Abstract

Chemotherapy for ovarian cancer often causes severe side effects. As candidates for combretastatin A4 (CA4) prodrug for ovarian cancer prodrug monotherapy (PMT), we designed and synthesized two β-galactose-conjugated CA4s (CA4-βGals), CA4-βGal-1 and CA4-βGal-2. CA4 was liberated from CA4-βGals by β-galactosidase, an enzyme more strongly expressed in ovarian cancer cells than normal cells. CA4-βGal-2, which has a self-immolative benzyl linker between CA4 and the β-galactose moiety, was more cytotoxic to ovarian cancer cell lines than CA4-βGal-1 without a linker. Therefore, CA4-βGal-2 can serve as a platform for the design and manufacture of prodrugs for ovarian cancer PMT.
Keywords: Antitumor agent, prodrug monotherapy, ovarian cancer, tubulin polymerization inhibitor, β-galactosidase, combretastatin A4
Ovarian cancer is called a “silent killer”, and its late diagnosis reduces the overall cure rate.1 Metastasis of ovarian cancer cells to the peritoneal cavity occurs easily because no anatomical barrier exists between them.2 Chemotherapy for stage 3 patients (in whom ovarian cancer cells have metastasized to the abdomen) revealed that intraperitoneal delivery of cisplatin and paclitaxel is more effective than intravenous delivery of those drugs. However, the intraperitoneal delivery method causes more side effects than the intravenous delivery method.3 This indicates that chemotherapy for advanced-stage ovarian cancer patients requires antitumor agents that exhibit selective cytotoxicity to cancer cells.
In prodrug monotherapy (PMT), a nontoxic prodrug is administered, which releases a cytotoxic drug in response to enzymatic activity enhanced in tumor tissues, realizing cancer cell selective chemotherapy.4 It is known that β-galactosidase activity is enhanced in some cancer cell lines, such as ovarian,5 breast,6 colon,6 and larynx7 cancers and gliomas.8 Utilizing this characteristic, Asanuma et al. developed a fluorescence probe that is transformable into a fluorescent dye by β-galactosidase and applied it to the visualization of small peritoneal metastases in mouse models of ovarian cancer.9 Based on this result, we hypothesized that noncytotoxic molecules that are transformed into cytotoxic molecules by β-galactosidase are suitable prodrugs for ovarian cancer PMT.
We focused on combretastatin A4 (CA4), a potent tubulin polymerization inhibitor, because microtubule disrupting agents, such as paclitaxel, are used for ovarian cancer chemotherapy. CA4 is a natural product isolated from the bark of the African willow tree Combretum caffrum.10 Structure–activity relationship studies of CA4 revealed that the three methoxy substituents on ring A, the cis-stilbene moiety, and hydrogen bond donors, such as the hydroxy group on ring B, are necessary for CA4 to exert potent cytotoxicity (Figure 1a).11 Based on this knowledge, we speculated that the cytotoxicity of CA4 would be weakened by substituting a β-galactose moiety for the hydroxy group on ring B. In this letter, we report the design and syntheses of β-galactose-conjugated CA4s (CA4-βGals) for ovarian cancer PMT and their potent cytotoxicity to ovarian cancer cell lines.
Figure 1.
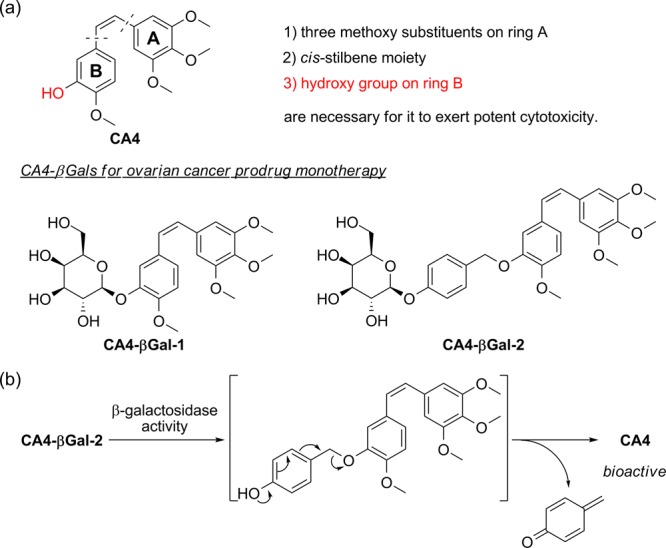
(a) Structures of CA4-βGal-1 and CA4-βGal-2 based on the structure–activity relationships of CA4. (b) Release pathway of CA4 from CA4-βGal-2.
