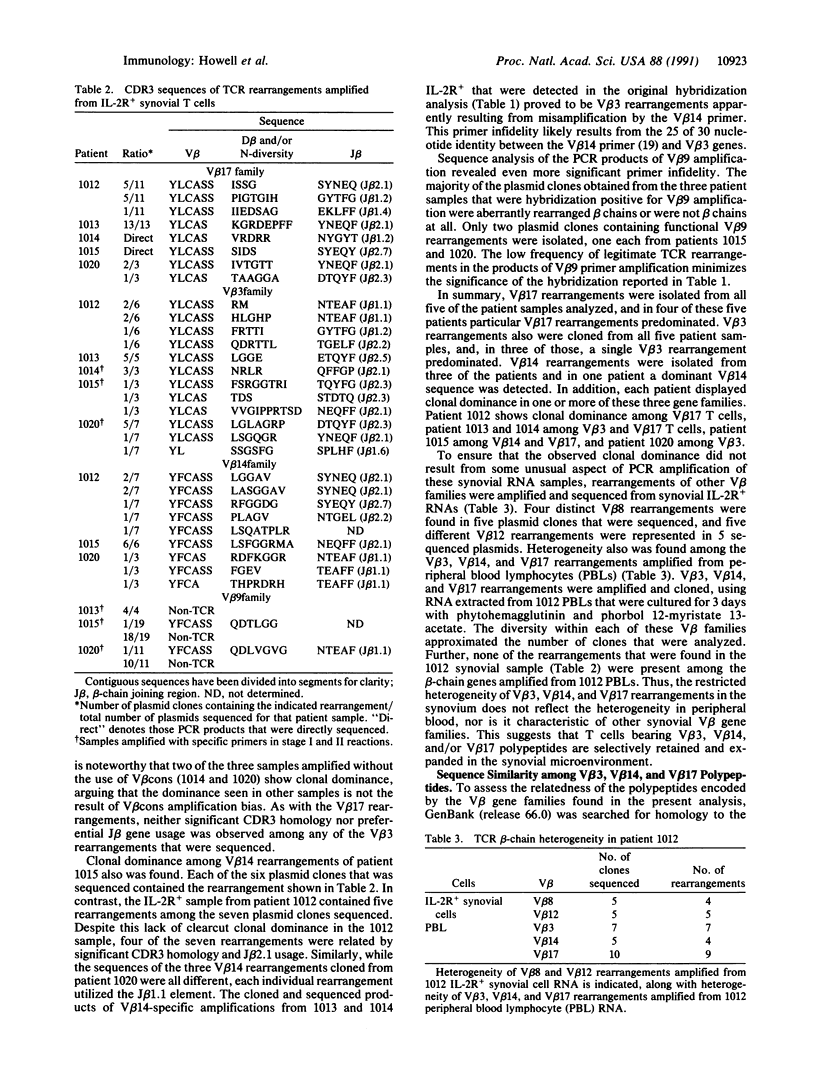
Abstract
Rheumatoid arthritis (RA) is a disease affecting the synovial membranes of articulating joints that is thought to result from T-cell-mediated autoimmune phenomena. T cells responsible for the pathogenesis of RA are likely present in that fraction of synovial T cells that expresses the interleukin 2 receptor (IL-2R), one marker of T-cell activation. We report herein an analysis of T-cell receptor (TCR) beta-chain gene expression by IL-2R-positive synovial T cells. These T cells were isolated from uncultured synovial tissue specimens by using IL-2R-specific monoclonal antibodies and magnetic beads, and TCR beta-chain transcription was analyzed by PCR-catalyzed amplification using a panel of primers specific for the human TCR beta-chain variable region (V beta). Multiple V beta gene families were found to be transcribed in these patients samples; however, three gene families, V beta 3, V beta 14, and V beta 17, were found in a majority of the five synovial samples analyzed, suggesting that T cells bearing these V beta s had been selectively retained in the synovial microenvironment. In many instances, the V beta 3, V beta 14, or V beta 17 repertoires amplified from an individual patient were dominated by a single rearrangement, indicative of clonal expansion in the synovium and supportive of a role for these T cells in RA. Of note is a high sequence similarity between V beta 3, V beta 14, and V beta 17 polypeptides, particularly in the fourth complementarity-determining region (CDR). Given that binding sites for superantigens have been mapped to the CDR4s of TCR beta chains, the synovial localization of T cells bearing V beta s with significant CDR4 homology indicates that V beta-specific T-cell activation by superantigen may play a role in RA.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell J. I., Denney D., Jr, Foster L., Belt T., Todd J. A., McDevitt H. O. Allelic variation in the DR subregion of the human major histocompatibility complex. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6234–6238. doi: 10.1073/pnas.84.17.6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmester G. R., Jahn B., Gramatzki M., Zacher J., Kalden J. R. Activated T cells in vivo and in vitro: divergence in expression of Tac and Ia antigens in the nonblastoid small T cells of inflammation and normal T cells activated in vitro. J Immunol. 1984 Sep;133(3):1230–1234. [PubMed] [Google Scholar]
- Burns F. R., Li X. B., Shen N., Offner H., Chou Y. K., Vandenbark A. A., Heber-Katz E. Both rat and mouse T cell receptors specific for the encephalitogenic determinant of myelin basic protein use similar V alpha and V beta chain genes even though the major histocompatibility complex and encephalitogenic determinants being recognized are different. J Exp Med. 1989 Jan 1;169(1):27–39. doi: 10.1084/jem.169.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazenave P. A., Marche P. N., Jouvin-Marche E., Voegtlé D., Bonhomme F., Bandeira A., Coutinho A. V beta 17 gene polymorphism in wild-derived mouse strains: two amino acid substitutions in the V beta 17 region greatly alter T cell receptor specificity. Cell. 1990 Nov 16;63(4):717–728. doi: 10.1016/0092-8674(90)90138-5. [DOI] [PubMed] [Google Scholar]
