Highlights
-
•
[THg] was measured in hair segments of women, reflecting exposure during pregnancy.
-
•
[THg] was noted to be significantly different by hair segment (three per individual).
-
•
Median hair [THg] in pregnant women from Baja California Sur, Mexico was 1.52 μg g−1.
-
•
Many (72%) of the participants had hair [THg] above 1 μg g−1.
-
•
Results suggest associations between [THg] and fish intake, BMI and tobacco exposure.
Keywords: Mercury, Hair, Piscivory, Tobacco, Pregnancy
Abstract
Seafood provides essential polyunsaturated fatty acids (PUFA) and other nutrients to pregnant women and their fetus(es) while a diet rich in finfish can be a major pathway of monomethyl mercury (MeHg+) exposure. We measured total mercury concentration ([THg]) in hair samples provided by 75 women in Baja California Sur (BCS) to assess its relationship with age, parity, tobacco smoke exposure, and diet based on survey methodologies. Generalized linear models (GLM) were used to explain the possible association of the different variables with [THg] in hair. Median [THg] in hair was 1.52 μg g−1, ranging from 0.12 to 24.19 μg g−1 and varied significantly by segment. Approximately 72% (54/75) of those evaluated exceed 1 μg g−1 [THg] and 8% (6/75) exceed 5 μg g−1 [THg] in hair. Although frequency of fish consumption contributed significantly to explaining hair [THg], fish consumption only explained 43% of [THg] in a GLM incorporating tobacco exposure and body mass index. This study establishes possible relationships among multiple potential sources of exposure and other factors related to [THg] in hair of women in the prenatal period. A more detailed examination of other sources of exposure and factors contributing to [THg] is warranted.
1. Introduction
The state of Baja California Sur (BCS), Mexico, is geographically bounded by the Sea of Cortes (east) and the Pacific Ocean (west), and has the largest coastline of any state in Mexico. Fish and shellfish are important dietary components for women of child-bearing age in BCS [1]. Fish consumption is particularly advantageous for pregnant women as it contains high concentrations of omega 3 (ω3) polyunsaturated fatty acids (PUFA), and amino acids that are essential for the developing fetal brain ([2], [3]). However, a diet rich in finfish may be reasonably regarded as a major pathway of exposure to mercury (Hg) [4], [5] and other contaminants.
Mercury exists in three general forms with different bioavailability and toxicity profiles: elemental (Hg0), inorganic (typically divalent, Hg+2), and organic Hg (e.g., monomethyl mercury, MeHg+) as discussed in Trasande et al. [6]. It is well known that MeHg+ concentration can increase with increasing trophic level, a phenomenon referred to as biomagnification [5]. Several reports have described the Hg concentrations in BCS coastal sediments [7], [8], [9]. Total Hg concentration ([THg]) has been reported for biological samples from BCS coast predators such as blue sharks and yellowfin tuna with [THg] up to 1.69 ± 0.18 μg g−1 and 0.15 ± 0.10 μg g−1, respectively, in muscle of the largest specimens [10], [11].
Exposure to MeHg+ from a diet rich in fish, or any other sources, during the pre-natal stage could be associated with serious effects on the central nervous system [12]. Once the mother is exposed to and absorbs MeHg+, it readily crosses the placenta, and reaches the fetal brain [5], where neuronal division and migration can be inhibited causing a disruption of the cerebral structure [13]. The Faroe Islands cohort study [14] documented adverse neurodevelopmental effects of MeHg+ exposure in fetuses, including language, attention, and memory deficits. The lowest observed adverse effect level (LOAEL) from that cohort was determined to be 58 μg L−1 of mercury in the blood of mothers of the group of children reported to have neurodevelopmental deficiencies. This was divided by an uncertainty factor of 10, resulting in a maternal blood [THg] of 5.8 μg L−1, which was further converted to an estimated maternal hair [THg] of approximately 1 μg g−1 associated with a daily intake of 0.1 μgmercury kgbody weight−1 day−1 ([15], [16]). However, the studies from the Faroe Islands, where the diet included pilot whales, are more likely to be confounded by concurrent exposure to other contaminants such as organochlorines (e.g., PCBs) than other populations studied [e.g., Seychelles Islands, Davidson et al. [17]].
Many studies have assessed exposure to Hg using different biological matrices (blood, hair, urine, and breast milk) ([18], [19], [1]). Hair is an excellent biomarker of exposure to Hg because of the capacity to indicate contamination over periods of weeks or months [20]. Hair incorporates circulating elements like Hg, especially the organic form of MeHg+, through the follicle during growth [20], [21], [22]. In humans, the rate of hair growth is approximately one centimeter per month [22]. Therefore, the exposure to Hg in pregnant women can be non-invasively monitored during the full gestation period using strategic study designs related to analyses of select hair segments. This information may suggest if products such as fish and shellfish consumed by the mothers could contribute to Hg exposure over time. The objective of the present study was to determine [THg] in hair segments of mothers living in Baja California Sur (BCS) and the potential relationship to age, parity, marine diet, and tobacco exposure. This manuscript is not intended to be a risk assessment or provide consumption advice.
2. Materials and methods
2.1. Sampling
Samples of occipital scalp hair were collected from women (n = 114) in BCS, Mexico, following the established sample collection procedure [22]. Sampling was performed during July to December 2011, and subjects were classified into one of three groups (n = 38 each) according to parity: GI (primipara); GII (two partum); GIII (three or more partum). During the first interview, informed consent and hair samples were collected on the day of discharge from the hospital. At the second interview, 7–10 days postpartum, the survey was administered and additional biological matrices collected. At this step, 43 of the women either did not want to give more information or could not be found. Overall, there were 97 samples with partial data and 75 with full information: GI (n = 27); GII (n = 23); GIII (n = 25). The 114 hair samples were shipped to the Wildlife Toxicology Laboratory (WTL) at the University of Alaska Fairbanks (UAF) for determination of [THg], and carbon and nitrogen (C, N) stable isotopes values. Stable isotope values are reported separately (Bentzen et al., companion paper). The research project, CONACYT-SALUD 2010-C01-140272 (also known as “Antioxidant response in breast milk to the presence of chemical contamination”), was approved by the Baja California Sur Chapter of the National Mexican Academy for Bioethics.
2.2. Exposure assessment: fish and shellfish consumption questionnaire
Demographic and epidemiological data were collected through a survey. The questionnaire requested information on age, parity (1st, 2nd or 3rd or more), body weight, and height. Weight and height were used to calculate the body mass index [BMI = weight (kg)/height squared (m2)]. General food consumption data covered 30 days prior to hair sample collection. No information was obtained about meal portion size, recipes, or preparation methods. Finfish and shellfish intake frequency data were grouped into four categories: not consumed; consumed once a month; consumed once every two weeks; and consumed more than twice a week. Information about tobacco smoke exposure was also requested and categorized as: smoker; passive exposure; and non-smoker. Informed consent was obtained from all participants.
