Abstract
Background
Concurrent chemoradiotherapy (concurrent CRT) to treat head and neck cancer is associated with significant reductions of weight, mobility, and quality of life (QOL). An intervention focusing on functional exercise may attenuate these losses.
Methods
We allocated patients to a 14-week functional resistance and walking program designed to maintain physical activity during cancer treatment (MPACT group; n = 11), or to usual care (control group; n = 9). Outcomes were assessed at baseline, and 7 and 14 weeks.
Results
Compared to controls, the MPACT participants had attenuated decline or improvement in several strength, mobility, physical activity, diet, and QOL endpoints. These trends were statistically significant (p < .05) in knee strength, mental health, head and neck QOL, and barriers to exercise.
Conclusion
In this pilot study of patients with head and neck cancer undergoing concurrent CRT, MPACT training was feasible and maintained or improved function and QOL, thereby providing the basis for larger future interventions with longer follow-up.
Keywords: head and neck cancer, physical activity, quality of life, radiation therapy, functional mobility
INTRODUCTION
Head and neck cancer affects more than 52,000 Americans yearly, accounting for 5% of cancers worldwide, and with a 5-year overall survival of 57%.1 Historically, most head and neck cancer occurs in older men with a history of significant tobacco and alcohol exposure. However, recent evidence suggests a shift in the epidemiology of head and neck cancer with a rise in the incidence of human papillomavirus (HPV)-positive disease among non-smokers who present at an earlier age. Although HPV-positive cancers are associated with more extensive nodal disease, they generally respond better to treatment and are associated with improved overall survival resulting in an ever-growing number of cancer survivors.2,3 Given the higher rates of tumor response and better prognosis in HPV-positive cancers, the focus in head and neck cancer research has changed from attempting to increase tumor response rates to identifying strategies that can decrease long-term treatment-related effects.
Treatment of head and neck cancer with concurrent chemoradiotherapy (CRT) with curative intent may cause side effects leading to deterioration of long-term quality of life (QOL) and disability that persists years after treatment. Head and neck cancer and its treatment can severely impact the ability to eat, speech, physical appearance, and emotional well-being.4,5 These side effects may worsen body image, self-esteem, and social interactions in patients with head and neck cancer.6,7 To improve the QOL of patients with cancer, cost-effective clinical paradigms that promote overall well-being, including lifestyle changes, are essential.8
Patients with head and neck cancer often experience significant unintentional weight loss despite adequate nutritional support.9,10 A large percentage of this weight loss is in lean body (muscle) mass.8,11 Muscle mass loss is associated with disability, deficits in functional mobility, and a decrease in physical activity level, as well as reduced QOL and worse overall patient prognosis.12–18 Moreover, muscle mass loss occurs despite aggressive nutritional interventions to maintain body weight,10 and dietary interventions alone increase fat mass with no effect on lean body mass.17 Patients with head and neck cancer are generally less active than other patients with cancer, suggesting that sedentary behavior may worsen muscle mass loss through muscle disuse and decondition-ing.12 Exercise and a focus on physical activity may counteract this unintentional weight loss, muscle mass loss, and attenuate the decline in QOL.
Exercise training has been demonstrated to improve strength, function, and QOL in patients undergoing treatment for cancers other than head and neck cancer, including breast, prostate, lung, and hematologic malignancies.19–28 However, many of those studies have focused on patients with breast and prostate cancer, who often are in better health at baseline, whose definitive treatment may not be as debilitating, and who do not suffer as much muscle mass loss compared with patients with head and neck cancer. At diagnosis, patients with head and neck cancer present with low baseline physical activity levels that remain low after treatment, with more than half of patients unable to return to work after treatment.18,29 Finally, whereas most studies have focused on exercise in cancer treatment survivors, initiating exercise interventions during definitive treatment may be more optimal,21–23,25,28 thereby providing the potential to limit treatment side effects and provide techniques that can accelerate recovery.
We conducted a pilot controlled trial to assess the feasibility and efficacy of a functional resistance and walking exercise intervention (maintaining physical activity during cancer treatment [MPACT]) both during concurrent CRT and in short-term follow-up after treatment. Our primary purposes were to assess the benefits of MPACT on muscle strength, functional mobility, and self-reported QOL in patients with head and neck cancer. Our hypothesis was that, compared to usual care controls, participants allocated to the MPACT intervention would exhibit less concurrent CRT-induced decline and accelerated post-concurrent CRT recovery in a number of key measures, including muscle strength, functional mobility, and QOL. Secondary aims were to assess the benefit of MPACT on other key endpoints, including body mass index (BMI), lean body mass, diet, activity (both self-reported and objectively measured), sleep, concurrent CRT toxicity, and barriers to exercise. Data from this study can be used to guide a more extensive intervention with longer-term follow-up to demonstrate not just attenuation of the concurrent CRT effect, but also long-term maintenance and enhancement of post-concurrent CRT gains.
PATIENTS AND METHODS
Participants
Eligibility criteria included patients 40 years or older with American Joint Committee on Cancer stage II to IV head and neck squamous cell carcinoma who were beginning first-line concurrent CRT without surgery and who were capable of understanding and adhering to the protocol requirements. Exclusion criteria included: substantial dementia (Folstein Mini-Mental State Examination score of <24 of 30), active cardiopulmonary disease, acute medical conditions not related to their head and neck cancer, such as acute flare-up of a joint condition or infection, refusal of a percutaneous endoscopic gastrostomy (PEG) tube for supplementary nutrition if determined as necessary by the treating physician, active treatment for another cancer, exercising at a moderate intensity for more than 150 minutes per week, or receiving physical therapy at the time of enrollment. Age 40 was used as the minimum given the relatively small number of younger patients who present with head and neck cancer, especially at the Veterans Affairs (VA) treating facility.
Design and participant allocation
After institutional review board approved informed consent, participants completed baseline assessments (see below) and were then allocated to MPACT or control groups after stratification by site, VA Ann Arbor Health Care System or University of Michigan (UM). Then, using a computerized algorithm, allocation to the MPACT or control group was based on a minimization procedure,30 which minimized the imbalance between the MPACT and control groups in key potential confounding variables, namely age, sex, and functional mobility (represented by comfortable gait speed).
Using intensity-modulated radiotherapy, patients were treated with platinum-based concurrent CRT to a total dose of 70 Gray. Concurrent CRT was delivered over 7 weeks to the primary tumor and bilateral neck. Chemotherapy was given weekly at the VA and every 3 weeks at UM as tolerated by the patient. As per standard of care, all participants were seen by the attending physician and care team at least weekly. All participants met with a dietician before the initiation of concurrent CRT, and then again at the discretion of the attending physician if the participant experienced greater than a 5% to 10% decrease in BMI. Only 1 participant (a control) utilized a PEG tube at baseline. All participants were reassessed at weeks 7 and 14 (see below), the latter coinciding with a standard follow-up visit with the attending physician and care team.
Assessments
Key factors thought to change with concurrent CRT and the MPACT intervention, including muscle strength, functional mobility, self-reported QOL, and physician-reported concurrent CRT toxicity, were assessed at baseline, 7 weeks, and 14 weeks. In order to determine feasibility for future studies and after initial accrual of participants in both groups, a subsequent participant recruited into the study group wore an actigraph for 7 days (objective physical activity assessment) and completed a 7-day diet journal (nutrition assessment). Assessors were blinded to experimental group allocation.
Muscle strength
Elbow flexion and knee extension strength (in Newton-meters) were assessed on the dominant side using an isokinetic dynamometer and standard positioning techniques (Biodex Medical Systems, Shirley, NY). Grip strength (in kilograms) was assessed on the right side using a hand dynamometer (Patterson Medical, Bolingbrook, IL). The best of 2 trials was used as the outcome unless the difference between the first and second trials was >10%, in which case a third trial was done.