- Chatila M. K., Pandolfi F., Stamenkovich I., Kurnick J. T. Clonal dominance among synovial tissue-infiltrating lymphocytes in arthritis. Hum Immunol. 1990 Jun;28(2):252–257. doi: 10.1016/0198-8859(90)90026-l. [DOI] [PubMed] [Google Scholar]
- Choi Y. W., Herman A., DiGiusto D., Wade T., Marrack P., Kappler J. Residues of the variable region of the T-cell-receptor beta-chain that interact with S. aureus toxin superantigens. Nature. 1990 Aug 2;346(6283):471–473. doi: 10.1038/346471a0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cole B. C., Kartchner D. R., Wells D. J. Stimulation of mouse lymphocytes by a mitogen derived from Mycoplasma arthritidis (MAM). VIII. Selective activation of T cells expressing distinct V beta T cell receptors from various strains of mice by the "superantigen" MAM. J Immunol. 1990 Jan 15;144(2):425–431. [PubMed] [Google Scholar]
- Duke O., Panayi G. S., Janossy G., Poulter L. W. An immunohistological analysis of lymphocyte subpopulations and their microenvironment in the synovial membranes of patients with rheumatoid arthritis using monoclonal antibodies. Clin Exp Immunol. 1982 Jul;49(1):22–30. [PMC free article] [PubMed] [Google Scholar]
- Feldmann M., Londei M., Leech Z., Brennan F., Savill C., Maini R. N. Analysis of T cell clones in rheumatoid arthritis. Springer Semin Immunopathol. 1988;10(2-3):157–167. doi: 10.1007/BF01857221. [DOI] [PubMed] [Google Scholar]
- Friedman S. M., Crow M. K., Tumang J. R., Tumang M., Xu Y. Q., Hodtsev A. S., Cole B. C., Posnett D. N. Characterization of human T cells reactive with the Mycoplasma arthritidis-derived superantigen (MAM): generation of a monoclonal antibody against V beta 17, the T cell receptor gene product expressed by a large fraction of MAM-reactive human T cells. J Exp Med. 1991 Oct 1;174(4):891–900. doi: 10.1084/jem.174.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregersen P. K., Shen M., Song Q. L., Merryman P., Degar S., Seki T., Maccari J., Goldberg D., Murphy H., Schwenzer J. Molecular diversity of HLA-DR4 haplotypes. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2642–2646. doi: 10.1073/pnas.83.8.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregersen P. K., Silver J., Winchester R. J. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987 Nov;30(11):1205–1213. doi: 10.1002/art.1780301102. [DOI] [PubMed] [Google Scholar]
- Hart C., Schochetman G., Spira T., Lifson A., Moore J., Galphin J., Sninsky J., Ou C. Y. Direct detection of HIV RNA expression in seropositive subjects. Lancet. 1988 Sep 10;2(8611):596–599. doi: 10.1016/s0140-6736(88)90639-3. [DOI] [PubMed] [Google Scholar]
- Herman A., Croteau G., Sekaly R. P., Kappler J., Marrack P. HLA-DR alleles differ in their ability to present staphylococcal enterotoxins to T cells. J Exp Med. 1990 Sep 1;172(3):709–717. doi: 10.1084/jem.172.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell M. D., Winters S. T., Olee T., Powell H. C., Carlo D. J., Brostoff S. W. Vaccination against experimental allergic encephalomyelitis with T cell receptor peptides. Science. 1989 Nov 3;246(4930):668–670. doi: 10.1126/science.2814489. [DOI] [PubMed] [Google Scholar]
- Janeway C. A., Jr Self superantigens? Cell. 1990 Nov 16;63(4):659–661. doi: 10.1016/0092-8674(90)90130-7. [DOI] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Yagi J., Conrad P. J., Katz M. E., Jones B., Vroegop S., Buxser S. T-cell responses to Mls and to bacterial proteins that mimic its behavior. Immunol Rev. 1989 Feb;107:61–88. doi: 10.1111/j.1600-065x.1989.tb00003.x. [DOI] [PubMed] [Google Scholar]
