Abstract
The concept that developmental events shape adult health and disease was sparked by the recognition of a link between maternal undernutrition and coronary disease in adults. From that beginning, a new field—the developmental origins of health and disease—emerged, and attention has focused on the effects of a wide array of developmental perturbations. Exposure to endocrine-disrupting chemicals has been of particular interest, and a ubiquitous environmental contaminant bisphenol A (BPA) has become the endocrine-disrupting chemical poster child. Bisphenol A has been the subject of intense investigation for nearly two decades, and exposure effects have been described in hundreds of experimental, epidemiological, and clinical studies. From the standpoint of reproductive health, the findings are particularly important, as they suggest that the ovary, testis, and reproductive tract in both sexes are targets of BPA action. The findings and the media and regulatory attention garnered by them have generated increasing public concern and resulted in legislative bans on BPA in some countries. The subsequent introduction of BPA-free products, although a masterful marketing strategy, is in reality only the beginning of a new and complex chapter of the BPA story. In this review we attempt to summarize what we have learned about the reproductive effects of BPA, present the reasons why studying the effects of this chemical in humans is no longer sufficient, and outline the challenges that the growing array of next generation bisphenols represents to clinicians, researchers, federal agencies, and the general public.
Keywords: Bisphenol, reproductive health, epigenetic, testis, ovary, endocrine-disrupting chemicals
Aseries of experimental studies at the turn of the century suggested effects on both male and female reproduction as a result of bisphenol A (BPA) exposure (1–7). In the intervening 15 years, intense research effort has been devoted to understanding the levels and routes by which humans are exposed to BPA and the risks imposed by this exposure. Most attention has focused on fetal or neonatal exposure, and subtle changes induced during development have been linked to metabolic, behavioral, and reproductive abnormalities in adults, as well as to increases in reproductive cancers (8). These findings led to growing consumer awareness and concern about this ubiquitous endocrine-disrupting chemical (EDC), resulting in legislative bans on the use of BPA in some types of consumer products (e.g., baby bottles) in several countries including Canada, France, and the United States. As a result, new products have rapidly transformed the marketplace, with the appearance of “BPA-free” plastics, thermal paper, and linings of food and beverage containers. However, although the BPA-free label has proved a valuable marketing strategy, it provides no guarantee of a safer product; in most products, BPA has been replaced by other bisphenols.
We summarize the most recent data on the reproductive effects of BPA exposure in men and women and what we know about human exposure and metabolism. We also summarize emerging research on BPA analogues, the ramifications of the rapid emergence of these structural variants of BPA, and the challenges they present for researchers and clinicians.
THE TESTIS: BPA, TESTICULAR DYSGENESIS, AND RISKS TO SUBSEQUENT GENERATIONS
What is known at present as the Estrogen Hypothesis grew out of observations in men. Analyses of semen samples collected between 1938 and 1991 in the United States and Europe suggested a decline over time in semen volume and sperm number and morphology (9). These changes in gamete production appeared to correlate with increases in the incidence of male reproductive disorders, which included undescended testes, urethral malformations, and reproductive tract cancers (9) (reviewed by Skakkebaek et al. [10]). This constellation of male reproductive disorders—known as testicular dysgenesis syndrome (10)—was postulated to result from exposure during development to exogenous estrogens entering our daily lives through foods, consumer products, and pollution (11).
Experimental studies of a number of different environmental estrogens demonstrate that developmental exposure induces changes to the male reproductive tract and testes, providing support for the estrogen hypothesis (reviewed by Sweeney et al. [12] and Skakkebaek [13]). In addition, studies of different EDCs including BPA suggest that the effects of exposure are not limited to the exposed individual, but may affect subsequent generations. The multigenerational and transgenerational inheritance of abnormal phenotypes induced by EDCs has been the subject of several recent reviews (e.g., Xin et al. [14] and Stel et al. [15]). These effects on subsequent generations are thought to result from epigenetic changes but, as discussed later, the link between exposure and altered epigenetic regulation remains unclear and is the subject of intense current investigation.
In the male, effects induced by fetal and early postnatal BPA exposure include changes in sperm and semen quality, hormonal imbalance, and malformations of the reproductive tract (reviewed by Peretz et al. [16]). For example, in rodents, exposure during testis development has been suggested to alter Sertoli cell number (17, 18), Leydig cell function (as evidenced by changes in steroidogenesis [19–21]), and to decrease sperm production (1). Importantly, errors occurring during reproductive tract development prime the male for adult diseases, including cancers of the testis and prostate (22, 23).
Several reports indicate that the deleterious effects of BPA may be transmitted to subsequent, unexposed generations (reviewed in Xin et al. [14]). Because exposure can directly affect the sperm produced, it is easy to understand how phenotypic effects can manifest in the offspring of exposed males. Effects that persist in unexposed generations (i.e., in the grandsons of exposed males) are more difficult to comprehend. This type of transgenerational inheritance not only requires epigenetic changes in the germline of the exposed male, but failure to erase these changes during germline reprogramming in the subsequent generation (see Xin et al. [14] for an excellent recent review). Metabolic, behavioral, and testicular phenotypes resulting from gestational exposure to BPA (24, 25) or to mixtures of plastic chemicals (26) have been reported to be transmitted to unexposed sons and grandsons, suggesting that exposure during specific stages of development may induce transgenerational effects.
Changes that endure across generations are typically thought to result from DNA mutations, but the reproducibility of transgenerational phenotypes induced by BPA and other EDCs implicate alterations to the epigenome rather than genetic mutations. Epigenetic modifications include changes in DNA methylation, histone post-translational modifications, and noncoding RNAs, and the mechanism(s) through which BPA induces transgenerational phenotypes remain unknown. Although several reports suggest that BPA exposure can induce changes in DNA methylation patterns in somatic tissues ([27–29]; reviewed in Xin et al. [14]), and a single study (26) has reported DNA methylation changes in mature sperm two generations after exposure, at present no study has demonstrated the induction of specific germline changes and their persistence across generations.
