Abstract
We performed a comparative literature review, to elucidate the major features of the Takotsubo (stress) cardiomyopathy (TCM) collected in last 25 years.
TCM is characterized by left- or biventricular apical ballooning with a clinical presentation, electrocardiographic abnormalities, and biomarker profils similar to those seen in acute myocardial infarction. Epidemiological studies have shown that TCM is more common in postmenopausal women; however exact figures are not available. The underlying aetiology is still largely undetermined. Elevated catecholamine levels, lack of estrogen, disturbed myocardial fatty acid metabolism and plaque rupture with spontaneous thrombolysis are potentially discussed mechanisms responsible for inducing a prolonged stunned myocardium. Strong emotional or physical stress is the most frequently described trigger in the literature. Therapy recommendations include appropriate antiplatelet treatment, β-blockers and ACE inhibitors. The abnormal kinetics usually resolve or improve within a month and carry a favorable prognosis in most cases. However, all the suspected complications of an acute myocardial infarction, including cardiogenic shock or lethal arrhythmias, may still occur.
Keywords: Acute left ventricular apical ballooning, ampulla cardiomyopathy, broken heart syndrome, stress-related cardiomyopathy, takotsubo cardiomyopathy
COMPARISON WITH SURGERY
Transient acute left-ventricular apical ballooning in the absence of significant coronary artery lesions, also known as stress-induced cardiomyopathy, broken heart syndrome, or takotsubo cardiomyopathy (TCM), was first described by Hikaru Sato et al. in 1990 [1]. It was termed takotsubo by Sato (tako: octopus; tsubo: pot) due to the resemblance of the patient’s left ventricle to a wide-based and close-necked clay jar used by Japanese fishermen to catch octopus. Later, cases of TCM were also reported in the West. Epidemiological data from Europe, Australia and the United States confirmed that TCM was a globally occurring syndrome and not a geographically isolated phenomenon, as previously thought [2-5]. A few authors even believe that TCM is underrepresented in medical literature and that most cases are not diagnosed due to the lack of awareness of the disease. The purpose of this paper is to draw attention to the clinical picture of TCM, its characteristics, and to review related studies and relevant case reports. Finally, this review will also attempt to summarize the clinical management strategies of TCM.
DEFINITION
TCM is characterized by an acute ventricular apical ballooning, which has not yet been fully elucidated [6]. The observed kinetic dysfunction mainly affects the left ventricle or in some cases the right ventricle as well [6, 7]. Signs of dynamic obstruction of the left ventricular outflow tract can be observed, including pressure gradient, acceleration of blood flow and systolic cardiac murmurs [8-10]. As a rule, there is nearly a complete restoration of the contraction abnormality in the majority of cases within four weeks [11-13]. If the apical systolic ballooning is secondary to a known cause, such as cerebrovascular disease or phaeochromocytoma, the condition is named takotsubo-like myocardial dysfunction, which should be differentiated from idiopathic TCM [6].
EPIDEMIOLOGY
TCM mostly affects postmenopausal women. The rate of incidence in Japan has been estimated at 1-2% of all patients hospitalized for suspected ST segment elevation myocardial infarction (STEMI) [13, 14]. In a recently published study by the members of the International Takotsubo Registry, Templin et al. presented data from 1750 patients with TCM. From these, almost 89.8% of all examined patients were women (mean age 66.8 years) [15]. The retrospective study by Hertting et al. examined almost 17000 cases, which underwent diagnostic coronary angiography and exhibited left ventricular apical ballooning in the presence of normal coronary arteries [2]. Thirty-two patients fulfilled the diagnostic criteria of TCM (0.2% incidence). Most of them were women (>90%) with a median age of 67 years. Park et al. carried out a prospective echocardiographic study of stress-induced cardiomyopathy in 92 intensive-care patients hospitalized due to a non-cardiac disease and without any previous cardiovascular history [12]. Twenty-six patients (28%) were found to have left ventricular apical ballooning. Wittstein et al. described a severe but reversible left ventricular dysfunction in 19 patients without coronary pathology [11]. Of these, 95% were women and the median age was 63 years. Since TCM was diagnosed retrospectively in most studies [2-3], it is assumed that the rate of incidence is underestimated due to lack of awareness of the disease. As mentioned above, our group could confirm in a recently published multicentre and prospective study in over 1750 TCM patients that TCM is a disorder that occurs world wide, and that the increasing number of reports and international studies contributes to the heightened awareness of this pathological phenomenon [15].
