Abstract
The recent outbreak of the Zika virus attracts worldwide attention probably because the most recently affected country (Brazil) will host the 2016 Olympic Game. Zika virus infected cases are now spreading to many other countries and its infection might be linked to some severe medical sequelae. Since its first isolation from the infected monkey in 1947 in Uganda, only a few studies had been taken until recent outbreak. According to the history of referenced publications, there is a 19-year gap from 1989 to 2007. This might be because only mild diseases were diagnosed from Zika virus infected populations. Obviously, the recent reports that Zika virus infection is probably associated with microcephaly of the neonates makes us reevaluate the medical significance of the viral pathogen. It can be transmitted sexually or by mosquito biting. Sexual transmission of the Zika virus distinguishes it from other members of the Genus Flavivirus. Detailed information of the Zika virus is needed through a thorough investigation covering basic, epidemical, subclinical and clinical studies. Here, we reviewed the published information of Zika virus.
Keywords: Zika virus, Flavivirus, Congenital infection, Outbreak, Microcephaly
Core tip: Zika virus is gaining new ground with the recent outbreaks that are starting to expand worldwide. While normally transmitted by the mosquito, other routes of transmission are being discovered. Also, other medical complications are being detected with Zika virus infections. These recent findings require the scientific community to thoroughly examine Zika virus to better understand it so that better diagnostic options, treatment, and preventative measures can be developed. In order to beat Zika virus, we must understand its history and outbreak patterns as well as gain a full understanding of all clinical manifestations associated with this virus.
INTRODUCTION
The Zika virus, together with the West Nile virus, Yellow fever virus, Japanese encephalitis virus, Dengue fever virus, and many other classified and unclassified viruses, forms the genus Flavivirus that belongs to family Flaviviridae. The family Flaviviridae consists of many other viruses that are summarized in a 2010 review[1]. This family of viruses have enveloped icosahedral capsid that contains a single strand RNA genome (about 11000 nucleotides) with positive sense[2]. Therefore, the infected viral RNA can be directly translated to a large polyprotein precursor, which is co- and post-translationally processed by viral and cellular proteases into structural and non-structural proteins. The three structural proteins are critical for the formation of envelop and capsid, and the seven non-structural (NS) proteins play important roles in virus replication. The three structural proteins are envelope, E; membrane precursor, PrM; and capsid, C. The seven NS proteins include NS1, NS2a, NS2b, NS3, NS4a, NS4b, and NS5 (Figure 1). The names, location in the infected cells, and functions of viral proteins are listed in Table 1. The members of the genus Flavivirus are characterized by similarities in genomic structure, viral protein function, pathogenesis and transmission.
Figure 1.
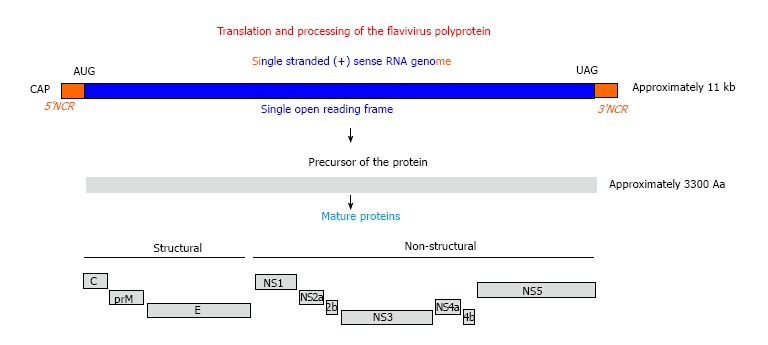
Genomic structure and gene production of Flavivirus. AUG: Translation start codon; UAG: Translation stop codon; NCR: Non coding RNA sequence; kb: Kilo base; Aa: Amino acid.
Table 1.
