Abstract
In vivo maturation (IVM) of human oocytes is a technique used to increase the number of usable oocytes for in vitro fertilization (IVF) and represents a necessity for women with different ovarian pathologies. During IVM the oocytes progress from the germinal vesicle stage (GV) through the metaphase II and during this journey both nuclear and cytoplasmic rearrangements must be obtained to increase the probability to get viable and healthy zygotes/embryos after IVF. As the successful clinical outcomes of this technique are a reality, we wanted to investigate the causes behind oocytes maturation arrest. For obvious ethical reasons, we were able to analyze only few human immature oocytes discarded and donated to research by transmission electron microscopy showing that, as in the mouse, they have different chromatin and cytoplasmic organizations both essential for further embryo development.
Key words: Human oocytes, human antral compartment, lipid droplets, SN oocytes, NSN oocytes, cytoplasmic lattices, transmission electron microscopy
Introduction
Oocyte quality is an essential parameter driving embryonic development through resumption and meiotic maturation. This process leads to the ability to be fertilized and to orchestrate a successful maternal-to-zygote transition, preceding to the embryo implantation. Oocyte quality encompasses several functional activities (DNA transcription and protein translation, metabolism, etc.) and cellular (cytoplasmic) organizations, many of which are still under investigations.
Most of the embryonic developmental success, both in vivo and in vitro, relies on the quality of the starting biological reagent: the oocyte and, in particular, the immature germinal vesicle (GV) oocyte. Most mammals (with some exceptions like cats)1 have two main kinds of GV oocytes residing in the ovarian antral compartment: the largest group named ‘surrounded nucleolus’ (SN; nearly 70-80%) represented by oocytes that likely naturally ovulate, is able to sustain the full embryo development after maturation and fertilization both in vivo and in vitro, while the smallest group named ‘not surrounded nucleolus’ (NSN; the remaining 20-30%), arrests the development at the 2/4-cell stage, if in vitro matured and fertilized.2
To date, the only way to distinguish between the NSN and SN type oocyte relies on the different patterns of Hoechst33342 positive heterochromatin staining around their nucleolus, hence their name.3 This invasive staining cannot be, for obvious ethical and safety reasons, used by operators of assisted reproduction techniques (ART); so it becomes important to find a non-invasive way to distinguish among SN and NSN oocytes whenever in vitro maturation (IVM) is the only approach to increase the number of usable oocytes. Patients at risk for ovarian hyperstimulation syndrome, polycystic ovarian syndrome, with estrogen sensitive cancer or with limited time for ovarian hyperstimulation are good candidates for IVM4,5 and, although the implantation and pregnancy rates are less than the conventional in vitro fertilization (IVF), the possibility of selecting only the oocytes with a SN phenotype would increase its success by 30%.
Recently, we showed that the developmental arrest of the NSN oocyte-derived embryos is due to the reduced expression of MATER and ribosomal proteins, strictly associated with lack of cytoplasmic lattices (CPLs).6 Based on these premises, we wanted to investigate the absence/presence of CPLs in the cytoplasm of human SN and NSN oocytes, because the identification of morphological markers (ideally with noninvasive techniques) defining each of the many developmental steps driving the oocyte-egg transition will greatly further our understandings of the whole process of oogenesis and beyond. Also, this will certainly raise the ability of biologists, physicians and veterinarians to choose the ‘correct’ oocyte, able to become a ‘good’ egg and a ‘good’ embryo, nowadays intrinsically tied to the operator’s decision although supported, for example, by observations obtained with a minimal invasive mechanical measurement at the zygote stage.7
Materials and Methods
Source of human oocytes
Discarded, immature human GV oocytes we obtained from consenting patients going through IVF with intra-cytoplasmic sperm injection (ICSI) at the Fertility and Reproductive Health Clinic at Stanford Medicine. Only mature metaphase II (MII) oocytes are injected during the ICSI procedure. Although most oocytes retrieved after standard gonadotropin induced superovulation are mature, it is not uncommon to have some oocytes remaining at the GV stage. De-identification of samples was performed according to the Stanford University Institutional Review Board approved protocol #10466 entitled ‘The RENEW Biobank’ and #13984 entitled ‘The Use of Nonviable, Abnormal, or Unusable Human Oocytes or Preembryos for Technique Development, Quality Control and Improvement, Staff Training, and investigational research to advance the field of in-vitro fertilization.’ A total of 62 oocytes have been analyzed in this study. The age of the donors was 32.6±4.1.
