Abstract
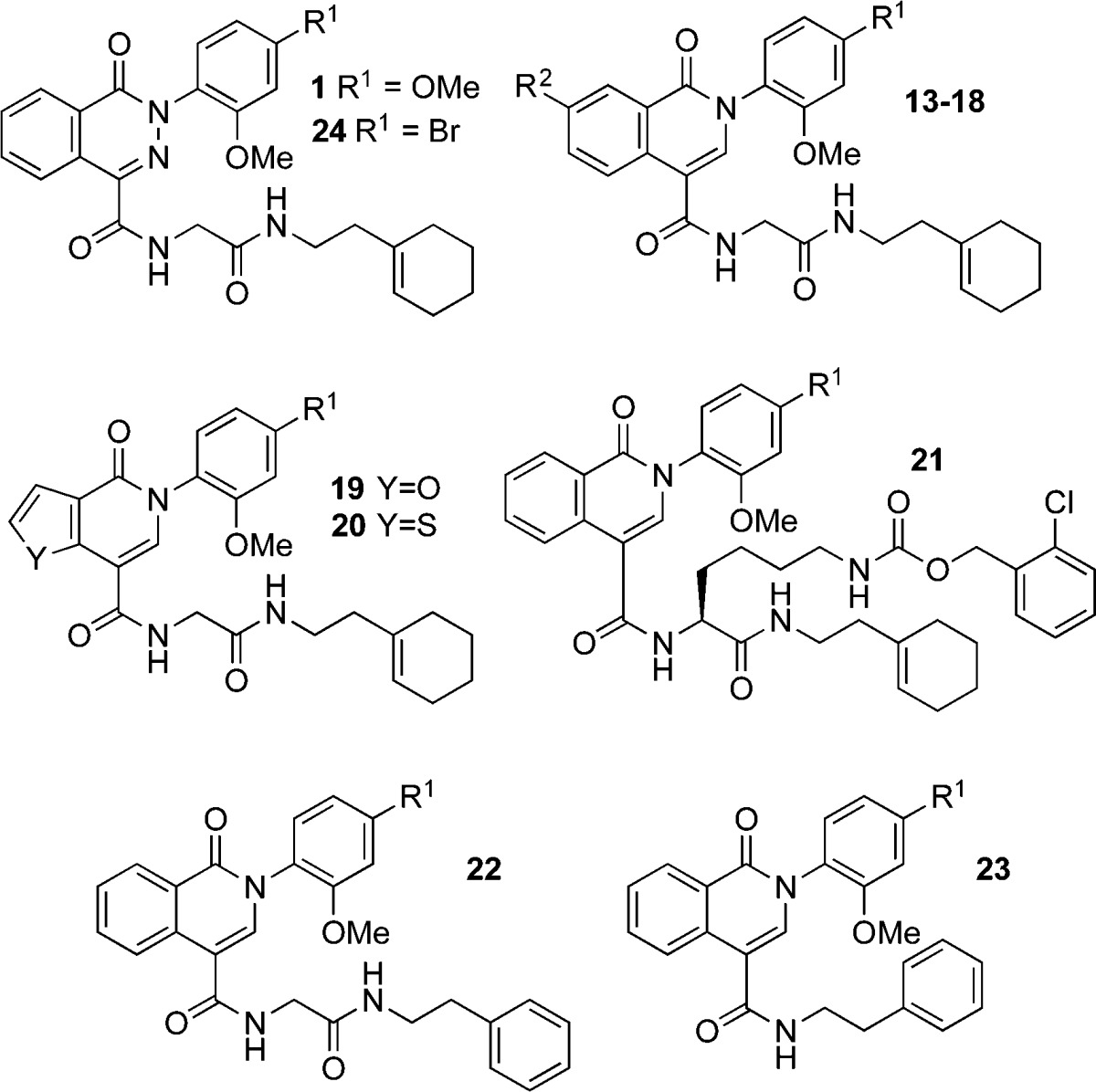
Four phthalazinones (CIDs 22334057, 22333974, 22334032, 22334012) and one isoquinolone (CID 5224943) were previously shown to be potent enhancers of antifungal activity of fluconazole against Candida albicans. Several even more potent analogues of these compounds were identified, some with EC50 as low as 1 nM, against C. albicans. The compounds exhibited pharmacological synergy (FIC < 0.5) with fluconazole. The compounds were also shown to enhance the antifungal activity of isavuconazole, a recently FDA approved azole antifungal. Isoquinolone 15 and phthalazinone 24 were shown to be active against several resistant clinical isolates of C. albicans.
Keywords: Candida albicans, antifungal agents, fluconazole, synergy, phthalazinone, isoquinolone
Azoles continue to be the drug of choice for many types of invasive fungal infections, acting on a key enzyme, sterol 14α-demethylase, in the ergosterol biosynthesis pathway. The azole family of antifungals has evolved continuously since the initial introduction of ketoconazole in the 1980s1 with the goal of achieving high affinity toward the fungal P450 14α-demethylase, low affinity toward human CYP enzymes,2,3 and, more recently, evasion of fungal resistance mechanisms.4 Fluconazole, introduced in 1990, proved well-tolerated in patients and has been used ubiquitously to treat invasive candidiasis.1 The emergence of fluconazole resistance has led to the increasing use of echinocandins and the development of third-generation azoles (voriconazole, posoconazole, isavuconazole) with higher affinity.5 Of the third-generation drugs, posoconazole and voriconazole work against a broader range of fungal pathogens but are more expensive and have other disadvantages: posoconazole has a less flexible dosing and absorption profile than fluconazole; voriconazole may be ineffective against strains that have already developed resistance toward fluconazole.6 Newer azole drugs like albaconazole and fosravuconazole are still in development.