AETIOLOGY AND PATHOGENESIS
In TCM, there is an expanded reversible myocardial stunning [9, 16, 17], whose origin has not been fully elucidated. Frequently described triggers include sudden strong emotional or physical stress, as for instance exhibited during a car crash, unexpected reunion, death or loss of a close relative or friend, armed robbery, fear of medical interventions, public speaking and appearance before court [15, 18-20]. In addition there is evidence that the incidence of TCM rises in association with large scale disasters (e.g. earthquakes), as is the case for acute coronary syndroms and sudden cardiac death [21]. Tsuchihashi et al. found that some serious diseases, such as an epileptic seizure, a chronic obstructive pulmonary disease exacerbation or an asthma attack usually precede the manifestation of TCM [13]. The study by Hertting et al. comprising of 32 TCM patients, showed that almost every other patient had chronic obstructive pulmonary disease (COPD) or bronchial asthma [2]. This leads to the assumption that COPD and bronchial asthma might increase the risk of TCM. In this context, the group by Pelliccia and colleagues recently reviewed relevant predisposing factors in 1109 patients with documented TCM that may result in the onset of TCM [22]. The most common comorbidities which they reported were psychological disorders (24%; range, 0-49%), pulmonary diseases (15%; range, 0-22%), and malignancies (10%; range, 4%-29%).
Nef et al. examined myocardial biopsies taken during the acute phase of left ventricular impairment as well as probes taken after functional recovery [26]. They found typical signs of catecholamine toxicity: areas of fibrotic response with widened interstitial space containing formation of cellular debris and contraction bands of sarcomeres. Furthermore electron microscopy revealed numerous vacuoles of different sizes and contents and loss of contractile material. Periodic acid-Schiff's reagent (PAS) staining showed remarkable intracellular accumulation of glycogen. Signs of oncotic and apoptotic cell death were absent. Probes taken after functional recovery showed nearly complete reversibility (Fig. 1).
Fig. (1).
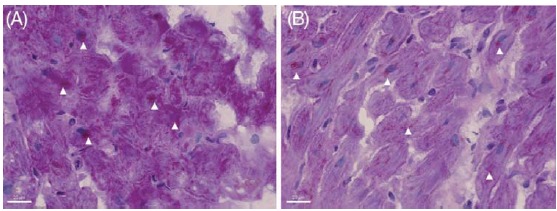
Periodic acid-Schiff's reagent staining (arrows) shows remarkable intracellular accumulation of glycogen in TCM (A). After functional recovery only small amounts of glycogen, particularly around the nuclei of myocytes (arrows), were documented (B). Scale bar indicates 20µm. Nef et al. with permission [26].
Ueyama et al. was able to induce acute left-ventricular ballooning in rats under immobilization stress and reversed it by a combined blockage of α- and β-adrenoreceptors [27]. This was the reason to assume that myocardial stunning, and therefore TCM, can be induced by stimulation of cardiac adrenoreceptors. Moreover, myocardial scintigraphy with 123I metaiodobenzylguanidine in TCM patients showed a significant sympathetic cardiac dysfunction, which is a very likely explanation of the neurogenic myocardial stunning [16].
Other authors presume that the catecholamine-induced hyperkinesia of the basal segments can cause a dynamic obstruction of the left-ventricular outflow tract. This, in turn, is the cause of apical ballooning. The result is higher apical and anterior wall tension. Due to the increased need for oxygen, lower systemic blood pressure and the related reduced coronary perfusion, myocardial ischemia (stunning) can lead to regional disorders of wall motion with corresponding changes in the ST-intervals on the surface ECG [9]. A counter-argument is the lack of echocardiographic or angiographic documentation of an intraventricular gradient in most patients with TCM. However, the time interval between the onset of symptoms and the imaging of the intraventricular gradients differed substantially between cases [9, 10, 28-37].
Impaired myocardial fatty acid: Using myocardial single-photon emission computed tomography (SPECT) with thallium201 and 123I-BMIPP (iodine-123-beta-methyl-p-iodophenyl penta-decanoic acid), Kurisu et al. found a serious prolonged defect in the myocardial fatty acid metabolism in 14 patients with TCM, especially during the earlier stages [17]. They assumed that a disorder of the fatty acid metabolism could lead to the stunned myocardium and, thus to left-ventricular dysfunction.
Reduction of oestrogen levels: In an animal model, it was shown that the extent of stress-induced ventricular dysfunction and tachycardia in female rats following ovarectomy could be reduced significantly by application of oestradiol [38]. This was a reason to assume that the lower oestrogen levels may be crucial for the higher incidence of TCM in postmenopausal women.
Ruptured coronary plaque: Ibanez et al. prospectively examined in five TCM patients the hypothesis that a ruptured coronary plaque with angiographically insignificant coronary atherosclerosis and normal coronary flow could be part of the underlying aetiology [39]. They found a dominant left anterior descending artery (LAD) with long diaphragmal extension in all patients. Intravascular ultrasound examination (IVUS) showed solitary ruptured non-occlusive atherosclerotic plaques in the central section of the LAD in all patients. These findings support the theory that an acute coronary syndrome (ACS) with spontaneous thrombolysis could be the cause of TCM in some patients. The reason for this is assumed to be extended myocardial stunning in the circulation area of the dominant LAD.
