Abstract
Immune thrombocytopenia (ITP) is a common autoimmune bleeding disorder. Despite considerable investigation, the pathogenesis of ITP remains incompletely understood, and for many patients, effective therapy is still unavailable. Using murine models and in vitro studies of human blood samples, we recently identified a novel Fc-independent platelet clearance pathway, whereby antibody-mediated desialylated platelets can be cleared in the liver via asialoglycoprotein receptors, leading to decreased response to standard first-line therapies targeting Fc-dependent platelet clearance. Here, we evaluated the significance of this finding in 61 ITP patients through correlation of levels of platelet desialylation with the efficacy of first-line therapies. We found that desialylation levels between different responses to treatment groups were statistically significant (p < 0.01). Importantly, correlation analysis indicated response to treatment and platelet desialylation were related (p < 0.01), whereby non-responders had significantly higher levels of platelet desialylation. Interestingly, we also found secondary ITP and certain non-ITP thrombocytopenias also exhibited significant platelet desialylation compared to healthy controls. These findings designate platelet desialylation as an important biomarker in determining response to standard treatment for ITP. Furthermore, we show for the first time platelet desialylation in other non-ITP thrombocytopenias, which may have important clinical implications and deserve further investigation.
Electronic supplementary material
The online version of this article (doi:10.1186/s13045-017-0413-3) contains supplementary material, which is available to authorized users.
Keywords: Platelet, Immune thrombocytopenia, Antibody, Desialylation, Steroid and IVIG therapy
Introduction
Immune thrombocytopenia (ITP) is a common clinical bleeding disorder characterized by an immune-mediated clearance of autologous platelets, predominantly through autoantibodies targeting platelet surface receptors GPIIbIIIa and/or GPIb-IX and clearance by phagocytic cells in the reticuloendothelial system via Fcγ-receptors [1–4]. Low platelet counts place ITP patients at risk for severe bleeding including fatal intracranial hemorrhage. Most therapies for ITP including first-line corticosteroids and immunoglobulin G (IVIG), and last resort splenectomy, mainly target the Fc-dependent clearance pathway via blocking/attenuating Fc-Fcγ-R interaction or removal of putative site of platelet clearance [4]. However, the pathogenesis and mechanisms of therapies remain poorly understood and around 15–20% of ITP patients are inexplicably refractory to first-line therapies, and around 10% are refractory to splenectomy [5, 6]. In recent years, murine models and large cohort human studies report antibody specificity (i.e., anti-GPIIbIIIa versus anti-GPIb-IX) may play a significant role in dictating response to therapy in ITP [7–9]; whereby presence of anti-GPIb-IX antibodies results in decreased response to corticosteroids and IVIG [7–9]. Most recently, we reported that anti-GPIbα and some anti-GPIIbIIIa antibodies in humans induced platelet desialylation leading to Fc-independent platelet clearance in the liver via hepatic asialoglycoprotein Ashwell-Morell receptors [10], suggesting antibody-mediated desialylation may be one of the underlying mechanisms behind resistance to standard ITP therapies [8, 9, 11].
In the present study, we sought to address whether increased platelet desialylation was correlated with decreased response to treatment in ITP patients (Additional file 1: Supplementary Material). The platelets of randomly and consecutively enrolled 61 patients diagnosed with primary ITP were tested for desialylation prior to the indicated treatments (Table 1). Fluorescein-conjugated lectins Ricinus communis agglutinin I (RCA-1) and Erythrina cristagalli lectin (ECL) were used to detect desialylated galactose and β-GlcNAc residues via flow cytometry. We found the platelets of ITP patients had significantly higher desialylation as measured by both RCA-1 and ECL binding compared to those of healthy blood donors (p < 0.05) (Fig. 1). The 61 ITP patients subsequently underwent standard first-line therapy independent of platelet desialylation and MAIPA assays. After 1 month of treatment, there were 26 complete responders (CR), 21 responders (R), and 14 non-responders (NR) (Table 1). Retrospective data analysis using Kruskal-Wallis rank sum test revealed NR patients had significantly higher platelet desialylation, as compared to the CR and R groups (p < 0.01). Correlation analysis indicated that efficacy and the desialylation level are related (RCA-1 r = 0.395, p < 0.01; ECL r = 0.391, p < 0.01). The higher desialylation, the poorer the efficacy of therapy observed.
