Abstract
Objective
To estimate the economic burden of vision loss and eye disorders in the United States population younger than 40 years in 2012.
Design
Econometric and statistical analysis of survey, commercial claims, and census data.
Participants
The United States population younger than 40 years in 2012.
Methods
We categorized costs based on consensus guidelines. We estimated medical costs attributable to diagnosed eye-related disorders, undiagnosed vision loss, and medical vision aids using Medical Expenditure Panel Survey and MarketScan data. The prevalence of vision impairment and blindness were estimated using National Health and Nutrition Examination Survey data. We estimated costs from lost productivity using Survey of Income and Program Participation. We estimated costs of informal care, low vision aids, special education, school screening, government spending, and transfer payments based on published estimates and federal budgets. We estimated quality-adjusted life years (QALYs) lost based on published utility values.
Main Outcome Measures
Costs and QALYs lost in 2012.
Results
The economic burden of vision loss and eye disorders among the United States population younger than 40 years was $27.5 billion in 2012 (95% confidence interval, $21.5–$37.2 billion), including $5.9 billion for children and $21.6 billion for adults 18 to 39 years of age. Direct costs were $14.5 billion, including $7.3 billion in medical costs for diagnosed disorders, $4.9 billion in refraction correction, $0.5 billion in medical costs for undiagnosed vision loss, and $1.8 billion in other direct costs. Indirect costs were $13 billion, primarily because of $12.2 billion in productivity losses. In addition, vision loss cost society 215 000 QALYs.
Conclusions
We found a substantial burden resulting from vision loss and eye disorders in the United States population younger than 40 years, a population excluded from previous studies. Monetizing quality-of-life losses at $50 000 per QALY would add $10.8 billion in additional costs, indicating a total economic burden of $38.2 billion. Relative to previously reported estimates for the population 40 years of age and older, more than one third of the total cost of vision loss and eye disorders may be incurred by persons younger than 40 years.
Disorders of the eye and resulting vision loss impose a significant burden on the United States, both economically and socially. In addition to medical costs, the debilitating nature of vision loss results in major indirect and nonmedical costs because of decreased productivity, quality of life, and independence among those affected. In recent years, several studies have estimated the medical and overall economic costs of vision loss and eye disorders, but in the United States, these studies have been restricted to adults 40 years of age or older.1–5 Rein et al3 estimated the 2004 annual Unites States economic cost of four major age-related eye diseases at $35.4 billion, including $19.1 billion in nonmedical costs. Frick et al2 estimated largely complementary costs, including medical costs attributable to low vision ($5.5 billion per year) and the value of lost quality of life ($10.5 billion per year) in the United States in 2004. A Prevent Blindness America report based on both of these studies estimated the total annual cost of vision problems in United States adults at $51.4 billion per year in 2004.6 To our knowledge, the economic burden among the United States population younger than 40 years has not been estimated previously.
In this analysis, we estimated the economic burden of vision loss and eye disorders in the United States population younger than 40 years, including children from birth through 17 years of age and adults 18 through 39 years of age. We followed the consensus guidelines for research on the cost of vision loss that were developed under the auspices of the Association for Research in Vision and Ophthalmology in 2010.7 These guidelines delineate definitions for analysis perspectives and specific cost categories that should be included in economic studies of vision loss. We included direct and indirect costs resulting from uncorrectable vision loss, refractive errors, and diagnosed disorders of the eye and ocular adnexa. We also reported the impact of vision loss on quality-of-life losses and estimated the monetized value of this burden.
Methods
We estimated the prevalence of vision loss and the treated prevalence of diagnosed eye and vision-related disorders. Costs were estimated for each category listed by consensus guidelines. Direct costs include medical care attributable to diagnosed disorders, medical vision aids, undiagnosed vision loss, low-vision aids or devices, special education, school screening, and federal assistance programs. Indirect costs include productivity losses of adults, productivity losses of children’s caregivers, transfer payments (not included in total), and deadweight loss from transfer payments. Costs also are reported from the payer’s perspective, including government, private insurance, and patient costs. All prices and costs were adjusted to 2012 United States dollars using the Consumer Price Index for nonmedical costs and medical components of the Consumer Price Index for medical expenses. United States population values are based on the 2010 census.
Prevalence of Vision Loss and Diagnosed Disorders
We estimated the prevalence of vision loss based on autorefractor-corrected visual acuity in the better-seeing eye as measured in the National Health and Nutrition Examination Survey (NHANES) from 2005 through 2008. Visual acuity thresholds for mild and moderate vision impairment and blindness are worse than 20/40, worse than 20/80, and worse than 20/200, respectively. Respondents who did not have an acuity test because of self-reported blindness were included in the prevalence of blindness. No nationally representative data exist on the prevalence of corrected bilateral vision loss among children younger than 12 years. We estimated the prevalence of vision loss among this population by adjusting the NHANES prevalence for 12 to 17 years using age-specific incidence of severe impairment and blindness as identified in United Kingdom surveillance data.8 In the sensitivity analysis, we assessed the impact of this assumption for children younger than 12 years by measuring the impact of varying the prevalence between 0 and the full rate observed among children 12 to 17 years of age.
