Abstract
Dengue is one of the most common arthropod-borne viral diseases in humans and it is a leading cause of illness and death in the tropical and subtropical regions of the world. It is thought to account for 400 million cases annually among approximately 3.97 billion people at risk of infection in 128 endemic countries. Despite the global prevalence of the disease, the availability of a vaccine is limited in most countries in the endemic areas. Most endemic countries in South America, South East Asia and Africa serve as attractive touristic sites for people from non-endemic countries who become infected and export the virus to dengue-free regions. Dengue fever typically resembles malaria and in endemic countries most cases of dengue are treated as presumptive malaria. Consequently, routine dengue diagnosis among persons with fever will offer early treatment and reduce the burden of the disease. Also, routine testing among travellers from endemic countries will reduce importation and prevent the geographical expansion of dengue. In this essay, we seek to highlight the usefulness of routine dengue testing in endemic countries.
Keywords: Dengue virus, Endemic, Mosquito, Vector-borne
Core tip: Dengue is an emerging arborvirus infection currently endemic in 128 countries in the world. In the absence of routine vaccination and specific antivirals, the main method to reduce the burden of dengue is to reduce the vector population, educate people on protective measures and timely laboratory identification. Unfortunately this routine laboratory investigation is currently neglected in most endemic countries and most cases of fevers are often misconstrued as malaria. This review provides a comprehensive summary of dengue infection and highlights the fact that routine dengue diagnosis will reduce the burden and global expansion of dengue.
INTRODUCTION
Dengue virus (DENV) is the most common arthropod-borne viral disease in humans and it is endemic in most tropical and sub-tropical countries[1]. It has been designated a major international public health concern by the World health Organization (WHO) as it accounts for 400 million cases annually among 3.97 billion people at risk of infection[1,2]. Previous phylogenetic analysis suggests that there are four distinct DENV serotypes (DENV 1 to 4)[3,4]. However, a 5th serotype associated with milder disease was isolated in 2013 in Malaysia[5]. Over the years, DENV has spread from less than 9 endemic countries to presently about 128 endemic countries[6,7]. Factors such as unrestricted large-scale international travel and trade, urbanization, global warming, virus and vector evolution contributed to its rapid spread to other regions of the World[8].
The main arthropod vectors for the transmission of DENVs are Aedes aegypti and Aedes albopictus mosquitoes are predominant in both tropical and sub-tropical regions of the world[9,10]. Infected individuals may be asymptomatic or may present with dengue fever (DF) - a mild febrile illness, dengue hemorrhagic fever (DHF) - a life-threatening complication, or dengue shock syndrome (DSS). The incubation period is between 3-15 d following an infected blood meal. Rare cases of human - human transmission via needle stick injuries, contaminated blood products, donor organs and vertical transmission from infected mother to an unborn child have been documented[11]. Dengue endemicity in 128 countries makes it a year round occurrence with peak prevalence during the rainy season when environmental conditions are optimal for the Aedes vector breeding[12]. As a result, epidemics are common during the rainy season when the vector population is high and the chances for human exposure to mosquito bites is increased[13]. In the absence of routine vaccination and specific antivirals, the main method to reduce the burden of dengue is to reduce the vector population, educate people on protective measures such as spraying of insecticides and wearing protective clothing[7,8,14]. DF typically resembles malaria and in endemic countries most cases of dengue are treated as presumptive malaria. Therefore, routine and differential diagnosis of dengue will provide a basis for evidence-based treatment and reduce the irrational use of antimalarial or antibiotics to treat febrile diseases. Routine screening in endemic countries will provide a better estimate on the burden of dengue disease for public health action.
DENGUE EPIDEMIOLOGY
Dengue is currently regarded as the most important arboviral disease internationally as over half of the world’s population lives in dengue endemic countries[7]. A global estimate suggests about 50-200 million cases of dengue with 500000 episodes of DHF/DSS occur annually culminating in about 20000 dengue related deaths[15,16]. The determining factors of dengue epidemiology trends include, but not limited to: (1) rapid urban population growth and density due to rural to urban migration; (2) poor sewage disposal system and land use pattern; (3) global warming; and (4) trade necessitating movement of people[17]. It is now known that every WHO region has evidence of dengue transmission[18]. Almost 75% of the world’s population at risk of dengue, live in South East Asia (SEA) and the Western Pacific region and the disease is the leading cause of hospitalization and death in children from these regions (Figure 1)[18]. Dengue is also recognized as an emerging infection in the Eastern Mediterranean region with multiple outbreaks occurring in Pakistan, Yemen, and Saudi Arabia[19]. Almost all countries in the Americas are now hyperendemic for dengue with epidemics occurring every three-to-five years especially in Latin America[16,18]. Due to the significant endemicity of malaria throughout Africa, the majority of “febrile illnesses” including dengue is likely to be mistreated as malaria. This negatively affects our understanding of the epidemiology of dengue in the region. Dengue is indeed underreported in Africa and is not a notifiable disease to WHO by most countries from the continent. A review of the subject by Amarasinghe et al[20] suggested that dengue is endemic in 34 countries in Africa and that the four main dengue serotypes circulate in Africa with serotype 2 responsible for most epidemics. Although the threat of dengue is rare in Europe, imported cases by European travellers to and from endemic countries continue to rise (Table 1). A report suggests the importation of dengue to 13 European countries by returning travellers[8].