It is known that ACS is not only caused by critical stenosis of the coronary arteries but also by a plaque rupture following cap fracture or superficial intimal erosion at a non-critical stenosis of the coronary arteries, which is not detected by angiography as the acute thrombus is dissolved [40, 41].
The hypothesis suggested by Ibanez et al. could however, not be proved by later performed studies [39]. In a series of 10 TCM patients in whom IVUS was conducted, Haghi et al. found no evidence of plaque rupture [42]. Another study including 11 patients, who underwent coronary angiography and IVUS could not identify a culprit lesion. Furthermore, the epicardial course of the LAD did not match the extent of the left ventricular apical ballooning [43].
AUTONOMIC NERVOUS SYSTEM IN THE ELDERLY
Since the heart is comprehensively innervated by neural structures, including sympathetic and parasympathetic input, the influence of the local cardiac autonomic nervous system has to be considered in particular to draw a complete picture regarding cardiovascular events. The knowledge that heart muscle cells age dependently control the autonomic nervous system due to autocrine / paracrine mechanisms including the expression and secretion of acetylcholine and nerve growth factor, leading to a sympathetic hyperinnervation in the elderly, underlines this assumption [44, 45]. In regard to this, further attention has to be given to the fact, that the occurrence of TCM is not only gender dependent in most cases, TCM occurs predominantly in the elderly. Cases of younger patients are relative rare in the literature.
Myocarditis hypothesis: Myocarditis with different afflictions of various myocardial regions has also been discussed. This hypothesis is rather unlikely considering the unspecific biopsy findings and the absence of prior infections and inflammation parameters in most cases described [8, 20, 46]. Furthermore, the presence of myocarditis excludes TCM according to recent guidelines for diagnosis of TCM recommended by the Research Committee of Idiopathic Cardiomyopathy. This is the reason why Myocarditis was not included in Box 1 as a presumed pathogenesis of TCM.
CLINICAL FEATURES
The typical clinical picture of TCM reflects angina pectoris with dyspnea (Box 2). In the acute phase, pulmonary oedema, respiratory failure, cardiogenic shock and arrhythmias, such as atrial fibrillation with fast ventricular response, ventricular tachycardia or ventricular fibrillation, can occur (Box 2) [46-51]. A lethal ventricular rupture was also reported in association with TCM [28]. Moreover, an acute left ventricular mural thrombus can develop, which in turn, can cause thromboembolic complications, such as renal infarction or acute cerebral ischemia [51-53].
Typically, symptoms occur a few hours after strong emotional or physical stress [12-14, 18]. In this context, a higher incidence of TCM has been observed in patients with an asthma attack or exacerbation of chronic obstructive pulmonary disease [2,13]. TCM has also been reported in association with other diseases, such as epilepsy, urosepsis, pneumonia and intensive care diseases [12, 13].
Regarding the latter, Park et al. showed that intensive care patients with left ventricular apical ballooning (LVAB) more frequently have sepsis (62% vs. 14%, P<0.001) and hypotension, a lower 2-month survival rate and a higher consumption of inotropic substances in comparison to patients without LVAB [12].
In addition, to the knowledge that some infectious diseases like pneumonia or urosepsis can affect the heart and its functions crucially, evidence is present that some central nervous system disorders (e.g. epilepsy, intracranial haemorrhage or head trauma) may also impair cardiac functions due to numerous anatomical and physiological links between the brain and the heart, the so called brain-heart axis. This specific interaction has been thoroughly reviewed by Finsterer and colleagues [54].
ELECTROCARDIOGRAPHIC FINDINGS
Mostly, the initial phase of acute ST elevation and its return to normalcy is followed by progressive T wave inversion with a maximum negative peak after three days. After that, the T waves flatten out and remain isoelectric for a few days. Two to three weeks later, deep negative T waves form again. The corrected Q-T interval becomes prolonged during the first negative phase of T waves and shorter as the T waves become shallower. Occasionally, the ECG changes are confined to ST depression and the development of Q waves [36, 46]. Apart from that, the time course of the ECG changes in cases of TCM (Fig. 2) is similar to that seen in patients with STEMI and early revascularisation with percutaneous coronary intervention (PCI) [6, 47].
Fig. (2).
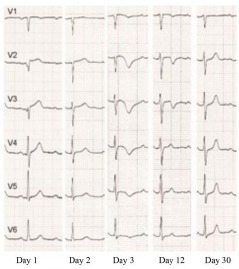
Electrocardiographic changes in the leads V1-V6 in a TCM patient from day 1 after the onset of symptoms.