To estimate the treated prevalence of diagnosed eye and vision disorders, we identified International Classification of Diseases 9th Revision (ICD-9) diagnosis codes related to eye and vision conditions.9 We included a broad range of eye and vision disorders, including disorders and diseases of the eye, visual function disorders, conjunctivitis, eye injuries and burns, and disorders of ocular adnexa, including the eyelids, the orbit, and the lacrimal system. We then estimated the treated prevalence of each code as a primary diagnosis using pooled data from the 2003 through 2008 Medical Expenditure Panel (MEPS) conditions file.
Medical and Other Health Costs
We calculated costs attributable to diagnosed eye-related disorders, costs attributable to self-reported low vision in the absence of a diagnosed eye disorder, and medical vision aid costs, including glasses and contact lenses, using 2003 through 2008 MEPS data. To identify relative costs of individual eye disorder diagnoses, we analyzed private insurance claims for individual ICD-9 codes in MarketScan claims data, which represent a subset of the total costs captured in MEPS data.
We estimated the medical costs attributable to diagnosed disorders of the eye and ocular adnexa and undiagnosed vision loss econometrically on 2003 through 2008 MEPS pooled event file data for persons younger than 40 years. We used a general linear model with γ distribution and log link to achieve the best fit.10 Because general linear models are multiplicative models, separately estimating costs for individual or groups of conditions may lead to double counting of costs when the presence of one condition increases the treatment costs of another. We controlled for possible double counting by using a process to adjust results such that the model would predict 100% of costs when summing across all possible combinations of chronic conditions in MEPS.11 The first part of the 2-part model used a logistic equation to estimate the probability of positive medical expenditures. The dependent variable in the second part was total medical expenditures excluding medical vision aid and optometrist visit costs, which we estimated separately. The primary independent variables were the presence of any eye-related, ocular adnexa, or vision-related ICD-9 diagnosis (eye disorders) and self-reported low vision in the absence of a vision diagnosis (undiagnosed vision loss). Other independent control variables included sociodemographic indicators and the comorbidities diabetes and hypertension. We independently estimated costs based on payer: private insurance, public payers (such as Medicaid), and patient out-of-pocket costs.
The MEPS collects self-reported costs for optometry visits and the cost for medical vision aids (including glasses and contact lenses) separately from other medical costs. We found that only a very small proportion of these costs would be predicted by the presence of a diagnosed eye or vision disorder or by self-reported low vision. Therefore, we calculated the total cost of optometry visits and medical vision aids for all respondents younger than 40 years in MEPS regardless of any diagnosis or self-reported low vision. We combined the cost of optometry visits with the cost of diagnosed vision disorders and separately reported the cost of medical vision aids.
Although overall costs are estimated using MEPS, these data could not provide statistically significant estimates of relative costs of individual diagnoses. To estimate these, we analyzed the 2008 MarketScan Commercial Claims and Encounters Database to estimate the annual cost of outpatient claims directly related to each eye disorder diagnosis code. MarketScan data are not nationally representative and do not include claims filed under most vision plans, which may include ophthalmologic services and most optometry and refractive error-related costs, but can provide an accurate measure of private insurance claims for individual medical diagnoses. We multiplied the average per-person, per–ICD-9 cost for each age group by the prevalence of this diagnosis identified in MEPS data and reported the proportion of medical costs filed under each diagnosed condition.
Low-Vision Aids and Devices
Low-vision aids include personal, home, and work devices adapted for use by persons with low vision. We estimated United States—specific low-vision aid device use for children and young adults with vision loss based on the prevalence of vision loss and incremental rates of demand identified in France; to our knowledge, these data are not available elsewhere.12 We then multiplied these use rates by the estimated United States cost of low-vision aids and devices.13 We estimated the cost of guide dogs for the blind by allocating a previous estimate of the cost of guide dogs for all ages in the United States based on an assumption of equal allocation of guide dog placement to the blind across all ages and adjusting costs for inflation.14
Caregivers
We estimated the cost of the additional informal care required for children with vision impairment and blindness. We applied relative rates of informal care resulting from vision loss identified in France to estimates of the time required to care for any children based on age as measured in the American Time Use Survey.13 We valued the resulting incremental informal care time based on the United States average wage rate. We did not include any costs for long-term care placement resulting from vision loss.
