Abstract
Objective
To demonstrate the diagnostic potentials of MRI, diffusion weighted imaging (DWI), and apparent diffusion coefficient (ADC) mapping in the detection of parotid masses correlated to the histopathological results.
Methods
Study design was retrospective. Fifteen patients with parotid gland masses were included as the study group and contralateral normal parotis glands of same patients were taken as the control group. Patients with bilateral parotid gland tumors were excluded, 7 right-sided and 8 left-sided parotid masses were included in the research. The study took place at the Department of Radiology, Ankara, Turkey, between May 2012 and September 2014.
Results
Apparent diffusion coefficient measurements of 15 parotis tumors in 1000 and 750 sec/mm2 b-values with comparison to the contralateral normal gland parenchyma were demonstrated. Neurofibromas was predicted as the highest, and lipomas as the lowest ADC values. Pleomorphic adenomas, Warthin’s tumor, and normal parotid parenchyma indicate significant statistical differences from each other on the basis of mean ADC values (p<0.05).
Conclusion
The DWI and ADC mapping of parotis gland could aid in the differential diagnosis of benign and malignant masses.
Parotid glands offer a large variety of different histologic neoplasms and the MRI explain the morphology and extent of the parotid tumors. However, routine MRI could not easily differentiate parotid tumors, especially histologic subtypes, which might influence the surgical procedure and preoperative characterization of tumors.1-3 Fine-needle aspiration cytology (FNAC) was a valuable tool in the pre-operative diagnosis of parotid gland tumors, but might cause spread of tumor cells, which could lead to increased recurrence rate, for pleomorphic adenomas and malignant parotid tumors, might also cause overlapping in the differentiation of some benign and malignant lesions such as differentiating pleomorphic adenomas from mucoepidermoid carcinomas and basal cell adenomas from basal cell adenocarcinomas.1-6
Diffusion weighted imaging (DWI) and apparent diffusion coefficient (ADC) mapping has been specified to the microscopic motions of water molecules in the tissue and identify water balance between intra-extra cellular compartment.2,5,7 Diffusion weighted imaging is a high MRI technique that supplies information about the organization, density, microstructure, and microcirculation of tissues.1-7 Apparent diffusion coefficient, as a quantitative parameter, determines the specific diffusion capacity of a biological tissue via combining capillary perfusion and water diffusion in the extracellular extravascular space.1,4,8 Previous studies1-4,6,8 stated that DWI-ADC mapping, a non-invasive approach, might be used to define the tumors of head and neck such as parotid gland tumors, and shows highest diagnostic potential to characterize the lesions and define the histologic subtypes of parotid gland tumors.
In this retrospective study, our aim is to utilize the diagnostic potentials of MRI-DWI and ADC-mapping in the detection of parotid masses correlated to the histopathological results.
Methods
This study was approved by the institutional ethic committee and written consents were taken from all patients. This medical research was acquired according to the Helsinki declaration. Study design was retrospective. Fifteen patients with parotid gland masses were included as the study group and contralateral normal parotis glands of same patients were taken as the control group. Patients with bilateral parotid gland tumors were excluded, 7 right-sided and 8 left-sided parotid masses were included in the research. Exclusion criteria for this retrospective research were bad image quality on MRI and DWI, presence of bilateral parotid masses, and unwillingness of patients to be included in this research.
The MRI of patients was performed between May 2012 and September 2014. The study took place at the Department Radiology, Ankara, Turkey. After MRI, histopathologic examinations of parotid masses were handled by either aspiration or true-cut biopsy, and all lesions were removed surgically in order to strengthen and confirm the exact diagnosis.
