Abstract
In long-term survivors of allogeneic hematopoietic cell transplantation (HCT), chronic graft-versus-host disease (GVHD) is the major cause of morbidity and mortality and a major determinant of quality of life. Chronic GVHD responds poorly to current immunosuppressive drugs, and while T-cell depletion may be preventative, this gain is offset by increased relapse rates. A significant impediment to progress in treating chronic GVHD has been the limitations of existing animal models. The goal of this study was to develop a reproducible, comprehensive model of chronic GVHD in the dog. Ten recipient dogs received 920 cGy total body irradiation, infusion of marrow, and an infusion of buffy coat cells from a dog leukocyte antigen (DLA) mismatched unrelated donor. Postgrafting immunosuppression consisted of methotrexate (days 1, 3, 6, 11) and cyclosporine. The duration of cyclosporine administration was limited to 80 days instead of the clinically used 180 days. This was done in order to contain costs since chronic GVHD was expected to develop at earlier time points. All recipients were given ursodiol for liver protection. One dog had graft failure while 9 dogs showed stable engraftment. Eight of the 9 developed de novo chronic GVHD. Dogs progressed with clinical signs of chronic GVHD over a period of 43 to 164 (median 88) days after discontinuation of cyclosporine. Target organs showed the spectrum of chronic GVHD manifestations that are typically seen clinically. These included lichenoid changes of the skin, fasciitis, ocular involvement (xerophthalmia), conjunctivitis, bronchiolitis obliterans, salivary gland involvement, gingivitis, esophageal involvement, and hepatic involvement. Peripheral blood lymphocyte surface antigen expression of CD28 and ICOS was elevated in dogs with GHVD compared to normal dogs but not significantly so. Serum levels of IL-8 and MCP-1 in GVHD affected dogs at time of euthanasia were elevated, while levels of IL-15 were depressed compared to normal dogs. Results indicate that the canine model is well suited for future studies aimed at preventing or treating chronic GVHD.
Keywords: chronic GVHD, canine model
Introduction
Chronic graft-vs-host disease (GVHD), first reported in the 1970s for human patients undergoing allogeneic hematopoietic cell transplantation (HCT) [1-4], has remained a major determinant of morbidity and mortality. Its manifestations resemble those of autoimmune, systemic collagen, and vascular diseases. Among patients undergoing transplantation for hematologic malignancies, a beneficial graft-vs.-tumor (GVT) effect has been described that has a significant association with chronic GVHD [5]. However, this benefit is offset by recurrent, often fatal bacterial and fungal infections due to the impaired immune function both from chronic GVHD itself and from the extended immunosuppressive treatment. The reported cumulative incidences of chronic GVHD range from 25% to 50% in survivors of allogeneic transplantation [6]. Chronic GVHD responds only slowly and often incompletely to current immunosuppressive drugs, with the median duration of treatment among surviving patients ranging from 2.5 to 3 years [7-9]. The mortality rate associated with chronic GVHD is approximately 25%. One way of reducing the incidence of chronic GVHD has been through T-cell depletion, which can either be accomplished in vitro by removing T-cells from the grafts or in vivo by treating patients with anti-thymocyte globulin, an antibody to CD52, or post-transplant cyclophosphamide [10-13]. However, the benefit from decreasing the risk of chronic GVHD by T-cell depletion may be offset by an increased risk of relapse [10]. So, the challenge is to retain the beneficial GVT effect of chronic GVHD, while significantly shortening the current, lengthy immunosuppressive treatment and its associated high risk of morbidity and mortality.
The first report on treatment of patients with chronic GVHD using combinations of steroids and other immunosuppressive agents was published in 1981 [14]. Since then, treatment efforts of chronic GVHD have been characterized by a lack of progress despite intense clinical investigations in the form of Phase I/II and randomized, controlled Phase III clinical trials [15-18]. This lack of progress against chronic GVHD has been disappointing and has not been helped by the limitations of existing animal (mostly murine) models of chronic GVHD [19]. The existing models do not replicate the full spectrum of the clinical disease and, to date, have not produced clinical advances comparable to those achieved in acute GVHD. We described a chronic GVHD model in allografted dogs in 1982 [20] but did not pursue these observations because of competing priorities and the belief that the chronic GVHD problem would be resolved in humans before canine studies could get underway, which was clearly an incorrect assessment. In light of the lack of success of human trials described above, we redeveloped a canine model of chronic GVHD which we describe in this report. A reproducible model of chronic GVHD in a clinically, highly relevant large animal will set the stage for a systematic evaluation of specific biological reagents directed at T-cell checkpoints for more effective and definitive treatment of chronic GVHD.
Materials and Methods
Experimental animals
Random-bred litters of beagles and mini-mongrel cross-breeds were raised at the Fred Hutchinson Cancer Research Center (Fred Hutch), Seattle WA. The dogs weighed from 8.3 to 15.3 (median 10.6) kg and were 6.5 to 15 (median 9.3) months old. They were observed for disease at least 20 days before study. The Institutional Care and Use Committee of the Fred Hutch approved the research protocols and the American Association for the Accreditation of Laboratory Animal Care certified the facility. Ten donors and ten recipients were unrelated for at least 5 generations and were mismatched for highly polymorphic major histocompatibility complex (dog leukocyte antigen [DLA]) class I and class II associated microsatellite markers [21, 22]. DLA mismatching was confirmed by direct sequencing for DLA-DRB1 alleles [23].
HCT
On day 0, HCT recipients were conditioned with a single dose of 920 cGy total body irradiation (TBI) delivered at a rate of 7 cGy/minute from a high-energy linear accelerator (Varian Clinac 6, Palo Alto, CA) (Figure 1). Within 4 hours after TBI, the recipients were given an intravenous (i.v.) infusion of 2.0 to 5.2 × 108 (median 4.2) nucleated donor marrow cells/kg. Twenty-four hours later the recipients were given an IV infusion of 0.2 to 3.5 × 108 (median 1.4) peripheral blood mononuclear cells/kg obtained by COBE apheresis from the marrow donor. Postgrafting immunosuppression consisted of i.v. methotrexate (MTX), 0.4 mg/kg/day on days 1, 3, 6, and 11, and cyclosporine (CSP), given twice daily starting on day -1 through 80 at a dose of 7.5–15 mg/kg, adjusted to maintain a blood CSP level between 100 to 300 ng/ml. Marrow recipients were given ursodiol (0.75 mg/kg, twice daily, days -1 to 80) for protection of the liver. All dogs were given standard postgrafting care including constant rate infusion of lactated Ringers solution while receiving MTX. Fevers were treated as sepsis and dogs were given antibiotics. Hematopoietic engraftment was assessed by chimerism studies using microsatellite markers [24, 25].