Special Education
The Individuals with Disabilities Education Act requires states to provide free intervention and education programs for children with disabilities, including blindness, from birth through 21 years of age. We estimated the number of children enrolled in special education because of low vision based on the American Printing House for the Blind registry of students who receive assistance through the Act to Promote Education of the Blind. We then multiplied this value by the incremental cost of special education programs per student cited by the act, which was $11,102 in 2012-adjusted costs.15
School Vision Screening
We estimated the cost of child vision screening programs based on a national survey of statewide screening programs and an evaluation of 3 statewide screening programs (Naser N, Hartmann EE. Comparison of state guidelines and policies for vision screening and eye exams: preschool through early childhood. Poster presented at: ARVO Annual Meeting, April 27, 2008; Fort Lauderdale, FL).16 We estimated in-school screening costs by multiplying the grade-level population of each state for each grade identified as a screening target by the estimated per-student screening cost for school-based acuity and stereopsis screening. For preschool screening, we assumed screening would target 3-year-olds with screening rates and costs based on those achieved in 2 preschool screening programs administered by Prevent Blindness America.16
Federal Assistance Programs
We included the budgetary cost of federal programs that provide services for the blind, including the American Printing House for the Blind, the National Library Services for the Blind, and the Committee for Purchase from People who are Blind or Severely Disabled. We allocated the cost of these programs based on the target age of the programs and the proportion of blind among persons younger than 40 years.
Transfers, Tax Losses, and Deadweight Loss
We included the budgetary impact and estimated deadweight loss associated with federal transfers and tax deduction programs, including Social Security Disability Insurance, Supplemental Security Income, and the Supplemental Nutrition Assistance Program. We estimated the reduction in federal income tax revenue resulting from the income tax deduction for blind individuals based on the employment rates and median household earnings of blind individuals 18 to 39 years of age. We also estimated deadweight loss associated with federal Social Security Disability Insurance and Supplemental Security Income transfer programs by assuming that 38% of transfer payments are lost because of allocative inefficiency.17,18
Productivity Losses
Productivity losses include the value of labor lost because of blindness and moderate vision impairment. We identified median household income by self-reported vision function from Survey of Income and Program Participation data for persons 18 to 39 years of age. We assumed that self-reported difficulty seeing words was analogous to visual impairment and that self-reported inability to see words in print or blindness was analogous to blindness. We estimated productivity losses by multiplying the number of blind and moderately impaired persons 18 to 39 years of age by the average reduction in median income associated with persons with moderate impairment or blindness identified in the survey.
Loss of Well-Being
We estimated the impact of low vision on personal well-being based on average utility values for normal, impairment, and blind vision reported by 12 published articles. We excluded the only study we found estimating utility values of vision loss for children because it was based on only 24 children and reported utility loss values far greater than were identified by the adult-based estimates.19
We adjusted the utility values based on age-specific background utilities.20 We then multiplied the adjusted utility values by the prevalent visually impaired and blind population and calculated quality-adjusted life year (QALY) losses based on the reduction from normal vision utilities. Because of a lack of data for the younger population, we do not adjust the QALYs for excess mortality because of low vision. We estimated the monetary value of loss of well-being based on a commonly cited societal valuation of $50 000 per QALY.2,21
Sensitivity Analyses
We conducted sensitivity analyses to investigate the impact of parameter uncertainty on overall results. In a 1-way sensitivity analysis, we varied individual major parameters between a low to high range based on the 95% confidence interval of the parameter estimate when available and a 50% range when a confidence interval was not available. We also conducted a probabilistic sensitivity analysis to estimate the 95% confidence interval of total costs. In the probabilistic sensitivity analysis, we simultaneously varied all major parameters in the analysis based on random draws from each parameter’s respective distribution in a Monte Carlo simulation and reported the 2.5 and 97.5 percentile cost observations (the credible interval) as the 95% confidence interval.22
Results
Prevalence of Vision Loss and Diagnosed Disorders
Among persons younger than 40 years, the prevalence of best-corrected vision impairment and blindness was 1.30% (Table 1). The prevalence of visual impairment was 1.12% for mild impairment (<20/40–20/80) and 0.12% for moderate impairment (<20/80–20/200). The prevalence of blindness (<20/200) was low: 0.10% among adults 18 to 39 years of age and only 0.01% among children. More than 2 million persons younger than 40 years in the United States have uncorrectable vision impairment, and another 98 000 are blind.
Table 1.
