Abstract
Objectives
To assess the performance of Xpert MTB/RIF, an automated molecular test for Mycobacterium tuberculosis (MTB) and resistance to rifampin (RIF), against smear microscopy and culture method for diagnosis of MTB infection.
Methods
This is a retrospective analysis of 103 respiratory and 137 non-respiratory patient specimens suspected of tuberculosis at King Khalid University Hospital, Riyadh, Kingdom of Saudi Arabia performed between April 2014 and March 2015. Each sample underwent smear microscopy, mycobacterial culture, and GeneXpert MTB/RIF test.
Results
Fifteen out of 103 respiratory samples were smear and culture positive, whereas 9 out of 137 non-respiratory samples were smear positive. Out of 9 smear positive specimens, 8 were also culture positive. All 15 culture positive respiratory samples were detected by Xpert MTB/RIF (sensitivity and positive predictive value [PPV]=100%). Similarly, all 8 culture positive non-respiratory specimens were identified by Xpert MTB/RIF (sensitivity 100%; PPV 88.8%). The Xpert MTB/RIF detected only one false positive result in 88 smear negative respiratory specimens (specificity 98.9%; negative predictive value [NPV]= 100%). All 125 smear negative non-respiratory specimens tested negative by culture and Xpert MTB/RIF (sensitivity, specificity, PPV, NPV= 100%).
Conclusion
The performance of Xpert MTB/RIF was comparable to the gold standard culture method for identification of MTB in both respiratory and non-respiratory clinical specimens.
Laboratory confirmed diagnosis of active tuberculosis (TB) is pivotal for management of disease and is an effective public health intervention. The current gold standard for confirmation of TB is mycobacterial culture, which is both labor-intensive and time consuming. Depending on the method used, it may take up to 8 weeks to confirm the diagnosis. Although smear microscopy is a simple and rapid method for detection of Mycobacterium tuberculosis (MTB), it lacks sensitivity, negative predictive value (NPV), and requires multiple sputum samples.1 While smear microscopy is most widely used for diagnosis of TB, only 45% of TB cases were diagnosed by a positive smear representing only 28% of the estimated global disease burden.1 Despite improvements in automated culture methods such as the mycobacterial growth indicator tube (MGIT) system now available in the clinical microbiology laboratory, the search for a reliable, rapid and simplified method of diagnosing MTB in resource limited regions is ongoing.
Recently, a number of molecular diagnostic devices have been recommended for rapid diagnosis of MTB by the World Health Organization (WHO) including the Xpert MTB/RIF (Cepheid Inc., Sunnyvale, California, USA) as a diagnostic test.2 This assay has also been cleared by the Food Drug Administration (FDA) for diagnosis of MTB.3 This cartridge-based rapid, automated, semi-quantitative real-time polymerase chain reaction (RT-PCR) assay simultaneously detects organisms in MTB complex, and the MTB rifampin (RIF) resistance gene in clinical samples within 2 hours. The Xpert MTB/RIF assay requires a minimal sample processing and hands-on time, yet is highly sensitive in detecting MTB in a single specimen.4 Performance of Xpert MTB/RIF for detection of MTB complex revealed an overall pooled sensitivity of 89% and a specificity of 99%.5 Furthermore, due to its high diagnostic accuracy, Xpert MTB/RIF has been recommended by FDA as an initial stand-alone test to replace smear microscopy.3
While the performance of Xpert MTB/RIF in the detection of MTB complex in respiratory samples is well described, its performance in extra-pulmonary clinical specimens has not been well characterized with lower sensitivity and specificity in some studies.6,7 Extra-pulmonary MTB infection remains a diagnostic challenge not only due to the low number of bacteria, but also due to invasive procedures often required for sample collection. Recent studies focusing on diagnosis of extra-pulmonary MTB revealed that Xpert MTB/RIF detection rate was variable with lower sensitivity in different extra-pulmonary tissues; lymph node (sensitivity 83%), cerebrospinal fluid (CSF) (sensitivity 80%), and pleural fluid (sensitivity 46%).8 These studies suggest that Xpert MTB/RIF performs better in respiratory samples compared to non-respiratory specimens possibly due to lower burden of mycobacteria. The aim of our study was to evaluate the efficiency and reliability of Xpert MTB/RIF system for detection of MTB in pulmonary and extra-pulmonary clinical samples in the patient population seen at our hospital.