Because the transmission of disease phenotypes to subsequent generations necessitates passage through the germline, a recent report (30) of permanent changes induced in spermatogonial stem cells is particularly interesting. Specifically, perinatal exposure to BPA or ethinyl E2 (EE) was reported to induce meiotic perturbations that could be traced back to changes in the spermatogonial stem cells of exposed males (30). Changes induced in spermatogonial stem cells will not only be transmitted to all spermatogenic descendants produced during the lifetime of the male, but can be passed to the next generation through mature sperm. Thus, epigenetic changes to the spermatogonial stem cell itself may be responsible for transmission of disease phenotypes in unexposed descendants. However, we currently have no understanding of the mechanisms that allow epimutations in the germline induced by BPA or other EDCs to escape the genomewide epigenetic reprogramming that occurs during fetal and perinatal development.
THE OVARY: VULNERABILITY AT MULTIPLE STAGES
Experimental data suggest that the ovary is sensitive to BPA exposure during at least three distinct stages of development, and involving multiple processes, including meiotic chromosome dynamics, follicle development and growth, and epigenetic reprogramming (reviewed by Peretz et al. [16]). Data from experimental studies suggest that maternal exposure during midgestation coinciding with the onset of oogenesis in the fetal ovary can alter synapsis and/or recombination between meiotic chromosomes in oocytes from mice, monkeys, and cultured human fetal ovaries (31–33). Importantly, the subtle meiotic changes induced during this fetal stage of oogenesis increase the frequency of chromosomally abnormal eggs and embryos in the adult (31). Exposure at a slightly later stage of development (late gestation in the rhesus monkey and immediately after birth in the mouse) disrupts the formation of ovarian follicles, leading to the generation of multioocyte follicles and unenclosed oocytes (32, 34, 35), altering viable follicle populations in the adult. Given these experimental data, the expectation for adult women exposed in utero would be, as follows: [1] an increase in pregnancy failure and birth defects, if exposure coincides with the onset of meiosis in the fetal ovary, and [2] a decreased ovarian reserve and potential shortening of the reproductive lifespan, if exposure occurs at the time of follicle formation. However, because records of an adult’s in utero exposure are currently unattainable, the potential effects of human exposure can only be extrapolated from animal data.
In addition to developmental exposure, experimental data provide compelling evidence that adult exposure can adversely affect later stages of oocyte development, including follicle growth and meiotic maturation. Studies using high and low doses of BPA suggest a nonlinear dose response relationship between BPA exposure and follicle growth. Specifically, BPA has been reported to inhibit steroidogenesis and growth of mouse follicles in vitro (36, 37), although lower concentrations have been reported to accelerate follicle growth and alter the epigenetic reprogramming that occurs in the oocyte at this time ([38] and reviewed in Peretz et al. [16]). Similarly, high urinary BPA concentrations in women have been linked to decreases in peak E2 levels (39), antral follicle numbers (40), oocyte yield, and success in the assisted reproduction technology (ART) setting (39, 41).
Ample evidence suggests that oocyte meiotic maturation also can be affected by BPA exposure. The first reported effect of BPA on the oocyte was of an exposure to the periovulatory follicle and an effect on meiotic chromosome alignment and segregation that increased the incidence of chromosomally abnormal eggs in mice (7). Numerous subsequent studies (reviewed in Peretz et al. [16] and see [42–44]) have provided support for the detrimental effects of BPA on oocyte maturation. Thus, there is ample evidence that the periovulatory oocyte in a range of species—including humans—is vulnerable to the effects of BPA exposure. Further, these data, in conjunction with the data on meiotic effects from fetal exposure, perfectly recapitulate what we know about the human maternal age effect on aneuploidy: events during pre- and postnatal development influence the likelihood of producing a chromosomally abnormal egg (45).
Our understanding of the mechanisms through which BPA exerts its effects on the ovary and oocyte remains rudimentary. However, estrogen receptor β (ER-β) and aryl hydrocarbon receptor-mediated pathways have been implicated in fetal meiosis and adult follicle growth, respectively (31, 37). Importantly, experimental data suggest that BPA exposure also interferes with the epigenetic reprogramming of the female germline that occurs during follicle growth (38, 46) and in the early preimplantation embryo ([47] and reviewed in Eichenlaub-Ritter and Pacchierotti [48]). Although direct links between BPA, receptor signaling, and epigenetic modifications in the oocyte have not yet been delineated, the role of the epigenome in fetal development and adult disease makes this is a critical area of investigation.
In summary, evidence from a variety of studies suggests that multiple stages of oocyte development can be adversely affected by BPA exposure. Further, there is growing evidence that times of chromatin remodeling and imprint establishment during gametogenesis in both sexes are particularly vulnerable.
HUMAN BPA EXPOSURE: WHAT WE KNOW AT PRESENT
For many years, the consumption of contaminated food and beverages was assumed to be the major route of human exposure to BPA. Because BPA that is swallowed is subject to rapid first-pass metabolism by the liver, it was argued that exposure to the bioactive parent compound would be brief and negligible, therefore BPA posed little risk to humans. Data from biomonitoring studies, however, have been inconsistent with this assumption, reporting higher levels of bioactive BPA in human serum than expected for BPA absorbed from the gastrointestinal tract (49–51). It has been argued that these studies were flawed, and the reported levels of unconjugated BPA resulted from contamination ([52]; for a review, see Vom Saal and Welshons [50]). As detailed later, issues surrounding the measurement of BPA and the control of contamination have been resolved and, importantly, growing evidence from human and experimental studies suggests that nonoral routes contribute significantly to human BPA exposure (50, 51, 53–55).