Figure 1.
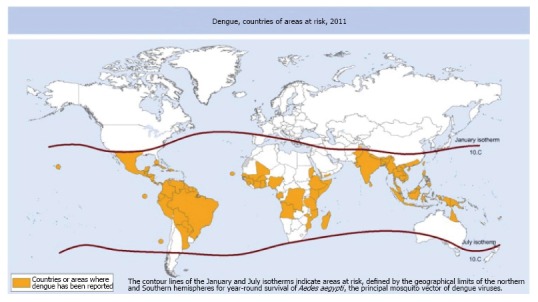
Countries or areas of the world where dengue was reported in 2011, as per data collected by the World Health Organization. Reprinted with permission from Murray et al[8]. The boundaries and names shown and the designations used on this map do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted and dashed lines on maps represent approximate borderlines for which there may not yet be full agreement.
Table 1.
Randomly selected articles revealing dengue importation by travelers from endemic countries
| Year | Import country | Source country | No. of cases | Age group | Serotype | Assay | Ref. |
| 2010 | France | Benin | 1 | 40s | Unknown | IgG/IgM seology | Gautret et al[54] |
| 2001-2009 | Denmark | Southeast Asia, South Asia, Central America, Africa, Caribbean, South America | 114 | 6-79 | DENV 1, 2, 3 and 4 | IgG/IgM serology, PCR | Vinner et al[55] |
| 2010 | Italy | Caribbean, India, Indonesia, Brazil, Thailand, Venezuela, Nicaragua-Honduras | 17 | 16-63 | DENV 1, 3 | IgG/IgM immunofluorescence, PCR | Pierro et al[56] |
| 2013 | France | Guadeloupe | 1 | 50s | DENV 2 | PCR | Marchand et al[57] |
| 2010 | France, Sweden | Tanzania | 5 | 41-69 | DENV 3 | PCR | Gautret et al[58] |
| 2012 | Germany, United Kingdom | Madeira | 42 | 20-73 | Unknown | Unknown | Frank et al[59] |
| 2007-2009 | Sweden | Thailand | 100 | Unknown | DENV 2 | Unknown | Heddini et al[60] |
| 2009 | Italy | Senegal | 1 | 40s | DENV 3 | PCR | Nisii et al[61] |
| 2012 | Finland | Madeira | 5 | 50-60 | DENV 3 | IgG/IgM, NS1 and PCR | Huhtamo et al[62] |
| 2013 | Germany | Japan | 1 | 50s | Unknown | IgG/IgM, NS1 and PCR | Schmidt-Chanasit et al[63] |
| 2010 | Germany | Croatia | 1 | 72 | Unknown | IgG/IgM, NS1 and PCR | Schmidt-Chanasit et al[64] |
DENV: Dengue virus; PCR: Polymerase chain reaction.
CLINICAL ASPECTS OF DENGUE INFECTION
Dengue infection may present as a mild asymptomatic infection to severe illness that may lead to death in some cases. The disease may start as an undifferentiated febrile illness (UF), which may culminate to a diverse and complicated clinical condition such as: DF, DHF, DSS[21]. Clinically, UF illness mimics malaria and other tropical fevers and in the absence of specific serological testing, UF illness could easily be misdiagnosed or labeled as fever of unknown origin. DF is considered to be a mild disease because death is rarely reported, but may be associated with high fever, severe headache, pain behind the eyes, muscle and bone or joint pains, nausea, vomiting, rash and skin haemorrhages. Leukopenia and thrombocytopenia may also occur. The clinical presentation of DHF is similar to DF but the latter is characterized by plasma leakage resulting from alteration in microvascular permeability. The plasma leakage occurs into the pleural and peritoneal cavities that may result in pleural effusion and ascites. Typical presentation of DHF includes high fever, haemorrhagic phenomena, thrombocytopenia, hepatomegaly and circulatory failure. On the other hand, the clinical features of DSS are also similar to those of DHF but the plasma leakage is so severe that the patient develops shock[21,22]. Other signs of circulatory failure such as the skin becoming cool, blotchy, and congested; circumoral cyanosis may be observed. Also, the patients may initially be lethargic, then become restless and then rapidly enter a critical stage of shock. DSS is usually characterized by weak pulse with narrowing of the pulse, hypotension with cold, clammy skin and restlessness. Death may occur in the absence of appropriate treatment.