LABORATORY FINDINGS
The troponin and creatine kinase (CK, CK MB) serum levels might be normal or disproportionately low compared to the extent of myocardial akinesia [16, 20]. The rise of Troponin can be missing in up to one third of the cases [13]. In analogy to cardiac insufficiency, the plasma brain natriuretic peptide (BNP) can be elevated during the acute phase [16]. D-dimer (cleavage products of fibrinogen) can also be elevated. If clinical signs of deep venous thrombosis or pulmonary embolism are present, further diagnostic tests should be undertaken to rule them out. The reason for elevated D-dimer levels is assumed to be intravascular coagulation with secondary hyperfibrinolysis owing to plaque rupture with spontaneous lysis [20, 50].
DIAGNOSIS
Bybee et al. proposed four criteria to establish the diagnosis of TCM [30], which were recently recommended by The Research Committee of Idiopathic Cardiomyopathy to be integrated into the guidelines for the diagnosis of TCM (Box 3) [6].
So far, the gold standard to distinguish TCM from STEMI or ACS is coronary angiography with ventriculography. In cases of TCM, an urgent coronary angiogram is usually performed. It can help to rule out acute coronary occlusion or relevant stenosis of coronary arteries (Fig. 3).
Fig. (3).
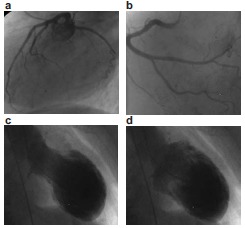
Typical angio- and ventriculogram during takotsubo cardiomyopathy. Top: Angiogram without relevant coronary stenosis, a) left coronary artery, b) right coronary artery. Bottom: Left ventricle with apical ballooning, c) systole, d) diastole.
Ventriculography demonstrates apical ballooning with hyperkinetic basal segments (Fig. 3). The contraction abnormality does not match to the distribution of a single coronary circulation area. Thereby, a pressure gradient can be occasionally measured in the left ventricle [31-33, 37].
Recently, a new variant of TCM with apical sparing in 14 patients has been reported [55]. Clinical presentation and time course were similar to the classical form of TCM. Both, ventriculography and echocardiogram showed an apical ballooning with hypercontraction of the basal segments during the acute phase [7]. In some cases, a left ventricular gradient with a higher velocity in the basal segments can be measured [6, 9, 10]. Furthermore, not only left ventricular but also biventricular infestation has been reported [7]. The left ventricular ejection fraction (LVEF) varied between 20% and 40%. The left ventricular dysfunction usually resolves within two to five weeks after onset. Residual contraction reductions can persist in some patients [3, 11-13].
In addition to the histological findings described by Nef et al. [26], further unspecific findings of myocardial biopsy in TCM patients were reported, such as slight interstitial lymphoid infiltration, slight interstitial fibrosis, mild myocardial atrophy and a small number of mononuclear cells [7, 8, 11, 46, 48]. In our view, myocardial biopsy in TCM patients can only be considered if there is a good reason to suspect concomitant myocarditis. In a typical presentation of TCM, myocardial biopsy is not required to confirm the diagnosis or to suggest a treatment tactic.
THERAPY
There are still no official guidelines for the treatment of TCM. The recommended management is based on the outcomes of case reports and published studies (Box 4). There is no evidence to prove the efficacy of the following recommended therapies from randomized control trials.
All patients presenting chest pain and/or ST elevation, new symptoms of ischemia or elevated troponin levels should receive the common therapy for acute myocardial infarction. Urgent coronary angio- and ventriculography are the examinations of choice. These recommended approaches might confirm the diagnosis, avoid fibrinolysis, and also enable revascularisation in cases of acute coronary occlusion. If an angiogram is not available, fibrinolytic therapy should not be withheld if indicated, because most patients with chest pain and ST elevation in the anterior leads suffer from an acute occlusion of the LAD. Furthermore, neither the echocardiographic nor the ECG changes or even the clinical picture can accurately differentiate between TCM and STEMI.
Adequate platelet inhibition with aspirin or clopidogrel, depending on the clinical context, is recommended [10, 13, 20]. In patients with left ventricular mural thrombus or with distinct akinesia, a short-term therapeutic anticoagulation treatment with heparin or oral vitamin-K antagonists is indicated until the left ventricular dysfunction improves or the thrombus resolves [20, 52, 53]. Beta-blockers should be administered during the acute phase. The LV dysfunction warrants the application of ACE inhibitors or angiotensin receptor blockers [13, 20]. In the acute phase and in NYHA III to IV, aldosterone antagonists should be considered. By inhibiting the pro-inflammatory action of aldosterone on the myocardium, remodelling during the acute phase may be antagonized. Given the fact that TCM can reoccur, permanent therapy with aggregation inhibitors and β-blockers should be considered [20, 55].