Human studies of adult subjects, although limited in number, provide direct evidence that the duration of BPA exposure depends on the route by which it enters the body: BPA that is swallowed (i.e., as a capsule or in soup) is rapidly metabolized (56, 57), but metabolism of BPA absorbed dermally and sublingually may be less efficient (54). Data from dogs (58), sheep (59), and monkeys (51, 60) suggest that the duration of exposure to unconjugated BPA is influenced by how long food containing BPA is held in the mouth, because BPA absorbed sublingually or from buccal tissues circumvents rapid first-pass metabolism. Similarly, sustained levels of bioactive BPA in human blood were reported when BPA was absorbed through the skin from the handling of thermal receipt paper (54). In summary, the combined data from human and experimental studies suggest that the level and duration of exposure to unconjugated BPA—which, until recently, was assumed to be the only bioactive form—depend on the route of exposure. In addition, data from studies in nonhuman primates suggest that BPA metabolism is altered by pregnancy, changes during the course of gestation (51), and that BPA rapidly crosses the placenta (51, 61). Notably, levels of unconjugated BPA found in the fetal compartment are sufficient to induce developmental effects in experimental studies (32, 51, 62–66).
Questions about the validity of BPA measurements in human serum due to concerns surrounding the analytical methodology used and the potential for sample contamination prompted the National Institutes of Health, National Institutes of Environmental Health Sciences to undertake a collaborative “round robin” study (49). Four laboratories participated, with the goal of establishing reliable protocols for the reproducible measurement of BPA in body fluids and tissues. This study demonstrated that contamination could be detected and eliminated from collection materials and regents through screening. It also led to the development of new methodology for the direct analysis of BPA in human biospecimens (67) and the ability to reliably and accurately measure unconjugated BPA and its two major metabolites, BPA glucuronide and BPA sulfate. The ability to identify individual metabolites using direct detection methods makes it possible to detect metabolic differences among individuals, identify allelic variants (e.g., in metabolic enzymes) that affect BPA metabolism, and to determine whether specific genetic variants render an individual more vulnerable to the effects of exposure (67). This is particularly important in light of recent data suggesting that the glucuronidated form exhibits estrogenic bioactivity (68).
The implications of the advances in our understanding of BPA metabolism and the effect of exposure route, coupled with new concerns about the bioactivity of metabolites, are serious. Estimates of human exposure made in the past decade have been based on multiple erroneous assumptions: that most of our exposure is oral, that BPA is rapidly metabolized, and that metabolites are harmless. Thus, human exposure has been significantly underestimated. As detailed later, however, other recent developments have served to expand concern beyond BPA.
BPA-FREE: NEW PROBLEMS, NOT SOLUTIONS
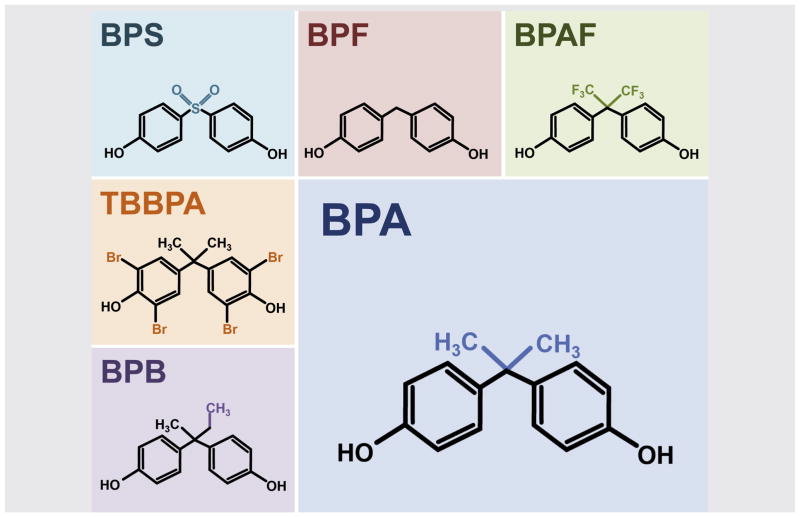
Nearly a decade after the initial reports of detrimental effects of developmental BPA exposure, consumer concern spurred bans in several countries on the use of BPA in specific products, most commonly products for infants and toddlers. Growing public awareness has fueled consumer demand for “BPA-free” products, including plastics, thermal paper, and linings of food and beverage containers. In most products, however, the BPA-free label provides no guarantee of a safer product, as BPA has been replaced by other bisphenols (Fig. 1). As discussed, the rapid emergence of these structural variants of bisphenol has important ramifications. Although the metabolism and endocrine disrupting properties of these new compounds have not been characterized, they have already begun to emerge as biocontaminants.
FIGURE 1.
Structural comparison of bisphenol A (BPA) and replacement bisphenols. The five common bisphenols in current use, Bisphenol-B (BPB), bisphenol-F (BPF), bisphenol-S (BPS), bisphenol-AF (BPAF), and tetrabromobisphenol-A (TBBPA), are structural analogues of BPA. All except TBBPA are structurally identical to BPA, with simple modifications of the BPA side chain.