DENGUE AMONG TRAVELLERS TO ENDEMIC COUNTRIES
The contribution of dengue expansion through international travel and intercontinental movement of goods is on the rise[23]. As the global community trades and travel more and more, so too do communicable and vector-borne diseases. Most dengue-endemic countries are popular touristic destinations and the frequency of international travel to these regions plays a role in the infection and transmission of the disease. With increasing growing markets and international trade in Africa, Asia and Latin America, the risk of dengue infection by travelers is high. It was observed in 2011 that air travel frequency was 40-times higher compared to the frequency during mid 20th century[24]. Human travel to endemic areas as well as travel of infected persons to non-endemic areas is the main driver in the global transmission and expansion of the disease. Overcrowded airports located in most tropical countries serve as ideal breeding ground and distribution source of dengue viruses and travelers contribute in the importation of the disease (Table 1)[25]. Other globalization factors such as international transport of cargo and goods, especially via commercial sea shipment also contribute in the importation or exportation of the dengue’s primary and secondary vectors, Aedes aegypti and Aedes albopictus, respectively[26]. The transatlantic transport of used cars and tires has been linked with the introduction of exotic mosquitoes from America to Europe, which contributed to other vector-borne disease epidemics[27,28].
ASSESSING DENGUE DISEASE BURDEN IN ENDEMIC COUNTRIES
While geographical expansion of dengue and its vector are evident, the true burden of the disease is underestimated due to lack of an efficient public health surveillance system for dengue. Dengue diagnosis is not routinely performed in endemic countries and most febrile illnesses are treated as presumptive malaria or fever of unknown origin. Also, most dengue cases are asymptomatic and go undetected and infected persons do not seek medical attention. Consequently, the number of dengue cases is underreported and the disease burden is grossly underestimated. However, DF, DHF and DSS cause significant humanitarian and economic hardship and it is suggested that about 3.97 billion people living in 128 endemic countries globally are at risk of dengue[7,29]. The disability adjusted life year (DALY) lost due to dengue infection globally was 700000 per year in 2009 while an estimate of aggregate annual cost of dengue was USD 2.1 billion in the Americas in 2000-2007[15,30-32]. Prior to 1990, dengue was endemic in only 9 countries but the disease is currently endemic in 128 countries across Africa, the Americas, the Eastern Mediterranean, SEA and the Western Pacific regions. A study involving twelve countries in the SEA region from 2001 to 2010 suggest an annual economic burden of US $950 million amongst the studied nation[8]. Overall, due to inadequate disease surveillance, low level of reporting, low case fatality rate, lack of routine diagnosis, the true incidence and burden of the disease is unclear.
DENGUE AND MALARIA ENDEMICITY
Mosquitoes are widespread in most tropical and sub-tropical regions of the world. Dengue vectors as well as those responsible for the transmission of yellow fever, chikungunya (Aedes spp) and those responsible for malaria (Anopheles spp) are known to be well established in these regions. Dengue - malaria co-infection has been recognized as an important clinical problem in endemic regions[33]. Vector expansion is driven partly by population growth, unplanned urbanization, crowded humans settlements and inadequate water, sewage and waste management[24]. These factors with the lack of effective vector control programs increase the exposure of humans to the disease vectors. Concurrent dengue and malaria co-infection has been reported in many areas of the world with predominance in the Americas, Asian tropical and sub-Saharan Africa regions[34-36]. The profound endemicity of both diseases with similar and overlapping clinical presentations often lead to misdiagnosis or misinterpretation as mono infections[37]. We previously reported that 10% of malaria patients in Ibadan, Nigeria had active dengue infection. Also, all malaria patients were positive for dengue IgG antibodies which is suggestive of a previous infection[33]. This concomitant dengue/malaria co-infection is consistent with the endemicity of both infections in the region. Despite this endemicity, routine diagnosis of dengue is often neglected and more focus is on malaria. Dengue misdiagnosis or under-diagnosis poses a great risk of increased morbidity and mortality in endemic countries[38]. Therefore, routine dengue diagnosis is very essential in endemic countries as misdiagnosis or lack of diagnosis is likely to have tremendous public health consequences in the general management of febrile conditions in these regions.
DENGUE DIAGNOSTIC METHODS
Dengue diagnosis is relevant in epidemiological surveillance, outbreak control, routine diagnosis in endemic countries among people with febrile diseases as well as diagnosis among travellers visiting or returning from endemic countries. There are several diagnostic assays such as virus culture, RNA detection, antigen detection and serology. These assays are associated with many advantages and disadvantages as well as different level of specificity and sensitivity.
Virus culture
This is usually done by inoculation of samples (serum, plasma or buffy coat) into mosquitoes cell lines such as C6/36 and AP61 or mammalian cell lines such as Vero and LLC-MK2 cells[39]. Sucking mice inoculated intra-cerebrally have also been used for the isolation of DENV[40]. Autopsy tissues from spleen, liver, thymus and lymph nodes have been used to isolate the virus from fatal cases[3]. After virus isolation, serotype identification is achieved by immunofluorescence using serotype-specific monoclonal antibodies. Despite the sensitivity of this method, the routine use is limited in endemic countries due to the fact that it requires improved laboratory safety capacity. The assay is also labor intensive and time consuming requiring adequate professional training. Also, virus detection is effective mostly during the early stages of the infection. It is therefore thought that, for better results, cultures should be performed using patient sample collected during the acute phase of the infection that may contain high viral copies. Acute infection is associated with rapid viral replication with high viral load that peaks before the onset of symptoms[3]. Therefore, timing of sample collection is very important for reliable test results.