2.3. Washing hair samples
Hair samples were prepared for [THg] segmental analyses to assess potential temporal variability (analysis of multiple segments per hair sample). The proximal end (segment) of the samples was identified and marked with thread. Each hair sample was tied with white thread every 3–4 cm to prevent tangling during washing. Samples were immersed in a 1% solution of Triton X-100® for 15–20 min to remove external contamination, then followed by an initial 10 min immersion in ultra-pure water (NANOpure Model D4751, Barnstead International, Dubuque, Iowa); then a 5 min immersion, with three additional sequential immersions. Cleaned samples were placed in 4 oz polyethylene bags and freeze-dried for 48 h.
2.4. Subsampling hair
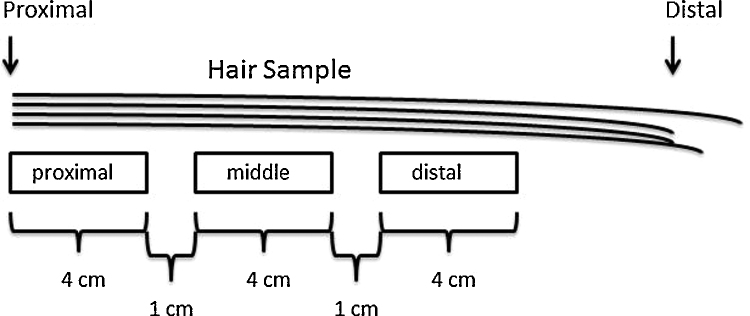
Each scalp hair specimen was subsampled in three locations (segments 3–4 cm long) along the length of the hair. Initial subsamples were 3 cm, but in some cases this did not result in sufficient sample mass for duplicate [THg] measurements. Consequently, sub-sample length was increased to 4 cm. Initially, sub-samples (3 cm) were cut from the proximal, middle, and distal segments of the intact hair samples. This resulted in three distinct periods of growth from each sample with the consistent proximal segment always representing recent hair growth (3–4 months). The growth time between the two distinct segments was variable depending on the initial length of the sample. Distal samples were occasionally of inadequate mass for replicate [THg] measurement as hair length was uneven. Most hair samples were at least 15 cm long, so subsequent segmental analysis included three 4 cm long segments starting at the proximal end, with 1 cm between each segment; thereby, incorporating the most recent 14 cm of hair growth (Fig. 1).
Figure 1.
Subsampling of hair samples incorporating 14 cm of hair growth beginning proximal to the scalp. Subsamples included approximately 4 cm of each of proximal, middle, and distal segments with 1 cm between segments.
2.5. Mercury analysis
Concentration of total Hg ([THg]) was measured in clean, dry hair segments using a Direct Mercury Analyzer (DMA80 Milestone Inc., Shelton, Connecticut; US EPA method 7473; [23], [24], [25]). Individual segments were cut into small pieces, thoroughly mixed, and analyzed in triplicate (6–15 mg per measurement) when sufficient mass was available. When hair mass was insufficient for triplicate analyses single or duplicate measurements were made. The minimum detection limit ranged from 0.067–0.167 μg g−1 of THg depending on sample mass. Quality control included liquid calibration standards and certified hair standard reference materials in each measurement run. Recoveries (mean ± S.D.) were 96.4 ± 3.0% (0.1 μg g−1 liquid standard), 99.1 ± 6.0 (1 μg g−1 liquid standard), 92.9 ± 2.9% (IAEA 086, human hair, 0.573 μg g−1), 102.2 ± 3.6% (NIES 13, human hair, 4.42 ± 0.2 μg g−1), and 96.6 ± 2.1% (IAEA 085, human hair spiked with MeHg+, 23.2 μg g−1).
2.6. Statistical analysis
Descriptive and summary statistics were calculated including means, medians, percentiles (10th and 90th), and percentages. Initially, mixed models were used in a repeated measures analysis (Proc MIXED) to examine whether [THg] varied by number of previous pregnancies and hair segment. This method was chosen since [THg] was measured at multiple points along the hair as “segments” for each individual and these measurements are likely more closely correlated than measurements taken from different individuals. Additionally, unequally-spaced and missing data do not pose a problem for the mixed model [26]. The first-order ante dependence covariance structure was used, as it allows for unequal variances over time and unequal correlations.
Due to the non-normal distribution of [THg] in hair, as shown by the Kolmogorov-Smirnov test, the medians of [THg] were used for between-groups comparisons (Kruskal–Wallis) with significance set at α < 0.05. A generalized linear model (GLM) was used to identify the explanatory variables that contribute to the [THg] measured in the hair samples, using the Poisson error distribution and a log canonical link function [27], [28]. The explanatory variables considered for modeling were age, BMI, number of pregnancies, fish and seafood intake, and tobacco exposure, all variables that in previous studies [1], [29] have been suggested to contribute to [THg]. Predictive models for [THg] were fitted in terms of the explanatory variables with fish intake, seafood intake, and tobacco exposure considered as factor variables included in the GLM. The simplification and selection of the minimal adequate model starting with the maximal model including all the variables of interest was done using the backward/forward stepwise procedure, evaluating all the alternative models by testing the contribution of each variable in turn (p ≤ 0.05), and the change in the residual deviance at each step time [28], [30]. The deviance criterion is a measure of the goodness-of-fit of the model to the data [28]. Finally, the distribution of deviance residuals of the minimal-fitted model was evaluated as a diagnostic method and model validation [28]. Equations for the minimal-fitted models were generated in terms of the explanatory variables with significant contribution to the [THg] in hair.
3. Results
3.1. Demographic characteristics
[THg] was measured in hair segments of 75 women. Participant age ranged from 17 to 44 years (mean = 26.3 ± 8.1 years). Of the total, 27 women were in their 1st pregnancy (gestation) (GI) (average age 22.5 ± 4.3 years), 23 in the 2nd pregnancy (GII) (26.5 ± 10.9 years), and 25 in their 3rd or more pregnancy (GIII) (30.3 ± 6.2 years) (Table 1). Most of the women (n = 42, 56%) work at home. The maternal age was significantly correlated with the number of pregnancies: R = 0.54, p ≤ 0.01.
Table 1.
Demographic (age, occupation), morphometric (body mass index), total mercury concentration in hair (by segment and total), tobacco exposure, and fish/seafood consumption information for mothers who participated and submitted hair, by parity (GI, GII, GIII)a.