The list of bisphenol analogues in current use is long and growing. Presently, Bisphenol-S, F, B, and AF are among the most common in consumer goods (for a review, see Caballero-Casero et al. [69]), although this statement will likely be out of date by the time it is published. Their rapid introduction makes understanding the physiological effects of these chemicals a daunting task. Bisphenol A, for example, was originally characterized as a weak estrogen based on its binding affinity for the classic ERs but, through extensive investigation, has proved to be a remarkable chemical chameleon. In mammalian cells, BPA exhibits many actions that vary according to dose, tissue, and developmental stage and include effects beyond strictly “estrogenic” and “antiestrogenic,” downstream of several different receptor types (70, 71). At present, little is known about individual replacement bisphenols but, because structural variation can alter receptor-binding affinity (72), it seems likely that biological effects will vary among bisphenols. However, the limited data from reproductive and other tissues provide preliminary evidence that, like BPA, other bisphenols induce developmental effects ([73–75]; reviewed in Rochester and Bolden [76]). For example, BPS and BPF exposure exert effects similar to those reported for BPA on testis gene expression and steroidogenesis in male rats (77); BPAF exposure disrupts mouse oocyte maturation to a similar or greater extent than BPA (42); studies in zebrafish show that the effects of BPS on the reproductive neuroendocrine pathway are similar to BPA (78). However, as with BPA, the nature of the specific effects of exposure undoubtedly depends on the developmental timing of exposure and the tissue examined. Thus, each new bisphenol will require extensive characterization. Each will also require the development of new analytical tools for accurate biomonitoring. Finally, and most importantly, the introduction of multiple structurally similar compounds presents a serious confounder to our efforts to understand the impact of BPA exposure on human reproductive health.
Recent profiling of bisphenols leaching from beverage containers detected, on average, 1.26 μg/L BPA in beverages along with an additional 1.00 μg/L BPF (79), and a study of bisphenol contamination in prepared foods yielded similar results (80). Thus, total bisphenol levels are likely substantially higher than levels of BPA alone, and understanding human exposure and the risks posed to human health requires a complete bisphenol profile. That is, the rapid emergence of replacement bisphenols not only complicates attempts to understand the risks posed by human BPA exposure, it amplifies the risk assessment problem because the risk posed by each new bisphenol is now a concern. Furthermore, because humans are exposed to many EDCs, an understanding of how bisphenols act and interact, not only with each other, but with other EDCs is essential. From studies in mice, we know that mixtures of estrogenic compounds can interfere with one another in vivo. Dietary phytoestrogens affect fetal but not maternal E2 levels (81), and can impact the dose-response relationship between BPA and effects on meiotic maturation in the periovulatory oocyte (82). Similarly, a recent study (83) in humans found that the correlation between high urinary BPA and poor ART success in women undergoing fertility treatment was influenced by the consumption of soy products. Thus, although they are structurally similar, bisphenols may exhibit markedly different receptor-binding behaviors and will not necessarily have additive effects in a mixture. Although sophisticated pathway modeling in silico may aid in parsing out the interactions of chemicals in mixtures, an understanding for each bisphenol of the pathway activation and downstream effects in individual tissues and during different developmental stages will be essential.
SUMMARY AND FUTURE DIRECTIONS
Data amassed from the study of BPA during the past two decades suggest that human exposure to this chemical is nearly constant and poses a major risk to male and female reproductive health. Increased consumer awareness and concern has led to the rapid replacement of BPA with other “next generation” bisphenols. Limited data available on the first group of these structurally similar replacement bisphenols suggest that their biological activity is similar to BPA and they are not safer alternatives to BPA. Tracking the emergence of new bisphenol contaminants, however, represents a major technical challenge that requires the development of new analytical tools. The challenges posed by this class of chemicals require more than new technology; the collaborative efforts of researchers, clinicians, and government agencies will be essential in understanding how humans are exposed to and metabolize bisphenols, the tissue and stage-specific effects of individual bisphenols, and the actions and interactions of mixtures of bisphenols and other EDCs in the body. Importantly, although the evidence of risk for BPA is significant and has prompted legislative action in a number of countries, the rapid release of new bisphenols onto the market undermines current and future risk assessment efforts. Thus, a critical and immediate societal challenge is the development of new methods of determining the biological actions and assessing the potential risk of chemicals before they are marketed.
Acknowledgments
Supported by grants from National Institute of Environmental Health Sciences F32 ES0206010 (C.V.S.), National Institute of Child Health and Human Development R01 HD083177 (P.A.H.), and National Institute of Environmental Health Sciences R01 ES013527 (P.A.H.).
The authors thank Terry Hassold for helpful comments on the manuscript; Crystal Lawson for help with the figure; and the contributions of numerous researchers in the field whose work could not be directly cited in this review due to space limitations.
Footnotes
C.V.S. has nothing to disclose. P.A.H. has nothing to disclose.