Oocyte chromatin evaluation
Oocytes in pre-equilibrated culture media (M2, Millipore) have been stained with a supravital concentration of Hoechst33342 (50 ng/µL) for 5 min at room temperature. Stained oocytes have been transferred in clean drops of M2 and quickly visualized under a fluorescence microscope (Leica DMI 6000 B) to detect the chromatin configuration. For transmission electron microscopy analysis 7 SN and 4 NSN have then been transferred to the bottom of a 2-mL tube containing the fixative solution and processed as specified in the following section.
Preparation of oocytes for transmission electron microscopy
Oocytes in M2 media were centrifuged at 5000 rpm for 20 min and pellets were processed for transmission electron microscopy. Fixation was performed by immersion through gentle replacement of the supernatant with 2.5% glutaraldehyde (EM grade) and 4% paraformaldehyde, with 0.1% tannic acid and 0.01 M MgCl2, in 0.1M sodium cacodylate buffer (pH 7.3) solution for 2 h at room temperature, followed by 4 h at 4°C. Oocytes were post-fixed for 1 h in osmium tetroxide 1.33% in 0.1 M s-collidine buffer and stained en bloc with 2% uranyl acetate and then dehydrated in a graded ethanol series. Finally, the specimens were embedded in epoxy resin Epon 812. Semithin (0.2 µm) and ultrathin (40-60 nm) sections were obtained using an ultra-microtome Reichert Ultracut S provided with a diamond knife. The semi-thin sections were stained with toluidine blue and ultrathin sections, after the collection on 200 mesh grids, were counterstained with lead citrate. Observations and electron micrographs were made using a Zeiss EM 10 transmission electron microscope operating at 80 kV with an objective aperture of 30 or 60 µm; images were recorded on Kodak 4489 Electron Image film and finally digitized on an Epson Perfection V750 Pro scanner at 1600 dpi.
Results
The chromatin configuration (NSN or SN type) of the human GV oocytes has been evaluated with a supravital Hoechst33342 staining (Figure 1) and the trend follows what happens in the mouse: a higher number of SN compared to NSN oocytes. In this study, we analyzed 62 antral human oocytes; two of them were probably apoptotic and their chromatin status was impossible to detect while among the remaining 60, 72.6% and 27.4% showed SN and NSN-like configurations, respectively. It is known that human GV oocytes can be divided into three/four categories depending on the distribution of heterochromatin around the nucleolus. In this way, partially surrounded nucleoli with or without threads of chromatin dispersed in the nucleoplasm resemble the NSN configuration in the mouse, while large nucleoli completely surrounded and with no chromatin around the nucleoplasm resemble the SN murine configuration.8-11 Here, we agreed to name the human GV oocytes according to the murine nomenclature.
Figure 1.
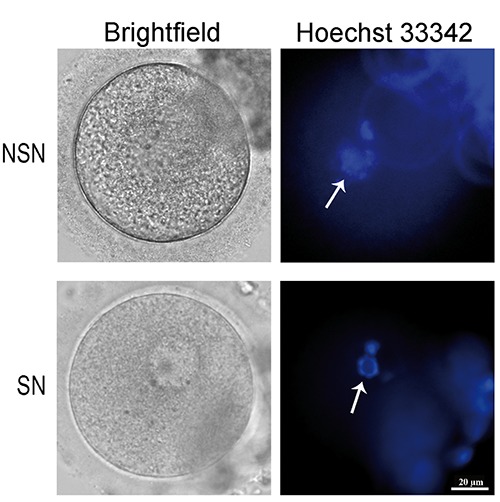
Human NSN and SN configuration oocytes. Representative images of bright field and Hoechst33342 imaged human NSN and SN oocytes (n=60). Arrows point to NSN and SN nucleoli, respectively.
Light microscopy evaluation (semi-thin sections)
Semi-thin sections from single oocytes (two peripheral, two tangential to the nuclear envelope and one equatorial) allowed us to describe the following differences among the oocytes. In particular: i) In NSN oocytes, the most significant aspect is the copious presence of vacuoli, likely lipid droplets (LD; as described later), mainly distributed in close proximity of the nucleus. Very few cytoplasmic organelles, mainly small mitochondria, appearing strongly stained, are detectable at the perinuclear level while their number increase at the cell periphery. Nucleoli are very well recognizable thanks to their strong basophyly; chromatin appears finely dispersed and just very few heterochromatin blocks are distinguishable (Figure 2 A-D, asterisk). ii) Instead, in SN oocytes very few vacuoli are scattered through all the cytoplasm and the organelles are uniformly distributed throughout the whole cytoplasmic area. Nucleoli are very well recognizable, as in the NSN oocytes, thanks to their strong basophyly; chromatin appears finely dispersed without any detectable heterochromatin blocks. A thin glycocalix is also easily detectable tightly tied to microvilli, which are uniformly distributed at the cellular surface (Figure 2 EH, arrows).