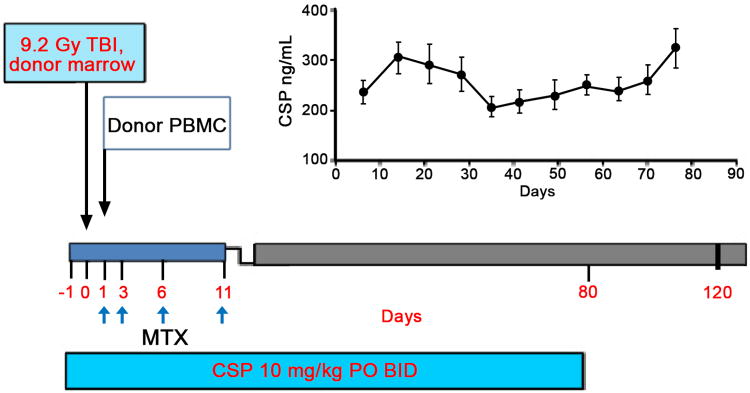
Figure 1. Treatment schema for induction of chronic GVHD in DLA-mismatched unrelated HCT recipients.
Recipients were given 920 cGy TBI and donor marrow on day 0. On day 1 recipients were injected with buffy coat cells from a COBE apheresis from their respective donor. Immunosuppression consisted of methotrexate (MTX) administered on days 1, 3, 6, 11 and cyclosporine (CSP) administered on days -1 through 80 at a dose to maintain serum levels between 100 and 300 ng/mL. The average CSP serum level for the 10 dogs on study is shown graphically in the top right portion of the figure. Ursodiol was given on days -1 through 80 to reduce the incidence of liver GVHD.
Evaluation of GVHD
A diagnosis of GVHD was based on clinical findings which included generalized skin ulcerations or scleroderma, facial edema, dry eye syndrome, erythema of the sclera, nasal occlusion, gingivitis, elevated liver enzymes, anorexia, vomiting and/or diarrhea. The dogs were monitored at a minimum twice daily and the progression of GVHD was recorded in a digital program DVMax (Veterinary Health Management Software, Westbrook, ME). Once GVHD had progressed to the point of diminished activity level, weight loss greater than 30%, failure to eat, signs of distress, or a requirement of critical care procedures were noted, the decision was made to euthanize the animal following establishing consensus of the principle investigator, clinical veterinarian, and animal technicians currently on hand. Following euthanasia, a complete necropsy was performed and representative tissues fixed in 10% buffered formalin, embedded in paraffin, cut, and stained with hematoxylin and eosin for evaluation by a pathologist (GS). Chronic GVHD was graded from mild to severe based on the degree of lymphocyte infiltration, and the degree of tissue damage (apoptosis, fibrosis, lichenoid formation and sclerodermatous foci).
Peripheral blood cell surface antigen expression
Peripheral blood was collected from dogs in 10% heparin, and centrifuged over Ficoll-Paque™-Plus (adjusted to 1.074 density with water) using standard methods. After washing in PBS +2% horse serum, the cells were resuspended in the same buffer and labeled with the following flurochrome conjugated mouse anti-canine specific antibodies: anti-CD3 (17.6F9), CD28 (1C6), and anti-ICOS (3F12) using standard labeling techniques. Commercial rat anti-canine CD4 and CD8-labeled antibodies were used for single and double staining (eBiosciences Inc., San Diego, CA). The cells were stained and analyzed for positive expression using BD Canto-2 flow cytometer (BD Biosciences, San Jose, CA). The percentage of positive cells was determined using FlowJo software (Ashland, OR).
MAP cytokine expression
Interleukin (IL)-2, IL-6, IL-8, IL-10, IL-15, tumor necrosis factor (TNF)-α and monocyte chemotactic protein (MCP)-1 levels were measured using CYTOMAG-90K Milliplex MAP kit (Millipore, Billerica, MA). Sera were collected from normal dogs and dogs with GVHD prior to euthanasia and were tested according to manufacturer's specifications. Levels of cytokines and chemokines were expressed in picograms/ml (pg/ml).
Results
Hematopoietic Cell Engraftment
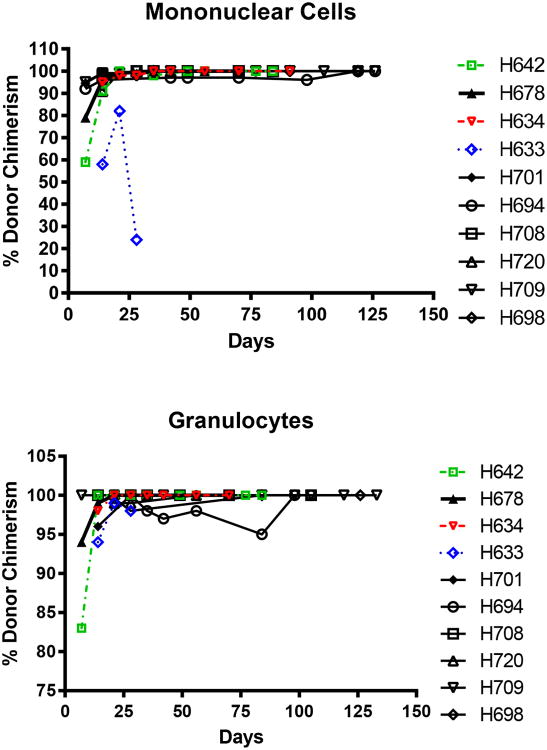
All 10 dogs had hematopoietic cell engraftment after HCT. Mononuclear cell and granulocyte counts recovered rapidly following nadirs on days 5 through 10; however, full granulocyte count recoveries for dogs H694 and H720 were delayed and platelet recoveries were also delayed to approximately day 50 (Figure 2). Donor cell chimerism (Figure 3) was apparent after one week and was sustained for both mononuclear cells and granulocytes. We assume the apparent loss in mononuclear chimerism for H633 after day 20 was likely due to the dilution effect of preceding multiple transfusions that were given due to thrombocytopenia. H633 died on day 30 due to a hemorrhagic cerebellar stroke and was excluded from further analysis. All dogs with the exception of H694 became febrile for a brief period of time (temperature > 39.2°C) between days 14 to 17 (median 15) but otherwise tolerated the protocol well. The transient temperature elevation was thought to be due to an engraftment syndrome [26].
Figure 2. Lymphocyte, granulocyte, and platelet recovery in dogs given DLA-mismatched, unrelated HCT.
