Abstract
Background
Epidemiologic studies suggest phthalate metabolite concentrations are associated with type 2 diabetes. GDM is a strong risk factor for type 2 diabetes. Little is known about phthalates and GDM risk factors (i.e. 1st trimester body mass index (BMI), gestational weight gain (GWG), and 2nd trimester glucose levels).
Methods
A total of 350 women participating in Lifecodes pregnancy cohort (Boston, MA), delivered at term and had pregnancy urinary phthalate metabolite concentrations. Nine specific gravity-adjusted urinary phthalate metabolites were evaluated. General linear regression was used to assess associations between quartiles of phthalate metabolites and continuous 1st trimester BMI and late 2nd trimester blood glucose. Linear mixed models were used for total GWG. Multivariable logistic regression was used for phthalate concentrations and categorized GWG and impaired glucose tolerance defined as glucose ≥ 140mg/dL based on a 50-gram glucose load test. Models were adjusted for potential confounders.
Results
There were no associations between 1st trimester urinary phthalate metabolite concentrations and 1st trimester BMI. Mono-ethyl phthalate (MEP) concentrations averaged across pregnancy were associated with a 2.17 increased odds of excessive GWG (95% CI: 0.98, 4.79). Second trimester MEP was associated with an increased odds of impaired glucose tolerance (adj. OR: 7.18; 95% CI: 1.97, 26.15). Di-2-ethylhexyl phthalate metabolite concentrations were inversely associated with impaired glucose tolerance (adj. OR: 0.25; adj. 95% CI: 0.08, 0.85).
Conclusions
Higher exposure to di-ethyl phthalate, the parent compound of MEP, may be associated with excessive GWG and impaired glucose tolerance; higher di-2-ethylhexyl phthalate was associated with reduced odds of impaired glucose tolerance.
Keywords: Phthalates, Gestational diabetes, Body mass index, Gestational weight gain, Impaired glucose tolerance, Pregnancy
1. Introduction
The incidence of gestational diabetes mellitus (GDM), traditionally defined as any type of glucose intolerance that first appears in pregnancy, has tripled in the last 20 years.[1–3] In fact, GDM now occurs in 7% of all pregnancies worldwide, with an incidence of up to 14% in some populations. As such, GDM is one of the most common complications of pregnancy.[1] Risk factors of GDM include pre-pregnancy obesity[4, 5] and high gestational weight gain (GWG) in early pregnancy.[6, 7] Elevated glucose levels in pregnancy are a hallmark of GDM, which is attributed to insufficient insulin production and glucose intolerance that results in hyperglycemia in pregnancy.[1, 8] Even among women without overt GDM, elevated glucose levels in pregnancy have been linked to adverse pregnancy outcomes.[9, 10] While lifestyle factors are involved with many GDM risk factors (i.e. pre-pregnancy obesity, GWG, and elevated glucose levels during pregnancy), a growing body of evidence suggests that environmental chemicals may also be involved in obesity, weight gain, and elevated glucose levels in non-pregnant populations.[11–14] Yet, few studies have evaluated the question of whether environmental chemical exposures during pregnancy could impact these factors.
Phthalates are one class of chemicals with evidence suggesting associations with obesity, weight gain, and elevated glucose levels in non-pregnant populations.[15–18] This class of environmental chemicals are ubiquitous and found in a variety of consumer products, including cosmetics and other personal care products.[19, 20] These chemicals are thought to increase the risk of obesity and alter glucose levels through their ability to bind to human proliferator activated receptors (PPAR) alpha and gamma.[21] By binding to PPAR alpha and gamma, phthalates may modulate target genes leading to alterations in hormones associated with adipogenesis and glucose metabolism.[21] While studies are still somewhat inconclusive on the associations for phthalates with obesity and diabetes,[22] some evidence suggest associations between higher BMI and elevated levels of mono-butyl phthalate (MBP) and mono(2-ethylhexyl) phthalate (MEHP), metabolites of di-butyl phthalate and di-2-ethylhexyl phthalate (DEHP), respectively.[23] A prospective cohort study of non-pregnant women found an association between more rapid weight gain and higher levels of MBP and mono-benzyl phthalate (MBzP), the latter being a metabolite of di-benzyl phthalate.[24] Also, a study found an association between these same, and several other phthalate metabolites and elevated glucose and insulin levels in non-pregnant women without diabetes.[15] Interestingly, one cross-sectional NHANES study found a 50%–100% increased odds of type 2 diabetes (T2DM) in non-pregnant women with higher concentrations of monobutyl phthalate (MBP), monobenzyl phthalate (MBzP), mono-3(carboxypropyl) phthalate, and di-2-ethylhexyl phthalate, in a representative sample of the U.S. population.[16] Given these findings in non-pregnant populations, evaluating whether these chemicals could impact body mass index, gestational weight gain, and glucose levels in pregnant populations has implications for future maternal and child health.
Only one study to our knowledge has evaluated phthalates and GDM and impaired glucose tolerance risk, finding that in ~2,000 women there was little association when evaluating urinary phthalate metabolites in early first trimester and risk of GDM assessed between second and third trimesters.[25] However, this study only assessed the exposure to phthalates, a non-persistent chemical, at one time-point. Also, they evaluated the association between urinary phthalate metabolites and overt GDM, without assessing risk factors related to GDM or actual glucose levels to determine whether these chemicals had an effect on GDM risk factors or elevated glucose levels in pregnancy.[25] Evaluating the relationship of these risk factors with urinary phthalate metabolites is important, given that pre-pregnancy obesity confers a 2- to 3-fold increased risk of GDM, while excessive GWG in early pregnancy is associated with an ~70% increased risk of GDM.[26, 27]
To this end, we evaluated the associations between urinary phthalate metabolites and risk factors associated with GDM. These associations were evaluated in a subset of a large multi-racial/ethnic U.S. pregnancy cohort. Specifically, we assessed first trimester urinary phthalate metabolites and first trimester BMI; average phthalate metabolite concentrations with period-specific and total GWG; and first and second trimester phthalate metabolite concentrations with later second trimester glucose levels.
2. Methods
2.1 Study population
Starting in 2006, the Lifecodes pregnancy cohort recruited pregnant women during the first trimester of pregnancy (at a median of 10 weeks gestation). (28) For inclusion into the cohort, all women had to plan to deliver at Brigham and Women’s Hospital (Boston, MA), be <15 weeks gestation at entry into the cohort, and could not be pregnant with >3 fetuses. Lifecodes study participants provided blood and urline samples at 4 different study visits. Participants completed a questionnaire, which queried sociodemographic and lifestyle factors.[28]
A nested case-control study was started in 2011 based on women who delivered between 2006 and 2008. Specific details of the case-control study have been previously published. Our study population was comprised of the control population from the nested case-control study, specifically those women who delivered at term (>37 weeks gestation; n=350 women).[29] Term births were selected for this study, to be able to assess the full-course of pregnancy, particularly for GDM-related factors, such as GWG. All women provided informed consent. This study was approved by the Partners Human Subjects Committee at Brigham and Women’s Hospital, and the University of Michigan’s Health Sciences Institutional Review Board.
2.2 Urinary Phthalate Metabolites
Spot urine samples were collected at the time of clinic visit and stored at −20C at the following median gestation weeks: visit 1: 9.9 weeks gestation; visit 2: 17.9 weeks gestation; visit 3: 26.1 weeks gestation; and visit 4: 35.3 weeks gestation. Among the 350 women, most had available data at all 4 time points, with 99% of women having samples available at visit 1; 87% at visit 2; 86% at visit 3, and 90% at visit 4.