We designed two CA4-βGals, CA4-βGal-1 and CA4-βGal-2, which release their β-galactose moieties and are transformed into CA4 in response to β-galactosidase activity (Figure 1b). In the case of CA4-βGal-1, a β-galactose moiety is directly introduced to the hydroxy group on ring B through a glycoside bond. In contrast, in the case of CA4-βGal-2, a self-immolative benzyl linker is inserted between CA4 and the β-galactose moiety. When the β-galactose moiety is removed, 1,6-benzyl elimination of the linker subsequently occurs, resulting in the formation of CA4.12,13 Some reports about prodrugs activated by a tumor-associated protease plasmin showed the importance of self-immolative linkers to avoid steric hindrance near the tripeptide substrate moiety for effective release of drugs.14−16 Therefore, we speculated that CA4-βGal-1 would be metabolized slowly by β-galactosidase because the methoxy group on ring B is located near the β-galactose moiety, whereas CA4-βGal-2 would be decomposed rapidly by β-galactosidase due to little steric hindrance near the β-galactose moiety.
CA4 was synthesized according to a reported scheme.17 Williamson ether syntheses of CA4 with 1 or 3 and removal of the acetyl groups using sodium methoxide produced CA4-βGal-1 and CA4-βGal-2, respectively (Scheme 1).
Scheme 1. Synthetic Sequence to CA4-βGal-1 and CA4-βGal-2.
Reagents and conditions: (a) CA4, NaH, THF/DMF (7:1), reflux, 23 h, yield 40%; (b) NaOMe, MeOH, 0 °C, 5 h, yield 41%; (c) CA4, NaH, THF/DMF (36:5), RT, 42 h, yield 71%; (d) NaOMe, MeOH, 0 °C, 3 h, yield 89%.
First, we evaluated the inhibitory activities of CA4-βGal-1 and CA4-βGal-2 against tubulin polymerization. As results, neither CA4-βGal-1 nor CA4-βGal-2 showed the tubulin polymerization inhibitory activity (Figure S1). These results are coincident with the structure–activity relationship of CA4 derivatives.
Next, we investigated whether CA4-βGals would be metabolized into CA4 by β-galactosidase. HPLC analyses revealed that β-galactosidase recognized both CA4-βGals as substrates and transformed them mainly into CA4 (Figure 2). In addition, under the same enzymatic reaction conditions, the yields of CA4 from CA4-βGal-1 were lower than those from CA4-βGal-2, and CA4-βGal-2 was more rapidly consumed by β-galactosidase than CA4-βGal-1 (Figure 2b). This difference probably results from the steric hindrance existing near their β-galactose moieties. This result demonstrates the suitability of the molecular design using 1,6-benzyl linker as a self-immolative benzyl linker to facilitate the access of β-galactosidase to the β-galactose moiety of a substrate.
Figure 2.

(a) HPLC profiles of enzymatic reactions of 0.2 mM CA4-βGal-1 (left column) and 0.2 mM CA4-βGal-2 (right column) with β-galactosidase (0.1 U) in 0.2 M PBS(−) (pH 7.4) at 37 °C. Reactions were monitored by measuring the absorption at 300 nm. (b) Yields of CA4 (left) and consumption of CA4-βGal-1 and CA4-βGal-2 (right) by β-galactosidase activity. Yields were determined on peak integrals.
Next, we evaluated the cytotoxicity levels of CA4-βGal-1, CA4-βGal-2, and CA4 in two ovarian cancer cell lines, OVCAR3 and OVK18, using a WST-8-based colorimetric cell cytotoxicity assay. The EC50 values of CA4-βGal-2 against OVCAR3 and OVK18 were 2.47 and 2.67 nM, respectively, and were equivalent to the EC50 values of CA4 against the same cell lines (3.45 and 1.99 nM, respectively). However, the EC50 values of CA4-βGal-1 against the same cell lines were 69.1 and 252 nM, respectively (Figure 3a, Table 1). These results revealed that CA4-βGal-2 exhibits higher cytotoxicity than CA4-βGal-1 in ovarian cancer cell lines. The enzymatic reaction rates of the two CA4-βGals in vitro might have led to these differences in cytotoxicity. The cellular permeability of CA4-βGals also might have affected their cytotoxicity. The estimated log P values of CA4-βGal-1 and CA4-βGal-2 are 1.5 and 3.1 (calculated by ChemBioDraw13), respectively, which indicate that CA4-βGal-2 is more hydrophobic than CA4-βGal-1. For compounds whose molecular weights are higher than 400, a certain level of hydrophobicity is required to enhance their cellular permeability.18 Therefore, CA4-βGal-2 is probably more cell-permeable than CA4-βGal-1.
