leukocyte telomere length with echocardio-graphic left ventricular mass: the Framingham heart study. Circulation. 2009;120:1195–1202. doi: 10.1161/CIRCULATIONAHA.109.853895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee DS, Gona P, Albano I, Larson MG, Benjamin EJ, Levy D, et al. A systematic assessment of causes of death after heart failure onset in the community: impact of age at death, time period, and left ventricular systolic dysfunction. Circ Heart Fail. 2011;4:36–43. doi: 10.1161/CIRCHEARTFAILURE.110.957480. [DOI] [PMC free article] [PubMed] [Google Scholar]