Virus RNA detection
Dengue RNA can be detected by polymerase chain reaction (PCR) from tissues, blood or sera collected during the acute phase of the infection using primers directed to serotype-specific regions of the genome[41,42]. Common genomic regions for PCR include; E, NS1, E/NS1, prM/E, NS5 and NS5/3. Viral load may be quantified by RT-PCR while strain typing could be performed by nucleotide sequencing and phylogenetic analysis[43]. However, it is essential that laboratories performing nested PCR take every precaution to prevent false-positive results that can occur as a result of contamination[40]. A recent development of real-time PCR enables a simpler and faster assay with less exposure to contamination during concurrent dengue detection and typing. The assay utilizes oligonucleotide primers and dual-labeled hydrolysis probes for in vitro qualitative detection of DENV serotypes in a singleplex or multiplex reactions. However, this method is hampered by late sample collection (> 5 d after onset of symptoms). Therefore a negative result does not preclude dengue diagnosis and samples should be subjected to an anti-IgM ELISA for laboratory confirmation of infection. Although highly sensitive, the method is financially prohibitive as most dengue endemic countries lack the capacity to perform such nucleic acid amplification test.
Antigen detection
The detection of viral antigen has emerged as a potential alternative to PCR and virus culture. The main antigen target is the non-structural protein 1 (NS1). The NS1 antigen is produced during viral replication and can be detected in patients with primary and secondary dengue infections up to 9 d after the onset of disease[44]. NS1 is secreted in all infected cells during the acute phase of the infection and the presence in blood stimulates a strong humoral response. Quantification of the NS1 is a prognostic maker for dengue disease and higher levels have been linked to progression to DHF[45]. A recent study that evaluates a rapid NS1 assay in both Vietnam and Malaysia revealed the following; in Vietnam the sensitivity and specificity of the test was 69.2% (95%CI: 62.8% to 75.6%) and 96% (95%CI: 92.2% to 99.8%) respectively. In Malaysia the performance was similar with 68.9% sensitivity (95%CI: 61.8% to 76.1%) and 96.7% specificity (95%CI: 82.8% to 99.9%) compared to RT-PCR[46].
Serological methods
Serology is based on screening for dengue IgG/IgM antibodies. It is suggested that IgM production occurs 4-8 d after the onset of fever and last for a couple of weeks. On the other hand, IgG production is low after primary infection but matures slowly within weeks and months and may last for several years[47]. ELISA-based IgM assays have become an important tool for the surveillance of dengue. Although these IgM-based assays are a useful diagnostic tool, results from these tests should be interpreted with caution. In addition, there is cross reactivity with other flaviviruses including West Nile virus (WNV), St. Louis encephalitis virus (SLE), Japanese encephalitis virus (JEV) and yellow fever virus (YFV). Therefore, during interpretation of results, patient’s past medical history, recent travel history, and vaccination record (especially yellow fever vaccination) should be reviewed in order to determine the likelihood that the current acute febrile illness is due to an infection with DENV. There may also be false-negative results due to an extended sero-conversion period[48]. The presence of anti-dengue IgM suggests recent infection while IgG antibody detection may be used for the classification of both primary and secondary infection[49]. That is a ratio of IgM/IgG greater than 1.78 represents primary infection and lesser than 1.78 represents secondary infections[48]. Also, the diagnostic value of IgA has been suggested and it has been shown that significantly higher levels of IgA antibodies occur in DHF/DSS than in DF cases[50]. The sensitivity and specificity of IgM-based assays is influenced by the quality of the antigen used and can vary greatly between commercially available products.
ROUTINE DENGUE DIAGNOSIS IN ENDEMIC COUNTRIES
In spite of the methods listed above, their routine use in dengue endemic countries is limited due to either lack of laboratory capacity or skilled personnel. An ideal routine diagnostic test in endemic countries would fulfill the ASSURED criteria: (1) Affordable by those at risk of infection; (2) Sensitive; (3) Specific; (4) User-friendly; (5) Rapid and robust; (6) Equipment-free; and (7) Delivered to those who need it. The recent developments in rapid point-of-care (POC) immunochromatograhic tests (ICT) offer hopes for improved diagnosis of early dengue infection[51]. ICT for the detection of DENV NS1 antigen, IgG, IgM, and IgA antibodies promises to offer tremendous opportunities for the rapid detection of DENV in clinical samples. These ICT are manufactured in lateral flow cassettes and strips and allows the flow of sample by capillary action[52]. Considering that the majority of patients in developing countries are treated in primary health centers without the availability of a laboratory, POC testing may offer a unique advantage in the routine diagnosis of DENV among febrile patients. The tests can be performed in approximately 10-15 min and requires no specialized equipment or training.
