Summary
An osseous Bankart lesion is commonly seen in patients with an anterior shoulder dislocation. It is defined as a detachment of the anteroinferior labrum associated with a glenoid rim fracture. Radiological studies are crucial not only for detecting glenoid bone defects but also for measuring the amount of bone loss. The precise quantification of the bony defect is crucial for the therapeutic desicion-making and clinical outcomes. Although we know that major glenoid bone loss requires surgical intervention, none of the studies performed so far answered the question what size of the defect should be an indication for open surgery procedures. Moreover, there is still no consensus on the exact percentage of glenoid loss that results in a higher risk of re-dislocations. In our opinion, there is a strong need for a consensus on universally accepted measuring techniques of the glenoid defect as well as on algorithms with validated glenoid bone loss threshold values for therapeutic decision-making. In this study, we review the techniques described so far in the literature and try to assess if any of these techniques should be treated as a leading method of detecting and quantifying osseous glenoid lesions.
MeSH Keywords: Glenoid Cavity; Imaging, Three-Dimensional; Magnetic Resonance Imaging; Shoulder Joint
Background
An osseous Bankart lesion is commonly seen in patients with an anterior shoulder dislocation and is defined as a detachment of the anetroinferior labrum associated with a glenoid rim fracture. In a recent anatomic study, the 3: 20 position has been found to be the most common location of the defect [1]. The glenoid defect may be a bone fracture caused by a primary injury or a bone erosion caused by repetitive episodes of subluxations and/or dislocations [1–3]. The glenoid bone defect has been proved to change the normal shoulder kinematics which results in anterior shoulder instability and further re-dislocations [4,5]. Radiological modalities are crucial not only for detecting glenoid bone defects but also for measuring the amount of bone loss. These measurements assist further surgical planing. The usefulness of available modalities fro the assessment of bone loss, ranging from classic radiographs, through CT with 2D and 3D reconstructions, MR, MR or CT-arthro, have been widely described. The usefulness of available modalities FOR the assessment of bone loss, ranging from classic radiographs, through CT with 2D and 3D reconstructions, MR, MR or CT-arthro, have been widely described.
Radiograms
When diagnosing anterior shoulder instability, the usual initial imaging modality is an anteroposterior radiograph of the shoulder. Radiographs of a stable shoulder present a sclerotic line which represents the normal anterior glenoid rim (Figure 1). The absence of that line for more than 5 mm from the inferior glenoid edge indicates bone lesion of the anterior glenoid rim (Figure 2). That sign is described as LSGL (loss of sclerotic glenoid line) [6]. Jankauskas et al. compared the LSGL sign on true AP radiographs with CT images. It appears that LSGL is moderately sensitive and highly specific for the detection of anterior osseous lesions. Several other projection of radiographs have been used for the imaging of anterior shoulder instability. The most common are Didiee, West Point and the so-called Barnegeau views [6,7]. For instance, in the West-Point view, the patient is in the prone-position with an abducted forearm, and the X-ray beam is angled 25° medially and anteriorly so that the anteroinferior glenoid rim is well demonstrated. The main advantage of the true AP radiograph is the fact that it is most commonly used, which makes it easily accessible in virtually every medical centre. It does not require any enforced forearm positions, which may be hard to perform by patients with a limited range of motion. However, the usefulness of this modality is limited in bilateral shoulder dislocations due to the lack of an intact contralateral side for comparison. Moreover, detecting small glenoid bone defects and a precise calculation can not be performed with this technique [4].
Figure 1.
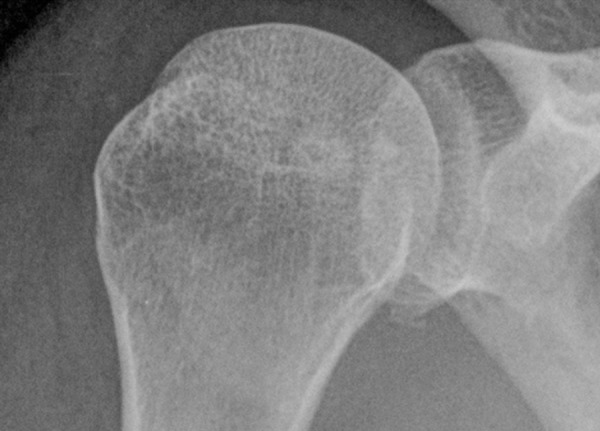
AP radiograph of a stable shoulder presents a preserved sclerotic line.
Figure 2.
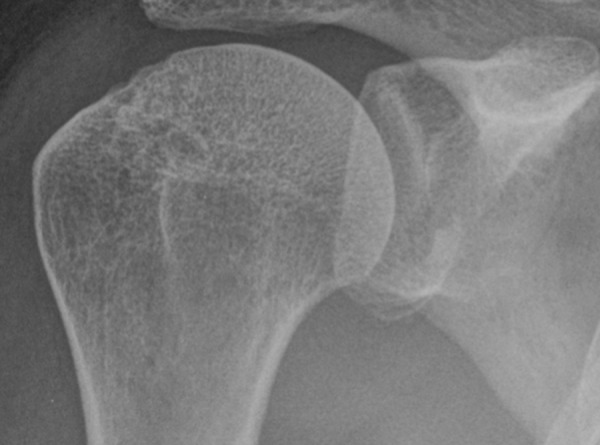
AP radiograph of the shoulder presents the loss of sclerotic glenoid line which indicates glenoid anterior rim bone lesion.