Roles of viral protein and RNA during viral infection in permissive cells
| Name of the vital material | Location in cell | Function |
| Viral genome + ssRNA (approximately 11000 nt) | Cytoplasm | Template for protein translation and for viral genome replication |
| Envelope, E (53 KDa)[52] | Cell membrane | Viral assembly, budding, attachment to target cells, and viral membrane fusion |
| Membrane precursor, PrM (20 KDa)[53] | Cell membrane | Facilitating E protein folding and trafficking, and virion maturation |
| Capsid, C (12 KDa)[54] | Cytoplasm | Virion maturation |
| NS1 (glycoprotein)[55] (46-55 kDa) | Endoplasmic reticulum | Subverting immune response |
| vesicular compartments, | virus-induced intracellular RNA | |
| cell surface | replication, neurovirulence | |
| NS2a (25 kDa)[56] | Transmembrane | Virus assembly, inhibit IFN-response |
| NS2b (14 kDa)[1,57] | Cytoplasm, nucleus | Viral protein cleavage |
| NS3 (69 kDa)[1] | Cytoplasm, nucleus | Viral protein cleavage, RNA |
| triphosphatase, mRNA capping, | ||
| RNA helicase | ||
| NS4a (16 kDa)[58] | Transmembrane | Viral RNA replication |
| NS4b (21.5 kDa)[59] | Integral membrane | Suppression of (IFN-α/β), |
| suppression of the host RNAi, | ||
| negatively regulate the helicase | ||
| function, viral replication | ||
| NS5 (103 kDa)[60,61] | Cytoplasm, nucleus | The RNA triphosphatase, |
| RNA-dependent RNA polymerase |
The large polyprotein precursor must be cleaved to generate actively functional proteins. The cleavage of the polyprotein precursor is a sophisticated process and is completed collaboratively by cellular proteases of the PACE (Paired basic Amino acid Cleaving Enzyme)-type or other Golgi-localized proteases and the viral serine protease embedded in the N-terminal domain of non-structural protein 3 (NS3Pro), which requires NS2b for its activity[1]. A distinct feature of genus Flavivirus from other genera of Flaviviridae is that the 5′-end of the (+)ssRNA genome of genus Flavivirus is decorated with an RNA cap structure (N7meGpppA2′Ome-RNA). 5’end capping of the viral RNA is as important as that for eukaryotic mRNAs, not only to initiate the process of translation but also to protect the viral RNA from degradation by endogenous RNA exonucleases. The protein translation happens immediately after the uncoating of viral particle in the cytoplasm. The (+)ssRNA genome is used as a template not only for gene expression but also for viral genome replication. Both viral RNA replication and gene translation occur in the cytoplasm. For RNA replication, viral NS proteins and cellular proteins interact to form a replication compartment (RC). During the period of viral RNA replication in the cytoplasm, the RC consists of morphologically distinct, membrane-bound compartments that also differ with respect to both function and NS proteins composition[3]. The NS3 and NS5 proteins are central to the viral RC, as together, they harbor most, if not all, of the catalytic activities required to both cap and replicate the viral RNA. Following replication, the protected genomic RNA is packaged by the C protein to form a capsid in a host-derived lipid bilayer in which the E protein is embedded and later integrated into viral envelope. The mature particles subsequently exit from the host cell by exocytosis.
REGIONAL ISOLATION OF ZIKA VIRUS
The Zika virus is phylogenetically close to Spondweni virus and a member of Flaviviridae family[4]. Comparative genomic analysis revealed that coding regions of pre-epidemic and epidemic strains of the Zika virus were similar with the exception of the NS2B. Bootscan analysis and multiple sequence alignment of the Asian lineage suggested that there may be genetic recombination of a fragment (nucleotides 4237-4528) of NS2B with that of the Spondweni virus[5].
African countries
In 1947, a group of scientists from United Kingdom led by Haddow et al[4] who were investigating yellow fever isolated Zika virus from a rhesus macaque with fever in the Zika Forest in Uganda[6,7]. The isolated viral strain has been stored in ATCC (ATCC® VR84™, MR 766) and the European Virus Archive (France) and is now still used for studies. The next important step was to find out whether the Zika virus is transmitted by mosquitos. First, Boorman et al[8] demonstrated that Zika virus can infect and replicate in mosquitos, providing experimental evidence that Zika virus may be transmitted by mosquitos. Later, the United Kingdom Flavivirus research group continued their studies of arboreal mosquitos as virus vectors in Uganda. They isolated 12 strains of Zika virus from Aedes (Stegomyia) africanus in the Zika forest[9]. Zika virus is apparently enzootic in Zika forest, and the evidence collected by Hoddow et al[9] suggested that Aedes africanus is the primary vector and that forest-dwelling monkeys and human are, on occasion, involved. It was not clear whether the mosquito transmitted the virus to other animals because no small mammal trapped in the forest showed serum antibody against the Zika virus. The Zika virus infection in humans was first reported in 1954[10]. It has also been experimentally demonstrated via volunteers that the Zika virus is able to infect humans[11]. In summary, results from these investigations suggest that the Zika virus is an arbovirus, transmitted by mosquitos and infects at least monkeys and humans.
Southern Asian countries
The first isolation of Zika virus in South-Eastern Asia was reported in 1969 in Malaysia[12]. Some years later, there was another report that the Zika virus was isolated from patients in Indonesia[13]. The event occurred during the end of the rainy season of 1977 when Aedes aegypti usually flourishes. Seven patients in central Java, Indonesia, appeared in the hospital with high fever, malaise, stomach ache, dizziness and anorexia. Data on these 7 Zika virus cases and several previously reported human infections indicated that clinical characteristics of infection with Zika virus appeared relatively mild, self-limiting, and nonlethal. It was suspected that the virus was transmitted by Aedes aegypti, which had been reported to be a probable vector in Malaysia[12]. A later investigation in Sabah, Malaysia, showed that the Zika virus infected 60 semi-captive and 84 free-ranging orangutans (Pongo pygmaeus pygmaeus)[14]. Another study conducted by the United States Naval Medical Research Unit No. 2 (NAMRU-2) isolated Zika virus in Cambodia in 2010[15]. This case was from a 3-year-old boy who had 4 d of fever, sore throat and cough as well a headache that lasted for 3 d. The studies conducted in southern Asia further confirmed that mosquitos are the vector and the primates might be the end host of viral infection.
The Zika virus has been also isolated from animals and human in other African countries. For examples, during the years 1964 to 1970, Moore et al[16] isolated 171 arboviruses of 15 different types from humans in Ibadan, Nigeria. Zika virus isolation rates also varied by season, with peaks in rainy seasons (June to August) and lows in dry seasons (January to February). Viruses were isolated from all age groups, with the majority from children one to four years old. The viruses isolated in largest numbers were chikungunya and yellow fever, which caused epidemics in 1969, and dengue types 1 and 2 and Tataguine, which are endemic in Ibadan. The Zika virus was isolated at a low rate. In 1999, three strains of the Zika virus were isolated as part of yellow fever studies in the Ivory Coast[17]. In 2010, it was reported that the Zika virus was isolated at a high rate in Cameroon. The research group investigated 102 sera from febrile patients (with negative laboratory findings for malaria and typhoid fever) at clinics in the Fako Division of Cameroon. The Zika virus was isolated at a rate of 11.4%, higher than that of any other members of Genus Flavivirus[18]. Therefore, following the time, the Zika virus has been spread throughout Africa.