Methods
All previous studies cited in this manuscript were reviewed by accessing the national library of medicine and Pubmed online database. This study did not involve human subjects or administration of any therapeutic agents. This is a retrospective study performed at King Khalid University Hospital, Riyadh, Saudi Arabia between April 2014 and March 2015. A total of 103 respiratory and 137 non-respiratory specimens were received by Mycobacteriology Laboratory with clinical suspicion of TB and were included in the study. Samples that were received outside the time period were excluded from the study. Respiratory samples included sputum, broncho-alveolar lavage (BAL), endotracheal aspirate, and pleural fluid. Non-respiratory (extra-pulmonary) samples included in our study were lymph node biopsy, peritoneal fluid, pus, and gastric aspirates. Each sample was processed for smear microscopy by Z-N and auramine-rhodamine stains and cultured in MGIT Bactec 960 liquid medium, Lowenstein-Jensen solid (LJ) pyruvate, and glycerol media. Time to detection (TTD) of growth of Mycobacteria was based on the date of earliest Bactec 960 instrument liquid culture positivity. The solid media was then incubated at 37°C in aerobic atmosphere until growth of mycobacteria on the culture medium. Identification of mycobacteria was based on colony morphology on the solid medium, colony pigmentation, rate of growth on the solid medium, results of biochemical tests including, the nitrate reductase test, Niacin test, and heat stable catalase and pyrazinamidase test. Differentiation of Mycobacterium tuberculosis complex, Mycobacterium bovis, Mycobacterium africanum, Mycobacterium microti and Bacillus Calmette-Guérin strain along with non-tuberculous mycobacteria (NTM) was achieved by the BD Probe Tec system (Becton Dickinson, USA) and growth of NTM on solid medium containing Para Amino Benzoic Acid (PNB). Biochemical tests were performed to identify Mycobacterium bovis.
The susceptibility of the MTB isolates from these specimens to the first line anti-TB drugs, streptomycin (STM), isoniazid (INH), RIF, and ethambutol (EMB), was assessed using BACTEC MGIT 960 SIRE Kit, (BD Biosciences, Sparks, MD, USA) following the manufacturer instructions. All isolates of Mycobacterium tuberculosis that grew on culture were saved at -80°C for further molecular investigations.
Xpert MTB/RIF.
The Xpert MTB/RIF RT-PCR assay was performed in accordance with the manufacturer’s instructions described in the package insert. Briefly, clinical samples were added to sample reagents using a ratio of 3:1. The specimen container was tightly closed and then manually agitated twice within a 15-min period at room temperature to inactivate possible contaminating bacteria. A 2 ml of the inactivated material (equivalent to 0.5 ml of decontaminated pellet) was then added to the Xpert MTB/RIF cartridge. All specimens that were MTB culture positive and Xpert MTB/RIF negative as well as specimens that were MTB culture negative and Xpert MTB/RIF positive were tested twice for confirmation of results. The last result obtained was used for the final analysis.
Statistical analysis
Data were collected in an excel sheet and were analyzed using the Statistical Package for Social Sciences Version 12.0 (SPSS Inc. Wacker Drive, Chicago, IL USA). A p-value of <0.05 was considered significant. The p-value was applied to compare sensitivity and specificity of Xpert MTB/RIF to the gold standard culture method for identification of TB in our study.
Results
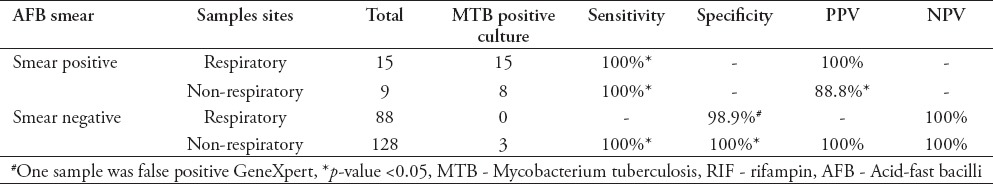
Fifteen specimens (14.5%) were MTB culture positive out of 103 respiratory samples, while only 11 (8%) samples were MTB culture positive from 137 non-respiratory samples tested. Table 1 compares the performance of Xpert MTB/RIF with culture results for detection of MTB. For the 15 smear positive and culture positive respiratory samples, Xpert MTB/RIF detected MTB consistently in all the 15 samples with a sensitivity of 100% and positive predictive value (PPV) of 100% (p<0.05). Among the 9 smear positive non-respiratory samples, 8 were culture positive. The Xpert MTB/RIF assay detected MTB in all 9 samples revealing one false positive result with a sensitivity of 100% and PPV of 88.8% (p<0.05). There were 88 smear negative respiratory samples and all tested negative by culture; whereas the Xpert MTB/RIF yielded one false positive result and 87 samples were true negative with specificity of 98.9% and a negative predictive value (NPV) of 100%. Among the 128 smear negative non-respiratory samples, MTB was confirmed in 3 samples, the remaining 125 samples were negative, both by culture and the Xpert MTB/RIF assay, with a sensitivity of 100%, specificity of 100%, PPV of 100%, and NPV of 100% (p<0.05).