- Janossy G., Panayi G., Duke O., Bofill M., Poulter L. W., Goldstein G. Rheumatoid arthritis: a disease of T-lymphocyte/macrophage immunoregulation. Lancet. 1981 Oct 17;2(8251):839–842. doi: 10.1016/s0140-6736(81)91107-7. [DOI] [PubMed] [Google Scholar]
- Jores R., Alzari P. M., Meo T. Resolution of hypervariable regions in T-cell receptor beta chains by a modified Wu-Kabat index of amino acid diversity. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9138–9142. doi: 10.1073/pnas.87.23.9138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff H., Binder A., Runge M., Meier B., Jacobs R., Busche K. Pathogenetic mechanisms in the Mycoplasma arthritidis polyarthritis of rats. Rheumatol Int. 1989;9(3-5):193–196. doi: 10.1007/BF00271879. [DOI] [PubMed] [Google Scholar]
- Kurosaka M., Ziff M. Immunoelectron microscopic study of the distribution of T cell subsets in rheumatoid synovium. J Exp Med. 1983 Oct 1;158(4):1191–1210. doi: 10.1084/jem.158.4.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald H. R., Schneider R., Lees R. K., Howe R. C., Acha-Orbea H., Festenstein H., Zinkernagel R. M., Hengartner H. T-cell receptor V beta use predicts reactivity and tolerance to Mlsa-encoded antigens. Nature. 1988 Mar 3;332(6159):40–45. doi: 10.1038/332040a0. [DOI] [PubMed] [Google Scholar]
- Marrack P., Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990 May 11;248(4956):705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- Martin R., Howell M. D., Jaraquemada D., Flerlage M., Richert J., Brostoff S., Long E. O., McFarlin D. E., McFarland H. F. A myelin basic protein peptide is recognized by cytotoxic T cells in the context of four HLA-DR types associated with multiple sclerosis. J Exp Med. 1991 Jan 1;173(1):19–24. doi: 10.1084/jem.173.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer C. J., de Graaff-Reitsma C. B., Lafeber G. J., Cats A. In situ localization of lymphocyte subsets in synovial membranes of patients with rheumatoid arthritis with monoclonal antibodies. J Rheumatol. 1982 May-Jun;9(3):359–365. [PubMed] [Google Scholar]
- Miltenburg A. M., van Laar J. M., Daha M. R., de Vries R. R., van den Elsen P. J., Breedveld F. C. Dominant T-cell receptor beta-chain gene rearrangements indicate clonal expansion in the rheumatoid joint. Scand J Immunol. 1990 Jan;31(1):121–126. doi: 10.1111/j.1365-3083.1990.tb02750.x. [DOI] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Nepom G. T., Byers P., Seyfried C., Healey L. A., Wilske K. R., Stage D., Nepom B. S. HLA genes associated with rheumatoid arthritis. Identification of susceptibility alleles using specific oligonucleotide probes. Arthritis Rheum. 1989 Jan;32(1):15–21. doi: 10.1002/anr.1780320104. [DOI] [PubMed] [Google Scholar]
- Offner H., Hashim G. A., Vandenbark A. A. T cell receptor peptide therapy triggers autoregulation of experimental encephalomyelitis. Science. 1991 Jan 25;251(4992):430–432. doi: 10.1126/science.1989076. [DOI] [PubMed] [Google Scholar]