β-agonists or inotropic substances should be avoided because of suspected sympathetic hyperactivity. There is no evidence for the last two recommendations. Nitrates are contraindicated if an intraventricular gradient is present, because nitrates can increase the gradient, which in turn impairs apical ballooning and leads to further haemodynamic aggravation.
Particular attention should also be given to the Q-T interval during the acute phase, since drugs such as amiodarone or sotalol can trigger torsade-de-pointes if depolarisation is extended further [13]. Intra-aortic balloon counterpulsation (IABP) may be beneficial during cardiogenic shock [20].
There is no recommendation in the literature available, related to the indications for implantable cardioverter defibrillator (ICD) as secondary prophylaxis during the acute phase of TCM or after ventricular fibrillation. In these cases, the indication for ICD-implantation should be made on basis of an individual assessment under the consideration of TCM-related reversible arrhythmogenic ECG changes. Under these conditions, the life jacket defibrillator, if required, can be provided to the patient in order to prevent the possibility of death due to malignant ventricular arrhythmias in the acute phase of the disease.
PROGNOSIS
Even if the prognosis of TCM patients is believed to be more favourable than STEMI patients, all the suspected complications of an acute myocardial infarction, including cardiogenic shock or ventricular fibrillation, can still occur [6]. With regards to the mortality rate of TCM, there is still a lack of adequate prospective studies. The deaths that have been reported in small-scale studies suggest that the mortality rate in TCM patients is clearly lower than that of patients after acute myocardial infarction. Two Japanese studies reported mortality rates of 1 in 25 (4%), and 2 in 88 (2.27%) patients [13, 14]. A French study reported one death among 10 TCM patients [56]. On the other hand, there was no reported mortality in 2 instances, an American study with a total of 19 patients [38] and a German one with a total of 6 patients [3].
SUMMARY
Since the first description of TCM in 1990 by Sato and colleagues, the awareness of this disorder rises constantly.
TCM mostly affects postmenopausal women and often occurs after strong emotional or physical stress. TCM should be considered as a differential diagnosis in patients with suspected STEMI and left ventricular dyskinesia without any concomitant epicardial coronary artery lesions. The typical clinical picture of TCM consists of angina pectoris, dyspnea and ECG changes mainly in the precordial leads V2-V6.
So far, the only reliable diagnostic method to distinguish TCM from STEMI is coronary angiography with ventriculography.
Platelet inhibition, β-blockers and ACE inhibitors are the main pillars of treatment. In patients with distinct akinesia, a short course of anticoagulation with heparin or oral vitamin-K antagonist is indicated in order to prevent formation of left ventricular thrombi. Drugs that may cause prolongation of the QT interval should be avoided, especially in the acute phase.
Although contraction abnormalities completely recover with supportive therapy, all suspected complications seen in acute myocardial infarction, including cardiogenic shock or lethal arrhythmias can occur in TCM patients.
ACKNOWLEDGEMENT
Declared none.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Sato H., Taiteishi H., Uchida T. Takotsubo-type cardiomyopathy due to multivessel spasm: Clinical aspect of myocardial injury: From ischemia to heart failure. Kagakuhyouronsha Tokyo. 1990;2:56–64. [Google Scholar]
- 2.Hertting K., Krause K., Härle T., et al. Transient left ventricular apical ballooning in a community hospital in Germany. Int. J. Cardiol. 2006;112:282–288. doi: 10.1016/j.ijcard.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Kleinfeldt T., Drawert S., Schneider H., et al. The “broken heart” syndrome--a rare clinical phenomenon. Dtsch. Med. Wochenschr. 2007;132:87–90. doi: 10.1055/s-2007-959293. [DOI] [PubMed] [Google Scholar]