| GI(n = 27) | GII(n = 23) | GIII(n = 25) | Total (n = 75) | |
|---|---|---|---|---|
| Age [years; mean (SD)] | 22.5 (4.3) | 26.6 (10.9) | 30.3 (6.2) | 26.3 (8.1) |
| Body mass index [kg/m2; mean (SD)] | 23.2 (3.7) | 28.6 (5.4) | 31.6 (7.4) | 29.6 (5.8) |
| Occupation [number (%)] | ||||
| Housewife | 14 (51.9) | 12 (52.2) | 16 (64.0) | 42 (56.0) |
| Sales | 6 (22.2) | 4 (17.4) | 3 (12.0) | 13 (17.3) |
| Farm | – | – | 2 (8.0) | 2 (2.6) |
| Office | 4 (14.8) | 7 (30.4) | 3 (12.0) | 14 (18.7) |
| Factory | 3 (11.1) | – | 1 (4.0) | 4 (5.3) |
| THg concentration (μg g−1 by hair segment) | ||||
| Proximal | ||||
| Mean (SD) | 2.7 (4.8) | 2.3 (3.6) | 1.8 (2.6) | 2.3 (3.8) |
| Median | 1.4 | 1.3 | 1.6 | 1.4 |
| Percentile 10 | 0.7 | 0.5 | 0.3 | 0.4 |
| Percentile 90 | 3.8 | 4.4 | 2.4 | 3.4 |
| Middle | ||||
| Mean (SD) | 3.1 (6.1) | 3.0 (5.0) | 2.2 (3.3) | 2.8 (4.9) |
| Median | 1.4 | 1.4 | 1.6 | 1.5 |
| Percentile 10 | 0.5 | 0.4 | 0.2 | 0.4 |
| Percentile 90 | 5.9 | 7.8 | 3.6 | 4.7 |
| Distal | ||||
| Mean (SD) | 2.5 (3.3) | 2.7 (3.8) | 2.1 (3.1) | 2.4 (3.3) |
| Median | 1.5 | 1.7 | 1.5 | 1.5 |
| Percentile 10 | 0.5 | 0.4 | 0.5 | 0.5 |
| Percentile 90 | 6.1 | 7.9 | 3.6 | 4.2 |
| Total | ||||
| Mean (SD) | 2.8 (4.7) | 2.6 (4.1) | 2.0 (3.0) | 2.5 (3.9) |
| Median | 1.6 | 1.5 | 1.6 | 1.5 |
| Percentile 10 | 0.5 | 0.5 | 0.3 | 0.5 |
| Percentile 90 | 5.3 | 6.5 | 2.9 | 3.8 |
| >1 μg g−1 [n (%)]b | 21 (77.8) | 17 (73.9) | 16 (64.0) | 54 (72.0) |
| <5 μg g−1 [n (%)]c | 25 (92.6) | 20 (86.9) | 24 (96) | 69 (92) |
| Tobacco exposure [n (%)] | ||||
| Smoker | 4 (14.8) | 3 (13.0) | 2 (6.45) | 9 (12.0) |
| Passive exposure | 5 (18.5) | 4 (17.4) | 7 (22.58) | 15 (20.0) |
| Non-smoker | 18 (66.7) | 16 (69.6) | 22 (70.97) | 51 (68.0) |
| Food consumption [n (%)] | ||||
| Fish | ||||
| None | 2 (7.4) | 2 (8.7) | 3 (12.0) | 7 (9.3) |
| Once a month | 6 (22.2) | 10 (43.5) | 12 (48.0) | 28 (37.3) |
| Once every 2 weeks | 15 (55.6) | 9 (39.1) | 7 (28.0) | 31 (41.3) |
| Two or more times a week | 4 (14.8) | 2 (8.7) | 3 (12.0) | 9 (12.0) |
| Seafood [n (%)] | ||||
| None | 9 (33.3) | 9 (39.1) | 5 (20.0) | 23 (30.7) |
| Once a month | 13 (48.1) | 8 (34.8) | 16 (64.0) | 37 (49.3) |
| Once every 2 weeks | 4 (14.8) | 5 (21.7) | 4 (16.0) | 13 (17.3) |
| Two or more times a week | 1 (3.7) | 1 (4.3) | – | 2 (2.7) |
There was no significant difference in BMI between GI (mean 23.2) and GII (mean 28.6) (sum of squares = 0.42, df = 1, F = 0.002 p = 0.96); neither between GI and GIII (mean 31.6) (sum of squares = 118.76, df = 1, F = 3.46, p = 0.07), nor between GII (mean 28.6) and GIII (mean 31.6) (sum of squares = 105.44, df = 1, F = 2.43, p = 0.12).
3.2. Frequency of tobacco use
Participants were asked about tobacco exposure; 12% (9/75) responded that they smoked more than one cigarette per day. Most of those who smoke were mothers in their first pregnancy 14.8% (4/27); or 5.3% of the 75 total participants. If they did not smoke, respondents were asked if someone else smokes in the household, at the office, or in some other enclosed space; 20% (15/75) answered affirmatively. A total of 68% (51/75) were not regularly exposed to tobacco smoke.
3.3. Frequency of fish and shellfish consumption
Respondents were asked about their fish and shellfish eating habits: a) fish intake; 7.6% never eat fish, 33.9% eat fish once a month, 41.3% eat fish once every 2 weeks, and 15.9% eat fish more than twice a week; b) shellfish intake; 30.7% (23/75) never eat shellfish, 49.3% (37/75) eat shellfish once a month, 17.3% (13/75) eat shellfish once every 2 weeks, and 2.7% (2/75) eat shellfish two or more times a week.
3.4. Total mercury concentrations ([THg])
For the total number of samples (75) a median [THg] in hair of 1.52 μg g−1, ranging from 0.12 to 24.19 μg g−1 was found. Seventy two percent of the women (54/75) exceeded the U.S. EPA recommended limit of 1 μg g−1 hair [THg]. For 77.8% (21/27) of GI women [THg] was greater than 1 μg g−1 hair.
Total Hg concentrations were significantly lower in the proximal hair segment than in the middle segment (−0.50, t = −3.35, p ≤ 0.01). [THg] did not differ between the middle and distal segments (0.30, t = 1.15, p = 0.25), or between the proximal and distal segments (−0.17, t = −0.98, p = 0.33).
Frequency of fish intake significantly contributed to the [THg] in the three hair segments (Table 2) (p < 0.01). In the middle segment, the median [THg] for those who never eat fish was 0.51 μg g−1, and those who eat fish two or more times a week was 2.13 μg g−1 (p < 0.01). Table 3 shows that fish intake in the GI (first pregnancy) group was the only exposure factor that significantly affected [THg], with a median [THg] for those who never eat fish of 0.46 μg g−1 and those who eat fish two or more times a week of 2.12 μg g−1 (p = 0.05).
Table 2.
Median comparison of total mercury concentration (μg g−1) in hair by segment (proximal, middle, distal, and total), related to tobacco exposure and fish and seafood consumption.
| Proximal | pa | Middle | p | Distal | p | Total | p | |
|---|---|---|---|---|---|---|---|---|
| Smoker | ||||||||
| Non-smoker | 1.5 | 1.4 | 1.5 | 1.5 | ||||
| Smoker | 1.4 | 0.95 | 1.5 | 0.89 | 1.6 | 0.9 | 1.6 | 0.97 |
| Passive exposure | 1.2 | 1.6 | 1.7 | 1.5 | ||||
| Food consumption | ||||||||
| Fish | ||||||||
| None | 0.5 | <0.01 | 0.5 | <0.01 | 0.5 | <0.01 | 0.6 | <0.01 |
| Once a month | 1.1 | 1.2 | 1.4 | 1.4 | ||||
| Once every 2 weeks | 1.7 | 1.6 | 1.6 | 1.7 | ||||
| Two or more times a week | 1.7 | 2.1 | 1.9 | 1.9 | ||||
| Seafood | ||||||||
| None | 1.1 | 0.14 | 1.3 | 0.2 | 1.6 | 0.22 | 1.3 | 0.24 |
| Once a month | 1.3 | 1.4 | 1.4 | 1.5 | ||||
| Once every 2 weeks | 1.8 | 1.7 | 2.1 | 2.0 | ||||
| Two or more times a week | 1.6 | 2.2 | 1.5 | 1.7 | ||||
a Kruskal–Wallis test
Table 3.
