Abstract
Zika virus (ZIKV) was identified as a cause of congenital disease during an explosive outbreak in the Americas and Caribbean in 2015. Because of the ongoing fetal risk from endemic disease and travel-related exposures, a vaccine to prevent viremia in women of child-bearing age and their partners is imperative. Vaccination with DNA expressing the prM and E proteins of ZIKV was immunogenic in mice and nonhuman primates, and protection against viremia after ZIKV challenge correlated with serum neutralizing activity. These data not only indicate DNA vaccination could be a successful approach to protect against ZIKV infection, but also suggest a protective threshold of vaccine-induced neutralizing activity that will prevent viremia following acute infection.
The emergence of Zika virus (ZIKV) in the Americas and the Caribbean follows a series of global threats to public health from mosquito-borne viral diseases over the last three decades. Because of the profound impact on individuals and society from a disabling congenital disease, WHO declared ZIKV infection a global health emergency in February 2016. Although it is likely that the incidence of ZIKV infection will decline significantly within 1–2 years (1), it is also likely to become endemic in tropical and subtropical regions with sporadic outbreaks and potential for spread into new geographical areas, as observed with other emerging arboviruses like West Nile (WNV) and chikungunya viruses. Therefore, unless immunity is established before child-bearing age, pregnant women will continue to be at risk for an infection that could harm their fetus. Further, because men can harbor ZIKV in semen for several months following a clinically unapparent infection and can sexually transmit virus to a pregnant partner (2), even women in nonendemic regions will have some ongoing risk if exposed to men who have traveled to endemic regions. These unique features of transmission and disease suggest there will be an ongoing need for a ZIKV vaccine to maintain a high level of immunity in the general population and in travelers to endemic regions to reduce the frequency of fetal infection.
To rapidly address the critical need for a preventive vaccine to curtail the current ZIKV outbreak in the Americas, we chose a gene-based vaccine delivery approach that leverages our prior experience with a DNA-based WNV vaccine (3). Advantages of DNA vaccines include the ability to rapidly test multiple candidate antigen designs, rapidly produce GMP material, an established safety profile in humans, and a relatively straightforward regulatory pathway into clinical evaluation.
Antigen design was guided by prior knowledge about humoral immunity to flaviviruses. Vaccine-elicited neutralizing antibodies (NAb) are associated with protection from flavivirus-mediated disease (4). Because the most potent monoclonal flavivirus NAbs map to conformational epitopes in domain III (DIII) of the E protein (5), or more complex quaternary epitopes that bridge between antiparallel E dimers or between dimer rafts arrayed on the virus surface (6, 7), our goal was to identify constructs that produced particles that faithfully capture the antigenic complexity of infectious virions. Expression of the structural proteins premembrane (prM) and envelope (E) are sufficient for the production and release of virus-like subviral particles (SVPs) with antigenic and functional properties similar to those of infectious virions (8, 9).
To identify promising vaccine candidates, prM-E constructs were synthesized and screened for expression and efficiency of particle release from transfected cells. prM-E sequences were inserted into a CMV-immediate early promoter-containing vector (VRC8400) evaluated clinically in several prior studies (3, 10, 11). These constructs are distinct from one reported in recent studies from Larocca et al. (12) and Abbink et al. (13) that was based on a Brazilian isolate (strain BeH815744) and did not express the first 93 amino acids of prM, encoding only the short M peptide that is the product of furin cleavage of prM during natural infection. Because prM plays a critical role in folding of the E protein and release of particles from cells (14, 15), it is not known how the antigenicity of that product compares to the prM-E product described here. The prM-E sequence in the current constructs was selected from a French Polynesian isolate (strain H/PF/2013) identical or highly related to strains circulating in the Americas. Neutralization studies using contemporary sera against temporally and geographically diverse strains indicate ZIKV exists as a single serotype, suggesting a single vaccine antigen will provide protection against all ZIKV strains (16). To improve expression, the ZIKV prM signal sequence was exchanged with the analogous region of Japanese encephalitis virus (JEV), as previously reported (17), to create vector VRC5283 (Fig. 1A). A second chimeric ZIKV/JEV prM-E construct, VRC5288, also encoding the JEV signal sequence, was designed with the final 98 amino acids of E, comprising the stem and transmembrane regions (ST/TM), exchanged with corresponding JEV sequences and previously shown to improve SVP secretion (18). Both vectors exhibited expression by mammalian cells (Fig 1B, right panel), with more efficient SVP release into supernatants by VRC5288 (Fig. 1B, right panel, and Fig. 1C) (19). Electron microscopic analysis of negative-stained purified VRC5288 SVP preparations revealed roughly spherical particles consistent with the appearance of other flavivirus SVPs (Fig. 1D) (8, 20).
Figure 1. ZIKV DNA vaccine design and characterization.
(A) Schematic representation of ZIKV genome and ZIKV DNA vaccine constructs VRC5283 and VRC5288. (B) Expression and secretion of ZIKV E was analyzed by Western blot of transfected 293T cell lysates and SVP precipitate pelleted from culture supernatants through a 20% sucrose cushion demonstrating that the VRC5288 construct secretes more particles than VRC5283. (C) Particle-capture ELISA quantifying the secretion of ZIKV SVP from transfected cells. (D) ZIKV subviral particles (SVP) were purified from the culture supernatant of VRC5288-transfected 293-F cells and subjected to negative staining and electron microscopy. SVP are labeled with arrowheads. The VRC8400 empty backbone plasmid vector was used as a control.
