Genome-wide analysis reveals the in vivo binding sites of Arabidopsis WRKY18, WRKY33, and WRKY40 transcription factors during early MTI and the consequences of WRKY18 and WRKY40 binding on transcriptional output.
Abstract
During microbial-associated molecular pattern-triggered immunity (MTI), molecules derived from microbes are perceived by cell surface receptors and upon signaling to the nucleus initiate a massive transcriptional reprogramming critical to mount an appropriate host defense response. WRKY transcription factors play an important role in regulating these transcriptional processes. Here, we determined on a genome-wide scale the flg22-induced in vivo DNA binding dynamics of three of the most prominent WRKY factors, WRKY18, WRKY40, and WRKY33. The three WRKY factors each bound to more than 1000 gene loci predominantly at W-box elements, the known WRKY binding motif. Binding occurred mainly in the 500-bp promoter regions of these genes. Many of the targeted genes are involved in signal perception and transduction not only during MTI but also upon damage-associated molecular pattern-triggered immunity, providing a mechanistic link between these functionally interconnected basal defense pathways. Among the additional targets were genes involved in the production of indolic secondary metabolites and in modulating distinct plant hormone pathways. Importantly, among the targeted genes were numerous transcription factors, encoding predominantly ethylene response factors, active during early MTI, and WRKY factors, supporting the previously hypothesized existence of a WRKY subregulatory network. Transcriptional analysis revealed that WRKY18 and WRKY40 function redundantly as negative regulators of flg22-induced genes often to prevent exaggerated defense responses.
INTRODUCTION
Plants are constantly exposed to a wide range of pathogens in their environment, but owing to their intricate and efficient basal defense system can often ward off such threats. Key to this successful defense is the ability of plants to recognize various conserved microbial structures, termed microbe-associated molecular patterns (MAMPs), through dedicated plasma membrane-localized pattern recognition receptors (PRRs), and to rapidly initiate intracellular signaling leading to MAMP-triggered immunity (MTI) (Monaghan and Zipfel, 2012; Schwessinger and Ronald, 2012; Newman et al., 2013; Vidhyasekaran, 2014; Li et al., 2016). FLS2 is currently the most intensively studied Arabidopsis thaliana PRR and is activated upon binding of bacterial flagellin or flg22, which is a conserved epitope present in the flagellin N terminus (Zipfel et al., 2004; Sun et al., 2013; Kadota et al., 2014). Upon flg22 perception, several immediate host responses can be observed, including the influx of H+ and Ca2+, the generation of reactive oxygen species, and the activation of calcium-dependent protein kinases and MAP kinase cascades (Vidhyasekaran, 2014). Subsequently, as with other ligand-PRR interactions, binding of flg22 to FLS2 results in rapid and massive transcriptional reprogramming within the host cell (Zipfel et al., 2004, 2006; Wan et al., 2008).
Transcriptional profiling has revealed that the expression of thousands of host genes is significantly altered during MTI. Wild-type Arabidopsis seedlings treated for 30 to 60 min with flg22 showed altered expression of more than 1000 genes and a rapid induction of gene sets classified to be involved in signal perception, signal transduction, and transcriptional regulation (Navarro et al., 2004; Zipfel et al., 2004). Prominent among the transcription factor (TF) genes that were induced by flg22 early on during MTI are members of the WRKY TF family. Fifteen WRKY TF genes were already strongly (>4-fold) induced 30 min after flg22 treatment in Arabidopsis seedlings including WRKY18 (>10-fold), WRKY33 (>15-fold), and WRKY40 (>20-fold) (Zipfel et al., 2004). The latter three WRKYs were also identified to be important functional HUBs within a proposed WRKY regulatory network (Choura et al., 2015). The potential importance of WRKY factors in modulating early MTI responses was further supported by the analysis of promoter sequences of flg22-induced genes, which revealed an overrepresentation of the W-box cis-acting DNA element, the consensus binding site of WRKY TFs (Navarro et al., 2004). Similarly, the W-box was overrepresented within promoters of the large group of early flg22-induced receptor-like kinase (RLK) genes (Zipfel et al., 2004).
WRKY factors have been demonstrated to fulfill essential regulatory functions to modulate pathogen-triggered cellular responses in numerous plant species (Rushton et al., 2010; Tsuda and Somssich, 2015). For instance, Arabidopsis WRKY33 is a key positive regulator of resistance against the necrotrophic fungi Alternaria brassicicola and Botrytis cinerea (Zheng et al., 2006; Birkenbihl et al., 2012). WRKY33 indirectly interacts with MAP kinase 4 via the VQ motif-containing protein MKS1. Upon flg22 treatment, WRKY33 is released from this complex and subsequently is associated with the promoter of the camalexin biosynthetic gene PAD3 (Qiu et al., 2008). Arabidopsis WRKY18 and WRKY40 act redundantly in negatively regulating resistance toward the obligate hemibiotrophic fungus Golovinomyces orontii (Pandey et al., 2010). Genetic studies on these two TFs have also clearly demonstrated that they have dual functions, namely, acting as negative regulators of MTI and as positive regulators of effector-triggered immunity mediated by the major resistance gene RPS4, as well as in resistance toward the herbivore Spodoptera littoralis (Xu et al., 2006; Lozano-Durán et al., 2013; Schön et al., 2013; Schweizer et al., 2013).
Recently, we succeeded in using chromatin immunoprecipitation-sequencing (ChIP-seq) to identify genome-wide binding sites of WRKY33 following infection of mature Arabidopsis leaves with the fungus B. cinerea 2100 (Liu et al., 2015). In this study, we aimed to define the genome-wide binding patterns of WRKY18, WRKY40, and WRKY33 during early MTI in Arabidopsis seedlings treated with the potent MAMP flg22 and to determine the consequence of the observed TF binding on the transcriptional output of the identified target genes.
RESULTS AND DISCUSSION
The function of transcription factors is mainly determined by the genomic sites they bind to and by the genes they regulate. Therefore, to investigate the function of WRKY18, WRKY40, and WRKY33 in early MTI, we determined genome-wide binding sites for those TFs upon flg22 treatment. For ChIP-seq, we used transgenic complementation lines of WRKY18 (WRKY18-HA), WRKY40 (WRKY40-HA), and WRKY33 (WRKY33-HA), which expressed the HA-epitope-tagged WRKY proteins under control of their native promoters in the respective knockout mutants (see Methods). In this way, we could avoid the problem that no appropriate antibodies exist against these WRKY factors and were able to use the same anti-HA antibody to determine the respective protein levels and to isolate proteo-DNA complexes containing the three WRKY proteins. Moreover, it was possible to use wild-type plants as negative controls, which are genotypically similar to the complementation lines and express the investigated WRKY genes without the HA-tag. To induce MTI and achieve a highly synchronous response to the elicitor, which is a precondition for the sensitive and clear detection of binding sites, we grew seedlings in liquid medium and treated them with the bacterial flagellin-derived peptide flg22 (Felix et al., 1999).
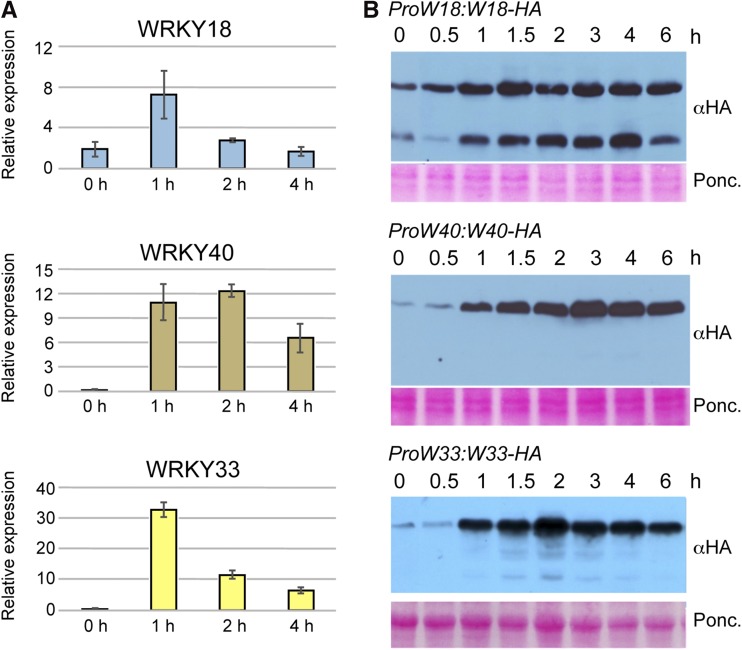
To investigate WRKY18, WRKY40, and WRKY33 expression and inducibility at the RNA level, wild-type seedlings were treated with 1 µM flg22 and samples taken for qRT-PCR analysis, either untreated or at 1, 2, and 4 h after treatment. All three WRKY genes were induced after 1 h flg22 treatment (Figure 1A). While WRKY18 was constitutively expressed with a moderate flg22-dependent increase at 1 h after treatment, WRKY40 and WRKY33 were strongly induced at 1 h with elevated RNA levels observed up to the 4 h time point.
Figure 1.
Induction of WRKY18, WRKY40, and WRKY33 by flg22 Treatment.
(A) RT-qPCR analysis of flg22-induced RNA levels. Total RNA was isolated from seedlings treated for 0, 1, 2, and 4 h with flg22 and analyzed by qPCR using gene-specific primers. Shown are the mean and sd (error bars) calculated from three biological replicates.
(B) Immunoblot analysis of flg22-induced protein levels. Protein extracts from seedlings of the HA-tagged complementation lines were treated for indicated times with flg22 and subsequently analyzed by immunoblot using an anti-HA antibody. Ponceau S staining served as loading control.
flg22-induced HA-tagged WRKY protein accumulation in the complementation lines followed the RNA expression patterns with a short delay (Figure 1B). For WRKY18, significant amounts of protein were visible in the noninduced state, the peak of protein abundance was at 1.5 h, and afterwards the protein level stayed high up to the 6 h time point. A second lower molecular weight protein band was consistently detected at all time points and was very likely a consequence of constant protein degradation in the plants. For WRKY40 and WRKY33, only very low protein levels were detected without elicitation. Upon flg22 treatment, their protein abundance strongly increased with peaks at 3 and 2 h after elicitation, respectively. Based on the kinetics of protein accumulation, the 2 h time point after flg22 treatment was chosen to determine early MTI-induced WRKY binding sites by ChIP-seq.
Analysis of WRKY18, WRKY40, and WRKY33 Binding Sites
In the course of this study, we established comprehensive lists of genome-wide binding sites for the three WRKY factors, WRKY18, WRKY40, and WRKY33, which provide a valuable source of information about general genome-wide in vivo W-box occupancy by WRKY factors, the inducibility of binding elicited by flg22, and genome-wide experimental evidence for the hypothesized WRKY regulatory network (Eulgem, 2006; Eulgem and Somssich, 2007; Chi et al., 2013; Choura et al., 2015). For our ChIP-seq experiments, we analyzed material from the three WRKY complementation lines before (0 h) and 2 h after flg22 treatment using the corresponding non-HA-antigen-containing wild-type plants as negative control.
DNA binding sites in each WRKY-HA line were determined separately for two biological replicates by comparing their observed sequencing read distribution with that of the similarly treated wild-type sample. Binding sites were scored as reproducible if the corresponding peak region overlapped with a peak region in the other replicate by at least 50% of the length of the smaller peak region. Furthermore, a binding site in the pooled samples of both replicates was counted as consistent if its peak region overlapped with a reproducible peak region in both of the original replicates by at least 50% of the length of the smaller region.
After 2 h of flg22 treatment, 1403 consistent binding sites were detected for WRKY18, 1622 for WRKY40, and 1208 binding sites for WRKY33 (Supplemental Data Set 1). Since many gene loci contained more than one binding site, the number of predicted target genes was lower, namely, 1290 for WRKY18, 1478 for WRKY40, and 1140 for WRKY33 (Table 1). For WRKY40 and WRKY33, the observed binding was almost exclusively dependent on flg22 treatment, while for WRKY18 binding to only 380 genes was defined as solely flg22 dependent. This was probably due to the detected constitutive protein levels in the WRKY18 complementation line, which appeared to also reflect the true situation, since elevated WRKY18 RNA levels were also observed prior to induction by flg22 in wild-type plants under our tested conditions (Figure 1A). Nevertheless, manual inspection of binding sites using the Integrative Genomics Viewer (IGV; Thorvaldsdóttir et al., 2013) revealed that most of the WRKY18 binding peaks visible at 0 h were significantly higher upon flg22 treatment. We found that half of the 209 consistent WRKY18 binding sites defined for 0 h had a more than 1.5 times higher ChIP score or enrichment score at 2 h (Supplemental Data Set 2). Taking this fact into account, these sites can also be considered to display flg22-dependent enrichment of WRKY18 binding, which is in agreement with the higher WRKY18 protein and RNA levels detected upon flg22 stimulation.
Table 1. Flg22-Induced WRKY18, WRKY40, and WRKY33 Binding Sites and Target Genes.
| TFs | Binding Sites | Total Target Genes | Induced Target Genes |
|---|---|---|---|
| WRKY18 | 1403 | 1290 | 380 |
| WRKY40 | 1622 | 1478 | 1477 |
| WRKY33 | 1208 | 1140 | 1104 |
In certain cases, our automated analysis pipeline failed to detect important target genes, for example, due to the applied stringent peak calling, or misassignments during the peak annotation step, or for neighboring genes on opposite strands that shared common overlapping promoter regions. To address these issues, we manually inspected the loci of some prominent defense genes not detected by the initial analysis in the IGV for obvious binding peaks. Genes that could be verified as binding targets in this way were added to the corresponding WRKY target lists. Using this strategy, we additionally classified the cysteine-rich receptor-like protein kinase gene CRK2, ISOCHORISMATE SYNTHASE1 (ICS1), the glycosyl hydrolase gene PEN2, and the cytochrome genes CYP83B1 and CYP71A13 as targets of WRKY40 (Supplemental Figures 1A to 1E). Moreover, we also rated the leucine-rich repeat serine/threonine protein kinase gene FLS2, which is the receptor for flg22, as a likely WRKY target gene, although the automated peak annotation assigned the corresponding binding region to a nearby tRNA gene (Supplemental Figure 1F).