Median comparison of total mercury concentration (μg g−1) in hair by number of pregnancy (GI, GII, GIII)a, related to tobacco exposure and fish and seafood consumption.
| GI | pb | GII | p | GIII | p | |
|---|---|---|---|---|---|---|
| Smoker | ||||||
| Non-smoker | 1.5 | 0.75 | 1.5 | 0.99 | 1.5 | 0.92 |
| Smoker | 1.4 | 1.3 | 1.7 | |||
| Passive exposure | 1.7 | 1.4 | 1.7 | |||
| Food consumption | ||||||
| Fish | ||||||
| None | 0.5 | 0.05 | 0.6 | 0.23 | 0.6 | 0.35 |
| Once a month | 1.2 | 1.5 | 1.4 | |||
| Once every 2 weeks | 1.7 | 1.4 | 2.0 | |||
| Two or more times a week | 2.1 | 2.4 | 1.6 | |||
| Seafood | ||||||
| None | 1.3 | 0.14 | 0.8 | 0.54 | 2.3 | 0.38 |
| Once a month | 1.2 | 1.6 | 1.4 | |||
| Once every 2 weeks | 6.4 | 1.4 | 1.8 | |||
| Two or more times a week | 1.9 | 1.6 | - | |||
a GI (primipara); GII (2 partum); GIII (3 partum)
b Kruskal–Wallis test
BMI was significantly and positively correlated with [THg] (R = 0.33, p ≤ 0.01). [THg] did not significantly vary by number of previous pregnancies (p = 0.82). Tobacco exposure did not affect [THg] in the bi-variate analysis.
3.5. Modeling analysis
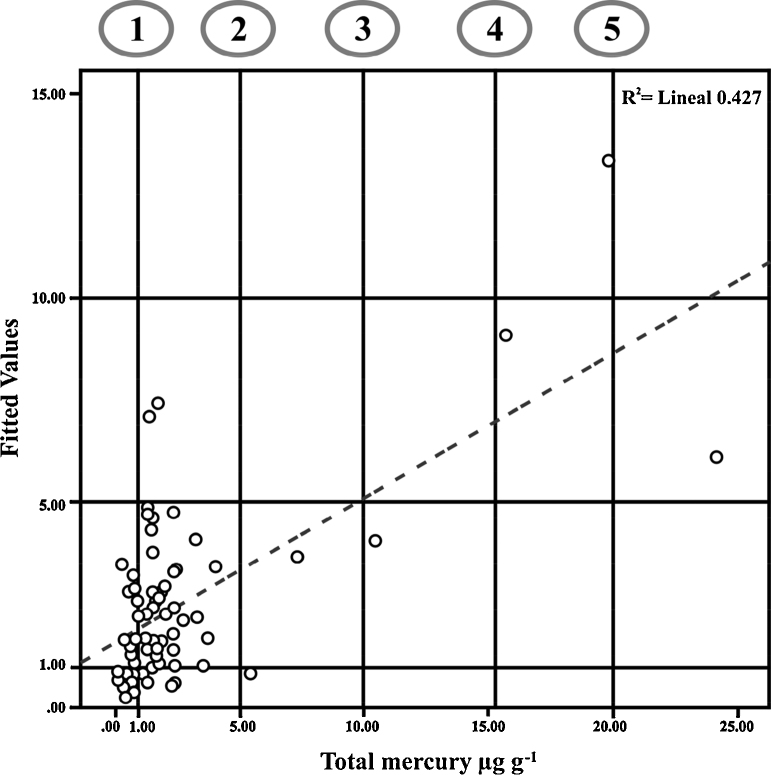
The minimal fitted model, generated by the GLM analysis, explained 43% of the [THg] in hair (Fig. 2). A relationship between fitted and observed values is shown in Fig. 2, where 28% of the samples showed levels under 1 μg g−1 [15], a relatively conservative guideline (a reference dose that is 10-fold less than the benchmark dose associated with an increased adverse effect), and 92% of the samples showed levels under the 5 μg g−1 threshold at which, for example, the Alaska Statewide Hair Mercury Biomonitoring Program (http://www.epi.alaska.gov/eh/biom/) has conducted individual follow up since 2002 [31].
Figure 2.
Relationship between fitted (generated by GLM analysis) and observed values of [THg] in hair of seventy five pregnant women from Baja California Sur, Mexico. Some published guidelines for hair mercury concentrations for humans are indicated and provided for general context only (i.e., to illustrate that a broad range of guidelines exists and not as a risk assessment): 1. U.S. Environmental Protection Agency [1 μg g−1; U.S. EPA [15]]; 2. Alaska Statewide Hair Mercury Biomonitoring Program [5 μg g−1; Hamade [31]]; 3. Health Canada [2–10 μg g−1 and > 10 μg−1 g−1; Feeley and Lo [45]; Legrand et al. [48]; NRC [46]]; 4. Agency for Toxic Substances and Disease Registry [15.3 μg g−1; Risher and DeWoskin [47]]; 5. World Health Organization [10-20 μg g−1; WHO [40]]. These guidelines are based on varying toxicological and Hg exposure assumptions and sources of data, represent varying levels of concern, and are not directly comparable.
The [THg] in hair was explained by the BMI, fish intake, and tobacco exposure. The coefficients generated by the GLM for [THg] were positively correlated to tobacco exposure, and negatively correlated to BMI and fish intake. The negative values of coefficients for fish intake are because the analysis considered as the control group, the one with lower risk of exposure (i.e., those who never eat fish) (Table 4). The equations for the [THg] were developed using the categories of tobacco exposure and fish intake according to the coefficients generated by the GLM (Table 5).
Table 4.
Coefficients fitted by the generalized linear model (GLM) with a Poisson error distribution for the total mercury (THg) concentration (μg g−1) in hair of pregnant women from Baja California Sur related to body mass index (BMI), frequency of fish consumption, and exposure to tobacco.
| Model | Variable | Unstandardized coefficients | z | p | Res. Dev.b (df c) Minimal Model | 95% Confidence interval of b | ||
|---|---|---|---|---|---|---|---|---|
| b | Std. error a | Lower bound | Upper bound | |||||
| THg d | (Intercept) | 3.242 | 0.434 | 7.465 | <0.001 | 129.6 (68) | 2.370 | 4.074 |
| BMI e | −0.085 | 0.013 | −6.578 | <0.001 | −0.110 | −0.060 | ||
| fish [Never] | −1.767 | 0.452 | −3.908 | <0.001 | −2.794 | −0.984 | ||
| Fish [>two times in a week] | −0.209 | 0.269 | −0.776 | 0.438 | −0.767 | 0.293 | ||
| Fish [Once in a month] | −0.781 | 0.181 | −4.306 | <0.001 | −1.147 | −0.433 | ||
| Non smoker | 0.322 | 0.270 | 1.195 | 0.232 | −0.177 | 0.887 | ||
| Passive | 0.879 | 0.291 | 3.017 | 0.003 | 0.332 | 1.481 | ||
Standard error
Deviance residual
Degrees of freedom
Total mercury concentration (μg g−1)
Body mass index
Table 5.