The development of improved azoles has been paralleled by the search for small molecules that enhance the antifungal effect of existing azoles,7−10 but the efforts have been met with limited success.11,12 A wide range of azole enhancers have been shown to exhibit antifungal synergy;13 two approved drugs, flucytosine14 and calcineurin inhibitors,15−19 have been shown to synergize with fluconazole against at low concentrations (<10 μg/mL) against strains of C. albicans but have limitations for general use. Against other species,20 flucytosine instead antagonizes the effect of fluconazole, so the benefits of flucytosine-azole combinations are not clear.21 Calcineurin inhibitors such as sirolimus and tacrolimus depend on human CYP enzymes for metabolic clearance; azole drugs like fluconazole exert off-target effects on these human CYP enzymes. Buildup of these calcineurin inhibitors in plasma increases risk of nephrotoxicity and, as immunosuppressants, may increase rates of infection from other pathogenic fungal species.22−24 Given the limitations of these existing azole synergizers, new potent alternatives are needed.
Lindquist, Schreiber, and co-workers carried out a screening campaign to identify small molecules that could enhance the antifungal effect of fluconazole against C. albicans.25,26 After an initial screen of over 300,000 compounds and a subsequent rescreening, 296 compounds were found to enhance the antifungal effect of fluconazole against a partially resistant clinical strain CaCi-8 without cytotoxicity against mammalian cells. Three of those compounds27,28,26 were selected for further optimization, but none of the resulting compounds (ML189, ML212, and ML229) were active below 0.7 μM against CaCi-8. Following up on another hit from the Lindquist–Schreiber screen, we recently reported an analogue called synazo-1 that could enhance the antifungal effect of fluconazole with an EC50 of 300 pM.29 Encouraged by the discovery of potent fluconazole synergizers we set out to identify new lead compounds, optimize their structures, study their synergy with other azoles, and assess their selectivity for C. albicans.
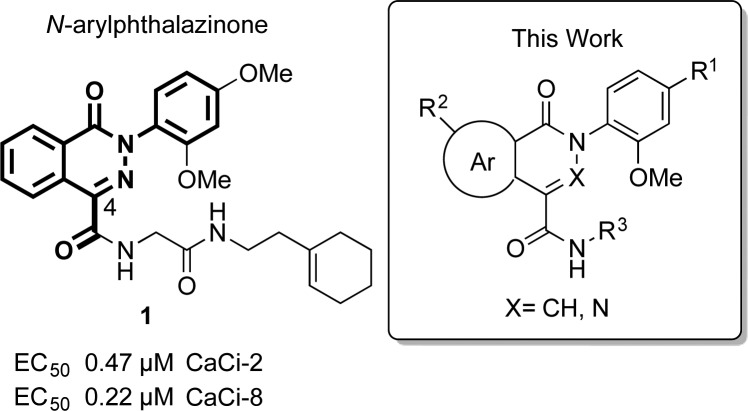
Among the compounds initially screened by Lindquist and co-workers were 233 structurally related N-arylphthalazinones (X = N, Figure 1) and N-arylisoquinolones (X = CH). Four phthalazinones and one isoquinolone were active in the initial screen. The most active compound of the five was phthalazinone 1 (CID 22334057), which showed an EC50 below 0.5 μM for C. albicans clincal isolates CaCi-8 and CaCi-2.
Figure 1.
Structure of lead phthalazinone (CID 22334057) and representative structure of analogues synthesized in this study.
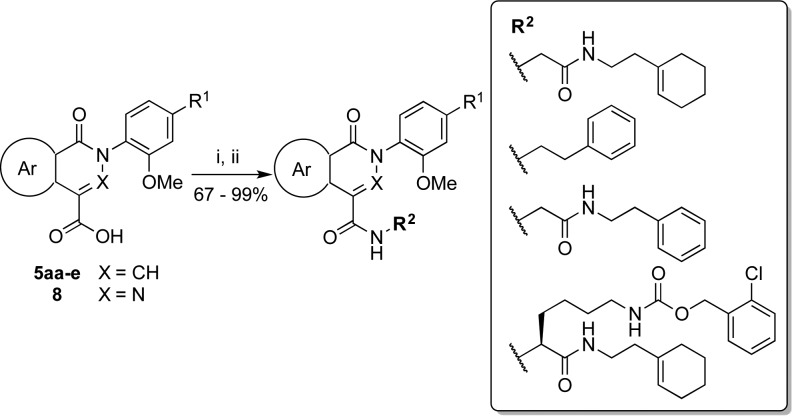
An initial analysis of the Lindquist data suggested that the biological activity of phthalazinones was sensitive to substituents on the N-phenyl ring and the C4 carboxamide side chain (Figure 1). In particular, a para substituent on the N-phenyl ring was favorable for activity, and an ortho substituent seemed essential. Additionally, the glycine linker was present in all of the active phthalazinones. The effect of modifications within the bicyclic core, at the atom X within the heterocycle or the fused benzo ring, were unclear. Analogues of the isoquinolone and phthalazinone compounds were therefore synthesized to explore chemical modification at three sites: (a) modification of the bicyclic core, including substitution of X (N versus CH), substitution of the fused benzo ring, or replacement of the benzo ring with a heterocycle; (b) substitution at the para position of the N-phenyl ring; (c) variation of the C4 carboxamide side chain.
