Abstract
BACKGROUND
Geographic clusters in prevalence and hospitalizations for COPD have been identified at national, state, and county levels. The study objective is to identify county-level geographic accessibility to pulmonologists for adults with COPD.
METHODS
Service locations of 12,392 practicing pulmonologists and 248,160 primary care physicians were identified from the 2013 National Provider Identifier Registry and weighted by census block-level populations within a series of circular distance buffer zones. Model-based county-level population counts of US adults ≥ 18 years of age with COPD were estimated from the 2013 Behavioral Risk Factor Surveillance System. The percentages of all estimated adults with potential access to at least one provider type and the county-level ratio of adults with COPD per pulmonologist were estimated for selected distances.
RESULTS
Most US adults (100% in urbanized areas, 99.5% in urban clusters, and 91.7% in rural areas) had geographic access to a primary care physician within a 10-mile buffer distance; almost all (≥ 99.9%) had access to a primary care physician within 50 miles. At least one pulmonologist within 10 miles was available for 97.5% of US adults living in urbanized areas, but only for 38.3% in urban clusters and 34.5% in rural areas. When distance increased to 50 miles, at least one pulmonologist was available for 100% in urbanized areas, 93.2% in urban clusters, and 95.2% in rural areas. County-level ratios of adults with COPD per pulmonologist varied greatly across the United States, with residents in many counties in the Midwest having no pulmonologist within 50 miles.
CONCLUSIONS
County-level geographic variations in pulmonologist access for adults with COPD suggest that those adults with limited access will have to depend on care from primary care physicians.
Keywords: COPD, epidemiology, geographic variation, pulmonologist
Over 15 million US adults are estimated to have been diagnosed with COPD,1–3 and many more may be undiagnosed. Declines in COPD prevalence, Medicare hospitalizations, and deaths since 1999 in the United States2 suggest considerable progress in the prevention of COPD, but > 6% of US adults continue to live with COPD and its complications while geographic clusters of persons with highest risk of COPD hospitalizations, health-care utilization, and death persist. Surveillance data from national and state surveillance systems show that there are geographic clusters of highest COPD prevalence, hospitalizations, and mortality in Southern states and states along the Mississippi River and Ohio River.1,2,4,5 Highest death rates from COPD have also been reported in multiple Western states.2 For COPD prevalence and Medicare hospitalizations, there are also counties with the highest levels in the nation even within states with much lower overall state levels.4,5
However, there is little information about geographic variations in the availability of health-care providers in areas with the highest number of adults with COPD. It has been predicted that the current aging US population will experience a shortage in the future of physicians and specialists such as pulmonologists.6,7 It is assumed that primary care physicians would provide care to persons with limited access to a pulmonologist; however, little is known about geographic access to that specialty. Therefore, we focused on the current geographic availability of either pulmonologists or primary care physicians in the United States and estimated whether adults with COPD would have access to these specialists based on geographic distance. We used a buffer approach to calculate potential population access to at least one physician specialty at selected distances.
Materials and Methods
Data used in this analysis includes physician address location extracted from the National Provider Identifier Registry (NPI); US Census 2010 block-level population counts and spatial data (polygons of blocks, counties, and states); and county-level estimates of individuals with COPD that were derived from the 2013 Behavioral Risk Factor Surveillance System (BRFSS). Because the NPI and Census information is available in the public domain and county COPD data were obtained by permission from the BRFSS, which removed personal identifiers, this study was exempt from human subject review.
US Population Counts
Census population counts were obtained from the US Census Bureau. Census blocks (N=11,155,486 in 2010) are the smallest unit of census geography; 44.4% were uninhabited in 2010; therefore, 6,207,027 blocks were retained for analysis. The census block was used as the initial geographic unit to calculate the population residing within each physician’s distance buffer and to aggregate data to higher geographic units, such as county and areas defined by urban/rural status. Each census block is identified by the US Census Bureau as belonging to an urbanized area (multiple census blocks with combined populations ≥50,000), urban cluster (multiple census blocks with combined populations of 2,500–49,999), or rural area (all remaining census blocks).8 Block-level 2010 population counts in urban/rural classifications and county-specific counts from the US Census Bureau were obtained to determine the number of all US adults ≥18 years of age who were living both within and outside of each physician’s distance buffer. These population counts were also used as weights to estimate the share of the population within overlapping locations for multiple physicians within each specialty type.
Adults With COPD
The BRFSS is a state-based, random-digit-dialed telephone survey of the noninstitutionalized US population ≥ 18 years of age, which is administered annually by all state health departments (including the District of Columbia) in collaboration with the Centers for Disease Control and Prevention to households with landline and cellular telephones.9 In 2013, a question about COPD was asked of 491,772 respondents10; 6.4% (an estimated 15.7 million) of US adults responded that they had ever been told by a doctor or other health professional that they had COPD, emphysema, or chronic bronchitis.3 The number of adults ≥ 18 years of age with COPD in each of the 3,143 US counties was estimated with BRFSS 2013 prevalence data and Census 2010 population counts using a small area estimation method that is described in detail elsewhere.4 In brief, COPD estimates were based on census block-level populations by age, race/ethnicity, and sex and then were aggregated to the county level. In the external validation study of this method, a correlation coefficient of 0.69 for COPD was observed between county-level model-based small area estimates and direct county-level prevalence measures.11
Physician Location
The NPI is issued by the Centers for Medicare Medicaid Services through the National Plan and Provider Enumeration System, is updated weekly, and has a unique 10-digit identification number for each covered health-care provider regardless of whether in a group or individual practice.12 We extracted pulmonologist data from the NPI data released on September 8, 2013, using the health-care taxonomy code 207RP1001X for physicians in pulmonary disease.13 Primary care physicians were identified by health taxonomy codes for geriatric medicine (207RG0300X), internal medicine/geriatric medicine (207QG0300X), internal medicine (207R00000X), family practice (207Q00000X), family practice/adult medicine (207QA0505X), or general practice (208D0000X).13 After excluding physicians practicing in US territories and outside the United States, the study identified 12,392 pulmonologists and 248,160 primary care physicians with practice locations in the 50 states and the District of Columbia. No information was available about the age, race/ethnicity, or board certification of providers.