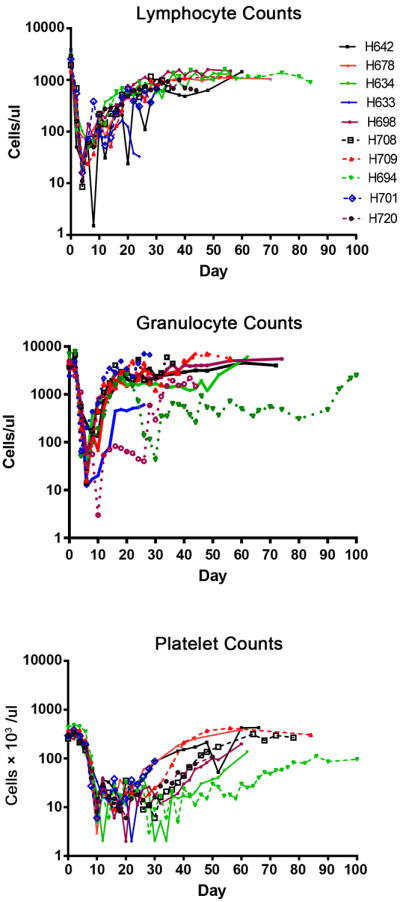
Figure 3. Percent donor cell chimerism of dogs transplanted with DLA-unmatched unrelated marrow and PBMC following 920 cGy TBI.
Chronic GVHD
Clinical course
Table 1 summarizes the outcome of nine evaluable dogs with sustained engraftment followed by development of GVHD. One dog, H701, developed acute GVHD and was euthanized on day 29. The remaining 8 dogs all developed de novo chronic GVHD without previous indications of acute GVHD. They progressed with clinical signs of chronic GVHD over a period of 43 to 164 (median 88) days. The time to euthanasia ranged from day 55 to 189 (median 103) days. Of note, the dogs were afebrile despite extensive skin GVHD.
Table 1. Chronic GVHD in dogs given DLA-mismatched, unrelated HCT after conditioning with 920 cGy. Recipients were given postgrafting immunosuppression consisting of methotrexate and cyclosporine.
| DOG | Appearance of chronic GVHD* (day) |
Day Euthanized |
Lung | Liver | Skin | Esophagus | Stomach | Small Bowel |
Large Bowel |
Diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|
| H642 | 66 | 74 | Bronch Glands | Triaditis | Trunk, Ear, Paw, Nose | Lichenoid and Ulceration | Fibrosis | WNL | WNL | Chronic GVHD |
| H634 | 88 | 103 | Inflammation Bronchiolitis | Hepatitis Triaditis | Mouth Epithelium | NA | NA | NA | NA | Chronic GVHD? |
| H678 | 81 | 91 | Early BO | Bile Ducts | Trunk, Ear, Paw, Nose | NA | Mild | Mild | mild | Chronic GVHD |
| H633 | NA | 30 | WNL | WNL | WNL | NA | WNL | WNL | Massive stroke | |
| H701 | 20 | 29 | WNL | Centrolobular Congestion | Nose | NA | WNL | GVHD 3-4 | Acute GVHD | |
| H694 | NA | 106 | Bronchiolitis | Lymphocytic Infiltration | Trunk, Ear, Paw, Nose | NA | NA | WNL | WNL | Chronic GVHD |
| H708 | 98 | 101 | Minimal | Triaditis | Trunk, Ear, Paw, Nose | Esophagitis | Grade 1 | WNL | WNL | Chronic GVHD |
| H720 | 43 | 55 | Bronchiolitis | Triaditis | Ear | NA | Atrophy | WNL | WNL | Chronic GVHD |
| H709 | 164 | 189 | Fibrotic | WNL | Trunk, Ear, Paw, Nose | Extensive Damage | WNL | WNL | WNL | Chronic GVHD |
| H698 | 114 | 121 | Fibrotic | Triaditis | Lichenoid | Fibrosis | Infiltrate | WNL | Infiltrate | Chronic GVHD |
day of initial clinical signs of GVHD after HCT (day =0).
Abbreviations: within normal limits (WNL), bronchiolitis obliterans (BO), not available (NA).
Organ involvement
Skin
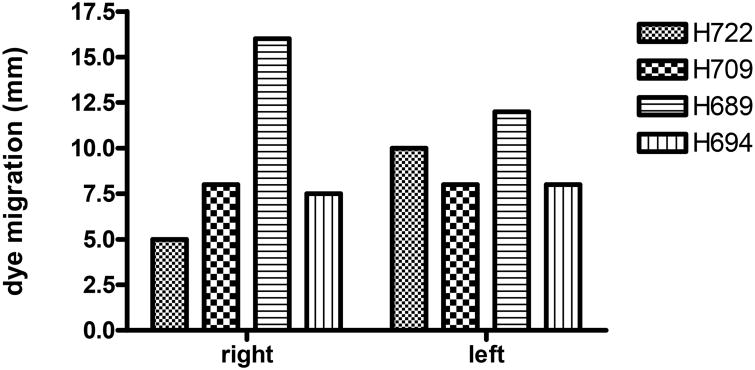
The data in Table 1 show the extent of chronic GVHD for each dog based on the affected tissues. The most common clinical diagnostic sign of chronic GVHD was skin involvement. This included erythema with progression to dry, scabbed lesions especially on the trunk and abdomen. Lichenoid formation occurred in 6 dogs in the skin from the nose, H709 (Figure 4A), ear, H708 (Figure 4B), paw, H708 (Figure 4C), and trunk, H709 (Figure 4D) all found to be positive for lymphocytic infiltration, epidermal atrophy, and fibrous remodeling. Additionally, hair follicles and sebaceous glands were common targets. In two dogs, H634 and H720, infiltration was less intense and localized to hair follicles of the ear. In addition, we observed lymphocytic infiltration and apoptosis of a nerve trunk in dog H709 (Figure 4A). The conjunctiva of the eye lids from several dogs was also pathognomonic for chronic GVHD. Conjunctivitis was observed in necropsy samples from H708, H698, H678, and H634. Lichenoid formation and destruction of goblet cells was seen in H678 and H720, respectively. In the case of H708, the hair follicles and sebaceous glands also showed lymphocytic infiltration and tissue destruction. The conjunctiva from H694 revealed chronic GVHD with involvement of sebaceous glands and parafollicular bulges with dense lymphocyte involvement. Additional evidence for chronic GVHD affecting tear production by the lacrimal gland and duct was provided by the Schirmer test strip method. The mean amount of tear production in healthy dogs (according to product insert) was 19 ± 5.33 millimeters. Mean tear production for the right and left eyes of four dogs with chronic GVHD, tested at time of euthanasia, was 9.1 ± 4.1and 9.1 ± 1.4 mm/min, respectively (Figure 5).
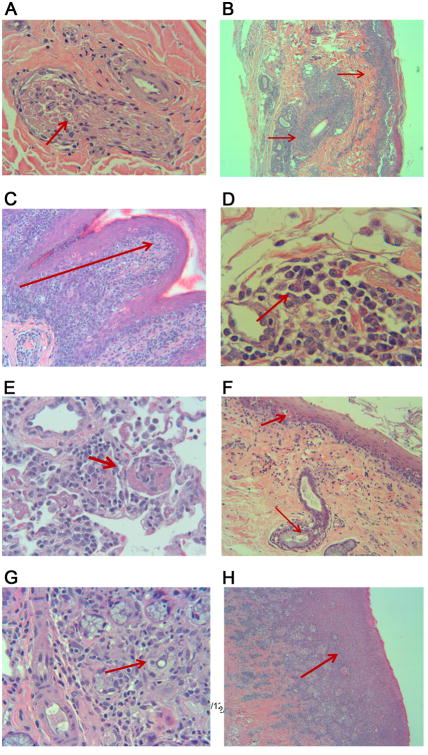
Figure 4. Histopathology of dogs with chronic GVHD at necropsy.