Table 1.
Platelet desialylation of different groups [M (P 25, P 75)]
| Age | Gender (M/F) | PLT (×109/L) | RCA-1 (%) | ECL (%) | |
|---|---|---|---|---|---|
| ITP (n = 61) | 43 ± 18 | 18/43 | 16.0 ± 12.5 | 1.60 (0.50,8.50) | 1.30 (0.30,5.05) |
| Efficacy grouping (n = 61) | |||||
| CR (n = 26) | 36 ± 16 | 4/22 | 16.1 ± 15.3 | 1.10 (0.30,2.05) | 0.85 (0.28,1.90) |
| R (n = 21) | 44 ± 19 | 10/11 | 17.2 ± 10.5 | 1.80 (0.65,5.75) | 1.00 (0.30,2.05) |
| NR (n = 14) | 52 ± 17 | 4/10 | 13.9 ± 9.7 | 32.95 (4.40,62.20) | 20.60 (2.83,34.68) |
| Antibody grouping (n = 33) | |||||
| Anti-GPIbα (+) (n = 9) | 39 ± 14 | 2/7 | 10.7 ± 5.3 | 2.50 (0.55,24.15) | 2.20 (0.45,13.85) |
| Single anti-GPIIbIIIa (+) (n = 14) | 35 ± 16 | 3/11 | 16.0 ± 14.5 | 0.55 (0.18,1.70) | 0.35 (0.10,1.90) |
| Double negative (n = 10) | 41 ± 17 | 5/5 | 14.9 ± 9.7 | 0.65 (0.10,5.50) | 1.15 (0.10,2.15) |
| CTD (n = 10) | 43 ± 20 | 3/7 | 20.3 ± 20.0 | 5.15 (1.63,28.85) | 2.20 (0.90,14.25) |
| MDS (n = 10) | 51 ± 27 | 3/7 | 29.3 ± 18.4 | 8.75 (1.30,14.03) | 5.60 (2.08,16.85) |
| AA (n = 6) | 31 ± 11 | 4/2 | 28.2 ± 9.6 | 0.75 (0.18,18.3) | 0.95 (0.10,3.05) |
| AML (n = 8) | 49 ± 19 | 4/4 | 19.4 ± 18.6 | 0.20 (0.13,0.80) | 0.03 (0.01,0.50) |
| Healthy control (n = 20) | 41 ± 12 | 10/10 | 197.7 ± 61.7 | 0.10 (0.10,0.30) | 0.00 (0.00,0.10) |
The platelets of primary ITP patients were collected prior to treatment. Fluorescin-conjugated lectins RCA-1 and ECL were used to detect desialylated galactose and β-GlcNAc residues on platelets via flow cytometry. Platelets from healthy blood donors (controls) and secondary ITP and non-ITP thrombocytopenic patients were also studied. Normal distribution measurement data is presented as mean ± SEM; skewed distribution measurement data is presented as M (P 25, P 75), in which M represents the median, P 25 and P 75 represent the 25th percentile and 75th percentile, respectively. Kruskal-Wallis rank sum test showed platelet desialylation is significantly higher in ITP patients as compared to that in healthy blood donors (RCA-1 Z = −4.918, p < 0.001; ECL Z = −5.512, p < 0.001). The course of therapies was independent from the platelet desialylation assays. The RCA-1 and ECL-positive platelets in non-responder (NR) group are significantly higher than those in complete responder (CR) and responder (R) groups (RCA-1 χ2 = 10.581, p < 0.01; ECL χ2 = 13.741, p < 0.005). No significant difference was observed between CR and R groups (p > 0.05). Correlation analysis indicated that as platelet desialylation increases, the efficacy of therapy decreases. Higher platelet desialylation in ITP patients with anti-GPIbα antibodies was observed as compared with other ITP patients although statistical difference was not reached (RCA-1 χ2 = 3.729, 0.16 > p > 0.05; ECL χ2 = 3.864, 0.15 > p > 0.05). Higher levels of platelet desialylation were also observed in patients of CTD (systemic lupus erythematosus, n = 6; sicca syndrome, n = 4) with thrombocytopenia; MDS and AA as compared with healthy controls (RCA-1 χ2 = 33.790, p < 0.001; ECL χ2 = 42.992, p < 0.001). There is no statistical difference in platelet desialylation between the AML patients and healthy donors (p > 0.05)
Fig. 1.