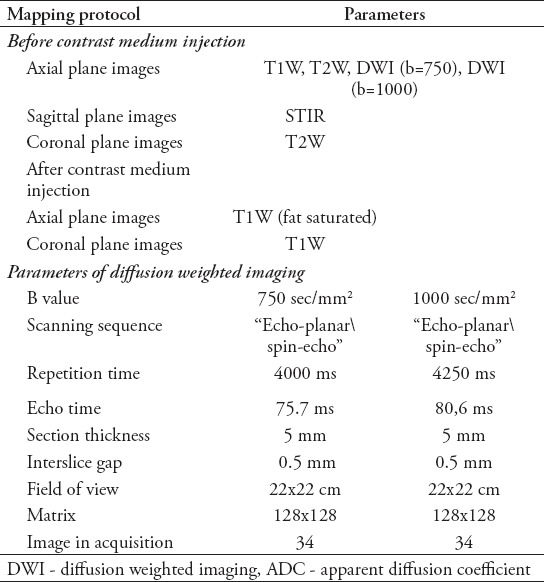
Magnetic resonance imaging was performed by 1.5 T MR scanner (General Electric, Signa HDx 1.5T, GE Medical Systems, Milwaukee, WI, USA) with 8 channel phased array head and neck coil, IV contrast agent was administered as 0.1 mmol/kg dosage with 3 mL/sec. injection rate. The MRI and DWI-ADC mapping protocol are presented in Table 1. Set of diffusion-weighted images were transferred to an independent work-station (Advantage Workstation 3.1; GE Medical Systems) in order to perform ADC maps. Region of interest (ROI) was handled in the masses and normal contralateral parenchyma; then, ADC measurements were acquired quantitatively from the mass regions distant from necrotic and cystic areas, with regard to enhanced images.
Table 1.
The MRI and DWI-ADC mapping protocol in 15 patients with 7 right-sided and 8 left-sided parotid masses.
Statistical analysis was performed by using Statistical Package for Social Sciences Version 15.0 (SPSS Inc., Chicago, IL, USA). Fisher’s Q-square test and Mann Whitney U-test were performed for statistical analysis of benign and malignant parotid masses, receiver operating curves (ROC) and area under curve (AUC) were also obtained to handle optimal cut-off values for distinguishing benign and malignant masses. The value of p<0.05 indicated significant statistical differences. The sensitivity, specificity, and AUC were calculated with 95% confidence interval (95%CI) for DWI-ADC mapping.
Results
Patients’ gender, age, and histopathologic types of parotid tumors is presented in Table 2. Eleven male and 4 female were included in the study. Their age ranges between 18 and 78 years (mean±SD 49±19.7). Four pleomorphic adenomas and Warthin’s tumor, 2 lipomas and metastasis of squamous cell carcinoma of lung, one mixt (epithelial/myoepithelial tumors) tumor-neurofibroma and reactive lymphoid hyperplasia cases were involved in this study.
Table 2.
Patients’ gender, age and histopathologic types of parotid tumors in 15 patients with 7 right-sided and 8 left-sided parotid masses.
The ADC measurements of 15 parotis tumors in 1000 and 750 seconds/mm2 b-values with comparison to the contralateral normal gland parenchyma were as follows: mean ADC values of parotid masses were measured as 1.014-926 for b-values: 750-1000, 863-761 for normal parenchyma. Apparent diffusion coefficient values of benign and malignant parotid masses were regarding to the control group of normal contralateral parenchyma (b=750 and b=1000). Mean ADC values for benign masses were 1016-931 for b-values: 750-1000 and 999-894 for malignant tumors.
Neurofibromas predicted the highest ADC values (2035-1979 for b-values: 750-1000), lipomas the lowest ADC values (320-330 for b-values: 750-1000). Cut-off values were: 1151-921 for Mixt tumor, 906-1271 for pleomorphic adenomas, and 772-711 for Warthin’s tumor under b-values: 750-1000. Pleomorphic adenomas and Warthin’s tumor, pleomorphic adenomas, and normal parotis parenchyma were differentiated from each other with statistical significance (p=0.021). In this study, the highest ADC value was in neurofibroma and the lowest ADC was in lipomas, then in Warthin’s tumor, next in metastasis of squamous cell carcinoma, and pleomorphic adenomas. Normal contralateral parotis gland parenchyma simulated significant statistical differences under non-parametric Mann-Whitney U-test (p=0.016).