Functional mobility
These assessments followed standard literature-based protocols. In addition to comfortable gait speed (in meters/second) over a 6-minute distance, the timed up and go (in seconds) was assessed as the time to rise from a chair, walk 3 minutes away, and then return to the chair and sit down31; the average of 2 trials was used for each outcome measure. For the 6-minute walk, participants were told to cover as much distance (in feet) as possible comfortably during a 6-minute hallway walk.
Self-reported quality of life
Participants completed the Medical Outcomes Study (MOS) Short Form-36 (SF-36), with analyses conducted of the summarized Physical and Mental domains as well as 8 subcomponents, including physical function, role-physical, role-mental, role-emotional, vitality, mental health, social functioning, pain, and general health.32 The 6-item MOS Sleep Problem Index (MOS-Sleep 6) was used to measure sleep initiation, maintenance, respiratory problems, adequacy, and somnolence, with higher scores indicating more sleep disturbance and worse sleep quality.33
Body mass index and lean body mass
Lean body mass (%) was calculated using dual-energy X-ray absorptiometry, as described previously.11
Self-reported physical activity
The Physical Activity Scale for the Elderly (PASE) questionnaire assessed the frequency of performing a variety of daily activities, including muscle strength/endurance, housework, sports, tasks involving standing or walking, and gardening. The PASE score was calculated using the Washburn approach,34 with higher scores indicating higher activity level. Missing data was handed by proportionally reweighting the remaining questions such that the sum of the weights was conserved.
Objectively assessed physical activity
A subgroup of participants wore an Actigraph GT3x+ accelerometer (Actigraph, Fort Walton Beach, FL) over the right hip for 7 consecutive days. Individuals were asked to remove the device during sleep, bathing, and any other aquatic-related activities. Actigraph data processing followed previously published standards.35 Non-wear time was defined as >60 consecutive minutes of zero activity (counts/minute), allowing for a tolerance spike of 2 minutes with activity counts per minute between 0 and 100.35 To determine valid days of wear (>10 hours/day of wear time) non-wear time was subtracted from 24 hours of data collected.36 Total activity counts per minute during wear time were classified into activity intensity levels using previously established standards (0–99 counts = sedentary; 100–759 counts = light; 760–1951 = lifestyle; ≥1952 = moderate to vigorous activity.37,38 The proportion of time spent in each activity intensity level was calculated by dividing minutes of intensity-specific movement by the total minutes of wear time. Further, each time a minute of activity broke the <100 to the >100 counts this activity was coded as a sedentary transition. For pilot purposes in the current article, we report proportions of sedentary and lifestyle time, as well as transitions in movement posture from sitting to standing.
Head and neck-specific quality of life
The 20-question Head and Neck Quality of Life questionnaire was used to assess disease-specific QOL across 4 domains: eating, communication, pain, emotion, as well as an additional single question assessing “waking up frequently at night” because of the patient’s head and neck condition.39 The analysis included both the individual questions as well as the overall domain scores, which were transformed to a 0 to 100 scale, with higher scores indicating worse problems.
Barriers to exercise
In a “Barriers to Exercise” survey, described previously,40 participants rated the frequency of 34 items that may have interfered with exercise in the past 4 weeks on a 1 to 5 Likert scale. Sample items included “lack of interest in exercise,” “lack of equipment,” “fatigue,” “family responsibilities,” as well as more treatment-specific items, including “difficulty swallowing,” “decreased food intake,” “dry mouth or throat,” and “need for tube feedings.” Higher scores indicated increased frequency that the perceived barrier interfered with exercise.
Smoking and alcohol use
Smoking habits were assessed using the Fagerstrom Nicotine Dependence Test, with higher scores associated with greater dependency.41 Alcohol use was assessed using the Alcohol Use Disorders Identification Test (AUDIT), with higher scores correlating with more problematic usage.42
Diet
A subgroup of participants was asked to keep 7-day diet journals through week 7. These diet journals were enumerated for servings of protein, dairy, grains, fruits and vegetables, and meal replacements. Daily totals in each category were calculated as was a “solid food” summary score that added daily servings of grains, protein, fruits, and vegetables.
Concurrent chemoradiotherapy toxicity
Data on adverse events were collected prospectively by the treating physician from the time of the initial concurrent CRT through the end of the study. Adverse events were identified based on descriptions and grading scales found in the revised National Cancer Institute Common Toxicity Criteria for Adverse Events.43 The proportion of patients experiencing toxicities of interest, defined as grade 3 or higher, blood count changes, mucositis, xerostomia, or dermatitis were assessed.
Maintain physical activity during cancer treatment intervention
The MPACT intervention was delivered at a clinical research center by a trainer after concurrent CRT initiation up to week 7 and then post-concurrent CRT at home, with weekly trainer telephone calls from weeks 8 to 14. The use of on-site plus follow-up telephonic support were consistent with the most effective mode of delivery of cancer rehabilitation programs.44 Controls received standard treatment, including active nutritional surveillance, but were neither encouraged nor discouraged to exercise. The initial 7-week MPACT program included initiation of a functional resistance training and a home walking program with concurrent CRT. The program followed American College of Sports Medicine prescription guidelines for patients with cancer and included strengthening, cardiovascular fitness, and physical activity components. Patients attended up to 3 sessions per week, lasting up to 1 hour as an eventual goal, including warmup, cool down, and rest periods. The warmup included rhythmic large muscle movements, such as marching and punching, whereas the cool down included leg, shoulder, and arm flexibility activities coupled with deep breathing. Exercise intensity and duration was sometimes modified as the effect of concurrent CRT intensified by the end of the seventh week, and later slowly increased as the effect waned. Participants were to perform the exercise program described below at a moderate intensity, as defined by 11 to 13 on a 20-point rate of perceived exertion scale.
Functional resistance training
The functional resistance training protocol was designed to minimize the loss of muscle mass associated with head and neck cancer and maintain and enhance physical activity levels during concurrent CRT. Upper body musculature were targeted through whole body exercises to optimize the training effect while reducing exercise fatigue and augmenting lower extremity function. Emphasis was placed on the development of a sustainable exercise habit, including safe independent performance of the functional resistance training and exercises to include exercise progression and integration into daily activities. Exercises included chest press in squat, wall push up, military press, side arm raises, biceps curl, shoulder shrugs, and calf raises. Weights included dumbbells and inserts into an ankle strap. Exercise duration and intensity were customized to the individual. The goal was to perform three 8 to 12 repetition sets of each functional resistance training exercise by the conclusion of the 7-week training period, to be customized in terms of repetitions and weights based on concurrent CRT side effects. Limited rest periods (eg, 2 minutes) were provided between sets to enhance the training effect.
Walking
For the cardiovascular and physical activity component, participants were given a pedometer, and the goal was to maintain step count based on the mean step count of the previous training week. Multiple short duration continuous walking periods were recommended, such as walking for 5 minutes 6 times throughout the day to achieve a total walking time of 30 minutes. Participants were also given information on optimal walking form, injury prevention, program progression, and ways to incorporate walking into daily life.
Home program
After the 7-week on-site training, MPACT participants were asked to integrate safe exercise activities into their own lifestyle through home activities. Participants were asked to perform their individualized functional resistance and walking program solely off-site for the last 7 weeks of the study (weeks 8–14). Each participant’s functional resistance in addition to walking and physical activity program was customized based on: (1) personal determinants (self-efficacy, benefits, and barriers); (2) physical activity preferences; (3) available community resources; and (4) health and environmental factors. The goal was for participants to maintain their physical activity for a minimum of 5 days a week and a minimum of 30 minutes per day, performed in bouts of 10 minutes or more, at a moderate intensity (rate of perceived exertion = 11–13). The MPACT trainer made weekly telephone calls to facilitate home program adherence through week 14. The trainer addressed participant concerns and barriers (such as weight loss, changes in medications, and symptoms such as fatigue) and then used participant strengths to identify tailored solutions to help each participant reach and/or maintain the exercise and physical activity goals. At week 11, when the participant returned for follow-up to the radiation oncology clinic, the trainer met with the participant to go over their individualized program and review the exercise technique.