Next we assessed the immunogenicity of each DNA candidate in BALB/c and C57BL/6 mice. Mice were immunized intramuscularly once with 50 μg of DNA in the quadriceps using electroporation as previously described (21). Serum was evaluated for binding to ZIKV SVPs (fig. S1A) and neutralizing activity using ZIKV reporter virus particles (RVPs) (fig. S1B–D) (16). Vaccination with either VRC5283 or VRC5288 elicited ZIKV-specific NAbs after a single immunization with titers up to 105 reciprocal EC50 serum dilution in C57BL/6 mice (fig. S1D). NAb titers were similar in mice vaccinated with 2, 10, or 50 μg DNA (fig. S2), and were of similar magnitude to titers induced by a previously described WNV DNA vaccine (fig. S3) (3).
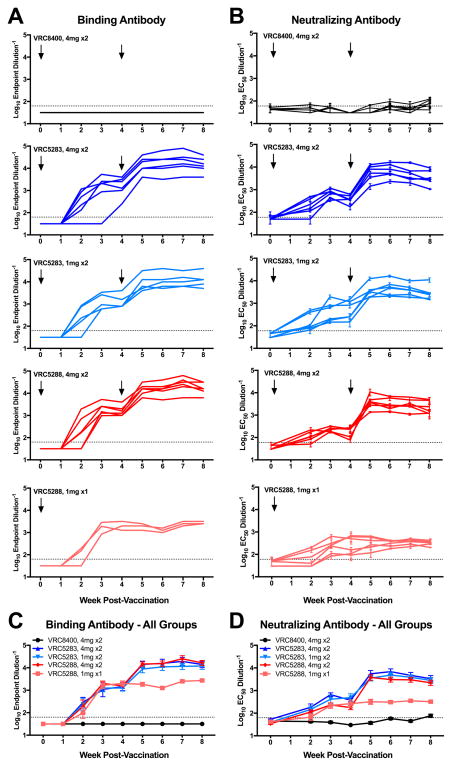
Immunogenicity in rhesus macaques was evaluated after delivering vaccine intramuscularly by a needle-free injection device (PharmaJet) (Fig. 2, fig. S4 and S5). Six animals per group received two 1 mg (VRC5283) or 4 mg (VRC5283 and VRC5288) doses of vaccine at 0 and 4 weeks, while one group received a single 1 mg dose of VRC5288 at week 0. After a single dose of DNA, binding and neutralizing antibody were detectable by week two and peaked at week three. All ZIKV vaccine groups had significantly higher NAb responses than macaques that received VRC8400 control vector when comparing area-under-the-curve (AUC) using a Kruskal-Wallis test (p=0.022, Fig. 2D). Macaques that received a single 1 mg dose of VRC5288 had significantly lower NAb titers than macaques that received two doses of either vaccine at either dose level (p=0.022). There were no significant differences in NAb titer between animals that received two doses of VRC5283 or animals that received two doses of VRC5288 comparing AUC. Sera collected at week 6 were also evaluated for NAb activity by the conventional focus-reduction neutralization test (FRNT) (22, 23) and a microneutralization (MN) assay (12, 13, 24). The results of both assays strongly correlated with EC50 RVP values (fig. S6, table S1), although the RVP assay was more sensitive as demonstrated by detection of neutralizating activity in macaques that received only a single 1 mg dose of VRC5288 compared to MN results (average week 6 EC50 reciprocal serum NAb titers of 322 versus <10 for RVP and MN assays, respectively). Further comparison of these values suggested MN values above the limit of detection corresponded more closely to EC90 RVP values (1.3-fold versus 9.6-fold average difference in RVP EC90/MN EC50 and RVP EC50/MN EC50 NAb titers, respectively, for all animals at week 6). These data indicate that both VRC5283 and VRC5288 elicit substantial ZIKV-specific NAb in macaques.
Figure 2. ZIKV DNA vaccines elicit robust binding and neutralizing antibodies in nonhuman primates.
Rhesus macaques (n=6/group) were either mock immunized with VRC8400 empty backbone expression plasmid or with VRC5283 or VRC5288 vaccine plasmids intramuscularly with the indicated doses and number of vaccinations. (A) Macaque sera were assayed weekly for ZIKV binding antibodies by ELISA. Each line represents the average titer of an individual animal from 1–2 technical duplicates and the dashed line indicates the limit of detection (reciprocal titer of 64). Any measurement below the limit of detection was assigned a value of half the limit of detection for graphing and statistical purposes. (B) The NAb response elicited by vaccination was analyzed using ZIKV reporter virus particles (RVPs). The dilution of sera required for half-maximal inhibition of virus infection (EC50) was estimated by non-linear regression analysis. Lines connect the average EC50 values of 2–5 independent experiments, each performed with duplicate technical replicates, for the individual monkeys in each group at each timepoint. Error bars denote the standard error of mean. The dotted line denotes the limit of confidence for the RVP assay (reciprocal titer of 60). Measurements below the limit of detection were assigned a value of 30. The average binding antibody (C) and NAb (D) responses for each vaccine group are shown. Error bars denote the standard error of the mean.