Figure 1.
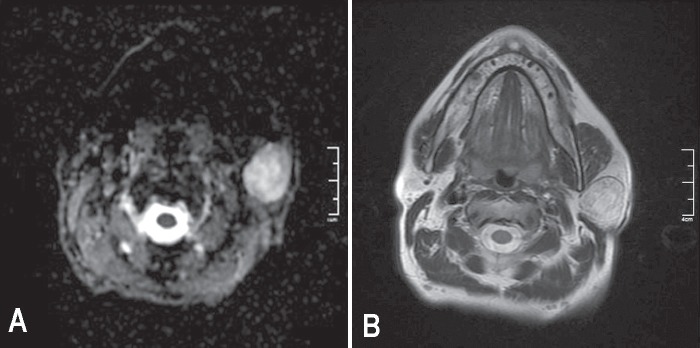
A) Axial ADC mapping image shows hyperintense left parotis mass. The biopsy indicated pleomorphic adenoma for this patient. B) T2W MR image indicating a left parotis mass of a patient with Warthin tumor.
Discussion
Magnetic resonance imaging is a powerful tool for defining the morphology, nature and extent of head and neck tumors as well as their relations to the normal adjacent anatomic structures pre-operatively, but in some routine MRI, it cannot reveal a clear differentiation between benign and malignant parotid tumors.2,5,9,10 Fine-needle aspiration cytology (FNAC) has some shortcomings in the exact diagnosis of parotid tumors, so different pre-operative imaging approaches have an important role in the evaluation of these tumors.2-5
The DWI-ADC mapping, has a great role in the characterization of parotid tumors, high potential to detect different histologic subtypes of parotid tumors, an inverse correlation between tumor cellularity, and ADC values had been previously reported.1-6,8,10 Previous studies2,3,7,11 reported that mean ADC values of benign lesions were significantly higher than those of malignant parotid tumors. However, ADC values alone might not be sufficient for the differentiation of benign and malignant tumors. Combination of MRI and DWI with ADC calculation may show higher diagnostic potential for determining specific histological subtypes of tumors when compared to the results of FNAC.1-11
Apparent diffusion coefficient values can also be affected not only by cellularity, but by extracellular components as well. For example, pleomorphic adenomas frequently exhibit myxoid, or chondroid matrices, leading to higher ADC values than the malignant tumors and Warthin’s tumor due to the extracellular matrix being rich in free water.2,3,9-12 Warthin’s tumor, a second most common benign tumor of parotid gland, has the highest microvascularity among all other parotid tumors, consider low ADC values that can overlap with those of malignant ones due to its hypercellular stroma, or its matrix composed of dense lymphoid tissue, which contributes to the low ADC values for Warthin’s tumor.1-3,9-13 As a result, tissue contrast of tumor is mostly affected degenerative alterations in the interstitial tissue such as the degree of differentiation of tumor cells, presence or absence of necrosis and cystic component, myxomatous alterations and diversity of tumor tissue components.2,9-14
Pleomorphic adenomas have a high risk of malignant transformation, and aggressive surgical approach has to be applied due to high risk of recurrence (lateral or complete parotid resection with high risk of facial nerve injury). Warthin’s tumor and myoepithelial adenomas have a low risk of malignant transformation or recurrences, thus, more conservative resections should be performed due to malignant transformation and suspected recurrences, pleomorphic adenomas, and Warthin’s tumor have to be differentiated accurately.2,9-11,14
There were also some technical difficulties and restrictions of DWI-ADC mapping of parotid gland which include low signal to noise ratio, chemical shift, magnetic susceptibility and motion artefacts. Thin slice selection, higher number of excitations use, application of presaturation bands and warning patients not to move their head and not to swallow might overcome these limitations.1,2,5,9,11 Eida et al9 presented that high ADC values of a parotid tumor supplied significant statistical differences in the differentiation of benign and malignant lesions, but they did not provide absolute ADC levels homogeneously and the major difficulty of their research was determining the average ADC values within a single tumor. Salivary