A total of nine commonly studied phthalate metabolites were measured, specifically, MBP (metabolite of dibutyl phthalate), mono-ethyl phthalate (MEP) (metabolite of diethyl phthalate), mono-isobutyl phthalate (MiBP) (metabolite of diisobutyl phthalate), MBzP (metabolite of benzyl butyl phthalate), mono-(3-carboxypropyl) phthalate (MCPP) (metabolite of di-n-octyl phthalate), MEHP, Mono-(2-ethyl-5-carboxypentyl) phthalate (MECPP), mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP), and mono-2-ethyl-5-oxohexyl phthalate (MEOHP) (these latter 4 are metabolites of di-2-ethylhexyl phthalate). Due to the high degree of correlation between DEHP metabolites,[29] we used a summary measure of DEHP based on the sum of the molar concentrations of four DEHP metabolites (MEHHP, MECPP, MEOHP, and MEHP). All phthalate metabolites were analyzed by National Science Foundation International, Inc. (Ann Arbor, MI) using protocol from the Centers for Disease Control and Prevention described elsewhere.[29, 30] Specifically, solid phase extraction and high performance liquid chromatography were used, along with tandem mass spectrometry.[29, 30] The limits of detection were in the low ranges; however, when levels were below the limit of detection, we used the standard method of dividing the limit of detection by the square root of two to assign values.[31]
We used specific gravity to account for urine concentration. We used the formula: Pc=P[(1.015−1)/SG−1] to account for urine volume.[29, 32] For this, Pc is the SG-adjusted concentration, P is the measured urinary concentration, SG is the specific gravity for the individual sample and 1.015 is the median SG over all samples.[4, 19] We excluded urine samples with SG>1.04, as these urines are outside of normal range of specific gravity (n=2 women).[33] For these analyses of phthalate metabolites, we evaluated each phthalate metabolite, as well as the DEHP summary measure, in quartiles based on the study population’s distribution. The lowest concentration (first quartile) was considered the referent category for all analyses. As an exploratory analyses, we assessed phthalate mixtures. First, we constructed a summary phthalate variable to evaluate those phthalates with anti-androgenic activity (i.e. molar sum of MBP, MBzP, MiBP, MEHHP, MECPP, MEOHP, and MEHP). Second, we constructed a summary phthalate variable to evaluate those phthalates whose source is primarily personal care products (i.e. molar sum of MEP and MBP). [34–36]
2.3 Outcomes
All outcome data was collected from study participants’ medical records. The specific outcomes of interests were first trimester BMI, period-specific and total GWG, and second trimester glucose levels.
2.3.1. First trimester BMI
We utilized first trimester BMI as a proxy of pre-pregnancy BMI. Data on BMI in first trimester was collected from the study participant’s medical record. Height and weight were abstracted and BMI was calculated as weight in kg/height in meters2. First trimester BMI was measured continuously (kg/m2), as well as categorized based on the National Heart Lung and Blood Institute’s criteria: <25 (reference), 25–<30, and 30+ (kg/m2).
2.3.2. GWG
GWG was defined as the difference between weight at a particular time point in pregnancy compared to weight at first prenatal visit. We defined total GWG as the difference between weight at delivery compared to first prenatal visit. Period-specific weight gain was defined as the difference between weight at a specified period compared to weight at the first prenatal visit. GWG was evaluated continuously, as well as by inadequate, adequate, and excessive total GWG based on the Institute of Medicine guidelines, which incorporate pre-pregnancy BMI. For this, we used first trimester BMI to categorize women into the appropriate categories.
2.3.3. Glucose levels in second and third trimesters
All study participants underwent a non-fasting 50-gram glucose load test (GLT) as a part of screening for GDM. This non-fasting test is the first step in determining whether a woman might have GDM based on the Carpenter-Coustan criteria for GDM diagnosis (the protocol and criteria used at Brigham and Women’s Hospital).[1] While the test is non-fasting, the GLT is a clinically-relevant tool used to determine glucose intolerance in pregnancy, with women having a glucose level ≥140mg/dL requiring further screening for possible GDM. For this study, continuous glucose levels were assessed. In addition, we evaluated categorized glucose levels, which for purposes of this study we defined this categorical glucose measure as impaired glucose tolerance if the GLT glucose value was ≥140mg/dL v. <140mg/dL. Due to small numbers, we did not assess GDM diagnosis with above-mentioned criteria (n=21 women); however these women were included in the group with impaired glucose tolerance (n=47 women). Based on screening test alone, among the 47 women with GLT glucose values ≥140mg/dL, 17 (36%) went on to be diagnosed with GDM.
2.3.4. Covariates
Based on risk factors of GDM and known predictors of urinary phthalate metabolite concentrations, we posited that maternal age,[37] race/ethnicity,[3] education,[38] smoking status,[39, 40] and alcohol use[38] were potential confounders for all three associations being tested (i.e. first trimester urinary phthalate metabolite levels and first trimester BMI; urinary phthalate metabolite levels and GWG; and first and early second trimester urinary phthalate metabolites and glucose levels in late second trimester). Maternal age was assessed categorically as <25, 25–<30 (reference), 30–<35, >=35. Race/ethnicity was evaluated as non-Hispanic white (reference), non-Hispanic black, Hispanic, Asian, unknown/other. We assessed maternal education as high school or less, technical school or some college, college graduate or higher (reference). Smoking status was categorized as current, past versus never (reference). Alcohol use during pregnancy was categorized as yes versus no (reference).
2.4. Statistical analysis
We evaluated the first trimester (baseline) distributions (median: 9.9 weeks gestation) of each phthalate metabolite by calculating SG-adjusted geometric means and 25th and 75th percentiles. To cross-sectionally assess the association between first trimester urinary phthalate metabolite concentrations and first trimester BMI, we used general linear regression. Least squares means of BMI were calculated for each quartile of phthalate metabolites and trend tests were conducted using the median metabolite level in each quartile as a continuous variable.
To longitudinally evaluate the association between urinary phthalate metabolite concentrations and GWG at specific time points, we evaluated weight gain during each period since the last phthalate measurement. Time periods consisted of 4 potential time points for this analysis (i.e. median times for visits 1–4: 9.9 weeks, 17.9 weeks, 26.1, and 35.3 weeks gestation). Since both maternal weight and urinary phthalate metabolites were measured on pregnant women multiple times, these weight trajectories were assessed in relation to corresponding period-specific phthalate exposure in quartiles using linear mixed model. This method allowed us to evaluate associations by incorporating information from the previous time points of phthalate concentrations in order to assess period-specific weight gain. An interaction term between period indicators and quartiles of phthalate metabolites was added to the model to examine whether GWG across pregnancy differed by urinary phthalate metabolite concentrations. Least squares means of weight gain were estimated at each time period for each phthalate quartile. In addition, we explored categorized GWG. We used multinomial logistic regression to estimate the odds of excess or inadequate GWG (versus adequate weight gain) across quartiles of average phthalate metabolite levels during pregnancy.
For the prospective association between urinary phthalate metabolites and second trimester glucose levels, we first examined the association of urinary phthalate metabolites at a median of 9.9 and 17.9 weeks gestation and continuous glucose levels based on the 50-gram GLT at 24–28 weeks gestation in separate models. We had an a priori hypothesis that the normal physiology of pregnancy, with its increasing insulin resistant state, could lead to sensitive windows for environmental exposures that are associated with glucose levels. Thus, first trimester and early second trimester were selected to evaluate possible sensitive periods, as well as to establish temporality between our exposure and the late second trimester glucose outcome. Using general linear regression, we calculated least squares means of glucose for each quartile of phthalate, as well as trend tests. As a secondary analysis, we used multivariable logistic regression to evaluate the association between first and early second trimester urinary phthalate metabolite concentrations and odds of glucose intolerance; we present only second trimester as a main finding of this secondary analysis. We calculated odds ratios and 95% confidence intervals.
The following potential confounders were assessed based on a priori hypothesized associations and if they altered the β for at least one of the urinary phthalate metabolites by more than 10%. For example, if the addition of education to the model evaluating the association between MEP and first trimester BMI resulted in a change in the β for MEP that was >10%, then education was included in all models for the association between phthalates and first trimester BMI. As such, the following variables were considered as potential confounders: age, race/ethnicity, education, alcohol consumption during pregnancy, and smoking status. We constructed two models: model 1 was unadjusted, while model 2 was multivariable adjusted for maternal age (<25, 25–30, 30–35, 35–40, >40), race/ethnicity (White, African-American, Asian, Hispanic, Other/Unknown), education (high school or less, technical school, some college, college graduates or higher), alcohol consumption during pregnancy (yes, no), and smoking status (never, past, current). For the analyses with GWG and GLT glucose levels as primary outcomes, first trimester BMI was added to model 2. As a sensitivity analysis, we also co-adjusted for all phthalate metabolites in a single model to evaluate the robustness of the associations found between certain urinary phthalate metabolites and GDM risk factors.