Prevalence of Vision Impairment and Blindness by Age Group in the National Health and Nutrition Examination Survey, 2005 through 2008
| Mild Impairment | Moderate Impairment | Blind | Total Vision Loss | |||||
|---|---|---|---|---|---|---|---|---|
| Age Group (yrs) | Prevalence (%) | Population* | Prevalence (%) | Population* | Prevalence (%) | Population* | Prevalence (%) | Population* |
| 0–17† | 1.07 | 775 | 0.10 | 76 | 0.01 | 6 | 1.16 | 857 |
| 95% CI | 0.58–1.22 | 434–903 | 0.01–0.20 | 6–145 | 0.00–0.03 | 0–20 | 0.59–1.44 | 440–1068 |
| 18–39 | 1.17 | 1078 | 0.14 | 128 | 0.10 | 92 | 1.41 | 1298 |
| 95% CI | 0.74–1.60 | 682–1473 | 0.02–0.26 | 16–241 | 0.01–0.34 | 6–316 | 0.77–2.21 | 704–2030 |
| Total younger than 40 | 1.12 | 1853 | 0.12 | 204 | 0.06 | 98 | 1.30 | 2155 |
| 95% CI | 0.67–1.43 | 1116–2376 | 0.01–0.23 | 22–386 | 0.01–0.20 | 6–336 | 0.69–1.87 | 1144–3098 |
CI = confidence interval.
Population in the thousands (n).
Based on prevalence for ages 12 to 17 years in National Health and Nutrition Examination Survey data. Prevalence for younger ages imputed based on incidence of blindness adjusted such that predicted prevalence at age 16 years equals the observed in National Health and Nutrition Examination Survey prevalence.
Based on self-reported conditions identified in MEPS data, the treated prevalence of diagnosed disorders of the eye and ocular adnexa, excluding disorders of refraction and accommodation, is 3.2% among people younger than 40 years, corresponding to almost 5.8 million persons (Table 2). Thus, almost 3 times as many persons younger than 40 years self-report an eye-related disorder than have uncorrectable vision loss. Children have a higher prevalence of diagnosed conditions than adults younger than 40 years, primarily because of disorders of the conjunctiva, which are the most prevalent conditions among children. Among adults 18 to 39 years of age, the conditions with highest prevalences were injuries and disorders of the globe.
Table 2.
Prevalence and Share of Medical Costs of Vision Disorder Diagnoses in the Medical Expenditure Panel Survey, 2003 through 2008
| 0–17 Years of Age | 18–39 Years of Age | Total Younger than 40 Years | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Condition* |
Prevalence (%)† |
Population‡ |
Cost (%) |
Prevalence (%)† |
Population‡ |
Cost (%) |
Prevalence (%)† |
Population‡ |
Cost (%) |
| Disorders of the globe | 0.67 | 499 | 22 | 0.45 | 417 | 17 | 0.57 | 916 | 19 |
| Injury and burns | 0.38 | 280 | 11 | 0.56 | 511 | 20 | 0.49 | 791 | 16 |
| Disorders of conjunctiva | 1.76 | 1302 | 17 | 0.54 | 493 | 8 | 1.42 | 1795 | 12 |
| Other eye disorders | 0.51 | 377 | 13 | 0.46 | 422 | 12 | 0.48 | 799 | 12 |
| Strabismus, binocular eye movements |
0.24 | 175 | 13 | 0.03§ | 27 | 2 | 0.21 | 202 | 7 |
| Visual disturbances | 0.26 | 196 | 5 | 0.17 | 160 | 9 | 0.22 | 356 | 7 |
| Blindness and low vision | 0.09 | 69 | 3 | 0.12 | 107 | 9 | 0.11 | 176 | 6 |
| Disorders of lacrimal system | 0.18 | 136 | 8 | 0.13 | 120 | 2 | 0.16 | 256 | 5 |
| Cataract | 0.01§ | 11 | 2 | 0.05 | 48 | 6 | 0.05 | 59 | 4 |
| Retinal detachment, defects, and disorders |
0.04 | 31 | 2 | 0.05 | 48 | 6 | 0.05 | 79 | 4 |
| Disorders of the eyelids | 0.16 | 121 | 3 | 0.19 | 174 | 4 | 0.18 | 295 | 4 |
| Glaucoma | 0.04§ | 28 | 1 | 0.11 | 97 | 3 | 0.09 | 125 | 2 |
| Disorders of optic nerve and visual pathways |
0.02§ | 14 | 1 | 0.03§ | 24 | 2 | 0.02§ | 38 | 1 |
| Total | 4.13 | 3063 | 100 | 2.62 | 2405 | 100 | 3.22 | 5887 | 100 |
Medical conditions exclude disorders of refraction and accommodation, and costs exclude claims filed to vision insurance plans.
Values do not sum because some individuals had multiple conditions.
Population in thousands.
Not statistically distinguishable from 0.