References
- 1.Vom Saal FS, Cooke PS, Buchanan DL, Palanza P, Thayer KA, Nagel SC, et al. A physiologically based approach to the study of bisphenol A and other estrogenic chemicals on the size of reproductive organs, daily sperm production, and behavior. Toxicol Ind Health. 1998;14:239–60. doi: 10.1177/074823379801400115. [DOI] [PubMed] [Google Scholar]
- 2.Howdeshell KL, Hotchkiss AK, Thayer KA, Vandenbergh JG, vom Saal FS. Exposure to bisphenol A advances puberty. Nature. 1999;401:763–4. doi: 10.1038/44517. [DOI] [PubMed] [Google Scholar]
- 3.Rubin BS, Murray MK, Damassa DA, King JC, Soto AM. Perinatal exposure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels. Environ Health Perspect. 2001;109:675–80. doi: 10.1289/ehp.01109675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakaue M, Ohsako S, Ishimura R, Kurosawa S, Kurohmaru M, Hayashi Y, et al. Bisphenol-A affects spermatogenesis in the adult rat even at a low dose. J Occup Health. 2001;43:185–90. [Google Scholar]
- 5.Suzuki A, Sugihara A, Uchida K, Sato T, Ohta Y, Katsu Y, et al. Developmental effects of perinatal exposure to bisphenol-A and diethylstilbestrol on reproductive organs in female mice. Reprod Toxicol. 2002;16:107–16. doi: 10.1016/s0890-6238(02)00005-9. [DOI] [PubMed] [Google Scholar]
- 6.Schonfelder G, Flick B, Mayr E, Talsness C, Paul M, Chahoud I. In utero exposure to low doses of bisphenol A lead to long-term deleterious effects in the vagina. Neoplasia. 2002;4:98–102. doi: 10.1038/sj.neo.7900212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunt PA, Koehler KE, Susiarjo M, Hodges CA, Ilagan A, Voigt RC, et al. Bisphenol a exposure causes meiotic aneuploidy in the female mouse. Curr Biol. 2003;13:546–53. doi: 10.1016/s0960-9822(03)00189-1. [DOI] [PubMed] [Google Scholar]
- 8.Rubin BS. Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects. J Steroid Biochem Mol Biol. 2011;127:27–34. doi: 10.1016/j.jsbmb.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992;305:609–13. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod. 2001;16:972–8. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- 11.Sharpe RM, Skakkebaek NE. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet. 1993;341:1392–5. doi: 10.1016/0140-6736(93)90953-e. [DOI] [PubMed] [Google Scholar]
- 12.Sweeney MF, Hasan N, Soto AM, Sonnenschein C. Environmental endocrine disruptors: Effects on the human male reproductive system. Rev Endocr Metab Disord. 2015;16:341–57. doi: 10.1007/s11154-016-9337-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skakkebaek NE. A brief review of the link between environment and male reproductive health: lessons from studies of testicular germ cell cancer. Horm Res Paediatr. 2016 doi: 10.1159/000443400. http://dx.doi.org/10.1159/000443400. [DOI] [PubMed]
- 14.Xin F, Susiarjo M, Bartolomei MS. Multigenerational and transgenerational effects of endocrine disrupting chemicals: A role for altered epigenetic regulation? Semin Cell Dev Biol. 2015;43:66–75. doi: 10.1016/j.semcdb.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stel J, Legler J. The role of epigenetics in the latent effects of early life exposure to obesogenic endocrine disrupting chemicals. Endocrinology. 2015;156:3466–72. doi: 10.1210/en.2015-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peretz J, Vrooman L, Ricke WA, Hunt PA, Ehrlich S, Hauser R, et al. Bisphenol A and reproductive health: update of experimental and human evidence, 2007–2013. Environ Health Perspect. 2014;122:775–86. doi: 10.1289/ehp.1307728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iida H, Maehara K, Doiguchi M, Mori T, Yamada F. Bisphenol A-induced apoptosis of cultured rat Sertoli cells. Reprod Toxicol. 2003;17:457–64. doi: 10.1016/s0890-6238(03)00034-0. [DOI] [PubMed] [Google Scholar]
- 18.Qi S, Fu W, Wang C, Liu C, Quan C, Kourouma A, et al. BPA-induced apoptosis of rat Sertoli cells through Fas/FasL and JNKs/p38 MAPK pathways. Reprod Toxicol. 2014;50:108–16. doi: 10.1016/j.reprotox.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 19.N’Tumba-Byn T, Moison D, Lacroix M, Lecureuil C, Lesage L, Prud’homme SM, et al. Differential effects of bisphenol A and diethylstilbestrol on human, rat and mouse fetal Leydig cell function. PLoS One. 2012;7:e51579. doi: 10.1371/journal.pone.0051579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akingbemi BT, Sottas CM, Koulova AI, Klinefelter GR, Hardy MP. Inhibition of testicular steroidogenesis by the xenoestrogen bisphenol A is associated with reduced pituitary luteinizing hormone secretion and decreased steroidogenic enzyme gene expression in rat Leydig cells. Endocrinology. 2004;145:592–603. doi: 10.1210/en.2003-1174. [DOI] [PubMed] [Google Scholar]
- 21.Salian S, Doshi T, Vanage G. Neonatal exposure of male rats to Bisphenol A impairs fertility and expression of sertoli cell junctional proteins in the testis. Toxicology. 2009;265:56–67. doi: 10.1016/j.tox.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Atanassova N, McKinnell C, Walker M, Turner KJ, Fisher JS, Morley M, et al. Permanent effects of neonatal estrogen exposure in rats on reproductive hormone levels, Sertoli cell number, and the efficiency of spermatogenesis in adulthood. Endocrinology. 1999;140:5364–73. doi: 10.1210/endo.140.11.7108. [DOI] [PubMed] [Google Scholar]
- 23.Howdeshell KL, Furr J, Lambright CR, Wilson VS, Ryan BC, Gray LE., Jr Gestational and lactational exposure to ethinyl estradiol, but not bisphenol A, decreases androgen-dependent reproductive organ weights and epididymal sperm abundance in the male long evans hooded rat. Toxicol Sci. 2008;102:371–82. doi: 10.1093/toxsci/kfm306. [DOI] [PubMed] [Google Scholar]