Figure 3.
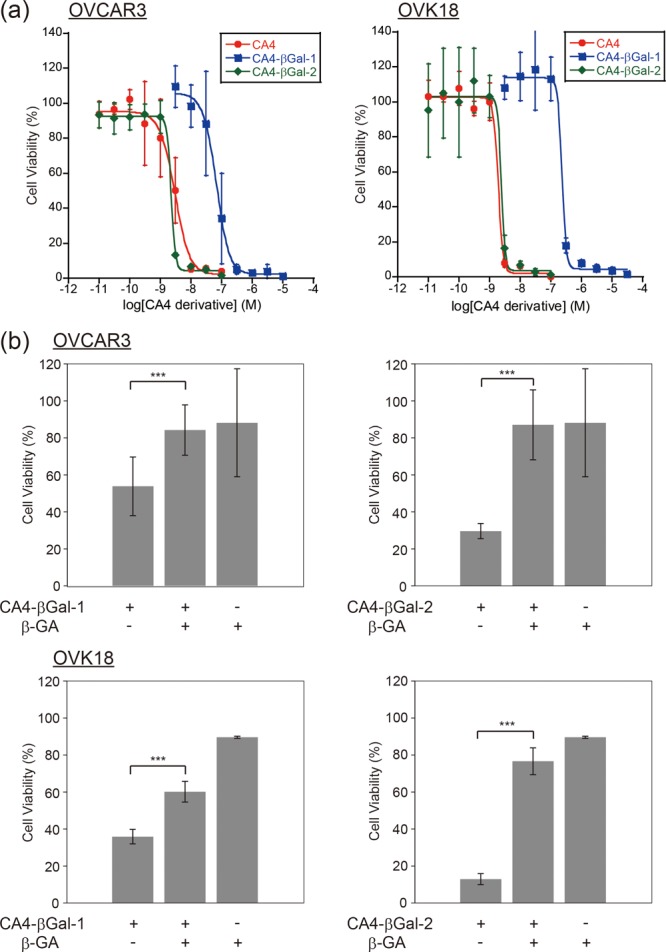
(a) Cell viability curves of ovarian cancer cell lines, OVCAR3 (left) and OVK18 (right), treated with CA4 and CA4-βGals. (b) Inhibition effects of intracellular β-galactosidase activity on cell viabilities of OVCAR3 (top) and OVK18 (bottom) with β-GA (1 mM). ***p < 0.01 by Welch’s t-test (n = 6 for each group).
Table 1. EC50 Values of CA4-βGals and CA4 against Ovarian Cancer Cell Linesa.
| EC50 (nM) |
|||
|---|---|---|---|
| cell line | CA4-βGal-1 | CA4-βGal-2 | CA4 |
| OVCAR3 | 69.06 ± 14.67 | 2.47 ± 0.21 | 3.45 ± 0.97 |
| OVK18 | 252.42 ± 17.56 | 2.67 ± 0.19 | 1.99 ± 0.05 |
Values represent the average and SE of six experiments performed in triplicate.
We also investigated whether the cytotoxicity levels of CA4-βGals are dependent on the intracellular β-galactosidase activity by introducing β-GA, a β-galactosidase inhibitor.19 When intracellular β-galactosidase activity was inhibited by 1 mM β-GA, the cytotoxicity levels of CA4-βGals in OVCAR3 and OVK18 were decreased substantially (Figure 3b). These results revealed that the cytotoxicity levels of CA4-βGals in ovarian cancer cell lines are dependent on intracellular β-galactosidase activity, and their high cytotoxicity levels are caused by CA4, the product of β-galactosidase enzymatic activity. The inhibition condition, 1 mM β-GA, suppressed β-galactosidase activity of OVK18 to that of normal cells.9 Therefore, these results also demonstrated that CA4-βGals disrupt ovarian cancer cells preferentially.