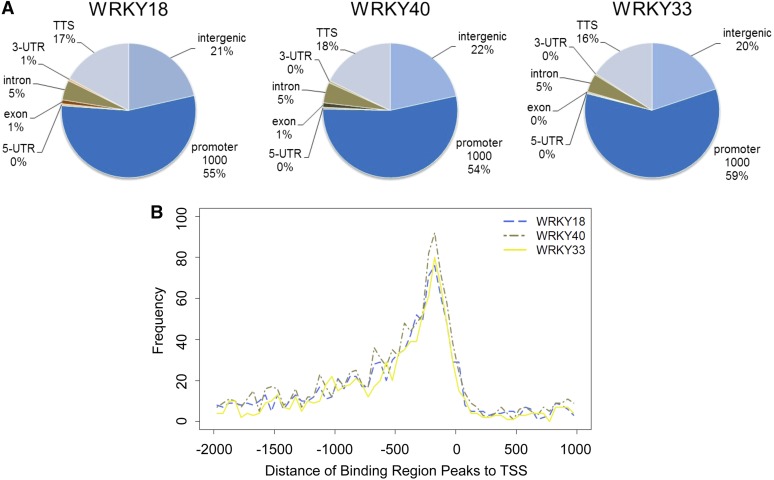
As anticipated for transcription factors, more than 97% of the binding sites for each of the studied WRKY TFs were located in noncoding DNA regions (Figure 2A), with a clear enrichment in the 1000-bp promoter regions (55%). About 5% of the peaks were located in introns, suggesting that some of these introns might also have regulatory capacity. For all three WRKY factors, the binding region peaks were located preferentially within the 400-bp region upstream of the transcription start site (TSS), with the highest frequency of peaks at around position −200 bp (Figure 2B).
Figure 2.
Distribution of flg22-Induced WRKY18, WRKY40, and WRKY33 Binding Regions in the Arabidopsis Genome.
(A) Prevalence of WRKY binding regions in different genomic categories. Promoters are defined as the 1000-bp region upstream of the TSS. TTS refers to the 1000-bp region downstream of the 3′UTR, and genome regions located in between a TTS and the promoter of the next gene are regarded as intergenic.
(B) Distance of WRKY binding region peaks to the transcription start site. The number of binding region peaks for each 50-bp region relative to the TSS is indicated.
The W-Box Is the Predominant WRKY Factor Binding Motif
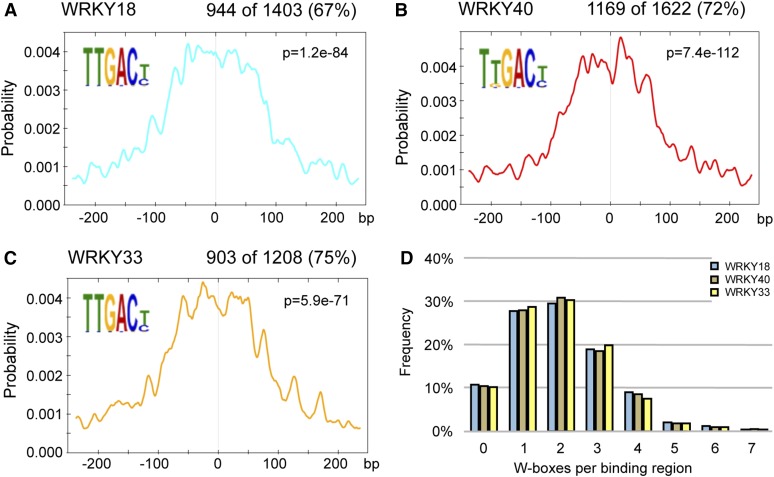
WRKY factors are known to bind to the nonpalindromic consensus nucleotide sequence TTGACT/C, termed the W-box. Within the 120-MB Arabidopsis Col-0 genome (TAIR10), 136,087 W-boxes can be identified, with equal numbers on the plus (68,029) and the minus (68,058) DNA strand, which averages roughly to one W-box per 880 bp. The W-box motifs are almost equally distributed among the regions classified as TSSs, transcription termination sites, exons (coding sequence), 5′ untranslated regions (UTRs), 3′UTRs, introns, and intergenic regions, again with no preference for the plus or minus strand (Supplemental Figure 2). To detect new or previously known binding motifs in the identified WRKY binding regions, we used the DREME/MEME software suite (Bailey, 2011; Machanick and Bailey, 2011) to perform stringent motif searches within a 500-bp region encompassing the peak summit of each binding region. The corresponding DREME searches for short motifs identified the W-box as the most frequently occurring motif for all three WRKY factors, with one or more W-boxes found in 67, 72, and 75% of the analyzed peak summit regions for WRKY18, WRKY40, and WRKY33, respectively (Figures 3A to 3C). This indicated that the W-box was the likely binding motif in most of the cases. For all three WRKY factors, the identified W-box motifs were preferentially located close to the binding peak summits; however, the probability curves did not display a single sharp central peak, as one would expect for a single binding site per binding region (Figures 3A to 3C). Consistent with this observation, the number of W-boxes per binding region (which can be larger than the 500 bp surrounding the peak summits analyzed in Figures 3A to 3C) was often higher than one for all tested WRKY factors (Figure 3D), with the number of observed W-boxes being independent of the sizes of the binding regions (Supplemental Figure 3). About 90% of the binding regions contained at least one W-box, and more than 60% contained two or more. Considering the average size of the binding regions of 730 bp, this clearly indicates an overrepresentation of W-boxes compared with the statistically expected 0.8 W-boxes per region. One prominent example for multiple WRKY factor binding sites within one promoter is the FLS2 locus, where 10 W-boxes within a 1-kb region with multiple binding peaks for all three WRKY factors were detected (Supplemental Figure 1F). For the identified binding regions, a 2-fold preference for the W-box sequence TTGACT over the TTGACC motif was observed, mirroring their genomic abundances.
Figure 3.
The W-Box Is the Predominant Motif within WRKY Binding Regions.
(A) to (C) Motif position probability graphs for WRKY18 (A), WRKY40 (B), and WRKY33 (C) established by CentriMo motif search (Bailey and Machanick, 2012). Indicated are the most frequent motif, its rate of occurrence, and the probability of this motif occurring at a given position relative to the binding peak summit (0) in the 500-bp binding regions. The included P value describes the significance for central enrichment.
(D) Distribution of W-box abundances in WRKY18, WRKY40, and WRKY33 binding regions.
Despite the predominance of the W-box motif, binding regions without W-boxes were also observed for all three WRKY factors. This may be indicative of additional W-box sequence-unrelated WRKY factor binding sites or alternatively sites of indirect WRKY binding via other DNA-associated proteins with different DNA binding specificities. When we performed comparable motif searches with the identified binding regions lacking W-boxes, no obvious consensus motifs could be detected.
WRKY18 and WRKY40 Can Bind Independently from Each Other
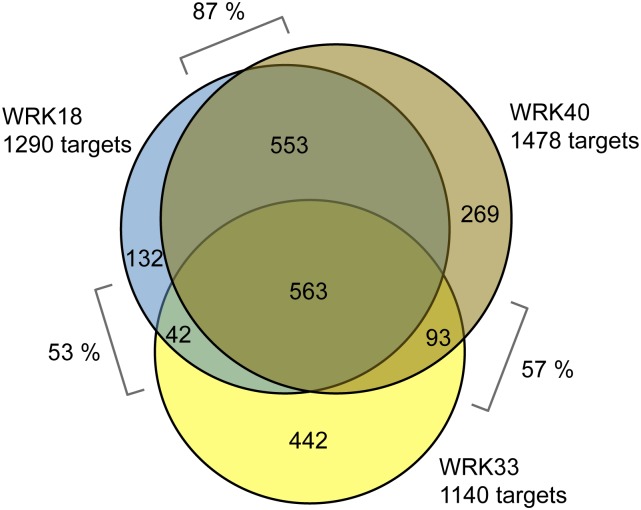
WRKY18 and WRKY40 were shown previously to form both homodimers and heterodimers that are capable of binding DNA and to be redundant in function (Xu et al., 2006; Pandey et al., 2010; Schön et al., 2013). When searching for overlaps between the identified target gene sets of the three WRKY factors, an almost complete overlap of target genes was detected between WRKY18 and WRKY40 after flg22 treatment (87%), while significantly fewer common targets were observed between WRKY33 and WRKY18 (53%) or WRKY40 (57%) (Figure 4; see Supplemental Data Set 3 for sector-specific gene lists).
Figure 4.
Overlap of WRKY18, WRKY40, and WRKY33 Target Gene Sets after 2 h flg22 Treatment.
Indicated are the number of target genes in each section and the fraction of overlapping genes between each pair of WRKY target gene sets with respect to the smaller set.
This could suggest that upon flg22 treatment WRKY18 and WRKY40 might have bound as heterodimers to the majority of their target sites, while WRKY33 binding occurred more independently. However, when WRKY18-HA or WRKY40-HA were individually expressed in a wrky18 wrky40 double mutant and ChIP-qPCR was performed on such samples, both factors were still able to bind to tested prominent target loci such as FLS2, BZIP60, and ERF-1 (Supplemental Figure 4). This observation indicates the ability of WRKY18 and WRKY40 to bind as either homodimers or monomers, which could also be the binding modes of WRKY18 in the noninduced state. The observed large overlap of WRKY factor target genes could be the result of simultaneous binding to closely spaced W-boxes within the same binding region. It is also possible that seemingly overlapping binding was the result of using whole seedlings for ChIP analyses, as such samples cannot resolve potential selective binding by different WRKY factors within specific tissues (e.g., roots and shoots) or cell types.
Binding of WRKY18, WRKY40, and WRKY33 to Promoters of Genes Implicated in MAMP-Triggered Immunity
To investigate the relevance of the identified WRKY target genes for plant defense responses, we performed a Gene Ontology (GO) term enrichment analysis using the GO tool on the TAIR webpage. We found that, compared with the corresponding prevalence in the complete genome, the target gene sets for WRKY18, WRKY40, and WRKY33 were most enriched for genes associated to the cellular component GO term plasma membrane, where perception of MAMPs and recognition of pathogens takes place. With respect to molecular functions, genes associated to the GO terms transferase activity, kinase activity, and receptor binding or activity were significantly overrepresented, and these functions are strongly linked to signal transduction to the nucleus upon recognition of pathogens or MAMPs. In agreement with this, the strongest enrichment among the WRKY target genes affected in biological processes was observed for genes connected to the GO terms response to stress, response to abiotic or biotic stimulus, and signal transduction (Supplemental Figure 5). This result indicates that upon elicitation all three WRKY factors showed a clear preference for binding gene loci involved in the early processes of MTI perception and signaling.
RLK Genes as Targets
Up to 75 receptor (-like) genes and 64 receptor (-like) kinase genes were identified as targets of WRKY18, WRKY40, and WRKY33 (Supplemental Data Set 4). Among these were genes encoding the prominent plasma membrane-bound MAMP receptors for flagellin (FLS2), chitin (CERK1 and LYM2), and lectin (CES101), as well as genes for the flg22-induced receptor kinase FRK1, the abscisic acid receptor PYL4, the phytosulfokin receptor 1, and four glutamate receptors (GLRs). Further identified WRKY targets were the gene encoding the receptor-associated kinase BIK1 that phosphorylates the reactive oxygen species-producing NADPH oxidase RBOHD (Macho and Zipfel, 2014) and the RBOHD gene itself, as well as genes encoding BIR1, which interacts with BAK1 to negatively regulate different plant resistance pathways (Gao et al., 2009); the Gα protein XLG2, which regulates immunity by direct interaction with FLS2 and BIK1 (Liang et al., 2016); AGB1, a heterotrimeric Gβ protein that acts together with XLG2 and AGG1/AGG2 (Gγ proteins) to attenuate proteasome-mediated degradation of BIK1; and PUB12, an E3 ubiquitin ligase involved in the negative regulation of FLS2 by degradation (Lu et al., 2011; Table 2).