- 4.Kurisu S., Inoue I., Kawagoe T., et al. Left ventricular apical thrombus formation in a patient with suspected tako-tsubo-like left ventricular dysfunction. Circ. J. 2003;67:556–558. doi: 10.1253/circj.67.556. [DOI] [PubMed] [Google Scholar]
- 5.Seth P.S., Aurigemma G.P., Krasnow J.M., et al. A syndrome of transient left ventricular apical wall motion abnormality in the absence of coronary disease: a perspective from the United States. Cardiology. 2003;100:61–66. doi: 10.1159/000073040. [DOI] [PubMed] [Google Scholar]
- 6.Kawai S., Kitabatake A., Tomoike H., et al. Takotsubo Cardiomyopathy Group Guidelines for Diagnosis of Takotsubo (Ampulla) Cardiomyopathy. Circ. J. 2007;71:990–992. doi: 10.1253/circj.71.990. [DOI] [PubMed] [Google Scholar]
- 7.Nyui N., Yamanaka O., Nakayama R., et al. “Tako-Tsubo” transient ventricular dysfunction: a case report. Jpn. Circ. J. 2000;64(9):715–719. doi: 10.1253/jcj.64.715. [DOI] [PubMed] [Google Scholar]
- 8.Abe Y., Kondo M., Matsuoka R., et al. Assessment of clinical features in transient left ventricular apical ballooning. J. Am. Coll. Cardiol. 2003;41:737–742. doi: 10.1016/s0735-1097(02)02925-x. [DOI] [PubMed] [Google Scholar]
- 9.Barriales Villa R., Bilbao Quesada R., Iglesias Río E., et al. Transient left ventricular apical ballooning without coronary stenoses syndrome: importance of the intraventricular pressure gradient. Rev. Esp. Cardiol. 2004;57:85–88. [PubMed] [Google Scholar]
- 10.Villareal R.P., Achari A., Wilansky S., et al. Anteroapical stunning and left ventricular outflow tract obstruction. Mayo Clin. Proc. 2001;76:79–83. doi: 10.4065/76.1.79. [DOI] [PubMed] [Google Scholar]
- 11.Wittstein I.S., Thiemann D.R., Lima J.A., et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N. Engl. J. Med. 2005;352:539–548. doi: 10.1056/NEJMoa043046. [DOI] [PubMed] [Google Scholar]
- 12.Park J.H., Kang S.J., Song J.K., et al. Left ventricular apical ballooning due to severe physical stress in patients admitted to the medical ICU. Chest. 2005;28:296–302. doi: 10.1378/chest.128.1.296. [DOI] [PubMed] [Google Scholar]
- 13.Tsuchihashi K., Ueshima K., Uchida T., et al. Transient left ventricular apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction. Angina Pectoris-Myocardial Infarction Investigations in Japan. J. Am. Coll. Cardiol. 2001;38:11–18. doi: 10.1016/s0735-1097(01)01316-x. [DOI] [PubMed] [Google Scholar]
- 14.Kawai S. Ampulla-shaped ventricular dysfunction or ampulla cardiomyopathy? Kokyu To Junkan. 2000;48:1237–1248. [Google Scholar]
- 15.Templin C., Ghadri J.R., Diekmann J., et al. Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. N. Engl. J. Med. 2015;373:929–938. doi: 10.1056/NEJMoa1406761. [DOI] [PubMed] [Google Scholar]
- 16.Akashi Y.J., Nakazawa K., Sakakibara M., et al. 123I-MIBG myocardial scintigraphy in patients with “takotsubo” cardiomyopathy. J. Nucl. Med. 2004;45:1121–1127. [PubMed] [Google Scholar]
- 17.Kurisu S., Inoue I., Kawagoe T., et al. Myocardial perfusion and fatty acid metabolism in patients with tako-tsubo-like left ventricular dysfunction. J. Am. Coll. Cardiol. 2003;41:743–748. doi: 10.1016/s0735-1097(02)02924-8. [DOI] [PubMed] [Google Scholar]
- 18.Ueyama T., Kasamatsu K., Hano T., et al. Emotional stress induces transient left ventricular hypocontraction in the rat via activation of cardiac adrenoceptors: a possible animal model of “tako-tsubo” cardiomyopathy. Circ. J. 2002;66:712–713. doi: 10.1253/circj.66.712. [DOI] [PubMed] [Google Scholar]
- 19.Owa M., Aizawa K., Urasawa N., et al. Emotional stress-induced “ampulla cardiomyopathy”: discrepancy between the metabolic and sympathetic innervation imaging performed during the recovery course. Jpn. Circ. J. 2001;65:349–352. doi: 10.1253/jcj.65.349. [DOI] [PubMed] [Google Scholar]
- 20.Said S.M., Buerke M., Schmidt H. Stress-related Cardiomyopathy: What do we know about tako-tsubo syndrome? Intensiv- Notf. behandl. 2007;32:167–176. [Google Scholar]
- 21.Sato M., Fujita S., Saito A., et al. Increased incidence of transient left ventricular apical ballooning (socalled “Takotsubo” cardiomyopathy) after the mid-Niigata Prefecture earthquake. Circ. J. 2006;70(8):947–953. doi: 10.1253/circj.70.947. [DOI] [PubMed] [Google Scholar]