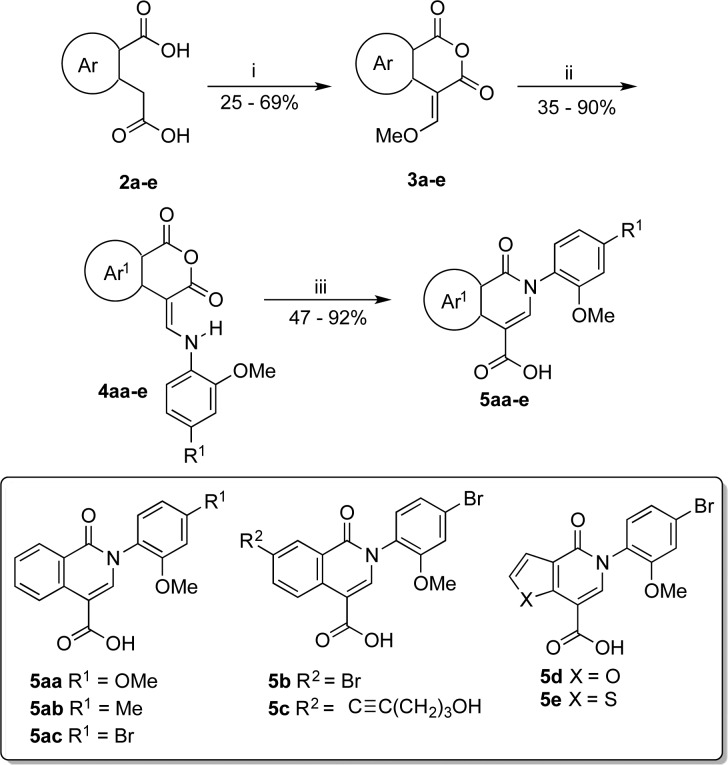
All analogues were synthesized by coupling heterocyclic carboxylic acids with amines. Isoquinolone analogues were synthesized from the corresponding dicarboxylic acids 2a–e following a route developed by Wolfbeis (Scheme 1).30 A one-step anhydride formation and formylation generated enol ethers 3a–e. Conjugate substitution of the methoxy group with various anilines generated the corresponding enamines 4aa–e. Ethoxide catalyzed the Wolfbeis rearrangement of anhydrides 4aa–e to isoquinolones 5aa–e.31
Scheme 1. Synthesis of Isoquinolone Carboxylic Acids.
Reagents and conditions: (i) 1.0 mol-equiv of CH(OCH3)3, Ac2O, 140 °C, 5 min-2.5 h; (ii) 1.0 mol-equiv of ArNH2, 1,4-dioxane, 23 °C, 1–4 h; (iii) 5.0 mol-equiv of NaOH, EtOH, 80 °C, 1–4 h.
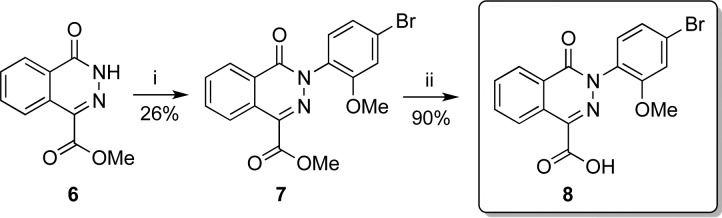
The original Dieckmann synthesis32 of N-arylphthalazinones was problematic due to the capricious reactivity of electron-rich arylhydrazines. N-Arylphthalazinone 7 was instead synthesized using a Cu-promoted cross-coupling of phthalazinone 6 with an arylboronic acid (Scheme 2). Ester 7 was saponified to afford carboxylic acid 8.
Scheme 2. Synthesis of Phthalazinone Carboxylic Acid.
Reagents and conditions: (i) 2 mol-equiv of Cu(OAc)2, 5 mol-equiv of pyridine, CH2Cl2, 23 °C, 24 h; (ii) 3.8 mol-equiv of LiOH, 3:1 THF/H2O, 23 °C, 16 h.
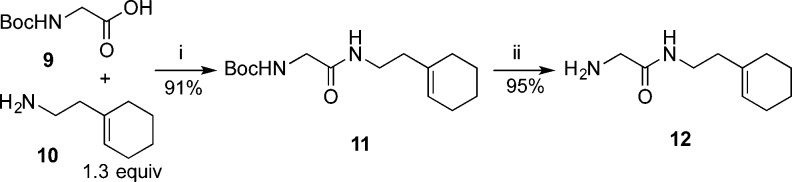
Several 2-amino acetamide fragments were synthesized in order to couple with carboxylic acids 5aa–e and 8 (Scheme 3). For illustrative purposes, the synthesis of amine 12 is described in Scheme 3. N-Boc-glycine 9 was coupled with 2-(1-cyclohexenyl)ethylamine 10 through a carbodiimide coupling reaction to afford amide 11 in good yield.33 Deprotection of the Boc group in the presence of the cyclohexenyl group, however, was found to be challenging. Common methods such as treatment with trifluoroacetic acid resulted in complete degradation of the starting material due to protonation of the alkene. Consequently, a thermal deprotection was employed to generate amine 12. Compound 11 was deproprotected by heating at reflux in water to remove the Boc group.34 However, related compounds with limited solubility in water were instead heated under reflux in ethylene glycol.35
Scheme 3. Representative Synthesis of 2-Amino Acetamide Analogues.