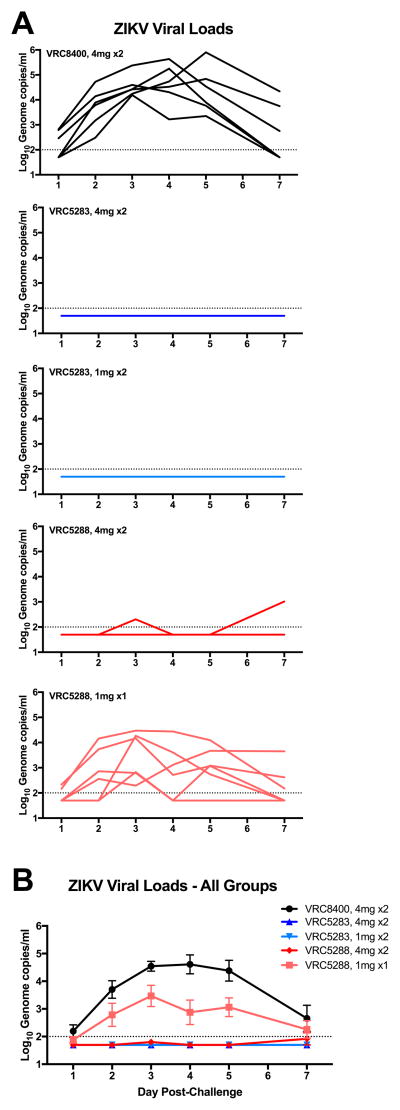
Eight weeks after the first immunization, all animals were challenged subcutaneously with 103 focus-forming units (FFU) of the Puerto Rican ZIKV strain PRVABC59 (GenBank KU501215.1) and blood was collected daily for quantitative PCR analysis of ZIKV genome copies in plasma (13, 19). Control animals showed peak virus load (VL) on day 3 or 4 between 104 and 106 genome copies/ml. Animals that received two doses of 4 mg or 1 mg of VRC5283 or 4 mg of VRC5288 were largely protected from viremia with 17 of 18 animals having no detectable viremia (Fig. 3A). One animal that received two 4 mg doses of VRC5288 had a low-level positive PCR in one of two assays performed on day 3 and another positive blip at day 7. All six animals that received a single dose of 1 mg of VRC5288 were viremic with peak VL on day 3 between 102 and 105 genome copies/ml. This VL was significantly reduced compared to animals that received two doses of VRC8400 control vector comparing AUC by a Wilcoxon Exact Test (two-sided p=0.041). The cutoff for low values has been established at <100 genome copies/ml, so it cannot be ruled out that low level viremia may have occurred in other animals.
Figure 3. ZIKV DNA vaccines reduce viremia in ZIKV-challenged rhesus macaques.
Eight weeks after the first vaccination, macaques were challenged with 103 FFU of ZIKV PRVABC59. (A) qPCR of the capsid gene was used to determine the genome copies/ml on days 1–5 and 7 post-challenge. Each line represents an individual animal. (B) Mean viral load after challenge in each group. Error bars represent the standard error of the mean. Dashed line indicates the limit of detection (100 copies/ml). Any value below the limit of detection was assigned a value half the limit of detection for graphing and AUC calculation.
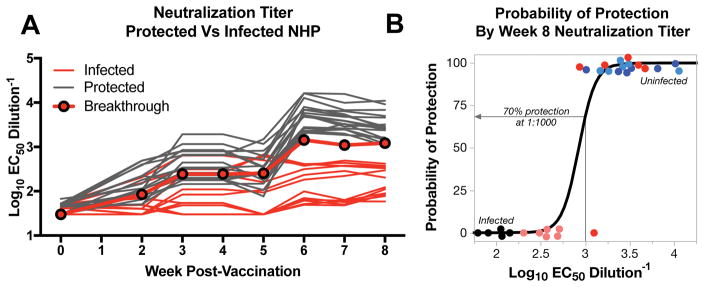
Seventeen of eighteen (94%) of animals that received two doses of vaccine had no detectable viremia post-challenge. The animal with blips above background at day 3 and 7 in the VRC5288 two-dose 4 mg group had a prechallenge reciprocal EC50 NAb titer of 1218, which was among the lowest titers of all two-dose vaccine groups (Fig. 4A). A probability analysis indicated that one could anticipate a 70% protection from viremia if a reciprocal EC50 serum NAb titer of 1000 is achieved in the RVP assay (Fig. 4B). This corresponds roughly to a reciprocal EC50 MN titer of ~100 (fig. S6) which is similar to the titer of NAb shown to prevent viremia in nonhuman primates passively treated with immune serum (13).
Figure 4. Protection from ZIKV challenge correlates with NAb titers present at challenge.
Animals that had detectable viremia post-challenge were analyzed with respect to pre-challenge NAb activity. (A) The reciprocal EC50 NAb titer of each animal is individually plotted to reflect whether infection occurred or not. Lines indicate individual animals. Protected (no detectable viremia) and infected (viremia detectable on two successive days) animals are represented by gray and red lines, respectively. The sole animal that received two 4 mg doses of VRC5288 and was found to have a low level of viremia on days 3 and 7 after challenge is denoted as “breakthrough” (black outlined dots). (B) The probability of infection (Logit) based on the reciprocal EC50 NAb titer is demonstrated and indicates that prevention of viremia would be expected in approximately 70% of animals with NAb titers >1000.
Animals receiving a single dose of 1 mg VRC5288 had prechallenge reciprocal EC50 NAb titers measured by the RVP assay between 203 and 417. The two animals with the highest NAb activity were the ones with delayed onset of viremia at day 3. MN titers at the 6-week timepoint, as noted above, were <10 in the 1 mg single dose group animals that uniformly had breakthrough infection (table S1). Therefore, the larger dynamic range of the RVP assay will allow a more precise definition of the protective threshold needed to prevent viremia in a particular model or against a particular challenge inoculum (fig. S5 and S7).
One concern routinely raised about vaccination against flaviviruses is the possibility of enhanced disease if there is incomplete or waning immunity, as observed in a subset of secondary dengue virus infections (25). In this study, the 1 mg single-dose group that received VRC5288 had low, sub-protective levels of NAb that resulted in breakthrough infections. In those animals, there were reduced levels of viremia compared to unvaccinated controls and no visible signs of illness or enhancement of replication. Retrospectively, we also determined that one animal in the mock-immunized control group and one in the single-dose 1 mg VRC5288 group with detectable levels of ZIKV antibody binding, but no neutralizing activity, had pre-existing WNV-specific NAbs (fig. S8). The levels of virus replication in these animals were near the group average and there was no evidence of disease enhancement in the setting of prior flavivirus exposure.