Economic Burden of Vision Loss and Disorders
We estimated the total economic burden of eye disorders among persons younger than 40 years in 2010 to be $27.5 billion per year in 2012 dollars (Table 3). Diagnosed disorders, including costs for optometry visits, totalled $7.3 billion. Medical vision aids, including glasses and contact lenses, cost $4.9 billion. Medical costs attributable to undiagnosed vision loss were $481 million. All other direct costs totalled $1.8 billion, with the largest components consisting of the cost of nonmedical aids and devices ($1 billion), followed by the cost of special education ($615 million). School screening programs cost approximately $92 million, and federal assistance programs added $42 million. Indirect costs, including productivity losses ($12.2 billion), informal care for children ($602 million), and deadweight loss from transfer payments ($188 million), totalled $13 billion.
Table 3.
Components of Cost by Perspective, Total Population Younger than 40 Years (in Millions of Dollars)
| Perspective | 0–17 Years of Age | 18–39 Years of Age | Total Younger than 40 Years |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Government | Insurance | Patient | All | Government | Insurance | Patient | All | ||
| Direct costs | |||||||||
| Diagnosed disorders | 633 | 1274 | 720 | 2623 | 693 | 2566 | 1112 | 4674 | 7297 |
| Medical vision aids | 252 | 312 | 909 | 1512 | 120 | 756 | 2491 | 3406 | 4918 |
| Undiagnosed vision | 13 | 18 | 14 | 44 | 135 | 205 | 115 | 437 | 481 |
| Low vision aids/devices | — | — | 402 | 402 | — | — | 623 | 623 | 1025 |
| Education | 615 | — | — | 615 | — | — | — | — | 615 |
| School screening | 95 | — | — | 95 | — | — | — | — | 95 |
| Assistance programs | 26 | — | — | 26 | 17 | — | — | 17 | 42 |
| Total direct costs | 1634 | 1604 | 2045 | 5315 | 966 | 3528 | 4340 | 9157 | 14 472 |
| Indirect costs | |||||||||
| Productivity loss | — | — | — | — | — | — | 12 213 | 12 213 | 12 213 |
| Caregivers | — | — | 602 | 602 | — | — | — | — | 602 |
| Entitlement programs* | 8 | — | — | — | 484 | — | — | — | — |
| Tax deduction* | — | — | — | — | 5 | — | — | — | — |
| Transfer deadweight loss | 3 | — | — | 3 | 184 | — | — | 184 | 188 |
| Total indirect costs | 12 | — | 602 | 605 | 674 | — | 12 213 | 12 398 | 13 003 |
| Total costs | 1646 | 1604 | 2646 | 5920 | 1639 | 3528 | 16 554 | 21 555 | 27 475 |
Transfer payments (Social Security Disability Insurance, Supplemental Security Income, and Supplemental Nutrition Assistance Programs) and tax losses are not included in the comprehensive perspective other than resulting deadweight loss, dashes indicate no costs.
Patients and their families bore 70% of the total economic burden of eye disorders and vision loss. Patients paid 25% of costs for diagnosed disorders but more than two thirds of the cost for medical vision aids. Private insurance, including vision plans, paid $5.1 billion in combined medical costs. Government paid $3.4 billion in total costs, including $1.8 billion in medical costs and $0.8 billion in assistance programs, transfer payments, and deadweight loss. Government spending also included $0.7 billion for special education and school screening, which was paid primarily through state and local governments.
Costs by Diagnosis and Provider
Table 2 shows the allocation of private medical insurance costs by diagnosis type based on MarketScan commercial insurance claims. These costs do not include claims filed to vision insurance plans, which may exclude most costs for disorders of refraction and accommodation, and thus we excluded this diagnosis. The highest-cost disorders for persons younger than 40 years were disorders of the globe (19%), followed by injuries and burns (16%), disorders of the conjunctiva (12%), and other eye disorders (12%). Among adults 18 to 39 years of age, injuries and burns were the most costly conditions, and disorders of conjunctiva accounted for only 8% of medical insurance costs. Strabismus accounted for 13% of costs for children but only 2% of costs among adults 18 to 39 years of age.
Quality of Life
We estimated that vision loss results in quality-of-life losses of 215 000 QALYs per year, including 81 000 QALYs among children and 134 000 QALYs among adults younger than 40 years (Table 4). A benchmark of $50 000 commonly is cited for societal willingness to pay per QALY gained by health care. Based on this benchmark, the total monetary value of the loss of well-being from vision impairment and blindness for the population younger than 40 years was $10.8 billion, including $4.1 billion for children and $6.7 billion among adults younger than 40 years.
Table 4.