glands are small organs so only larger lesions within this small organ might allow application of DWI-ADC mapping. Matsushima et al11 in contrast to Eida et al,9 found out no significant differences between benign and malignant lesions on the basis of ADC values and stated that mean ADC values increased with the degree of extracellular components. They concluded that ADC levels alone were not enough to differentiate benign and malignant parotis tumors. Habermann et al2 regarded that according to ADC values alone, pleomorphic adenomas were distinguishable from all other masses, except for myoepithelial adenomas and ADC levels of Warthin’s tumor were statistically different from all other tumor subtypes except for mucoepidermoid carcinomas, acinic cell carcinomas and basal cell carcinomas. Ikeda et al7 revealed that average ADC values of Warthin’s tumor was significantly lower than all included malignant lesions. Prades et al15 concluded that morphologic appearances of tumors were very promising for determining whether the tumor is benign or malignant and combining of DWI-ADC map with those morphologic features non-invasively might improve the results. They predicted 83% sensitivity for detecting pleomorhic adenomas and 85% sensitivity for Warthin’s tumor via combined use of ADC values and morphologic factors. In our research, pleomorphic adenomas were differentiated from Warthin’s tumor and from normal parenchyma on the basis of mean ADC values, regarded significant statistical differences (p=0.043, p=0.021). All other histologic subtypes of tumors and normal parenchyma predicted no statistical differences from each other(p=0.371, p=0.630); thus, 8 of the parotid lesions in this research (pleomorphic adenoma, Warthin’s tumor) were differentiated statistically with significance. As this was a preliminary study which included 15 parotid masses, 8 out of 15 (53%) of tumors were distinguished with statistical accuracy. Malignant masses (2 metastatic tumors) were not differentiated from pleomorphic adenomas, Warthin’s tumor and normal parenchyma statistically (p=0.165, p=0.365).
Study limitations
First, the research was only preliminary, further prospective studies had to be carried out with high number of cases and with a wider range of histologic subtypes of tumors to validate the results of this study. Second, there is only a small number of cases and therefore only a small number of different subtypes of benign and malignant tumors were participated in the research which might degrade the confidential statistical results. Third, ADC values might vary after including more patients for every subgroup which could lead to bias and unsatisfactory validity of statistics. Forth, DWI-ADC mapping was organized with b-values of 750 and 1000 sec/mm2, higher b-value use, for example 2000-3000, with higher SNR might improve the results of this preliminary study.
In conclusion, discrimination between benign and malignant masses of parotid gland was so important for surgical procedure planning and conventional imaging modalities might be insufficient for this differentiation. DWI and ADC mapping of parotis gland could aid in this differential diagnosis of masses. Qualitative and quantitative results of both sequences for parotid masses have to be validated in larger prospective studies, so that both high MR imaging approach can be included in the routine Parotis MR Imaging protocol. This research aids to the relevant literature and implies for future searches via emphasizing the quantitative ADC values in the differentiation of malignant and benign parotis masses, prospective researches with a high number of cases may easily reveal beneficial information for the diagnosis of parotis mass lesions.