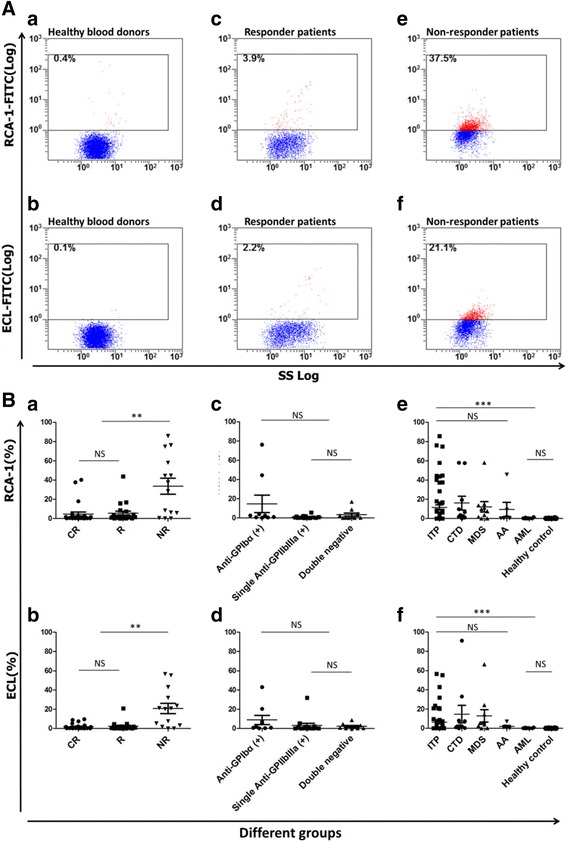
A Platelet desialylation in representative patients and healthy controls. The RCA-1 and ECL binding to platelets from healthy blood donors (a, b), responder (c, d), and non-responder patients (e, f) were examined by flow cytometry. The representative dot plots from each group are shown. B Platelet desialylation in different patient groups and healthy controls. The RCA-1 and ECL levels (Mean ± SEM) in complete responders (CR), responders (R) and non-responders (NR) (a, b); anti-GPIbα antibody positive (+) group, single anti-GPIIb/IIIa antibody (+) group, and double negative group (c, d); and in the thrombocytopenias/controls (e, f) (ITP, CTD, MDS, AA, AML, and healthy controls) were examined by flow cytometry. Each point represents the level of platelet desialylation of an individual patient or healthy blood donor (**p < 0.01; ***p < 0.001)
To test whether the presence of anti-GPIbα antibodies is associated with the platelet desialylation, we detected antibody using MAIPA in the available 33 patient samples collected prior to treatment. We observed a two- to sixfold increased platelet desialylation in patients with anti-GPIbα antibodies (n = 9) compared to that in patients with anti-GPIIbIIIa (n = 14) or without detectable antibodies (n = 10) (Table 1 and Fig. 1B). However, statistical significance was not reached, which is likely due to small sample size. Future larger studies should be useful in determining direct correlation between anti-GPIbα antibody positivity with platelet desialylation.