- Owhashi M., Heber-Katz E. Protection from experimental allergic encephalomyelitis conferred by a monoclonal antibody directed against a shared idiotype on rat T cell receptors specific for myelin basic protein. J Exp Med. 1988 Dec 1;168(6):2153–2164. doi: 10.1084/jem.168.6.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullen A. M., Potts W., Wakeland E. K., Kappler J., Marrack P. Surprisingly uneven distribution of the T cell receptor V beta repertoire in wild mice. J Exp Med. 1990 Jan 1;171(1):49–62. doi: 10.1084/jem.171.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullen A. M., Wade T., Marrack P., Kappler J. W. Identification of the region of T cell receptor beta chain that interacts with the self-superantigen MIs-1a. Cell. 1990 Jun 29;61(7):1365–1374. doi: 10.1016/0092-8674(90)90700-o. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Walsh P. S., Levenson C. H., Erlich H. A. Genetic analysis of amplified DNA with immobilized sequence-specific oligonucleotide probes. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6230–6234. doi: 10.1073/pnas.86.16.6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamenkovic I., Stegagno M., Wright K. A., Krane S. M., Amento E. P., Colvin R. B., Duquesnoy R. J., Kurnick J. T. Clonal dominance among T-lymphocyte infiltrates in arthritis. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1179–1183. doi: 10.1073/pnas.85.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd J. A., Acha-Orbea H., Bell J. I., Chao N., Fronek Z., Jacob C. O., McDermott M., Sinha A. A., Timmerman L., Steinman L. A molecular basis for MHC class II--associated autoimmunity. Science. 1988 May 20;240(4855):1003–1009. doi: 10.1126/science.3368786. [DOI] [PubMed] [Google Scholar]
- Urban J. L., Kumar V., Kono D. H., Gomez C., Horvath S. J., Clayton J., Ando D. G., Sercarz E. E., Hood L. Restricted use of T cell receptor V genes in murine autoimmune encephalomyelitis raises possibilities for antibody therapy. Cell. 1988 Aug 12;54(4):577–592. doi: 10.1016/0092-8674(88)90079-7. [DOI] [PubMed] [Google Scholar]
- Vandenbark A. A., Hashim G., Offner H. Immunization with a synthetic T-cell receptor V-region peptide protects against experimental autoimmune encephalomyelitis. Nature. 1989 Oct 12;341(6242):541–544. doi: 10.1038/341541a0. [DOI] [PubMed] [Google Scholar]
- Wordsworth B. P., Lanchbury J. S., Sakkas L. I., Welsh K. I., Panayi G. S., Bell J. I. HLA-DR4 subtype frequencies in rheumatoid arthritis indicate that DRB1 is the major susceptibility locus within the HLA class II region. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10049–10053. doi: 10.1073/pnas.86.24.10049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wucherpfennig K. W., Ota K., Endo N., Seidman J. G., Rosenzweig A., Weiner H. L., Hafler D. A. Shared human T cell receptor V beta usage to immunodominant regions of myelin basic protein. Science. 1990 May 25;248(4958):1016–1019. doi: 10.1126/science.1693015. [DOI] [PubMed] [Google Scholar]
- Wuilmart C., Urbain J. An additional hypervariable region encoded by V gene segments occurs in Tcr V beta at a location compatible with its involvement in Tcr active site--a general model for alloreactivity. Mol Immunol. 1991 Sep;28(9):931–941. doi: 10.1016/0161-5890(91)90178-m. [DOI] [PubMed] [Google Scholar]
- Zaller D. M., Osman G., Kanagawa O., Hood L. Prevention and treatment of murine experimental allergic encephalomyelitis with T cell receptor V beta-specific antibodies. J Exp Med. 1990 Jun 1;171(6):1943–1955. doi: 10.1084/jem.171.6.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]