Fitted models for the total mercury (THg) concentration in hair of pregnant women from Baja California Sur based on levels of exposure to tobacco and frequency of fish consumption.
| Median | Median | ||||
|---|---|---|---|---|---|
| Variable | Tobacco | Fish intake | Model | THg* measured | THg fitted model |
| Mercury | Smoker | Never | THg =1.475−0.085BMI** | nd*** | nd |
| Once in a month | THg = e2.460−0.085BMI | 3.37 | 1.12 | ||
| Once in 2 weeks | THg = e3.242−0.085BMI | 1.23 | 2.34 | ||
| Greater than 2 times in a week | THg = e3.033−0.085BMI | 1.56 | 1.60 | ||
| Passive | Never | THg = e2.354−0.085BMI | 0.75 | 1.10 | |
| Once in a month | THg = e3.340−0.085BMI | 1.53 | 2.53 | ||
| Once in 2 weeks | THg = e4.121−0.085BMI | 7.55 | 6.16 | ||
| Greater than 2 times in a week | THg = e3.912−0.085BMI | nd | nd | ||
| Non Smoker | Never | THg = e1.797−0.085BMI | 0.73 | 0.67 | |
| Once in a month | THg = e2.783−0.085BMI | 1.38 | 1.19 | ||
| Once in 2 weeks | THg = e3.564−0.085BMI | 3.38 | 3.57 | ||
| Greater than 2 times in a week | THg = e3.355−0.085BMI | 2.14 | 2.13 |
*Total mercury concentration (μg g−1)
**Body mass index
***Not determined
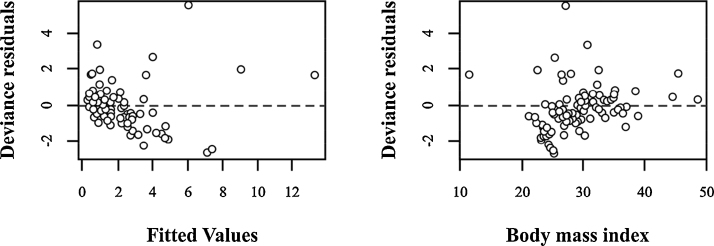
For any given equation of linear regression generated, different values of intercepts were found in the population sampled [32]. The intercepts help to explain the [THg] using the categories of tobacco exposure and fish intake. The model explained an increment in the median of the fitted values of [THg] in those women (smoker, passive, or non-smoker) who included fish in their diet with a frequency of once in two weeks or as frequently as two or more times a week ([THg] > 2.5 μg g−1, Table 5). The women, whether exposed or not to tobacco, who never consumed fish were the group with the lower median [THg] levels in hair ([THg] < 1.12 μg g−1, Table 5). In general, the median of the fitted values generated by the GLM were higher than the [THg] measured in hair (Table 5). Age, pregnancy number, and shellfish consumption did not contribute to explaining [THg] in hair. The residuals of the model showed an evident homoscedasticity in the distribution suggesting constant variance, as expected for a fitted model (Fig. 3).
Figure 3.
Residual plots of the minimal adequate model for body mass index of women inhabiting in Baja California Sur.
4. Discussion
4.1. Use of hair and segmental analysis
Human hair has an average growth rate of 1–1.5 cm per month [22]. The three segments of hair analyzed in this study reflect approximately the 12 month period prior to parturition, and suggests a chronic exposure to Hg by most of the women. The difference in concentrations of [THg] between two of the three segments may be due to seasonal variations in dietary exposure [20]. Possibly, the greatest exposure factor for [THg] in hair of women in BCS is fish consumption. In the state of Veracruz, Guentzel et al. [33] demonstrated seasonality in the diet, with consumption of predatory fish during the rainy season, and an increase in the consumption of benthic fish during the dry season; which is reflected as an increase in [THg] in hair during the rainy season. This relationship between [THg] and the consumption of large predatory fish has been described by various authors ([4], [5], [34], [35]). In BCS, the majority of local fisheries are based on predatory fish species [36], with potential for relatively high [THg]. For example, in muscle samples from the largest specimens, mean [THg] in blue shark was 1.69 ± 0.18 μg g−1 and in yellowfin tuna was 0.15 ± 0.10 μg g−1 [10], [11]. This may explain, to a certain degree, the relationship between frequency of fish consumption and increased [THg], a situation observed in the GI group. Approximately 70% (19/27) of women in the GI group eat fish at least once in two weeks up to more than twice a week. Although portion sizes are unknown, the same range of frequency of consumption is high in comparison to the GIII at 40% (10/25).
4.2. Importance of assessing pregnant women
The development of the nervous system begins in the first weeks of gestation and consists of a series of processes that occur in a predetermined sequence and depend upon each other. Interference with one of these processes can also affect later phases of development [37]. This explains the importance of the period and duration (timing) of exposure to Hg in the organogenesis and cerebral histogenesis, the effects of which can be expressed later in life, including in the adult stage ([12], [37]).
The main drivers for addressing Hg exposure in this study are associated with vulnerability of the fetal cerebrum, as the period studied is comprised of the entire pregnancy. Chronic exposure of the fetal nervous system to Hg can produce alterations in its development ([4], [14], [37]). These lesions can present themselves in the cerebral structure with focal necrosis of the cortical and cerebelluous neurons, with destruction of the perifocal glial cells, or in the cerebral function, with interference in the process of migration of the cortical and subcortical neuronal layers ([13], [37]).
4.3. Comparison to recommendation limits and other populations
In this study, we report our data relative to some published guidelines ranging from 1 to 20 μg g−1 [THg] in hair (Fig. 2) to put these data into context (not a risk assessment). These hair guidelines represent various data sources, assumptions, and levels of concern and illustrate the wide range of advisory information available. Many recommendation limits related to fish intake have been reported in the literature based on [THg] in hair (and/or blood). Guidelines of acceptable daily intake of mercury generated from hair or blood [THg] also use a variety of models, assumptions and correction factors and range from 0.1 to ≥0.8 μg kg−1 day−1 [U.S. EPA, 0.1 μg kg−1 day −1; Alaska Statewide Hair Mercury Biomonitoring Program, 0.56 μg kg−1 day−1; Health Canada, 0.2 μg kg−1 day−1; Agency for Toxic Substances and Disease Registry, 0.3 μg kg−1 day−1; and World Health Organization, ≥ 0.8 μg kg−1 day−1 for more detail, see Hamade [31]]. Our results showed that 72% of the hair samples contained [THg] above 1 μg g−1 [15] with fewer samples (8%) above 5 μg g−1 [31]. Similar results are reported in previous studies of women with high fish consumption in coastal populations [22], [33], [34], [35], [38]. In Bachok, Malaysia, 72% of hair samples analyzed showed levels above 1 μg g−1 [34]. In Japan, 70% of hair samples from women showed levels above those recommended by the U.S. EPA [38]. In Mexico, levels above 1 μg g−1 were reported in 58% of women from the Veracruz population on the coast of the Gulf of Mexico [33]. The coastal population of Veracruz, in contrast to that in Baja California Sur, is not geographically isolated. This may allow for greater inclusion of different protein sources in the women's diet. There is, however, a discrepancy from results reported by Trasande et al. [6] in Chapala, Jalisco, in the central region of Mexico. Those data show that only 27.2% of women were found with average [THg] levels in hair above 1 μg g−1 even though this population consumes freshwater fish, which were proven to contain relatively high [THg] [6].