Figure 2.
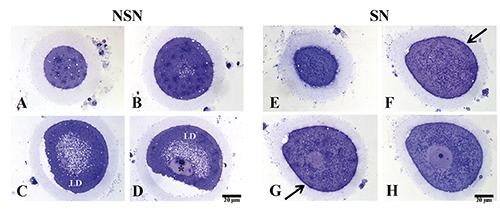
A-D) Representative images of semi-thin sections of discarded human NSN oocyte; asterisk refers to heterochromatin blocks; LD, lipid droplets. E-H) Representative images of semi-thin sections of discarded human SN oocyte; arrows point to thin glycocalix distributed at the cellular surface.
Many LD have been observed in the NSN cytoplasm as already shown in the mouse,6 and this can be one of the causes related to the developmental arrest of NSN oocytes. Interestingly, the number of LD residing in the SN cytoplasm is very low suggesting that other factors may have caused its maturation arrest and inability to mature to MII.
Transmission electron microscopy
Images obtained with transmission electron microscopy (TEM) showed: i) cytoplasm of human NSN oocytes is dispersed with an abundant amorphous component. Organelles are mainly represented by small roundish mitochondria with a low electron dense matrix and a low number of cristae mostly distributed at the periphery. Mitochondria with microvacuolized matrix and both external and internal membrane protrusion, similar to superficial blebs, are abundant. These structures are lacking of mitochondrial matrix (Figure 3A, asterisk). These features are associated with degenerative mitochondrial damage. Endoplasmic reticulum is rare. Some endoplasmic reticulum profiles with associated ribosomes and polyribosomes are rare too. Vacuolar structures are very abundant and their ultrastructure is variable (Figure 3 B-C). Likely, they are made by lipids, proteins and glycoproteins components related to cytoplasmic autolysis processes. Residual ghosts of mitochondrial lysis are also visible at higher magnification (40.000X, Figure 3D). These structures can be considered as secondary lysosomes which are performing active intracellular digestion of cytoplasmic components. No cytoplasmic lattices or structures similar to them have been seen. ii) cytoplasm of human SN oocytes is very well preserved, with a homogenous matrix with a normal medium-electron density. Mitochondrial vacuolization is rare, limited to some protrusions of the external mitochondrial membrane (Figure 3 E-F, asterisks). Golgi apparatus is very well represented with stacks of flattened cisternae (Figure 3E, black arrow). At higher magnification, profiles of smooth/rough endoplasmic reticulum (Figure 3G, black arrows) and cytoplasmic lattices (Figure 3H, black arrows) are visible.
Figure 3.
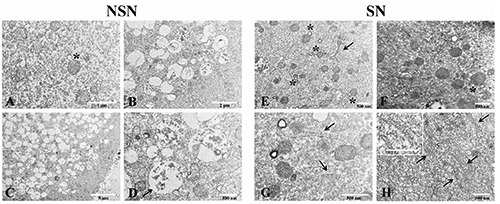
A-D) Transmission electron microscopy images of discarded human NSN oocyte; asterisk refers to mitochondria with microvacuolized matrix and both external and internal membrane protrusion; arrow points to residual ghosts of mitochondrial lysis. E-H) Transmission electron microscopy images of discarded human SN oocyte. Asterisks refer to mitochondrial vacuolar protrusions; arrow in E points to Golgi apparatus and arrows in G refer to smooth/rough reticulum; cytoplasmic lattices are visible in H (arrows); insert in H shows a magnification of CPLs.
Discussion
To our knowledge, this is the first time that sorted human antral oocytes have been studied to seek cytological markers essential to sustain their perspective embryo development. We already tried several types of microscopic observations of murine antral oocytes without adding any staining or invasive manipulation (two photon microscopy and polarized light techniques; personal communication), but we were not able to appreciate any difference between SN and NSN oocytes.
Many authors believe that NSN oocytes do not normally reside in the pre-ovulatory follicles because they represent a transition stage leading to the formation of the SN oocytes.8,10 Although this is probably what normally happens in vivo, we cannot ignore that a small percentage of NSN within their pre-ovulatory follicles can be aspirated for further IVM procedures. Although we are aware that healthy immature oocytes might have slightly different characteristics, we think that our results are throwing a stone in the wide sea of knowledge about the physiology of human immature oocytes that still remains to be discovered. Also, thanks to this opportunity, we could check the chromatin status of human GV oocytes showing that 27.4% are NSN type, therefore unable to progress beyond the 2/4-cell stage of embryo development. This is a further evidence of the urgency of finding life-compatible markers/features enabling to choose only the oocytes (i.e., SN type) able to support the entire pre-implantation embryo development. Thus, more efforts should focus on non-invasive techniques and tests to help physicians in selecting (in a very limited period of time) the healthy oocyte to be matured and further fertilized in vitro.