For the exploratory analysis of phthalate mixtures (i.e. the summary of anti-androgenic phthalates and summary of phthalates with a primary source of personal care products), we utilized the same set of models specified for each GDM outcome with adjustment for the same set of potential confounders. All analyses were performed with SAS 9.4 (SAS Institute Inc., Cary, NC). We hypothesized that higher concentrations of specific phthalate metabolites, namely those previously found to be associated with increased insulin resistance in non-pregnant populations (i.e. MBzP, MCPP, and metabolites of DEHP)[15, 16, 18] would be associated with increased first trimester BMI, GWG, and elevated glucose levels in pregnancy. Further, we hypothesized that early second trimester might be a sensitive time period for phthalate exposure as it related to late second trimester glucose levels.
3. Results
Table 1 describes the sociodemographic and GDM risk factors for the study population. On average, the study population was 31.9 years of age, most were college-educated (86.6%), never smokers (94.6%), and did not use alcohol during pregnancy (94.6%). The mean first trimester maternal BMI was 25.9, with 46% of women being overweight or obese. For GWG about one-third of women had inadequate, adequate, and excessive GWG based on IOM guidelines. Weight gain in the first trimester had a median of 2.3kg, while GWG was similar in second and third trimesters at 4.5kg. Median glucose levels based on the second trimester 50-gram glucose load testing was 106mg/dL, with a range from 62mg/dL to 213mg/dL. A total of 47 women (16.0%) had glucose levels from the GLT≥140 mg/dL, which for this analysis is considered impaired glucose tolerance.
Table 1.
Urinary phthalate metabolite levels at baseline by study population characteristics
| MEP | MnBP | MiBP | MBzP | MCPP | ∑DEHP | ||
|---|---|---|---|---|---|---|---|
| SG-adjusted geometric means (25th percentiles, 75th percentiles) | |||||||
| Maternal age | N (%)1 | ||||||
| <25 yrs | 42 (12) | 319.2 (92.5,1042.) |
27.3 (16.5, 39.2) |
10.1 (6.9, 16.0) |
15.5 (7.0, 35.9) |
2.4 (1.2, 3.9) |
0.3 (0.2, 0.6) |
| 25 –<30 yrs | 68 (19) | 147.9 (58.9, 357.0) |
17.1 (10.8, 26.8) |
7.7 (4.7, 12.3) |
8.4 (3.6, 15.8) |
2.0 (1.2, 2.9) |
0.4 (0.2, 0.7) |
| 30 –<35 yrs | 138 (39) | 112.4 (45.4, 243.2) |
15.8 (9.9, 22.2) |
6.9 (4.2, 10.7) |
6.2 (3.3, 12.9) |
2.1 (0.9, 3.6) |
0.5 (0.2, 0.8) |
| ≥ 35 yrs | 102 (29) | 114.0 (41.0, 295.7) |
15.6 (10.6, 22.4) |
6.3 (4.3, 9.9) |
5.0 (2.7, 8.1) |
2.1 (1.1, 2.6) |
0.4 (0.2, 1.0) |
| Race/ethnicity | |||||||
| Caucasian | 206 (59) | 98.7 (41.0, 240.0) |
15.5 (10.5, 21.2) |
6.0 (4.2, 9.6) |
5.4 (3.1, 9.1) |
2.0 (1.0, 3.1) |
0.4 (0.2, 0.8) |
| African- American |
55 (16) | 286.0 (136.6, 468.3) |
22.1 (11.8, 39.1) |
10.2 (6.0, 17.3) |
10.2 (4.2, 24.5) |
3.2 (1.3, 5.6) |
0.4 (0.2, 0.8) |
| Hispanic | 50 (14) | 247.4 (72.5, 630.0) |
21.7 (14.1, 33.9) |
8.6 (4.5, 14.2) |
11.6 (4.9, 23.9) |
2.0 (0.9, 2.9) |
0.4 (0.2, 0.7) |
| Unknown/Other | 39 (11) | 116.2 (30.8, 277.5) |
14.9 (10.1, 24.9) |
9.2 (5.7, 15.4) |
7.7 (3.0, 15.5) |
1.7 (1.0, 2.6) |
0.3 (0.2, 0.5) |
| Education | |||||||
| Less than HS | 47 (13) | 328.6 (104.5, 1035.9) |
28.3 (17.3, 44.9) |
10.2 (7.2, 15.4) |
16.9 (6.8, 38.4) |
2.9 (1.3, 4.0) |
0.3 (0.1, 0.5) |
| Technical school/some college |
152 (43) | 135.9 (50.8, 322.5) |
16.5 (10.8, 24.3) |
6.7 (4.1, 11.9) |
6.3 (3.0, 13.4) |
2.0 (0.9, 3.1) |
0.4 (0.2, 0.7) |
| College graduate or higher |
151 (43) | 101.4 (40.9, 254.3) |
15.2 (9.8, 21.3) |
6.9 (4.6, 10.0) |
5.7 (3.3, 8.7) |
1.9 (1.0, 2.7) |
0.5 (0.2, 1.2) |
| Alcohol use during pregnancy | |||||||
| No | 331 (95) | 135.9 (47.1, 345.0) |
17.2 (10.9, 25.5) |
7.1 (4.5, 11.1) |
7.0 (3.4, 13.7) |
2.0 (1.0, 3.0) |
0.4 (0.2, 0.8) |
| Yes | 19 (5) | 119.0 (55.1, 148.9) |
15.5 (9.7, 32.2) |
8.4 (3.9, 25.3) |
5.3 (3.5, 7.0) |
3.1 (1.1, 3.6) |
1.2 (0.5, 3.0) |
| Smoking status | |||||||
| Never | 331 (95) | 132.5 (47.2, 328.6) |
16.8 (10.8, 25.3) |
7.1 (4.4, 11.1) |
6.6 (3.3, 12.4) |
2.0 (1.0, 2.9) |
0.4 (0.2, 0.8) |
| Past | 8 (2) | 188.2 (48.9, 1039.4) |
19.9 (7.7, 37.5) |
9.7 (4.0, 22.2) |
17.5 (6.0, 49.2) |
2.9 (1.8, 4.6) |
0.3 (0.2, 0.9) |
| Current | 11 (3) | 183.4 (75.6, 470.2) |
27.0 (16.2, 61.2) |
8.0 (6.5, 10.2) |
14.2 (6.9, 26.7) |
3.2 (1.4, 5.9) |
0.4 (0.2, 0.8) |
| Maternal BMI | |||||||
| <25 | 187 (54) | 113.0 (42.5, 295.7) |
16.7 (10.5, 25.1) |
7.0 (4.4, 10.5) |
6.3 (3.1, 13.2) |
1.9 (0.9, 2.7) |
0.4 (0.2, 0.8) |
| 25–<30 | 94 (27) | 132.8 (45.8, 277.5) |
15.3 (10.8, 23.5) |
7.6 (4.6, 13.6) |
6.6 (3.2, 10.2) |
2.0 (1.1, 3.1) |
0.5 (0.2, 1.1) |
| >-30 | 65 (19) | 221.1 (78.3, 551.6) |
20.9 (12.0, 28.9) |
7.4 (5.6, 11.6) |
9.1 (5.1, 15.8) |
2.6 (1.2, 3.9) |
0.5 (0.2, 0.7) |
| GWG | |||||||
| Adequate | 121 (36) | 118.7 (45.4, 246.2) |
17.0 (11.0, 25.3) |
7.1 (4.4, 10.8) |
7.0 (3.6, 14.8) |
2.1 (1.0, 3.1) |
0.5 (0.2, 1.0) |
| Excess | 112 (33) | 139.5 (55.5, 364.4) |
16.6 (10.7, 23.7) |
7.1 (4.5, 11.8) |
6.9 (3.5, 11.5) |
2.0 (1.1, 3.5) |
0.4 (0.2, 0.8) |
| Inadequate | 107 (31) | 145.4 (48.7, 434.2) |
17.4 (10.9, 27.1) |
7.4 (4.4, 11.1) |
6.7 (2.9, 15.1) |
2.1 (0.9, 2.8) |
0.4 (0.2, 0.7) |
| Impaired glucose tolerance | |||||||
| GLT ≥140 mg/dL |
47 | 157.1 (49.0, 323.5) |
16.1 (9.7, 25.1) |
7.2 (4.4, 11.8) |
5.9 (3.6, 13.6) |
1.8 (0.9, 2.8) |
0.5 (0.2, 1.4) |
| GLT <140 mg/dL |
251 | 132.9 (49.2, 324.5) |
17.6 (11.0, 26.9) |
7.3 (4.5, 11.9) |
7.4 (3.3, 14.8) |
2.3 (1.1, 3.6) |
0.4 (0.2, 0.8) |
Number may not sum up to 350 due to missing values
Abbreviations: BMI—body mass index; GWG—gestational weight gain; GLT—glucose load test
Table 1 also provides information on baseline urinary phthalate metabolite concentrations by sociodemographic and lifestyle factors. Younger and less educated women had higher concentrations of MEP, MnBP, MiBP. Non-whites had higher concentrations of MEP, MnBP, and MBzP. Women who did not use alcohol during pregnancy had slightly higher MEP concentrations. Women who were ever smokers or who were obese had higher concentrations of MEP, MnBP, and MBzP. While a greater proportion of those without impaired glucose tolerance based on the GLT had lower MBzP concentrations, more women with higher concentrations of MEP had impaired glucose tolerance, as well as excessive or inadequate weight gain.