Table 2. Selected Receptor and Receptor-Related Target Genes of WRKY18, WRKY40, and WRKY33.
| Score ChIP | |||||
|---|---|---|---|---|---|
| Gene ID |
Gene Name |
WRKY18 |
WRKY40 |
WRKY33 |
Gene Product Description |
| AT3G21630 | CERK1 | 22.5 | 29.5 | 30.4 | Chitin elicitor receptor kinase 1 |
| AT3G16030 | CES101 | 56.2 | 56.2 | 23.8 | Lectin receptor kinase CES101 |
| AT4G23130 | CRK5 | 16.2 | 17.8 | – | Cysteine-rich receptor-like protein kinase 5 |
| AT4G23220 | CRK14 | 63.1 | 66.1 | 34.6 | Cysteine-rich receptor-like protein kinase 14 |
| AT4G23250 | CRK17 | 23.6 | 34.3 | 16.6 | Cysteine-rich receptor-like protein kinase 17 |
| AT4G23260 | CRK18 | 21.1 | 23.2 | 11.3 | Cysteine-rich receptor-like protein kinase 18 |
| AT4G23280 | CRK20 | 31.2 | 44.6 | – | Putative cysteine-rich receptor-like protein kinase 20 |
| AT4G21400 | CRK28 | 22.9 | 21.5 | 23.7 | Cysteine-rich receptor-like protein kinase 28 |
| AT5G46330 | FLS2 | 36.0 | 36.0 | 22.6 | LRR receptor like kinase |
| AT2G39660 | BIK1 | 23.8 | 30.7 | – | Serine/threonine-protein kinase BIK1 |
| AT5G48380 | BIR1 | 21.5 | 25.5 | 20.4 | Leucine-rich repeat receptor-like protein kinase |
| AT4G34390 | XLG2 | 19.3 | 18.6 | 17.5 | Extra large GTP-binding protein 2 |
| AT4G34460 | AGB1 | 10.8 | – | 16.3 | Guanine nucleotide-binding protein subunit β |
| AT2G28830 | PUB12 | 29.1 | 38.1 | 11.3 | Plant U-box 24 protein |
| AT4G18710 | BIN2 | 26.3 | 26.0 | 16.1 | Shaggy-related protein kinase η |
| AT5G47910 | RBOHD | – | 12.3 | – | Respiratory burst oxidase-D |
| AT2G19190 | FRK1 | 12.7 | 17.5 | 14.5 | Flg22-induced receptor kinase 1 |
| AT5G48410 | GLR1.3 | – | – | 20.8 | Glutamate receptor 1.3 |
| AT5G11210 | GLR2.5 | 12.1 | – | 14.2 | Glutamate receptor 2.5 |
| AT2G29120 | GLR2.7 | 16.3 | 20.3 | – | Glutamate receptor 2.7 |
| AT2G29100 | GLR2.9 | – | 18.8 | 20.7 | Glutamate receptor 2.9 |
| AT5G01560 | LECRKA4.3 | 20.8 | 25.6 | – | Lectin-domain containing receptor kinase A4.3 |
| AT2G17120 | LYM2 | 32.3 | 44.2 | – | LysM domain-containing GPI-anchored protein 2 |
| AT1G73080 | PEPR1 | 32.7 | 33.9 | 18.0 | LRR receptor-like protein kinase PEPR1 |
| AT5G64890 | PROPEP2 | 18.9 | 20.3 | 21.2 | Elicitor peptide 2 |
| AT5G64905 | PROPEP3 | 26.1 | 35.0 | 40.3 | Elicitor peptide 3 |
| AT1G09970 | RLK7 | 13.0 | 15.2 | 16.3 | Leucine-rich receptor-like protein kinase LRR XI-23 |
| AT2G02220 | PSKR1 | – | – | 24.0 | Phytosulfokin receptor 1 |
| AT2G38310 | PYL4 | 13.4 | 16.6 | – | Abscisic acid receptor PYL4 |
| AT1G65790 | RK1 | – | 19.1 | 11.6 | Receptor kinase 1 |
| AT1G65800 | RK2 | 30.5 | 34.1 | – | Receptor kinase 2 |
| AT4G21380 | RK3 | 20.0 | 25.3 | 14.5 | Receptor kinase 3 |
| AT5G60900 | RLK1 | 37.8 | 43.8 | 15.5 | Receptor-like protein kinase 1 |
| AT1G16150 | WAKL4 | 30.6 | 35.9 | 20.2 | Wall-associated kinase-like 4 |
| AT1G16160 | WAKL5 | 20.2 | 21.1 | 16.3 | Wall-associated receptor kinase-like 5 |
Indicated are ChIP scores for the respective WRKY factor and target gene at 2 h flg22 treatment. Dashes indicate ChIP scores below the threshold level.
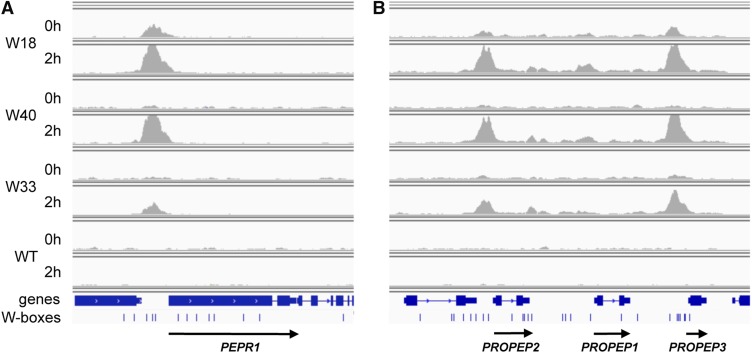
Interestingly, genes encoding components of damage-associated molecular pattern (DAMP) signaling, including the main receptor PEPR1, the expressed DAMP ligands PROPEP2 and PROPEP3, but not the nonexpressed PROPEP1 (Figure 5; Bartels and Boller, 2015), and RLK7, the receptor of PIP1 peptide signaling (Hou et al., 2014), were bound by all three WRKY factors upon flg22 treatment. This suggests that besides WRKY33, which positively regulates expression of PROPEP2 and PROPEP3 (Logemann et al., 2013), also the flg22-induced WRKY18 and WRKY40 factors provide a mechanistic and functional link between MTI and DAMP-triggered immunity. Additionally, several genes encoding receptors of the membrane-bound TIR-NBS-LRR class resistance proteins, usually active during effector-triggered immunity, were identified as WRKY targets during MTI (Supplemental Data Set 1). Taken together, these findings are in full agreement with earlier predictions that numerous RLK genes would be targets of WRKY factors, based on the overrepresentation of W-box elements within their respective promoters (Zipfel et al., 2004).
Figure 5.
WRKY18, WRKY40, and WRKY33 Binding to the PEPR1, PROPEP2, and PROPEP3 Loci.
IGV images of the PEPR1 (A) and PROPEP1-3 loci (B). Binding of WRKYs is visualized by read coverage histograms indicating sequencing read accumulation before (0 h) or after flg22 treatment (2 h). Wild-type samples served as negative control. The three lower tracks show the corresponding gene structures, position of W-boxes, and the direction of transcription (arrows).
The perception of MAMPs by dedicated receptors triggers various signaling pathways that ultimately give rise to appropriate transcriptional responses within the nucleus. In many cases, such signaling is executed by kinases, transferases, and redox-active and reactive proteins through posttranslational modification of proteins within signaling cascades. Protein kinase genes were identified as putative targets of all three WRKY factors, being 2-fold overrepresented among the target genes compared with their abundance within the genome. Besides the receptor kinase genes, we additionally identified ∼110 kinase genes as WRKY targets, most of which belong to the large families of proteins classified as kinase-like proteins or concanavalin A-like lectin kinase proteins. Furthermore, there were also several kinases from MAP kinase cascades and calcium-dependent protein kinases identified as WRKY targets, both of which are important signaling components leading to multiple defense responses (Tena et al., 2011; Supplemental Data Set 4). In the case of WRKY40 and WRKY33, all detected binding was dependent on flg22 treatment.
WRKY Targets Connected to Hormone Pathways
Numerous studies have shown that treatment with pathogens or MAMPs induces hormone signaling and causes the rapid production of plant hormones as well as upregulation of hormone response genes (Robert-Seilaniantz et al., 2011; Pieterse et al., 2012). In particular, the ethylene, salicylic acid, and jasmonic acid pathways constitute major defense pathways that are highly interconnected, thereby enabling the plant to quickly adapt to changing biotic environments (Tsuda et al., 2009).
Ethylene Pathway
The induction of the ethylene (ET) pathway is one of the earliest events during MTI (Broekgaarden et al., 2015). Two hours after flg22 treatment, we observed induced binding of WRKY18, WRKY40, and WRKY33 to the promoters of key genes in this pathway (Table 3). Among the observed WRKY targets were genes encoding the enzymes of ET biosynthesis ACS2, ACS6, and ACS8, as well as MKK9, which was reported to function upstream of MPK3/MPK6 in the activation of ACS2 and ACS6 by phosphorylation and binding of WRKY33 (Yoo et al., 2008; Li et al., 2012). Also, the ACC oxidase gene ACO2, which catalyzes the final step in the biosynthesis of ET, was a target of the WRKY proteins. Other prominent target genes were EIN2, encoding an essential transducer of ET signaling that stabilizes the key ET-dependent gene activator EIN3 (Alonso et al., 1999), and as targets of WRKY18 and WRKY40, the negative regulators of ET signaling EBF1 and EBF2 (Gagne et al., 2004). In addition, 34 ET-responsive transcription factor genes (ERFs) were found to be targets of the three WRKY factors. Among them were positive regulators like ERF1, ERF-1, ERF2, ERF5, and ERF6 and the negative regulator ERF4 (Huang et al., 2016). Furthermore, the ERF gene ORA59 encoding a major integrator of ET and jasmonic acid (JA) signaling (Pré et al., 2008), was identified as an flg22-induced target of WRKY18 and WRKY40. ORA59 was previously shown also to be a direct target of WRKY33 upon B. cinerea infection (Birkenbihl et al., 2012; Liu et al., 2015). Additional targets of all three WRKYs were MYB51, which connects ET signaling with indole glucosinolate biosynthesis, and ABA REPRESSOR1 (ABR1), encoding an ERF factor that negatively regulates abscisic acid (ABA)-activated signaling pathways, thereby possibly influencing ET-ABA signal antagonism (Pandey et al., 2005; Clay et al., 2009).
Table 3. Selected ET Pathway-Related Target Genes of WRKY18, WRKY40, and WRKY33.
| Score ChIP | |||||
|---|---|---|---|---|---|
| Gene ID |
Gene Name |
WRKY18 |
WRKY40 |
WRKY33 |
Gene Product Description |
| AT1G73500 | MKK9 | 28.9 | 26.3 | – | MAP kinase kinase 9 |
| AT1G01480 | ACS2 | 27.3 | 36.4 | – | 1-Aminocyclopropane-1-carboxylate synthase 2 |
| AT4G11280 | ACS6 | 60.9 | 90.0 | 29.9 | 1-Aminocyclopropane-1-carboxylate synthase 6 |
| AT4G37770 | ACS8 | 23.4 | 22.3 | 30.5 | 1-Aminocyclopropane-1-carboxylate synthase 8 |
| AT1G62380 | ACO2 | 18.6 | 12.5 | – | Aminocyclopropanecarboxylate oxidase |
| AT2G25490 | EBF1 | 33.8 | 35.0 | – | EIN3-binding F-box protein 1 |
| AT5G25350 | EBF2 | 73.4 | 67.7 | 22.8 | EIN3-binding F-box protein 2 |
| AT5G03280 | EIN2 | 39.6 | 40.6 | – | Ethylene-insensitive protein 2 |
| AT4G17500 | ERF-1 | 54.0 | 65.7 | 41.1 | Ethylene-responsive transcription factor 1A |
| AT3G23240 | ERF1 | 21.8 | 20.9 | – | Ethylene-responsive transcription factor 1B |
| AT5G47220 | ERF2 | 15.4 | 17.4 | – | Ethylene-responsive transcription factor 2 |
| AT3G15210 | ERF4 | 16.2 | 22.3 | – | Ethylene-responsive transcription factor 4 |
| AT5G47230 | ERF5 | – | 15.3 | 24.2 | Ethylene-responsive transcription factor 5 |
| AT4G17490 | ERF6 | 21.5 | 23.2 | – | Ethylene-responsive transcription factor 6 |
| AT1G06160 | ORA59 | 21.8 | 22.5 | – | Ethylene-responsive transcr. factor ERF094 |
| AT5G64750 | ABR1 | 65.7 | 82.4 | 17.2 | Ethylene-responsive transcription factor ABR1 |
| AT1G18570 | MYB51 | 19.2 | 40.6 | 26.8 | myb domain protein 51 |
Indicated are ChIP scores for the respective WRKY factor and target gene at 2 h flg22 treatment. Dashes indicate ChIP scores below the threshold level.
The observed binding of WRKY factors to the promoters of numerous genes encoding components of distinct parts of the pathway, including ET biosynthesis, ET signaling, and transcriptional regulation of the ET response, suggests that these WRKY TFs participate in the regulation of the entire pathway at various levels.
Salicylic Acid Pathway
Among the identified WRKY target genes associated with the salicylic acid (SA) pathway were ICS1, encoding a key SA biosynthetic enzyme (Garcion et al., 2008); EDS1, encoding a major component required for host resistance toward various pathogens and an upstream regulator of SA signaling; SAG101, coding for a key EDS1 interacting protein; EDS5, encoding a multidrug transporter required for SA accumulation upon pathogen challenge (Serrano et al., 2013); the SA receptor genes NPR3 and NPR4 (Fu et al., 2012); NIMIN1, encoding a negative regulator of NPR1 function (Weigel et al., 2005); PBS3, encoding an amino acid conjugating GH3 family member regulating SA levels (Okrent et al., 2009); and the resistance gene SNC1, encoding an immune receptor acting in the EDS1 pathway and requiring SA signaling (Li et al., 2001). Furthermore, several MYB and WRKY TF genes involved in the regulation of downstream SA responses were identified as targets (Table 4).