Each physician was geocoded to the practice location street address in separate analyses for pulmonologists and primary care physicians. The geocoding methodology has been described and illustrated in detail elsewhere.14 A series of circular buffers were created around each practice location using a geographic information system for selected buffer distances (5, 10, 15, 20, 30, and 50 miles) to represent Euclidean (straight-line) distance. The buffer approach used in this study is similar to the two-step floating catchment area method,15,16 except that street addresses were geocoded in this study rather than using driving distance. In this study, Euclidean (straight-line) distances of 5 or 10 miles were assumed to be reasonable proxies for travel by public transportation in urban areas; 15-, 20-, and 30-mile distances were assumed to approximate 30- to 45-min travel time by automobile, and a 50-mile distance was assumed to approximate 1 h of travel by automobile, which might be more typical of travel time from a rural area to a regional medical center. For nonemergency service, both straight-line distance and driving distance provide similar precision.17 Because some physicians may practice in very close proximity to one another or in a group practice, each physician may have a buffer zone that overlaps that of others.
Access to a Physician Specialty
Access to a physician specialty was calculated separately for pulmonologists and primary care physicians. The methodology for calculating access to a physician specialty has been described in detail elsewhere.14 For any census block(s) located within a selected buffer distance, all of the adult population was defined as having potential access to that specialty. We summarized the total block population (Pd) inside each individual buffer zone and distributed the physician to each person 1/Pd living inside the buffer. Because a census block may be located in multiple physicians’ buffer zones, the share of a specialist for each person is the sum of 1/Pd1 + 1/Pd2 + … + 1/Pdn, where n is the number of those specialists within the given distance. Then, we calculated the number of specialists for each census block as the product of each person’s share in that block and the total population (Pb) of that block as Db = Pd (1/Pd1 + 1/Pd2 + … + 1/Pdn). We summarized the number of each census block’s specialists within a county to obtain the number of specialists for that county Dc = (Db1 + Db2 + … + Dbm), where m represents the number of blocks within a county. Finally, we summarized the census block population within and outside the buffer zone for each county to calculate the percentage of adults that had access to at least one specialist at the county level. Because each census block also is characterized by the Census Bureau as urban or rural, we also calculated the percentage of US adults that had access to at least one specialist in urbanized areas, urban clusters, and rural areas.
Ratio of Adults With COPD Per Pulmonologist
We calculated the ratio (Rc) of the estimated number (Cc) of adults ≥18 years of age with COPD to the number of pulmonologists (Dc) for each county (Rc = Cc/Dc) within each selected distance. We mapped the county-level ratio within a 50-mile buffer zone to illustrate the ratio of persons with COPD at the county level who have access to at least one pulmonologist within a ≤ 1-h drive. For those blocks that did not fall inside any pulmonologist’s buffer zone, the number of pulmonologists for that block was zero. For a county that did not have any blocks inside any pulmonologist’s buffer zone, the population in that county was defined as having no available pulmonologists within the selected distance.
Results
Among the 234.5 million adults ≥ 18 years of age residing in the 50 states or District of Columbia, 71.1% resided in urbanized areas, 9.5% resided in urban clusters, and 19.4% resided in rural areas. Table 1 shows the cumulative percentage of the US adult population with potential access to at least one pulmonologist and at least one primary care physician in 2013 by distance and urban/rural characteristics. Overall, 79.7% of US adults had access to a pulmonologist within 10 miles, 90.3% had access within 20 miles, and 98.4% had access within 50 miles. These high percentages were influenced by the greater proportion of the US population living in urbanized areas. In urbanized areas, 97.5% had access to at least one pulmonologist within 10 miles, 99.5% had access within 15 miles, and 100% had access within 50 miles. However, adults living in urban clusters and rural areas were much less likely to have a pulmonologist available within short driving distances. Within 10 miles at least one pulmonologist was available to only 38.3% of those living in urban clusters and 34.5% of those living in rural areas. Only 52.1% in urban clusters and 54.5% in rural areas had a pulmonologist within 20 miles. However, 93.2% of those living in urban clusters and 95.2% of those living in rural areas had access to at least one pulmonologist within 50 miles. In contrast, 100% of persons living in urbanized areas, 99.5% of those living in urban clusters, and 91.7% of those living in rural areas had access to at least one primary care physician within 10 miles (Table 1). At least 99.9% of persons living in urban clusters had access to a primary care physician within 15 miles, and 99.9% of those living in rural areas had access to one within 50 miles.
TABLE 1.