Computed Tomography
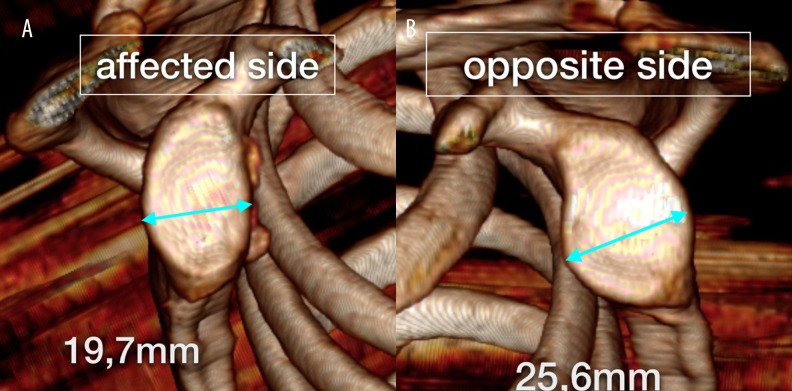
A typical feature seen in reformatted en face images of the glenoid fossa is a flattening of the anterior counter. Griffith et al. claim that en face images are more useful in quantifying anterior bone loss than 3D reconstructions, because good-quality 3D reconstructions are not as easily achievable as en face glenoid views [8]. In the same study, the authors proposed the two following measuring techniques as the best indicators of glenoid bone loss. The first is the difference between the maximum width of the glenoid fossa in both the unstable and the contralateral healthy shoulder (Figure 3). The second technique measures the maximum glenoid width and length, which allows to calculate a width to length ratio. Flattening of the anterior curvature with an unchanged glenoid length results in decreased values of this ratio. The referential value for stable shoulders equals approximately 0,7 [8].
Figure 3.
(A) CT 3D reconstruction of the glenoid surface of both shoulders. Affected side: the bone loss in the anterior part of the glenoid is present. (B) Opposite side: the surface of the unaffected glenoid is visualised with unchanged maximum width.
The principle of measuring bone defects on sagittal en-face views is based on the fact that the inferior aspect of the glenoid resembles a circle. The circle can be drawn along the posterior, anterior and inferior margins of the glenoid [9]. The best-fit circle method led to several different measuring techniques.
The method introduced by Baudi et al., i.e. the Pico method, is based on CT scanning of both shoulders to provide oblique sagittal images of the healthy and the affected glenoid surfaces. By drawing two identical circumferential areas on the inferior parts of both glenoids, it is possible to measure the missing part of the circle in the affected glenoid and express that area as the size of the defect. The calculating formula was: surface D/surface A ×100, where surface D refers to the size of the missing part of the circle in the affected glenoid and surface A refers to the size of the circle in the healthy glenoid (both areas measured in square millimetres). Intra- and inter-observer readability in the Pico method has been found to be very good [2,10].
3D-CT reconstructions with the humeral head removed have been introduced by Sugaya et al. as another radiological modality to visualise glenoid fossa morphology and to measure bone defect size. In the inferior part of the glenoid, the authors drew a circle which fit the outer counter of the rim. If a bone fragment was present, the authors quantified its size by calculating the ratio between the fragment area and assumed an area of the lower circular portion of the glenoid (Figure 4). In case there is no free osseous fragment, the missing part of the circle resembles the defect (Figure 5) [11]. The main differences between 3D (Sugaya) and 2D (Pico) techniques is that the 2D (Pico) reconstructions require both shoulders to be scanned. According to Griffith at al., the crucial criteria for the analysis of glenoid fossa on CT images require comparisons with the healthy contralateral side, which makes the 2D-CT reconstructions useful for only unilateral glenoid rim lesions [12].
Figure 4.
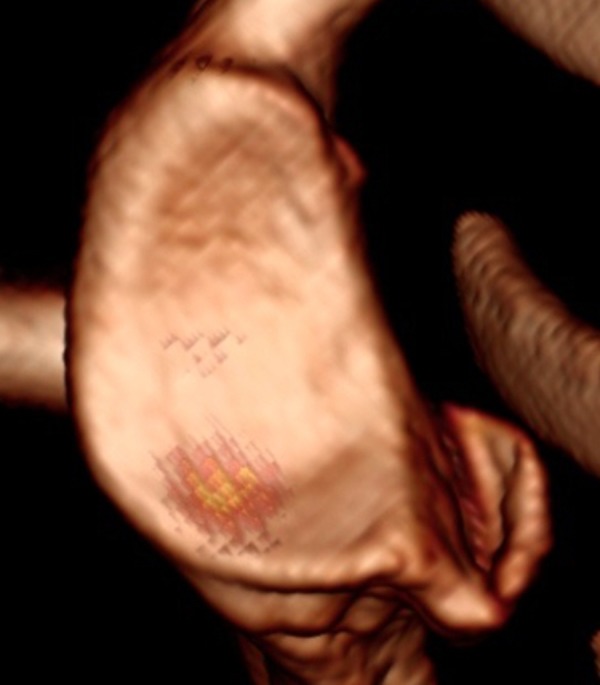
CT 3D reconstruction of the glenoid surface on sagittal en-face view. The bone loss of the anterior part of glenoid is present with a large bone fragment.