Table 1.
Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of Xpert MTB/RIF on various sample types as it compared to mycobacterium culture.
Discussion
While a number of studies assessed the Xpert MTB/RIF assay in different populations, there are only limited data in the gulf countries.9 Our findings indicate higher sensitivity for this assay than previously reported in Saudi patients.9
The performance of the Xpert MTB/RIF assay with 100% sensitivity is comparable to the gold standard as noted in this study. These observations are in agreement with findings from previous reports1,10 assessing the sensitivity of the Xpert MTB/RIF assay for the diagnosis of smear positive pulmonary MT B. Previous studies10 suggest that the Xpert MTB/RIF assay has a sensitivity of approximately 71.4% for smear negative respiratory samples, which is significantly less than the sensitivity observed in our study especially in non-respiratory specimens. This may be attributed to the quality of the specimen, or to the differences in patient demographics. Nevertheless, the ability of this assay to identify smear negative MTB is of great clinical value as increasing numbers of smear negative cases are being encountered among TB patients co-infected with human immunodeficiency virus (HIV), particularly in resource limited laboratories. While culture method is considered as the gold standard for diagnosis of smear positive pulmonary MTB infection, we found that the Xpert MTB/RIF assay substantially increased MTB detection with higher sensitivity in smear positive than smear negative patients, which is consistent with a meta-analysis by Steingart et al.5 A review of Xpert MTB/RIF performance in a multi-country study found sensitivity of 83% among culture positive TB cases and 98% in smear negative and culture positive patients with MTB.11 Smear negative TB cases are associated with delayed or failure of diagnosis, poor treatment outcomes, risk of transmission, and death. Introducing the Xpert MTB/RIF assay early in diagnostic workflow can potentially improve detection and shorten turn-around time in the laboratory. Recent reports suggest that Xpert MTB/RIF assay increases the rate of detection of RIF resistance and also decreases unnecessary empiric treatment among smear negative pulmonary MTB.12
Diagnosing extra-pulmonary TB infection presents a significant challenge likely due to lower numbers of the organisms that can be recovered in the specimen. In our study, we found that the Xpert MTB/RIF assay performs well in detecting extra-pulmonary TB from different sites (lymph node biopsy, peritoneal fluid, pus and gastric aspirates). The detection rate of MTB among smear negative non-respiratory specimens by the Xpert MTB/RIF assay varies between studies with sensitivity rate of 20-47.7%.1,10 As noted earlier, Xpert MTB/RIF has a higher sensitivity for detecting MTB in lymph nodes (83%) compared to other specimens. This could be due to the high mycobacterial burden during infection as well as ease of accessing the site for specimen collection. These findings are important in the diagnosis of extra-pulmonary TB specifically in the pediatric age group where up to 60% of patients with extra-pulmonary TB develop cervical lymphadenitis.13 Furthermore, since the diagnosis of MTB in children is often delayed due to non-specific clinical features and difficult expectoration, the Xpert MTB/RIF assay stands out as a useful method in the diagnosis of pediatric MTB.14
While neither FDA, nor Conformité Européene ie European Conformity (CE) approved Xpert MTB/RIF for diagnosis of extra-pulmonary MTB, the World Health Organization released a policy statement recommending Xpert MTB/RIF for diagnosis of extra-pulmonary MTB and RIF resistance in children.4 This decision was based on emerging evidence from a meta-analysis of 85 peer reviewed publications that Xpert MTB/RIF performed favorably in detecting extra-pulmonary infections including lymph nodes, CSF, and body fluids. These recommendations are consistent with another meta-analysis in which 27 peer-reviewed publications collectively suggest that Xpert MTB/RIF accurately detects extra pulmonary TB in children and adults.11
Xpert MTB/RIF assay reported one false positive result in the present study. Patients with false positive results are liable to receive avoidable anti-TB therapy. False positivity of Xpert MTB/RIF assay results has been reported previously and has been attributed to the presence of dead MTB in the test samples particularly among previously treated patients.15 Due to the lack of access to patient records, information regarding previous therapy with anti-TB drugs could not be retrieved. Careful history taking with special emphasis on previous treatment with anti-TB drugs appears to be critical in avoiding false positive results.
In conclusion, results of our study are consistent with the existing data and enhance the performance of Xpert MTB/RIF assay in detection of MTB in respiratory as well as non-respiratory samples in Saudi patients. We provide further evidence that Xpert/RIF system has superior sensitivity, specificity, and predictive values which can positively impact clinical outcomes. Xpert/RIF allows accurate and early diagnosis of TB infection and the shorter turnaround time provides opportunity for timely therapeutic intervention, which is beneficial not only for the patient, but also for any possible contacts. Our findings support the continued use of the Xpert MTB/RIF assay in the Saudi population and recommend further large scale studies in future.