Cumulative Percentage of Adults ≥ 18 Years of Age With Access to At Least One Pulmonologist or Primary Care Physician, by Distance and Urban/Rural Characteristics: United States, 2013
| Euclidean Distance, Miles | Total (N = 234.5 Million) |
Urbanized Area (n = 166.7 Million) |
Urban Cluster (n = 22.2 Million) |
Rural Areas (n = 45.6 Million) |
|---|---|---|---|---|
| Pulmonologist | ||||
| 5 | 64.6 | 84.3 | 28.3 | 10.4 |
| 10 | 79.7 | 97.5 | 38.3 | 34.5 |
| 15 | 86.2 | 99.5 | 52.1 | 54.5 |
| 20 | 90.3 | 99.8 | 64.2 | 68.4 |
| 30 | 95.3 | 99.9 | 81.5 | 85.1 |
| 50 | 98.4 | 100.0 | 93.2 | 95.2 |
| Primary care physician | ||||
| 5 | 92.6 | 99.8 | 97.4 | 63.9 |
| 10 | 98.3 | 100.0 | 99.5 | 91.7 |
| 15 | 99.5 | 100.0 | 99.9 | 97.7 |
| 20 | 99.8 | 100.0 | 99.9 | 99.0 |
| 30 | 99.9 | 100.0 | 100.0 | 99.7 |
| 50 | 100.0 | 100.0 | 100.0 | 99.9 |
Data are presented as percentages or as otherwise indicated
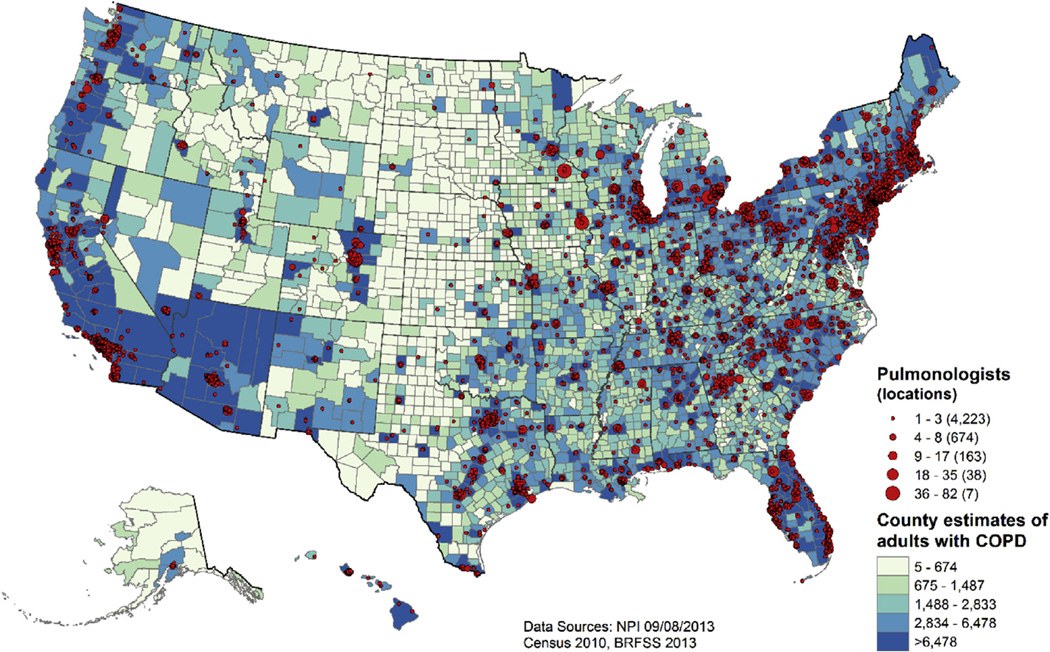
Among the 12,392 US pulmonologists who self-identified in the September 2013 NPI as providing pulmonary care to adults, 82.7% were men and 18.8% had solo practices. Most pulmonologists (92.9%) were located in urban areas, whereas 5.0% practiced in urban clusters and 2.1% practiced in rural areas. Figure 1 shows the location of US pulmonologists in 2013. The 12,392 pulmonologists were identified as practicing in 5,105 separate locations. The seven largest numbers of pulmonologists with practice locations clustered in the same location were observed in Rochester, Minnesota (n = 82); Denver, Colorado (n = 63); Boston, Massachusetts (n = 53); Philadelphia, Pennsylvania (n = 46); Cleveland, Ohio (n = 45); Ann Arbor, Michigan (n = 43); and Iowa City, Iowa (n = 40). In general, however, most locations (82.7%) included only one to three pulmonologists. State-level numbers of pulmonologists ranged from 12 in Wyoming and 15 in Alaska to 1,104 in New York and 1,259 in California.
Figure 1.
Locations of 12,392 US pulmonologists and quintiles of county estimates of the 15.7 million adults ≥ 18 years of age with diagnosed COPD: United States, 2013.
Practice location is obviously influenced by population density—particularly the density of available patients. Figure 1 also depicts pulmonologist practice locations overlaid with quintiles of county-level estimated numbers of US adults ≥ 18 years of age with diagnosed COPD. The estimated number of adults with diagnosed COPD in 2013 ranged from five in Kalawao County, Hawaii, to 398,279 in Los Angeles County, California. Although there are small numbers of pulmonologist locations in the Midwest and in the Rocky Mountain regions, there also appear to be fewer persons with diagnosed COPD living in those areas.