3.1. Phthalate metabolite concentrations and BMI in first trimester
In table 2, we present the cross-sectional findings of the SG-adjusted urinary phthalate metabolite concentrations at baseline (median 9.9 weeks gestation) and first trimester BMI (measured at the same time period). In the unadjusted models, we found a significant linear trend for higher BMI among those women with the highest concentrations of MEP and MBzP. However, after adjustment, associations did not remain. In particular, adjustment for maternal race/ethnicity appeared to be the primary driver for this attenuation of associations for 1st trimester MEP and 1st trimester BMI. On the other hand, maternal education had the most impact on the attenuation of the association between MBzP and BMI.
Table 2.
Association of body mass index with quartiles of phthalate metabolites at baseline
| SG-adjusted phthalate metabolites |
Median1 | Least squares means of baseline body mass index Overall population |
|
|---|---|---|---|
| Unadjusted | Multivariable2 | ||
| MEP | |||
| Q1 | 27.5 | 24.4 (23.2, 25.6) | 26.2 (24.0, 28.4) |
| Q2 | 74.0 | 26.1 (24.9, 27.3) | 27.5 (25.2, 29.8) |
| Q3 | 181.8 | 26.5 (25.3, 27.7) | 26.8 (24.7, 29.0) |
| Q4 | 818.5 | 27.0 (25.8, 28.2) | 27.6 (25.4, 29.8) |
| p for trend | <0.01 | 0.17 | |
| MnBP | |||
| Q1 | 7.9 | 25.5 (24.3, 26,7) | 26.9 (24.7, 29.2) |
| Q2 | 13.2 | 24.9 (23.7, 26.1) | 26.4 (24.1, 28.6) |
| Q3 | 19.8 | 26.6 (25.4, 27.8) | 27.2 (25.0, 29.4) |
| Q4 | 40.3 | 26.9 (25.7, 28.1) | 27.2 (25.0, 29.4) |
| p for trend | 0.04 | 0.51 | |
| MiBP | |||
| Q1 | 3.3 | 25.5 (24.3, 26.7) | 26.7 (24.5, 29.0) |
| Q2 | 5.6 | 25.2 (24.0, 26.5) | 26.6 (24.4, 28.9) |
| Q3 | 8.9 | 26.2 (24.9, 27.4) | 27.1 (24.9, 29.3) |
| Q4 | 17.4 | 27.0 (25.8, 28.2) | 27.2 (25.0, 29.4) |
| p for trend | 0.05 | 0.48 | |
| MBzP | |||
| Q1 | 2.2 | 24.6 (23.4, 25.9) | 26.0 (23.7, 28.3) |
| Q2 | 4.3 | 25.7 (24.5, 26.9) | 26.8 (24.5, 29.1) |
| Q3 | 8.6 | 26.6 (25.4, 27.8) | 27.7 (25.5, 29.9) |
| Q4 | 26.3 | 27.0 (25.8, 28.2) | 26.9 (24.8, 29.0) |
| p for trend | <0.01 | 0.51 | |
| MCPP | |||
| Q1 | 0.69 | 24.9 (23.7, 26.1) | 26.1 (23.8, 28.4) |
| Q2 | 1.28 | 25.6 (24.4, 26.8) | 26.5 (24.3, 28.7) |
| Q3 | 2.02 | 26.8 (25.6, 28.0) | 27.5 (25.3, 29.8) |
| Q4 | 7.27 | 26.6 (25.4, 27.8) | 27.4 (25.3, 29.5) |
| p for trend | 0.04 | 0.11 | |
| ∑DEHP | |||
| Q1 | 0.12 | 25.4 (24.1, 26.6) | 26.2 (24.0, 28.3) |
| Q2 | 0.24 | 26.4 (25.2, 27.6) | 27.3 (25.1, 29.5) |
| Q3 | 0.53 | 26.4 (25.2, 27.6) | 27.5 (25.2, 29.7) |
| Q4 | 2.09 | 25.8 (24.5, 27.0) | 27.2 (25.0, 29.4) |
| p for trend | 0.83 | 0.33 | |
µmol/L for the two DEHP measures, and µg/L for the other phthalate metabolites
Adjusted for maternal age, race/ethnicity, education, smoking, alcohol drinking
3.2. Phthalate metabolite concentrations and GWG
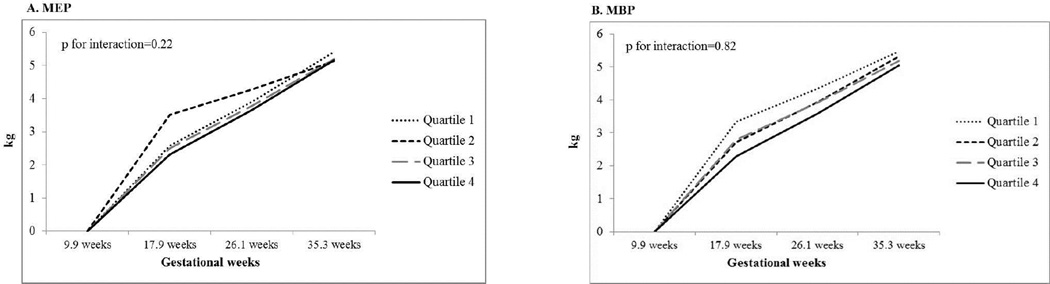
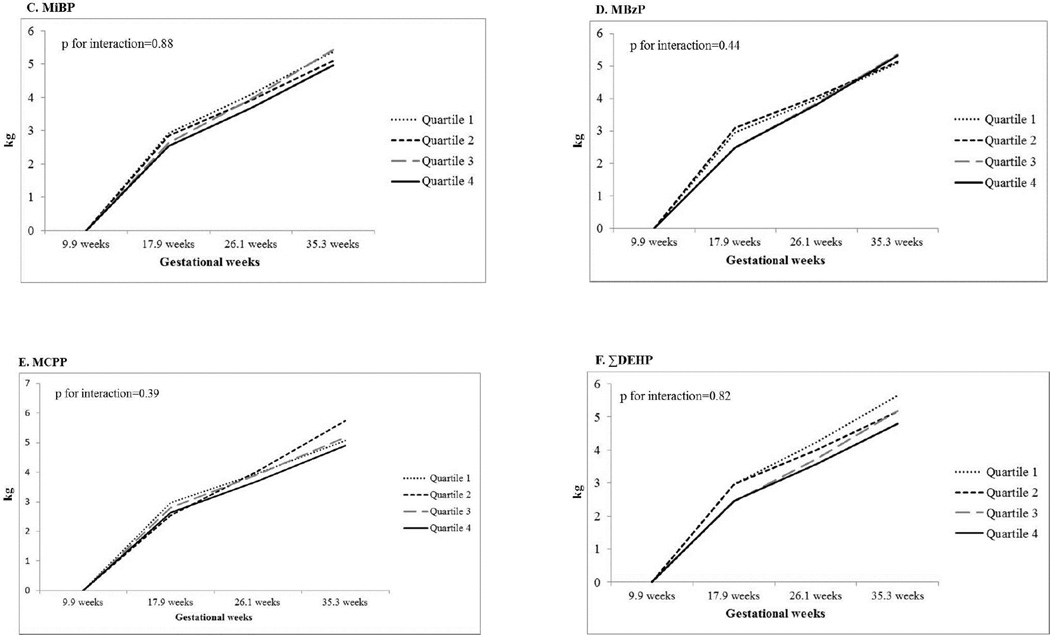
Figure 1, panels A–F, presents the longitudinal associations between SG-adjusted urinary phthalate metabolite concentrations across pregnancy and the period-specific weight gain during pregnancy. All phthalate metabolites, including the summary measure of the DEHP metabolites, showed little evidence for an association with period-specific continuous GWG. When exploring categorical weight gain based on IOM guidelines, we found women with higher average concentrations of MEP across pregnancy had over a 2-fold increased odds of excessive GWG relative to women with the lowest concentrations of MEP (Supplemental Table 1). This association was non-monotonic with the second and fourth quartiles having higher odds of excess GWG compared to the lowest quartile. Adjustment for maternal age, race/ethnicity, education, smoking, alcohol drinking and baseline BMI slightly attenuated the odds of excessive GWG (adj. OR for Q2: 2.09; 95% CI: 0.96, 4.53 and adj. OR for Q4: 2.17; 95% CI: 0.98, 4.79). Higher average concentrations of MnBP were associated with a reduced odds of excessive GWG. Women in the highest quartile had 0.39 the odds of excessive GWG compared to women in the lowest quartile (95% CI: 0.17, 0.88).