- 24.Susiarjo M, Xin F, Bansal A, Stefaniak M, Li C, Simmons RA, et al. Bisphenol a exposure disrupts metabolic health across multiple generations in the mouse. Endocrinology. 2015;156:2049–58. doi: 10.1210/en.2014-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolstenholme JT, Goldsby JA, Rissman EF. Transgenerational effects of prenatal bisphenol A on social recognition. Horm Behav. 2013;64:833–9. doi: 10.1016/j.yhbeh.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PLoS One. 2013;8:e55387. doi: 10.1371/journal.pone.0055387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weinhouse C, Bergin IL, Harris C, Dolinoy DC. Stat3 is a candidate epigenetic biomarker of perinatal Bisphenol A exposure associated with murine hepatic tumors with implications for human health. Epigenetics. 2015;10:1099–110. doi: 10.1080/15592294.2015.1107694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci U S A. 2007;104:13056–61. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laing LV, Viana J, Dempster EL, Trznadel M, Trunkfield LA, Uren Webster TM, et al. Bisphenol A causes reproductive toxicity, decreases dnmt1 transcription, and reduces global DNA methylation in breeding zebrafish (Danio rerio) Epigenetics. 2016;11:526–38. doi: 10.1080/15592294.2016.1182272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vrooman LA, Oatley JM, Griswold JE, Hassold TJ, Hunt PA. Estrogenic exposure alters the spermatogonial stem cells in the developing testis, permanently reducing crossover levels in the adult. PLoS Genet. 2015;11:e1004949. doi: 10.1371/journal.pgen.1004949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Susiarjo M, Hassold TJ, Freeman E, Hunt PA. Bisphenol A exposure in utero disrupts early oogenesis in the mouse. PLoS Genet. 2007;3:e5. doi: 10.1371/journal.pgen.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hunt PA, Lawson C, Gieske M, Murdoch B, Smith H, Marre A, et al. Bisphenol A alters early oogenesis and follicle formation in the fetal ovary of the rhesus monkey. Proc Natl Acad Sci U S A. 2012;109:17525–30. doi: 10.1073/pnas.1207854109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brieno-Enriquez MA, Robles P, Camats-Tarruella N, Garcia-Cruz R, Roig I, Cabero L, et al. Human meiotic progression and recombination are affected by Bisphenol A exposure during in vitro human oocyte development. Hum Reprod. 2011;26:2807–18. doi: 10.1093/humrep/der249. [DOI] [PubMed] [Google Scholar]
- 34.Wang W, Hafner KS, Flaws JA. In utero bisphenol A exposure disrupts germ cell nest breakdown and reduces fertility with age in the mouse. Toxicol Appl Pharmacol. 2014;276:157–64. doi: 10.1016/j.taap.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou C, Wang W, Peretz J, Flaws JA. Bisphenol A exposure inhibits germ cell nest breakdown by reducing apoptosis in cultured neonatal mouse ovaries. Reprod Toxicol. 2015;57:87–99. doi: 10.1016/j.reprotox.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peretz J, Gupta RK, Singh J, Hernandez-Ochoa I, Flaws JA. Bisphenol A impairs follicle growth, inhibits steroidogenesis, and downregulates rate-limiting enzymes in the estradiol biosynthesis pathway. Toxicol Sci. 2011;119:209–17. doi: 10.1093/toxsci/kfq319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ziv-Gal A, Craig ZR, Wang W, Flaws JA. Bisphenol A inhibits cultured mouse ovarian follicle growth partially via the aryl hydrocarbon receptor signaling pathway. Reprod Toxicol. 2013;42:58–67. doi: 10.1016/j.reprotox.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trapphoff T, Heiligentag M, El Hajj N, Haaf T, Eichenlaub-Ritter U. Chronic exposure to a low concentration of bisphenol A during follicle culture affects the epigenetic status of germinal vesicles and metaphase II oocytes. Fertil Steril. 2013;100:1758–67. e1. doi: 10.1016/j.fertnstert.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 39.Mok-Lin E, Ehrlich S, Williams PL, Petrozza J, Wright DL, Calafat AM, et al. Urinary bisphenol A concentrations and ovarian response among women undergoing IVF. Int J Androl. 2010;33:385–93. doi: 10.1111/j.1365-2605.2009.01014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Souter I, Smith KW, Dimitriadis I, Ehrlich S, Williams PL, Calafat AM, et al. The association of bisphenol—a urinary concentrations with antral follicle counts and other measures of ovarian reserve in women undergoing infertility treatments. Reprod Toxicol. 2013;42:224–31. doi: 10.1016/j.reprotox.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ehrlich S, Williams PL, Missmer SA, Flaws JA, Ye X, Calafat AM, et al. Urinary bisphenol A concentrations and early reproductive health outcomes among women undergoing IVF. Hum Reprod. 2012;27:3583–92. doi: 10.1093/humrep/des328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakano K, Nishio M, Kobayashi N, Hiradate Y, Hoshino Y, Sato E, et al. Comparison of the effects of BPA and BPAF on oocyte spindle assembly and polar body release in mice. Zygote. 2016;24:172–80. doi: 10.1017/S0967199415000027. [DOI] [PubMed] [Google Scholar]