- 22.Pelliccia F., Parodi G., Greco C., et al. Comorbidities Frequency in Takotsubo Syndrome: An International Collaborative Systematic Review Including 1109 Patients. Am. J. Med. 2015;128:11–19. doi: 10.1016/j.amjmed.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 23.Spinelli L., Trimarco V., Di Marino S., et al. L41Q polymorphism of the G protein coupled receptor kinase 5 is associated with left ventricular apical ballooning syndrome. Eur. J. Heart Fail. 2010;12(1):13–16. doi: 10.1093/eurjhf/hfp173. [DOI] [PubMed] [Google Scholar]
- 24.Santulli G., Campanile A., Spinelli L., et al. G protein-coupled receptor kinase 2 in patients with acute myocardial infarction. Am. J. Cardiol. 2011;107(8):1125–1130. doi: 10.1016/j.amjcard.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Santulli G. Adrenal signaling in heart failure: something more than a distant ship's smoke on the horizon. Hypertension. 2014;63(2):215–216. doi: 10.1161/HYPERTENSIONAHA.113.02382. [DOI] [PubMed] [Google Scholar]
- 26.Nef H.M., Möllmann H., Kostin S., et al. Tako-Tsubo cardiomyopathy: intraindividual structural analysis in the acute phase and after functional recovery. Eur. Heart J. 2007;28:2456–2464. doi: 10.1093/eurheartj/ehl570. [DOI] [PubMed] [Google Scholar]
- 27.Ueyama T. Emotional stress-induced Tako-tsubo cardiomyopathy: animal model and molecular mechanism. Ann. N. Y. Acad. Sci. 2004;1018:437–444. doi: 10.1196/annals.1296.054. [DOI] [PubMed] [Google Scholar]
- 28.Akashi Y.J., Tejima T., Sakurada H., et al. Left ventricular rupture associated with Takotsubo cardiomyopathy. Mayo Clin. Proc. 2004;79:821–824. doi: 10.4065/79.6.821. [DOI] [PubMed] [Google Scholar]
- 29.Barrera-Ramirez C.F., Jimenez-Mazuecos J.M., Alfonso F. Apical thrombus associated with left ventricular apical ballooning. Heart. 2003;89:927. doi: 10.1136/heart.89.8.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bybee K.A., Prasad A., Barsness G.W., et al. Clinical characteristics and thrombolysis in myocardial infarction frame counts in women with transient left ventricular apical ballooning syndrome. Am. J. Cardiol. 2004;94:343–346. doi: 10.1016/j.amjcard.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 31.Kyuma M., Tsuchihashi K., Shinshi Y., et al. Effect of intravenous propranolol on left ventricular apical ballooning without coronary artery stenosis (ampulla cardiomyopathy): three cases. Circ. J. 2002;66:1181–1184. doi: 10.1253/circj.66.1181. [DOI] [PubMed] [Google Scholar]
- 32.Matsuoka H., Kawakami H., Koyama Y., et al. “Takotsubo” cardiomyopathy with a significant pressure gradient in the left ventricle. Heart Vessels. 2000;15:203. doi: 10.1007/s003800070024. [DOI] [PubMed] [Google Scholar]
- 33.Matsuoka K., Nakayama S., Okubo S., et al. Transient cerebral ischemic attack induced by transient left ventricular apical ballooning. Eur. J. Intern. Med. 2004;15:393–395. doi: 10.1016/j.ejim.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 34.Matsuoka K., Okubo S., Fujii E., et al. Evaluation of the arrhythmogenecity of stress-induced “Takotsubo cardiomyopathy” from the time course of the 12-lead surface electrocardiogram. Am. J. Cardiol. 2003;92:230–233. doi: 10.1016/s0002-9149(03)00547-2. [DOI] [PubMed] [Google Scholar]
- 35.Peraira Moral J.R., Segovia Cubero J., Oteo Domínguez J.F., et al. A case of transient left ventricular apical ballooning with an unusual complication. Rev. Esp. Cardiol. 2002;55:1328–1332. [PubMed] [Google Scholar]
- 36.Yamasa T., Ikeda S., Ninomiya A., et al. Characteristic clinical findings of reversible left ventricular dysfunction. Intern. Med. 2002;41:789–792. doi: 10.2169/internalmedicine.41.789. [DOI] [PubMed] [Google Scholar]
- 37.Ueyama T., Senba E., Kasamatsu K., et al. Molecular mechanism of emotional stress-induced and catecholamine-induced heart attack. J. Cardiovasc. Pharmacol. 2003;41(Suppl. 1):S115–S118. [PubMed] [Google Scholar]
- 38.Ueyama T., Hano T., Kasamatsu K., et al. Estrogen attenuates the emotional stress-induced cardiac responses in the animal model of Tako-tsubo (Ampulla) cardiomyopathy. J. Cardiovasc. Pharmacol. 2003;42(Suppl. 1):S117–S119. doi: 10.1097/00005344-200312001-00024. [DOI] [PubMed] [Google Scholar]