Statistical analysis
Baseline
Participant characteristics were compared at baseline between MPACT and controls, using a 2-tailed t test for continuous variables (including functional mobility and SF-36 measures), Fisher’s exact test for non-ordered categorical variables, and Cochrane–Armitage trend test for ordinal variables (eg, head and neck cancer-specific QOL individual questions or barriers to exercise).
Baseline versus 7 and 14 weeks
Changes in measures between baseline and week 7 or 14 were calculated for each patient and compared between the MPACT and control treatment groups. Per the protocol, a 1-sided t test was used to compare treatment groups with respect to mean change from baseline to 7 or 14 weeks for continuous outcomes. Mean change from baseline in Likert scale variables (such as the head and neck cancer QOL and Barriers to Exercise) was compared between treatment groups using the Cochrane–Armitage trend tests. All available data at each timepoint were used rather than selecting only patients who completed all timepoints. Statistical significance was set at p < .05. All statistical analyses were performed using R 3.0.1 statistical software (http://www.r-project.org/).
RESULTS
Participant enrollment and attrition
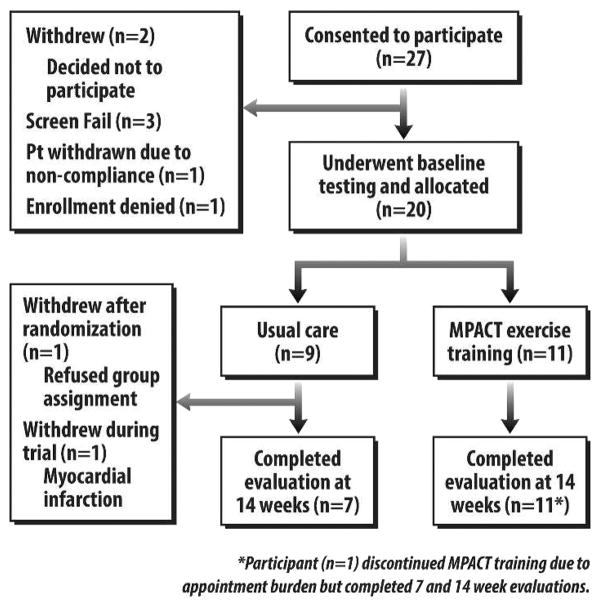
Of the 27 consented participants, 7 did not complete the program because they either withdrew, failed to meet screening criteria, or were deemed noncompliant before baseline testing and group allocation (see Figure 1). Ultimately 20 patients were allocated, 11 to the MPACT group and 9 to the control group. Seventeen patients (10 in the MPACT group and 7 in the control group) completed 14 weeks on-study. One MPACT participant discontinued all exercise sessions in the first week because of difficulty with multiple appointments, but continued to be assessed and was therefore included in the final analysis. Of the 2 control withdrawals, 1 refused the control group assignment and the second suffered a myocardial infarction. Excluding the MPACT participant who discontinued exercise in the first week, MPACT participants completed an average of 15.2 sessions of a maximum 21 offered (72% attendance rate). The most common reason for not attending a session was cancer-related or treatment-related toxicity.
FIGURE 1.
Study flow diagram.
Participant baseline characteristics
Table 1 shows the baseline characteristics for the 7 control and 11 MPACT participants who completed the 14-week testing. Eleven of these 18 were veterans. Mean age was 57 years for both groups and all patients were male except for a single UM female MPACT patient. The majority of patients had oropharyngeal primary tumors, including the base of the tongue and tonsil. There were no significant differences between the 2 groups in chemotherapy, tumor stage, baseline PEG use, BMI, lean body mass, alcohol use (AUDIT score), smoking (Fagerstrom score), or Karnofsky score. Baseline muscle strength and functional mobility were similar between groups, except for significantly higher knee extension strength in controls versus MPACT (p = .04). There were no significant differences at baseline in the SF-36 scores or subscores, MOS Sleep-6, and physical activity based on the PASE questionnaire. The baseline to 7 and 14 week changes in assessment outcomes are presented in Table 2.
TABLE 1.
Baseline participant characteristics.
| Characteristics | MPACT (n = 11) | Controls (n = 7) | p value |
|---|---|---|---|
| Age, y | 57 (7) | 57 (7) | .9 |
| Institution | |||
| VA | 7 | 4 | 1.0 |
| UM | 4 | 3 | |
| Tumor location | |||
| Larynx | 1 | 0 | 1.0 |
| Nasopharynx | 1 | 0 | |
| Oropharynx | 8 | 6 | |
| Unknown primary | 1 | 1 | |
| Stage | |||
| Stage III | 4 | 0 | .1 |
| Stage IV | 7 | 7 | |
| Chemotherapy | |||
| Platinum only | 9 | 5 | 1.0 |
| Taxol + platinum | 2 | 2 | |
| BMI | 30 (5) | 32 (3) | .4 |
| Percent lean body mass | 62 (9) | 62 (5) | 1.0 |
| Karnofsky performance score | |||
| <90 | 1 | 3 | .2 |
| ≥90 | 8 | 3 | |
| NA | 2 | 1 | |
| 6-min walk distance, feet | 1400 (243) | 1530 (233) | .3 |
| Elbow flexion, N-m | 54 (16) | 60 (22) | .6 |
| Knee extension, N-m | 154 (44) | 224 (67) | .04* |
| Grip strength, kg | 42 (8) | 45 (9) | .5 |
| Timed up and go, sec | 8 (3) | 8 (1) | .6 |
| Gait speed, m/s | 1.3 (0.2) | 1.5 (0.2) | .8 |
| MOS sleep score | 34 (19) | 41 (20) | .5 |
| PASE | 147 (90) | 150 (116) | 1.0 |
| SF-36 | 64 (22) | 56 (22) | .5 |
| Physical | 67 (20) | 56 (23) | .3 |
| Mental | 61 (29) | 60 (26) | .9 |
| Physical function | 82 (19) | 69 (26) | .3 |
| Role-physical | 50 (39) | 36 (45) | .5 |
| Role-emotional | 64 (46) | 57 (54) | .8 |
| Vitality | 58 (23) | 54 (21) | .7 |
| Mental health | 62 (27) | 67 (28) | .7 |
| Social functioning | 60 (33) | 61 (38) | 1.0 |
| Pain | 50 (31) | 31 (8) | .1 |
| General health | 58 (18) | 55 (17) | .8 |
| AUDIT | 4 (4) | 3 (4) | 1.0 |
| Fagerstrom, smoking | 2 (4) | 3 (4) | .9 |
| Barriers to exercise | |||
| Lack of interest | 2.6 (1.3) | 2.8 (1.8) | .7 |
| Exercise is boring | 2.2 (1.3) | 2.1 (1.7) | 1.0 |
| Hand neck cancer-related QOL | |||
| Domain: emotion | 47 (28) | 38 (28) | .5 |
| Domain: communication | 23 (23) | 44 (34) | .2 |
| Domain: eating | 23 (20) | 38 (35) | .3 |
| Domain: pain | 37 (28) | 49 (24) | .3 |
Abbreviations: MPACT, maintain physical activity during cancer treatment; VA, Veterans Affairs; UM, University of Michigan; BMI, body mass index; NA, not applicable; N-m, Newton-meter; m/s, meters/second; MOS, Medical Outcomes Study; PASE, Physical Activity Scale for the Elderly; SF-36, Short Form Health Survey; AUDIT, Alcohol Use Disorders Identification Test; QOL, quality of life.
Numbers represent the number of patients (n) or mean value (SD). All 18 patients (everyone who began and did not subsequently withdraw from the study) are included, regardless of whether or not they completed all sessions
p < .05.