View from top left to bottom right, H709 nasal skin and cutaneous nerve trunk with arrow showing inflammation and apoptosis of a nerve bundle (A). H708 ventral surface ear skin showing dense lymphocytic infiltration around a hair follicle and along the surface epithelium (B). H708 paw skin with arrow indicating inflammation with multiple apoptotic basal keratinocytes from the shoulder of the rete ridge to the surface epithelium (C). H709 trunk skin biopsy with eosinophilic fasciitis resulting in sclerodermatous changes with apparent early sclerosis at the right side of the image (D). H694 lung tissue with initial signs of bronchiolitis obliterans (E). H709 esophagus with damaged epithelium, esophageal gland, and duct (F). H694 salivary gland with sialadenitis as evidenced by lymphocytic destruction and fibrosis of glandular tissues (G). H708 gingival tissue with severe gingivitis and lichenoid formation (H).
Figure 5. Evidence of chronic GVHD of the lacrimal glands.
Dogs were evaluated for tear production at or within two days of euthanasia using the Schirmer's Test. Strips were placed under the lower lid of each eye for 1 minute, the dye front was measured immediately afterward.
Lung
Abnormal histopathology of the lung is also found in chronic GVHD. Lung tissues were examined from all 10 dogs. Findings in 9 dogs ranged from normal (H701) to bronchopenia (H720), to chronic lymphocytic infiltration with fibrosis (H708, H709, H698), and bronchiolar infiltration (H642, H634). Clear indications of early to mild bronchiolitis obliterans, a useful diagnostic feature of chronic GVHD, were observed in two dogs, H678 and H694 (Figure 4E). Of note, lung samples from H701, diagnosed with acute GVHD, revealed no signs of pathology within the lung.
Liver
The liver is a common target of GVHD. Because ursodiol is commonly administered in human patients to mitigate chemo-radiation therapy damage and GVHD of the liver, we chose to add this reagent to the treatment protocol. Nonetheless, histopathological evidence of chronic liver GVHD was apparent upon necropsy with triaditis and small bile duct damage in 5 of the 8 dogs. Liver enzymes were elevated in dog H720 a week before euthanasia and in H694 two days before euthanasia (data not shown).
Gastrointestinal tract
In general, the small and large bowel autopsy specimens collected from dogs diagnosed with chronic GVHD showed either minor lymphocytic infiltrate or were unremarkable. In two of the dogs, there were signs of chronic GVHD in the stomach evidenced by gastric atrophy (H720, H708). However, in the small bowel of the dog diagnosed with acute GVHD (H701), we observed a complete replacement of the mucosal surface of the small bowel by a thick layer of granulation tissue consistent with grade III-IV damage. Autopsy samples of the esophagus from dogs with chronic GVHD revealed a significant amount of inflammatory activity [27]. In every dog diagnosed for which necropsy samples of the esophagus were available (dogs H698, H642, H709, H708), tissue damage was extensive and included severe esophagitis with involvement of esophageal glands and ducts (H709, Figure 4F), and lichenoid involvement with ulceration of the squamous epithelium. The oral mucosal tissue frequently showed signs of chronic GVHD ranging from focal evidence in the basal layers of the epithelium (H634) to lichenoid changes in the basal layers of the epithelium and the epidermal mucosal junction (H678, H709, H698, H708 (Figure 4G).
Salivary glands
In human patients, sialadenitis is a common feature of chronic GVHD [28]. In 6 of the 9 dogs, evidence of salivary gland inflammation ranged from unremarkable or minimal (H720, H708) to ductal damage (H634) with chronic inflammation and obliterated gland lobules due to interstitial fibrosis (H709, H698, H694 (Figure 4H).
Overall comparison of findings in current and historical dogs
The overall clinical and histopathological findings in current MTX/CSP/ursodiol-treated dogs are compared in Table 2 to previously reported findings of long-term survivors among dogs given a 102-day course of monotherapy with MTX [25]. In the current study, dogs developed chronic GVHD without prior development of acute GVHD. In the study by Atkinson et al. [29], 10 of 80 transplanted dogs with hematopoietic grafts from DLA-nonidentical littermates showed sustained engraftment and developed chronic GVHD. Among the 10 dogs, 6 had recovered from acute GVHD, 2 dogs progressed to chronic GVHD from acute GVHD and 2 dogs developed de novo chronic GVHD. The median days to onset, the length of survival, and the target organs for chronic GVHD were similar in the two studies. In the present study, we observed an even distribution of pathology between skin, liver, and gastrointestinal tract when taking into account the esophagus as a target of chronic GVHD. In the historical study, the esophagus was not examined, possibly resulting in a lower reported incidence of GVHD of the intestinal tract. The notable difference between the two studies is the incidence of multi-organ involvement was the norm in the current study while in the historical study chronic GVHD was primarily observed only in the skin or liver. This finding is likely due to the addition of cyclosporine and ursodiol in the current study to a short course of MTX.
Table 2. Clinical and histopathological characteristics of chronic GVHD in dogs given 920 cGy TBI followed by unrelated DLA-mismatched HCT*.
| Study | Current (n=8) | Historical (n=10) [29] |
|---|---|---|
| Median days of onset (range) | 91 (29-199) | 124 (59-150) |
| Median days of survival (range) | 98 (38-224) | 150 (69-275) |
| Organ involvement (no. of dogs) | ||
| Skin | 7 | 6 |
| Liver | 6 | 6 |
| Gastrointestinal tract | 6 | 2 |
| Multiple organ involvement (no. of dogs) | ||
| Skin, liver, gut | 4 | 1 |
| Skin, liver | 3 | 1 |
| Skin, gut | 1 | 0 |
| Liver, gut | 0 | 1 |
| Skin only (no. of dogs) | 0 | 4 |
| Liver only | 0 | 3 |
| Gut only | 0 | 0 |
Current dogs received a short course of MTX and 80 days of CSP while historical dogs were selected long-term survivors given a 102-day course of MTX [29].