- 43.Ferris J, Mahboubi K, MacLusky N, King WA, Favetta LA. BPA exposure during in vitro oocyte maturation results in dose-dependent alterations to embryo development rates, apoptosis rate, sex ratio and gene expression. Reprod Toxicol. 2016;59:128–38. doi: 10.1016/j.reprotox.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 44.Machtinger R, Combelles CM, Missmer SA, Correia KF, Williams P, Hauser R, et al. Bisphenol-A and human oocyte maturation in vitro. Hum Reprod. 2013;28:2735–45. doi: 10.1093/humrep/det312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagaoka SI, Hassold TJ, Hunt PA. Human aneuploidy: mechanisms and new insights into an age-old problem. Nat Rev Genet. 2012;13:493–504. doi: 10.1038/nrg3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santangeli S, Maradonna F, Gioacchini G, Cobellis G, Piccinetti CC, Dalla Valle L, et al. BPA-induced deregulation of epigenetic patterns: Effects on female zebrafish reproduction. Sci Rep. 2016;6:21982. doi: 10.1038/srep21982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Susiarjo M, Sasson I, Mesaros C, Bartolomei MS. Bisphenol a exposure disrupts genomic imprinting in the mouse. PLoS Genet. 2013;9:e1003401. doi: 10.1371/journal.pgen.1003401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eichenlaub-Ritter U, Pacchierotti F. Bisphenol A effects on mammalian oogenesis and epigenetic integrity of oocytes: a case study exploring risks of endocrine disrupting chemicals. Biomed Res Int. 2015;2015:698795. doi: 10.1155/2015/698795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vandenberg LN, Gerona RR, Kannan K, Taylor JA, van Breemen RB, Dickenson CA, et al. A round robin approach to the analysis of bisphenol A (BPA) in human blood samples. Environ Health. 2014;13:25. doi: 10.1186/1476-069X-13-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vom Saal FS, Welshons WV. Evidence that bisphenol A (BPA) can be accurately measured without contamination in human serum and urine, and that BPA causes numerous hazards from multiple routes of exposure. Mol Cell Endocrinol. 2014;398:101–13. doi: 10.1016/j.mce.2014.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vom Saal FS, VandeVoort CA, Taylor JA, Welshons WV, Toutain PL, Hunt PA. Bisphenol A (BPA) pharmacokinetics with daily oral bolus or continuous exposure via silastic capsules in pregnant rhesus monkeys: relevance for human exposures. Reprod Toxicol. 2014;45:105–16. doi: 10.1016/j.reprotox.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Churchwell MI, Camacho L, Vanlandingham MM, Twaddle NC, Sepehr E, Delclos KB, et al. Comparison of life-stage-dependent internal dosimetry for bisphenol A, ethinyl estradiol, a reference estrogen, and endogenous estradiol to test an estrogenic mode of action in Sprague Dawley rats. Toxicol Sci. 2014;139:4–20. doi: 10.1093/toxsci/kfu021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stahlhut RW, Welshons WV, Swan SH. Bisphenol A data in NHANES suggest longer than expected half-life, substantial nonfood exposure, or both. Environ Health Perspect. 2009;117:784–9. doi: 10.1289/ehp.0800376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hormann AM, vom Saal FS, Nagel SC, Stahlhut RW, Moyer CL, Ellersieck MR, et al. Holding thermal receipt paper and eating food after using hand sanitizer results in high serum bioactive and urine total levels of bisphenol A (BPA) PLoS One. 2014;9:e110509. doi: 10.1371/journal.pone.0110509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thayer KA, Taylor KW, Garantziotis S, Schurman SH, Kissling GE, Hunt D, et al. Bisphenol A, bisphenol S, and 4-hydroxyphenyl 4-isoprooxyphenylsulfone (BPSIP) in urine and blood of cashiers. Environ Health Perspect. 2016;124:437–44. doi: 10.1289/ehp.1409427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Volkel W, Colnot T, Csanady GA, Filser JG, Dekant W. Metabolism and kinetics of bisphenol a in humans at low doses following oral administration. Chem Res Toxicol. 2002;15:1281–7. doi: 10.1021/tx025548t. [DOI] [PubMed] [Google Scholar]
- 57.Teeguarden JG, Twaddle NC, Churchwell MI, Yang X, Fisher JW, Seryak LM, et al. 24-hour human urine and serum profiles of bisphenol A: evidence against sublingual absorption following ingestion in soup. Toxicol Appl Pharmacol. 2015;288:131–42. doi: 10.1016/j.taap.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 58.Gayrard V, Lacroix MZ, Collet SH, Viguie C, Bousquet-Melou A, Toutain PL, et al. High bioavailability of bisphenol A from sublingual exposure. Environ Health Perspect. 2013;121:951–6. doi: 10.1289/ehp.1206339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guignard D, Gauderat G, Gayrard V, Lacroix MZ, Picard-Hagen N, Puel S, et al. Characterization of the contribution of buccal absorption to internal exposure to bisphenol A through the diet. Food Chem Toxicol. 2016;93:82–8. doi: 10.1016/j.fct.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 60.Taylor JA, vom Saal FS, Welshons WV, Drury B, Rottinghaus G, Hunt PA, et al. Similarity of bisphenol A pharmacokinetics in rhesus monkeys and mice: relevance for human exposure. Environ Health Perspect. 2011;119:422–30. doi: 10.1289/ehp.1002514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Patterson TA, Twaddle NC, Roegge CS, Callicott RJ, Fisher JW, Doerge DR. Concurrent determination of bisphenol A pharmacokinetics in maternal and fetal rhesus monkeys. Toxicol Appl Pharmacol. 2013;267:41–8. doi: 10.1016/j.taap.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 62.Chapalamadugu KC, Vandevoort CA, Settles ML, Robison BD, Murdoch GK. Maternal bisphenol a exposure impacts the fetal heart transcriptome. PLoS One. 2014;9:e89096. doi: 10.1371/journal.pone.0089096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Calhoun KC, Padilla-Banks E, Jefferson WN, Liu L, Gerrish KE, Young SL, et al. Bisphenol A exposure alters developmental gene expression in the fetal rhesus macaque uterus. PLoS One. 2014;9:e85894. doi: 10.1371/journal.pone.0085894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van Winkle LS, Murphy SR, Boetticher MV, VandeVoort CA. Fetal exposure of rhesus macaques to bisphenol a alters cellular development of the conducting airway by changing epithelial secretory product expression. Environ Health Perspect. 