Table 4. Selected SA Pathway-Related Target Genes of WRKY18, WRKY40, and WRKY33.
| Score ChIP |
|||||
|---|---|---|---|---|---|
| Gene ID | Gene Name | WRKY18 | WRKY40 | WRKY33 | Gene Product Description |
| AT1G74710 | ICS1 | 51.0 | 75.0 | – | Isochorismate sythase 1 |
| AT3G48090 | EDS1 | 26.1 | 24.9 | – | Enhanced disease susceptibility 1 protein |
| AT4G39030 | EDS5 | 27.5 | 32.5 | 11.5 | Enhanced disease susceptibility 5, drug transport |
| AT1G02450 | NIMIN1 | 18.9 | 20.5 | – | Protein NIM1-interacting 1 |
| AT4G16890 | SNC1 | 17.4 | 19.3 | 15.9 | Protein SUPPRESS. OF npr1-1, CONSTITUT. 1 |
| AT5G45110 | NPR3 | – | 14.2 | – | NPR1-like protein 3 |
| AT4G19660 | NPR4 | – | 14.9 | – | NPR1-like protein 4 |
| AT5G13320 | PBS3 | 47.4 | 44.2 | – | Auxin-responsive GH3 family protein |
| AT5G14930 | SAG101 | – | – | 21.2 | Senescence-associated protein 101 |
| AT2G47190 | MYB2 | – | 15.9 | – | myb domain protein 2 |
| AT3G61250 | MYB17 | 15.1 | – | – | myb domain protein 17 |
| AT1G74650 | MYB31 | 41.5 | 51.8 | – | myb domain protein 31 |
| AT1G18570 | MYB51 | 19.2 | 40.6 | 26.8 | myb domain protein 51 |
| AT1G68320 | MYB62 | 21.3 | – | – | myb domain protein 62 |
| AT5G62470 | MYB96 | 40.7 | 48.2 | – | myb domain protein 96 |
| AT5G65790 | MYB68 | 26.8 | 30.1 | – | myb domain protein 68 |
| AT4G31800 | WRKY18 | 229.4 | 97.6 | 21.1 | WRKY transcription factor 18 |
| AT5G24110 | WRKY30 | 24.0 | 26.0 | 25.2 | WRKY DNA-binding protein 30 |
| AT5G22570 | WRKY38 | 21.9 | 22.7 | 12.5 | Putative WRKY transcription factor 38 |
| AT4G23810 | WRKY53 | 14.9 | 18.2 | 29.5 | Putative WRKY transcription factor 53 |
| AT2G25000 | WRKY60 | 78.2 | 62.5 | – | Putative WRKY transcription factor 60 |
| AT5G01900 | WRKY62 | – | 12.5 | – | Putative WRKY transcription factor 62 |
Indicated are ChIP scores for the respective WRKY factor and target gene at 2 h flg22 treatment. Dashes indicate ChIP scores below the threshold level.
JA Pathway
Several genes that encode functions related to JA signaling were also identified as direct targets of the three tested WRKY factors. The two JA biosynthetic genes LIPOXYGENASE3 (LOX3) and ALLENE OXIDE CYCLASE3 (AOC3) were targets, as was the amidohydrolase gene ILL6 that contributes to the turnover of active JA-isoleucine upon wounding (Widemann et al., 2013). Additional targets included JAZ6, coding for a repressor of JA signaling (Chini et al., 2007); BOP2, encoding a paralog of NPR1 essential for pathogen resistance induced by methyl jasmonate (Canet et al., 2012); and the transcription factor genes ORA59, WRKY50, and WRKY51. The WRKY50 and WRKY51 TFs negatively regulate JA responses following SA treatment or under low 18:1 fatty acid conditions (Gao et al., 2011; Table 5).
Table 5. Selected JA Pathway-Related Target Genes of WRKY18, WRKY40, and WRKY33.
| Score ChIP |
|||||
|---|---|---|---|---|---|
| Gene ID | Gene Name | WRKY18 | WRKY40 | WRKY33 | Gene Product Description |
| AT1G72450 | JAZ6 | 30.4 | 30.9 | 38.6 | Jasmonate-ZIM-domain protein 6 or AT1g72460 |
| AT1G17420 | LOX3 | 28.5 | 30.4 | – | Lipoxygenase 3 |
| AT3G25780 | AOC3 | – | 10.3 | 10.1 | Allene oxide cyclase 3 |
| AT1G15520 | PDR12 | 23.1 | 32.3 | 21.5 | ABC transporter G family member 40 |
| AT2G41370 | BOP2 | 17.6 | 19.9 | 10.4 | Ankyrin repeat and BTB/POZ domain-cont. protein |
| AT1G06160 | ORA59 | 14.2 | 12.2 | – | Ethylene-responsive transcription factor ERF094 |
| AT5G26170 | WRKY50 | – | 14.6 | – | Putative WRKY transcription factor 50 |
| AT5G64810 | WRKY51 | 19.2 | 22.1 | 15.8 | Putative WRKY transcription factor 51 |
Indicated are ChIP scores for the respective WRKY factor and target gene at 2 h flg22 treatment. Dashes indicate ChIP scores below the threshold level.
Tryptophan-Derived Secondary Metabolite Genes as Targets
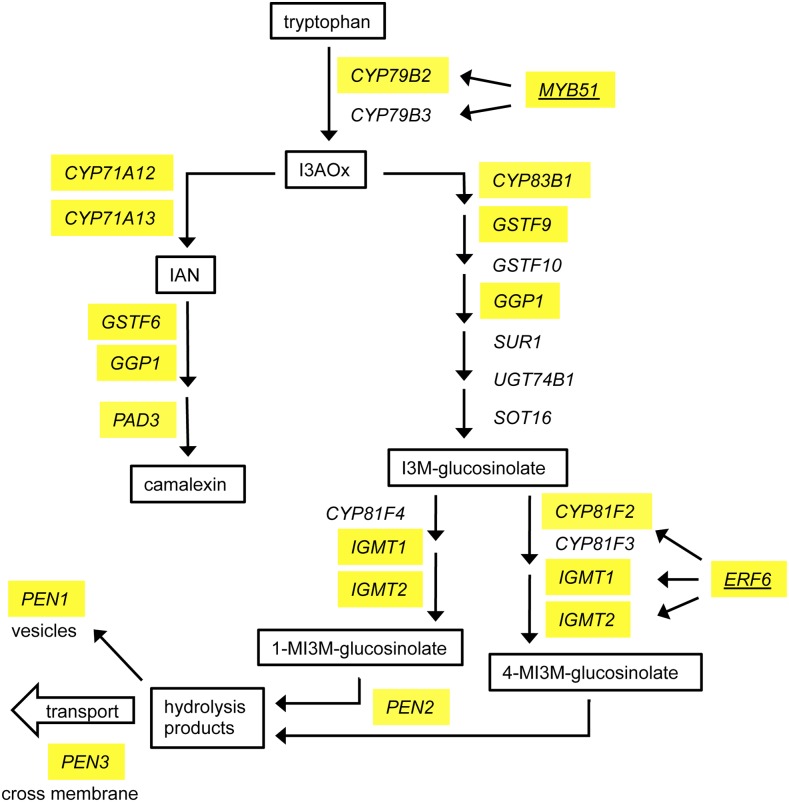
As was the case with the aforementioned signaling- and hormone pathway-associated target genes, the tested WRKY factors bound to the promoters of genes encoding diverse functional activities related to the indole secondary metabolites glucosinolates and camalexin, possibly to achieve a more synchronized and robust control of the entire defense system. We detected binding to genes encoding transcriptional regulators as well as biosynthesis enzymes and genes related to the transport of the metabolites. The indolic glucosinolate pathway, emanating from tryptophan, branches at indole-3-acetaldoxime (I3AOx) toward either the production of the phytoalexin camalexin or the generation of indole glucosinolates (Sønderby et al., 2010). In the common part of the pathway, as well as in the downstream branches, genes encoding biosynthetic enzymes were detected as flg22-dependent targets of WRKY18, WRKY40, and WRKY33 (Table 6), including previously reported targets of WRKY33 upon infection with B. cinerea (Mao et al., 2011; Birkenbihl et al., 2012; Liu et al., 2015), but also several new gene loci. Among the identified targets were CYP79B2, encoding the enzyme that participates in converting tryptophan to I3AOx; and CYP71A12, CYP71A13, GSTF6, GGP1, and PAD3, coding for enzymes subsequently converting I3AOx to indole-3-acetonitrile and finally to camalexin (Figure 6).
Table 6. Selected Target Genes of WRKY18, WRKY40, and WRKY33 Associated with Indole Glucosinolate and Camalexin.
| Score ChIP |
|||||
|---|---|---|---|---|---|
| Gene ID | Gene Name | WRKY18 | WRKY40 | WRKY33 | Gene Product Description |
| AT4G30530 | GGP1 | Yes | Yes | Yes | γ-Glutamyl-peptidase 1 |
| AT1G02930 | GSTF6 | 16.9 | 18.1 | 19.2 | Glutathione S-transferase 6 |
| AT2G30860 | GSTF9 | – | – | 11.9 | Glutathione S-transferase 9 |
| AT4G31500 | CYP83B1 | Yes | Yes | 16.1 | Cytochrome P450 83B1 |
| AT4G39950 | CYP79B2 | 19.5 | 25.1 | 23.1 | Tryptophan N-hydroxylase 1 |
| AT5G57220 | CYP81F2 | 31.0 | 43.4 | 15.0 | Cytochrome P450 81F2 |
| AT2G30770 | CYP71A13 | Yes | Yes | Yes | Cytochrome P450 71A13 |
| AT2G30750 | CYP71A12 | Yes | 14.1 | 20.7 | Cytochrome P450 71A12 |
| AT3G26830 | PAD3 | 28.4 | 36.2 | 27.8 | Cytochrome P450 71B15 |
| AT3G11820 | PEN1 | 19.1 | 18.5 | 14.7 | Syntaxin-121, vesicle traffic |
| AT2G44490 | PEN2 | 38.9 | 31.7 | 36.4 | β-Glucosidase 26 |
| AT1G59870 | PEN3 | 107.2 | 137.8 | 64.7 | ABC transporter G family member 36 |
| AT1G18570 | MYB51 | 19.2 | 40.6 | 26.8 | myb domain protein 51 |
| AT4G17490 | ERF6 | 21.5 | 23.2 | – | Ethylene-responsive transcript. factor 6 |
| AT1G21100 | IGMT1 | 25.0 | 24.1 | 15.1 | O-methyltransferase-like protein |
| AT1G21110 | IGMT3 | 18.7 | 17.0 | 10.4 | O-methyltransferase family protein |
| AT1G21120 | IGMT2 | 22.5 | 25.4 | 17.2 | O-methyltransferase family protein |
| AT1G21130 | IGMT4 | 31.4 | 37.3 | 11.8 | O-methyltransferase-like protein |
Indicated are ChIP scores for the respective WRKY factor and target gene at 2 h flg22 treatment. Manual inspection of the binding sites in the IGV browser identified additional targets that were included (and marked with “yes”; compare to Supplemental Figure 1). Dashes indicate ChIP scores below the threshold level.
Figure 6.
WRKY Factor Binding to Genes Related to Secondary Metabolism.
WRKY18, WRKY40, and WRKY33 bind to genes involved in biosynthesis and transport of secondary metabolites camalexin and indole-glucosinolates (biosynthetic pathways adapted from Sønderby et al. [2010], Pfalz et al. [2011], and Møldrup et al. [2013]). Identified WRKY18, WRKY40, or WRKY33 target genes are indicated in yellow. TFs are underlined.
Among the genes encoding the indole-glucosinolate core structure-forming enzymes, which convert I3AOx to indol-3-yl-methyl glucosinolate, CYP83B1, GSTF9, and GGP1 were identified as WRKY18 and/or WRKY40 and/or WRKY33 target genes. In addition, the genes coding for CYP81F2 and the two indole-glucosinolate methyltransferase genes IGMT1 and IGMT2, which establish secondary modifications leading to 4(and 1)-methoxy-indol-3-yl-methyl glucosinolate (4MI3MG) (Pfalz et al., 2011), were identified as WRKY18, WRKY40, and WRKY33 targets. The genes encoding CYP81F3 and CYP81F4, both also reported to be capable of modifying the indole ring of indol-3-yl-methyl glucosinolate (Pfalz et al., 2011), were not targeted by the WRKY factors. Among the targets of the three tested WRKY TFs were the atypical myrosinase gene PEN2, the syntaxin gene PEN1, and the ABC transporter gene PEN3, involved in the production or transport of toxic metabolites derived from 1MI3MG or 4MI3MG (Assaad et al., 2004; Xu et al., 2016). Further WRKY target genes were the flg22-induced TFs MYB51, also named HIGH GLUCOSINOLATE1 (HIG1), and ERF6 that regulate the production of I3AOx or 4MI3MG via direct binding to the promoters of the biosynthetic genes CYP79B2/CYP79B3 (Frerigmann and Gigolashvili, 2014), or CYP81F2, IGMT1, and IGMT2 (Xu et al., 2016), respectively.
Transcription Factor Genes as Targets
A further predominant functional class of WRKY18, WRKY40, and WRKY33 targets upon flg22 treatment were transcription factor genes. We found that nearly 10% of all target genes of the tested WRKY TFs encode transcription factors, which is markedly higher than their relative abundance within the Arabidopsis genome (∼6%), and an indication that these WRKYs are master regulators of plant immunity. Among the targeted genes, AP2/ERF TF genes were highly overrepresented (Table 7), which again reflects the importance of ET signaling in early MTI (Broekgaarden et al., 2015).
Table 7. WRKY18, WRKY40, and WRKY33 Binding to Genes from Selected TF Families Related to Stress Response at 2 h flg22 Treatment.
| TF Families | WRKY18 | WRKY40 | WRKY33 | Genome |
|---|---|---|---|---|
| Total TFs | 129/10.0%** | 156/10.5%** | 102/9.0%** | 1700/5.6% |
| AP2/ERF | 27/19.6%** | 34/24.6%** | 13/9.4%* | 138 |
| NAC | 6/6.3% | 8/8.3% | 5/5.2% | 96 |
| MYB | 12/9.2%* | 14/10.7%* | 1/9.2%* | 131 |
| bHLH | 10/6.2% | 12/7.5% | 7/4.3% | 161 |
| WRKY | 22/30.6%** | 27/37.5%** | 20/27.8%** | 72 |
Indicated are the numbers of gene loci of a TF gene family bound by the respective WRKY factor and their fraction in percentage of the entire TF gene family. Single and double asterisks indicate P < 0.05 and P < 0.0001, respectively, in a hypergeometric test for enrichment. The column “Genome” lists the total numbers of genes within the Arabidopsis genome (agris, http://arabidopsis.med.ohio-state.edu/AtTFDB/).