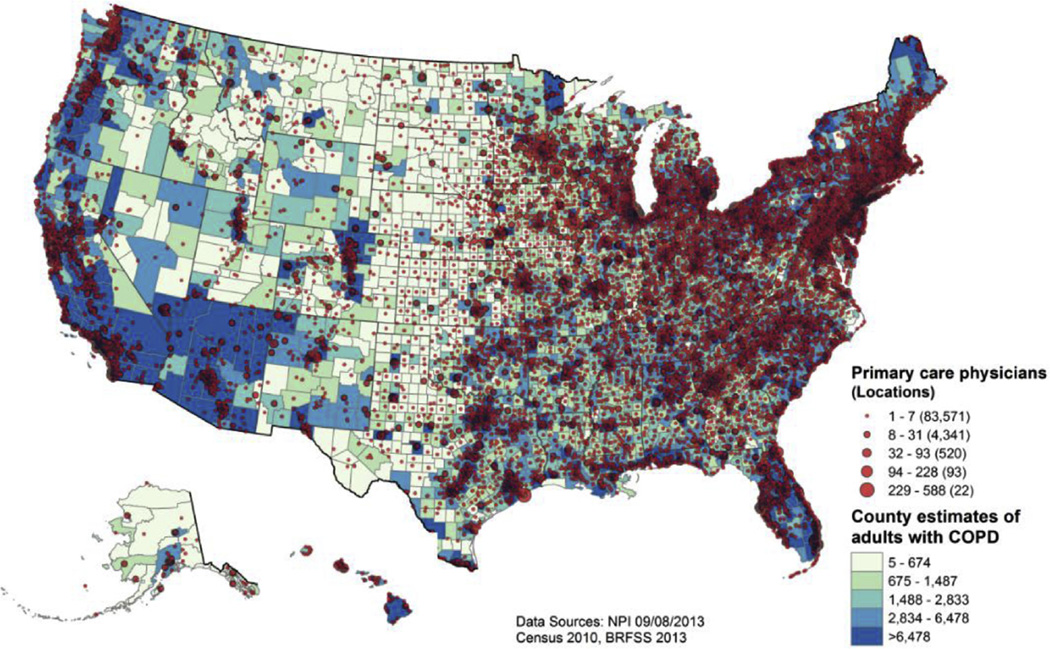
Among the 248,160 US primary care physicians who self-identified in the September 2013 NPI as providing care to adults, 62.7% were men and 24.5% had solo practices. The self-identified specialties included internal medicine (48.0%), family practice (45.1%), general practice (4.1%), geriatric medicine (1.5%), family practice/adult medicine (0.8%), and internal medicine/geriatric medicine (0.6%). Most primary care physicians (81.8%) were located in urban areas, whereas 11.2% practiced in urban clusters and 6.9% practiced in rural areas.
Figure 2 shows the location of all US primary care physicians in 2013 in relation to the same quintiles of adults with COPD, as shown in Figure 1 for pulmonologists. The 248,160 primary care physicians were identified as practicing in 88,547 separate locations, with Boston, Massachusetts, having the largest numbers of primary care physicians at two separate addresses (n = 588 and n = 468) followed by one address in Rochester, Minnesota (n = 486). In general, however, most locations (94.3%) included only one to seven primary care physicians. State-level numbers of primary care physicians ranged from 400 in Wyoming and 641 in Vermont to 15,549 in Texas and 26,844 in California.
Figure 2.
Locations of 248,160 US primary care physicians and quintiles of county estimates of the 15.7 million adults ≥ 18 years of age with diagnosed COPD: United States, 2013.
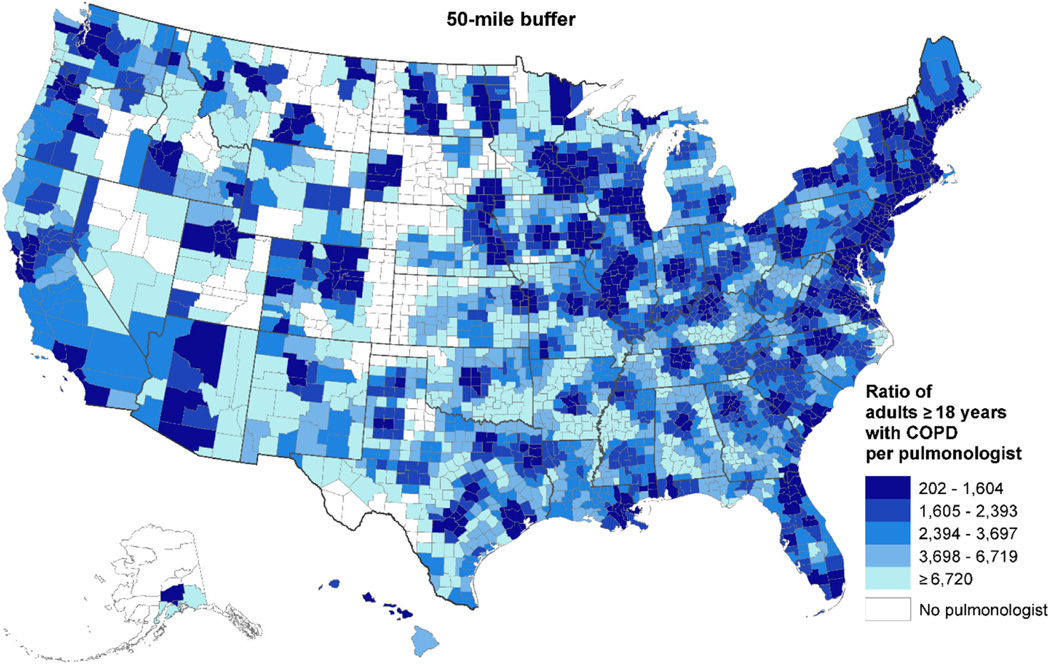
Figure 3 shows the ratio of the estimated number of adults with COPD per pulmonologist at the county level for a 50-mile circular buffer. Darker shades represent smaller patient/pulmonologist ratios because of the larger number of pulmonologists available within that 50-mile buffer zone. Figure 3 also shows that there were some counties in Midwestern states, in some Western states, and counties along the Rio Grande that had no pulmonologist within 50 miles. In remote areas with a pulmonologist within 50 miles, the ratio of adults with COPD reached to ≥ 6,720 per pulmonologist.
Figure 3.
County-level ratio of adults ≥ 18 years of age with COPD per pulmonologist within 50 miles: United States, 2013.