- 39.Ibanez B., Navarro F., Cordoba M., et al. Tako-tsubo transient left ventricular apical ballooning: is intravascular ultrasound the key to resolve the enigma? Heart. 2005;91:102–104. doi: 10.1136/hrt.2004.035709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Libby P. Act local, act global: inflammation and the multiplicity of “vulnerable” coronary plaques. J. Am. Coll. Cardiol. 2005;45:1600–1602. doi: 10.1016/j.jacc.2005.02.058. [DOI] [PubMed] [Google Scholar]
- 41.Nissen S.E. Pathobiology, not angiography, should guide management in acute coronary syndrome/non-ST-segment elevation myocardial infarction: the non-interventionist’s perspective. J. Am. Coll. Cardiol. 2003;41(4) Suppl. S:103S–112S. doi: 10.1016/s0735-1097(02)02775-4. [DOI] [PubMed] [Google Scholar]
- 42.Haghi D., Roehm S., Hamm K., et al. Takotsubo cardiomyopathy is not due to plaque rupture: an intravascular ultrasound study. Clin. Cardiol. 2010;33:307–310. doi: 10.1002/clc.20747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delgado G.A., Truesdell A.G., Kirchner R.M., et al. An angiographic and intravascular ultrasound study of the left anterior descending coronary artery in takotsubo cardiomyopathy. Am. J. Cardiol. 2011;108:888–891. doi: 10.1016/j.amjcard.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 44.Saygili E., Kluttig R., Rana O.R., et al. Age-related regional differences in cardiac nerve growth factor expression. Age (Dordr.) 2012;34:659–667. doi: 10.1007/s11357-011-9262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rana O.R., Schauerte P., Kluttig R., et al. Acetylcholine as an age-dependent non-neuronal source in the heart. Auton. Neurosci. 2010;156:82–89. doi: 10.1016/j.autneu.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 46.Akashi Y.J., Nakazawa K., Sakakibara M., et al. The clinical features of takotsubo cardiomyopathy. QJM. 2003;96:563–573. doi: 10.1093/qjmed/hcg096. [DOI] [PubMed] [Google Scholar]
- 47.Kurisu S., Inoue I., Kawagoe T., et al. Time course of electrocardiographic changes in patients with tako-tsubo syndrome: comparison with acute myocardial infarction with minimal enzymatic release. Circ. J. 2004;68:77–81. doi: 10.1253/circj.68.77. [DOI] [PubMed] [Google Scholar]
- 48.Kurisu S., Sato H., Kawagoe T., et al. Tako-tsubo-like left ventricular dysfunction with ST-segment elevation: a novel cardiac syndrome mimicking acute myocardial infarction. Am. Heart J. 2002;143:448–455. doi: 10.1067/mhj.2002.120403. [DOI] [PubMed] [Google Scholar]
- 49.Said S.M., Hahn J., Schmeisser A., et al. Takotsubo cardiomyopathy: diagnosis and therapy. Kardiologe. 2009;3:211–219. [Google Scholar]
- 50.Said S.M., Albouaini K., Herold J., et al. Takotsubo syndrome from original description up to now. Med. Klin. 2009;104:434–440. doi: 10.1007/s00063-009-1092-9. [DOI] [PubMed] [Google Scholar]
- 51.Schneider B., Stein I. Tako-tsubo-like transient left ventricular dysfunction: Prevalence and clinical findings in a western population. Circulation. 2004;110(Suppl. III):III-697. [Google Scholar]
- 52.Sasaki N., Kinugawa T., Yamawaki M., et al. Transient left ventricular apical ballooning in a patient with bicuspid aortic valve created a left ventricular thrombus leading to acute renal infarction. Circ. J. 2004;68:1081–1083. doi: 10.1253/circj.68.1081. [DOI] [PubMed] [Google Scholar]
- 53.Tibrewala A.V., Moss B.N., Cooper H.A. A rare case of tako-tsubo cardiomyopathy complicated by a left ventricular thrombus. South. Med. J. 2006;99:70–73. doi: 10.1097/01.smj.0000197509.72076.21. [DOI] [PubMed] [Google Scholar]
- 54.Finsterer J., Wahbi K. CNS-disease affecting the heart: Brain–heart disorders. J. Neurol. Sci. 2014;345:8–14. doi: 10.1016/j.jns.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 55.Abdulla I., Kay S., Mussap C., et al. Apical sparing in tako-tsubo cardiomyopathy. Intern. Med. J. 2006;36:414–418. doi: 10.1111/j.1445-5994.2006.01095.x. [DOI] [PubMed] [Google Scholar]
- 56.Lipiecki J., Durel N., Decalf V., et al. Transient left ventricular apical ballooning or the tako-tsubo syndrome. Arch. Mal. Coeur Vaiss. 2005;98:275–280. [PubMed] [Google Scholar]