Interestingly, we also observed significant platelet desialylation in patients with non-ITP thrombocytopenias including connective tissue diseases (CTD), myelodysplastic syndrome (MDS), and aplastic anemia (AA) (p < 0.001) but not acute myeloid leukemia (AML) compared to healthy controls (Table 1 and Fig. 1B). Notably, although RCA-1 and ECL measures different types of deglycosylation, we did not observe significant difference between these two assays, suggesting either of them can be used for the potential diagnosis and prognosis.
In summary, our data demonstrates for the first time that the higher level of platelet desialylation is correlated with non-response to the first-line ITP therapies (likely also splenectomies; Additional file 1: Supplementary Material). These findings not only suggest that platelet desialylation is a useful biomarker in predicting response to treatment in clinical ITP but positions sialidase inhibitors, such as Tamiflu [12], as a potential novel therapeutic in the treatment of ITP as well as other thrombocytopenias.
Additional file
Supplementary Material. (DOCX 49 kb)
Acknowledgements
The authors would like to thank Mr. Thomas McKeown, Miss Xun Fu, and Miss Jade Sullivan for editing the manuscript.
Funding
This work was supported by the Canadian Institutes of Health Research (MOP 97918, MOP 119540, MOP 119551); Canadian Blood Services-Canadian Institutes of Health Research partnership fund (CIHR-BUC201403-HN-326400); by the Special Research Funding for the Doctoral Program (NO. 20103420110001), Ministry of Education, People’s Republic of China; and by the Foundation of the Second Affiliated Hospital and the Hematological Research Center, Anhui Medical University, People’s Republic of China. June Li is a recipient of Ph.D. Graduate Fellowship from Canadian Blood Services. Miao Xu is a recipient of the State Scholarship Fund from the China Scholarship Council and Ontario Trillium Scholarship, Canada.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Authors’ contributions
LT planned and carried out the experiments, analyzed the data, and prepared the manuscript. QZ supervised the clinical studies and analyzed the data. LJ and MX analyzed the data and prepared the manuscript. JW, YP, HW, QT, and YC carried out the experiments and analyzed the data. AJGJ, JP, and MH joined the international collaboration group meetings, analyzed the data and contributed to the preparation of manuscript. HN and ZZ (PIs) supervised the research, analyzed the data, and prepared the manuscript. LT and QZ contributed equally to this work and should be acknowledged as co-first authors. Both Dr. HN and Dr. ZZ should be acknowledged as co-corresponding authors. All authors read and approved the final manuscript.
Authors’ information
The contact information for the corresponding authors:
Heyu Ni, M.D.; Ph.D, Professor, Department of Laboratory Medicine and Pathobiology, Department of Medicine, and Department of Physiology, University of Toronto; Scientist of Canadian Blood Services Centre for Innovation; Platform Director for Hematology, Cancer and Immunological Diseases, St. Michael’s Hospital, Room 420, LKSKI - Keenan Research Centre, 209 Victoria Street, Toronto, Ontario, M5B 1W8, CANADA. Tel: 1-416-847-1738; Email: nih@smh.ca
Zhimin Zhai, M.D.; PhD. Professor, Director of Department of Hematology, The Second Affiliated Hospital and the Hematological Research Center, Anhui Medical University, Hefei 230601, China. Tel: 86-138-5514-7434; Email: zzzm889@163.com
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study has been approved by the Ethic Board of Anhui Medical University (No. 20131038). Patients in this study all signed informed consents to clinical data use at enrolment.
Abbreviations
- AA
Aplastic anemia
- AML
Acute myeloid leukemia
- CR
Complete responder
- CTD
Connective tissue disease
- ECL
Erythrina cristagalli lectin
- FITC
Fluorescein isothiocyanate
- ITP
Immune thrombocytopenia
- IVIG
Intravenous immunoglobulin G
- MAIPA
Monoclonal antibody immobilization of platelet antigen assay
- MDS
Myelodysplastic syndrome
- NR
Non-responder
- PRP
Platelet-rich plasma
- R
Responder
- RCA-1
Ricinus communis agglutinin I
Contributor Information
Heyu Ni, Phone: 1-416-847-1738, Email: nih@smh.ca.