Discussion
This study provides a broad picture of the geographic availability of the pulmonologist workforce in the United States at many different access distances by linking physician locations from the 2013 NPI with population counts from the 2010 US Census and estimates of adults with diagnosed COPD from the 2013 BRFSS. This study observed that all adults in urbanized areas, where 71% of the current US adult population reside, have access to at least one pulmonologist within 50 miles. Approximately 7% of adults living in urban clusters and 5% in rural areas do not have a pulmonologist available within 50 miles; this represents approximately 3.7 million US adults (1.5 million in urban clusters and 2.2 million in rural areas) without a pulmonologist who would be available within a 1-h driving distance. In each state, pulmonologists were located primarily in urban areas or in counties with the greatest population density, including the greatest density of adults estimated to have diagnosed COPD based on 2013 BRFSS prevalence.
What is not clear is whether pulmonologist location impacts the density of persons with diagnosed COPD such that an additional unknown proportion of adults with undiagnosed COPD might be likely to live in counties in the Midwest and some Rocky Mountain areas that are outside a 50-mile buffer zone. This speculation is fueled by a previous observation of the highest and third highest quartiles of 2009 to 2010 death rates with COPD as the underlying cause of death in states such as Montana, Wyoming, Idaho, Nebraska, Colorado, and New Mexico2—states with many counties showing the lowest prevalence of diagnosed COPD4 and, as shown in this study, the lowest estimated numbers of adults with diagnosed COPD. In a study of 2 million Medicare patients in Alaska, Idaho, North Carolina, South Carolina, and Washington, those living in small rural locations had to travel 33.4 average miles for 42.5 average minutes to receive a pulmonary function test, whereas those in urban areas traveled 8.4 average miles for 13.0 min for the test.18 Such distances in rural areas may discourage persons with COPD symptoms from seeking a COPD diagnostic test.
Fortunately, this study shows that at least one primary care physician was available within 10 miles for 99.5% of US adults living in urban clusters and within 20 miles for 99.0% of adults living in rural areas. However, there is concern about the accuracy of COPD diagnosis and the classification of airway obstruction among primary care physicians. For example, in a study of 1,205 patients with a primary care diagnosis who were admitted for pulmonary rehabilitation between 2007 and 2010 in Rotherham, England, 20% of spirometry results reported on the referral form were inconsistent with a diagnosis of COPD; among patients diagnosed with COPD and referred by respiratory specialists, only 6.5% had an inconsistent spirometry result.19 Among those patients with a primary care referral diagnosis of COPD that had an inconsistent spirometry result, the most common subsequent diagnosis was asthma.19 Remote reporting of spirometry tests from primary care physicians to a pulmonary specialist in the United Kingdom has been shown to be feasible and resulted in more accurate interpretations.20
It is not established that all adults with COPD in the United States require specialist care from a pulmonologist because there do not seem to be clinical practice guidelines that have assessed evidence about the necessity for such specialty care. Clinical practice guidelines for the diagnosis and management of COPD are provided for all clinicians who manage patients with COPD.21 There is limited recent information about the diagnoses and characteristics of US patients seen, services provided, or the proportion of time spent in ambulatory vs inpatient service among US pulmonologists. Most such information may be outdated. In a 2005 survey of 1,911 patients with COPD who were identified from 12 medical practices across Minnesota, almost two-thirds reported a generalist as having primary responsibility for their COPD care.22 In the same report, 60% of the patients with COPD also reported having spirometry in the last year; however, claims data suggested that only < 20% of patients with COPD had a medical claim for spirometry.22 Among all 5.6 million ambulatory visits for any cause in 1994 to 1995 among Medicare beneficiaries in the state of Washington, pulmonologists provided care to only 2% and averaged 222.0 Medicare patients per pulmonologist.23 Among pulmonologists in that study, almost 30% of outpatient diagnoses were COPD, emphysema, and chronic bronchitis, and 36.0% were out-of-domain diagnoses; therefore, pulmonologists also reported providing 30.8% of their outpatient practice to majority-of-care for their patients.23 Results from a 10% stratified random sample of physicians from the 1996 American Medical Association Physician Masterfile demonstrated that pulmonologists provided < 10% of all ambulatory pulmonary care in the United States; most (67.8%) pulmonary patients seen in office visit settings were < 65 years of age.6 In contrast, hospitalizations for COPD, pneumonia, and respiratory failure accounted for 80.5% of all pulmonary inpatient days; 66.8% of all inpatient pulmonary days were incurred by patients ≥65 years of age, with pulmonologists more likely to care for patients with COPD, asthma, and respiratory failure than for those with pneumonia.6 In that study, pulmonologists also spent 67.1% of their nonintensive care unit clinical time providing pulmonary services.6
It is also unclear whether current outcomes of patients with COPD in the United States differ between those treated by pulmonologists and those treated by primary care physicians. In one US study of patients with COPD hospitalized with severe COPD from 1989 to 1994, patients seen by pulmonologists compared with those seen by generalists were younger, had more severe acute disease, and had worse estimated survival on admission, but survival at 30 days did not differ significantly after adjustment for differences in case mix.24 Whether patients with COPD seen by a pulmonologist in the United States are more likely to receive recommended therapies and show greater adherence than those seen by primary care physicians in actual practice also is not established; however, it could be assumed that pulmonologists would be more likely to have greater awareness of new treatment modalities. In a Barcelona, Spain, study of 346 patients with moderate-to-severe COPD who had been admitted with COPD exacerbations to four hospitals in 1997 through 1998, those being controlled by a general practitioner were less likely to receive pharmacologic and nonpharmacologic treatments and less likely to perform inhalation maneuvers correctly compared with those being