4.4. Context with respect to well-studied populations (Faroe and Seychelles)
The degree of neuropsychological deficits in memory and language depends on several factors, according to the epidemiological studies of pre- and postnatal exposure to Hg of children in the Seychelles Islands [17] and pre-natal exposure of children of the Faroe Islands [12], [14]: a) Hg levels in fish—the children of the Seychelles were consuming fish with lower concentrations of Hg, as compared to the Faroe Islands ([12], [14], [17], [37]), b) frequency—the ingestion of fish is 10–12 meals week−1 in the Seychelles Islands, in comparison to 2–3 meals week−1 in the Faroe Islands [14], [17], c) other factors and intakes—the Seychelles Islands have a tropical climate and different species of fish. As such, the population of these islands has greater access to fruits and vegetables, in comparison to the population of the Faroe Islands where more tubers and red meat are consumed. Moreover, the inhabitants of the Faroe Islands include toothed whales in their diet that are rich in polychlorinated biphenyls (and other organohalogens) and numerous heavy metals [37]. The population of the Seychelles Islands shares some characteristics with the population in this study; both are tropical, both incorporate marine protein through consumption of fish but not marine mammals, and both have a greater ability, when compared to the inhabitants of the Faroe Islands, to include fruits and vegetables in their diet. It is hard to suggest which guidelines Mexico may have to adopt for the BCS region, because they can range from the U.S. EPA recommendation for Hg ingestion of no greater than 0.1 μg kg−1 body weight day−1, which translates to 1 μg g−1 in hair ([39], [15], [16]), to the recommendation of the World Health Organization of 20 μg g−1 in hair [40].
4.5. Mathematical modelling
The statistical models used are simplified representations, and describe possible associations between the dependent variable ([THg]) and the independent variables (BMI, exposure to tobacco smoke, ingestion of fish) with a probabilistic component, which involves the inclusion of variability due to unknown random factors [27], [30]. Although the ingestion of fish seems to be the main variable that participates in the explanation of [THg] in the hair of the women in BCS, through multi-variable analysis, a possible association with other factors was identified. The co-variables adjusting the [THg] in the model were BMI, fish consumption (never and once a month), and tobacco exposure (passive exposure) (Table 4). Although, there is no relationship between [THg] and smoking status (Table 2), when developing the generalized linear models, exposure to tobacco smoke adjusts the model in conjunction with fish consumption and BMI in 43% of the explained [THg] in hair. Tobacco exposure is positively related to [THg] in hair, especially in the passive exposure. A similar situation was previously reported in Spanish children [41], in which a decrease in [THg] related to BMI was reported. The outcomes of this study, namely passive smoking contributing to hair [THg] with no influence from smoking status, parallel the results of Park et al. [42]. Possible explanations for this are the contribution of heavy metals in the smoke impregnating the hair of the passive smoker, and/or activation of detoxification processes [cytochrome P450, glutathione S-transferase, for further discussion see Gaxiola-Robles et al. [29]; Gaxiola-Robles et al. [1]] in those women who do smoke. The combined findings indicate that BMI interacts with heavy metal toxicants in a manner that may alter toxicodynamics within the body that reduces [THg] in hair [42], [43]. In addition, there is likely an interaction between BMI and/or tobacco exposure that requires further investigation related to [THg] in hair that is independent of fish consumption. Therefore, the actual [THg] associated to frequency of fish intake may be lower than initially assumed because of possible BMI and tobacco physiologically-based interactions.
The data from this study suggest that the ingestion of fish is a key factor, along with smoke exposure and BMI, in determining [THg] in hair of pregnant women. Nevertheless, there are other factors which were not analyzed, but which might be related to the results reported in this study. These include those cited in the literature: beauty products such as creams to lighten the skin tone, hair dyes, home remedies, and dental fillings with amalgam, among many others [5]. The benefit of including fish in the diet (PUFAs, selenium) must be weighed and prioritized against the possible negative effects related to Hg. In order to be able to properly develop recommendations for fish consumption (species, portions, frequency), future studies must be directed toward the recognition of fish species and mass consumed (portion size) at the local level, and their possible contribution to the levels of Hg.
When using the GLM, it is important before modeling to assess the correlation among the explanatory variables in order to avoid the effect of multicollineality and to get consistency in the fit models independent of the simplification procedures used [44]. The predictive power of the models fits solely for observations within the same range of data analyzed, and should be assessed with validation tests using new, independent data [30].
5. Conclusions
Frequency of fish consumption contributed significantly to explaining hair [THg]. However, based on the GLM, and considering the other significant co-variables (BMI and tobacco exposure) it explains only 43% of the [THg]. As the contribution of fish consumption frequency to [THg] is relatively low, it is necessary to assess other factors which may be contributing to exposure: dental amalgams, use of creams to lighten the skin, and other factors that were not included in the present study. In particular, a more detailed assessment of the mass of the fish meal and type of fish (e.g., predatory) may prove as, or more, important than fish meal frequency. The GLM is a practical tool for identifying the variables that contribute to the explanation of the exposure to Hg during pregnancy. It allows for establishing the possible relationship between multiple potential sources of exposure and [THg] in hair of women in the prenatal period. The variables that were found in this study to have significant relationships with [THg] were hair segment sampled, BMI, tobacco exposure, and the ingestion of fish; which deserve a focused and intensive follow up at the physiologic and genomic levels. In all models created, the frequent ingestion of fish (more than once every two weeks) showed increases in the averages of the adjusted values of [THg].
Transparency document
Acknowledgments
This project was funded by grants from CONACYT–Salud (2010-C01-140272) and CIBNOR (PC2.0, PC0.10, PC0.5). This study would not have been possible without the assistance of some current and former members of the Wildlife Toxicology Laboratory and School of Fisheries and Ocean Sciences at the University of Alaska Fairbanks. University of Alaska personnel were partially supported through the Center for Alaska Native Health Research by Award Number P20RR016430 from the National Center for Research Resources and through the IDeA Network of Biomedical Research Excellence Award Number P20GM103395 from the National Institute of General Medical Sciences of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Footnotes
Available online 17 October 2014
Contributor Information
Ramón Gaxiola-Robles, Email: r.gaxiolar@gmail.com.
Rebecca Bentzen, Email: rlmcguire@alaska.edu.
Tania Zenteno-Savín, Email: tzenteno04@cibnor.mx.
Vanessa Labrada-Martagón, Email: vlabrada@gmail.com.
J. Margaret Castellini, Email: rlmcguire@alaska.edu, maggie.c@alaska.edu.
Alfredo Celis, Email: alfredo_celis@yahoo.com.
Todd O’Hara, Email: tmohara@alaska.edu.
Lía Celina Méndez-Rodríguez, Email: lmendez04@cibnor.mx.