Vaccine development for ZIKV must be specific and guided by an expanded understanding of ZIKV virology, pathogenesis, immunity, and transmission. It must also be strategic, matching technical and manufacturing feasibility with the target populations that will benefit most from vaccination. In addition, to achieve both rapid deployment and long-term protection, it should be staged. This means that a rapid response to the global health emergency may require a different vaccine approach than the longer term goal of achieving durable immunity in the general population as ZIKV becomes a sporadic, endemic infection. Both VRC5288 and VRC5283 will be evaluated in humans. A Phase 1 clinical trial (NCT02840487) of VRC5288 has launched to test a variety of regimens and doses for safety and immunogenicity. These trials represent the initial efforts to define the level of vaccine-induced NAbs required for prevention of ZIKV viremia. Establishing a functional serological correlate of sterilizing immunity is key for leveraging the information gained from efficacy trials from one candidate vaccine to the next. The Phase 1 clinical trials with VRC5288 and VRC5283 are being designed in parallel with other groups who will evaluate a purified, protein-based, whole-inactivated ZIKV vaccine (ZPIV) or live-attenuated vaccine approaches. These studies and others to evaluate alternative antigen designs, delivery approaches, and combination vaccine regimens will provide safety and immunogenicity data in humans that will inform the next steps of vaccine development and provide options for achieving both the short-term goal of identifying an intervention to protect women of child-bearing age in the current ZIKV outbreak, and the long-term goal of vaccinating the general population of endemic regions and travelers to those regions.
Supplementary Material
Fig. S1. Immunogenicity of VRC5283 and VRC5288 DNA vaccine candidates in mice. The binding and neutralizing antibody response in mice elicited by vaccination with ZIKV DNA vaccine candidates was analyzed using an ELISA (A) and ZIKV RVPs (B–D), respectively. Groups of ten BALB/c and C57BL/6 mice were immunized with one 50 μg dose of VRC5283 or VRC5288 vaccine and bled weekly for serological studies. (A) Binding antibodies were assayed for each mouse, at each of the indicated timepoints, using a particle-based ELISA. Bars represent group mean and associated error bars denote standard error of the mean. Dots represent titers for individual animals. (B–D) To assess NAb responses, ZIKV strain H/PF/2013 RVPs were mixed with serial four-fold dilutions of serum for 1 h at 37°C prior to being added to Raji-DCSIGNR cells. After 48 h, GFP-positive infected cells were quantitated by flow cytometry. The dilution of sera required for half-maximal inhibition of virus infection (EC50) was estimated by non-linear regression analysis. Representative dose-response neutralization profiles are shown for individual mice immunized with VRC5288 (B) or VRC5283 (C) DNA vaccine candidates. The neutralizing activity of sera collected 56 days post-vaccination (closed circles) is shown relative to sera collected 59 days post-vaccination from a mouse vaccinated with a control construct, VRC4974 (open circles). VRC4974 is identical to VRC5283 with the exception of a three amino acid deletion at the amino terminus of prM that prevents SVP particle release. Error bars reflect the range of two technical replicates, present even when not visible. (D) The EC50 serum neutralization titer was determined for each mouse, at each of the indicated timepoints. Dots denote the titers for individual animals (n=1). Bars and associated error bars denote the group mean neutralization titer and standard error of the mean, respectively.
Fig. S2. Immunogenicity of increasing doses of VRC5283 and VRC5288 vaccine candidates in mice. ZIKV H/PF/2013 RVPs were mixed with four-fold serial dilutions of sera collected and pooled from four mice 21 days post-vaccination with 2, 10, or 50 μg of VRC5283 or VRC5288, and from sera collected 59 days post-vaccination with a control construct, VRC4974. VRC4974 is identical to VRC5283 with the exception of a three amino acid deletion at the amino terminus of prM that prevents SVP release. Immune complexes were incubated for 1 h at 37°C prior to being added to Raji-DCSIGNR cells. After 48 h, GFP-positive infected cells were quantitated by flow cytometry and the results analyzed by non-linear regression. Error bars denote the range of technical duplicates, present even when not visible.
Fig. S3. Neutralization of WNV and ZIKV RVPs by DNA vaccine-immune sera. WNV NY99 (A) and ZIKV H/PF/2013 (B) RVPs were mixed with four-fold serial dilutions of sera pooled from four mice 14 days post-vaccination with a single 50 μg dose of WNV (VRC8111), ZIKV (VRC5283 and VRC5288) or MERS (VRC3593) DNA constructs. Immune complexes were incubated for 1 h at 37°C prior to being added to Raji-DCSIGNR cells. After 48 h, GFP-positive infected cells were quantitated by flow cytometry and the results analyzed by non-linear regression. Dose-response neutralization curves from a representative experiment of two independent assays are shown. Error bars denote the range of technical duplicates.