CA4 exhibits potent inhibition of tubulin polymerization and disrupts intracellular microtubule architecture.10 To elucidate whether the cytotoxicity of CA4-βGals to ovarian cancer cells is caused by the disruption of intracellular microtubule architecture, we evaluated intracellular α-tubulin and DNA, which were stained with suitable fluorescent dyes, with a confocal fluorescence microscope after incubating OVCAR3 with CA4-βGal-1 or CA4-βGal-2 for 24 h. When OVCAR3 was incubated with CA4-βGal-2 at a concentration below its EC50 (1 nM), contraction of cytoskeleton was observed, but intracellular microtubule architecture was maintained (Figure 4c, left). When CA4-βGal-2 above its EC50 was added (10 nM), we observed disruption of microtubule architecture in addition to contraction of cytoskeleton. Some cells showed nuclear contraction and fragmentation (Figure 4c, right). Similar abnormal morphological changes of microtubule architecture, cytoskeleton, and nucleus were observed in OVCAR3 treated with 10 nM CA4 for 24 h (Figure 4b). We also observed similar morphological changes in the case of CA4-βGal-1 (Figure S2). These cytoskeletal and nuclear changes are characteristic of apoptosis. These findings demonstrate that the cytotoxicity of CA4-βGals to ovarian cancer cells is a result of the disruption of microtubule architecture induced by CA4, which is produced from CA4-βGals by intracellular β-galactosidase activity, and the subsequent apoptosis.
Figure 4.

Fluorescence microscopy images of the ovarian cancer cell line OVCAR3 (a) treated with CA4 at 10 nM (b) and CA4-βGal-2 at 1 and 10 nM (c). Nuclei were directly stained with DAPI (blue), and α-tubulin was detected with Alexa Fluor 647 (red) conjugated with secondary antibody. Scale bars, 10 μm.
In conclusion, we designed and synthesized two CA4-βGals for ovarian cancer PMT, CA4-βGal-1 and CA4-βGal-2, and demonstrated that CA4-βGal-2 exhibited more potent cytotoxicity to ovarian cancer cell lines than CA4-βGal-1. This result indicates the suitability of introducing the self-immolative 1,6-benzyl linker in the design of prodrugs for ovarian cancer PMT. Because high β-galactosidase activity is reported in not only ovarian cancer but also other malignant tumors,6−9 CA4-βGal-2 can be also used as a prodrug candidate for PMT for these malignant tumors. Our design strategy for CA4-βGal-2 is valuable for the development of prodrugs for PMT targeting various cancers.
Acknowledgments
We thank Prof. Yasuteru Urano for measurements of high-resolution mass spectra.
Glossary
ABBREVIATIONS
- PMT
prodrug monotherapy
- CA4
combretastatin A4
- HPLC
high-performance liquid chromatography
- WST-8
water-soluble tetrazolium salt-8
- EC50
half maximal effective concentration
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.6b00427.
Chemical synthesis and characterization of target compounds; protocols of biological assays (PDF)
Author Contributions
‡ T.D. and K.T. contributed equally to this work. T.D. and K.T. designed this research; T.D. and K.T. performed experiments and analyzed data; N.S. and Y.O. supervised this research; and T.D., K.T., N.S., and Y.O. cowrote the manuscript.
This research was supported in part by Chiba University Research Promotion Program (a grant to T.D.), by The Mochida Memorial Foundation for Medical and Pharmaceutical Research (a grant to T.D.), by the Ministry of Education, Culture, Sports, Science and Technology of Japan Grant-in-Aid for Scientific Research (KAKENHI) (a grant 16K18909 to T.D.), and JSPS KAKENHI Grants-in-Aid for Scientific Research (a grant 26460032 to N.S.).
The authors declare no competing financial interest.