More and more Zika virus strains have been isolated from humans worldwide[17]. Studies conducted in Nigeria during 1971-1975 isolated the Zika virus from humans. Serological experiments showed that 40% of the persons tested had neutralizing antibody to the Zika virus[16,19]. The infected populations were detected in other African countries such as Uganda, Tanzania, Egypt, Central African Republic, Sierra Leone, and Gabon, and in parts of Asia, including India, Malaysia, the Philippines, Thailand, Vietnam, and Indonesia[20]. Table 2 lists the strains that have been sequenced. The data from the viral genomic analysis support the hypothesis that the Zika viruses can be classified by origin into the Southern-Eastern Asian type and African type (Table 2). Other isolates might be derived from these types.
Table 2.
Origin of the types Zika viruses
| Isolation region | Isolation year | Accession # | Strain | Ref. |
| Malaysia | 1966 | HQ234499 | P6-740 | Haddow et al[4] |
| Micronesia | 2007 | EU545988 | N/A | Lanciotti et al[23] |
| Cambodia | 2010 | JN860885 | FSS13025 | Haddow et al[4] |
| Thailand | 2016 | KU681082 | H.sapiens-tc/PHL/2012/CPC-0740 | unpublished |
| Philippines | 2016 | KU681081 | H.sapiens-tc/THA/2014/SV0127 | unpublished |
| China | 2016 | KU744693 | VE Ganxian | unpublished |
| China | 2016 | KU740184 | GD01 | unpublished |
| Nigeria | 1968 | HQ234500 | IBH 30656 | Haddow et al[4] |
| Senegal | 1984 | HQ234501 | ArD 41519 | Haddow et al[4] |
| Uganda | 1947 | HQ234498 | MR766 | Haddow et al[4] |
| Uganda | 2004 | NC012532 | N/A | Kuno et al[62] |
| CAR | 2014 | KF268948 | ARB13565 | Berthet et al[63] |
| CAR | 2014 | KF268949 | ARB15076 | Berthet et al[63] |
| CAR | 2014 | KF268950 | ARB7701 | Berthet et al[63] |
| Senegal | 2001 | KF383119 | ArD158084 | Faye et al[2] |
| Senegal | 2001 | KF383118 | ArD157995 | Faye et al[2] |
| Senegal | 2001 | KF383117 | ArD128000 | Faye et al[2] |
| Senegal | 2001 | KF383116 | ArD7117 | Faye et al[2] |
| Brazil | 2016 | KU497555 | Brazil-ZKV2015 | Calvet et al[64] |
| Brazil | 2016 | KU707826 | SSABR1 | Costa et al[65] |
| Brazil | 2016 | KU527608 | Natal RGN | Mlakar et al[48] |
| Brazil | 2016 | KU501215 | PRVABC59 | Lanciotti et al[23] |
| Brazil | 2016 | KU321639 | ZikaSPH2015 | Staples et al[66] |
| Brazil | 2016 | KU312312 | Z1106033 | Enfissi et al[67] |
| France | 2014 | KJ776791 | H/PF/2013 | Baronti et al[68] |
| Martinique | 2016 | KU647676 | Martinique_PaRi_2015 | Baronti et al[68] |
| Haiti | 2014 | KU509998 | Haiti/1225/2014 | Lednicky et al[69] |
CAR: Central African Republic; N/A: Not applicable.
ZIKA VIRUS OUTBREAKS AND CLINICAL COMPLICATIONS
The Zika virus has been considered as a benign pathogen, causing asymptomatic or mild infections. Currently, there is no serological test that can clearly distinguish the Zika virus from other Flaviviruses. Diagnostic tests for Zika include RT-PCR, an IgM ELISA, and a plaque reduction neutralization test (PRNT). Some commercial tests have only become recently available[21]. Even a report from Olson et al[13] in 1981 that a cluster of 7 people with serologic evidence of the Zika virus illness in Indonesia did not attract serious attention and was not considered an outbreak due to the mildness of the associated illness. Later on, the same arbovirus research group performed a serological study that showed that 9/71 (13%) human volunteers in Lombok, Indonesia, had neutralizing antibodies to the Zika virus[22]. However, no serious cases were reported. The first outbreak of Zika virus-caused diseases was reported in 2007 on Yap Island of Micronesia. In April 2007, physicians on Yap Island characterized the disease with rash, conjunctivitis, arthralgia, arthritis, and fever. The disease affected 99 patients in 2 mo. A comprehensive study that combined analysis of patient samples, serological testing and real-time RT-PCR revealed the genetic and serological properties of the Zika virus epidemic[23]. The studies suggested that the 2007 Yap Island Zika virus is distantly related to African subclades and may be spread from Southeast Asia and the Pacific. Duffy et al[24] later conducted an extensive study on the Yap Island Zika virus outbreak. From 185 patients, 49 had been confirmed with the Zika virus illness, only 5 were excluded from Zika virus infection, and all others were suspected of Zika virus infection. They used survey studies in a large population, and estimated that 73% of the population of the Yap Island was infected with the Zika virus during the epidemic outbreak. Therefore, the outbreak on Yap Island in 2007 suggested that Zika virus infection has been spread outside of Africa and Asia[17]. Of course, whether or not the Zika virus was imported from Africa or Asia or other places remains to be verified.