Mechanistic studies
Lymphocyte surface antigen expression
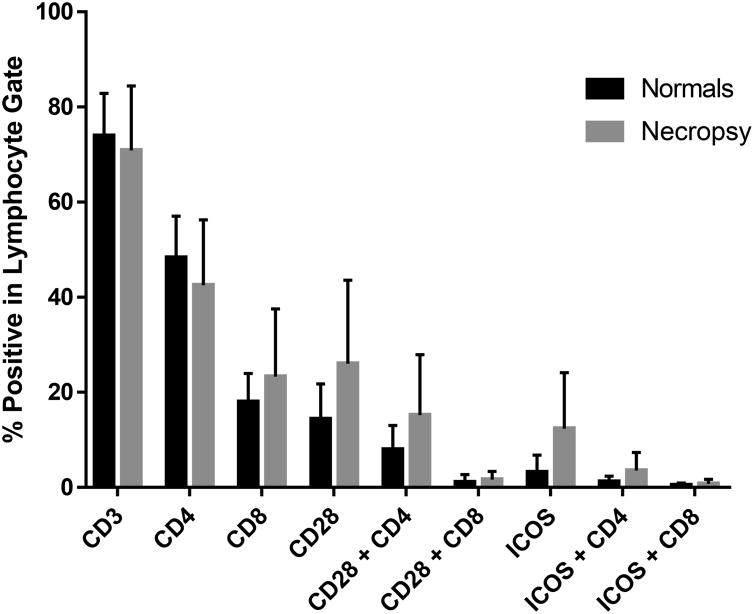
Cell surface antigen expression may uncover novel targets for therapy and provide information regarding the pathogenesis of chronic GVHD [30]. During the course of our investigation, peripheral blood samples were collected and mononuclear cells were purified from 21 normal untreated dogs and a minimum of 8 dogs transplanted for the generation of chronic GVHD. For the latter, blood was collected at time of euthanasia and flow cytometry was used to delineate cell surface antigen expression in both populations. As shown in Figure 6, there was a tendency towards increased expression of CD28, and ICOS (particularly on CD4+ lymphocytes). In addition, CD8+ cell numbers from dogs with GVHD showed a trend towards increased numbers over normal untreated dogs. Significant differences in these comparisons were not attainable due to low sample size.
Figure 6. Cell surface expression of selected antigens in transplanted dogs at time of euthanasia for GVHD and normal healthy dogs.
Peripheral blood mononuclear cells were isolated over Ficoll-Paque using standard methods. Cells were stained with fluorochrome labeled antibodies and analyzed by flow cytometery. Data represents mean and standard deviation of cell surface antigen expression of 21 normal dogs and 8-11 GVHD dogs sampled at time of euthanasia.
Cytokine and chemokine expression
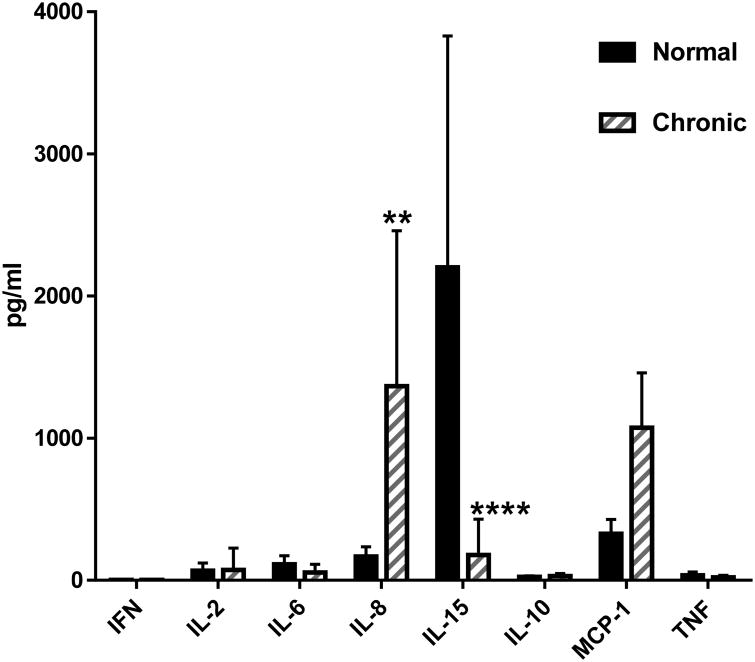
Serum levels of select cytokines and chemokines were analyzed from normal dogs and dogs transplanted for GVHD at the time of euthanasia (Figure 7). Using a MAP cytokine assay, we analyzed serum levels of IFN-g, IL-2, IL-6, IL-8, IL-15, IL-10, MCP-1, and TNF-a. Of these, serum levels of IL-8 were significantly increased and MCP-1 levels were elevated, while levels of IL-15 were significantly decreased in dogs with chronic GVHD compared to normal untreated dogs.
Figure 7. MAP Canine Cytokine Assay of serum from normal (N=4) or dogs with chronic GVHD (N=6).
Serum levels of IL-8 were greater in GVHD affected dogs while levels of IL-15 were lower compared to normal dogs (significance tested using the F test (** p< 0.0046, **** P<0.0001). The levels of MCP-1 in the serum from dogs with chronic GVHD were significantly greater than normal control dogs at p<0.0023 using an unpaired T test with Welch's correction.
Discussion
Chronic GVHD has remained a serious complication after human HCT. Progress towards effective treatment of chronic GVHD has been hampered by poorly understood pathophysiology, complexity of disease manifestations, and the early lack of standardized diagnostics, a problem which has since been addressed by the establishment of NIH consensus criteria in 2005 [31]. Another major impediment has been the lack of research progress in existing animal models, as reviews have highlighted [19, 32]. This particular concern suggests new animal models should be explored.
The canine GVHD model has as its foundation DLA-mismatched unrelated HCT which has been used to identify multiple immunosuppressive drug combinations for the purpose of controlling GVHD, which are now in clinical use [20, 29, 33-35]. Here, we administered a clinically used MTX/CSP regimen for GVH control, kept CSP blood levels at 100–300 ng/mL, but modified the regimen by shortening the time of CSP administration from 180 days to 80 days. Extending CSP administration to the customary 180 days was deemed cost prohibitive in this canine model. We anticipated de novo chronic GVHD manifestations to develop between 90 and 200 days following HCT, an expectation that was met in the majority of current dogs. Preliminary studies indicated the length of treatment with MTX was critical for the development of chronic GVHD. Eliminating the day 11 dose of MTX routinely resulted in acute GVHD. Importantly, clinical and histopathological manifestations of chronic GVHD, which are commonly seen in the clinical setting, were captured in the current canine study. These include lichenoid changes of the skin, along with fasciitis, ocular involvement (xerophthalmia), along with abnormal Schirmer test, conjunctivitis, bronchiolitis obliterans, salivary gland involvement, gingivitis, esophageal involvement, and hepatic involvement. No correlation could be found between dose of buffy coat cells and the occurrence of acute or chronic GVHD or the extent of organ involvement. Limited mechanistic studies identified cell surface expression of several antigens including the previously identified increased expression of ICOS in dogs with chronic GVHD [36]. However, ongoing, as yet unpublished longitudinal studies have shown that ICOS expression can be variable and at times highly significantly upregulated. ICOS expression has been associated with chronic GVHD in human patients but in mice was only seen on lymphocytes during acute GVHD [37, 38].