controlled by a pneumologist.25 In a study of 80 US patients with a medical record diagnosis of COPD who were being followed at outpatient pulmonary and patient care clinics in 2001 to 2002, care (use of pulmonary function tests and bronchodilators) was more likely to be consistent with Global Initiative for Chronic Obstructive Lung Disease guidelines when specialists were the usual source of care, but generalists were more likely to perform better with smoking cessation and vaccine prophylaxis.26 A more recent international survey in 2013 that included 151 US primary care physicians and 49 US respiratory specialists assessed physician knowledge and application of Global Initiative for Chronic Obstructive Lung Disease diagnosis and treatment recommendations.27 In that survey, US respiratory specialists compared with US primary care physicians were more likely to be aware of the 2011 Global Initiative for Chronic Obstructive Lung Disease global strategy guidelines (98% vs 56%, respectively); were more likely to report using spirometry (100% vs 84%, respectively) and bronchodilator responsiveness (80% vs 66%, respectively) and less likely to use peak expiratory flow (35% vs 63%, respectively) to establish a COPD diagnosis; and were more likely to be concordant with first- or second-choice treatment options (67% vs 21%, respectively) in survey scenarios.27 These findings were an improvement over results for both primary care physicians and pulmonologists from an earlier 2003 to 2004 US physician survey.28 Common barriers to the diagnosis and treatment of COPD by primary care physicians are reported to be failure of patients to report COPD symptoms; presence of multiple morbidities; low physician confidence; low outcome expectancy; time constraints; lack of knowledge and inadequate training in COPD diagnosis and management; and low belief in benefits of pulmonary rehabilitation and new therapies.29–31
A strength of this study is that the buffer approach used defined distance zones, which avoids problems when aggregating data according to existing census geographic units, such as states or counties, that vary in size and population heterogeneity.32 The buffer approach also accounts for potential cross-boundary health care-seeking behaviors in the United States, which are not confined within geopolitical boundaries, especially for residents in isolated rural areas, those living near county or state boundaries lines, or those seeking more specialized care at regional medical centers. Because Euclidean distance was used as a proxy for travel distance, however, geographic accessibility to a pulmonologist at various distances may be overestimated in rural or mountain areas where road networks are more limited and less straight than in urban areas. This study can only assess geographic availability and distance to pulmonologists and primary care physicians. There are many other barriers to access to care related to patient finances, insurance problems, transportation issues, low patient knowledge and awareness about COPD, disabilities, and caregiver support that cannot be assessed in the current analyses.
Racial/ethnic disparity is also an important issue in considering barriers to health-care access. In the 2013 BRFSS, the age-adjusted prevalence of COPD was highest among American Indian/Alaska Natives (10.2%) and non-Hispanic adults reporting multiple races (10.7%) in comparison with non-Hispanic whites (6.3%), non-Hispanic blacks (6.5%), Hispanics (4.1%), and Asians (2.0%).3 Among adults ≥ 25 years of age, blacks in comparison with whites had lower aged-adjusted rates (per 10,000) for physician office visits (539.2 vs 571.1), higher ED visit rates (118.2 vs 78.6), and higher hospital visit rates (34.9 vs 30.5) for COPD as a first-listed diagnosis in 2009 to 2010.2 Among Medicare enrollees ≥ 65 years of age in 2010, hospital rates (per 1,000 Medicare enrollees) for COPD as the first-listed diagnosis were higher among Native Americans (13.2) and non-Hispanic blacks (12.4) than non-Hispanic whites (11.3), Hispanics (9.7), and Asians (4.8).2 However, death rates (per 100,000) for COPD as the underlying cause in 2010 were highest among non-Hispanic whites (70.2) and American Indian/Alaska Natives (62.9) compared with non-Hispanic blacks (41.8), Hispanics (28.5), and Asian/Pacific Islanders (19.0).2 Although these national statistics suggest that COPD may not be underdiagnosed among American Indian/Alaska Native populations, which may have access to tribal clinics and the Indian Health Service clinics in rural locations, the current study is limited in the ability to ascertain whether underdiagnosis and delayed treatment of COPD is more likely to occur among Hispanics and non-Hispanic blacks residing in rural areas.
There are other limitations in this study. First, the NPI from the Centers for Medicare and Medicaid Services may under- or overestimate pulmonology practices compared with other physician workforce datasets, such as the American Medical Association Masterfile or professional organization membership lists. In the NPI, all covered health-care providers must obtain a unique NPI number and provide only one location address to facilitate electronic transmission of claims and other health-care information.33 The public use registry does not provide information about multiple practice sites, board certification status, physician age or race/ethnicity, interventional pulmonology status, or the proportion of time usually divided between office practice, hospital or administrative duties, intensive care units, research, or teaching—factors which may impact the true availability of a pulmonologist in actual practice and the likelihood that an adult with COPD potentially would seek care from the nearest pulmonologist. For example, at least 45 US address locations had 18 to 82 pulmonologists per site and may have represented academic medical centers or teaching hospitals. Therefore, many of these pulmonologists in this high-density setting may have been in critical care or other subspecialties, may have had teaching or administrative responsibilities, and were not actually available for the general population of patients with COPD. Second, estimations of numbers of adults with diagnosed COPD are derived from BRFSS prevalence measures that are dependent on self-reported information during a telephone survey. Such information cannot be validated with medical records, and severity of disease cannot be determined. This is of particular importance if less severe cases are less likely to need access to a pulmonologist. Therefore, ratios of potential patients per pulmonologist may be overestimated in terms of need. However, the unique strength of this study is the ability to describe geographic variations in the availability of pulmonologists at selected distances.