Another Zika outbreak occurred between Oct. 2013 and Feb. 2014 in French Polynesia - like Yap Island, another island in the Pacific Ocean. In the very beginning of the outbreak, a mild dengue-like illness was observed in the patients within a family (consisting of wife, husband and their son-in-law). The symptoms included low fever (< 38 °C), asthenia, wrist and fingers arthralgia, headache, rash, and conjunctivitis. The RT-PCR test confirmed that it was a Zika virus infection[25]. The epidemic has been spread to a large population as reported by the syndromic surveillance network (6630 suspected Zika virus infection cases), 333 of which were confirmed by real-time RT-PCR as Zika virus infections. Symptoms of most Zika virus infection cases are mild and self-limited (mean duration of symptoms is 3-6 d)[25-27]. No hospitalizations for acute infection have been reported. In contrast to the outbreak in Yap Island, some severe complications were seen in this outbreak: The first case of Guillain-Barré syndrome (GBS) was found immediately after a Zika virus infection[28], and another case of vertical transmission from an infected pregnant woman to the baby was reported in this outbreak[29].
The spread of Zika virus from the outbreak of French Polynesia has been reported. Two Japanese travelers were confirmed to be infected with the Zika virus after they returned from a trip to French Polynesia during the time of the outbreak[30]. In addition, it was found to have spread to other Pacific Islands including New Caledonia, Cook Islands, Easter Island, Vanuatu, and Solomon Islands[31]. The introduction of the Zika virus from French Polynesia into New Caledonia caused another outbreak in New Caledonia in 2014[32]; The first cases of Zika virus infection were confirmed in November 2013, and they were imported from French Polynesia. By the end of 2014, a total of 1383 cases were confirmed in a laboratory[32]. Consequently, an outbreak in New Caledonia was declared. Thus far, introduction of the Zika virus from French Polynesia to other countries has been continuously reported.
Between 1947 and 2006, < 20 cases of Zika virus infection have been reported[5]. There have been recent reports of imported cases of Zika virus infections in 18 travelers returning to the Netherlands from Surinam, which is in South America near the northern border of Brazil, and the Dominican Republic[33], 13 infections were imported from Venezuela, Fiji/Samoa, or Suriname to China[34], and 4 infections were imported from Brazil to Portugal[35]. Autochthonous cases were reported in places such as Mexico[36], Colombia[37], and Easter Island, which was the first outbreak (51 cases) reported in a territory of the Americas in early 2014[38].
The recent outbreak in Brazil has attracted the most attention due to not only its growing infected population but also its likely enhanced severity of the clinical sequelae. In March of 2015, Zanluca et al[39] from the Molecular Virology Laboratory of Carlos Chagas Institute, Oswaldo Cruz Institute, state of Paraná, Brazil, detected the Zika virus genome by RT-PCR from 8 out of 21 acute-phase serum specimens from the patients with dengue-like symptoms. This is the first report of Zika virus outbreak in Brazil. Later, another group reported a similar detection of Zika virus cases (8 out 24 samples) by RT-PCR[40]. The virus has been assumed to have been imported from French Polynesia either by the travelers during the time of the World Cup[39] or by the teams from the Va’a World Sprint Championship canoe race that was held in Rio de Janeiro, Brazil[41]. It has been reported that the virus is carried by the travelers to other countries[42]. Genomic sequencing has been conducted to analyze the similarities between different strains isolated historically. Phylogenetic studies showed that the Brazilian strain is closely related to the one from French Polynesia, and the French Polynesia strain is likely derived from Yap Island. These strains all belong to the Asian lineage[41].
The severe clinical sequelae caused by Zika virus infection include the following. First, during the outbreak of the Zika virus in French Polynesia, the Zika virus was detected from the semen of a patient, which brought out the presumption that the Zika virus might be transmitted sexually[43]. Several cases of Zika virus infected patients have been reported to be sexually transmitted[44]. This observation implies another transmission route for the Zika virus other than through mosquito. Secondly, the Zika virus was reported to be transmitted vertically (from the infected mother to the fetus). This is a major problem for patients infected by Zika virus because the virus directly results in birth defects. Again, the first cases of congenital Zika virus infection were found during the French Polynesia outbreak[29]. Thirdly, it was reported to be related to some severe syndromes like GBS[28,45]. In addition, Zika virus infection might have been associated with microcephaly[46-51]. However, after more detailed and accurate experimental studies and clinical analysis, the number of Zika-related microcephaly dropped quickly. Therefore, all the linkages to the severe diseases are still informative not conclusive. Systemic research in different aspects for Zika virus is needed to assure that the clinical findings are explained and understood.