Figure 1.
Multivariate-adjusted least squares means of the association between average phthalate metabolite concentrations and period-specific gestational weight gain during pregnancy and
3.3. Phthalate metabolite concentrations and blood glucose levels
In Table 3, we present the longitudinal associations between SG-adjusted urinary phthalate metabolites at two time points (median gestation of 9 weeks and 17 weeks) and blood glucose levels from the GLT conducted at the second or third trimesters (median: 26.1 weeks). Other than an association between 1st trimester SG-adjusted urinary MCPP concentrations and glucose levels, we found little evidence to suggest associations between any of the phthalate metabolites and continuous glucose levels.
Table 3.
Associations of blood glucose levels with quartiles of urinary phthalate metabolites measured at 8–10 and 16–18 gestation weeks
| Measured between 8–10 weeks | Measured between 16–18 weeks | |||
|---|---|---|---|---|
| Phthalate metabolites | Unadjusted | Multivariable2 | Unadjusted | Multivariable2 |
| Least geometric means of blood glucose (mg/dL) | ||||
| MEP | ||||
| Q1 | 111 (105, 117) | 113 (103, 125) | 106 (100, 112) | 109 (100, 120) |
| Q2 | 109 (104, 115) | 114 (103, 125) | 109 (103, 115) | 112 (102, 124) |
| Q3 | 114 (108, 120) | 115 (105, 126) | 108 (102, 114) | 110 (99, 121) |
| Q4 | 105 (99, 110) | 107 (98, 118) | 112 (106, 118) | 116 (105, 128) |
| p for trend | 0.29 | 0.25 | 0.2 | 0.19 |
| MnBP | ||||
| Q1 | 111 (105, 117) | 115 (104, 126) | 107 (102, 113) | 111 (100, 123) |
| Q2 | 110 (105, 116) | 113 (103, 124) | 109 (103, 115) | 113 (103, 125) |
| Q3 | 112 (106, 118) | 114 (103, 125) | 110 (104, 116) | 114 (104, 125) |
| Q4 | 105 (100, 111) | 109 (99, 119) | 108 (102, 114) | 108 (98, 119) |
| p for trend | 0.19 | 0.13 | 0.9 | 0.92 |
| MiBP | ||||
| Q1 | 111 (106, 117) | 114 (103, 125) | 108 (103, 114) | 111 (100, 122) |
| Q2 | 109 (104, 115) | 111 (101, 123) | 107 (101, 112) | 109 (99, 120) |
| Q3 | 110 (105, 116) | 114 (103, 125) | 106 (100, 112) | 110 (100, 121) |
| Q4 | 108 (102, 114) | 110 (100, 121) | 114 (108, 121) | 114 (104, 126) |
| p for trend | 0.46 | 0.43 | 0.19 | 0.17 |
| MBzP | ||||
| Q1 | 105 (100, 111) | 108 (98, 119) | 104 (98, 110) | 106 (96, 118) |
| Q2 | 114 (109, 121) | 118 (108, 130) | 110 (105, 117) | 113 (102, 126) |
| Q3 | 109 (104, 115) | 112 (102, 122) | 112 (106, 118) | 114 (103, 125) |
| Q4 | 110 (104, 115) | 111 (102, 122) | 109 (103, 115) | 110 (101, 121) |
| p for trend | 0.66 | 0.68 | 0.28 | 0.42 |
| MCPP | ||||
| Q1 | 111 (105, 117) | 114 (103, 126) | 108 (102, 114) | 110 (99, 121) |
| Q2 | 113 (107, 119) | 116 (106, 128) | 108 (102, 114) | 110 (100, 121) |
| Q3 | 111 (105, 117) | 112 (102, 124) | 106 (101, 112) | 109 (99, 120) |
| Q4 | 104 (99, 110) | 107 (98, 118) | 113 (107, 119) | 115 (105, 126) |
| p for trend | 0.08 | 0.05 | 0.21 | 0.21 |
| ∑DEHP | ||||
| Q1 | 108 (102, 113) | 110 (100, 120) | 114 (108, 120) | 117 (106, 129) |
| Q2 | 114 (108, 120) | 115 (105, 127) | 107 (101, 113) | 110 (100, 121) |
| Q3 | 105 (100, 111) | 108 (98, 118) | 106 (100, 112) | 109 (99, 120) |
| Q4 | 112 (106, 117) | 114 (104, 126) | 108 (102, 114) | 110 (100, 121) |
| p for trend | 0.72 | 0.49 | 0.23 | 0.16 |
Adjusted for maternal age, race/ethnicity, education, smoking, alcohol drinking and baseline BMI
When evaluating the association between urinary phthalate metabolite concentrations and categorized impaired glucose tolerance (GLT ≥140mg/dL), we only found an association with second trimester and this outcome. (First trimester data not shown). Specifically, we found that women with the highest concentrations of 2nd trimester MEP had a 7-foldincreased odds of impaired glucose tolerance relative to women with the lowest concentrations of MEP (adj. OR: 7.18; 95% CI: 1.97, 26.15) (Table 4). This association became somewhat stronger when we adjusted for gestational age at time of GLT. When exploring phthalate mixtures coming from personal care products (i.e. MEP and MBP), we found even stronger associations, with women in the highest quartile having a 10.7 increased odds of impaired glucose tolerance relative to women in the first quartile (95% CI: 2.63, 43.99). On the other hand, women with the highest 2nd trimester concentrations of ΣDEHP had a significantly reduced odds of impaired glucose tolerance, with this group having 0.25 the odds of impaired glucose tolerance compared to women in the lowest ΣDEHP quartile (95% CI: 0.08, 0.85). Associations were not as strong when evaluating phthalate mixtures with anti-androgenic activity (adj. OR for Q4 v. Q1: 0.50; 95% CI: 0.16, 1.51).
Table 4.