PREVENTION AND CONTROL OF DENGUE
In the absence of specific antivirals and a vaccine, the main method of dengue control is to reduce the vector population and to educate people in endemic countries as well as travellers to these regions on basic protection measures such as wearing protective clothing and the use of anti-insecticide sprays.
Effective dengue control in endemic countries also requires more governmental, public and other stakeholder commitment and intervention at all levels. We recommend the following areas for action: Institution of policies for dengue to be a notifiable disease and the provision of POC diagnosis of dengue in febrile patients in endemic regions. Vector reduction activities by sustained environment and space spraying with larvicide as complementary measure. Unfortunately, there is lack of key indicator measurements for vector control programs at national surveillance systems in most tropical countries. Mobilization for public awareness on dengue control and the need to establish a sustained and integrated disease surveillance-response information and knowledge generation programs in vulnerable countries[53]. Effective waste disposal and water supply system management to reduce vector breeding grounds. Operational research is needed to generate evidence-based and cost-effective knowledge for innovative policies to outwit dengue from the region. Innovative approach on genetically modified mosquitoes to reduce vector population and interrupt transmission. Availability of the dengue vaccine, Dengvaxia (Sanofi Pasteur) for use in 9 to 45 years old persons in hotspots areas as well as accelerating dengue drug discovery and the availability of treatment.
CONCLUSION
This review outlined some of the basic public health issues associated with dengue control in endemic countries. Despite the increasing contribution of DENV as a major cause of febrile disease in developing countries, there is a high rate of misdiagnosis and underreporting from endemic countries, as well as lack of routine surveillance and public health prioritization. In summary, we suggest the need for public health commitment to include dengue as a notifiable disease, implement routine laboratory diagnosis and personnel training in endemic countries. Also, dengue NS1 ICTs or IgM antibody tests should be available at all primary health care centers to enable early detection of cases. Travellers visiting dengue endemic countries should be fully informed on symptoms of dengue and strongly urged to do a dengue test prior to departure or immediately after entering their own country, if they suspect infection by the virus, to reduce the risk of importation of the disease.
Footnotes
Conflict-of-interest statement: The authors whose names are listed below certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speaker’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Manuscript source: Invited manuscript
Specialty type: Virology
Country of origin: Canada
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: October 11, 2016
First decision: November 14, 2016
Article in press: December 9, 2016
P- Reviewer: Bonilauri P, Garg RK, Kim ST, Santos-Lopez G S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
References
- 1.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gubler DJ. The global emergence/resurgence of arboviral diseases as public health problems. Arch Med Res. 2002;33:330–342. doi: 10.1016/s0188-4409(02)00378-8. [DOI] [PubMed] [Google Scholar]
- 3.Bäck AT, Lundkvist A. Dengue viruses - an overview. Infect Ecol Epidemiol. 2013:3. doi: 10.3402/iee.v3i0.19839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mustafa MS, Agrawal VK. Dengue Vaccine: The Current Status. Med J Armed Forces India. 2008;64:161–164. doi: 10.1016/S0377-1237(08)80065-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mustafa MS, Rasotgi V, Jain S, Gupta V. Discovery of fifth serotype of dengue virus (DENV-5): A new public health dilemma in dengue control. Med J Armed Forces India. 2015;71:67–70. doi: 10.1016/j.mjafi.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ebi KL, Nealon J. Dengue in a changing climate. Environ Res. 2016;151:115–123. doi: 10.1016/j.envres.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 7.Brady OJ, Gething PW, Bhatt S, Messina JP, Brownstein JS, Hoen AG, Moyes CL, Farlow AW, Scott TW, Hay SI. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis. 2012;6:e1760. doi: 10.1371/journal.pntd.0001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murray NE, Quam MB, Wilder-Smith A. Epidemiology of dengue: past, present and future prospects. Clin Epidemiol. 2013;5:299–309. doi: 10.2147/CLEP.S34440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCall PJ, Lenhart A. Dengue control. Lancet Infect Dis. 2008;8:7–9. doi: 10.1016/S1473-3099(07)70298-0. [DOI] [PubMed] [Google Scholar]