TABLE 2.
Mean (SE) change in assessment outcome at weeks 7 and 14.
| Measure | Mean (SE) change at 7 wks
|
Mean (SE) change at 14 wks
|
||
|---|---|---|---|---|
| MPACT (n = 11) | Control (n = 7) | MPACT (n = 11) | Control (n = 7) | |
| Physical performance | ||||
| Knee extension strength, N-m | 1 (11) | −36 (16)* | −4 (7) | −46 (14)† |
| 6-min walk distance, feet | −71 (36) | −166 (93) | 60 (40) | −19 (89) |
| Comfortable gait speed, m/s | −0.08 (0.04) | −0.14 (0.09) | 0.02 (0.04) | −0.05 (0.06) |
| Timed up and go (s) | 0.7 (0.8) | −0.07 (0.7) | −0.7 (0.6) | −0.2 (0.6) |
| Quality of life and sleep | ||||
| Overall SF-36 | −19 (5) | −24 (5) | 4 (4) | −6 (8) |
| SF-36 physical summary | −24 (6) | −22 (8) | 1 (4) | −3 (8) |
| SF-36 mental summary | −12 (7) | −26 (6) | 9 (4) | −9 (12) |
| SF-36 subscale: vitality | −19 (7) | −33 (3)* | 7 (5) | −9 (10) |
| SF-36 subscale: mental health | 3 (4) | −16 (7)* | 15 (4) | −1 (6)† |
| MOS sleep index | −5 (5) | 8 (6) | −14 (5) | −1 (11) |
| BMI and lean body mass | ||||
| BMI | −2.9 (0.6) | −3 (0.5) | −3.9 (0.7) | −4.6 (0.6) |
| Lean body mass, percent | 0.2 (0.5) | 1.0 (0.7) | 4.7 (1.5) | 4.0 (0.9) |
| Self-reported physical activity | ||||
| PASE | −65 (24) | −96 (35) | 42 (18) | −10 (31) |
| Objective physical activity | (n = 4) | (n = 3) | (n = 4) | (n = 3) |
| Proportion of lifestyle min | −0.03 (0.01) | −0.07 (0.03) | 0.00 (0.01) | −0.03 (0.01) |
| Proportion of sedentary min | 0.04 (0.02) | 0.08 (0.02) | −0.02 (0.02) | 0.06 (0.03) |
| No. of sit-to-stand transitions | 11.5 (7.9) | −2.7 (5.2) | 6.7 (8.9) | 1.8 (2.1) |
Abbreviations: MPACT, maintain physical activity during cancer treatment; N-m, Newton-meter; m/s, meters/second; SF-36, Short Form Health Survey; MOS, Medical Outcomes Study; BMI, body mass index; PASE, Physical Activity Scale for the Elderly.
Refers to p < .05 when comparing MPACT vs controls from baseline to 7-weeks.
Refers to p < .05 when comparing MPACT vs controls from baseline to 14-weeks.
Muscle strength
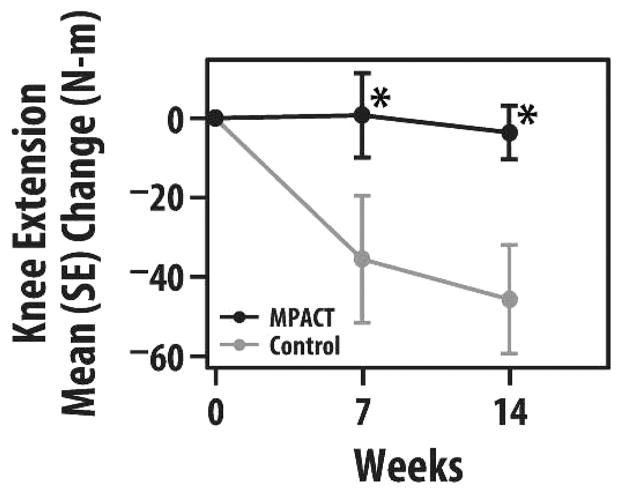
Knee extension tended to be maintained for all 14 weeks in MPACT versus a decline in controls mostly by the seventh week, resulting in a significant mean 14-week decrease of 4 Newton-meters (N-m) for MPACT and 46 N-m for controls (p < .05; Figure 2). There was no group difference in baseline to 7 or 14 week change in elbow flexion or grip strength.
FIGURE 2.
Group mean (SE) change in knee extension strength (in Newton-meters) at 7 and 14 weeks (maintain physical activity during cancer treatment [MPACT] = 11; controls = 7). *p < .05.
Functional mobility
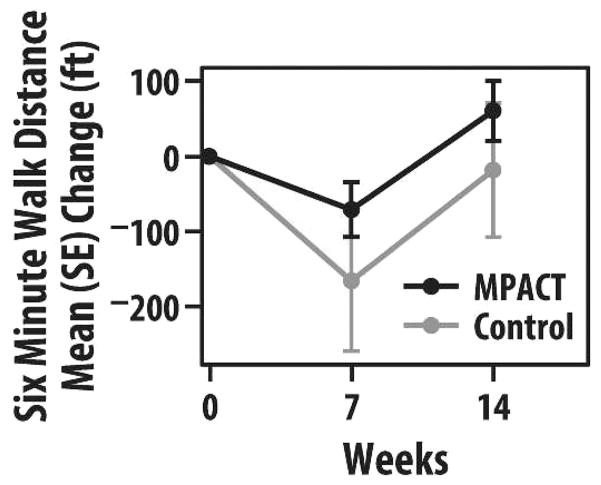
The 6-minute walk test showed a trend toward less decline in MPACT than controls at 7 weeks (decrease of 71 feet in MPACT vs 166 feet in controls). MPACT tended to improve at 14 weeks versus baseline (by 60 feet), whereas controls decreased by 19 feet, although these differences were not statistically significant (Figure 3). Comfortable gait speed in MPACT and controls followed a trend at 7 and 14 weeks similar to the 6-minute walk, but timed up and go did not.
FIGURE 3.
Group mean (SE) change in the 6-minute walk distance (in feet) at 7 and 14 weeks (maintain physical activity during cancer treatment [MPACT] = 11; controls = 7).
Quality of life and sleep
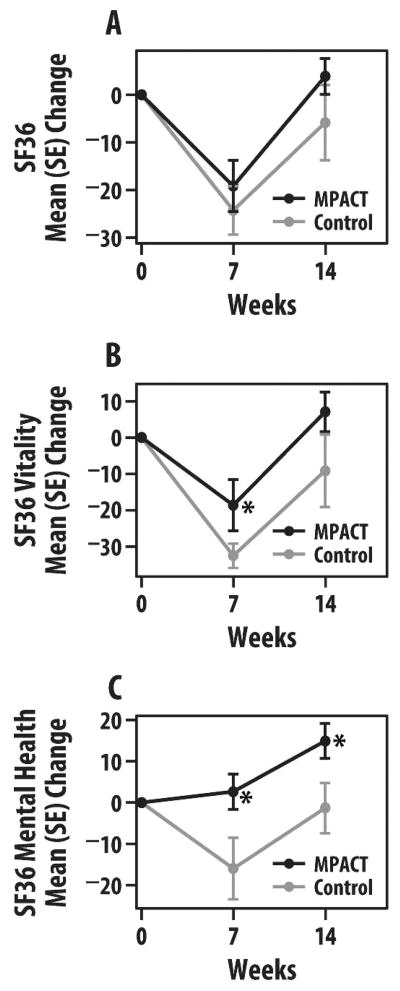
QOL measured by SF-36 tended to show less decline and even improvement in MPACT versus controls when evaluating changes from baseline to 7 and 14 weeks. In MPACT versus controls and compared to baseline, overall SF-36 scores tended to decline less at 7 weeks (−19 vs −24) and improved at 14 weeks (+4 vs −6; Figure 4A). The SF-36 physical summary measure declined similarly in both groups at 7 weeks but the MPACT group returned to baseline at 14 weeks compared to a continued deficit in controls (−3). The SF-36 mental summary score declined less in MPACT than controls by 7 weeks (−12 vs −26) and improved in MPACT versus continued decline in controls (+9 vs −9). Of the other subscales evaluated that showed group differences, the SF-36 vitality subscale measuring energy and fatigue showed similar trends at 7 weeks (−19 for MPACT and −33 for controls; p < .05) and 14 weeks (+7 vs −9; Figure 4B). In addition, the SF-36 mental health subscale showed significant improvement in MPACT versus decline in controls at 7 weeks (+3 vs −16; p < .05) and 14 weeks (+15 vs −1; p < .05; Figure 4C). The MOS Sleep Index tended to show continuous improvement in sleep disturbance in the MPACT group versus controls at weeks 7 (−5 vs +8) and 14 (−14 vs −1).