A clear picture of the current geographic availability of pulmonologists in the United States is important for resource planning and intervention strategy development. The goals of COPD management strategies are to reduce declines in lung function, improve exercise tolerance and health-related quality of life, prevent and treat exacerbations, and reduce hospitalizations and mortality.21 Most care provided to persons with COPD, particularly in urban clusters and rural areas, may be performed by primary care physicians, physician assistants, nurse practitioners, and respiratory therapists. Although results vary about the efficacy and improvement in outcomes about such intervention strategies in the management of COPD,34–37 important general solutions to filling gaps in the geographic availability of specialists may include promoting chronic disease self-management interventions for patients with COPD, promoting telemonitoring strategies for daily symptom reporting by patients, coordinating services (patient-sharing) with other health-care providers who frequently share patients with COPD in claims data submissions for multiple comorbid conditions, and improving opportunities so that primary care providers can manage the patient with COPD with high-quality care while consulting with or making referrals to a pulmonologist.
Acknowledgments
FUNDING/SUPPORT: The authors have reported to CHEST that no funding was received for this study.
ABBREVIATIONS
- BRFSS
Behavioral Risk Factor Surveillance System
- NPI
National Provider Identifier Registry
Footnotes
Author contributions: J. B. C. had full access to all of the data in the study and takes responsibility for the content of the manuscript, the integrity of the data, and the accuracy of the analysis. J. B. C. contributed to the study concept and design, interpretation of data, drafting of the manuscript, critical revisions of the manuscript for important intellectual content, and study supervision. H. L. contributed to the study concept and design; acquisition, analysis, and interpretation of data; spatial statistical analysis; provision of technical support; drafting of the manuscript; and critical revisions of the manuscript for important intellectual content. X. Z. contributed to the study concept and design; acquisition, analysis, and interpretation of data; small area estimation statistical analysis; drafting of the manuscript; and critical revisions of the manuscript for important intellectual content. J. B. H. contributed to the study concept and design, drafting of the manuscript, critical revisions of the manuscript for important intellectual content, and study supervision.
Financial/nonfinancial disclosures: None declared. Work was performed at the Centers for Disease Control and Prevention, Atlanta, Georgia. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Centers for Disease Control and Prevention. Chronic obstructive pulmonary disease among adults—United States, 2011. MMWR Morb Mort Wkly Rep. 2012;61(46):938–943. [PubMed] [Google Scholar]
- 2.Ford ES, Croft JB, Mannino DM, Wheaton AG, Zhang X, Giles WH. COPD surveillance—United States, 1999–2011. Chest. 2013;144(1):284–305. doi: 10.1378/chest.13-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wheaton AG, Cunningham TJ, Ford ES, Croft JB. Employment and activity limitations among adults with chronic obstructive pulmonary disease—United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64(11):289–295. [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X, Holt JB, Lu H, Wheaton AG, Ford ES, Greenlund KJ, Croft JB. Multilevel regression and poststratification for small-area estimation of population health outcomes: a case study of chronic obstructive pulmonary disease prevalence using the behavioral risk factor surveillance system. Am J Epidemiol. 2014;179(8):1025–1033. doi: 10.1093/aje/kwu018. [DOI] [PubMed] [Google Scholar]
- 5.Holt JB, Zhang X, Presley-Cantrell L, Croft JB. Geographic disparities in chronic obstructive pulmonary disease (COPD) hospitalization among Medicare beneficiaries in the United States. Int J COPD. 2011;61:321–328. doi: 10.2147/COPD.S19945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Angus DC, Kelley MA, Schmitz RJ, White A, Popovich J. Current and projected workforce requirements for care of the critically ill and patients with pulmonary disease: Can we meet the requirements of an aging population? JAMA. 2000;284(21):2762–2770. doi: 10.1001/jama.284.21.2762. [DOI] [PubMed] [Google Scholar]
- 7.Bureau of Health Professions. Physician Supply and Demand: Projections in 2020. Washington, DC: Health Resources and Services Administration; 2006. [Google Scholar]
- 8.US Census Bureau. 2010 census urban and rural classification and urban area criteria. [Accessed August 21, 2015];US Census Bureau website. http://www.census.gov/geo/reference/ua/urban-rural-2010.html.
- 9.Centers for Disease Control and Prevention. Behavioral risk factor surveillance system: survey data and documentation. [Accessed August 21, 2015];Centers for Disease Control and Prevention website. http://www.cdc.gov/brfss/data_documentation/index.htm.
- 10.Centers for Disease Control and Prevention. BRFSS 2013 survey data and documentation. [Accessed August 21, 2015];Centers for Disease Control and Prevention website. http://www.cdc.gov/brfss/annual_data/annual_2013.html.