Of value was our limited MAP cytokine assay. We observed increased levels of IL-8 and MCP-1 and decreased levels of IL-15 compared to serum samples collected from dogs without GVHD. A review by Pidala et al. [39] stated that most studies on cytokine levels in chronic GVHD reported inflammatory and immunomodulatory markers such as IL-8 to be elevated. Similarly, IL-8 levels are increased in tear production by patients with chronic GVHD [40]. Cytokine mapping up to 100 days after HCT was performed on children and showed MCP-1 increased dramatically on weeks 2 to 4 [41]. Also, low levels of IL-15 in the dog may correlate with the human clinical setting. In a cohort of 153 HCT recipients those patients who presented with low levels of IL-15 on day 7 after transplantation had a 2.7-fold greater likelihood of developing significant chronic GVHD [40].
For decades, mice have been studied as animal model of GVHD [42]. Mice are genetically well-defined, inbred animals, which allow evaluating cell trafficking with in vivo imaging techniques, and dissection of mechanistic pathways with gene knock-in and knock-out methods. Table 3 shows three well-established chronic GVHD mouse models, the sclerodermatous [42-46], the autoantibody-mediated or lupus like model, and the murine obliterans model [47-51]. Several differences between the murine studies and the clinical and canine settings are immediately obvious. Generally, murine reports described H-2-mismatched, parent → F1 hybrid transplantations using spleen cells. F1 hybrids received no conditioning therapy, and none received postgrafting immunosuppression. The non-H-2-mismatched spleen grafts involved only one donor recipient strain combination, B10.D2 → BALB/c. While these recipients received TBI conditioning, on the order of 6–10 Gy, they also did not receive postgrafting immunosuppression. In all studies, the time to development of chronic GVHD after spleen grafting was short, ranging from 14 to 49 days after transplantation. Importantly, the chronic GVHD manifestations were restricted and narrower than those seen in the random-bred human or canine settings. For example, the B10.D2 → BALB/c grafts resulted in fibrosis/scleroderma in either skin/lung, skin, or parotid gland. The parent → F1 grafts resulted in lupus-like symptoms, manifested as either lymphadenopathy, auto-antibodies, or hyperglobulinemia. One study involving C57/BL/6 → B10.BR grafts showed bronchiolitis obliterans [52]. Another study also involving C57/BL/6 → B10.BR grafts showed bronchiolitis obliterans which manifested Itself by decreased pulmonary function as early as day 28 after transplantation along with pulmonary and hepatic IgG2c deposition and fibrosis [51].
Table 3. Common mouse chronic GVHD models.
| Donor | Recipient | Genetics | Conditioning (TBI in Gy) | Stem Cells | Postgraft Immuno-suppression | Target Organs | Time to GVHD (weeks) | Outcome | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Pro-fibrotic (scleroderma) model | |||||||||
| B10.D2 | BALB/c | H2d (minor) | 7 | marrow+ spleen | none | skin, lungs | 2 to 3 | fibrosis, scleroderma | [42, 43] |
| B10.D2 | BALB/c | H2d (minor) | 6 to 10 | spleen | none | skin | 3 to 7 | scleordema | [44, 45] |
| B10.D2 | BALB/c | H2d (minor) | 8.5 | spleen | none | parotid SG | 3 | sialadenitis | [46] |
|
| |||||||||
| Lupus-like model | |||||||||
| DBA.2 | (B6D2)F1 | H2d/H2b (major) | none | marrow+ spleen | none | NA | 4 to 7 | lymphadenopathy | [47] |
| DBA.2 | (B6D2)F1 | H2d/H2 (major) | none | spleen | none | NA | NA | hyper γ-globulinemia | [48, 49] |
| C57Bl/6 | (B6D2)F1 | H2b/H2bd (major) | none | spleen | none | antibody deposition in kidney | 2 to 4 | anti-ss and ds DNA | [50] |
| BALB/c | (CB6)F1 | H2d/H2bd | none | spleen | none | antibody deposition in kidney | 2 to 4 | autoantibodies | [50] |
|
| |||||||||
| B. obliterans model | |||||||||
| C57Bl/6 | B10.BR | H2b/H2k (major) | Cy+7.5 | marrow (T-depl) spleen | none | lung, colon. liver | 4 to 7 | bronchiolitis. obliterans | [51] |
Abbreviations: salivary gland (SG); total body irradiation (TBI); Gray (Gy); Bronchiolitis (B); cyclophosphamide (Cy); not available (NA).
Taken together, the murine manifestations of chronic GVHD are in part dependent on the mouse strain combinations used, they occur very early after transplantation at times when acute GVHD is seen in humans or in the canine model, and they present in a more restricted and narrower form than those encountered clinically in humans and preclinically in random bred dogs. As shown in the current and in preceding studies, dogs experiencing chronic GVHD present the entire spectrum of the disease that is seen clinically. Thus, the canine model is ideally positioned for the conduct of studies aimed at preventing or treating this serious immunologic complication after allogeneic HCT.
Highlights.
We have developed a protocol that consistently produces de novo chronic GVHD in the clinically relevant hematopoietic cell transplantation canine model.
We have shown that the esophagus is targeted in the canine chronic GVHD in a manner similar to that of the human.
We have shown that the outbred canine model possesses a broad spectrum of manifestations of chronic GVHD that are typically seen clinically.
Initial mechanistic studies revealed that levels of select chemokines and cytokines are similarly affected in the canine as in the clinical setting.
Acknowledgments
The authors wish to thank Alix McPhearson and the Fred Hutchinson Cancer Research Center's Animal Research Technicians for their assistance with animal care, Stacy Zellmer and Debe Higginbotham for DLA typing and chimerism analysis, Bonnie Larson and Helen Crawford for manuscript preparation.
Funding: This work was supported by grants R21 OD010489, P30 CA15704, and P01 CA78902 from the National Institutes of Health, Bethesda, MD.