Even more pronounced was the binding of all three WRKY factors to gene loci of their own gene family. WRKY33 binding to the WRKY33 locus was previously reported (Mao et al., 2011). Here, we found that all three WRKY factors bound to about one-third of all WRKY gene promoters, including their own promoters, indicating extensive cross- and autoregulation within the WRKY TF family upon flg22 elicitation. Of the 32 WRKY genes bound by the three WRKY factors in total, all but three showed significantly upregulated gene expression (see below and Supplemental Table 1) after 2 h of flg22 treatment. This preferred binding to the regulatory regions of the WRKY TF gene family was observed earlier for WRKY33 after infection of Arabidopsis with the necrotrophic fungus B. cinerea (Liu et al., 2015). In fact, despite distinct modes of elicitation, nearly the same set of WRKY genes reported by Liu et al. (2015) to be targeted by WRKY33 were also found in our study (Supplemental Table 1). These findings suggest that there is a core set of WRKY factors that is activated upon elicitation by different stimuli and add support to the hypothesis that WRKY factors form a complex subregulatory network during the establishment of rapid host defense responses (Eulgem, 2006; Eulgem and Somssich, 2007; Chi et al., 2013; Choura et al., 2015).
Transcriptional Changes upon flg22 Treatment
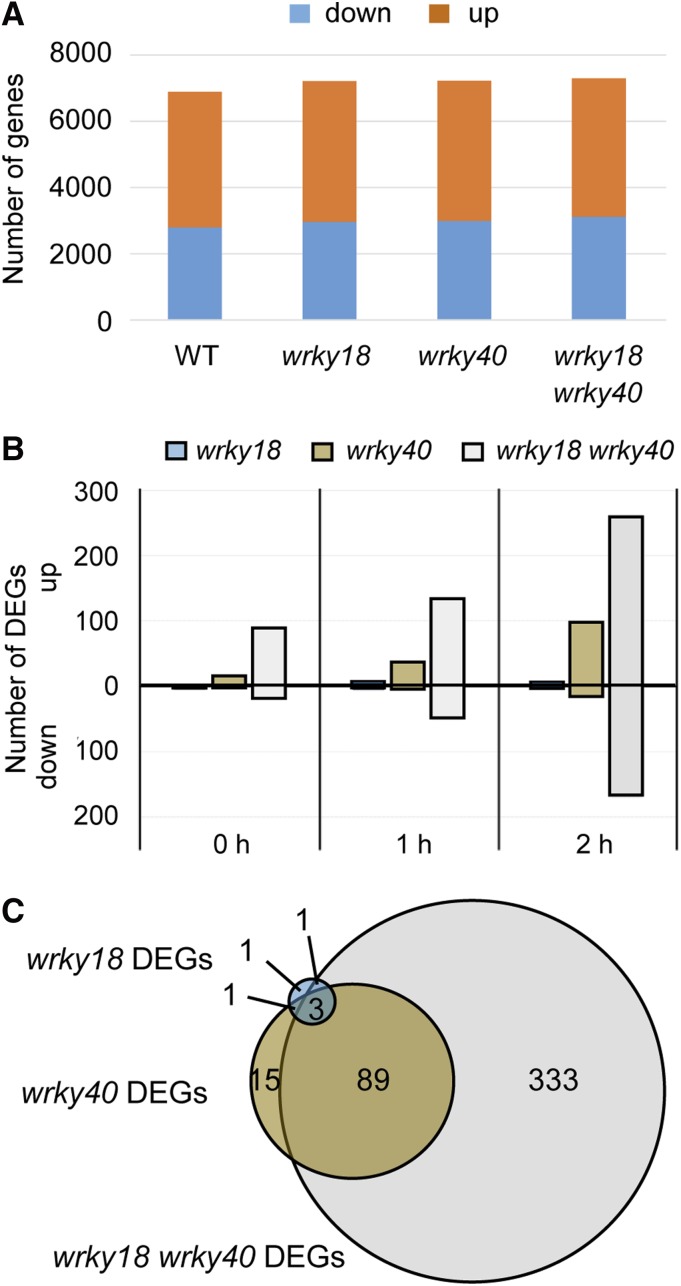
WRKY18 and WRKY40 have been reported to act as negative regulators of MTI (Xu et al., 2006). To investigate the genome-wide effect of these two TFs in transcriptional changes induced by flg22 treatment, RNA-seq experiments were performed in wild-type plants and wrky18, wrky40, and wrky18 wrky40 mutant lines. As for the ChIP-seq experiments, seedlings of each genotype were independently grown for biological replicates in liquid medium and treated with flg22 for 2 h or left untreated. Consistent with previous publications, massive transcriptional changes were observed upon elicitation (Navarro et al., 2004; Zipfel et al., 2004, 2006). About 7000 genes in each genotype were altered in their expression upon 2 h flg22 treatment compared with untreated seedlings, by at least 2-fold (absolute fold change [FC] ≥ 2) at a false discovery rate (FDR) of <0.05 (Supplemental Data Set 5). In all lines, ∼4000 genes were upregulated and 3000 downregulated upon flg22 treatment (Table 8, Figure 7A).
Table 8. Numbers of Genes Altered in Their Expression in the Respective Genotype upon flg22 Treatment.
| Genotype | Total | Up | Down |
|---|---|---|---|
| Wild type | 6892 | 4099 | 2793 |
| wrky18 | 7208 | 4244 | 2964 |
| wrky40 | 7222 | 4230 | 2992 |
| wrky18 wrky40 | 7297 | 4179 | 3118 |
Figure 7.
DEGs in wrky18, wrky40, and wrky18 wrky40 Plants.
(A) Number of significantly (FC ≥ 2, FDR < 0.05) up- or downregulated genes at 2 h flg22 treatment compared with 0 h in the indicated genotype.
(B) Number of up- and downregulated DEGs (FDR < 0.05, FC ≥ 2) in the respective mutant lines compared with the wild type at 0, 1, or 2 h after flg22 treatment.
(C) Overlap of the identified sets of DEGs in the respective mutant lines relative to the wild type at 2 h after flg22 treatment. The number of DEGs in each section is indicated.
GO term analysis of the genes with altered expression levels upon flg22 treatment revealed (Supplemental Figure 6) (1) rather small differences in GO term enrichment between the different genotypes, probably due to the high number of differentially expressed genes upon flg22 treatment and the relatively low number of genotype-specific differentially expressed genes (data not shown). (2) The terms unknown cellular components, unknown molecular functions, and unknown biological processes were clearly underrepresented among the flg22-affected genes in the wild type, reflecting that genes involved in MAMP-triggered immunity have been extensively investigated. (3) The most obvious differences in GO term enrichment could be observed between upregulated and downregulated genes upon flg22 treatment. Thus, the downregulated genes were strongly enriched for genes associated to the cellular component terms chloroplast and plastid, which were underrepresented among upregulated genes. Moreover, the upregulated genes showed a markedly stronger enrichment for genes associated to the molecular function kinase activity and the biological processes response to stress and signal transduction, while the downregulated genes showed stronger enrichment of genes associated to cell organization and biogenesis and developmental processes. Together, this discrimination in GO terms mirrors the well-described tradeoff between plant defense and growth during MTI (Huot et al., 2014; Li et al., 2015).
Differentially Expressed Genes in wrky18, wrky40, and wrky18 wrky40
When we searched for genotype-specific differentially expressed genes (DEGs) in the WRKY mutant lines compared with the wild type (absolute FC ≥ 2, FDR < 0.05), we identified in untreated and flg22-treated wrky18 seedlings only three and six differentially regulated genes, respectively, with one of them being WRKY18. In wrky40 seedlings under the same conditions, 14 and 108 DEGs were identified, while in the wrky18 wrky40 double mutant, 112 and 426 genes, respectively, were affected (Figure 7B; Supplemental Data Set 6). The low number of DEGs in the wrky18 and wrky40 single mutants compared with the much higher number detected in the double mutant demonstrates the functional redundancy of WRKY18 and WRKY40 at this stage of MTI, with WRKY40 seemingly acting more independent than WRKY18. This redundancy was further confirmed by the high overlap between wrky40 and wrky18 wrky40 DEGs, which was 85% with respect to the wrky40 gene set, and by the finding that four out of the six wrky18 DEGs also appeared in the wrky40 gene set (Figure 7C; see Supplemental Data Set 7 for sector-specific gene lists). Functional redundancy of WRKY18 and WRKY40 was previously demonstrated both in their role as negative regulators of resistance toward the powdery mildew fungus G. orontii (Pandey et al., 2010) and in positively regulating AvrRPS4 effector-triggered immunity against Pseudomonas syringae (Schön et al., 2013). Two hours after flg22 treatment, markedly more DEGs were upregulated than downregulated in wrky40 (88%) and wrky18 wrky40 (61%) compared with the wild type (Table 9, Figure 7B), indicating that WRKY40, and potentially WRKY18 acting redundantly with WRKY40, were mainly negatively affecting gene expression.
Table 9. Numbers of DEGs in the Respective Genotype Compared with the Wild Type.
| 0 h | 2 h | |||||
|---|---|---|---|---|---|---|
| Genotype |
Total |
Up |
Down |
Total |
Up |
Down |
| wrky18 | 3 | 0 | 3 | 6 | 3 | 3 |
| wrky40 | 15 | 12 | 3 | 108 | 96 | 12 |
| wrky18 wrky40 | 112 | 92 | 20 | 426 | 259 | 167 |
Differentially Regulated Direct Target Genes
About 55% of all identified WRKY18, WRKY40, and WRKY33 target genes belonged to the gene set showing altered expression in wild-type seedlings after flg22 treatment, which is clearly higher than the expected 23% based on the proportion of genes altered in expression in the wild type relative to all annotated Arabidopsis genes. About 90% of these target genes with altered expression levels were upregulated upon flg22 treatment (Supplemental Figure 7 and Supplemental Data Set 8), suggesting that these WRKY factors play a positive regulatory role in reprogramming the transcriptome at this stage of MTI. This apparent discrepancy, that these WRKY factors are negative regulators of most of the differentially expressed genes identified between the wild type and the respective mutant on the one hand, while their flg22-dependent binding is mainly observed to upregulated genes in wild-type plants on the other hand, suggests that they may function to negatively counterbalance the positive effect of other TFs acting at such loci. Candidates for such activators are SARD1, CBP60g, and CHE. SARD1 and CBP60g belong to the family of calmodulin binding TFs, while CHE is a member of the TCP family. All three TFs have been shown to positively influence defense gene expression and to regulate MTI responses (Truman and Glazebrook, 2012; Zheng et al., 2015). Recent ChIP-seq studies have determined genome-wide binding sites for SARD1 during MTI and demonstrated substantial redundancy with CBP60g binding (Sun et al., 2015). Comparison between the SARD1 data and our WRKY target gene set revealed a substantial overlap despite differences in the experimental setups employed (Supplemental Figure 8 and Supplemental Data Set 9). Of the total 392 common targets, 29 are DRTs of WRK18 and WRKY40 and all of these DRTs are upregulated upon flg22 treatment in the wrky18 wrky40 double mutant (Supplemental Table 2). One of the common directly regulated target genes, ICS1, has been shown to be a direct and positively regulated target not only of SARD1, CBP60g, and CHE (TCP21), but also of other TFs including TCP8 and the NAC TF NTL9 (Wang et al., 2015; Zheng et al., 2015), while in our experiments it was significantly downregulated by WRKY18 and WRKY40. Similarly, we see a clear negative effect of the WRKY factors on the expression of the receptor-like kinase gene CRK5. CRK5 expression is strongly induced upon flg22 treatment and it is a direct target of WRKY18 and WRKY40, but not WRKY33 (Supplemental Data Sets 1 and 5). Previous cotransfection assays performed in tobacco (Nicotiana benthamiana) leaves revealed that expression of a reporter gene construct driven by the CRK5 promoter was suppressed by W-box-dependent promoter binding of WRKY18 or WRKY40 (Lu et al., 2016). ALD1 and CRK45 are two additional genes that are direct negatively regulated targets of WRKY18 or WRKY40 but are also targets of SARD1. The ald1 mutant was shown to be compromised in basal resistance and negatively affected in early flg22 responses, while elevated expression of ALD1 enhanced basal resistance (Song et al., 2004; Cecchini et al., 2015). CRK45 mutants also showed enhanced basal susceptibility to P. syringae, whereas overexpression lines thereof increased basal resistance (Zhang et al., 2013). Thus, the proper coordinate regulation of defense genes appears rather complex and very likely involves dynamic binding of both positive and negative transcriptional regulators to fine-tune expression and to ensure robust responsiveness to the stimulus.
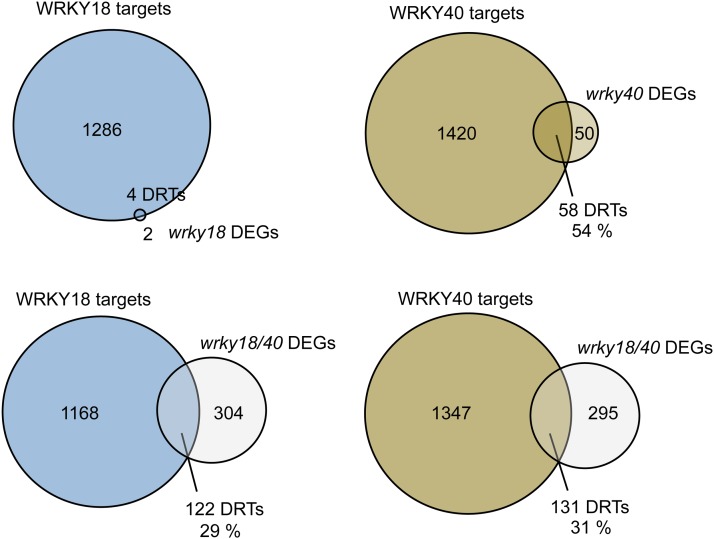
To identify directly regulated target genes (DRTs) of WRKY18 and WRKY40, we searched for DEGs between the wild type and the mutant lines wrky18, wrky40, and wrky18 wrky40 that overlapped with the target genes of the respective WRKY factor as identified by ChIP-seq. As the VENN diagram in Figure 8 demonstrates, four loci out of the six DEGs detected in wrky18 seedlings were bound by WRKY18 after flg22 treatment, including WRKY18 itself. Fifty-eight (54%) of the wrky40 DEG loci were bound by WRKY40, with binding detected only after induction by flg22. In the WRKY40 DRT set, WRKY40 was also found, hinting toward possible feedback regulation via WRKY18 and WRKY40. Of the DEGs identified in wrky18 wrky40 seedlings, 122 (29%) and 131 (31%) gene loci were identified as WRKY18 and WRKY40 direct targets, respectively (see sector specific gene lists in Supplemental Data Sets 10 and 11). The overlap between the WRKY18 and WRKY40 DRTs in wrky18 wrky40 was 117 genes, including almost all WRKY18 DRTs (Supplemental Data Set 12), again supporting the notion of a strong functional redundancy of the two WRKY factors and possibly binding to DNA as heterodimers as was previously proposed (Xu et al., 2006). In the same study, WRKY60 was also shown to be capable of forming heterodimers with WRKY18 and WRKY40, and WRKY60 is also a DRT of both. However, its role in MTI remains unclear since expression of this gene is not influenced by flg22, based also on our own RNA-seq data or by other MAMPs including elf18, chitin, and oligogalacturonides, according to numerous perturbation studies available at Genvestigator (https://genevestigator.com/gv/index.jsp).