FUTURE DIRECTIONS
Even though the world has noticed the emergence of Zika virus infection, time is needed to achieve understanding of its pathogenesis, prevention, and treatment. A previously systemic study is lacking, so the Zika virus, from now on, will be another member of Genus Flavivirus to be the center of virological research. The following aspects may be very important in the near future: Animal model for Zika virus infection: It will help researchers understand whether and how Zika virus causes neural disorder through interfering with the neural progenitor cell/neural stem cell (NPC/NSC) proliferation and differentiation; vaccine development: Like all other viruses, the best and most effective way to prevent viral infection is by vaccine. Some successful experience in Dengue virus and yellow fever virus may be useful towards developing the Zika vaccine; transmission prevention. Viral transmission needs to be studied, such as whether and how semen components enhance viral infection.
Footnotes
Supported by a Charles and Mary Latham Fund (Q.T.), No. NIH/NIAID SC1AI112785 (Q.T.); and National Institute on Minority Health and Health Disparities of the National Institutes of Health, No. G12MD007597.
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
Manuscript source: Unsolicited manuscript
Specialty type: Virology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
Peer-review started: May 12, 2016
First decision: June 14, 2016
Article in press: August 15, 2016
P- Reviewer: Arriagada GL, Cunha C, De Berardinis P, Ghiringhelli PD, Giannecchini S S- Editor: Qiu S L- Editor: A E- Editor: Lu YJ
References
- 1.Bollati M, Alvarez K, Assenberg R, Baronti C, Canard B, Cook S, Coutard B, Decroly E, de Lamballerie X, Gould EA, et al. Structure and functionality in flavivirus NS-proteins: perspectives for drug design. Antiviral Res. 2010;87:125–148. doi: 10.1016/j.antiviral.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faye O, Freire CC, Iamarino A, Faye O, de Oliveira JV, Diallo M, Zanotto PM, Sall AA. Molecular evolution of Zika virus during its emergence in the 20(th) century. PLoS Negl Trop Dis. 2014;8:e2636. doi: 10.1371/journal.pntd.0002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mackenzie J. Wrapping things up about virus RNA replication. Traffic. 2005;6:967–977. doi: 10.1111/j.1600-0854.2005.00339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haddow AD, Schuh AJ, Yasuda CY, Kasper MR, Heang V, Huy R, Guzman H, Tesh RB, Weaver SC. Genetic characterization of Zika virus strains: geographic expansion of the Asian lineage. PLoS Negl Trop Dis. 2012;6:e1477. doi: 10.1371/journal.pntd.0001477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu Z, Chan JF, Tee KM, Choi GK, Lau SK, Woo PC, Tse H, Yuen KY. Comparative genomic analysis of pre-epidemic and epidemic Zika virus strains for virological factors potentially associated with the rapidly expanding epidemic. Emerg Microbes Infect. 2016;5:e22. doi: 10.1038/emi.2016.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dick GW. Zika virus. II. Pathogenicity and physical properties. Trans R Soc Trop Med Hyg. 1952;46:521–534. doi: 10.1016/0035-9203(52)90043-6. [DOI] [PubMed] [Google Scholar]
- 7.Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46:509–520. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- 8.Boorman JP, Porterfield JS. A simple technique for infection of mosquitoes with viruses; transmission of Zika virus. Trans R Soc Trop Med Hyg. 1956;50:238–242. doi: 10.1016/0035-9203(56)90029-3. [DOI] [PubMed] [Google Scholar]
- 9.Haddow AJ, Williams MC, Woodall JP, Simpson DI, Goma LK. Twelve isolations of zika virus from aedes (stegomyia) africanus (theobald) taken in and above a uganda forest. Bull World Health Organ. 1964;31:57–69. [PMC free article] [PubMed] [Google Scholar]
- 10.Macnamara FN. Zika virus: a report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans R Soc Trop Med Hyg. 1954;48:139–145. doi: 10.1016/0035-9203(54)90006-1. [DOI] [PubMed] [Google Scholar]
- 11.Bearcroft WG. Zika virus infection experimentally induced in a human volunteer. Trans R Soc Trop Med Hyg. 1956;50:442–448. [PubMed] [Google Scholar]
- 12.Marchette NJ, Garcia R, Rudnick A. Isolation of Zika virus from Aedes aegypti mosquitoes in Malaysia. Am J Trop Med Hyg. 1969;18:411–415. doi: 10.4269/ajtmh.1969.18.411. [DOI] [PubMed] [Google Scholar]
- 13.Olson JG, Ksiazek TG. Zika virus, a cause of fever in Central Java, Indonesia. Trans R Soc Trop Med Hyg. 1981;75:389–393. doi: 10.1016/0035-9203(81)90100-0. [DOI] [PubMed] [Google Scholar]
- 14.Kilbourn AM, Karesh WB, Wolfe ND, Bosi EJ, Cook RA, Andau M. Health evaluation of free-ranging and semi-captive orangutans (Pongo pygmaeus pygmaeus) in Sabah, Malaysia. J Wildl Dis. 2003;39:73–83. doi: 10.7589/0090-3558-39.1.73. [DOI] [PubMed] [Google Scholar]