Footnotes
Financial Disclosure Statement: The authors have no primary financial relationships with any company that has a direct financial interest in the subject matter or products discussed in the submitted manuscript, or with a company that produces a competing product.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Storb R, Thomas ED, Weiden PL, et al. Aplastic anemia treated by allogeneic bone marrow transplantation: A report on 49 new cases from Seattle. Blood. 1976;48:817–841. [PubMed] [Google Scholar]
- 2.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. American Journal of Medicine. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 3.Gratwohl AA, Moutsopoulous HM, Chused TM, et al. Sjögren-type syndrome after allogeneic bone-marrow transplantation. Annals of Internal Medicine. 1977;87:703–706. doi: 10.7326/0003-4819-87-6-703. [DOI] [PubMed] [Google Scholar]
- 4.Graze PR, Gale RP. Chronic graft versus host disease: A syndrome of disordered immunity. American Journal of Medicine. 1979;66:611–620. doi: 10.1016/0002-9343(79)91171-9. [DOI] [PubMed] [Google Scholar]
- 5.Weiden PL, Sullivan KM, Flournoy N, Storb R, Thomas ED the Seattle Marrow Transplant T. Antileukemic effect of chronic graft-versus-host disease. Contribution to improved survival after allogeneic marrow transplantation. New England Journal of Medicine. 1981;304:1529–1533. doi: 10.1056/NEJM198106183042507. [DOI] [PubMed] [Google Scholar]
- 6.Storb R, Gyurkocza B, Storer BE, et al. Graft-versus-host disease and graft-versus-tumor effects after allogeneic hematopoietic cell transplantation. Journal of Clinical Oncology. 2013;31:1530–1538. doi: 10.1200/JCO.2012.45.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stewart BL, Storer B, Storek J, et al. Duration of immunosuppressive treatment for chronic graft-versus-host disease. Blood. 2004;104:3501–3506. doi: 10.1182/blood-2004-01-0200. [DOI] [PubMed] [Google Scholar]
- 8.Flowers MED, Inamoto Y, Carpenter PA, et al. Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood. 2011;117:3214–3219. doi: 10.1182/blood-2010-08-302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolff D, Gerbitz A, Ayuk F, et al. Consensus conference on clinical practice in chronic graft-versus-host disease (GVHD): first-line and topical treatment of chronic GVHD. Biology of Blood and Marrow Transplantation. 2010;16:1611–1628. doi: 10.1016/j.bbmt.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 10.Wagner JE, Thompson JS, Carter SL, Kernan NA. Effect of graft-versus-host disease prophylaxis on 3-year disease-free survival in recipients of unrelated donor bone marrow (T-cell Depletion Trial): a multi-centre, randomised phase II-III trial. Lancet. 2005;366:733–741. doi: 10.1016/S0140-6736(05)66996-6. [DOI] [PubMed] [Google Scholar]
- 11.Marmont AM, Horowitz MM, Gale RP, et al. T-cell depletion of HLA-identical transplants in leukemia. Blood. 1991;78:2120–2130. [PubMed] [Google Scholar]
- 12.Luznik L, O'Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, post-transplantation cyclophosphamide. Biology of Blood and Marrow Transplantation. 2008;14:641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mielcarek M, Furlong T, O'Donnell PV, et al. Posttransplantation cyclophosphamide for prevention of graft-versus-host disease after HLA-matched mobilized blood cell transplantation. Blood. 2016;127:1502–1508. doi: 10.1182/blood-2015-10-672071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sullivan KM, Shulman HM, Storb R, et al. Chronic graft-versus-host disease in 52 patients: adverse natural course and successful treatment with combination immunosuppression. Blood. 1981;57:267–276. [PubMed] [Google Scholar]
- 15.Koc S, Leisenring W, Flowers MED, et al. Thalidomide for treatment of patients with chronic graft-versus-host disease. Blood. 2000;96:3995–3996. [PubMed] [Google Scholar]
- 16.Arora M, Wagner JE, Davies SM, et al. Randomized clinical trial of thalidomide, cyclosporine, and prednisone versus cyclosporine and prednisone as initial therapy for chronic graft-versus-host disease. Biology of Blood & Marrow Transplantation. 2001;7:265–273. doi: 10.1053/bbmt.2001.v7.pm11400948. [DOI] [PubMed] [Google Scholar]
- 17.Koc S, Leisenring W, Flowers MED, et al. Therapy for chronic graft-versus-host disease: a randomized trial comparing cyclosporine plus prednisone versus prednisone alone. Blood. 2002;100:48–51. doi: 10.1182/blood.v100.1.48. [DOI] [PubMed] [Google Scholar]
- 18.Martin PJ, Storer BE, Rowley SD, et al. Evaluation of mycophenolate mofetil for initial treatment of chronic graft-versus-host disease. Blood. 2009;113:5074–5082. doi: 10.1182/blood-2009-02-202937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu YW, Gress RE. Murine models of chronic graft-versus-host disease: insights and unresolved issues (Review) Biology of Blood & Marrow Transplantation. 2008;14:365–378. doi: 10.1016/j.bbmt.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deeg HJ, Storb R, Weiden PL, et al. Cyclosporin A and methotrexate in canine marrow transplantation: engraftment, graft-versus-host disease, and induction of tolerance. Transplantation. 1982;34:30–35. doi: 10.1097/00007890-198207000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Wagner JL, Burnett RC, Storb R. Molecular analysis of the DLA DR region. Tissue Antigens. 1996;48:549–553. doi: 10.1111/j.1399-0039.1996.tb02668.x. [DOI] [PubMed] [Google Scholar]
- 22.Wagner JL, Burnett RC, DeRose SA, Francisco LV, Storb R, Ostrander EA. Histocompatibility testing of dog families with highly polymorphic microsatellite markers. Transplantation. 1996;62:876–877. doi: 10.1097/00007890-199609270-00032. [DOI] [PubMed] [Google Scholar]
- 23.Wagner JL, Works JD, Storb R. DLA-DRB1 and DLA-DQB1 histocompatibility typing by PCR-SSCP and sequencing (Brief Communication) Tissue Antigens. 1998;52:397–401. doi: 10.1111/j.1399-0039.1998.tb03063.x. [DOI] [PubMed] [Google Scholar]
- 24.Hilgendorf I, Weirich V, Zeng L, et al. Canine haematopoietic chimerism analyses by semiquantitative fluorescence detection of variable number of tandem repeat polymorphism. Veterinary Research Communications. 2005;29:103–110. doi: 10.1023/b:verc.0000047486.01458.c5. [DOI] [PubMed] [Google Scholar]
- 25.Graves SS, Hogan W, Kuhr CS, et al. Stable trichimerism after marrow grafting from 2 DLA-identical canine donors and nonmyeloablative conditioning. Blood. 2007;110:418–423. doi: 10.1182/blood-2007-02-071282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y, Huang XJ, Liu KY, et al. Infusion-related febrile reaction after haploidentical stem cell transplantation in children is associated with higher rates of engraftment syndrome and acute graft-versus-host disease. Pediatr Transplant. 2015;19:918–924. doi: 10.1111/petr.12586. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen JA, Law RM, Fiman KH, Roberts CA. Esophageal lichen planus: a case report and review of the literature. World J Gastroenterol. 2013;19:2278–2281. doi: 10.3748/wjg.v19.i14.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shulman HM, Sale GE, Lerner KG, et al. Chronic cutaneous graft-versus-host disease in man. American Journal of Pathology. 1978;91:545–570. [PMC free article] [PubMed] [Google Scholar]