Footnotes
References
- 1.Inci E, Hocaoglu E, Kilickesmez O, Aydin S, Cimilli T. Quantitative diffusion-weighted MR imaging in the differential diagnosis of parotid gland tumors: Is it a useful technique? Turkiye Klinikleri J Med Sci. 2010;30:1339–1345. [Google Scholar]
- 2.Habermann CR, Arndt C, Graessner J, Diestela L, Petersenb KU, Reitmeierc F, et al. Diffusion-weighted echo-planar MR imaging of primary parotid gland tumors: is a prediction of different histologic subtypes possible? AJNR Am J Neuroradiol. 2009;30:591–596. doi: 10.3174/ajnr.A1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshino N, Yamada I, Ohbayashi N, Honda E, Ida M, Kurabayashi T, et al. Salivary glands and lesions: evaluation of apparent diffusion coefficients with split echo diffusion-weighted MR imaging--initial results. Radiology. 2001;221:837–842. doi: 10.1148/radiol.2213010131. [DOI] [PubMed] [Google Scholar]
- 4.Yabuuchi H, Fukuya T, Tajima T, Hachitanda Y, Tomita K, Koga M. Salivary gland tumors: diagnostic value of gadolinium-enhanced dynamic MR imaging with histopathologic correlation? Radiology. 2003;226:345–354. doi: 10.1148/radiol.2262011486. [DOI] [PubMed] [Google Scholar]
- 5.Yabuuchi H, Matsuo Y, Kamitani T, Setoguchi T, Okafuji T, Soeda H, et al. Parotid gland tumors: can addition of diffusion-weighted MR imaging to dynamic contrast-enhanced MR imaging improve diagnostic accuracy in characterization? Radiology. 2008;249:909–916. doi: 10.1148/radiol.2493072045. [DOI] [PubMed] [Google Scholar]
- 6.Takashima S, Noguchi Y, Okumura T, Aruga H, Kobayashi T. dynamic MR imaging in the head and neck. Radiology. 1993;189:813–821. doi: 10.1148/radiology.189.3.8234709. [DOI] [PubMed] [Google Scholar]
- 7.Ikeda M, Motoori K, Hanazawa T, Nagai Y, Yamamoto S, Ueda T, et al. Warthin tumor of the parotid gland: diagnostic value of MR imaging with histopathologic correlation. AJNR Am J Neuroradiol. 2004;25:1256–1262. [PMC free article] [PubMed] [Google Scholar]
- 8.Alibek S, Zenk J, Bozzato A, Lell M, Grunewald M, Anders K, et al. The value of dynamic MRI studies in parotid tumors. Acad Radiol. 2007;14:701–710. doi: 10.1016/j.acra.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Eida S, Sumi M, Sakihama N, Takahashib H, Nakamuraa T. Apparent diffusion coefficient mapping of salivary gland tumors: prediction of the benignancy and malignancy. AJNR Am J Neuroradiol. 2007;28:116–121. [PMC free article] [PubMed] [Google Scholar]
- 10.Eida S, Sumi M, Nakamura T. Multiparametric magnetic resonance imaging for the differentiation between benign and malignant salivary gland tumors. J Magn Reson Imaging. 2010;31:673–679. doi: 10.1002/jmri.22091. [DOI] [PubMed] [Google Scholar]
- 11.Matsushima N, Maeda M, Takamura M, Takeda K. Apparent diffusion coefficients of benign and malignant salivary gland tumors. Comparison to histopathological findings. J Neuroradiol. 2007;34:183–189. doi: 10.1016/j.neurad.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Martínez Barbero, Rodríquez Jiménez I, Martin Noguerol T, Luna Alcalá A. Utility of MRI diffusion techniques in the evaluation of tumors of the head and neck. Cancers (Basel) 2013;5:875–889. doi: 10.3390/cancers5030875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sumi M, Takagi Y, Uetani M, Morikawa M, Hayashi K, Kabasawa H, et al. Diffusion-weighted echoplanar MR imaging of the salivary glands. AJR Am J Roentgenol. 2002;178:959–965. doi: 10.2214/ajr.178.4.1780959. [DOI] [PubMed] [Google Scholar]
- 14.Balcik C, Akan H, Incesu L. Evaluating of parotid gland tumors according to Diffusion-Weighted MRI. Eur J Gen Med. 2014;11:77–84. [Google Scholar]
- 15.Prades JM, Oletski A, Faye MB, Dumollard JM, Timoshenko AP, Veyret C, et al. Parotid gland masses: diagnostic value of MR imaging with histopathologic correlations. Morphologie. 2007;91:44–51. doi: 10.1016/j.morpho.2007.05.003. [DOI] [PubMed] [Google Scholar]