This study was however limited by being a single center study and relatively small numbers of total specimens. Therefore, the findings here may not be applicable to a wider local population. Large scale studies including data from multiple centers across the Kingdom should be performed to validate our findings in this study.
References
- 1.Ryan GJ, Shapiro HM, Lenaerts AJ. Improving acid fast fluorescent staining for the detection of mycobacteria using a new nucleic acid staining approach. Tuberculosis (Edinb) 2014;94:511–518. doi: 10.1016/j.tube.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Rapid implementation of the Xpert MTB/RIF diagnostic test. Geneva: World Health Organization; 2011. [Google Scholar]
- 3.Division of Microbiology Devices FDA/CDC. Revised device labeling for the Cepheid Xpert MTB/RIF assay for detecting Mycobacterial tuberculosis. MMWR Morbidity Mortality Weekly Reports. 2015;64:193. [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF assay for the diagnosis of pulmonary and extrapulmonary TB in adults and children: policy update. Geneva: WHO; 2013. [PubMed] [Google Scholar]
- 5.Steingart KR, Schiller I, Horne DJ, Pai M, Boehme CC, Dendukuri N. Xpert MTB/RIF assay for pulmonary tuberculosis and rifampin resistance in adults. Cochrane Database Syst Rev. 2014;(1):CD009593. doi: 10.1002/14651858.CD009593.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McNerney R, Zumla A. Impact of the Xpert MTB/RIF diagnostic test for tuberculosis in countries with a high burden of disease. Curr Opin Pulm Med. 2015;21:304–308. doi: 10.1097/MCP.0000000000000161. [DOI] [PubMed] [Google Scholar]
- 7.Nataraj G, Kanade S, Mehta P. Xpert MTB/RIF for improved case detection of extra pulmonary TB in a tertiary care setting in urban India. Int J Tuberc Lung Dis. 2016;20:890–894. doi: 10.5588/ijtld.15.0849. [DOI] [PubMed] [Google Scholar]
- 8.Dekinger CM, Schumacher SG, Boehme CC, Dendukuri N, Pai M, Steingart KR. Xpert MTB/RIF assay for the diagnosis of extrapulmonary tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2014;44:435–446. doi: 10.1183/09031936.00007814. [DOI] [PubMed] [Google Scholar]
- 9.Al-Ateah SM, Al-Dowaidi MM, El-Khizzi NA. Evaluation of direct detection of Mycobacterium tuberculosis complex in respiratory and non-respiratory clinical specimens using the Cepheid Gene Xpert®system. Saudi Med J. 2012;33:1100–1115. [PubMed] [Google Scholar]
- 10.Bunsow E, Ruiz-Serrano MJ, Roa P, Kestler M, Viedma D, Bouza E. Evaluation of GeneXpert MTB/RIF for the detection of Mycobacterium tuberculosis and resistance to rifampicin in clinical specimens. J Infect. 2014;68:338–343. doi: 10.1016/j.jinf.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 11.Maynard-Smith L, Larke N, Peters JA, Lawn SD. Diagnostic accuracy of the Xpert MTB/RIF assay for extra-pulmonary and pulmonary tuberculosis when testing non-respiratory samples: a systematic review. BMC Infect Dis. 2014;14:709. doi: 10.1186/s12879-014-0709-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim YW, Seong MW, Kim TS, Yoo CG, Kim YW, Han SK, Yim JJ. Evaluation of Xpert MTB/RIF assay: diagnosis and treatment outcomes in Rifampin resistant tuberculosis. Int J Tuberc Lung Dis. 2015;19:1261–1221. doi: 10.5588/ijtld.15.0183. [DOI] [PubMed] [Google Scholar]
- 13.Neelakantan S, Nair PP, Emmanuel RV, Agarwal K. Diversities in presentation of extrapulmonary tuberculosis. BMJ Case Rep. 2013;28:2013. doi: 10.1136/bcr-2013-008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perez-Velez CM, Marias BJ. Tuberculosis in Children. N Engl J Med. 2012;367:348–361. doi: 10.1056/NEJMra1008049. [DOI] [PubMed] [Google Scholar]
- 15.Boyles TH, Hughes J, Cox V, Burton R, Meintjes G, Mendelson M. False-positive Xpert®MTB/RIF assays in previously treated patients: need for caution in interpreting results. Int J Tuberc Lung Dis. 2014;18:876–878. doi: 10.5588/ijtld.13.0853. [DOI] [PubMed] [Google Scholar]