- 15.Heang V, Yasuda CY, Sovann L, Haddow AD, Travassos da Rosa AP, Tesh RB, Kasper MR. Zika virus infection, Cambodia, 2010. Emerg Infect Dis. 2012;18:349–351. doi: 10.3201/eid1802.111224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore DL, Causey OR, Carey DE, Reddy S, Cooke AR, Akinkugbe FM, David-West TS, Kemp GE. Arthropod-borne viral infections of man in Nigeria, 1964-1970. Ann Trop Med Parasitol. 1975;69:49–64. doi: 10.1080/00034983.1975.11686983. [DOI] [PubMed] [Google Scholar]
- 17.Hayes EB. Zika virus outside Africa. Emerg Infect Dis. 2009;15:1347–1350. doi: 10.3201/eid1509.090442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fokam EB, Levai LD, Guzman H, Amelia PA, Titanji VP, Tesh RB, Weaver SC. Silent circulation of arboviruses in Cameroon. East Afr Med J. 2010;87:262–268. doi: 10.4314/eamj.v87i6.63085. [DOI] [PubMed] [Google Scholar]
- 19.Fagbami AH. Zika virus infections in Nigeria: virological and seroepidemiological investigations in Oyo State. J Hyg (Lond) 1979;83:213–219. doi: 10.1017/s0022172400025997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saluzzo JF, Ivanoff B, Languillat G, Georges AJ. [Serological survey for arbovirus antibodies in the human and simian populations of the South-East of Gabon (author’s transl)] Bull Soc Pathol Exot Filiales. 1982;75:262–266. [PubMed] [Google Scholar]
- 21.Saiz JC, Vázquez-Calvo Á, Blázquez AB, Merino-Ramos T, Escribano-Romero E, Martín-Acebes MA. Zika Virus: the Latest Newcomer. Front Microbiol. 2016;7:496. doi: 10.3389/fmicb.2016.00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olson JG, Ksiazek TG, Gubler DJ, Lubis SI, Simanjuntak G, Lee VH, Nalim S, Juslis K, See R. A survey for arboviral antibodies in sera of humans and animals in Lombok, Republic of Indonesia. Ann Trop Med Parasitol. 1983;77:131–137. doi: 10.1080/00034983.1983.11811687. [DOI] [PubMed] [Google Scholar]
- 23.Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, Stanfield SM, Duffy MR. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 2008;14:1232–1239. doi: 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, Pretrick M, Marfel M, Holzbauer S, Dubray C, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360:2536–2543. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- 25.Cao-Lormeau VM, Roche C, Teissier A, Robin E, Berry AL, Mallet HP, Sall AA, Musso D. Zika virus, French polynesia, South pacific, 2013. Emerg Infect Dis. 2014;20:1085–1086. doi: 10.3201/eid2006.140138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Musso D, Nilles EJ, Cao-Lormeau VM. Rapid spread of emerging Zika virus in the Pacific area. Clin Microbiol Infect. 2014;20:595–596. doi: 10.1111/1469-0691.12707. [DOI] [PubMed] [Google Scholar]
- 27.Musso D, Nhan T, Robin E, Roche C, Bierlaire D, Zisou K, Shan Yan A, Cao-Lormeau VM, Broult J. Potential for Zika virus transmission through blood transfusion demonstrated during an outbreak in French Polynesia, November 2013 to February 2014. Euro Surveill. 2014;19 doi: 10.2807/1560-7917.es2014.19.14.20761. [DOI] [PubMed] [Google Scholar]
- 28.Oehler E, Watrin L, Larre P, Leparc-Goffart I, Lastere S, Valour F, Baudouin L, Mallet H, Musso D, Ghawche F. Zika virus infection complicated by Guillain-Barre syndrome--case report, French Polynesia, December 2013. Euro Surveill. 2014;19 doi: 10.2807/1560-7917.es2014.19.9.20720. [DOI] [PubMed] [Google Scholar]
- 29.Besnard M, Lastere S, Teissier A, Cao-Lormeau V, Musso D. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill. 2014;19:pii: 20751. [PubMed] [Google Scholar]
- 30.Kutsuna S, Kato Y, Takasaki T, Moi M, Kotaki A, Uemura H, Matono T, Fujiya Y, Mawatari M, Takeshita N, et al. Two cases of Zika fever imported from French Polynesia to Japan, December 2013 to January 2014 [corrected] Euro Surveill. 2014;19:pii: 20683. doi: 10.2807/1560-7917.es2014.19.4.20683. [DOI] [PubMed] [Google Scholar]
- 31.Musso D, Cao-Lormeau VM, Gubler DJ. Zika virus: following the path of dengue and chikungunya? Lancet. 2015;386:243–244. doi: 10.1016/S0140-6736(15)61273-9. [DOI] [PubMed] [Google Scholar]