Supplementary Material
References
- Cannistra S. A. Cancer of the ovary. N. Engl. J. Med. 2004, 351, 2519–2529. 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- Bast R. C.; Hennessy B.; Mills G. B. The biology of ovarian cancer: new opportunities for translation. Nat. Rev. Cancer 2009, 9, 415–428. 10.1038/nrc2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. K.; Bundy B.; Wenzel L.; Huang H. Q.; Baergen R.; Lele S.; Copeland L. J.; Walker J. L.; Burger R. A. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N. Engl. J. Med. 2006, 354, 34–43. 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- Bosslet K.; Czech J.; Hoffmann D. A novel one-step tumor-selective prodrug activation system. Tumor Target. 1995, 1, 45–50. [Google Scholar]
- Chatterjee S. K.; Bhattacharya M.; Barlow J. J. Glycosyltransferase and glycosidase activities in ovarian cancer patients. Cancer Res. 1979, 39, 1943–1951. [PubMed] [Google Scholar]
- Bosmann H. B.; Hall T. C. Enzyme activity in invasive tumors of human breast and colon. Proc. Natl. Acad. Sci. U. S. A. 1974, 71, 1833–1837. 10.1073/pnas.71.5.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewska E.; Borzym-Kluczyk M.; Rzewnicki I.; Wojtowicz J.; Rogowski M.; Pietruski J. K.; Czajkowska A.; Sieskiewicz A. Possible role of α-mannosidase and β-galactosidase in larynx cancer. Wspolczesna Onkol. 2012, 16, 154–158. 10.5114/wo.2012.28795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wielgat P. Activity of lysosomal exoglycosidases in human gliomas. J. Neuro-Oncol. 2006, 80, 243–249. 10.1007/s11060-006-9188-z. [DOI] [PubMed] [Google Scholar]
- Asanuma D.; Sakabe M.; Kamiya M.; Yamamoto K.; Hiratake J.; Ogawa M.; Kosaka N.; Choyke P. L.; Nagano T.; Kobayashi H.; Urano Y. Sensitive β-galactosidase-targeting fluorescence probe for visualizing small peritoneal tumors in vivo. Nat. Commun. 2015, 6, 6463. 10.1038/ncomms7463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit G. R.; Cragg G. M.; Herald D. L.; Schmidt J. M.; Lohavanijaya P. Isolation and structure of combretastatin. Can. J. Chem. 1982, 60, 1374–1376. 10.1139/v82-202. [DOI] [Google Scholar]
- Lin C. M.; Singh S. B.; Chu P. S.; Dempcy R. O.; Schmidt J. M.; Pettit G. R.; Hamel E. Interactions of tubulin with potent natural and synthetic analogs of the antimitotic agent combretastatin: a structure-activity study. Mol. Pharmacol. 1988, 34, 200–208. [PubMed] [Google Scholar]
- Meyer Y.; Richard J.-A.; Delest B.; Noack P.; Renard P.-Y.; Romieu A. A comparative study of the self-immolation of para-aminobenzylalcohol and hemithioaminal-based linkers in the context of protease-sensitive fluorogenic probes. Org. Biomol. Chem. 2010, 8, 1777–1780. 10.1039/b926316k. [DOI] [PubMed] [Google Scholar]
- Alouane A.; Labruère R.; Le Saux T.; Schmidt F.; Jullien L. Self-immolative spacers: kinetic aspects, structure-property relationships, and applications. Angew. Chem., Int. Ed. 2015, 54, 7492–7509. 10.1002/anie.201500088. [DOI] [PubMed] [Google Scholar]
- Chakravarty P. K.; Carl P. L.; Weber M. J.; Katzenellenbogen J. A. Katzenellenbogen. Plasmin-activated prodrugs for cancer chemotherapy. 2. Synthesis and biological activity of peptidyl derivatives of doxorubicin. J. Med. Chem. 1983, 26, 638–644. 10.1021/jm00359a004. [DOI] [PubMed] [Google Scholar]
- de Groot F. M. H.; de Bart A. C. W.; Verheijen J. H.; Scheeren H. W. Synthesis and biological evaluation of novel prodrugs of anthracyclines for selective activation by the tumor-associated protease plasmin. J. Med. Chem. 1999, 42, 5277–5283. 10.1021/jm9910472. [DOI] [PubMed] [Google Scholar]
- de Groot F. M. H.; Loos W. J.; Koekkoek R.; van Berkom L. W. A.; Busscher G. F.; Seelen A. E.; Albrecht C.; de Bruijn P.; Scheeren H. W. Elongated multiple electronic cascade and cyclization spacer systems in activatible anticancer prodrugs for enhanced drug release. J. Org. Chem. 2001, 66, 8815–8830. 10.1021/jo0158884. [DOI] [PubMed] [Google Scholar]
- Fürst R.; Zupkó I.; Berényi A.; Ecker G. F.; Rinner U. Synthesis and antitumor-evaluation of cyclopropyl-containing combretastatin analogs. Bioorg. Med. Chem. Lett. 2009, 19, 6948–6951. 10.1016/j.bmcl.2009.10.064. [DOI] [PubMed] [Google Scholar]
- Waring M. J. Lipophilicity in drug discovery. Expert Opin. Drug Discovery 2010, 5, 235–248. 10.1517/17460441003605098. [DOI] [PubMed] [Google Scholar]
- Guo W.; Hiratake J.; Ogawa K.; Yamamoto M.; Ma S.-J.; Sakata K. β-D-Glycosylamidines: potent, selective, and easily accessible β-glycosidase inhibitors. Bioorg. Med. Chem. Lett. 2001, 11, 467–470. 10.1016/S0960-894X(00)00706-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