2013;121:912–8. doi: 10.1289/ehp.1206064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Elsworth JD, Jentsch JD, Vandevoort CA, Roth RH, Redmond DE, Jr, Leranth C. Prenatal exposure to bisphenol A impacts midbrain dopamine neurons and hippocampal spine synapses in non-human primates. Neurotoxicology. 2013;35:113–20. doi: 10.1016/j.neuro.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tharp AP, Maffini MV, Hunt PA, VandeVoort CA, Sonnenschein C, Soto AM. Bisphenol A alters the development of the rhesus monkey mammary gland. Proc Natl Acad Sci U S A. 2012;109:8190–5. doi: 10.1073/pnas.1120488109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gerona RR, Pan J, Zota AR, Schwartz JM, Friesen M, Taylor JA, et al. Direct measurement of Bisphenol A (BPA), BPA glucuronide and BPA sulfate in a diverse and low-income population of pregnant women reveals high exposure, with potential implications for previous exposure estimates: a crosssectional study. Environ Health. 2016;15:50. doi: 10.1186/s12940-016-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boucher JG, Boudreau A, Ahmed S, Atlas E. In vitro effects of Bisphenol A beta-D-Glucuronide (BPA-G) on adipogenesis in human and murine preadipocytes. Environ Health Perspect. 2015;123:1287–93. doi: 10.1289/ehp.1409143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Caballero-Casero N, Lunar L, Rubio S. Analytical methods for the determination of mixtures of bisphenols and derivatives in human and environmental exposure sources and biological fluids. A review Anal Chim Acta. 2016;908:22–53. doi: 10.1016/j.aca.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 70.Wetherill YB, Akingbemi BT, Kanno J, McLachlan JA, Nadal A, Sonnenschein C, et al. In vitro molecular mechanisms of bisphenol A action. Reprod Toxicol. 2007;24:178–98. doi: 10.1016/j.reprotox.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 71.Shanle EK, Xu W. Endocrine disrupting chemicals targeting estrogen receptor signaling: identification and mechanisms of action. Chem Res Toxicol. 2011;24:6–19. doi: 10.1021/tx100231n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Okada H, Tokunaga T, Liu X, Takayanagi S, Matsushima A, Shimohigashi Y. Direct evidence revealing structural elements essential for the high binding ability of bisphenol A to human estrogen-related receptor-gamma. Environ Health Perspect. 2008;116:32–8. doi: 10.1289/ehp.10587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pisapia L, del Pozzo G, Barba P, Caputo L, Mita L, Viggiano E, et al. Effects of some endocrine disruptors on cell cycle progression and murine dendritic cell differentiation. Gen Comp Endocrinol. 2012;178:54–63. doi: 10.1016/j.ygcen.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 74.Svajger U, Dolenc MS, Jeras M. In vitro impact of bisphenols BPA, BPF, BPAF and 17beta-estradiol (E2) on human monocyte-derived dendritic cell generation, maturation and function. Int Immunopharmacol. 2016;34:146–54. doi: 10.1016/j.intimp.2016.02.030. [DOI] [PubMed] [Google Scholar]
- 75.Li Y, Burns KA, Arao Y, Luh CJ, Korach KS. Differential estrogenic actions of endocrine-disrupting chemicals bisphenol A, bisphenol AF, and zearalenone through estrogen receptor alpha and beta in vitro. Environ Health Perspect. 2012;120:1029–35. doi: 10.1289/ehp.1104689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rochester JR, Bolden AL. Bisphenol S and F: a systematic review and comparison of the hormonal activity of Bisphenol A substitutes. Environ Health Perspect. 2015;123:643–50. doi: 10.1289/ehp.1408989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eladak S, Grisin T, Moison D, Guerquin MJ, N’Tumba-Byn T, Pozzi-Gaudin S, et al. A new chapter in the bisphenol A story: bisphenol S and bisphenol F are not safe alternatives to this compound. Fertil Steril. 2015;103:11–21. doi: 10.1016/j.fertnstert.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 78.Qiu W, Zhao Y, Yang M, Farajzadeh M, Pan C, Wayne NL. Actions of Bisphenol A and Bisphenol S on the reproductive neuroendocrine system during early development in zebrafish. Endocrinology. 2016;157:636–47. doi: 10.1210/en.2015-1785. [DOI] [PubMed] [Google Scholar]
- 79.Regueiro J, Wenzl T. Determination of bisphenols in beverages by mixed-mode solid-phase extraction and liquid chromatography coupled to tandem mass spectrometry. J Chromatogr A. 2015;1422:230–8. doi: 10.1016/j.chroma.2015.10.046. [DOI] [PubMed] [Google Scholar]
- 80.Regueiro J, Wenzl T. Development and validation of a stable-isotope dilution liquid chromatography-tandem mass spectrometry method for the determination of bisphenols in ready-made meals. J Chromatogr A. 2015;1414:110–21. doi: 10.1016/j.chroma.2015.08.037. [DOI] [PubMed] [Google Scholar]
- 81.Ruhlen RL, Howdeshell KL, Mao J, Taylor JA, Bronson FH, Newbold RR, et al. Low phytoestrogen levels in feed increase fetal serum estradiol resulting in the “fetal estrogenization syndrome” and obesity in CD-1 mice. Environ Health Perspect. 2008;116:322–8. doi: 10.1289/ehp.10448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Muhlhauser A, Susiarjo M, Rubio C, Griswold J, Gorence G, Hassold T, et al. Bisphenol A effects on the growing mouse oocyte are influenced by diet. Biol Reprod. 2009;80:1066–71. doi: 10.1095/biolreprod.108.074815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chavarro JE, Minguez-Alarcon L, Chiu YH, Gaskins AJ, Souter I, Williams PL, et al. Soy intake modifies the relation between urinary bisphenol A concentrations and pregnancy outcomes among women undergoing assisted reproduction. J Clin Endocrinol Metab. 2016;101:1082–90. doi: 10.1210/jc.2015-3473. [DOI] [PMC free article] [PubMed] [Google Scholar]