Quality-of-Life Losses
| Quality-of-Life Measure |
0–17 Years of Age |
18–39 Years of Age |
Total Younger than 40 Years |
|---|---|---|---|
| QALY losses | |||
| Visual impairment | 79 799 | 110 534 | 190 333 |
| Blindness | 1663 | 23 177 | 24 840 |
| Total QALYs lost | 81 462 | 133 711 | 215 173 |
| Monetary value of quality-of-life losses |
|||
| $50 000 per QALY* | $4073 | $6686 | $10 759 |
QALY = quality-adjusted life year.
Units of measure for the first 3 rows are QALYs.
Monetary costs are in millions.
Sensitivity Analyses
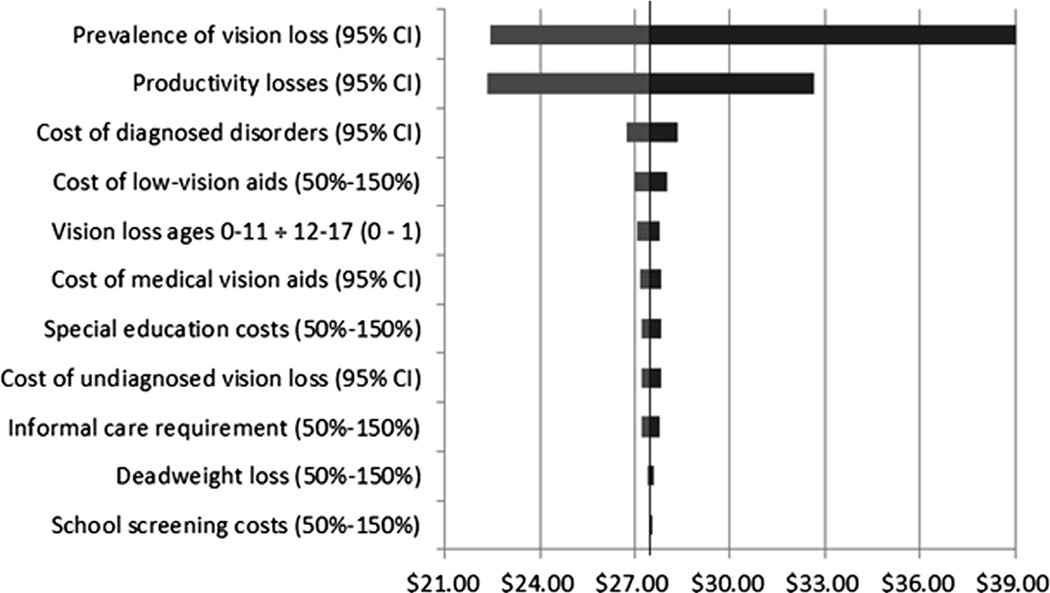
The results of the 1-way sensitivity analysis are shown in Figure 1. This tornado diagram shows the range of total economic burden associated with the range of each parameter or parameter category. Results were most sensitive to the prevalence of vision impairment and blindness, followed by the reduction in productivity associated with self-reported vision loss. On the basis of the probabilistic sensitivity analysis, we estimated a 95% confidence interval of $21.5 billion to $37.2 billion in total costs.
Figure 1.
Graph showing the results of the univariate sensitivity analysis. Bars represent the range of total burden associated with changes in the respective parameter from minimum to maximum values. Costs are shown as billions of dollars. Distributions denoted by CI represent 95% confidence intervals; others represent uniform distributions within the range 50% to 150% of the baseline estimate.
Discussion
This study provides the first estimate of the economic burden of vision loss and eye disorders among children and adults younger than 40 years in the United States. We estimated the economic burden of disorders of the eye, disorders of the ocular adnexa, and vision loss in this population to be $27.5 billion in 2012 dollars, including $5.3 billion for children younger than 18 years and $21.2 billion incurred by adults 18 to 39 years of age. Monetizing the cost of quality-of-life losses at $50 000 per QALY increased the total burden by $10.8 billion, bringing the total cost of vision loss and eye disorders to $38.2 billion for the United States population younger than 40 years, including $10 billion for children and $28.2 billion for adults 18 to 39 years of age. Based on this total, among the entire population younger than 40 years, the per-person cost of vision loss and eye disorders was $230. Diagnosed disorders cost $1370 per each person with a diagnosis. All other costs totalled $9400 per person with vision loss, including $40 400 per person blind.
Previous studies estimated the economic and quality-of-life burden of eye disorders and vision loss among the United States population 40 years of age or older to be $51.4 billion in 2004. Updating these costs for general and medical inflation implied a cost of $66.6 billion in 2012 dollars. Although methodologic differences complicate direct comparison with this study, combining this total with our results yielded a total burden of $104.8 billion in 2012 dollars for the entire United States population, including $56.8 billion in direct costs and $48 billion in indirect costs. It is possible that the true total cost is even higher because the estimates for the population 40 years of age or older are based on the cost of only 4 eye disorders and do not include most costs of refraction correction and because updating these costs based on the Consumer Price Index will not account fully for newly available therapies and treatments.