Figure 8.
DRTs of WRKY18 and WRKY40.
DRTs were identified by the overlaps of the WRKY18 and WRKY40 target gene sets with the sets of DEGs compared with the wild type in wrky18, wrky40, and wrky18 wrky40 mutants at 2 h after flg22 treatment. Indicated are the number of target genes, DEGs, and DRTs in the respective sections and the fraction DEGs identified as DRTs in each comparison.
About 10% of the WRKY18 or WRKY40 direct target genes showed altered expression upon flg22 treatment in a WRKY18- or WRKY40-dependent manner in the wrky18 wrky40 double mutant. This relatively poor correlation between TF binding and transcriptional response of target genes has been consistently observed in genome-wide ChIP studies (for possible explanations, see Heyndrickx et al. [2014]). Conversely, ∼30% of the DEGs between wrky18 wrky40 and the wild type identified in this study were bound by WRKY18 or WRKY40. The limited overlap could be due to the dynamics of TF binding, which is often transient and cannot be resolved by analyzing one time point by ChIP-seq as performed here, or to the requirement of additional factors besides TF binding to alter gene expression.
GO analysis of the WRKY18 and WRKY40 DRTs revealed further enrichment of GO terms related to MAMP perception and signaling compared with the overall set of target genes (Supplemental Figure 9). This again is indicative of the two WRKY factors preferentially regulating genes important for early MTI. This further enrichment was observed for the cellular component term plasma membrane, the molecular function terms kinase activity and transcription factor activity, and the biological functions response to stress, response to abiotic and biotic stimulus, and signal transduction.
Among the 117 DRTs common for WRKY18 and WRKY40 were 25 genes encoding kinases, 20 of them receptor and receptor-like kinases (including RK1, RK2, RLK1, CRK20, CES101, and WAKL4) and together with MKK9 potential perception and signaling components, 16 genes for TFs, mainly ERF-type factors important in early MTI and stress-responsive WRKY factors, five genes for VQ motif-containing proteins that can interact with specific WRKY proteins and positively influence their binding to DNA (Jing and Lin, 2015), and seven genes for cytochrome P450 proteins, all of them, together with IGMT1, catalyzing the biosynthesis of secondary metabolites (Bak et al., 2011). Additionally, the three SA pathway-related genes, ICS1, PBS3, and NIMIN1, are DRTs of WRKY18 and WRKY40.
As noted above, nearly all of these genes and most of the other DRTs, were flg22-dependently upregulated in wrky18 wrky40 compared with the wild type, indicating that WRKY18 and WRKY40 often fulfill negative regulatory functions in combination with positive regulators at target sites, thereby very likely ensuring proper spatiotemporal transcriptional outputs (Table 10; Supplemental Data Set 7). This negative function in early MAMP-induced responses is in accordance with wrky18 wrky40 double mutants being less susceptible to the virulent bacterium Pst DC3000 than the wild type (Xu et al., 2006) and with WRKY40 acting together with BZR1 in balancing the trade-off between defense and growth (Lozano-Durán et al., 2013). Moreover, it is also consistent with a recent large genome-wide study on ABA-treated Arabidopsis seedlings revealing that highly upregulated genes are targeted by multiple TFs and that they act in concert to dynamically modulate transcription and to rapidly restore basal expression levels after stimulation (Song et al., 2016).
Table 10. DRTs at 2 h flg22 of WRKY18 and/or WRKY40 in wrky18, wrky40, and wrky18 wrky40.
In summary, we have defined the genome-wide in vivo binding sites of three Arabidopsis WRKY TFs during early MTI. Our study revealed that the two related WRKY factors WRKY18 and WRKY40 targeted nearly the same set of genes during this response, resulting in the altered expression of an almost identical subgroup of genes consistent with these WRKY factors being in part functionally redundant. Moreover, although WRKY33 targets additional unique loci, in many cases it targets the same promoters, or even the same DNA sites as WRKY18 and WRKY40. An additional key finding was that these three WRKY factors target numerous genes encoding key components of both MAMP and DAMP perception and signaling, providing a mechanistic link between these two functionally interconnected basal defense pathways (Albert, 2013; Bartels and Boller, 2015). Finally, our data imply that WRKY18 and WRKY40 are negative-acting regulatory components of numerous flg22-induced early response genes that act together with TF activators to modulate expression in a robust manner. The comprehensive data provide valuable insights that should prove helpful in our endeavor to define the genome-wide transcriptional regulatory network affected by WRKY TFs in the course of plant immunity.
METHODS
Plant Material
For all experiments, seedlings of the Arabidopsis thaliana ecotype Columbia (Col-0) or mutants in the Col-0 background were used. Besides wild-type plants, insertion mutants for WRKY18 (GABI_328G03), WRKY40 (SLAT collection of dSpm insertion lines; Shen et al., 2007), and WRKY33 (GABI_324B11) were used. The double mutant wrky18 wrky40 was obtained by crossing the corresponding single mutants (Shen et al., 2007). The ProWRKY33:WRKY33-HA complementation line in the wrky33 mutant was described earlier (Birkenbihl et al., 2012). To generate the ProWRKY18:WRKY18-HA complementation line, the 5.9-kb genomic fragment (Chromosome 4: nucleotides 15378866 to 15384809), consisting of the 4.4-kb upstream region up to the next gene and the 1.5-kb intron containing coding region without the stop codon was amplified by PCR, and cloned into the vector pAM-KAN-HA in front of the sequence encoding the HA-epitope tag. For ProWRKY40:WRKY40-HA, the 8.7-kb fragment (Chromosome 1: nucleotides 30376646 to 30385353), consisting of a 7.2-kb upstream region and 1.5-kb coding region was used. In both cases, the 3′UTR was replaced by a 35S CaMV terminator sequence. All oligonucleotides used in this study are described in Supplemental Table 3. The constructs were transformed via Agrobacterium tumefaciens into the respective single mutants.
Successfully transformed plants were identified by kanamycin selection. To express the same genomic constructs in the wrky18 wrky40 double mutant, they were cloned into a pAMP-HYG-HA vector conferring hygromycin resistance to the transformed plants. To confirm functionality of the constructs, transformants were tested for their ability to render resistant wrky18 wrky40 plants susceptible to the powdery mildew fungus Golovinomyces orontii (Pandey et al., 2010).
Plant Growth and flg22 Treatment
To establish seedling cultures, seeds were surface sterilized with ethanol and subsequently grown in 1× MS medium supplemented with 0.5% sucrose and 0.1% claforan. After 12 d in a light chamber at long-day conditions (16 h light/8 h dark) illuminated by daylight-white fluorescence lamps (FL40SS ENW/37; Osram), the seedlings were treated with flg22 by replacing the growth medium with medium containing 1 µM flg22.
ChIP-Seq and ChIP-qPCR Experiments
For the ChIP experiments, two independent biological replicates were created at different times with the wild type and the complementation lines WRKY18-HA, WRKY40-HA, and WRKY33-HA. For each line and treatment, 2 g of whole seedlings was collected from separate cultures 2 h after the medium exchange, either without flg22 (0 h) or with medium containing flg22 (2 h), and processed separately as described previously (Birkenbihl et al., 2012), following the modified protocol of Gendrel et al. (2005). The immunoprecipitation was performed using a rabbit polyclonal anti-HA antibody (Sigma-Aldrich; catalog number H6908). The precipitated DNA was purified with the Qia quick PCR purification kit (Qiagen) and processed further for ChIP-seq. To prepare the ChIP-seq libraries, the DNA was first amplified by two rounds of linear DNA amplification (LinDA; Shankaranarayanan et al., 2011) and then libraries were constructed with the NEBNext ChIP-seq library construction kit (New England Biolabs). The libraries were sequenced at the Max Planck Genome Centre Cologne with an Illumina HiSeq 2500, resulting in 7 to 20 million 100-bp single-end reads per sample.
ChIP-Seq Data Analysis
ChIP-seq data processing and analysis was performed as described by Liu et al. (2015) using the TAIR10 Arabidopsis reference genome (http://www.arabidopsis.org). The ChIP-seq data created in this study have been deposited at the Gene Expression Omnibus (GEO) repository (GSE85922).
To identify potential WRKY binding regions (“peak regions”), the QuEST peak calling program (version 2.4; Valouev et al., 2008) was used as described by Liu et al. (2015) to search for genomic DNA regions enriched in sequencing reads in the WRKY complementation lines compared with the corresponding wild-type samples. The peak calling was performed separately for the two biological replicates, the mapped reads of both replicates were pooled, and peaks were called for the pooled samples. Peaks were annotated with respect to nearby gene features in TAIR10 using the annotatePeaks.pl function from the Homer suite (Heinz et al., 2010) with default settings. Consistent peaks between the replicates were identified as described by Liu et al. (2015), i.e., peak regions were counted as consistent, if they were found to be overlapping between the both replicates as well as the pooled sample (by at least 50% of the smaller region).
To search for conserved binding motifs in the consistent WRKY binding regions, for each consistent peak the 500-bp sequence surrounding the peak maximum was extracted and submitted to the online version of MEME-ChIP (Machanick and Bailey, 2011). MEME-ChIP was run with default settings, but a custom background model derived from the Arabidopsis genome was provided and “any number of repetitions” of a motif was allowed. To extract the number/percentage of peak regions that contain a certain motif, the online version of FIMO (Grant et al., 2011) was run with the peak sequences and the motif of interest (either MEME/DREME output or known W-box motif) as input and a P value threshold of 0.001. To identify all locations of the W-box motif (TTGACT/C) in the complete Arabidopsis genome, the R function ‘matchPattern’ (Pagès, 2016; https://bioconductor.org/packages/release/bioc/html/BSgenome.html) was used.
RNA Extraction and Quantitative RT-PCR
For RT-qPCR and the RNA-seq experiments (see below), three independent biological replicates were used: Whole seedlings of the indicated genotypes for each treatment were separately grown in three parallel liquid culture sets and processed separately. Total RNA was extracted from 100 mg of untreated or flg22-treated seedlings using the TRI reagent solution (Applied Biosystems; AM9738) following the manufacturer’s instructions. The RNA was treated with DNase I (Roche) and purified using the RNeasy MiniElute cleanup kit (Qiagen). For RT-qPCR, the RNA was reverse transcribed with an oligo(dT) primer to produce cDNA using the SuperScript first-strand synthesis system for RT-PCR following the manufacturer’s protocol (Invitrogen). cDNA corresponding to 2.5 ng of total RNA was subjected to qPCR with gene-specific primers (Supplemental Table 3) using the Brilliant SYBR Green qPCR core reagent kit (Stratagene). The qPCRs were performed on the iQ5 Multicolor Real-Time PCR detection system (Bio-Rad) with two technical replicates in the same run and the three biological replicates in different runs. The data were analyzed using the DDCt method (Livak and Schmittgen, 2001), and RNA levels are indicated as relative expression compared with the endogenous reference gene At4g26410 (Czechowski et al., 2005). Error bars represent the sd of the three biological replicates.
Immunoblot Analysis
To analyze the levels of HA-tagged WRKY proteins from seedlings, total proteins were extracted with Laemmli buffer and equal amounts separated by 8% SDS-PAGE. The blot was probed with a monoclonal rat antibody against the HA-tag (Roche; cat. no. 1867423) and developed with a secondary, peroxidase-conjugated goat anti-rat antibody (Sigma-Aldrich; A9037) using the ECL system according to standard protocols.
RNA-Seq Experiment
For RNA-seq, DNase I-treated and purified RNAs from three biological replicates (as described for RT-qPCR) were used. Libraries for mRNA sequencing were constructed using the TrueSeq RNA sample preparation Kit (Illumina), and the three biological replicates for each condition were sequenced at the Max Planck Genome Centre Cologne with an Illumina HiSeq 2500, resulting in 23 to 44 million 100-bp single-end reads per sample. The obtained reads were mapped to the Arabidopsis genome as described by Liu et al. (2015). The RNA-seq data created in this study have been deposited at the GEO repository (GSE85923).