FIGURE 4.
Group mean (SE) change in Short Form Health Survey (SF-36) scores at 7 and 14 weeks. (A) Total SF-36 score; (B) vitality subscore; and (C) mental health subscore (maintain physical activity during cancer treatment [MPACT] = 11; controls = 7). *p < .05.
Head and neck-specific quality of life
Notable changes in head and neck-specific QOL survey items were in the same direction. When we examined the 21 items individually, one statistically significant finding was in emotional problems: MPACT participants reported fewer problems than controls when comparing 7 weeks to baseline (p < .05). No differences between the MPACT and the control groups were found in the change in overall domain scores. Baseline domain scores were higher than previously described, largely driven by mean baseline scores for the VA patients (emotion, 55; communication, 42; eating, 38; and pain, 52) that were significantly higher for every domain than for the UM patients (emotion, 24; communication, 14; eating, 13; and pain, 26), consistent with prior results.45–47
Body mass index and lean body mass
BMI declined by an average of 3 points by week 7 and 4 to 5 points by week 14 in both the MPACT and control groups. Lean body mass tended to improve slightly by 7 weeks in MPACT (0.2%) and controls (1%) and more substantially by week 14 (5% and 4%, respectively).
Self-reported and objectively assessed physical activity
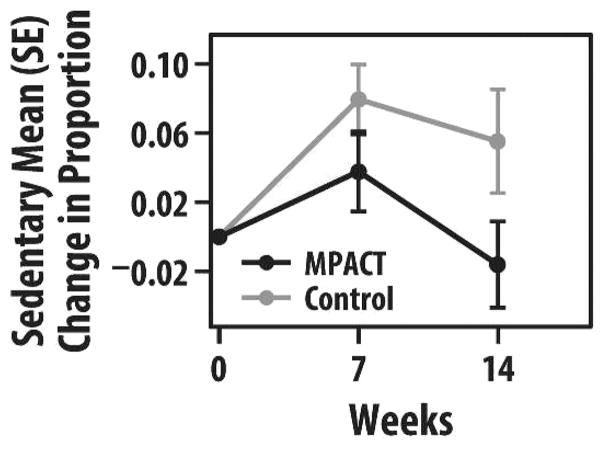
Using the PASE, the decline in self-reported physical activity was more attenuated at 7 weeks and tended to show greater improvement at 14 weeks compared to baseline in MPACT versus controls (7 weeks, −65 vs −96; 14 weeks, +42 vs −10 points, respectively). Objective physical activity monitoring via actigraph assessment was available on a subsample (4 MPACT and 3 controls) of participants. MPACT versus controls decreased less in the proportion of time spent in lifestyle activity by week 7 (3% vs 7% reduction, respectively), with MPACT returning back to baseline levels by week 14. The proportion of time spent in sedentary behavior increased less in the MPACT group versus controls by week 7 (4% vs 8% increase, respectively), and actually decreased overall by 2% in the MPACT group by week 14 (Figure 5). Postural transitions of sit to stand increased by 10 in the MPACT group by week 7, with some retention of this increase through week 14, compared to essentially no change in sit to stand transitions in the controls.
FIGURE 5.
Group mean (SE) change in proportion of time spent in sedentary behavior, as measured by actigraphy, at 7 and 14 weeks (maintain physical activity during cancer treatment [MPACT] = 4; controls = 3).
Barriers to exercise
Of the 34 barriers assessed, most showed no differences in change between groups, with 2 notable exceptions. Lack of interest in exercise as a barrier tended to be unchanged at 7 weeks in MPACT but was significantly more of a barrier in the controls (0 vs +1.6; p < .05). Exercise being classified as boring was also more of a barrier at 7 weeks in the controls than in the MPACT group (+0.8 vs −0.6; p < .05).
Diet
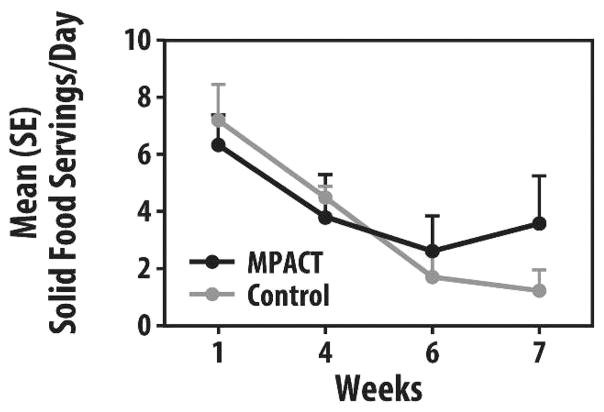
Complete diet journals were available on a sub-sample (5 MPACT and 3 controls). Fruit and vegetable intakes at study start were about 2 to 3 servings/day, well below the United States Department of Agriculture (USDA) recommendation of 7 to 11/day, and dropped greatly as use of meal replacements increased. The decrease in fruit and vegetable consumption was less pronounced in the MPACT group and tended to be better maintained versus the control group, as seen in Table 3. There also was a trend for MPACT to return to solid foods more quickly than controls at week 7 (Figure 6).
TABLE 3.
Mean (SE) dietary journal assessments in the maintain physical activity during cancer treatment group (n = 5) and the control group (n = 3).
| Measure | Wk 1 | Wk 4 | Wk 6 | Wk 7 | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| MPACT | Control | MPACT | Control | MPACT | Control | MPACT | Control | |
| Fruit and vegetable servings | 1.8 (0.6) | 2.9 (0.6) | 1.1 (0.4) | 1.7 (0.5) | 1.2 (0.6) | 0.7 (0.5) | 1.4 (0.8) | 0.5 (0.3) |
| Solid food servings | 6.3 (1.0) | 7.2 (1.3) | 3.8 (1.5) | 4.5 (0.4) | 2.6 (1.2) | 1.7 (0.9) | 3.6 (1.7) | 1.2 (0.7) |
| Meal replacements | 1.1 (0.8) | 0.4 (0.3) | 3.3 (1.6) | 1.4 (0.6) | 3.4 (1.4) | 3.5 (1.4) | 2.5 (0.8) | 3.3 (1.3) |
Abbreviation: MPACT, maintain physical activity during cancer treatment.
FIGURE 6.
Group mean (SE) solid food servings per day at 1, 4, 6, and 7 weeks (maintain physical activity during cancer treatment [MPACT] = 5, control = 3).
Concurrent chemoradiotherapy toxicity
Reports of concurrent CRT toxicity over the 14-week period did not differ between groups.
DISCUSSION
In this pilot controlled trial of patients with head and neck cancer, we demonstrated the feasibility of a functional resistance and walking exercise intervention (MPACT) provided during 7 weeks of concurrent CRT and during 7 weeks of follow-up. Once enrolled, MPACT participants, despite the concurrent CRT, completed 72% of the on-site exercise sessions. Ten of 11 of the MPACT participants (91%) completed the full 14-week program, with the 11th participant discontinuing training at week 1.