Fig. S4. Immunogenicity of VRC5283 and VRC5288 vaccine candidates in nonhuman primates. The NAb response in macaques elicited by vaccination with ZIKV DNA vaccine candidates was analyzed using ZIKV RVPs as described in Figure 2. Representative dose-response neutralization profiles are shown for individual animals immunized with VRC5283 (A) or VRC5288 (B) DNA vaccine candidates. The neutralizing activity of sera collected 7 weeks post-vaccination (W7, closed circles) is shown relative to pre-immune sera from the same animal (PRE, open circles). Error bars reflect the range of two technical replicates, present even when not visible. (C) The EC50 serum neutralization titer was determined for each animal, at each of the indicated timepoints. Dots denote the average titers for individual animals, calculated from 2–5 independent experiments. Bars and associated error bars denote the group mean neutralization titer and standard deviation, respectively. The dotted line denotes the limit of confidence for the RVP assay (defined by the highest concentration of sera used in the assay); samples with titers <60 are reported at half the limit of detection (1:30).
Fig. S5. Magnitude of the neutralizing antibody response elicited in vaccinated nonhuman primates as a function of pre-immune titers. The NAb response in macaques elicited by vaccination with ZIKV DNA vaccine candidates was analyzed using ZIKV RVPs as described in Figure 2. The data presented represents the fold-change in the EC50 titer of sera collected at the indicated time post-vaccination as compared to the pre-immune titer of that same animal (Post-vaccination EC50/Pre-immune EC50). Lines represent individual animals and connect the fold-change values calculated from average EC50 NAb titers at each timepoint that are representative of 2–5 independent experiments, each performed with duplicate technical replicates. In each panel, the area under the curve for the line connecting group mean fold-change values is shaded gray. The dotted line denotes four standard deviations from pre-immune EC50 NAb titers. Note that the scales of the left-most and right-most panels have a smaller range than the middle three panels. Note that the scales of the top-most and bottom-most panels have a smaller range than the middle three panels. Presentation of the NAb data in this format emphasizes the sensitivity of the RVP neutralization assay, particularly in the one dose, 1 mg VRC5288 group. Animals in this particular dose group had undetectable NAb titers as measured by the MN assay (fig. S6 and table S1).
Fig. S6. Comparison of serum neutralization titers determined by three distinct assays. The neutralizing potency of nonhuman primate sera collected 6 weeks after vaccination was determined by three ZIKV neutralization assays: reporter virus particles (RVP), microneutralization (MN), or focus reduction neutralization test (FRNT). Sera from all 30 animals comprising all five vaccination groups were tested in the RVP and MN assays. A subset of monkeys, the 12 animals that received two doses of 4 mg VRC5283 or VRC5288, was assessed via FRNT. Neutralization titers for individual serum samples tested using the indicated assays are plotted on the x- and y-axis. Shown are comparisons of RVP EC50 versus MN EC50 (A), RVP EC90 versus MN EC50 (B), and RVP EC50 versus FRNT EC50 (C). RVP EC50 and EC90 values represent the average of 2–4 independent experiments performed with duplicate technical replicates, FRNT EC50 values represent the average of 1–4 independent experiments performed with duplicate technical replicates, and MN EC50 values represent a single experiment. Error bars reflect the standard deviation. The correlation between independent measurements was evaluated by Spearman’s correlation.
Fig. S7. Correlation of pre-challenge serum NAb activity with peak viremia. The level of peak viremia on day 3 is inversely related to the prechallenge serum NAb titer. This correlation remained significant when the day of viremia was varied, and when restricted to viremic animals (Spearman Rho= −0.856, p<0.0001). Viremic animals are shown as red dots, completely protected animals as grey dots and the breakthrough animal from the group that received 2×4mg of VRC5288 is outlined in black. Grey box indicates a NAb titer <1000 reciprocal EC50 serum dilution.
Fig. S8. Prior WNV infection does not protect against or enhance ZIKV infection. Retrospectively, it was determined that one animal in the mock-immunized control group (A8V016) and one animal in the single-dose 1 mg VRC5288 group (A5V033) with detectable ZIKV binding, but non-neutralizing antibody had pre-existing WNV-specific NAbs. WNV NY99 RVPs were mixed with serial dilutions of a potently neutralizing WNV mAb E16 (A), week 0 sera from macaque A8V016 (B), macaque A5V033 (C), and macaques A13V061, A13V190, and 21412 (D). Immune complexes were incubated for 1 h at 37°C prior to being added to Raji-DCSIGNR (top panels) or FcγR+ K562 (bottom panels) cells to detect neutralizing and enhancing activity, respectively. After 48 h, GFP-positive infected cells were quantitated by flow cytometry and the Raji-DCSIGNR results analyzed by non-linear regression. Error bars in A, B, and the top panels of C and D denote the range of duplicate technical replicates from a single assay. Data presented in the bottom panels of C and D was not performed in duplicate. Results are representative of 1–2 independent assays. The ability to both neutralize and enhance infection of WNV RVPs indicates prior WNV exposure in macaques A8V016 and A5V033. Negative results for the three macaques shown in panel (D) are representative of the remaining animals in the study. Viral loads of animals vaccinated with two 4 mg doses of VRC8400 (E) or one 1 mg dose of VRC5288 (F) on day 1–7 after challenge. Macaques A8V016 and A5V033 are shown in purple and orange, respectively, demonstrating no protection from or enhancement of ZIKV infection.
Table S1. Comparison of the EC50 neutralization titers of nonhuman primate sera collected 6-weeks post-vaccination determined by three distinct assays.