Reagents and conditions: (i) 1.3 mol-equiv of EDC, 1.7 mol-equiv of HOBT, 5.5 mol-equiv of DIPEA, THF, 23 °C, 16 h; (ii) H2O, 100 °C, 16 h.
Carboxylic acids 5aa–e and 8 were converted to the corresponding acid chlorides under Vilsmeier conditions and then directly coupled with amines in the presence of triethylamine (Scheme 4) to afford analogues 13–15 and 17–24 in good yields (Table 1).36
Scheme 4. Coupling of Carboxylic Acid and Amine Fragments.
Reagents and conditions: (i) 1.5 mol-equiv of oxalyl chloride, 10 mol % DMF, CH2Cl2, 0 °C, 2 h; (ii) 1.2 mol-equiv of Et3N, CH2Cl2, 0–23 °C, 30 min.
Table 1. Antifungal Activities for Phthalazinone and Isoquinolone Analogues Against C. albicans in the Presence of Fluconazolea.
| compd | R1 | R2 | EC50 (μM) |
|---|---|---|---|
| 1 | OMe | 0.015 ± 0.002 | |
| 13 | OMe | H | 0.033 ± 0.005 |
| 14 | Me | H | 0.065 ± 0.02 |
| 15 | Br | H | 0.003 ± 0.001 |
| 16 | C≡C(CH2)3OH | H | >100 |
| 17 | Br | Br | 0.003 ± 0.001 |
| 18 | Br | C≡C(CH2)3OH | >100 |
| 19 | Br | >10 | |
| 20 | Br | 0.230 ± 0.01 | |
| 21 | Br | 0.023 ± 0.008 | |
| 22 | Br | 0.020 ± 0.006 | |
| 23 | Br | 0.220 ± 0.05 | |
| 24 | Br | 0.001 ± 0.0003 |
Cells incubated with 0.25 μg/mL fluconazole.
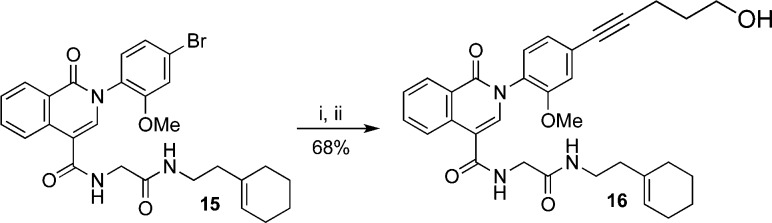
To test the steric demands around the N-phenyl group the bromine substituent of isoquinolone 15 was converted to the corresponding ω-hydroxyalkyne using a palladium-catalyzed Sonogashira coupling (Scheme 5).
Scheme 5. Derivatization of Analogue 15 via Sonogashira Coupling.
Reagents and conditions: (i) 1.2 mol-equiv of 4-pentyn-1-ol, 5 mol % Pd(Cl)2(PPh3)2, 5 mol % CuI, 3:2 DMF/Et3N, 100 °C, 45 min.
We determined the enhancement of antifungal activity for all of the new analogues against a susceptible strain of C. albicans, HLY4123, derived from the common laboratory strain CAI4.29 In the presence of a constant, nonlethal concentration of fluconazole (0.25 μg/mL), the analogues inhibited fungal cell growth in a dose-dependent fashion, from which EC50 values were determined (Table 1).
Against HLY4123, phthalazinone 1 demonstrated promising activity (EC50 0.015 μM) in the presence of 0.25 μg/mL fluconazole. The isostere, isoquinolone 13, exhibited a similar EC50 of 0.033 μM suggesting that both the isoquinolone and phthalazinone cores are competent scaffolds for eliciting antifungal activity. Only one of five active compounds in the initial Lindquist–Schreiber screen possessed an isoquinolone core, so it was unclear which structural features most influenced antifungal activity. We set out to examine the isoquinolone core further by making modifications to the N-phenyl ring and benzo ring of the core, as well as the C4 carboxamide side chain.
First, we explored the effect of substitution at the para position of the N-phenyl ring (R1). A decrease in activity was observed when methoxy (isoquinolone 13) was replaced by a methyl group (isoquinolone 14) at this position.
A bromine atom at the para position led to a surprising improvement in activity, with bromo-analogue 15 exhibiting 10-fold better activity than methoxy-analogue 13, and also better activity than phthalazinone 1. Substitution at R1 with an alcohol-terminated alkyne abolishes activity, suggesting that larger or more rigid substituents are detrimental at this position.
After establishing the importance of the bromine substituent in isoquinolone 15, we proceeded to evaluate the arene ring fused to the central heterocycle. Substitution with bromine at the C7 position of the benzo ring (R2) resulted in activity of analogue 17 comparable to that of 15. As with R1, substitution at R2 with an alcohol-terminated alkyne abolished activity. The sensitivity to substituents at R1 and R2 suggests that both positions play an important role in binding to the biological target.
Attempts to replace the benzo group with a smaller furano (analogue 19) or thiopheno (analogue 20) group resulted in a significant loss of potency. Even so, the thiophene analogue 20 showed significantly better activity than its electron-rich furan isostere 19.