Our findings show that only human SN oocytes do contain CPLs in their cytoplasm, as in the mouse,6 thus laying the groundwork for further investigations. Also, the abundance of LD in the cytoplasm of human NSN oocytes can be related with one the causes of their inability to naturally mature to MII in vivo, for this reason, the quantification of LD in oocytes6,12,13 and early embryos13 can be an indicator of developmental potential.
In turn, the achievement of a clear functional topography of the oocyte has to be analyzed to decipher genotype-to-phenotype relationships and can be exploited for biological discovery and therapeutic target identifications; it may also help to explain a considerable component of the yet undiscovered genetics associated with human oocyte diseases.
Furthermore, besides providing rooms for further researches, these data are endowed with ethical and philosophical implications useful to fuel the biopolitics discussion on the embryo status.
Acknowledgements
We thank the members of the Stanford Fertility and Reproductive Health Clinic for assistance with experiments. This study was funded by the San Matteo Foundation for Health, Hospitalization and Care (to MM) and a Siebel stem cells fellowship (to MW).
References
- 1.Comizzoli P, Pukazhenthi BS, Wildt DE. The competence of germinal vesicle oocytes is unrelated to nuclear chromatin configuration and strictly depends on cytoplasmic quantity and quality in the cat model. Human Reprod 2011;26:2165-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Debey P, Szollosi MS, Szollosi D, Vautier D, Girousse A, Besomber D. Competent mouse oocytes isolated from antral follicles exhibit different chromatin organization and follow different maturation dynamics. Mol Reprod Dev 1993;36:59-74. [DOI] [PubMed] [Google Scholar]
- 3.Monti M, Redi CA. Isolation and characterization of mouse antral oocytes based on nucleolar chromatin organization. J Vis Exp 2016;107:e53616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. In vitro maturation: a committee opinion. Fertil Steril 2013;99: 663-6. [DOI] [PubMed] [Google Scholar]
- 5.Strowitzki T. In vitro maturation (IVM) of human oocytes. Arch Gynecol Obstet 2013;88:971-5. [DOI] [PubMed] [Google Scholar]
- 6.Monti M, Zanoni M, Calligaro A, Ko MSH, Mauri P, Redi CA. Developmental arrest and mouse antral not-surrounded nucleolus oocytes. Biol Reprod 2013;88:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yanez LZ, Han J, Behr BB, Reijo Pera RA, Camarillo DB. Human oocyte developmental potential is predicted by mechanical properties within hours after fertilization. Nat Comm 2016;7: 10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Combelles CMH, Cekleniak NA, Racowsky C, Albertini DF. Assesment of nuclear and cytoplasmic maturation in in-vitro matured human oocytes. Hum Reprod 2002;17:1006-16. [DOI] [PubMed] [Google Scholar]
- 9.Miyara F, Migne C, Dumont-Hassan M, Le Meur A, Cohen-Bacrie P, Aubriot FX, et al. Chromatin configuration and transcriptional control in human and mouse oocytes. Mol Reprod Dev 2003; 64:458-70. [DOI] [PubMed] [Google Scholar]
- 10.Coticchio G, Dal Canto M, Mignini Renzini M, Guglielmo MC, Brambillasca F, Turchi D, et al. Oocyte maturation: gamete-somatic cells interaction, meiotic resumption, cytoscheletal dynamics and cytoplasmic reorganization. Human Reprod Update 2015; 21:427-54. [DOI] [PubMed] [Google Scholar]
- 11.Bonnet-Garnier A, Feuerstein P, Chebrout M, Fleurot R, Jan H-U, Debey P, et al. Genome organization and epi-genetic marks in mouse germinal vesicle oocytes. Int J Dev Biol 2012;56: 877-87. [DOI] [PubMed] [Google Scholar]
- 12.Ami D, Mereghetti P, Natalello A, Doglia SM, Zanoni M, Redi CA, et al. FTIR spectral signatures of mouse antral oocytes: molecular markers of oocyte maturation and developmental competence. Biochim Biophys Acta 2011;1813:1220-9. [DOI] [PubMed] [Google Scholar]
- 13.Bradley J, Pope L, Masia F, Sanusi R, Langbein W, Swann K, et al. Quantitative imaging of lipids in live mouse oocytes and early embryos using CARS microscopy. Development 2016; 143:2238-47. [DOI] [PMC free article] [PubMed] [Google Scholar]