Acknowledgments
We thank A. Fauci, H. Marston, J. Ledgerwood, S. Whitehead, N. Michael and M. Crank for scientific advice and comments; J. Stein and M. Young for coordinating collaborations; A. Cook and A. Dodson for the macaque work; K. Leung, L. Wang, W. Shi, K. Foulds, M. Donaldson, B. Fisher, A. Creanga, D. Scorpio, and B. Dekosky for laboratory and animal study support; M. Diamond for ZIKV-specific antibodies prior to publication; N. Bourne and A. Barrett for scientific advice and murine experiments; E. Moseley for serological assays; B. Hartman for help with figure optimization; R. Larocca and A. Badamchi-Zadeh for murine experiments, and N. Mercado for shipping and coordination. The data presented in this manuscript are tabulated in the main paper and in the supplementary materials. This work was supported by intramural funding from the National Institute of Allergy and Infectious Diseases; startup funding from the Department of Diagnostic Medicine and Pathobiology, College of Veterinary Medicine, Kansas State University (D.L.V.); and federal funds from the Frederick National Laboratory for Cancer Research, NIH, under contract HHSN261200800001E. Leidos Biomedical Research, Inc. (Y.T.).
Footnotes
REFERENCES AND NOTES
- 1.Lessler J, et al. Assessing the global threat from Zika virus. Science. 2016;353:aaf8160. doi: 10.1126/science.aaf8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrower J, et al. Sexual Transmission of Zika Virus and Persistence in Semen, New Zealand, 2016. Emerging infectious diseases. 2016:22. doi: 10.3201/eid2210.160951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ledgerwood JE, et al. A West Nile virus DNA vaccine utilizing a modified promoter induces neutralizing antibody in younger and older healthy adults in a phase I clinical trial. The Journal of infectious diseases. 2011;203:1396–1404. doi: 10.1093/infdis/jir054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitehead SS, Blaney JE, Durbin AP, Murphy BR. Prospects for a dengue virus vaccine. Nature reviews Microbiology. 2007;5:518–528. doi: 10.1038/nrmicro1690. [DOI] [PubMed] [Google Scholar]
- 5.Pierson TC, Fremont DH, Kuhn RJ, Diamond MS. Structural insights into the mechanisms of antibody-mediated neutralization of flavivirus infection: implications for vaccine development. Cell host & microbe. 2008;4:229–238. doi: 10.1016/j.chom.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dejnirattisai W, et al. A new class of highly potent, broadly neutralizing antibodies isolated from viremic patients infected with dengue virus. Nature immunology. 2015;16:170–177. doi: 10.1038/ni.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rouvinski A, et al. Recognition determinants of broadly neutralizing human antibodies against dengue viruses. Nature. 2015 doi: 10.1038/nature14130. [DOI] [PubMed] [Google Scholar]
- 8.Ferlenghi I, et al. Molecular organization of a recombinant subviral particle from tick-borne encephalitis virus. Mol Cell. 2001;7:593–602. doi: 10.1016/s1097-2765(01)00206-4. [DOI] [PubMed] [Google Scholar]
- 9.Chang GJ, Davis BS, Hunt AR, Holmes DA, Kuno G. Flavivirus DNA vaccines: current status and potential. Ann N Y Acad Sci. 2001;951:272–285. [PubMed] [Google Scholar]
- 10.Catanzaro AT, et al. Phase I clinical evaluation of a six-plasmid multiclade HIV-1 DNA candidate vaccine. Vaccine. 2007;25:4085–4092. doi: 10.1016/j.vaccine.2007.02.050. [DOI] [PubMed] [Google Scholar]
- 11.Ledgerwood JE, Graham BS. DNA vaccines: a safe and efficient platform technology for responding to emerging infectious diseases. Hum Vaccin. 2009;5:623–626. doi: 10.4161/hv.8627. [DOI] [PubMed] [Google Scholar]
- 12.Larocca RA, et al. Vaccine protection against Zika virus from Brazil. Nature. 2016;536:474–478. doi: 10.1038/nature18952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abbink P, et al. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science. 2016 doi: 10.1126/science.aah6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Konishi E, Mason PW. Proper maturation of the Japanese encephalitis virus envelope glycoprotein requires cosynthesis with the premembrane protein. Journal of virology. 1993;67:1672–1675. doi: 10.1128/jvi.67.3.1672-1675.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allison SL, Stadler K, Mandl CW, Kunz C, Heinz FX. Synthesis and secretion of recombinant tick-borne encephalitis virus protein E in soluble and particulate form. Journal of virology. 1995;69:5816–5820. doi: 10.1128/jvi.69.9.5816-5820.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dowd KA, et al. Broadly Neutralizing Activity of Zika Virus-Immune Sera Identifies a Single Viral Serotype. Cell Rep. 2016;16:1485–1491. doi: 10.1016/j.celrep.2016.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis BS, et al. West Nile virus recombinant DNA vaccine protects mouse and horse from virus challenge and expresses in vitro a noninfectious recombinant antigen that can be used in enzyme-linked immunosorbent assays. Journal of virology. 2001;75:4040–4047. doi: 10.1128/JVI.75.9.4040-4047.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang GJ, et al. Enhancing biosynthesis and secretion of premembrane and envelope proteins by the chimeric plasmid of dengue virus type 2 and Japanese encephalitis virus. Virology. 2003;306:170–180. doi: 10.1016/s0042-6822(02)00028-4. [DOI] [PubMed] [Google Scholar]
- 19.Materials and methods are available as supplementary materials on Science Online.