Neither contraction of the benzo ring nor expansion by substitution improved activity. Therefore, we directed our attention to the C4 carboxamide side chain. Using analogue 15 again as a reference point, we evaluated the effect of substitution at the glycine α-carbon. The activity of ClCbz-lysine analogue 21 was only slightly attenuated relative to glycine analogue 15, revealing some tolerance for bulky substituents at this position of the side chain. We then prepared phenethyl amide 22 to test the importance of the cyclohexenylethyl group. The activity of cyclohexenylethyl analogue 15 was superior to that of the aromatic phenethyl analogue 22. Shortened analogue 23, missing the glycine linker, was 70-fold less active than full-length analogue 15.
Phthalazinone 24 was slightly more active than the isosteric bromophenyl isoquinolone 15. With an EC50 of 1 nM, bromophenyl phthalazinone 24 was the most active of all compounds tested.
Lead compound 1, isoquinolone 15, and phthalazinone 24 showed potent activity even at fluconazole concentrations of 0.05 μg/mL, 5-fold lower than that used in Table 1. The EC50 values were measured as 49, 24, and 7 nM, respectively.
Next, highly active bromophenyl analogues 15 and 24 were tested against five fluconazole-resistant clinical isolates of C. albicans (Table 2). Resistance in these isolates has been linked to multiple factors, including overexpression and point mutations in ERG11,37 the gene encoding sterol 14α-demethylase. Point mutations in ERG11 may contribute to fluconazole resistance by reducing binding affinity to the enzyme active site.38 In some cases, the isolates also exhibit gain-of-function mutations in UPC2, a transcription factor regulating ERG11 expression. The isolates also show varying levels of CDR1, CDR2, and MDR1 ression, implicating efflux pumps as an important source of fluconazole resistance.
Table 2. Strains Used in This Study.
| species | strain | relevant characteristics or genotype | ref | ||||
|---|---|---|---|---|---|---|---|
| Candida albicans | HLY4123 | ERG3-GFP | Premachandra29 | ||||
| Candida glabrata | BG2 | WT | Fidel39,40 | ||||
| Cryptococcus neoformans var. grubii | H99 | WT | Broad Institute41 | ||||
| expression (fold)a | |||||||
| Candida albicans | clinical isolate | mutations | ERG11 | CDR1 | CDR2 | MDR1 | Flowers37 |
| 17 | ERG11A114S,Y257H | 1.6 | 2.7 | 23.9 | 0.8 | ||
| 26 | ERG11G307S,G448R | 1.0 | 0.9 | 0.5 | 2.6 | ||
| 33 | ERG11F145L,E266D | 1.7 | 16.2 | 1465.6 | 4.1 | ||
| 36 | UPC2Y642F | 12.6 | 6.8 | 201.5 | 1.5 | ||
| 45 | ERG11E266D,G464S, UPC2A646V | 3.5 | 4.8 | 147.8 | 15.5 | ||
Fold increase relative to expression levels averaged across three unrelated fluconazole-susceptible strains.
In dose-dependent broth microdilution assays, analogues 15 and 24 showed potent activities (EC50 < 1 μM) against all resistant isolates. The EC50 values were determined by varying the analogue concentration while keeping the fluconazole concentration fixed. The fluconazole MIC50 was used and was determined for each isolate individually. Compound 24 exhibited comparable or better activity than compound 15 against the isolates tested (Table 3). At present, the origin of fluconazole synergy is uncertain. Many factors contribute to fluconazole resistance in these isolates, and we did not observe a correlation of compound activity with one specific resistance mechanism, such as levels of CDR1 expression, among the isolates we tested here. Therefore, we are unable to attribute the synergistic effect of compounds 15 and 24 with fluconazole to any single variable. Investigation into the possible causes of fluconazole synergy is ongoing.
Table 3. Antifungal Activities of Analogues 15 and 24 against Resistant Isolates of C. albicans in the Presence of Fluconazole.
| EC50a |
|||
|---|---|---|---|
| isolate | [Flu]b | 15 | 24 |
| 17 | 12 | 74 ± 10 | 39 ± 3 |
| 26 | 32 | 11 ± 1 | 17 ± 2 |
| 33 | 64 | 371 ± 23 | 72 ± 6 |
| 36 | 64 | 32 ± 4 | 20 ± 2 |
| 45 | 96 | 170 ± 20 | 65 ± 15 |
EC50 (nM) expressed as arithmetic mean ± SD of three independent experiments.
Measured MIC50 values for fluconazole alone; EC50 values were determined at the fluconazole concentrations listed.
Compounds 1, 15, and 24 were also tested against Candida glabrata BG239,40 and Cryptococcus neoformans var. grubii H9941 and showed no activity up to 30 μM at their MIC50 values (128 and 4.0 μg/mL, respectively). It is not clear why analogues 15 and 24 are active against C. albicans but not C. glabrata or Cryptococcus neoformans. We are currently investigating this issue.
Isavuconazole is one of the newest azole drugs, approved by FDA in 2015. We tested the potency of compounds 15 and 24 against HLY4123 and the highly resistant CaCi-45 in the presence of isavuconazole (Table 4). Compounds 15 and 24 showed potent antifungal activity with isavuconazole at much lower concentrations than was required for fluconazole.