- 32.Dupont-Rouzeyrol M, O’Connor O, Calvez E, Daurès M, John M, Grangeon JP, Gourinat AC. Co-infection with Zika and dengue viruses in 2 patients, New Caledonia, 2014. Emerg Infect Dis. 2015;21:381–382. doi: 10.3201/eid2102.141553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duijster JW, Goorhuis A, van Genderen PJ, Visser LG, Koopmans MP, Reimerink JH, Grobusch MP, van der Eijk AA, van den Kerkhof JH, Reusken CB, et al. Zika virus infection in 18 travellers returning from Surinam and the Dominican Republic, The Netherlands, November 2015-March 2016. Infection. 2016;44:797–802. doi: 10.1007/s15010-016-0906-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y, Chen W, Wong G, Bi Y, Yan J, Sun Y, Chen E, Yan H, Lou X, Mao H, et al. Highly diversified Zika viruses imported to China, 2016. Protein Cell. 2016;7:461–464. doi: 10.1007/s13238-016-0274-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zé-Zé L, Prata MB, Teixeira T, Marques N, Mondragão A, Fernandes R, Saraiva da Cunha J, Alves MJ. Zika virus infections imported from Brazil to Portugal, 2015. IDCases. 2016;4:46–49. doi: 10.1016/j.idcr.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jimenez Corona ME, De la Garza Barroso AL, Rodriguez Martínez JC, Luna Guzmán NI, Ruiz Matus C, Díaz Quiñonez JA, Lopez Martinez I, Kuri Morales PA. Clinical and Epidemiological Characterization of Laboratory-Confirmed Autochthonous Cases of Zika Virus Disease in Mexico. PLoS Curr. 2016;8:pii: ecurrents.outbreaks.a2fe1b3d6d71e24ad2b5afe982824053. doi: 10.1371/currents.outbreaks.a2fe1b3d6d71e24ad2b5afe982824053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Camacho E, Paternina-Gomez M, Blanco PJ, Osorio JE, Aliota MT. Detection of Autochthonous Zika Virus Transmission in Sincelejo, Colombia. Emerg Infect Dis. 2016;22:927–929. doi: 10.3201/eid2205.160023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zanluca C, Dos Santos CN. Zika virus - an overview. Microbes Infect. 2016;18:295–301. doi: 10.1016/j.micinf.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 39.Zanluca C, Melo VC, Mosimann AL, Santos GI, Santos CN, Luz K. First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz. 2015;110:569–572. doi: 10.1590/0074-02760150192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Campos GS, Bandeira AC, Sardi SI. Zika Virus Outbreak, Bahia, Brazil. Emerg Infect Dis. 2015;21:1885–1886. doi: 10.3201/eid2110.150847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Musso D. Zika Virus Transmission from French Polynesia to Brazil. Emerg Infect Dis. 2015;21:1887. doi: 10.3201/eid2110.151125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zammarchi L, Tappe D, Fortuna C, Remoli ME, Günther S, Venturi G, Bartoloni A, Schmidt-Chanasit J. Zika virus infection in a traveller returning to Europe from Brazil, March 2015. Euro Surveill. 2015;20 doi: 10.2807/1560-7917.es2015.20.23.21153. [DOI] [PubMed] [Google Scholar]
- 43.Musso D, Roche C, Robin E, Nhan T, Teissier A, Cao-Lormeau VM. Potential sexual transmission of Zika virus. Emerg Infect Dis. 2015;21:359–361. doi: 10.3201/eid2102.141363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hills SL, Russell K, Hennessey M, Williams C, Oster AM, Fischer M, Mead P. Transmission of Zika Virus Through Sexual Contact with Travelers to Areas of Ongoing Transmission - Continental United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:215–216. doi: 10.15585/mmwr.mm6508e2. [DOI] [PubMed] [Google Scholar]
- 45.Wise J. Study links Zika virus to Guillain-Barré syndrome. BMJ. 2016;352:i1242. doi: 10.1136/bmj.i1242. [DOI] [PubMed] [Google Scholar]
- 46.Barreto ML, Barral-Netto M, Stabeli R, Almeida-Filho N, Vasconcelos PF, Teixeira M, Buss P, Gadelha PE. Zika virus and microcephaly in Brazil: a scientific agenda. Lancet. 2016;387:919–921. doi: 10.1016/S0140-6736(16)00545-6. [DOI] [PubMed] [Google Scholar]
- 47.de Paula Freitas B, de Oliveira Dias JR, Prazeres J, Sacramento GA, Ko AI, Maia M, Belfort RJr. Ocular Findings in Infants With Microcephaly Associated With Presumed Zika Virus Congenital Infection in Salvador, Brazil. JAMA Ophthalmol. 2016 doi: 10.1001/jamaophthalmol.2016.0267. Feb 9; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mlakar J, Korva M, Tul N, Popović M, Poljšak-Prijatelj M, Mraz J, Kolenc M, Resman Rus K, Vesnaver Vipotnik T, Fabjan Vodušek V, et al. Zika Virus Associated with Microcephaly. N Engl J Med. 2016;374:951–958. doi: 10.1056/NEJMoa1600651. [DOI] [PubMed] [Google Scholar]
- 49.Stratton SJ. Zika Virus Association with Microcephaly: The Power for Population Statistics to Identify Public Health Emergencies. Prehosp Disaster Med. 2016;31:119–120. doi: 10.1017/S1049023X16000170. [DOI] [PubMed] [Google Scholar]
- 50.Ventura CV, Maia M, Bravo-Filho V, Góis AL, Belfort R. Zika virus in Brazil and macular atrophy in a child with microcephaly. Lancet. 2016;387:228. doi: 10.1016/S0140-6736(16)00006-4. [DOI] [PubMed] [Google Scholar]
- 51.Werner H, Fazecas T, Guedes B, Lopes Dos Santos J, Daltro P, Tonni G, Campbell S, Araujo Júnior E. Intrauterine Zika virus infection and microcephaly: correlation of perinatal imaging and three-dimensional virtual physical models. Ultrasound Obstet Gynecol. 2016;47:657–660. doi: 10.1002/uog.15901. [DOI] [PubMed] [Google Scholar]