Odds ratios and 95% confidence intervals for 2nd trimester levels of phthalate metabolites in quartiles and impaired glucose tolerance
| Impaired Glucose Tolerance1 | ||
|---|---|---|
| Phthalate metabolites |
Unadjusted | Multivariable2 |
| MEP | ||
| Q1 | Ref | Ref |
| Q2 | 1.93 (0.61, 6.09) | 2.14 (0.57, 8.10) |
| Q3 | 2.18 (0.70, 6.77) | 2.53 (0.71, 8.97) |
| Q4 | 3.59 (1.22, 10.55) | 7.18 (1.97, 26.15) |
| p for trend | 0.02 | <0.01 |
| MBP | ||
| Q1 | Ref | Ref |
| Q2 | 1.00 (0.37, 2.70) | 0.95 (0.31, 2.91) |
| Q3 | 1.13 (0.43, 2.99) | 1.26 (0.43, 3.68) |
| Q4 | 1.27 (0.49, 3.29) | 1.14 (0.37, 3.51) |
| p for trend | 0.58 | 0.74 |
| MiBP | ||
| Q1 | Ref | Ref |
| Q2 | 0.46 (0.15, 1.43) | 0.47 (0.14, 1.58) |
| Q3 | 0.77 (0.28, 2.10) | 1.16 (0.38, 3.54) |
| Q4 | 1.79 (0.75, 4.31) | 1.79 (0.62, 5.16) |
| p for trend | 0.1 | 0.18 |
| MBzP | ||
| Q1 | Ref | Ref |
| Q2 | 1.13 (0.43, 2.99) | 1.17 (0.40, 3.48) |
| Q3 | 1.13 (0.43, 2.99) | 1.16 (0.39, 3.49) |
| Q4 | 1.13 (0.43, 2.99) | 1.28 (0.40, 4.07) |
| p for trend | 0.82 | 0.69 |
| MCPP | ||
| Q1 | Ref | Ref |
| Q2 | 0.57 (0.21, 1.58) | 0.47 (0.14, 1.52) |
| Q3 | 0.88 (0.34, 2.23) | 0.97 (0.34, 2.77) |
| Q4 | 0.98 (0.39, 2.45) | 0.96 (0.34, 2.71) |
| p for trend1 | 0.76 | 0.7 |
| ∑DEHP | ||
| Q1 | Ref | Ref |
| Q2 | 0.64 (0.25, 1.63) | 0.46 (0.15, 1.40) |
| Q3 | 0.73 (0.29, 1.80) | 0.74 (0.27, 2.04) |
| Q4 | 0.48 (0.18, 1.30) | 0.25 (0.08, 0.85) |
| p for trend | 0.2 | 0.06 |
Defined as GLT>140mg/dL from 50-gram, non-fasting glucose load tests as first step in Carpenter-Coustan GDM screening test
Adjusted for age, race/ethnicity, education baseline BMI, alcohol drinking, and smoking
In two additional secondary analyses, we found that MEP and impaired glucose tolerance appeared to be stronger when evaluated in overweight/obese women. On the other hand, stronger associations were seen for ΣDEHP metabolites and impaired glucose tolerance in older women. Mutual adjustment for MEP and ΣDEHP metabolites in the same model yielded similar associations. Further, adjustments for all phthalate metabolites yielded similar associations for DEHP and impaired glucose tolerance, but attenuated associations for MEP and impaired glucose tolerance.
4. Discussion
In this study of pregnancy urinary phthalate metabolites and GDM risk factors (first trimester BMI, GWG, and second or third trimester glucose levels from the GLT), we consistently found an association between higher concentrations of MEP and two of the three GDM risk factors studied. Specifically, higher MEP concentrations were associated excess GWG and significantly increased odds of impaired glucose tolerance (GLT≥140 mg/dL). On the other hand, MnBP, MCPP and ΣDEHP were associated with reduced odds of GDM risk factors. Specifically, higher MnBP was associated with reduced odds of excessive GWG, while higher MCPP and ΣDEHP concentrations were associated with reduced odds of continuous blood glucose and impaired glucose tolerance, respectively. Other urinary phthalate metabolites did not show associations with GDM risk factors. Exploratory mixtures analyses suggested stronger associations for those phthalates whose source was personal care products and anti-androgenic phthalates with impaired glucose tolerance. With phthalates being ubiquitous, these findings suggest the need to further explore the role of phthalates and their impact on excess gestational weight gain and impaired glucose tolerance in pregnancy.
Limited information is available for the association between pregnancy phthalate exposure and risk factors of GDM, specifically, pre-pregnancy BMI, GWG, and pregnancy glucose levels. In non-pregnant populations, several studies have shown conflicting associations between elevated urinary phthalate metabolite concentrations and increased adult BMI.[23, 41, 42] For example, Stahlhut et al only evaluated men in relation to phthalate metabolites and BMI using a representative sample of the U.S. population (National Health and Nutrition Examination survey 1999–2002), finding a positive association between higher phthalate levels and elevated BMI.[42] On the other hand, Hatch al studied women and men between 20 and 59 years of age from the same representative sample of the U.S. population.[41] They found a slight increased association between MEP and MEHHP and BMI, but inverse associations between MBP and MEHP with BMI among non-pregnant women. They also found a null association between MBzP and BMI for this same group of women. Another study by Yaghjyan et al, which was from the same study population, as Hatch et al, but incorporated one additional cycle of NHANES found modest associations between certain phthalate metabolites and BMI in a non-pregnant population.[23, 41] Our lack of associations for phthalate metabolite concentrations and first trimester BMI are somewhat in line with several of the findings from these other studies of non-pregnant women from the U.S. for the associations between urinary phthalate metabolites and BMI.
Few studies have evaluated the association between phthalates and weight gain in women. A recent study in Nurses’ Health Study I and II non-pregnant participants found a modest association between higher urinary levels of MBzP and MnBP and weight gain over a ten-year period of time.[24] In our study, we saw little evidence to suggest that pregnancy phthalate metabolite concentrations were associated with increased cumulative or period-specific continuous GWG in pregnancy. However, we did find that MnBP was associated with a significant monotonic reduction in excess gestational weight gain. These differing findings between the present study and the study conducted in the Nurses’ Health Study cohorts might be attributed to differences in urinary phthalate concentrations in the two study populations, as well as changes in how these chemicals may alter weight gain based on pregnancy state.
Interestingly, we found that MEP was associated with excess GWG based on IOM guidelines for total weight gain across pregnancy. The biological mechanism by which the current study’s finding that MEP is associated with excessive GWG is unclear. While higher MEP has been associated with sex hormone binding globulin and increased testosterone levels in certain populations, these studies were conducted at different time points outside of pregnancy and within all male populations.[43, 44] Future studies will need to further evaluate the possible association between MEP and excessive weight gain during pregnancy, with implications for adverse maternal and child health outcomes.[45, 46]
The association between urinary phthalate metabolites and glucose levels has been explored in the context of non-pregnant populations more extensively.[15, 18] In a previous study, higher concentrations of MiBP, MCPP and ΣDEHP metabolites were associated with elevations in glucose levels.[15] Several studies have reported an association between phthalate metabolite concentrations and diabetes in non-pregnant populations,[16–18] finding associations between certain phthalate metabolites and diabetes. Few studies have evaluated the role of phthalates on glucose-related outcomes in pregnancy, including continuous glucose levels and glucose intolerance. The one recently published study evaluating phthalates and GDM risk found no association,[25] but this study did not evaluate continuous glucose levels. While GDM is an important and clinically-relevant diagnosis, elevated glucose levels in pregnancy, without overt diabetes, is associated with a variety of maternal and child health outcomes, including risk of preeclampsia,[9, 47] depression,[10] and childhood obesity.[9, 48] Furthermore, the only study published on urinary phthalate metabolites and GDM evaluated a one-time measurement in 1st trimester.[25] Timing of exposure may be particularly important as insulin resistance and potential for glucose intolerance increases as pregnancy progresses. More research is needed to understand these possible sensitive windows of environmental chemical exposure and glucose levels. The present study found associations between 2nd trimester MEP and impaired glucose tolerance, but not with continuous glucose. Furthermore, we found an inverse association between higher concentrations of 2nd trimester ΣDEHP and reduced odds of impaired glucose tolerance. This latter finding counters previous studies conducted in non-pregnant populations evaluating diabetes and insulin resistance.[15, 16] Future studies will need to confirm this association with impaired glucose tolerance and evaluate GDM looking across multiple pregnancy time points.
Phthalates are thought to be associated with obesity and glucose levels through their ability to bind to peroxisome proliferator-activated receptor (PPAR) gamma and alpha.[21, 49] While the former involves upregulation of adipocyte production, the latter involves potential changes in lipid handling and beta cell functioning, with important implications for the insulin-resistant state of pregnancy.[50] Little is known about phthalates effect on beta cell functioning in pregnant women. It is possible that the findings of reduced risk of excessive GWG and impaired glucose tolerance with MnBP and ΣDEHP, respectively are attributed to these PPAR-associated pathways. While MnBP and ΣDEHP may operate by binding to PPAR alpha or gamma, di-ethyl phthalate is not known to interact with the PPARs. Several studies suggest that MEP is associated with both sex hormone binding globulin and testosterone levels.[43, 44] Another study suggests that MEP has estrogenic activity.[51] As such, it is possible that MEP could alter GWG or impaired glucose tolerance through hormonal pathways, but these exact mechanisms need to be further investigated.