Table 4. Antifungal Activitiesa of Analogues 15 and 24 against C. albicans in the Presence of Isavuconazole.
| isolate |
||
|---|---|---|
| compd | HLY4123 ([isa] = 0.001 μg/mL)b | CaCi-45 ([isa] = 0.5 μg/mL)b |
| 15 | 6 ± 0.2 | 110 ± 20 |
| 24 | 4 ± 0.2 | 13 ± 4 |
EC50 (nM) expressed as arithmetic mean ± SD of three independent experiments.
MIC values
At 30 μM or below, the analogues do not significantly affect fungal growth in the absence of azoles. Checkerboard assays revealed that compounds 15 and 24 are true synergizers with fluconazole, exhibiting fractional inhibitory concentration indices of less than 0.17 and 0.12, respectively. These indices are well below the upper-limit of 0.5 for pharmacological synergy (Supporting Information, 29–30).
All analogues were also tested for cytotoxicity against 3T3 mammalian fibroblasts. No cytotoxicity was observed up to 10 μM, indicating high selectivity for inhibiting C. albicans cell growth over that of mammalian fibroblasts.
In order to assess the potential for compounds 15 and 24 as drug leads, the physicochemical properties were calculated using FAF-Drugs3 (Supporting Information, 32). The bromine atom is beneficial for activity but puts the molecular weight over 500. Both compounds exhibit favorable characteristics according to other criteria including tPSA, predicted bioavailability, and predicted induction of phospholipidosis but were slightly lipophilic based on logD.
In conclusion we have designed, synthesized, and studied phthalazinone and isoquinolone analogues of lead compound 1 (CID 22334057), which was previously shown to be active against C. albicans in the presence of fluconazole. Most of the analogues synthesized showed highly potent activities against a susceptible strain of C. albicans, HLY4123. Two compounds, isoquinolone analogue 15 and the other a phthalazinone analogue 24, were selected for further studies and shown to be active against clinical isolates with high resistance to fluconazole. Checkerboard assays confirmed compounds 15 and 24 as true synergizers of fluconazole. Also, compounds 15 and 24 were shown to increase the efficacy of the new azole drug, isavuconazole.
Acknowledgments
We are grateful to Dr. David Rogers for providing us with resistant clinical isolates of C. albicans used for this study. We are also grateful to Dr. Zhibin Guan for assistance with the mammalian cytotoxicity assays.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.6b00355.
Complete experimental details on synthetic and bioassay methods (PDF)
Author Contributions
∥ A.D.M. and I.D.U.A.P. contributed equally to this work.
This work was supported by the NIH R01 AI099190.
The authors declare no competing financial interest.
Supplementary Material
References
- Maertens J. A. History of the development of azole derivatives. Clin. Microbiol. Infect. 2004, 1, 1–10. 10.1111/j.1470-9465.2004.00841.x. [DOI] [PubMed] [Google Scholar]
- Mast N.; Zheng W.; Stout C. D.; Pikuleva I. A. Antifungal Azoles: Structural Insights into Undesired Tight Binding to Cholesterol-Metabolizing CYP46A1. Mol. Pharmacol. 2013, 84, 86–94. 10.1124/mol.113.085902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonios I.; Bennett J. Update on Azole Antifungals. Semin. Respir. Crit. Care Med. 2008, 29, 198–210. 10.1055/s-2008-1063858. [DOI] [PubMed] [Google Scholar]
- Lass-Flörl C. Triazole antifungal agents in invasive fungal infections: a comparative review. Drugs 2011, 71, 2405–2419. 10.2165/11596540-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Mast N.; Zheng W.; Stout C. D.; Pikuleva I. A. Antifungal Azoles: Structural Insights into Undesired Tight Binding to Cholesterol-Metabolizing CYP46A1. Mol. Pharmacol. 2013, 84, 86–94. 10.1124/mol.113.085902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panackal A.; Gribskov J.; Staab J.; Kirby A.; Rinaldi M.; Marr K. Clinical significance of azole antifungal drug cross-resistance in Candida glabrata. J. Clin. Microbiol. 2006, 44, 1740–1743. 10.1128/JCM.44.5.1740-1743.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFleur M.; Lucumi E.; Napper A.; Diamond S.; Lewis K. Novel high-throughput screen against Candida albicans identifies antifungal potentiators and agents effective against biofilms. J. Antimicrob. Chemother. 2011, 66, 820–826. 10.1093/jac/dkq530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer M.; Griffiths E.; Blakely K.; Wildenhain J.; Ejim L.; Rossi L.; De Pascale G.; Curak J.; Brown E.; Tyers M.; Wright G. D. Cross-species discovery of syncretic drug combinations that potentiate the antifungal fluconazole. Mol. Syst. Biol. 2011, 21, 1–14. 10.1038/msb.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins N.; Spitzer M.; Yu T.; Cerone R.; Averette A.; Bahn Y.; Heitman J.; Sheppard D.; Tyers M.; Wright G. An Antifungal Combination Matrix Identifies a Rich Pool of Adjuvant Molecules that Enhance Drug Activity against Diverse Fungal Pathogens. Cell Rep. 2015, 13, 1481–1492. 10.1016/j.celrep.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.; Wang L.; Li Y.; Liu J.; An M.; Zhu S.; Cao Y.; Jiang Z.; Zhao M.; Cai Z.; Dai L.; Ni T.; Liu W.; Chen S.; Wei C.; Zang C.; Tian S.; Yang J.; Wu C.; Zhang D.; Liu H.; Jiang Y. Structural optimization of berberine as a synergist to restore antifungal activity of fluconazole against drug-resistant Candida albicans. ChemMedChem 2014, 9, 207–216. 10.1002/cmdc.201300332. [DOI] [PubMed] [Google Scholar]