- 29.Atkinson K, Shulman HM, Deeg HJ, et al. Acute and chronic graft-versus-host disease in dogs given hemopoietic grafts from DLA-nonidentical littermates: two distinct syndromes. American Journal of Pathology. 1982;108:196–205. [PMC free article] [PubMed] [Google Scholar]
- 30.Pidala J, Sarwal M, Roedder S, Lee SJ. Biologic markers of chronic GVHD. Bone Marrow Transplantation. 2013;49:324–331. doi: 10.1038/bmt.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and Staging Working Group report. Biology of Blood and Marrow Transplantation. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Socie G, Ritz J, Martin PJ. Current challenges in chronic graft-versus-host disease. Biology of Blood and Marrow Transplantation. 2010;16(Suppl):S146–S151. doi: 10.1016/j.bbmt.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 33.Atkinson K, Storb R, Weiden PL, Deeg HJ, Gerhard-Miller L, Thomas ED. In vitro tests correlating with presence or absence of graft- vs -host disease in DLA nonidentical canine radiation chimeras: evidence that clonal abortion maintains stable graft-host tolerance. Journal of Immunology. 1980;124:1808–1814. [PubMed] [Google Scholar]
- 34.Deeg HJ, Storb R, Appelbaum FR, Kennedy MS, Graham TC, Thomas ED. Combined immunosuppression with cyclosporine and methotrexate in dogs given bone marrow grafts from DLA-haploidentical littermates. Transplantation. 1984;37:62–65. doi: 10.1097/00007890-198401000-00017. [DOI] [PubMed] [Google Scholar]
- 35.Yu C, Seidel K, Nash RA, et al. Synergism between mycophenolate mofetil and cyclosporine in preventing graft-versus-host disease among lethally irradiated dogs given DLA-nonidentical unrelated marrow grafts. Blood. 1998;91:2581–2587. [PubMed] [Google Scholar]
- 36.Sato M, Storb R, Loretz C, et al. Inducible costimulator (ICOS) up-regulation on activated T cells in graft-versus-host disease after dog leukocyte antigen-nonidentical hematopoietic cell transplantation: a potential therapeutic target. Transplantation. 2013;96:34–41. doi: 10.1097/TP.0b013e318295c025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li J, Semple K, Suh WK, et al. Roles of CD28, CTLA4, and inducible costimulator in acute graft-versus-host disease in mice. Biology of Blood & Marrow Transplantation. 2011;17:962–969. doi: 10.1016/j.bbmt.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fujimura J, Takeda K, Kaduka Y, et al. Contribution of B7RP-1/ICOS co-stimulation to lethal acute GVHD. Pediatric Transplantation. 2010;14:540–548. doi: 10.1111/j.1399-3046.2009.01279.x. [DOI] [PubMed] [Google Scholar]
- 39.Pidala J, Chai X, Kurland BF, et al. Analysis of gastrointestinal and hepatic chronic graft-versus-host disease manifestations on major outcomes: a Chronic Graft-Versus-Host Disease Consortium study. Biology of Blood & Marrow Transplantation. 2013;19:784–791. doi: 10.1016/j.bbmt.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cocho L, Fernandez I, Calonge M, et al. Biomarkers in ocular chronic graft versus host disease: tear cytokine- and chemokine-based predictive model. Invest Ophthalmol Vis Sci. 2016;57:746–758. doi: 10.1167/iovs.15-18615. [DOI] [PubMed] [Google Scholar]
- 41.DiCarlo J, Agarwal-Hashmi R, Shah A, et al. Cytokine and chemokine patterns across 100 days after hematopoietic stem cell transplantation in children. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2014;20:361–369. doi: 10.1016/j.bbmt.2013.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, McCormick LL, Desai SR, Wu C, Gilliam AC. Murine sclerodermatous graft-versus-host disease, a model for human scleroderma: cutaneous cytokines, chemokines, and immune cell activation. Journal of Immunology. 2002;168:3088–3098. doi: 10.4049/jimmunol.168.6.3088. [DOI] [PubMed] [Google Scholar]
- 43.McCormick LL, Zhang Y, Tootell E, Gilliam AC. Anti-TGF-beta treatment prevents skin and lung fibrosis in murine sclerodermatous graft-versus-host disease: a model for human scleroderma. Journal of Immunology. 1999;163:5693–5699. [PubMed] [Google Scholar]
- 44.Jaffee BD, Claman HN. Chronic graft-versus-host disease (GVHD) as a model for scleroderma. I. Description of model systems. Cellular Immunology. 1983;77:1–12. doi: 10.1016/0008-8749(83)90001-1. [DOI] [PubMed] [Google Scholar]
- 45.Claman HN, Jaffee BD, Huff JC, Clark RA. Chronic graft-versus-host disease as a model for scleroderma. II. Mast cell depletion with deposition of immunoglobulins in the skin and fibrosis. Cellular Immunology. 1985;94:73–84. doi: 10.1016/0008-8749(85)90086-3. [DOI] [PubMed] [Google Scholar]
- 46.Levy S, Nagler A, Okon S, Marmary Y. Parotid salivary gland dysfunction in chronicgraft-versus-host disease (cGVHD): a longitudinal study in a mouse model. Bone Marrow Transplant. 2000;25:1073–1078. doi: 10.1038/sj.bmt.1702383. [DOI] [PubMed] [Google Scholar]
- 47.Slayback DL, Dobkins JA, Harper JM, Allen RD. Genetic factors influencing the development of chronic graft-versus-host disease in a murine model. Bone Marrow Transplant. 2000;26:931–938. doi: 10.1038/sj.bmt.1702661. [DOI] [PubMed] [Google Scholar]
- 48.Via CS, Sharrow SO, Shearer GM. Role of cytotoxic T lymphocytes in the prevention of lupus-like disease occurring in a murine model of graft-vs-host disease. J Immunol. 1987;139:1840–1849. [PubMed] [Google Scholar]
- 49.De Wit D, Van Mechelen M, Zanin C, et al. Preferential activation of Th2 cells in chronic graft-versus-host reaction. Journal of Immunology. 1993;150:361–366. [PubMed] [Google Scholar]
- 50.Tschetter JR, Mozes E, Shearer GM. Progression from acute to chronic disease in a murine parent-into-F1 model of graft-versus-host disease. J Immunol. 2000;165:5987–5994. doi: 10.4049/jimmunol.165.10.5987. [DOI] [PubMed] [Google Scholar]
- 51.Srinivasan M, Flynn R, Price A, et al. Donor B-cell alloantibody deposition and germinal center formation are required for the development of murine chronic GVHD and bronchiolitis obliterans. Blood. 2012;119:1570–1580. doi: 10.1182/blood-2011-07-364414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flynn R, Du J, Veenstra RG, et al. Increased T follicular helper cells and germinal center B cells are required for cGVHD and bronchiolitis obliterans. Blood. 2014;123:3988–3998. doi: 10.1182/blood-2014-03-562231. [DOI] [PMC free article] [PubMed] [Google Scholar]