Zhimin Zhai, Phone: 86-138-5514-7434, Email: zzzm889@163.com.
References
- 1.Rodeghiero F, Michel M, Gernsheimer T, et al. Standardization of bleeding assessment in immune thrombocytopenia: report from the International Working Group. Blood. 2013;121:2596–2606. doi: 10.1182/blood-2012-07-442392. [DOI] [PubMed] [Google Scholar]
- 2.Cines DB, Bussel JB, Liebman HA, et al. The ITP syndrome: pathogenic and clinical diversity. Blood. 2009;113:6511–6521. doi: 10.1182/blood-2009-01-129155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lazarus AH, Semple JW, Cines DB. Innate and adaptive immunity in immune thrombocytopenia. Semin Hematol. 2013;50(Suppl 1):S68–70. doi: 10.1053/j.seminhematol.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J, van der Wal DE, Zhu L, et al. Fc-independent phagocytosis: implications for IVIG and other therapies in immune-mediated thrombocytopenia. Cardiovasc Hematol Disord Drug Targets. 2013;13:50–58. doi: 10.2174/1871529X11313010006. [DOI] [PubMed] [Google Scholar]
- 5.Provan D, Newland A. Fifty years of idiopathic thrombocytopenic purpura (ITP): management of refractory itp in adults. Br J Haematol. 2002;118:933–944. doi: 10.1046/j.1365-2141.2002.03669.x. [DOI] [PubMed] [Google Scholar]
- 6.Vianelli N, Galli M, de Vivo A, et al. Efficacy and safety of splenectomy in immune thrombocytopenic purpura: long-term results of 402 cases. Haematologica. 2005;90:72–77. [PubMed] [Google Scholar]
- 7.Webster ML, Sayeh E, Crow M, et al. Relative efficacy of intravenous immunoglobulin G in ameliorating thrombocytopenia induced by antiplatelet GPIIbIIIa versus GPIbalpha antibodies. Blood. 2006;108:943–946. doi: 10.1182/blood-2005-06-009761. [DOI] [PubMed] [Google Scholar]
- 8.Zeng Q, Zhu L, Tao L, et al. Relative efficacy of steroid therapy in immune thrombocytopenia mediated by anti-platelet GPIIbIIIa versus GPIbalpha antibodies. Am J Hematol. 2012;87:206–208. doi: 10.1002/ajh.22211. [DOI] [PubMed] [Google Scholar]
- 9.Peng J, Ma SH, Liu J, et al. Association of autoantibody specificity and response to intravenous immunoglobulin G therapy in immune thrombocytopenia: a multicenter cohort study. J Thromb Haemost. 2014;12:497–504. doi: 10.1111/jth.12524. [DOI] [PubMed] [Google Scholar]
- 10.Li J, van der Wal DE, Zhu G, et al. Desialylation is a mechanism of Fc-independent platelet clearance and a therapeutic target in immune thrombocytopenia. Nat Commun. 2015;6:7737. doi: 10.1038/ncomms8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Callum JL, Lin Y, et al. Severe platelet desialylation in a patient with glycoprotein Ib/IX antibody-mediated immune thrombocytopenia and fatal pulmonary hemorrhage. Haematologica. 2014;99:e61–63. doi: 10.3324/haematol.2013.102897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shao L, Wu Y, Zhou H, et al. Successful treatment with oseltamivir phosphate in a patient with chronic immune thrombocytopenia positive for anti-GPIb/IX autoantibody. Platelets. 2015;26:495–497. doi: 10.3109/09537104.2014.948838. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material. (DOCX 49 kb)
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.