- Chaturvedi V.; Ramani R.; Andes D.; Diekema D.; Pfaller M.; Ghannoum M.; Knapp C.; Lockhart S.; Ostrosky-Zeichner L.; Walsh T.; Marchillo K.; Messer S.; Welshenbaugh A.; Bastulli C.; Iqbal N.; Paetznick V.; Rodriguez J.; Sein T. Multilaboratory testing of two-drug combinations of antifungals against Candida albicans, Candida glabrata, and Candida parapsilosis. Antimicrob. Agents Chemother. 2011, 55, 1543–1548. 10.1128/AAC.01510-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee P.; Sheehan D.; Hitchcock C. Combination treatment of invasive fungal infections. Clin. Microbiol. Rev. 2005, 18, 163–194. 10.1128/CMR.18.1.163-194.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J.; Ren B.; Tong Y.; Dai H.; Zhang L. Synergistic combinations of antifungals and anti-virulence agents to fight against Candida albicans. Virulence 2015, 6, 362–71. 10.1080/21505594.2015.1039885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girmenia C.; Venditti M.; Martino P. Fluconazole in combination with flucytosine in the treatment of fluconazole-resistant Candida infections. Diagn. Microbiol. Infect. Dis. 2003, 46, 227–31. 10.1016/S0732-8893(03)00064-6. [DOI] [PubMed] [Google Scholar]
- Onyewu C.; Blankenship J.; Del Poeta M.; Heitman J. Ergosterol biosynthesis inhibitors become fungicidal when combined with calcineurin inhibitors against Candida albicans, Candida glabrata, and Candida krusei. Antimicrob. Agents Chemother. 2003, 47, 956–964. 10.1128/AAC.47.3.956-964.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S.; Li Y.; Guo Q.; Shi C.; Yu J.; Ma L. In vitro inter- actions between tacrolimus and azoles against Candida albicans determined by different methods. Antimicrob. Agents Chemother. 2008, 52, 409–417. 10.1128/AAC.01070-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uppuluri P.; Nett J.; Heitman J.; Andes D. Synergistic effect of calcineurin inhibitors and fluconazole against Candida albicans biofilms. Antimicrob. Agents Chemother. 2008, 52, 1127–1132. 10.1128/AAC.01397-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denardi L.; Mario D.; Loreto É.; Santurio J.; Alves S. Synergistic effects of tacrolimus and azole antifungal compounds in fluconazole-susceptible and fluconazole-resistant Candida glabrata isolates. Braz. J. Microbiol. 2015, 46, 125–129. 10.1590/S1517-838246120120442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach W.; Schell W.; Blankenship J.; Onyewu C.; Heitman J.; Perfect J. In vitro interactions between antifungals and immunosuppressants against Aspergillus fumigatus. Antimicrob. Agents Chemother. 2004, 48, 1664–1669. 10.1128/AAC.48.5.1664-1669.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Te Dorsthorst D.; Verweij P.; Meletiadis J.; Bergervoet M.; Punt N.; Meis J.; Mouton J. In vitro interaction of flucytosine combined with amphotericin B or fluconazole against thirty-five yeast isolates determined by both the fractional inhibitory concentration index and the response surface approach. Antimicrob. Agents Chemother. 2002, 46, 2982–2989. 10.1128/AAC.46.9.2982-2989.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M.; MacDougall C.; Ostrosky-Zeichner L.; Perfect J.; Rex J. Combination Antifungal Therapy. Antimicrob. Agents Chemother. 2004, 48, 693–715. 10.1128/AAC.48.3.693-715.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuypers D.; Claes K.; Evenepoel P.; Vanrenterghem Y. Clinically Relevant Drug Interaction Between Voriconazole and Tacrolimus in a Primary Renal Allograft Recipient. Transplantation 2006, 81, 1750–1752. 10.1097/01.tp.0000226080.71764.8c. [DOI] [PubMed] [Google Scholar]
- Imbert S.; Bresler P.; Boissonnas A.; Gauthier L.; Souchet L.; Uzunov M.; Leblond V.; Mazier D.; Nguyen S.; Fekkar A. Calcineurin inhibitors impair neutrophil activity against Aspergillus fumigatus in allogenic hematopoietic stem cell transplant recipients. J. Allergy Clin. Immunol. 2016, 138, 860. 10.1016/j.jaci.2016.02.026. [DOI] [PubMed] [Google Scholar]
- Dodds-Ashley E. Management of drug and food interactions with azole antifungal agents in transplant recipients. Pharmacotherapy 2010, 30, 842–854. 10.1592/phco.30.8.842. [DOI] [PubMed] [Google Scholar]
- National Center for Biotechnology Information. Summary of Broad Institute MLPCN Reversing Antifungal Drug Resistance Project. https://pubchem.ncbi.nlm.nih.gov/assay/assay.cgi?aid=2007 (accessed Mar 11, 2015).