- 20.Arora U, Tyagi P, Swaminathan S, Khanna N. Virus-like particles displaying envelope domain III of dengue virus type 2 induce virus-specific antibody response in mice. Vaccine. 2013;31:873–878. doi: 10.1016/j.vaccine.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 21.Wang L, et al. Evaluation of candidate vaccine approaches for MERS-CoV. Nature communications. 2015;6:7712. doi: 10.1038/ncomms8712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okuno Y, Igarashi A, Fukai K. Neutralization tests for dengue and Japanese encephalitis viruses by the focus reduction method using peroxidase-anti-peroxidase staining. Biken J. 1978;21:137–147. [PubMed] [Google Scholar]
- 23.Zhao H, et al. Structural Basis of Zika Virus-Specific Antibody Protection. Cell. 2016;166:1016–1027. doi: 10.1016/j.cell.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas SJ, et al. A phase II, randomized, safety and immunogenicity study of a re-derived, live-attenuated dengue virus vaccine in healthy adults. The American journal of tropical medicine and hygiene. 2013;88:73–88. doi: 10.4269/ajtmh.2012.12-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guzman MG, Alvarez M, Halstead SB. Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: an historical perspective and role of antibody-dependent enhancement of infection. Archives of virology. 2013;158:1445–1459. doi: 10.1007/s00705-013-1645-3. [DOI] [PubMed] [Google Scholar]
- 26.Davis CW, et al. West Nile virus discriminates between DC-SIGN and DC-SIGNR for cellular attachment and infection. Journal of virology. 2006;80:1290–1301. doi: 10.1128/JVI.80.3.1290-1301.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baronti C, et al. Complete coding sequence of zika virus from a French polynesia outbreak in 2013. Genome Announc. 2014;2 doi: 10.1128/genomeA.00500-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin JE, et al. A West Nile virus DNA vaccine induces neutralizing antibody in healthy adults during a phase 1 clinical trial. The Journal of infectious diseases. 2007;196:1732–1740. doi: 10.1086/523650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pierson TC, et al. A rapid and quantitative assay for measuring antibody-mediated neutralization of West Nile virus infection. Virology. 2006;346:53–65. doi: 10.1016/j.virol.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 30.Pierson TC, et al. The stoichiometry of antibody-mediated neutralization and enhancement of West Nile virus infection. Cell host & microbe. 2007;1:135–145. doi: 10.1016/j.chom.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ansarah-Sobrinho C, Nelson S, Jost CA, Whitehead SS, Pierson TC. Temperature-dependent production of pseudoinfectious dengue reporter virus particles by complementation. Virology. 2008;381:67–74. doi: 10.1016/j.virol.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dowd KA, DeMaso CR, Pierson TC. Genotypic Differences in Dengue Virus Neutralization Are Explained by a Single Amino Acid Mutation That Modulates Virus Breathing. MBio. 2015;6 doi: 10.1128/mBio.01559-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.VanBlargan LA, et al. The type-specific neutralizing antibody response elicited by a dengue vaccine candidate is focused on two amino acids of the envelope protein. PLoS pathogens. 2013;9:e1003761. doi: 10.1371/journal.ppat.1003761. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Immunogenicity of VRC5283 and VRC5288 DNA vaccine candidates in mice. The binding and neutralizing antibody response in mice elicited by vaccination with ZIKV DNA vaccine candidates was analyzed using an ELISA (A) and ZIKV RVPs (B–D), respectively. Groups of ten BALB/c and C57BL/6 mice were immunized with one 50 μg dose of VRC5283 or VRC5288 vaccine and bled weekly for serological studies. (A) Binding antibodies were assayed for each mouse, at each of the indicated timepoints, using a particle-based ELISA. Bars represent group mean and associated error bars denote standard error of the mean. Dots represent titers for individual animals. (B–D) To assess NAb responses, ZIKV strain H/PF/2013 RVPs were mixed with serial four-fold dilutions of serum for 1 h at 37°C prior to being added to Raji-DCSIGNR cells. After 48 h, GFP-positive infected cells were quantitated by flow cytometry. The dilution of sera required for half-maximal inhibition of virus infection (EC50) was estimated by non-linear regression analysis. Representative dose-response neutralization profiles are shown for individual mice immunized with VRC5288 (B) or VRC5283 (C) DNA vaccine candidates. The neutralizing activity of sera collected 56 days post-vaccination (closed circles) is shown relative to sera collected 59 days post-vaccination from a mouse vaccinated with a control construct, VRC4974 (open circles). VRC4974 is identical to VRC5283 with the exception of a three amino acid deletion at the amino terminus of prM that prevents SVP particle release. Error bars reflect the range of two technical replicates, present even when not visible. (D) The EC50 serum neutralization titer was determined for each mouse, at each of the indicated timepoints. Dots denote the titers for individual animals (n=1). Bars and associated error bars denote the group mean neutralization titer and standard error of the mean, respectively.
Fig. S2. Immunogenicity of increasing doses of VRC5283 and VRC5288 vaccine candidates in mice. ZIKV H/PF/2013 RVPs were mixed with four-fold serial dilutions of sera collected and pooled from four mice 21 days post-vaccination with 2, 10, or 50 μg of VRC5283 or VRC5288, and from sera collected 59 days post-vaccination with a control construct, VRC4974. VRC4974 is identical to VRC5283 with the exception of a three amino acid deletion at the amino terminus of prM that prevents SVP release. Immune complexes were incubated for 1 h at 37°C prior to being added to Raji-DCSIGNR cells. After 48 h, GFP-positive infected cells were quantitated by flow cytometry and the results analyzed by non-linear regression. Error bars denote the range of technical duplicates, present even when not visible.
Fig. S3. Neutralization of WNV and ZIKV RVPs by DNA vaccine-immune sera. WNV NY99 (A) and ZIKV H/PF/2013 (B) RVPs were mixed with four-fold serial dilutions of sera pooled from four mice 14 days post-vaccination with a single 50 μg dose of WNV (VRC8111), ZIKV (VRC5283 and VRC5288) or MERS (VRC3593) DNA constructs. Immune complexes were incubated for 1 h at 37°C prior to being added to Raji-DCSIGNR cells. After 48 h, GFP-positive infected cells were quantitated by flow cytometry and the results analyzed by non-linear regression. Dose-response neutralization curves from a representative experiment of two independent assays are shown. Error bars denote the range of technical duplicates.