References
- 1.Gaxiola-Robles R., Zenteno-Savín T., Labrada-Martagón V., Celis A., Acosta-Vargas B., Méndez-Rodríguez L.C. Concentraciones de mercurio en leche de mujeres del noroeste de México; posible asociación a la dieta, tabaco y otros factores maternos. Nutrición Hospitilaria. 2013;28:934–942. doi: 10.3305/nh.2013.28.3.6447. [DOI] [PubMed] [Google Scholar]
- 2.Innis S.M. Fatty acids and early human development. Early Hum. Dev. 2007;83:761–766. doi: 10.1016/j.earlhumdev.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Prospéro-García O. Méndez Díaz M, Alvarado Capuleño I, Pérez Morales M, López Juárez J, Ruiz Contreras AE. Inteligencia para la alimentación, alimentación para la inteligencia. Salud Mental. 2013;36:101–107. [Google Scholar]
- 4.Acosta-Saavedra L.C., Moreno M.E., Rodriguez-Kessler T., Luna A., Arias-Salvatierra D., Gomez R. Environmental exposure to lead and mercury in Mexican children: a real health problem. Toxicol. Mech. Methods. 2011;21:656–666. doi: 10.3109/15376516.2011.620997. [DOI] [PubMed] [Google Scholar]
- 5.Ortega-García J.A., Ferrís-Tortajada J., Cánovas-Conesa A., Garcia-Castell J. Neurotóxicos medioambientales (y II). Metales: efectos adversos en el sistema nervioso fetal y posnatal. Acta Pediatrica Espanola. 2005;63:182–192. [Google Scholar]
- 6.Trasande L., Cortes J.E., Landrigan P.J., Abercrombie M.I., Bopp R.F., Cifuentes E. Methylmercury exposure in a subsistence fishing community in Lake Chapala, Mexico: an ecological approach. Environ. Health. 2010;9:1. doi: 10.1186/1476-069X-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gutierrez-Galindo E.A., Casa-Beltran D.A., Muños-Barbosa A., Daesslé L.W., Segovia-Zavala J.A., Macías-Zamora J.V. Distribution of mercury in superficial sediment from Todos Santos Bay, Baja California, Mexico. Bull. Environ. Contam. Toxicol. 2008;80:123–127. doi: 10.1007/s00128-007-9329-x. [DOI] [PubMed] [Google Scholar]
- 8.Kot F.S., Green-Ruiz C., Paez-Osuna F., Shumilin E.N., Rodriguez-Meza D. Distribution of mercury in sediments from La Paz lagoon, Peninsula of Baja California, Mexico. Bull. Environ. Contam. Toxicol. 1999;63:45–51. doi: 10.1007/s001289900946. [DOI] [PubMed] [Google Scholar]
- 9.Rodríguez-Meza G.D., Shumilin E., Sapozhnikov D., Méndez-Rodríguez L.C., Acosta-Vargas B. Evaluación geoquímica de elementos mayoritarios y oligoelementos en los sedimentos de Bahía Concepción (B.C.S. México) Boletín de la Sociedad Geológica Mexicana. 2009;61:57–72. [Google Scholar]
- 10.Barrera-García A., O’Hara T., Galván-Magaña F., Méndez-Rodríguez L.C., Castellini J.M., Zenteno-Savín T. Oxidative stress indicators and trace elements in the blue shark (Prionace glauca) off the east coast of the Mexican Pacific Ocean. Comparative Biochemistry and Physiology. C. Toxicol. Pharmacol. 2012;156:59–66. doi: 10.1016/j.cbpc.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Ordiano-Flores A., Rosiles-Martinez R., Galvan-Magana F. Biomagnification of mercury and its antagonistic interaction with selenium in yellowfin tuna Thunnus albacares in the trophic web of Baja California Sur, Mexico. Ecotox. Environ. Safe. 2012;86:182–187. doi: 10.1016/j.ecoenv.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 12.Grandjean P., Weihe P., Nielsen J.B. Methylmercury: significance of intrauterine and postnatal exposures. Clin. Chem. 1994;40:1395–1400. [PubMed] [Google Scholar]
- 13.Choi B.H., Lapham L.W., Amin-Zaki L., Saleem T. Abnormal neuronal migration, deranged cerebral cortical organization, and diffuse white matter astrocytosis of human fetal brain: a major effect of methylmercury poisoning in utero. J. Neuropath. Exp. Neur. 1978;37:719–733. doi: 10.1097/00005072-197811000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Debes F., Budtz-Jorgensen E., Weihe P., White R.F., Grandjean P. Impact of prenatal methylmercury exposure on neurobehavioral function at age 14 years. Neurotoxicol. Teratol. 2006;28:536–547. doi: 10.1016/j.ntt.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 15.U.S. EPA. 1997. Mercury study report to Congress, Office of Air Quality Planning & Standards and Offices of Research and Development, Environmental Protection Agency, International Programme on Chemical Safety (IPCS)http://www.epa.gov/ttn/oarpg/t3/reports/volume1.pdf Vol 1., Executive Summary, 95 pp. [Google Scholar]
- 16.U.S. EPA. 2004. What You Need to Know about Mercury in Fish and Shellfish. Environmental Protection Agency, Water: Outreach and Communication.http://water.epa.gov/scitech/swguidance/fishshellfish/outreach/advice_index.cfm [Google Scholar]
- 17.Davidson P.W., Leste A., Benstrong E., Burns C.M., Valentin J., Sloane-Reeves J. Fish consumption, mercury exposure, and their associations with scholastic achievement in the Seychelles Child Development Study. Neurotoxicology. 2010;31:439–447. doi: 10.1016/j.neuro.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carrier G., Bouchard M., Brunet R.C., Caza M. A toxicokinetic model for predicting the tissue distribution and elimination of organic and inorganic mercury following exposure to methyl mercury in animals and humans. II. Application and validation of the model in humans. Toxicol. Appl. Pharm. 2001;171:50–60. doi: 10.1006/taap.2000.9113. [DOI] [PubMed] [Google Scholar]
- 19.Castillo-Castañeda P.C., Gaxiola-Robles R., Méndez- Rodríguez L.C., Zenteno-Savín T. Defensas antioxidantes en leche materna en relación al número de gestas y a la edad de las madres. Nutrición Hospitalaria. 2014;30 doi: 10.3305/nh.2014.30.3.7623. In Press. [DOI] [PubMed] [Google Scholar]
- 20.Lieske C.L., Moses S.K., Castellini J.M., Klejka J., Hueffer K., O’Hara T.M. Toxicokinetics of mercury in blood compartments and hair of fish-fed sled dogs. Acta Vet. Scand. 2011;53:66. doi: 10.1186/1751-0147-53-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Budtz-Jorgensen E., Grandjean P., Jorgensen P.J., Weihe P., Keiding N. Association between mercury concentrations in blood and hair in methylmercury-exposed subjects at different ages. Environ. Res. 2004;95:385–393. doi: 10.1016/j.envres.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 22.McDowell M.A., Dillon C.F., Osterloh J., Bolger P.M., Pellizzari E., Fernando R. Hair mercury levels in U.S. children and women of childbearing age: reference range data from NHANES 1999-2000. Environ. Health Perspect. 2004;112:1165–1171. doi: 10.1289/ehp.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castellini J.M., Rea L.D., Lieske C.L., Beckmen K.B., Fadely B.S., Maniscalco J.M. Mercury concentrations in hair from neonatal and juvenile Steller Sea Lions (Eumetopias jubatus): implications based on age and region in this northern Pacific marine sentinel piscivore. EcoHealth. 2012;9:267–277. doi: 10.1007/s10393-012-0784-4. [DOI] [PubMed] [Google Scholar]