RNA-Seq Data Analysis
The mapped RNA-seq reads were transformed into a read count per gene per sample using the htseq-count script (s = reverse, t = exon) in the package HTSeq (Anders et al., 2015). Genes with <100 reads in all samples together were discarded; subsequently, the count data of the remaining genes were TMM normalized and log2 transformed using functions ‘calcNormFactors’ (R package EdgeR; Robinson et al., 2010) and ‘voom’ (R package limma; Law et al., 2014). To be able to analyze differential gene expression both between treated and untreated samples within each genotype and between the different mutants (wrky18, wrky40, and wrky18 wrky40) and the wild-type genotype (Col-0), a linear model with the explanatory variable ‘genotype_treatment’ (i.e., encoding both genotype and treatment information) was fitted for each gene using the function lmFit (R package limma). Subsequently, moderated t tests were performed over the different contrasts of interest, comparing flg22-treated with untreated samples within each genotype (four contrasts) and comparing each of the mutants with the wild type within each treatment (six contrasts). In all cases, the resulting P values were adjusted for false discoveries due to multiple hypothesis testing via the Benjamini-Hochberg procedure. For each contrast, we extracted a set of significantly differentially expressed genes between the tested conditions (adjusted P value ≤ 0.05, |log2FCΙ ≥ 1).
GO Analysis
For GO analysis, the corresponding tool on the TAIR webpage (http://www.arabidopsis.org/tools/bulk/go/index.jsp) was used. The numbers of genes associated to each GO term in the identified sets of ChIP-seq targets, transcriptionally regulated genes (DEGs), and DRTs for each WRKY factor were transformed to relative abundances within each data set and compared with the relative abundance of the respective GO term in the whole genome. To assess statistical significance of the observed enrichment or underrepresentation of each GO term in the different analyzed gene sets, the P values were calculated using a hypergeometric test.
Accession Numbers
The ChIP-seq data and the RNA-seq data created in this study have been deposited at the GEO repository with the accession numbers GSE85922 and GSE85923, respectively. The related GEO Super Series Number is GSE85924.
Supplemental Data
Supplemental Figure 1. IGV images of WRKY18, WRKY40, and WRKY33 binding to the CRK2, ICS1, PEN2, CYP83B1, CYP71A13, and FLS2 loci.
Supplemental Figure 2. Distribution of W-box locations in the Arabidopsis genome.
Supplemental Figure 3. The number of identified W-boxes per binding region is independent of the region size for WRKY18, WRKY40, and WRKY33.
Supplemental Figure 4. Binding of WRKY18 or WRKY40 to selected target genes in the wrky18, wrky40, or wrky18 wrky40 mutant.
Supplemental Figure 5. GO analysis of WRKY18, WRKY40, and WRKY33 target genes.
Supplemental Figure 6. GO analysis of genes that exhibit flg22-induced changes of gene expression in wild-type seedlings.
Supplemental Figure 7. Up- and downregulated WRKY target genes upon 2 h flg22 treatment.
Supplemental Figure 8. Target genes common to SARD1 and WRKY18, WRKY40, and WRKY33.
Supplemental Figure 9. GO analysis of WRKY18 and WRKY40 target genes and their directly regulated targets in wrky18 wrky40.
Supplemental Table 1. WRKY18, WRKY40, and WRKY33 binding to WRKY genes and fold changes of expression of these targets in wild-type plants upon flg22 treatment.
Supplemental Table 2. WRKY18 and WRKY40 DRTs in wrky18 wrky40 that are also bound by SARD1.
Supplemental Table 3. Primers used for cloning, RT-qPCR, and ChIP-qPCR.
Supplemental Data Set 1. Genome-wide binding sites and target genes of WRKY18, WRKY40, and WRKY33 detected after 2 h flg22 treatment.
Supplemental Data Set 2. Increased enrichment of WRKY18 at target genes defined by clearly higher binding scores at 2 h than at 0 h flg22.
Supplemental Data Set 3. Overlap between WRKY18, WRKY40, and WRKY33 target gene sets after 2 h flg22 treatment (corresponds to VENN diagram Figure 4).
Supplemental Data Set 4. WRKY18, WRKY40, and WRKY33 target genes related to the GO term kinase activity.
Supplemental Data Set 5. flg22-responsive genes in wild-type plants and wrky18, wrky40, and wrky18 wrky40 mutants.
Supplemental Data Set 6. Differentially expressed genes upon flg22 treatment in wrky18, wrky40, or wrky18 wrky40 mutants compared with wild-type plants.
Supplemental Data Set 7. Overlap of DEGs in wrky18, wrky40, or the wrky18 wrky40 mutant compared with wild-type plants upon flg22 treatment (corresponds to VENN diagram Figure 7C).
Supplemental Data Set 8. Up- and downregulated target and nontarget genes in response to flg22 treatment (corresponds to VENN diagram in Supplemental Figure 7).
Supplemental Data Set 9. Target genes common to SARD1 and WRKY18, WRKY40, and WRKY33 (corresponds to VENN diagram in Supplemental Figure 8).
Supplemental Data Set 10. WRKY18 and WRKY40 directly regulated target genes (corresponding to VENN diagrams in Figure 8).
Supplemental Data Set 11. Expression and binding data of WRKY18 and WRKY40 DRTs.
Supplemental Data Set 12. Directly regulated targets common to WRKY18 and WRKY40.
Acknowledgments
This work was supported by a Deutsche Forschungsgemeinschaft grant in the framework of SFB670 Cell Autonomous Immunity (to I.E.S. and B.K.).
AUTHOR CONTRIBUTIONS
R.P.B. conceived and designed the study, conducted experiments, acquired data, analyzed and interpreted data, and drafted and revised the manuscript. B.K. analyzed and interpreted data, and drafted and revised the manuscript. I.E.S. conceived and designed the study, analyzed and interpreted data, and drafted and revised the manuscript.
Glossary
- MAMP
microbe-associated molecular pattern
- PRR
pattern recognition receptor
- MTI
MAMP-triggered immunity
- TF
transcription factor
- ChIP-seq
chromatin immunoprecipitation-sequencing
- IGV
Integrative Genomics Viewer
- TSS
transcription start site
- UTR
untranslated region
- GO
Gene Ontology
- DAMP
damage-associated molecular pattern
- ET
ethylene
- JA
jasmonic acid
- ABA
abscisic acid
- SA
salicylic acid
- I3AOx
indole-3-acetaldoxime
- 4MI3MG
4(and 1)-methoxy-indol-3-yl-methyl glucosinolate
- FC
fold change
- FDR
false discovery rate
- DEG
differentially expressed gene
- DRT
directly regulated target gene
- GEO
Gene Expression Omnibus
References
- Albert M. (2013). Peptides as triggers of plant defence. J. Exp. Bot. 64: 5269–5279. [DOI] [PubMed] [Google Scholar]
- Alonso J.M., Hirayama T., Roman G., Nourizadeh S., Ecker J.R. (1999). EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284: 2148–2152. [DOI] [PubMed] [Google Scholar]
- Anders S., Pyl P.T., Huber W. (2015). HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics 31: 166–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaad F.F., Qiu J.-L., Youngs H., Ehrhardt D., Zimmerli L., Kalde M., Wanner G., Peck S.C., Edwards H., Ramonell K., Somerville C.R., Thordal-Christensen H. (2004). The PEN1 syntaxin defines a novel cellular compartment upon fungal attack and is required for the timely assembly of papillae. Mol. Biol. Cell 15: 5118–5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey T.L. (2011). DREME: motif discovery in transcription factor ChIP-seq data. Bioinformatics 27: 1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey T.L., Machanick P. (2012). Inferring direct DNA binding from ChIP-seq. Nucleic Acids Res. 40: e128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak S., Beisson F., Bishop G., Hamberger B., Höfer R., Paquette S., Werck-Reichhart D. (2011). Cytochromes P450. The Arabidopsis Book 9: e0144, doi/10.1199/tab.0144. [DOI] [PMC free article] [PubMed]
- Bartels S., Boller T. (2015). Quo vadis, Pep? Plant elicitor peptides at the crossroads of immunity, stress, and development. J. Exp. Bot. 66: 5183–5193. [DOI] [PubMed] [Google Scholar]
- Birkenbihl R.P., Diezel C., Somssich I.E. (2012). Arabidopsis WRKY33 is a key transcriptional regulator of hormonal and metabolic responses toward Botrytis cinerea infection. Plant Physiol. 159: 266–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekgaarden C., Caarls L., Vos I.A., Pieterse C.M.J., Van Wees S.C.M. (2015). Ethylene: Traffic controller on hormonal crossroads to defense. Plant Physiol. 169: 2371–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canet J.V., Dobón A., Fajmonová J., Tornero P. (2012). The BLADE-ON-PETIOLE genes of Arabidopsis are essential for resistance induced by methyl jasmonate. BMC Plant Biol. 12: 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchini N.M., Jung H.W., Engle N.L., Tschaplinski T.J., Greenberg J.T. (2015). ALD1 regulates basal immune components and early inducible defense responses in Arabidopsis. Mol. Plant Microbe Interact. 28: 455–466. [DOI] [PubMed] [Google Scholar]
- Chi Y., Yang Y., Zhou Y., Zhou J., Fan B., Yu J.-Q., Chen Z. (2013). Protein-protein interactions in the regulation of WRKY transcription factors. Mol. Plant 6: 287–300. [DOI] [PubMed] [Google Scholar]
- Chini A., Fonseca S., Fernández G., Adie B., Chico J.M., Lorenzo O., García-Casado G., López-Vidriero I., Lozano F.M., Ponce M.R., Micol J.L., Solano R. (2007). The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448: 666–671. [DOI] [PubMed] [Google Scholar]
- Choura M., Rebai A., Masmoudi K. (2015). Unraveling the WRKY transcription factors network in Arabidopsis thaliana by integrative approach. New Biol. 5: 55–61. [Google Scholar]
- Clay N.K., Adio A.M., Denoux C., Jander G., Ausubel F.M. (2009). Glucosinolate metabolites required for an Arabidopsis innate immune response. Science 323: 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czechowski T., Stitt M., Altmann T., Udvardi M.K., Scheible W.-R. (2005). Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol. 139: 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulgem T. (2006). Dissecting the WRKY web of plant defense regulators. PLoS Pathog. 2: e126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulgem T., Somssich I.E. (2007). Networks of WRKY transcription factors in defense signaling. Curr. Opin. Plant Biol. 10: 366–371. [DOI] [PubMed] [Google Scholar]
- Felix G., Duran J.D., Volko S., Boller T. (1999). Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 18: 265–276. [DOI] [PubMed] [Google Scholar]
- Frerigmann H., Gigolashvili T. (2014). MYB34, MYB51, and MYB122 distinctly regulate indolic glucosinolate biosynthesis in Arabidopsis thaliana. Mol. Plant 7: 814–828. [DOI] [PubMed] [Google Scholar]
- Fu Z.Q., Yan S., Saleh A., Wang W., Ruble J., Oka N., Mohan R., Spoel S.H., Tada Y., Zheng N., Dong X. (2012). NPR3 and NPR4 are receptors for the immune signal salicylic acid in plants. Nature 486: 228–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagne J.M., Smalle J., Gingerich D.J., Walker J.M., Yoo S.-D., Yanagisawa S., Vierstra R.D. (2004). Arabidopsis EIN3-binding F-box 1 and 2 form ubiquitin-protein ligases that repress ethylene action and promote growth by directing EIN3 degradation. Proc. Natl. Acad. Sci. USA 101: 6803–6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M., Wang X., Wang D., Xu F., Ding X., Zhang Z., Bi D., Cheng Y.T., Chen S., Li X., Zhang Y. (2009). Regulation of cell death and innate immunity by two receptor-like kinases in Arabidopsis. Cell Host Microbe 6: 34–44. [DOI] [PubMed] [Google Scholar]
- Gao Q.-M., Venugopal S., Navarre D., Kachroo A. (2011). Low oleic acid-derived repression of jasmonic acid-inducible defense responses requires the WRKY50 and WRKY51 proteins. Plant Physiol. 155: 464–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcion C., Lohmann A., Lamodière E., Catinot J., Buchala A., Doermann P., Métraux J.-P. (2008). Characterization and biological function of the ISOCHORISMATE SYNTHASE2 gene of Arabidopsis. Plant Physiol. 147: 1279–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendrel A.-V., Lippman Z., Martienssen R., Colot V. (2005). Profiling histone modification patterns in plants using genomic tiling microarrays. Nat. Methods 2: 213–218. [DOI] [PubMed] [Google Scholar]
- Grant C.E., Bailey T.L., Noble W.S. (2011). FIMO: scanning for occurrences of a given motif. Bioinformatics 27: 1017–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz S., Benner C., Spann N., Bertolino E., Lin Y.C., Laslo P., Cheng J.X., Murre C., Singh H., Glass C.K. (2010). Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38: 576–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyndrickx K.S., Van de Velde J., Wang C., Weigel D., Vandepoele K. (2014). A functional and evolutionary perspective on transcription factor binding in Arabidopsis thaliana. Plant Cell 26: 3894–3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou S., Wang X., Chen D., Yang X., Wang M., Turrà D., Di Pietro A., Zhang W. (2014). The secreted peptide PIP1 amplifies immunity through receptor-like kinase 7. PLoS Pathog. 10: e1004331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P.-Y., Catinot J., Zimmerli L. (2016). Ethylene response factors in Arabidopsis immunity. J. Exp. Bot. 67: 1231–1241. [DOI] [PubMed] [Google Scholar]
- Huot B., Yao J., Montgomery B.L., He S.Y. (2014). Growth-defense tradeoffs in plants: a balancing act to optimize fitness. Mol. Plant 7: 1267–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing Y., Lin R. (2015). The VQ motif-containing protein family of plant-specific transcriptional regulators. Plant Physiol. 169: 371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadota Y., Sklenar J., Derbyshire P., Stransfeld L., Asai S., Ntoukakis V., Jones J.D., Shirasu K., Menke F., Jones A., Zipfel C. (2014). Direct regulation of the NADPH oxidase RBOHD by the PRR-associated kinase BIK1 during plant immunity. Mol. Cell 54: 43–55. [DOI] [PubMed] [Google Scholar]