- 11.Zhang X, Holt JB, Yun S, Lu H, Greenlund KJ, Croft JB. Validation of multilevel regression and poststratification methodology for small area estimation of health indicators from the behavioral risk factor surveillance system. Am J Epidemiol. 2015;182(2):127–137. doi: 10.1093/aje/kwv002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Medicare and Medicaid Services. National Provider Identifier Standard (NPI) [Accessed August 21, 2015];Centers for Medicare and Medicaid Services website. https://www.cms.gov/Regulations-and-Guidance/HIPAA-Administrative-Simplification/NationalProvIdentStand/index.html?redirect=/NationalProvIdentStand/
- 13.Centers for Medicare and Medicaid Services. Taxonomy. [Accessed August 21, 2015];Centers for Medicare and Medicaid Services website. http://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/MedicareProviderSupEnroll/Taxonomy.html. [PubMed]
- 14.Lu H, Holt JB, Cheng YJ, Zhang X, Onufrak S, Croft JB. Population-based access to endocrinologists in the United States, 2012. BMC Health Serv Res. 2015;15:541. doi: 10.1186/s12913-015-1185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo W, Wang FH. Measures of spatial accessibility to health care in a GIS environment: synthesis and a case study in the Chicago region. Environ Plann B Plann Des. 2003;30:865–884. doi: 10.1068/b29120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delamater PL. Spatial accessibility in suboptimally configured health care systems: a modified two-step floating catchment area (M2SFCA) metric. Health Place. 2013;24:30–43. doi: 10.1016/j.healthplace.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Boscoe FP, Henry KA, Zdeb MS. A nationwide comparison of driving distance versus straight-line distance to hospitals. Prof Geogr. 2012;64(2):188–196. doi: 10.1080/00330124.2011.583586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan L, Hart LG, Goodman DC. Geographic access to health care for rural Medicare beneficiaries. J Rural Health. 2006;22(2):140–146. doi: 10.1111/j.1748-0361.2006.00022.x. [DOI] [PubMed] [Google Scholar]
- 19.Strong M, Green A, Goyder E, et al. Accuracy of diagnosis and classification of COPD in primary and specialist nurse-led respiratory care in Rotherham, UK: a cross-sectional study. Prim Care Respr J. 2014;23(1):67–73. doi: 10.4104/pcrj.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White P, Wong W, Fleming T, Gray B. Primary care spirometry: test quality and the feasibility and usefulness of specialist reporting. Br J Gen Pract. 2007;57(542):701–705. [PMC free article] [PubMed] [Google Scholar]
- 21.Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155(3):179–191. doi: 10.7326/0003-4819-155-3-201108020-00008. [DOI] [PubMed] [Google Scholar]
- 22.Heins-Nesvold J, Carlson A, King-Schultz L, Joslyn KE. Patient identified needs for a chronic obstructive pulmonary disease versus billed services for care received. Int J COPD. 2008;3(3):415–421. doi: 10.2147/copd.s1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenblatt RA, Hart LG, Baldwin L, Chan L, Schneeweiss R. The generalist role of specialty physicians: Is there a hidden system of primary care? JAMA. 1998;279(17):1364–1370. doi: 10.1001/jama.279.17.1364. [DOI] [PubMed] [Google Scholar]
- 24.Regueiro CR, Hamel MB, Davis RB, Desbiens N, Connors AF, Phillips RS. A comparison of generalist and pulmonologist care for patients hospitalized with severe chronic obstructive pulmonary disease: resource intensity, hospital costs, and survival. Am J Med. 1998;105(5):366–372. doi: 10.1016/s0002-9343(98)00290-3. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Aymerich J, Escarrabill J, Marrades RM, Monso E, Barreiro E, Anto JM. Differences in COPD care among doctors who control the disease: general practitioner vs. pneumologist. Respir Med. 2006;100(2):332–339. doi: 10.1016/j.rmed.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 26.Diaz-Fuentes G, Lakshmi V, Sy S, Escalera E. Chronic obstructive pulmonary disease: comparison of care by specialists and generalists in an inner-city hospital. Internet J Pulmonary Med. 2004;5:1. [Google Scholar]
- 27.Davis KJ, Landis SH, Oh YM, et al. Continuing to confront COPD international physician survey: physician knowledge and application of COPD management guidelines in 12 countries. Int J COPD. 2014;10:39–55. doi: 10.2147/COPD.S70162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barr RG, Celli BR, Martinez FJ, et al. Physician and patient perceptions in COPD: The COPD resource network needs assessment survey. Am J Med. 2005;118(12):1415.e9–1415.e17. doi: 10.1016/j.amjmed.2005.07.059. [DOI] [PubMed] [Google Scholar]
- 29.Yawn BP, Wollan PC. Knowledge and attitudes of family physicians coming to COPD continuing medical education. Int J COPD. 2008;3(2):311–317. doi: 10.2147/copd.s2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salinas GD, Williamson JC, Kalhan R, et al. Barriers to adherence to chronic obstructive pulmonary disease guidelines by primary care physicians. Int J COPD. 2011;6:171–179. doi: 10.2147/COPD.S16396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perez X, Wisnivesky JP, Lurslurchachai L, Kleinman LC, Kronish IM. Barriers to adherence to COPD guidelines among primary care providers. Respiratory Med. 2012;106(3):374–381. doi: 10.1016/j.rmed.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Openshaw S. Ecological fallacies and the analysis of areal census data. Environ Plan A. 1984;16(1):17–31. doi: 10.1068/a160017. [DOI] [PubMed] [Google Scholar]
- 33.Bindman AB. Using the National Provider Identifier for health care workforce evaluation. Medicare Medicaid Res Rev. 2013;3(3) doi: 10.5600/mmrr.003.03.b03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zwerink M, Brusse-Keizer M, Van der Valk PD, et al. Self management for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;3 doi: 10.1002/14651858.CD002990.pub3. CD002990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bolton CE, Waters CS, Peirce S, Elwyn G. Insufficient evidence of benefit: a systematic review of home telemonitoring for COPD. J Eval Clin Pract. 2011;17(6):1216–1222. doi: 10.1111/j.1365-2753.2010.01536.x. [DOI] [PubMed] [Google Scholar]
- 36.Goldstein RS, O’Hoski S. Telemedicine in COPD: time to pause. Chest. 2014;145(5):945–949. doi: 10.1378/chest.13-1656. [DOI] [PubMed] [Google Scholar]
- 37.Pollack CE, Lemke KW, Roberts E, Weiner JP. Patient sharing and quality of care: measuring outcomes of care coordination using claims data. Med Care. 2015;53(4):317–323. doi: 10.1097/MLR.0000000000000319. [DOI] [PMC free article] [PubMed] [Google Scholar]