- 52.Heinz FX, Mandl CW, Holzmann H, Kunz C, Harris BA, Rey F, Harrison SC. The flavivirus envelope protein E: isolation of a soluble form from tick-borne encephalitis virus and its crystallization. J Virol. 1991;65:5579–5583. doi: 10.1128/jvi.65.10.5579-5583.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li L, Lok SM, Yu IM, Zhang Y, Kuhn RJ, Chen J, Rossmann MG. The flavivirus precursor membrane-envelope protein complex: structure and maturation. Science. 2008;319:1830–1834. doi: 10.1126/science.1153263. [DOI] [PubMed] [Google Scholar]
- 54.Jones CT, Ma L, Burgner JW, Groesch TD, Post CB, Kuhn RJ. Flavivirus capsid is a dimeric alpha-helical protein. J Virol. 2003;77:7143–7149. doi: 10.1128/JVI.77.12.7143-7149.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muller DA, Young PR. The flavivirus NS1 protein: molecular and structural biology, immunology, role in pathogenesis and application as a diagnostic biomarker. Antiviral Res. 2013;98:192–208. doi: 10.1016/j.antiviral.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 56.Leung JY, Pijlman GP, Kondratieva N, Hyde J, Mackenzie JM, Khromykh AA. Role of nonstructural protein NS2A in flavivirus assembly. J Virol. 2008;82:4731–4741. doi: 10.1128/JVI.00002-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pastorino BA, Peyrefitte CN, Grandadam M, Thill MC, Tolou HJ, Bessaud M. Mutagenesis analysis of the NS2B determinants of the Alkhurma virus NS2B-NS3 protease activation. J Gen Virol. 2006;87:3279–3283. doi: 10.1099/vir.0.82088-0. [DOI] [PubMed] [Google Scholar]
- 58.McLean JE, Wudzinska A, Datan E, Quaglino D, Zakeri Z. Flavivirus NS4A-induced autophagy protects cells against death and enhances virus replication. J Biol Chem. 2011;286:22147–22159. doi: 10.1074/jbc.M110.192500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zou J, Xie X, Lee le T, Chandrasekaran R, Reynaud A, Yap L, Wang QY, Dong H, Kang C, Yuan Z, et al. Dimerization of flavivirus NS4B protein. J Virol. 2014;88:3379–3391. doi: 10.1128/JVI.02782-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grun JB, Brinton MA. Dissociation of NS5 from cell fractions containing West Nile virus-specific polymerase activity. J Virol. 1987;61:3641–3644. doi: 10.1128/jvi.61.11.3641-3644.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Laurent-Rolle M, Morrison J, Rajsbaum R, Macleod JM, Pisanelli G, Pham A, Ayllon J, Miorin L, Martínez-Romero C, tenOever BR, et al. The interferon signaling antagonist function of yellow fever virus NS5 protein is activated by type I interferon. Cell Host Microbe. 2014;16:314–327. doi: 10.1016/j.chom.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuno G, Chang GJ. Full-length sequencing and genomic characterization of Bagaza, Kedougou, and Zika viruses. Arch Virol. 2007;152:687–696. doi: 10.1007/s00705-006-0903-z. [DOI] [PubMed] [Google Scholar]
- 63.Berthet N, Nakouné E, Kamgang B, Selekon B, Descorps-Declère S, Gessain A, Manuguerra JC, Kazanji M. Molecular characterization of three Zika flaviviruses obtained from sylvatic mosquitoes in the Central African Republic. Vector Borne Zoonotic Dis. 2014;14:862–865. doi: 10.1089/vbz.2014.1607. [DOI] [PubMed] [Google Scholar]
- 64.Calvet G, Aguiar RS, Melo AS, Sampaio SA, de Filippis I, Fabri A, Araujo ES, de Sequeira PC, de Mendonça MC, de Oliveira L, et al. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. Lancet Infect Dis. 2016;16:653–660. doi: 10.1016/S1473-3099(16)00095-5. [DOI] [PubMed] [Google Scholar]
- 65.Costa F, Sarno M, Khouri R, de Paula Freitas B, Siqueira I, Ribeiro GS, Ribeiro HC, Campos GS, Alcântara LC, Reis MG, et al. Emergence of Congenital Zika Syndrome: Viewpoint From the Front Lines. Ann Intern Med. 2016;164:689–691. doi: 10.7326/M16-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Staples JE, Dziuban EJ, Fischer M, Cragan JD, Rasmussen SA, Cannon MJ, Frey MT, Renquist CM, Lanciotti RS, Muñoz JL, et al. Interim Guidelines for the Evaluation and Testing of Infants with Possible Congenital Zika Virus Infection - United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:63–67. doi: 10.15585/mmwr.mm6503e3. [DOI] [PubMed] [Google Scholar]
- 67.Enfissi A, Codrington J, Roosblad J, Kazanji M, Rousset D. Zika virus genome from the Americas. Lancet. 2016;387:227–228. doi: 10.1016/S0140-6736(16)00003-9. [DOI] [PubMed] [Google Scholar]
- 68.Baronti C, Piorkowski G, Charrel RN, Boubis L, Leparc-Goffart I, de Lamballerie X. Complete coding sequence of zika virus from a French polynesia outbreak in 2013. Genome Announc. 2014;Jun 5; 2 doi: 10.1128/genomeA.00500-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lednicky JA, Butel JS, Luetke MC, Loeb JC. Complete genomic sequence of a new Human polyomavirus 9 strain with an altered noncoding control region. Virus Genes. 2014;49:490–492. doi: 10.1007/s11262-014-1119-z. [DOI] [PubMed] [Google Scholar]