- 24.Knott K.K., Boyd D., Ylitalo G.M., O’Hara T.M. Concentrations of mercury and polychlorinated biphenyls in blood of Southern Beaufort Sea polar bears (Ursus maritimus) during spring: variations with lipids and stable isotopes ((15 N, (13 C) values. Can. J. Zool. 2011;89:999–1012. [Google Scholar]
- 25.Rea L.D., Castellini J.M., Correa L., Fadely B.S., O’Hara T.M. Maternal Steller sea lion diets elevate fetal mercury concentrations in an area of population decline. Sci. Total Environ. 2013;454-455:277–282. doi: 10.1016/j.scitotenv.2013.02.095. [DOI] [PubMed] [Google Scholar]
- 26.Wang L.A., Goonewardene Z. The use of mixed models in the analysis of animal experiments with repeated measures data. Can. J. Anim. Sci. 2004;84:1–11. [Google Scholar]
- 27.Lindsey J.K. Springer; New York: 1997. Applying Generalized Linear Models. [Google Scholar]
- 28.Zuur A.F., Ieno E.N., Walker N.J., Saveliev A.A., Smith G.M. Springer; New York, NY: 2009. Mixed Effects Models and Extensions in Ecology with R. p. 574. [Google Scholar]
- 29.Gaxiola-Robles R., Labrada-Martagón V., Celis de la Rosa A.J., Acosta-Vargas B., Méndez-Rodríguez L.C., Zenteno-Savín T. Interaction between mercury (Hg), arsenic (As) and selenium (Se) affects the activity of glutathione S-transferase in breast milk; possible relationship with fish and shellfish intake. Nutrición Hospitalaria. 2014;30:436–446. doi: 10.3305/nh.2014.30.2.7441. [DOI] [PubMed] [Google Scholar]
- 30.Labrada-Martagón V., Mendez-Rodriguez L.C., Mangel M., Zenteno-Savin T. Applying generalized linear models as an explanatory tool of sex steroids, thyroid hormones, and their relationships with environmental and physiologic factors in immature East Pacific green sea turtles (Chelonia mydas) Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2013;166:91–100. doi: 10.1016/j.cbpa.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 31.Hamade A.K. Fish consumption advice for Alaskans: A risk management strategy to optimize the public's health. July 21. Alaska Scientific Advisory Committee for Fish Consumption. Section of Epidemiology.; Division of Public Health, Department of Health and Social Services, State of Alaska; 2014. p. 78. [Google Scholar]
- 32.Kleinbaum D.G., Kupper L.L. Duxbury Press; Boston, Massachusetts, USA: 1978. Applied Regression Analysis and Other Multivariable Methods; p. 556. [Google Scholar]
- 33.Guentzel J.L., Portilla E., Keith K.M., Keith E.O. Mercury transport and bioaccumulation in riverbank communities of the Alvarado Lagoon System, Veracruz State, Mexico. Sci. Total Environ. 2007;388:316–324. doi: 10.1016/j.scitotenv.2007.07.060. [DOI] [PubMed] [Google Scholar]
- 34.Tengku-Hanidza T.I., Harun R., Juahir H., Zin S.M., Yasutake A., Tunku K.F. Hair mercury levels in relation to marine fish consumption among adults in Malaysia. EnvironmentAsia. 2010;3:175–185. [Google Scholar]
- 35.Xue J., Zartarian V.G., Liu S.V., Geller A.M. Methyl mercury exposure from fish consumption in vulnerable racial/ethnic populations: Probabilistic SHEDS-Dietary model analyses using 1999–2006 NHANES and 1990–2002 TDS data. Sci. Total Environ. 2012;414:373–379. doi: 10.1016/j.scitotenv.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 36.Erisman B.E., Paredes G.A., Plomozo-Lugo T., Cota-Nieto J.J., Hastings P.A., Aburto-Oropeza O. Spatial structure of commercial marine fisheries in Northwest Mexico. ICES J. Mar. Sci. 2011;68:564–571. [Google Scholar]
- 37.Ortega García J.A., Ferrís i Tortajada J., Canovas Conesa A., Apolinar Valiente E., Crehuá Gaudiza E., García i Castell J. Neurotóxicos medioambientales (I). Pesticidas: efectos adversos en el sistema nervioso fetal y posnatal. Acta Pediatrica Espanola. 2005;63:140–149. [Google Scholar]
- 38.Yasutake A., Matsumoto M., Yamaguchi M., Hachiya N. Current hair mercury levels in Japanese: survey in five districts. Tohoku J. Exp. Med. 2003;199:161–169. doi: 10.1620/tjem.199.161. [DOI] [PubMed] [Google Scholar]
- 39.Lincoln R.A., Shine J.P., Chesney E.J., Vorhees D.J., Grandjean P., Senn D.B. Fish consumption and mercury exposure among Louisiana recreational anglers. Environ. Health Perspect. 2011;119:245–251. doi: 10.1289/ehp.1002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.WHO. Environmental Health Criteria 101. World Health Organization. International Programme on Chemical Safety (IPCS); Geneva: 1990. Environmental health criteria for methylmercury: Evaluation of human health risks. [Google Scholar]
- 41.Freire C., Ramos R., Lopez-Espinosa M-J., Díez S., Vioque J., Ballester F. Hair mercury levels, fish consumption, and cognitive development in preschool children from Granada, Spain. Environ. Res. 2010;110:96–104. doi: 10.1016/j.envres.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 42.Park H.J., Kim D.S., Moon J.S., Yang W.H., Son B.S. Mercury level in hair of primary school children in Korea and China. Mol. Cell. Toxicol. 2008;43:235–245. [Google Scholar]
- 43.Barbosa A.C., Jardim W., Dorea J.G., Fosberg B., Souza J. Hair mercury speciation as a function of gender, age, and body mass index in inhabitants of the Negro River basin, Amazon, Brazil. Arch. Environ. Contam. Toxicol. 2001;40:439–444. doi: 10.1007/s002440010195. [DOI] [PubMed] [Google Scholar]
- 44.Katz M.H., Multivariable Analysis . Cambridge University Press; Cambridge: 2006. A Practical Guide for Clinicians. [Google Scholar]
- 45.Feeley M.M., Lo M-T. Risk Assessment for Mercury in Health Canada—Development of the Provisional Tolerable Daily Intake (pTDI) Value. In: Pilgrim W., Burgess N., Giguère M-F., editors. Proceedings of the Conference on Mercury in Eastern Canada and the Northeast States, September. 1998. pp. 21–23.http://www.eman-rese.ca/eman/reports/publications/98_mercury2/intro.html [Google Scholar]
- 46.NRC . National Research Council, National Academy Press; Washington, D.C.: 2000. Toxicological Effects of Methylmercury. [Google Scholar]
- 47.Risher J., DeWoskin R., Health Effects . Toxicological profile for mercury. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry (ATSDR); Atlanta, GA: 1999. Relevanec to public health; pp. 220–300. [Google Scholar]
- 48.Legrand M., Feeley M., Tikhonov C., Schoen D., Li-Muller A. Methylmercury blood guidance values for Canada. Can. J. Pub. Health. 2010;101:28–31. doi: 10.1007/BF03405557. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.