- 10.Murray JV, Jansen CC, De Barro P. Risk Associated with the Release of Wolbachia-Infected Aedes aegypti Mosquitoes into the Environment in an Effort to Control Dengue. Front Public Health. 2016;4:43. doi: 10.3389/fpubh.2016.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wagner D, de With K, Huzly D, Hufert F, Weidmann M, Breisinger S, Eppinger S, Kern WV, Bauer TM. Nosocomial acquisition of dengue. Emerg Infect Dis. 2004;10:1872–1873. doi: 10.3201/eid1010.031037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Wet N, Ye W, Hales S, Warrick R, Woodward A, Weinstein P. Use of a computer model to identify potential hotspots for dengue fever in New Zealand. N Z Med J. 2001;114:420–422. [PubMed] [Google Scholar]
- 13.Hales S, de Wet N, Maindonald J, Woodward A. Potential effect of population and climate changes on global distribution of dengue fever: an empirical model. Lancet. 2002;360:830–834. doi: 10.1016/S0140-6736(02)09964-6. [DOI] [PubMed] [Google Scholar]
- 14.Ooi EE, Goh KT, Gubler DJ. Dengue prevention and 35 years of vector control in Singapore. Emerg Infect Dis. 2006;12:887–893. doi: 10.3201/10.3201/eid1206.051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shepard DS, Coudeville L, Halasa YA, Zambrano B, Dayan GH. Economic impact of dengue illness in the Americas. Am J Trop Med Hyg. 2011;84:200–207. doi: 10.4269/ajtmh.2011.10-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shepard DS, Undurraga EA, Halasa YA, Stanaway JD. The global economic burden of dengue: a systematic analysis. Lancet Infect Dis. 2016;16:935–941. doi: 10.1016/S1473-3099(16)00146-8. [DOI] [PubMed] [Google Scholar]
- 17.Brady OJ, Smith DL, Scott TW, Hay SI. Dengue disease outbreak definitions are implicitly variable. Epidemics. 2015;11:92–102. doi: 10.1016/j.epidem.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferreira GL. Global dengue epidemiology trends. Rev Inst Med Trop Sao Paulo. 2012;54 Suppl 18:S5–S6. doi: 10.1590/s0036-46652012000700003. [DOI] [PubMed] [Google Scholar]
- 19.Rasheed SB, Butlin RK, Boots M. A review of dengue as an emerging disease in Pakistan. Public Health. 2013;127:11–17. doi: 10.1016/j.puhe.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Amarasinghe A, Kuritsk JN, Letson GW, Margolis HS. Dengue virus infection in Africa. Emerg Infect Dis. 2011;17:1349–1354. doi: 10.3201/eid1708.101515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalayanarooj S. Dengue classification: current WHO vs. the newly suggested classification for better clinical application? J Med Assoc Thai. 2011;94 Suppl 3:S74–S84. [PubMed] [Google Scholar]
- 22.Kalayanarooj S. Clinical Manifestations and Management of Dengue/DHF/DSS. Trop Med Health. 2011;39:83–87. doi: 10.2149/tmh.2011-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilder-Smith A, Gubler DJ. Geographic expansion of dengue: the impact of international travel. Med Clin North Am. 2008;92:1377–1390, x. doi: 10.1016/j.mcna.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Gubler DJ. Dengue, Urbanization and Globalization: The Unholy Trinity of the 21(st) Century. Trop Med Health. 2011;39:3–11. doi: 10.2149/tmh.2011-S05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gardner LM, Fajardo D, Waller ST, Wang O, Sarkar S. A predictive spatial model to quantify the risk of air-travel-associated dengue importation into the United States and europe. J Trop Med. 2012;2012:103679. doi: 10.1155/2012/103679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banu S, Hu W, Hurst C, Tong S. Dengue transmission in the Asia-Pacific region: impact of climate change and socio-environmental factors. Trop Med Int Health. 2011;16:598–607. doi: 10.1111/j.1365-3156.2011.02734.x. [DOI] [PubMed] [Google Scholar]
- 27.Napoli C, Salcuni P, Pompa MG, Declich S, Rizzo C. Estimated imported infections of Chikungunya and Dengue in Italy, 2008 to 2011. J Travel Med. 2012;19:294–297. doi: 10.1111/j.1708-8305.2012.00640.x. [DOI] [PubMed] [Google Scholar]
- 28.Sutherst RW. Global change and human vulnerability to vector-borne diseases. Clin Microbiol Rev. 2004;17:136–173. doi: 10.1128/CMR.17.1.136-173.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Senn N, Luang-Suarkia D, Manong D, Siba PM, McBride WJ. Contribution of dengue fever to the burden of acute febrile illnesses in Papua New Guinea: an age-specific prospective study. Am J Trop Med Hyg. 2011;85:132–137. doi: 10.4269/ajtmh.2011.10-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cattand P, Desjeux P, Guzman MG, Jannin J, Kroeger A, Medici A, Musgrove P, Nathan MB, Shaw A, Schofield CJ. Tropical Diseases Lacking Adequate Control Measures: Dengue, Leishmaniasis, and African Trypanosomiasis. In: Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, et al., editors. Disease Control Priorities in Developing Countries. 2nd edition. Washington (DC): World Bank; 2006. p. Chapter 23. [Google Scholar]