As hypothesized, compared with controls and in evaluating changes from baseline to 7 and 14 weeks, MPACT participants tended to exhibit less concurrent CRT-induced decline and even some improvement. These trends were found in nearly all measured domains, namely in muscle strength, functional mobility, QOL, sleep disturbance, head and neck-specific QOL, self-reported and objectively assessed physical activity, barriers to exercise, and diet. Despite the small sample size, statistically significant changes in MPACT versus controls were noted in knee strength, mental health and head and neck QOL, and barriers to exercise.
Trends in changes favoring MPACT over controls in muscle strength and functional mobility occurred especially in knee extension strength and 6-minute walk distance. Despite the inclusion of whole body functional exercises, the MPACT focus on upright exercise likely favored lower extremity strength, whereas the home program focus on increased walk time favored longer walk measures, such as the 6-minute walk (vs shorter walk measures such as gait speed). For the 6-minute walk, clinically meaningful changes in older adults have been proposed at 2 levels, small (20 minutes, 66 feet) and substantial (50 minutes, 164 feet).48 Thus, in the present study, MPACT participants exhibited a small meaningful decline at 7 weeks (−74 feet) and a small meaningful improvement at 14 weeks (+67 feet); controls exhibited substantial decline at 7 weeks (−166 feet) and minimal change (−3 feet) at 14 weeks.
Other studies during head and neck cancer treatment find analogous but sometimes conflicting training-induced changes in strength and functional mobility. Samuel et al49 utilized a combined 6-week resistance training and walking program during concurrent CRT and found similar results, with improvement in the 6-minute walk at 6 weeks of a median of 43 minutes versus a decline in the control group of a median of 96 minutes. In a small 12-week pilot of resistance-band training with a design analogous to the present study (6 weeks on-site during concurrent CRT and 6 weeks home-based after concurrent CRT), the main tendency for resistance training (n = 5) versus controls (n = 8) was to reduce the average time to rise from a chair, but there was a decline in back/leg extensor strength.50
Studies conducted posttreatment for cancer utilized various interventions to improve muscle strength and functional mobility. Physiotherapy-guided training in a single group 8-week trial improved the 6-minute walk distance51 compared to self-selected physical activity controls, whereas 12 weeks of resistance training improved lean body mass and strength but not functional mobility.52 No differences in BMI or lean body mass were seen in the present study between the control and intervention groups, possibly because the resistance training load was more functional and not at a high load (such as 80% of 1 repetition maxima). In summary, exercise programs providing both resistance and walking training might improve muscle strength and functional mobility in patients with head and neck cancer both during and after concurrent CRT, with the suggestion that there is likely to be a degree of training specificity necessary (ie, walking training to improve walking). Further controlled studies are needed with more prolonged follow-up50 to evaluate how well these changes are maintained.
Tendencies for less decline and even improvement favoring MPACT over controls were noted in QOL measures, both general (particularly SF-36 physical summary, mental summary, and vitality/fatigue scales) and head and neck cancer-specific (particularly emotional problems). Statistically significant changes favoring MPACT were found in the SF-36 mental health scale, waking at night, and lack of interest in exercise. Using the proposed Reliable Change Index (RCI; the change between pretreatment and post-treatment scores that would be statistically and clinically reliable53), these differences in SF-36 change from baseline between groups are reliable at 14 weeks for the physical summary score (RCI = 7), and at both 7 and 14 weeks for the mental summary score (RCI = 10), the vitality subscore (RCI = 10), and the mental health subscore (RCI = 11). Other studies also have found QOL-related improvement with exercise programs in head and neck cancer participants, and there was often a greater effect in the mental health component. In the study by Samuel et al,49 the intervention group median scores were stable (SF-36 physical component) or slightly improved (SF-36 mental component), whereas the controls decreased in both, most strikingly on the mental component. Using the Functional Assessment of Cancer Therapy (FACT) scales, Rogers et al54 found tendencies favoring intervention over control for FACT-G (general), physical well-being, emotional well-being, and functional well-being, as well as a blunting of increases in the fatigue subscale, with no changes for head and neck-specific scores (FACT-H&N). This blunting of the fatigue subscale and, in the present study, the SF-36 vitality/fatigue scale, is consistent with literature showing that exercise mitigates cancer-related fatigue.44 Note that improvement in QOL may have been facilitated by the telephone trainer support contact; multidisciplinary rehabilitation programs that contain a psychosocial as well as a physical component have been shown to improve SF-36 scores in cancer survivors in a large meta-analysis.55
Sleep disorders are another significant contributor to fatigue and decreased QOL in patients with cancer,56 and sleep quality is poorer in patients with head and neck cancer compared to a healthy population.57 In the present study, compared to controls, the MPACT group showed continuous improvement in this MOS Sleep-6. Factors in multiple domains likely influence sleep quality. Predictors of poor sleep quality among patients with head and neck cancer, both at baseline and at 1-year follow-up include pain, xerostomia, depression, the presence of a tracheostomy tube, comorbidities, and younger age,57 none of which seemed to differ between the present study groups. Obstructive sleep apnea has also been recently proposed as a contributor to sleep disorders and fatigue in patients with head and neck cancer.58 Previous work suggests that decreased sleep is an independent predictor of increased inflammatory marker levels, specifically interleukin-6.59 Elevated inflammatory markers are associated with cancer cachexia, muscle wasting, and radiation treatment51,52; on the other hand, exercise training may decrease inflammation as well as improve sleep in healthy older adults.60 The impact of exercise training on sleep and inflammatory markers in patients with head and neck cancer (and patients with cancer in general) undergoing definitive treatment has not been carefully studied and may be an important new area of research.
Compared with controls, the decline in self-reported physical activity tended to be more attenuated in the MPACT group at 7 weeks and improved more by week 14. As noted in a previous review, physical activity (and exercise) is compromised in patients with head and neck cancer.8 Enjoyment of physical activity, task efficacy (confidence), and any alcohol use are positively correlated with physical activity, whereas symptom index, comorbidity score, and perceived barriers are negatively correlated with physical activity.40 There was little difference in reported barriers to exercise, although the MPACT group reported no change in lack of interest, whereas the controls increased in lack of interest. One possible contributor to the continued interest and lack of difference in barriers may have been the support of the trainers, both on-site and in telephone follow-up. Providing feedback and support, and promoting knowledge and skills have been identified as key contributors to adherence in breast cancer strength training participants.61 Taken along with the improvement in SF-36 mental health measures, future studies utilizing MPACT will need to explore the importance of cognitive-behavioral support and outcomes.
In order to determine feasibility for future studies, both actigraphy and diet were analyzed in small subsets, and seem to be feasible and follow the trends noted above. Actigraphy provided important objective trends favoring MPACT in maintaining lifestyle physical activity and avoiding more sedentary activity, and in showing an increase in posture changes from sitting to standing. These outcomes help to verify the attainment of physical activity goals based on American College of Sports Medicine guidelines and provide a more objective outcome for use in future intervention studies. In terms of diet, the MPACT group may have maintained their nutrition better and returned to eating solid foods faster than the controls. This could reflect better physical function and recovery from treatment, but this observation as well as the influence of diet on the other outcomes, have to be confirmed in larger studies.
One important study limitation was the small sample size, which limited the statistical power, but the assessment and intervention paradigm are feasible. An additional limitation of the study was that HPV status was not available for every patient. Although we doubt that this would have changed the results of our study, it is possible that given the better prognosis of HPV-positive patients they are even more likely to benefit from a physical activity program. The lack of a detailed record of adherence to the home program was an additional limitation. Future studies supplementing self-reported physical activity with objective measures such as actigraphy can help verify the extent of actual physical activity performed, both as a check for adherence and as an objective outcome measure. Strengths include the use of a control group, measures validated for use in patients with cancer, an intervention that began with the start of cancer treatment and continued in early posttreatment follow-up, an intervention oriented to functional strength, walking, and physical activity, and use of both an on-site and home-based intervention.