This study has several limitations. First, we evaluated a healthier subset of a larger pregnancy cohort—those women who had term births. This allowed us to evaluate those factors specifically related to GDM risk, independent of preterm birth status, which has been found to be associated with higher concentrations of certain urinary phthalate metabolites.[29] However, this may have attenuated associations between phthalate metabolites and our outcomes of interest. Furthermore, several of the findings (i.e. impaired glucose tolerance) had wide confidence intervals due to small sample size and a lack of power. Future studies will need to evaluate this question in a larger study population to improve precision. Second, while we have multiple measurements of phthalate metabolites across pregnancy, all measurements are from one-time spot urines at the specified time periods within pregnancy. Studies in pregnant and non-pregnant populations found considerable variability for certain phthalate metabolites within a 24-hour period.[52, 53] This could result in exposure misclassification that could result in attenuation of association if the changes across time were independent of GDM risk factors. However, unlike other studies, we do have multiple measurements and fully utilize this information in our analyses. Third, we were underpowered to evaluate GDM as primary outcome in this analysis. Instead, we evaluated glucose from a 50-gram GLT as a primary outcome and an indicator of glucose intolerance in pregnancy. We present the impaired glucose tolerance based on the GLT screening test and categorized GWG findings in a supplemental table. Fourth, a variety of potential confounders were unavailable, including diet information which was not collected as a part of this cohort study. As such, we were unable to adjust for diet, a source of DEHP exposure. Higher exposure to diets contaminated with DEHP may also be linked to obesity and diabetes. However, in previous studies of phthalates and diabetes risk, diet did little to affect these associations in non-pregnant women.(15, 16) Future studies will need to evaluate these GDM risk factors, as well as clinically-diagnosed GDM, taking into account additional confounding factors, to determine whether higher concentrations of these chemicals across multiple time points in pregnancy might be associated with an increased risk of this pregnancy complication.
Despite these limitations, the study has several strengths. First, this is the first study to evaluate urinary phthalate metabolites and risk factors of GDM, namely first trimester BMI, GWG, and glucose levels during the second trimester of pregnancy. Given that several studies have shown an association with diabetes and its risk factors, this study provides important information about a common pregnancy complication that can affect the subsequent health of the mothers and their offspring. Second, we evaluated multiple measurements of phthalate exposure across pregnancy. These multiple measures allowed us to assess potential sensitive windows of time. This assessment is particularly important given the dynamic nature of insulin sensitivity and resistance across pregnancy. Third, we evaluated multiple risk factors of GDM to assess the impact of these chemicals on different factors that affect GDM risk. Fourth, we evaluated these associations with the ability to adjust for sociodemographic and lifestyle factors in a racially and socioeconomically diverse U.S. study population.
In conclusion, we found 2nd trimester MEP to be associated with two important GDM risk factors—excessive GWG and impaired glucose tolerance based glucose values from the GLT. MnBP, MCPP, and ΣDEHP were associated with reduced odds of GWG, lower 2nd trimester glucose levels, and impaired glucose tolerance, respectively. Other urinary phthalate metabolites had weak or no associations with risk factors of GDM. While di-butyl and di-2-ethylhexyl phthalate have declined in use,47 these strong inverse associations may warrant further investigation. The high prevalence of exposure to di-ethyl phthalate, primarily from personal care product use, and the positive associations with two major GDM risk factors may warrant further investigation. If replicated, reducing exposure to di-ethyl phthalate could reduce excessive GWG and impaired glucose tolerance in pregnant women, with implications for reducing GDM and its sequelae.
Supplementary Material
Highlights.
Higher 2nd trimester MEP concentrations were associated with a higher risk of impaired glucose tolerance and excessive gestational weight gain
Higher 2nd trimester ΣDEHP metabolite concentrations were inversely associated with continuous glucose levels in 2nd trimester
No associations were found for 1st trimester urinary phthalate metabolite concentrations and GDM risk factors
Acknowledgments
This research was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (K12HD051959), the National Institute of Environmental Health Sciences (R01ES018872, P30ES017885), and the National Heart Lung and Blood Institute (K24RR018613). Funding support for KKF was provided by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no competing financial interests.
Conflict of interest: The authors declare no conflict of interest.
References
- 1.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36(Suppl 1):S67–S74. doi: 10.2337/dc13-S067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dabelea D, et al. Increasing prevalence of gestational diabetes mellitus (GDM) over time and by birth cohort: Kaiser Permanente of Colorado GDM Screening Program. Diabetes Care. 2005;28(3):579–584. doi: 10.2337/diacare.28.3.579. [DOI] [PubMed] [Google Scholar]
- 3.Thorpe LE, et al. Trends and racial/ethnic disparities in gestational diabetes among pregnant women in New York City, 1990–2001. Am J Public Health. 2005;95(9):1536–1539. doi: 10.2105/AJPH.2005.066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chu SY, et al. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care. 2007;30(8):2070–2076. doi: 10.2337/dc06-2559a. [DOI] [PubMed] [Google Scholar]
- 5.Torloni MR, et al. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obes Rev. 2009;10(2):194–203. doi: 10.1111/j.1467-789X.2008.00541.x. [DOI] [PubMed] [Google Scholar]
- 6.Morisset AS, et al. Weight gain measures in women with gestational diabetes mellitus. J Womens Health (Larchmt) 2011;20(3):375–380. doi: 10.1089/jwh.2010.2252. [DOI] [PubMed] [Google Scholar]
- 7.Morisset AS, et al. Prevention of gestational diabetes mellitus: a review of studies on weight management. Diabetes Metab Res Rev. 2010;26(1):17–25. doi: 10.1002/dmrr.1053. [DOI] [PubMed] [Google Scholar]
- 8.Kjos SL, Buchanan TA. Gestational diabetes mellitus. N Engl J Med. 1999;341(23):1749–1756. doi: 10.1056/NEJM199912023412307. [DOI] [PubMed] [Google Scholar]
- 9.Group HSCR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 10.Huang T, et al. Pregnancy Hyperglycaemia and Risk of Prenatal and Postpartum Depressive Symptoms. Paediatr Perinat Epidemiol. 2015;29(4):281–289. doi: 10.1111/ppe.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alonso-Magdalena P, Quesada I, Nadal A. Endocrine disruptors in the etiology of type 2 diabetes mellitus. Nat Rev Endocrinol. 2011;7(6):346–353. doi: 10.1038/nrendo.2011.56. [DOI] [PubMed] [Google Scholar]
- 12.Casals-Casas C, Desvergne B. Endocrine disruptors: from endocrine to metabolic disruption. Annu Rev Physiol. 2011;73:135–162. doi: 10.1146/annurev-physiol-012110-142200. [DOI] [PubMed] [Google Scholar]
- 13.Polyzos SA, et al. The emerging role of endocrine disruptors in pathogenesis of insulin resistance: a concept implicating nonalcoholic fatty liver disease. Curr Mol Med. 2012;12(1):68–82. doi: 10.2174/156652412798376161. [DOI] [PubMed] [Google Scholar]