- 31.Hotez PJ, Fenwick A, Savioli L, Molyneux DH. Rescuing the bottom billion through control of neglected tropical diseases. Lancet. 2009;373:1570–1575. doi: 10.1016/S0140-6736(09)60233-6. [DOI] [PubMed] [Google Scholar]
- 32.Hotez PJ. The neglected tropical diseases and their devastating health and economic impact on the member nations of the Organisation of the Islamic Conference. PLoS Negl Trop Dis. 2009;3:e539. doi: 10.1371/journal.pntd.0000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oyero OG, Ayukekbong JA. High dengue NS1 antigenemia in febrile patients in Ibadan, Nigeria. Virus Res. 2014;191:59–61. doi: 10.1016/j.virusres.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 34.Deresinski S. Concurrent plasmodium vivax malaria and dengue. Emerg Infect Dis. 2006;12:1802. doi: 10.3201/eid1211.060341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaushik RM, Varma A, Kaushik R, Gaur KJ. Concurrent dengue and malaria due to Plasmodium falciparum and P. vivax. Trans R Soc Trop Med Hyg. 2007;101:1048–1050. doi: 10.1016/j.trstmh.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 36.Charrel RN, Brouqui P, Foucault C, de Lamballerie X. Concurrent dengue and malaria. Emerg Infect Dis. 2005;11:1153–1154. doi: 10.3201/eid1107.041352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yong LS, Koh KC. A case of mixed infections in a patient presenting with acute febrile illness in the tropics. Case Rep Infect Dis. 2013;2013:562175. doi: 10.1155/2013/562175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zaki SA. Malaria and dengue co-infection. Ann Indian Acad Neurol. 2011;14:141–142. doi: 10.4103/0972-2327.82821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosen L, Gubler D. The use of mosquitoes to detect and propagate dengue viruses. Am J Trop Med Hyg. 1974;23:1153–1160. doi: 10.4269/ajtmh.1974.23.1153. [DOI] [PubMed] [Google Scholar]
- 40.Peeling RW, Artsob H, Pelegrino JL, Buchy P, Cardosa MJ, Devi S, Enria DA, Farrar J, Gubler DJ, Guzman MG, et al. Evaluation of diagnostic tests: dengue. Nat Rev Microbiol. 2010;8:S30–S38. doi: 10.1038/nrmicro2459. [DOI] [PubMed] [Google Scholar]
- 41.Johnson BW, Russell BJ, Lanciotti RS. Serotype-specific detection of dengue viruses in a fourplex real-time reverse transcriptase PCR assay. J Clin Microbiol. 2005;43:4977–4983. doi: 10.1128/JCM.43.10.4977-4983.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu SJ, Lee EM, Putvatana R, Shurtliff RN, Porter KR, Suharyono W, Watts DM, King CC, Murphy GS, Hayes CG, et al. Detection of dengue viral RNA using a nucleic acid sequence-based amplification assay. J Clin Microbiol. 2001;39:2794–2798. doi: 10.1128/JCM.39.8.2794-2798.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chow VT, Chan YC, Yong R, Lee KM, Lim LK, Chung YK, Lam-Phua SG, Tan BT. Monitoring of dengue viruses in field-caught Aedes aegypti and Aedes albopictus mosquitoes by a type-specific polymerase chain reaction and cycle sequencing. Am J Trop Med Hyg. 1998;58:578–586. doi: 10.4269/ajtmh.1998.58.578. [DOI] [PubMed] [Google Scholar]
- 44.Dussart P, Labeau B, Lagathu G, Louis P, Nunes MR, Rodrigues SG, Storck-Herrmann C, Cesaire R, Morvan J, Flamand M, et al. Evaluation of an enzyme immunoassay for detection of dengue virus NS1 antigen in human serum. Clin Vaccine Immunol. 2006;13:1185–1189. doi: 10.1128/CVI.00229-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Libraty DH, Young PR, Pickering D, Endy TP, Kalayanarooj S, Green S, Vaughn DW, Nisalak A, Ennis FA, Rothman AL. High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J Infect Dis. 2002;186:1165–1168. doi: 10.1086/343813. [DOI] [PubMed] [Google Scholar]
- 46.Fry SR, Meyer M, Semple MG, Simmons CP, Sekaran SD, Huang JX, McElnea C, Huang CY, Valks A, Young PR, et al. The diagnostic sensitivity of dengue rapid test assays is significantly enhanced by using a combined antigen and antibody testing approach. PLoS Negl Trop Dis. 2011;5:e1199. doi: 10.1371/journal.pntd.0001199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rubens Costa Lima J, Rouquayrol MZ, Monteiro Callado MR, Florindo Guedes MI, Pessoa C. Interpretation of the presence of IgM and IgG antibodies in a rapid test for dengue: analysis of dengue antibody prevalence in Fortaleza City in the 20th year of the epidemic. Rev Soc Bras Med Trop. 2012;45:163–167. doi: 10.1590/s0037-86822012000200005. [DOI] [PubMed] [Google Scholar]