In summary, the MPACT functional resistance and walking exercise intervention for patients with head and neck cancer, both during concurrent CRT and afterward, seems feasible. The findings of attenuation in decline during concurrent CRT as well as improvements up through 14 weeks in the MPACT versus control groups suggest efficacy. Improvements tended to occur not just in physical but also in mental/behavioral outcomes, suggesting a broader effect of the program. Data from this study can be used to guide a more extensive intervention with longer-term follow-up to demonstrate long-term maintenance and enhancement of post-concurrent CRT recovery. The results of this study can guide care for patients with cancer treated with concurrent CRT and help address the long-term physical and psychosocial rehabilitation needs of cancer survivors.
Acknowledgments
The authors thank members of the research team who assisted with numerous aspects of planning and execution of the study: Deb Arnoldi, Michelle Baumgart, Brad Grincewicz, Jo Jennens, Gabriela Mueller, Martina Nabozny, Ayowale Oladeji, Eric Pear, Debbie Strasburg, Steven Kronenberg, and Erica Twardzik. We also thank all the patients who volunteered for this study.
References
- 1.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 2.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lassen P, Eriksen JG, Hamilton–Dutoit S, Tramm T, Alsner J, Overgaard J. Effect of HPV-associated p16INK4A expression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J Clin Oncol. 2009;27:1992–1998. doi: 10.1200/JCO.2008.20.2853. [DOI] [PubMed] [Google Scholar]
- 4.Duffy SA, Ronis DL, McLean S, et al. Pretreatment health behaviors predict survival among patients with head and neck squamous cell carcinoma. J Clin Oncol. 2009;27:1969–1975. doi: 10.1200/JCO.2008.18.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duffy SA, Ronis DL, Valenstein M, et al. Depressive symptoms, smoking, drinking, and quality of life among head and neck cancer patients. Psychosomatics. 2007;48:142–148. doi: 10.1176/appi.psy.48.2.142. [DOI] [PubMed] [Google Scholar]
- 6.Fingeret MC, Yuan Y, Urbauer D, Weston J, Nipomnick S, Weber R. The nature and extent of body image concerns among surgically treated patients with head and neck cancer. Psychooncology. 2012;21:836–844. doi: 10.1002/pon.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhoten BA, Murphy B, Ridner SH. Body image in patients with head and neck cancer: a review of the literature. Oral Oncol. 2013;49:753–760. doi: 10.1016/j.oraloncology.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Hunter KU, Jolly S. Clinical review of physical activity and functional considerations in head and neck cancer patients. Support Care Cancer. 2013;21:1475–1479. doi: 10.1007/s00520-013-1736-4. [DOI] [PubMed] [Google Scholar]
- 9.Chasen MR, Bhargava R. A descriptive review of the factors contributing to nutritional compromise in patients with head and neck cancer. Support Care Cancer. 2009;17:1345–1351. doi: 10.1007/s00520-009-0684-5. [DOI] [PubMed] [Google Scholar]
- 10.Jager–Wittenaar H, Dijkstra PU, Vissink A, et al. Changes in nutritional status and dietary intake during and after head and neck cancer treatment. Head Neck. 2011;33:863–870. doi: 10.1002/hed.21546. [DOI] [PubMed] [Google Scholar]
- 11.Jackson W, Alexander N, Schipper M, Fig L, Feng F, Jolly S. Characterization of changes in total body composition for patients with head and neck cancer undergoing chemoradiotherapy using dual-energy x-ray absorptiometry. Head Neck. 2014;36:1356–1362. doi: 10.1002/hed.23461. [DOI] [PubMed] [Google Scholar]
- 12.Lenk K, Schuler G, Adams V. Skeletal muscle wasting in cachexia and sarcopenia: molecular pathophysiology and impact of exercise training. J Cachexia Sarcopenia Muscle. 2010;1:9–21. doi: 10.1007/s13539-010-0007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mick R, Vokes EE, Weichselbaum RR, Panje WR. Prognostic factors in advanced head and neck cancer patients undergoing multimodality therapy. Otolaryngol Head Neck Surg. 1991;105:62–73. doi: 10.1177/019459989110500109. [DOI] [PubMed] [Google Scholar]
- 14.O’Neill M, Heron DE, Flickinger JC, Smith R, Ferris RL, Gibson M. Post-treatment quality-of-life assessment in patients with head and neck cancer treated with intensity-modulated radiation therapy. Am J Clin Oncol. 2011;34:478–482. doi: 10.1097/COC.0b013e3181f4759c. [DOI] [PubMed] [Google Scholar]
- 15.Pichard C, Kyle UG, Morabia A, Perrier A, Vermeulen B, Unger P. Nutritional assessment: lean body mass depletion at hospital admission is associated with an increased length of stay. Am J Clin Nutr. 2004;79:613–618. doi: 10.1093/ajcn/79.4.613. [DOI] [PubMed] [Google Scholar]
- 16.Rogers LQ, Courneya KS, Robbins KT, et al. Physical activity and quality of life in head and neck cancer survivors. Support Care Cancer. 2006;14:1012–1019. doi: 10.1007/s00520-006-0044-7. [DOI] [PubMed] [Google Scholar]
- 17.Silver HJ, Dietrich MS, Murphy BA. Changes in body mass, energy balance, physical function, and inflammatory state in patients with locally advanced head and neck cancer treated with concurrent chemoradiation after low-dose induction chemotherapy. Head Neck. 2007;29:893–900. doi: 10.1002/hed.20607. [DOI] [PubMed] [Google Scholar]
- 18.Taylor JC, Terrell JE, Ronis DL, et al. Disability in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 2004;130:764–769. doi: 10.1001/archotol.130.6.764. [DOI] [PubMed] [Google Scholar]
- 19.Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 20.McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. CMAJ. 2006;175:34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mishra SI, Scherer RW, Snyder C, Geigle PM, Berlanstein DR, Topaloglu O. Exercise interventions on health-related quality of life for people with cancer during active treatment. Clin Otolaryngol. 2012;37:390–392. doi: 10.1111/coa.12015. [DOI] [PubMed] [Google Scholar]
- 22.Monga U, Garber SL, Thornby J, et al. Exercise prevents fatigue and improves quality of life in prostate cancer patients undergoing radiotherapy. Arch Phys Med Rehabil. 2007;88:1416–1422. doi: 10.1016/j.apmr.2007.08.110. [DOI] [PubMed] [Google Scholar]
- 23.Schneider CM, Hsieh CC, Sprod LK, Carter SD, Hayward R. Effects of supervised exercise training on cardiopulmonary function and fatigue in breast cancer survivors during and after treatment. Cancer. 2007;110:918–925. doi: 10.1002/cncr.22862. [DOI] [PubMed] [Google Scholar]
- 24.Segal RJ, Reid RD, Courneya KS, et al. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J Clin Oncol. 2003;21:1653–1659. doi: 10.1200/JCO.2003.09.534. [DOI] [PubMed] [Google Scholar]
- 25.Courneya KS, McKenzie DC, Mackey JR, et al. Effects of exercise dose and type during breast cancer chemotherapy: multicenter randomized trial. J Natl Cancer Inst. 2013;105:1821–1832. doi: 10.1093/jnci/djt297. [DOI] [PubMed] [Google Scholar]
- 26.Quist M, Langer SW, Rørth M, Christensen KB, Adamsen L. “EXHALE”: exercise as a strategy for rehabilitation in advanced stage lung cancer patients: a randomized clinical trial comparing the effects of 12 weeks supervised exercise intervention versus usual care for advanced stage lung cancer patients. BMC Cancer. 2013;13:477. doi: 10.1186/1471-2407-13-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jarden M, Moller T, Kjeldsen L, et al. Patient activation through counseling and exercise–acute leukemia (PACE-AL)–a randomized controlled trial. BMC Cancer. 2013;13:446. doi: 10.1186/1471-2407-13-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henke CC, Cabri J, Fricke L, et al. Strength and endurance training in the treatment of lung cancer patients in stages IIIA/IIIB/IV. Support Care Cancer. 2014;22:95–101. doi: 10.1007/s00520-013-1925-1. [DOI] [PubMed] [Google Scholar]