At $104 billion, this estimate of the cost of vision loss and eye disorders is among the highest estimated costs for health conditions in the United States. A recent report estimated the burden of several of the costliest chronic diseases in the United States, but did not include eye- and vision-related conditions.23 Although subject to substantial methodological differences, our estimate of the direct costs of eye and vision disorders would have placed as the fifth costliest disease. This is in line with findings from Australia, where vision disorders are estimated to be the seventh costliest health condition.1
This study was subject to several limitations and assumptions. The prevalence of diagnosed disorders was based on self-reported conditions and verified medical encounters in MEPS data. Unlike other medical costs, the costs for optometry visits and medical vision aids were not verified by MEPS. The allocation of costs by diagnosis group were based on MarketScan commercial claims, which is not nationally representative and excludes most claims filed under vision insurance plans, out-of-pocket costs, and government insurance payments. The prevalence of visual impairment and blindness is based on autorefractor-corrected near distance acuity as measured by NHANES from 2005 through 2008. Contrast sensitivity and visual field were not assessed among participants younger than 40 years. The NHANES did not assess acuity among participants younger than 12 years. We imputed prevalence in this age group based on the incidence of blindness reported in the United Kingdom and the prevalence among older children in the NHANES data, which may introduce bias. We expect that this may have underestimated the prevalence of visual impairment at very young ages. The sensitivity analysis identified the prevalence of vision loss as the primary cause of uncertainty in results, almost entirely because of its impact on productivity losses. The QALY losses similarly are sensitive to the prevalence of vision loss. We found no data on the relative demand for assistive living devices or informal care resulting from vision loss for persons in the United States. We assumed that the relative impact on demand resulting from vision loss in the United States was identical to rates observed in Europe, which might have introduced bias. We did not include the cost of vision screening other than school and preschool screening, such as acuity chart screening during annual physicals or well-child checks. Finally, we did not include the monetized value of quality-of-life losses in our primary results because of limitations and uncertainty in the utility loss associated with vision loss, the monetary value of a QALY, and controversy over their inclusion in economic burden studies.
Not unlike many other chronic conditions, most of the costs of eye and vision problems are borne by older adults. However, we found that the burden of disease among the population younger than 40 years remained substantial, and many of these costs were the result of chronic and persistent conditions that will continue to accrue direct and indirect costs for the duration of an individual’s life. Recently published guidelines focused attention on the need to account for the many nonmedical and indirect costs of eye and vision problems. Future research on the economic burden of eye disorders and vision loss also should consider the burden faced by all age groups.
Acknowledgments
Supported by the Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, Georgia (grant no.: 200-2008-27958). The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, the National Opinion Research Center at the University of Chicago, RTI International, or Duke University.
Footnotes
Financial Disclosure(s):
The author(s) have no proprietary or commercial interest in any materials discussed in this article.
References
- 1.Taylor HR, Pezzullo ML, Keeffe JE. The economic impact and cost of visual impairment in Australia. Br J Ophthalmol. 2006;90:272–275. doi: 10.1136/bjo.2005.080986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frick K, Gower EW, Kempen J, Wolff JL. Economic impact of visual impairment and blindness in the United States. Arch Ophthalmol. 2007;125:544–550. doi: 10.1001/archopht.125.4.544. [DOI] [PubMed] [Google Scholar]
- 3.Rein DB, Zhang P, Wirth KE, et al. The economic burden of major adult visual disorders in the United States. Arch Ophthalmol. 2006;124:1754–1760. doi: 10.1001/archopht.124.12.1754. [DOI] [PubMed] [Google Scholar]
- 4.Frick KD, Foster A. The magnitude and cost of global blindness: an increasing problem that can be alleviated. Am J Ophthalmol. 2003;135:471–476. doi: 10.1016/s0002-9394(02)02110-4. [DOI] [PubMed] [Google Scholar]