- 14.Thayer KA, et al. Role of environmental chemicals in diabetes and obesity: a National Toxicology Program workshop review. Environ Health Perspect. 2012;120(6):779–789. doi: 10.1289/ehp.1104597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang T, et al. Gender and racial/ethnic differences in the associations of urinary phthalate metabolites with markers of diabetes risk: National Health and Nutrition Examination Survey 2001–2008. Environ Health. 2014;13(1):6. doi: 10.1186/1476-069X-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.James-Todd T, et al. Urinary phthalate metabolite concentrations and diabetes among women in the National Health and Nutrition Examination Survey (NHANES) 2001–2008. Environ Health Perspect. 2012;120(9):1307–1313. doi: 10.1289/ehp.1104717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lind PM, Zethelius B, Lind L. Circulating levels of phthalate metabolites are associated with prevalent diabetes in the elderly. Diabetes Care. 2012;35(7):1519–1524. doi: 10.2337/dc11-2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Svensson K, et al. Phthalate exposure associated with self-reported diabetes among Mexican women. Environ Res. 2011;111(6):792–796. doi: 10.1016/j.envres.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crinnion WJ. Toxic effects of the easily avoidable phthalates and parabens. Altern Med Rev. 2010;15(3):190–196. [PubMed] [Google Scholar]
- 20.Hauser R, Calafat AM. Phthalates and human health. Occup Environ Med. 2005;62(11):806–818. doi: 10.1136/oem.2004.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desvergne B, Feige JN, Casals-Casas C. PPAR-mediated activity of phthalates: A link to the obesity epidemic? Mol Cell Endocrinol. 2009;304(1–2):43–48. doi: 10.1016/j.mce.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 22.Goodman M, Lakind JS, Mattison DR. Do phthalates act as obesogens in humans? A systematic review of the epidemiological literature. Crit Rev Toxicol. 2014;44(2):151–175. doi: 10.3109/10408444.2013.860076. [DOI] [PubMed] [Google Scholar]
- 23.Yaghjyan L, et al. Associations of urinary phthalates with body mass index, waist circumference and serum lipids among females: National Health and Nutrition Examination Survey 1999–2004. Int J Obes (Lond) 2015;39(6):994–1000. doi: 10.1038/ijo.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song Y, et al. Urinary concentrations of bisphenol A and phthalate metabolites and weight change: a prospective investigation in US women. Int J Obes (Lond) 2014;38(12):1532–1537. doi: 10.1038/ijo.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shapiro GD, et al. Exposure to phthalates, bisphenol A and metals in pregnancy and the association with impaired glucose tolerance and gestational diabetes mellitus: The MIREC study. Environ Int. 2015;83:63–71. doi: 10.1016/j.envint.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 26.Hedderson MM, Darbinian JA, Ferrara A. Disparities in the risk of gestational diabetes by race-ethnicity and country of birth. Paediatr Perinat Epidemiol. 2010;24(5):441–448. doi: 10.1111/j.1365-3016.2010.01140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solomon CG, et al. A prospective study of pregravid determinants of gestational diabetes mellitus. JAMA. 1997;278(13):1078–1083. [PubMed] [Google Scholar]
- 28.McElrath TF, et al. Longitudinal evaluation of predictive value for preeclampsia of circulating angiogenic factors through pregnancy. Am J Obstet Gynecol. 2012;207(5):407 e1–407.e7. doi: 10.1016/j.ajog.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 29.Ferguson KK, McElrath TF, Meeker JD. Environmental phthalate exposure and preterm birth. JAMA Pediatr. 2014;168(1):61–67. doi: 10.1001/jamapediatrics.2013.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.CDC. (Centers for Disease Control and Prevention): Third National Report on Human Exposure to Environmental Chemicals. [cited 2011];2005 Available from: http://www.cdc.gov/exposurereport/
- 31.Hornung R, Reed L. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5:46–51. [Google Scholar]
- 32.Ferguson KK, et al. Variability in urinary phthalate metabolite levels across pregnancy and sensitive windows of exposure for the risk of preterm birth. Environ Int. 2014;70C:118–124. doi: 10.1016/j.envint.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boeniger MF, Lowry LK, Rosenberg J. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. Am Ind Hyg Assoc J. 1993;54(10):615–627. doi: 10.1080/15298669391355134. [DOI] [PubMed] [Google Scholar]
- 34.Braun JM, et al. Personal care product use and urinary phthalate metabolite and paraben concentrations during pregnancy among women from a fertility clinic. J Expo Sci Environ Epidemiol. 2013 doi: 10.1038/jes.2013.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kobrosly RW, et al. Socioeconomic factors and phthalate metabolite concentrations among United States women of reproductive age. Environ Res. 2012;115:11–17. doi: 10.1016/j.envres.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 36.Schettler T. Human exposure to phthalates via consumer products. Int J Androl. 2006;29(1):134–139. doi: 10.1111/j.1365-2605.2005.00567.x. discussion 181-5. [DOI] [PubMed] [Google Scholar]
- 37.Lao TT, et al. Maternal age and prevalence of gestational diabetes mellitus. Diabetes Care. 2006;29(4):948–949. doi: 10.2337/diacare.29.04.06.dc05-2568. [DOI] [PubMed] [Google Scholar]
- 38.Bouthoorn SH, et al. Low-educated women have an increased risk of gestational diabetes mellitus: the Generation R Study. Acta Diabetol. 2015;52(3):445–452. doi: 10.1007/s00592-014-0668-x. [DOI] [PubMed] [Google Scholar]
- 39.Gunton JE, et al. Cigarette smoking affects glycemic control in diabetes. Diabetes Care. 2002;25(4):796–797. doi: 10.2337/diacare.25.4.796-a. [DOI] [PubMed] [Google Scholar]
- 40.Sargeant LA, et al. Cigarette smoking and glycaemia: the EPIC-Norfolk Study. European Prospective Investigation into Cancer. Int J Epidemiol. 2001;30(3):547–554. doi: 10.1093/ije/30.3.547. [DOI] [PubMed] [Google Scholar]
- 41.Hatch EE, et al. Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: a cross-sectional study of NHANES data, 1999–2002. Environ Health. 2008;7:27. doi: 10.1186/1476-069X-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stahlhut RW, et al. Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insulin resistance in adult US. males. Environ Health Perspect. 2007;115(6):876–882. doi: 10.1289/ehp.9882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Main KM, et al. Human breast milk contamination with phthalates and alterations of endogenous reproductive hormones in infants three months of age. Environ Health Perspect. 2006;114(2):270–276. doi: 10.1289/ehp.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watkins DJ, et al. In utero and peripubertal exposure to phthalates and BPA in relation to female sexual maturation. Environ Res. 2014;134:233–241. doi: 10.1016/j.envres.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cedergren M. Effects of gestational weight gain and body mass index on obstetric outcome in Sweden. Int J Gynaecol Obstet. 2006;93(3):269–274. doi: 10.1016/j.ijgo.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 46.Crane JM, et al. The effect of gestational weight gain by body mass index on maternal and neonatal outcomes. J Obstet Gynaecol Can. 2009;31(1):28–35. doi: 10.1016/s1701-2163(16)34050-6. [DOI] [PubMed] [Google Scholar]
- 47.Rosenberg TJ, et al. Maternal obesity and diabetes as risk factors for adverse pregnancy outcomes: differences among 4 racial/ethnic groups. Am J Public Health. 2005;95(9):1545–1551. doi: 10.2105/AJPH.2005.065680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hillier TA, et al. Childhood obesity and metabolic imprinting: the ongoing effects of maternal hyperglycemia. Diabetes Care. 2007;30(9):2287–2292. doi: 10.2337/dc06-2361. [DOI] [PubMed] [Google Scholar]
- 49.Casals-Casas C, Feige JN, Desvergne B. Interference of pollutants with PPARs: endocrine disruption meets metabolism. Int J Obes (Lond) 2008;32(Suppl 6):S53–S61. doi: 10.1038/ijo.2008.207. [DOI] [PubMed] [Google Scholar]
- 50.Tsatsoulis A, Wyckoff J, Brown FM, editors. Sex Differences in Energy Balance, Body Composition, and Body Fat Distribution. New York: Humana Press/Springer Science+Business Media; 2009. Diabetes in Women: Pathphysiology and Therapy. [Google Scholar]
- 51.Kumar N, et al. Assessment of estrogenic potential of diethyl phthalate in female reproductive system involving both genomic and non-genomic actions. Reprod Toxicol. 2014;49:12–26. doi: 10.1016/j.reprotox.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 52.Fisher M, et al. Bisphenol A and phthalate metabolite urinary concentrations: Daily and across pregnancy variability. J Expo Sci Environ Epidemiol. 2015;25(3):231–239. doi: 10.1038/jes.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Preau JL, Jr, et al. Variability over 1 week in the urinary concentrations of metabolites of diethyl phthalate and di(2-ethylhexyl) phthalate among eight adults: an observational study. Environ Health Perspect. 2010;118(12):1748–1754. doi: 10.1289/ehp.1002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.