- 29.Jolly S, Peterson LA, Rozek LS, et al. Longitudinal changes in physical activity of head and neck squamous cell carcinoma patients. J Clin Oncol. 2013;(suppl) abstract 9607. [Google Scholar]
- 30.Taves DR. Minimization: a new method of assigning patients to treatment and control groups. Clin Pharmacol Ther. 1974;15:443–453. doi: 10.1002/cpt1974155443. [DOI] [PubMed] [Google Scholar]
- 31.Beauchet O, Fantino B, Allali G, Muir SW, Montero–Odasso M, Annweiler C. Timed Up and Go test and risk of falls in older adults: a systematic review. J Nutr Health Aging. 2011;15:933–938. doi: 10.1007/s12603-011-0062-0. [DOI] [PubMed] [Google Scholar]
- 32.Ware JE, Jr, Gandek B. Overview of the SF-36 health survey and the international quality of life assessment (IQOLA) project. J Clin Epidemiol. 1998;51:903–912. doi: 10.1016/s0895-4356(98)00081-x. [DOI] [PubMed] [Google Scholar]
- 33.Hays RD, Martin SA, Sesti AM, Spritzer KL. Psychometric properties of the medical outcomes study sleep measure. Sleep Med. 2005;6:41–44. doi: 10.1016/j.sleep.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 34.Washburn RA, Smith KW, Jette AM, Janney CA. The physical activity scale for the elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 35.Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 36.Mâsse LC, Fuemmeler BF, Anderson CB, et al. Accelerometer data reduction: a comparison of four reduction algorithms on select outcome variables. Med Sci Sports Exerc. 2005;37(11 Suppl):S544–S554. doi: 10.1249/01.mss.0000185674.09066.8a. [DOI] [PubMed] [Google Scholar]
- 37.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30:777–781. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 38.Matthew CE. Calibration of accelerometer output for adults. Med Sci Sports Exerc. 2005;37(11 Suppl):S512–S522. doi: 10.1249/01.mss.0000185659.11982.3d. [DOI] [PubMed] [Google Scholar]
- 39.Terrell JE, Nanavati KA, Esclamado RM, Bishop JK, Bradford CR, Wolf GT. Head and neck cancer-specific quality of life: instrument validation. Arch Otolaryngol Head Neck Surg. 1997;123:1125–1132. doi: 10.1001/archotol.1997.01900100101014. [DOI] [PubMed] [Google Scholar]
- 40.Rogers LQ, Courneya KS, Robbins KT, et al. Physical activity correlates and barriers in head and neck cancer patients. Support Care Cancer. 2008;16:19–27. doi: 10.1007/s00520-007-0293-0. [DOI] [PubMed] [Google Scholar]
- 41.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 42.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption–II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 43.Trotti A, Colevas AD, Setser A, et al. CTCAE v3. 0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 44.McNeely ML, Courneya KS. Exercise programs for cancer-related fatigue: evidence and clinical guidelines. J Natl Compr Canc Netw. 2010;8:945–953. doi: 10.6004/jnccn.2010.0069. [DOI] [PubMed] [Google Scholar]
- 45.Feng FY, Kim HM, Lyden TH, et al. Intensity-modulated chemoradiotherapy aiming to reduce dysphagia in patients with oropharyngeal cancer: clinical and functional results. J Clin Oncol. 2010;28:2732–2738. doi: 10.1200/JCO.2009.24.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vainshtein JM, Griffith KA, Feng FY, Vineberg KA, Chepeha DB, Eisbruch A. Patient-reported voice and speech outcomes after whole-neck intensity modulated radiation therapy and chemotherapy for oropharyngeal cancer: prospective longitudinal study. Int J Radiat Oncol Biol Phys. 2014;89:973–980. doi: 10.1016/j.ijrobp.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 47.Lin A, Kim HM, Terrell JE, Dawson LA, Ship JA, Eisbruch A. Quality of life after parotid-sparing IMRT for head-and-neck cancer: a prospective longitudinal study. Int J Radiat Oncol Biol Phys. 2003;57:61–70. doi: 10.1016/s0360-3016(03)00361-4. [DOI] [PubMed] [Google Scholar]
- 48.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 49.Samuel SR, Maiya GA, Babu AS, Vidyasagar MS. Effect of exercise training on functional capacity & quality of life in head & neck cancer patients receiving chemoradiotherapy. Indian J Med Res. 2013;137:515–520. [PMC free article] [PubMed] [Google Scholar]
- 50.Capozzi LC, Lau H, Reimer RA, McNeely M, Giese–Davis J, Culos–Reed SN. Exercise and nutrition for head and neck cancer patients: a patient oriented, clinic-supported randomized controlled trial. BMC Cancer. 2012;12:446. doi: 10.1186/1471-2407-12-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eades M, Murphy J, Carney S, et al. Effect of an interdisciplinary rehabilitation program on quality of life in patients with head and neck cancer: review of clinical experience. Head Neck. 2013;35:343–349. doi: 10.1002/hed.22972. [DOI] [PubMed] [Google Scholar]
- 52.Lønbro S, Dalgas U, Primdahl H, et al. Progressive resistance training rebuilds lean body mass in head and neck cancer patients after radiotherapy–results from the randomized DAHANCA 25B trial. Radiother Oncol. 2013;108:314–319. doi: 10.1016/j.radonc.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 53.Ferguson RJ, Robinson AB, Splaine M. Use of the reliable change index to evaluate clinical significance in SF-36 outcomes. Qual Life Res. 2002;11:509–516. doi: 10.1023/a:1016350431190. [DOI] [PubMed] [Google Scholar]
- 54.Rogers LQ, Anton PM, Fogleman A, et al. Pilot, randomized trial of resistance exercise during radiation therapy for head and neck cancer. Head Neck. 2013;35:1178–1188. doi: 10.1002/hed.23118. [DOI] [PubMed] [Google Scholar]
- 55.Scott DA, Mills M, Black A, et al. Multidimensional rehabilitation programmes for adult cancer survivors. Cochrane Database Syst Rev. 2013;3:CD007730. doi: 10.1002/14651858.CD007730.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roscoe JA, Kaufman ME, Matteson–Rusby SE, et al. Cancer-related fatigue and sleep disorders. Oncologist. 2007;12(Suppl 1):35–42. doi: 10.1634/theoncologist.12-S1-35. [DOI] [PubMed] [Google Scholar]
- 57.Shuman AG, Duffy SA, Ronis DL, et al. Predictors of poor sleep quality among head and neck cancer patients. Laryngoscope. 2010;120:1166–1172. doi: 10.1002/lary.20924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou J, Jolly S. Obstructive sleep apnea and fatigue in head and neck cancer patients. Am J Clin Oncol. 2014 doi: 10.1097/01.coc.0000436086.61460.cb. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 59.Duffy SA, Teknos T, Taylor JM, et al. Health behaviors predict higher interleukin-6 levels among patients newly diagnosed with head and neck squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2013;22:374–381. doi: 10.1158/1055-9965.EPI-12-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Santos RV, Viana VA, Boscolo RA, et al. Moderate exercise training modulates cytokine profile and sleep in elderly people. Cytokine. 2012;60:731–735. doi: 10.1016/j.cyto.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 61.McGuire R, Waltman N, Zimmerman L. Intervention components promoting adherence to strength training exercise in breast cancer survivors with bone loss. West J Nurs Res. 2011;33:671–689. doi: 10.1177/0193945910379004. [DOI] [PubMed] [Google Scholar]