- 5.Javitt JC, Zhou Z, Willke RJ. Association between vision loss and higher medical care costs in Medicare beneficiaries: costs are greater for those with progressive vision loss. Ophthalmology. 2007;114:238–245. doi: 10.1016/j.ophtha.2006.07.054. [DOI] [PubMed] [Google Scholar]
- 6.Prevent Blindness America. The economic impact of vision problems: the toll of major adult eye disorders, visual impairment, and blindness on the U.S. economy. Chicago, IL: Prevent Blindness America; 2007. [Accessed December 24, 2012]. Available at: http://www.preventblindness.net/site/DocServer/Impact_of_Vision_Problems.pdf. [Google Scholar]
- 7.Frick KD, Kymes SM, Lee PP, et al. Vancouver Economic Burden of Vision Loss Group. The cost of visual impairment: purposes, perspectives and guidance. Invest Ophthalmol Vis Sci. 2010;51:1801–1805. doi: 10.1167/iovs.09-4469. [DOI] [PubMed] [Google Scholar]
- 8.Rahi JS, Cable N British Childhood Visual Impairment Study Group (BCVISG) Severe visual impairment and blindness in children in the UK. Lancet. 2003;362:1359–1365. doi: 10.1016/S0140-6736(03)14631-4. [DOI] [PubMed] [Google Scholar]
- 9.Ganz ML, Xuan Z, Hunter DG. Prevalence and correlates of children’s diagnosed eye and vision conditions. Ophthalmology. 2006;113:2298–2306. doi: 10.1016/j.ophtha.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 10.Manning WG, Mullahy J. Estimating log models: to transform or not to transform? J Health Econ. 2001;20:461–494. doi: 10.1016/s0167-6296(01)00086-8. [DOI] [PubMed] [Google Scholar]
- 11.Trogdon JG, Finkelstein EA, Hoerger TJ. Use of econometric models to estimate expenditure shares. Health Serv Res. 2008;43:1442–1452. doi: 10.1111/j.1475-6773.2007.00827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brézin A, Lafuma A, Fagnani F, et al. Prevalence and burden of self-reported blindness, low vision, and visual impairment in the French community: a nationwide survey. Arch Ophthalmol. 2005;123:1117–1124. doi: 10.1001/archopht.123.8.1117. [DOI] [PubMed] [Google Scholar]
- 13.Lafuma A, Brézin A, Lopatriello S, et al. Evaluation of nonmedical costs associated with visual impairment in four European countries: France, Italy, Germany and the UK. Pharmacoeconomics. 2006;24:193–205. doi: 10.2165/00019053-200624020-00007. [DOI] [PubMed] [Google Scholar]
- 14.Wirth KE, Rein DB. The economic costs and benefits of dog guides for the blind. Ophthalmic Epidemiol. 2008;15:92–98. doi: 10.1080/09286580801939353. [DOI] [PubMed] [Google Scholar]
- 15.Chambers JG, Parrish TB, Harr JJ. What are we spending on special education services in the United States, 1999e2000. Washington, DC: United States Department of Education, Office of Special Education Programs; 2004. [Accessed December 24, 2012]. Available at: http://www.csef-air.org/publications/seep/national/advrpt1.pdf. [Google Scholar]
- 16.Rein DB, Wittenborn JS, Zhang X, et al. Vision Cost-effectiveness Study Group. The potential cost-effectiveness of amblyopia screening programs. J Pediatr Ophthalmol Strabismus. 2012;49:146–155. doi: 10.3928/01913913-20110823-02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallaway L, Vedder R. The impact of transfer payments on economic growth: John Stuart Mill versus Ludwig von Mises. Q J Austrian Econ. 2002;5:57–65. [Google Scholar]
- 18.Vedder R, Gallaway L. Some underlying principles of tax policy. Staff report, Office of the Chairman, Joint Economic Committee; 1998. [Google Scholar]
- 19.Chadha RK, Subramanian A. The effect of visual impairment on quality of life of children aged 3–16 years. Br J Ophthalmol. 2011;95:642–645. doi: 10.1136/bjo.2010.182386. [DOI] [PubMed] [Google Scholar]
- 20.Gold MR, Franks P, McCoy KI, Fryback DG. Toward consistency in cost-utility analyses: using national measures to create condition-specific values. Med Care. 1998;36:778–792. doi: 10.1097/00005650-199806000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Hirth RA, Chernew ME, Miller E, et al. Willingness to pay for a quality-adjusted life year: in search of a standard. Med Decis Making. 2000;20:332–342. doi: 10.1177/0272989X0002000310. [DOI] [PubMed] [Google Scholar]
- 22.Briggs A, Schulpher M, Claxton K. Decision Modelling for Health Economic Evaluation. New York: Oxford University Press; 2006. pp. 122–126. [Google Scholar]
- 23.DeVol R, Bedroussian A, Charuworn A, et al. Charting a New Course to Save Lives and Increase Productivity and Economic Growth. Santa Monica, CA: Milken Institute; 2007. [Accessed December 24, 2012]. An Unhealthy America: the Economic Burden of Chronic Disease; pp. 3–7. Available at: https://www.milkeninstitute.org/pdf/chronic_disease_report.pdf. [Google Scholar]