- Youngsaye W.; Hartland C.; Morgan B.; Ting A.; Nag P.; Vincent B.; Mosher C.; Bittker J.; Dandapani S.; Palmer M.; Whitesell L.; Lindquist S.; Schreiber S.; Munoz B. ML212: A small-molecule probe for investigating fluconazole resistance mechanisms in Candida albicans. Beilstein J. Org. Chem. 2013, 9, 1501–1507. 10.3762/bjoc.9.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngsaye W.; Vincent B.; Hartland C.; Morgan B.; Buhrlage S.; Johnston S.; Bittker J.; MacPherson L.; Dandapani S.; Palmer M.; Whitesell L.; Lindquist S.; Schreiber S.; Munoz B. Piperazinyl quinolines as chemosensitizers to increase fluconazole susceptibility of Candida albicans clinical isolates. Bioorg. Med. Chem. Lett. 2011, 21, 5502–5505. 10.1016/j.bmcl.2011.06.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngsaye W.; Dockendorff C.; Vincent B.; Hartland C.; Bittker J.; Dandapani S.; Palmer M.; Whitesell L.; Lindquist S.; Schreiber S. L.; Munoz B. Overcoming fluconazole resistance in Candida albicans clinical isolates with tetracyclic indoles. Bioorg. Med. Chem. Lett. 2012, 22, 3362–3365. 10.1016/j.bmcl.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premachandra I. D. U. A.; Scott K. A.; Shen C.; Wang F.; Lane S.; Liu H.; Van Vranken D. L. Potent Synergy between Spirocyclic Pyrrolidinoindolinones and Fluconazole against Candida albicans. ChemMedChem 2015, 10, 1672–1686. 10.1002/cmdc.201500271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfbeis O. Synthesen von Fluoreszenzfarbstoffen, 11 über die Umlagerung von Alkoxymethylen- und Aminomethylenhomophthalsäureanhydriden zu Isocumarinen bzw. Isochinolinonen. Liebigs Ann. Chem. 1981, 5, 819–827. 10.1002/jlac.198119810507. [DOI] [Google Scholar]
- Patil C. D.; Sadana A.; Deodhar S. Syntheses of 2-substituted-1,2-dihydro-1-oxo-4-isoquinoline-carboxamides and 4-amidoximino-2-substituted-1,2-dihydro-1-oxoisoquinolines as antimicrobial agents. J. Indian Chem. Soc. 1990, 67, 654–656. [Google Scholar]
- Dieckmann W.; Meiser W. Über Homophthalsäureester, Oxymethylen-homophthalsäureester und die aus ihm entstehenden Isocumarin-und Isocarbostyril-Derivate. Ber. Dtsch. Chem. Ges. 1908, 41, 3253–3269. 10.1002/cber.190804102277. [DOI] [Google Scholar]
- Liu B.; Yi W.; Zhang J.; Liu Q.; Liu Y.; Fan S.; Yu X. Synthesis and gene transfection of cyclen-based cationic lipids with asymmetric acyl-cholesteryl hydrophobic tails. Org. Biomol. Chem. 2014, 12, 3484–3492. 10.1039/c4ob00384e. [DOI] [PubMed] [Google Scholar]
- Zinelaabidine C.; Souad O.; Zoubir J.; Malika B.; Nour-Eddine A. A Simple and Efficient Green Method for the Deprotection of N-Boc in Various Structurally Diverse Amines under Water-mediated Catalyst-free Conditions. Int. J. Chem. 2012, 4, 73–79. 10.5539/ijc.v4n3p73. [DOI] [Google Scholar]
- Martin L.; Coello M.; Reyes B.; Rodriguez V.; Garranzo G.; Murcia P.; Francesch S.; Sanchez S.; Fernandez R.. Antitumoral dihydropyran-2-one compounds. WO Patent 2007 144 423, 2007.
- Liu Z.; Wang R.; Guo R.; Hu J.; Li R.; Zhao Y.; Gong P. Design, synthesis and biological evaluation of novel 6,7-disubstituted-4-phenoxyquinoline derivatives bearing 4-oxo-3,4-dihydrophthalazine-1-carboxamide moieties as c-Met kinase inhibitors. Bioorg. Med. Chem. 2014, 22, 3642–3653. 10.1016/j.bmc.2014.05.013. [DOI] [PubMed] [Google Scholar]
- Flowers S.; Barker K.; Berkow E.; Toner G.; Chadwick S.; Gygax S.; Morschhauser J.; Rogers D. Gain-of-Function Mutations in UPC2 Are a Frequent Cause of ERG11 Upregulation in Azole-Resistant Clinical Isolates of Candida albicans. Eukaryotic Cell 2012, 11, 1289–1299. 10.1128/EC.00215-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrilow A. G.; Martel C. M.; Parker J. E.; Melo N.; Lamb D. C.; Nes W. D.; Kelly D. E.; Kelly S. L. Azole Binding Properties of Candida albicans Sterol 14-α Demethylase (CaCYP51). Antimicrob. Agents Chemother. 2010, 54, 4235–4245. 10.1128/AAC.00587-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormack B. P.; Falkow S. Efficient homologous and illegitimate recombination in the opportunistic yeast pathogen Candida glabrata. Genetics. 1999, 151, 979–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidel P. L.; Cutright J. L.; Tait L.; Sobel J. D. A Murine Model of Candida glabrata Vaginitis. J. Infect. Dis. 1996, 173, 425–431. 10.1093/infdis/173.2.425. [DOI] [PubMed] [Google Scholar]
- Janbon G.; et al. Analysis of the Genome and Transcriptome of Cryptococcus neoformans var. grubii Reveals Complex RNA Expression and Microevolution Leading to Virulence Attenuation. PLoS Genet. 2014, 10, 1–26. 10.1371/journal.pgen.1004261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.