- Law C.W., Chen Y., Shi W., Smyth G.K. (2014). voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 15: R29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Meng X., Shan L., He P. (2016). Transcriptional regulation of pattern-triggered immunity in plants. Cell Host Microbe 19: 641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Meng X., Wang R., Mao G., Han L., Liu Y., Zhang S. (2012). Dual-level regulation of ACC synthase activity by MPK3/MPK6 cascade and its downstream WRKY transcription factor during ethylene induction in Arabidopsis. PLoS Genet. 8: e1002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Zhang J., Li J., Zhou G., Wang Q., Bian W., Erb M., Lou Y. (2015). Prioritizing plant defence over growth through WRKY regulation facilitates infestation by non-target herbivores. eLife 4: e04805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Clarke J.D., Zhang Y., Dong X. (2001). Activation of an EDS1-mediated R-gene pathway in the snc1 mutant leads to constitutive, NPR1-independent pathogen resistance. Mol. Plant Microbe Interact. 14: 1131–1139. [DOI] [PubMed] [Google Scholar]
- Liang X., Ding P., Lian K., Wang J., Ma M., Li L., Li L., Li M., Zhang X., Chen S., Zhang Y., Zhou J.-M. (2016). Arabidopsis heterotrimeric G proteins regulate immunity by directly coupling to the FLS2 receptor. eLife 5: e13568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Kracher B., Ziegler J., Birkenbihl R.P., Somssich I.E. (2015). Negative regulation of ABA signaling by WRKY33 is critical for Arabidopsis immunity towards Botrytis cinerea 2100. eLife 4: e07295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- Logemann E., Birkenbihl R.P., Rawat V., Schneeberger K., Schmelzer E., Somssich I.E. (2013). Functional dissection of the PROPEP2 and PROPEP3 promoters reveals the importance of WRKY factors in mediating microbe-associated molecular pattern-induced expression. New Phytol. 198: 1165–1177. [DOI] [PubMed] [Google Scholar]
- Lozano-Durán R., Macho A.P., Boutrot F., Segonzac C., Somssich I.E., Zipfel C. (2013). The transcriptional regulator BZR1 mediates trade-off between plant innate immunity and growth. eLife 2: e00983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D., Lin W., Gao X., Wu S., Cheng C., Avila J., Heese A., Devarenne T.P., He P., Shan L. (2011). Direct ubiquitination of pattern recognition receptor FLS2 attenuates plant innate immunity. Science 332: 1439–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K., Liang S., Wu Z., Bi C., Yu Y.-T., Wang X.-F., Zhang D.-P. (2016). Overexpression of an Arabidopsis cysteine-rich receptor-like protein kinase, CRK5, enhances abscisic acid sensitivity and confers drought tolerance. J. Exp. Bot. 67: 5009–5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machanick P., Bailey T.L. (2011). MEME-ChIP: motif analysis of large DNA datasets. Bioinformatics 27: 1696–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macho A.P., Zipfel C. (2014). Plant PRRs and the activation of innate immune signaling. Mol. Cell 54: 263–272. [DOI] [PubMed] [Google Scholar]
- Mao G., Meng X., Liu Y., Zheng Z., Chen Z., Zhang S. (2011). Phosphorylation of a WRKY transcription factor by two pathogen-responsive MAPKs drives phytoalexin biosynthesis in Arabidopsis. Plant Cell 23: 1639–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møldrup M.E., Salomonsen B., Geu-Flores F., Olsen C.E., Halkier B.A. (2013). De novo genetic engineering of the camalexin biosynthetic pathway. J. Biotechnol. 167: 296–301. [DOI] [PubMed] [Google Scholar]
- Monaghan J., Zipfel C. (2012). Plant pattern recognition receptor complexes at the plasma membrane. Curr. Opin. Plant Biol. 15: 349–357. [DOI] [PubMed] [Google Scholar]
- Navarro L., Zipfel C., Rowland O., Keller I., Robatzek S., Boller T., Jones J.D.G. (2004). The transcriptional innate immune response to flg22. Interplay and overlap with Avr gene-dependent defense responses and bacterial pathogenesis. Plant Physiol. 135: 1113–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman M.-A., Sundelin T., Nielsen J.T., Erbs G. (2013). MAMP (microbe-associated molecular pattern) triggered immunity in plants. Front. Plant Sci. 4: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okrent R.A., Brooks M.D., Wildermuth M.C. (2009). Arabidopsis GH3.12 (PBS3) conjugates amino acids to 4-substituted benzoates and is inhibited by salicylate. J. Biol. Chem. 284: 9742–9754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagès H. (2016). BSgenome: Infrastructure for Biostrings-based genome data packages and support for efficient SNP representation. R package version 1.40.1, https://bioconductor.org/packages/release/bioc/html/BSgenome.html. [Google Scholar]
- Pandey G.K., Grant J.J., Cheong Y.H., Kim B.G., Li L., Luan S. (2005). ABR1, an APETALA2-domain transcription factor that functions as a repressor of ABA response in Arabidopsis. Plant Physiol. 139: 1185–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey S.P., Roccaro M., Schön M., Logemann E., Somssich I.E. (2010). Transcriptional reprogramming regulated by WRKY18 and WRKY40 facilitates powdery mildew infection of Arabidopsis. Plant J. 64: 912–923. [DOI] [PubMed] [Google Scholar]
- Pfalz M., Mikkelsen M.D., Bednarek P., Olsen C.E., Halkier B.A., Kroymann J. (2011). Metabolic engineering in Nicotiana benthamiana reveals key enzyme functions in Arabidopsis indole glucosinolate modification. Plant Cell 23: 716–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse C.M.J., Van der Does D., Zamioudis C., Leon-Reyes A., Van Wees S.C.M. (2012). Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 28: 489–521. [DOI] [PubMed] [Google Scholar]
- Pré M., Atallah M., Champion A., De Vos M., Pieterse C.M.J., Memelink J. (2008). The AP2/ERF domain transcription factor ORA59 integrates jasmonic acid and ethylene signals in plant defense. Plant Physiol. 147: 1347–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J.-L., et al. (2008). Arabidopsis MAP kinase 4 regulates gene expression through transcription factor release in the nucleus. EMBO J. 27: 2214–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert-Seilaniantz A., Grant M., Jones J.D.G. (2011). Hormone crosstalk in plant disease and defense: more than just jasmonate-salicylate antagonism. Annu. Rev. Phytopathol. 49: 317–343. [DOI] [PubMed] [Google Scholar]
- Robinson M.D., McCarthy D.J., Smyth G.K. (2010). edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26: 139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton P.J., Somssich I.E., Ringler P., Shen Q.J. (2010). WRKY transcription factors. Trends Plant Sci. 15: 247–258. [DOI] [PubMed] [Google Scholar]
- Schön M., Töller A., Diezel C., Roth C., Westphal L., Wiermer M., Somssich I.E. (2013). Analyses of wrky18 wrky40 plants reveal critical roles of SA/EDS1 signaling and indole-glucosinolate biosynthesis for Golovinomyces orontii resistance and a loss-of resistance towards Pseudomonas syringae pv. tomato AvrRPS4. Mol. Plant Microbe Interact. 26: 758–767. [DOI] [PubMed] [Google Scholar]
- Schweizer F., Bodenhausen N., Lassueur S., Masclaux F.G., Reymond P. (2013). Differential contribution of transcription factors to Arabidopsis thaliana defence against Spodoptera littoralis. Front. Plant Sci. 4: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwessinger B., Ronald P.C. (2012). Plant innate immunity: perception of conserved microbial signatures. Annu. Rev. Plant Biol. 63: 451–482. [DOI] [PubMed] [Google Scholar]
- Serrano M., Wang B., Aryal B., Garcion C., Abou-Mansour E., Heck S., Geisler M., Mauch F., Nawrath C., Métraux J.-P. (2013). Export of salicylic acid from the chloroplast requires the multidrug and toxin extrusion-like transporter EDS5. Plant Physiol. 162: 1815–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankaranarayanan P., Mendoza-Parra M.-A., Walia M., Wang L., Li N., Trindade L.M., Gronemeyer H. (2011). Single-tube linear DNA amplification (LinDA) for robust ChIP-seq. Nat. Methods 8: 565–567. [DOI] [PubMed] [Google Scholar]
- Shen Q.-H., Saijo Y., Mauch S., Biskup C., Bieri S., Keller B., Seki H., Ulker B., Somssich I.E., Schulze-Lefert P. (2007). Nuclear activity of MLA immune receptors links isolate-specific and basal disease-resistance responses. Science 315: 1098–1103. [DOI] [PubMed] [Google Scholar]
- Sønderby I.E., Geu-Flores F., Halkier B.A. (2010). Biosynthesis of glucosinolates--gene discovery and beyond. Trends Plant Sci. 15: 283–290. [DOI] [PubMed] [Google Scholar]
- Song J.T., Lu H., McDowell J.M., Greenberg J.T. (2004). A key role for ALD1 in activation of local and systemic defenses in Arabidopsis. Plant J. 40: 200–212. [DOI] [PubMed] [Google Scholar]
- Song L., Huang S.-C., Wise A., Castanon R., Nery J.R., Chen H., Watanabe M., Thomas J., Bar-Joseph Z., Ecker J.R. (2016). A transcription factor hierarchy defines an environmental stress response network. Science 354: 598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T., Zhang Y., Li Y., Zhang Q., Ding Y., Zhang Y. (2015). ChIP-seq reveals broad roles of SARD1 and CBP60g in regulating plant immunity. Nat. Commun. 6: 10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Li L., Macho A.P., Han Z., Hu Z., Zipfel C., Zhou J.M., Chai J. (2013). Structural basis for flg22-induced activation of the Arabidopsis FLS2-BAK1 immune complex. Science 342: 624–628. [DOI] [PubMed] [Google Scholar]
- Tena G., Boudsocq M., Sheen J. (2011). Protein kinase signaling networks in plant innate immunity. Curr. Opin. Plant Biol. 14: 519–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorvaldsdóttir H., Robinson J.T., Mesirov J.P. (2013). Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief. Bioinform. 14: 178–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truman W., Glazebrook J. (2012). Co-expression analysis identifies putative targets for CBP60g and SARD1 regulation. BMC Plant Biol. 12: 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda K., Somssich I.E. (2015). Transcriptional networks in plant immunity. New Phytol. 206: 932–947. [DOI] [PubMed] [Google Scholar]
- Tsuda K., Sato M., Stoddard T., Glazebrook J., Katagiri F. (2009). Network properties of robust immunity in plants. PLoS Genet. 5: e1000772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valouev A., Johnson D.S., Sundquist A., Medina C., Anton E., Batzoglou S., Myers R.M., Sidow A. (2008). Genome-wide analysis of transcription factor binding sites based on ChIP-Seq data. Nat. Methods 5: 829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidhyasekaran P. (2014). PAMP Signals in Plant Innate Immunity: Signal Perception and Transduction. (Dordrecht, The Netherlands: Springer; ). [Google Scholar]
- Wan J., Zhang X.-C., Neece D., Ramonell K.M., Clough S., Kim S.Y., Stacey M.G., Stacey G. (2008). A LysM receptor-like kinase plays a critical role in chitin signaling and fungal resistance in Arabidopsis. Plant Cell 20: 471–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Gao J., Zhu Z., Dong X., Wang X., Ren G., Zhou X., Kuai B. (2015). TCP transcription factors are critical for the coordinated regulation of isochorismate synthase 1 expression in Arabidopsis thaliana. Plant J. 82: 151–162. [DOI] [PubMed] [Google Scholar]
- Weigel R.R., Pfitzner U.M., Gatz C. (2005). Interaction of NIMIN1 with NPR1 modulates PR gene expression in Arabidopsis. Plant Cell 17: 1279–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widemann E., Miesch L., Lugan R., Holder E., Heinrich C., Aubert Y., Miesch M., Pinot F., Heitz T. (2013). The amidohydrolases IAR3 and ILL6 contribute to jasmonoyl-isoleucine hormone turnover and generate 12-hydroxyjasmonic acid upon wounding in Arabidopsis leaves. J. Biol. Chem. 288: 31701–31714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Meng J., Meng X., Zhao Y., Liu J., Sun T., Liu Y., Wang Q., Zhang S. (2016). Pathogen-responsive MPK3 and MPK6 reprogram the biosynthesis of indole glucosinolates and their derivatives in Arabidopsis immunity. Plant Cell 28: 1144–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Chen C., Fan B., Chen Z. (2006). Physical and functional interactions between pathogen-induced Arabidopsis WRKY18, WRKY40, and WRKY60 transcription factors. Plant Cell 18: 1310–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S.-D., Cho Y.-H., Tena G., Xiong Y., Sheen J. (2008). Dual control of nuclear EIN3 by bifurcate MAPK cascades in C2H4 signalling. Nature 451: 789–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Han X., Shi R., Yang G., Qi L., Wang R., Li G. (2013). Arabidopsis cysteine-rich receptor-like kinase 45 positively regulates disease resistance to Pseudomonas syringae. Plant Physiol. Biochem. 73: 383–391. [DOI] [PubMed] [Google Scholar]
- Zheng X.Y., Zhou M., Yoo H., Pruneda-Paz J.L., Spivey N.W., Kay S.A., Dong X. (2015). Spatial and temporal regulation of biosynthesis of the plant immune signal salicylic acid. Proc. Natl. Acad. Sci. USA 112: 9166–9173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z., Qamar S.A., Chen Z., Mengiste T. (2006). Arabidopsis WRKY33 transcription factor is required for resistance to necrotrophic fungal pathogens. Plant J. 48: 592–605. [DOI] [PubMed] [Google Scholar]
- Zipfel C., Robatzek S., Navarro L., Oakeley E.J., Jones J.D.G., Felix G., Boller T. (2004). Bacterial disease resistance in Arabidopsis through flagellin perception. Nature 428: 764–767. [DOI] [PubMed] [Google Scholar]
- Zipfel C., Kunze G., Chinchilla D., Caniard A., Jones J.D.G., Boller T., Felix G. (2006). Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell 125: 749–760. [DOI] [PubMed] [Google Scholar]