Fig. S4. Immunogenicity of VRC5283 and VRC5288 vaccine candidates in nonhuman primates. The NAb response in macaques elicited by vaccination with ZIKV DNA vaccine candidates was analyzed using ZIKV RVPs as described in Figure 2. Representative dose-response neutralization profiles are shown for individual animals immunized with VRC5283 (A) or VRC5288 (B) DNA vaccine candidates. The neutralizing activity of sera collected 7 weeks post-vaccination (W7, closed circles) is shown relative to pre-immune sera from the same animal (PRE, open circles). Error bars reflect the range of two technical replicates, present even when not visible. (C) The EC50 serum neutralization titer was determined for each animal, at each of the indicated timepoints. Dots denote the average titers for individual animals, calculated from 2–5 independent experiments. Bars and associated error bars denote the group mean neutralization titer and standard deviation, respectively. The dotted line denotes the limit of confidence for the RVP assay (defined by the highest concentration of sera used in the assay); samples with titers <60 are reported at half the limit of detection (1:30).
Fig. S5. Magnitude of the neutralizing antibody response elicited in vaccinated nonhuman primates as a function of pre-immune titers. The NAb response in macaques elicited by vaccination with ZIKV DNA vaccine candidates was analyzed using ZIKV RVPs as described in Figure 2. The data presented represents the fold-change in the EC50 titer of sera collected at the indicated time post-vaccination as compared to the pre-immune titer of that same animal (Post-vaccination EC50/Pre-immune EC50). Lines represent individual animals and connect the fold-change values calculated from average EC50 NAb titers at each timepoint that are representative of 2–5 independent experiments, each performed with duplicate technical replicates. In each panel, the area under the curve for the line connecting group mean fold-change values is shaded gray. The dotted line denotes four standard deviations from pre-immune EC50 NAb titers. Note that the scales of the left-most and right-most panels have a smaller range than the middle three panels. Note that the scales of the top-most and bottom-most panels have a smaller range than the middle three panels. Presentation of the NAb data in this format emphasizes the sensitivity of the RVP neutralization assay, particularly in the one dose, 1 mg VRC5288 group. Animals in this particular dose group had undetectable NAb titers as measured by the MN assay (fig. S6 and table S1).
Fig. S6. Comparison of serum neutralization titers determined by three distinct assays. The neutralizing potency of nonhuman primate sera collected 6 weeks after vaccination was determined by three ZIKV neutralization assays: reporter virus particles (RVP), microneutralization (MN), or focus reduction neutralization test (FRNT). Sera from all 30 animals comprising all five vaccination groups were tested in the RVP and MN assays. A subset of monkeys, the 12 animals that received two doses of 4 mg VRC5283 or VRC5288, was assessed via FRNT. Neutralization titers for individual serum samples tested using the indicated assays are plotted on the x- and y-axis. Shown are comparisons of RVP EC50 versus MN EC50 (A), RVP EC90 versus MN EC50 (B), and RVP EC50 versus FRNT EC50 (C). RVP EC50 and EC90 values represent the average of 2–4 independent experiments performed with duplicate technical replicates, FRNT EC50 values represent the average of 1–4 independent experiments performed with duplicate technical replicates, and MN EC50 values represent a single experiment. Error bars reflect the standard deviation. The correlation between independent measurements was evaluated by Spearman’s correlation.
Fig. S7. Correlation of pre-challenge serum NAb activity with peak viremia. The level of peak viremia on day 3 is inversely related to the prechallenge serum NAb titer. This correlation remained significant when the day of viremia was varied, and when restricted to viremic animals (Spearman Rho= −0.856, p<0.0001). Viremic animals are shown as red dots, completely protected animals as grey dots and the breakthrough animal from the group that received 2×4mg of VRC5288 is outlined in black. Grey box indicates a NAb titer <1000 reciprocal EC50 serum dilution.
Fig. S8. Prior WNV infection does not protect against or enhance ZIKV infection. Retrospectively, it was determined that one animal in the mock-immunized control group (A8V016) and one animal in the single-dose 1 mg VRC5288 group (A5V033) with detectable ZIKV binding, but non-neutralizing antibody had pre-existing WNV-specific NAbs. WNV NY99 RVPs were mixed with serial dilutions of a potently neutralizing WNV mAb E16 (A), week 0 sera from macaque A8V016 (B), macaque A5V033 (C), and macaques A13V061, A13V190, and 21412 (D). Immune complexes were incubated for 1 h at 37°C prior to being added to Raji-DCSIGNR (top panels) or FcγR+ K562 (bottom panels) cells to detect neutralizing and enhancing activity, respectively. After 48 h, GFP-positive infected cells were quantitated by flow cytometry and the Raji-DCSIGNR results analyzed by non-linear regression. Error bars in A, B, and the top panels of C and D denote the range of duplicate technical replicates from a single assay. Data presented in the bottom panels of C and D was not performed in duplicate. Results are representative of 1–2 independent assays. The ability to both neutralize and enhance infection of WNV RVPs indicates prior WNV exposure in macaques A8V016 and A5V033. Negative results for the three macaques shown in panel (D) are representative of the remaining animals in the study. Viral loads of animals vaccinated with two 4 mg doses of VRC8400 (E) or one 1 mg dose of VRC5288 (F) on day 1–7 after challenge. Macaques A8V016 and A5V033 are shown in purple and orange, respectively, demonstrating no protection from or enhancement of ZIKV infection.
Table S1. Comparison of the EC50 neutralization titers of nonhuman primate sera collected 6-weeks post-vaccination determined by three distinct assays.