- 48.Schwartz E, Mileguir F, Grossman Z, Mendelson E. Evaluation of ELISA-based sero-diagnosis of dengue fever in travelers. J Clin Virol. 2000;19:169–173. doi: 10.1016/s1386-6532(00)00114-1. [DOI] [PubMed] [Google Scholar]
- 49.Blacksell SD, Jarman RG, Gibbons RV, Tanganuchitcharnchai A, Mammen MP, Nisalak A, Kalayanarooj S, Bailey MS, Premaratna R, de Silva HJ, et al. Comparison of seven commercial antigen and antibody enzyme-linked immunosorbent assays for detection of acute dengue infection. Clin Vaccine Immunol. 2012;19:804–810. doi: 10.1128/CVI.05717-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koraka P, Murgue B, Deparis X, Setiati TE, Suharti C, van Gorp EC, Hack CE, Osterhaus AD, Groen J. Elevated levels of total and dengue virus-specific immunoglobulin E in patients with varying disease severity. J Med Virol. 2003;70:91–98. doi: 10.1002/jmv.10358. [DOI] [PubMed] [Google Scholar]
- 51.Peeling RW, Mabey D. Point-of-care tests for diagnosing infections in the developing world. Clin Microbiol Infect. 2010;16:1062–1069. doi: 10.1111/j.1469-0691.2010.03279.x. [DOI] [PubMed] [Google Scholar]
- 52.Blacksell SD. Commercial dengue rapid diagnostic tests for point-of-care application: recent evaluations and future needs? J Biomed Biotechnol. 2012;2012:151967. doi: 10.1155/2012/151967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Badurdeen S, Valladares DB, Farrar J, Gozzer E, Kroeger A, Kuswara N, Ranzinger SR, Tinh HT, Leite P, Mahendradhata Y, et al. Sharing experiences: towards an evidence based model of dengue surveillance and outbreak response in Latin America and Asia. BMC Public Health. 2013;13:607. doi: 10.1186/1471-2458-13-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gautret P, Simon F, Hervius Askling H, Bouchaud O, Leparc-Goffart I, Ninove L, Parola P. Dengue type 3 virus infections in European travellers returning from the Comoros and Zanzibar, February-April 2010. Euro Surveill. 2010;15:19541. [PubMed] [Google Scholar]
- 55.Vinner L, Domingo C, Ostby AC, Rosenberg K, Fomsgaard A. Cases of travel-acquired dengue fever in Denmark 2001-2009. Clin Microbiol Infect. 2012;18:171–176. doi: 10.1111/j.1469-0691.2011.03543.x. [DOI] [PubMed] [Google Scholar]
- 56.Pierro A, Varani S, Rossini G, Gaibani P, Cavrini F, Finarelli AC, Macini P, Cagarelli R, Mattivi A, Angelini P, et al. Imported cases of dengue virus infection: Emilia-Romagna, Italy, 2010. Clin Microbiol Infect. 2011;17:1349–1352. doi: 10.1111/j.1469-0691.2011.03544.x. [DOI] [PubMed] [Google Scholar]
- 57.Marchand E, Prat C, Jeannin C, Lafont E, Bergmann T, Flusin O, Rizzi J, Roux N, Busso V, Deniau J, et al. Autochthonous case of dengue in France, October 2013. Euro Surveill. 2013;18:20661. doi: 10.2807/1560-7917.es2013.18.50.20661. [DOI] [PubMed] [Google Scholar]
- 58.Gautret P, Botelho-Nevers E, Charrel RN, Parola P. Dengue virus infections in travellers returning from Benin to France, July-August 2010. Euro Surveill. 2010;15:pii: 19657. [PubMed] [Google Scholar]
- 59.Frank C, Höhle M, Stark K, Lawrence J. More reasons to dread rain on vacation? Dengue fever in 42 German and United Kingdom Madeira tourists during autumn 2012. Euro Surveill. 2013;18:20446. doi: 10.2807/1560-7917.es2013.18.14.20446. [DOI] [PubMed] [Google Scholar]
- 60.Heddini A, Janzon R, Linde A. Increased number of dengue cases in Swedish travellers to Thailand. Euro Surveill. 2009;14:pii: 19111. doi: 10.2807/ese.14.05.19111-en. [DOI] [PubMed] [Google Scholar]
- 61.Nisii C, Carletti F, Castilletti C, Bordi L, Meschi S, Selleri M, Chiappini R, Travaglini D, Antonini M, Castorina S, et al. A case of dengue type 3 virus infection imported from Africa to Italy, October 2009. Euro Surveill. 2010;15:pii: 19487. [PubMed] [Google Scholar]
- 62.Huhtamo E, Korhonen E, Vapalahti O. Imported dengue virus serotype 1 from Madeira to Finland 2012. Euro Surveill. 2013;18:pii: 20405. [PubMed] [Google Scholar]
- 63.Schmidt-Chanasit J, Emmerich P, Tappe D, Gunther S, Schmidt S, Wolff D, Hentschel K, Sagebiel D, Schoneberg I, Stark K, et al. Autochthonous dengue virus infection in Japan imported into Germany, September 2013. Euro Surveill. 2014;19:pii: 20681. doi: 10.2807/1560-7917.es2014.19.3.20681. [DOI] [PubMed] [Google Scholar]
- 64.Schmidt-Chanasit J, Haditsch M, Schoneberg I, Gunther S, Stark K, Frank C. Dengue virus infection in a traveller returning from Croatia to Germany. Euro Surveill. 2010;15:pii: 19677. doi: 10.2807/ese.15.40.19677-en. [DOI] [PubMed] [Google Scholar]