Figure 5.
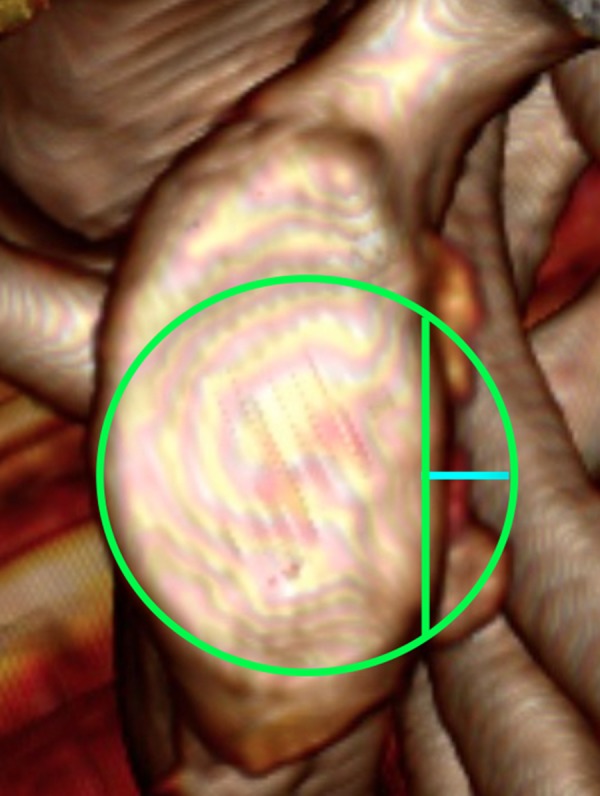
CT 3D reconstruction of the glenoid surface. Best fit circle method. Blue line presents the width of the missing bone fragment.
Another technique using 3D CT reconstructions is the glenoid index described by Chuang et al. The glenoid index is the ratio between the width of the injured glenoid and the width of the unaffected glenoid before an injury (Figure 6) [13]. This technique is similar to the Pico technique, where the shoulder before an injury is tantamount to the contralateral healthy shoulder described by Baudi. Finally, a unilateral quantification of the unstable shoulder in 3D CT images was used in Barchilon et al. study. These authors proposed a simple method of measuring the size of the glenoid defect by calculating the ratio between the depth and the radius of the best-fitted circle. The depth was defined as the line drawn from the centre of the circle to the injured anterior glenoid margin [14].
Figure 6.
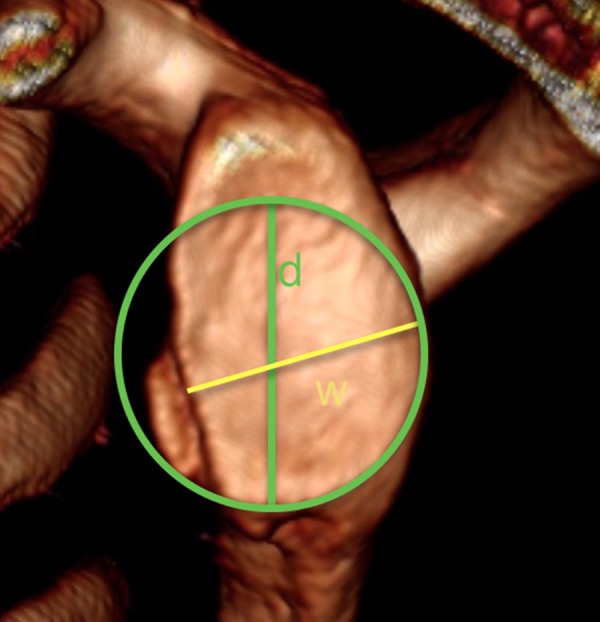
CT reconstruction of the glenoid surface. Best fit circle method with the use of Chuang measuring technique where „d” is the width of the unaffected glenoid and „w” represents the width of theinjured glenoid.
All methods that require measurements of the contralateral healthy glenoid assume that the size and shape of both glenoids are symmetrical. The exact side-to side symmetry has been proved by Lin Shi et al. [15]. These authors compared side-to-side glenoid shapes as well as sizes by detailed digital analysis of CT scans. They measured maximum glenoid length, width, area and circumference. None of these parameters showed significant statistical differences between both sides. This supports the use of the contralateral glenoid for comparisons.
CT is considered to be a highly sensitive and specific technique of detecting and quantifying glenoid bone loss. Moreover, it is considered the most accurate radiological modality for the visualization of the cortical glenoid rim [4,8,16]. Despite these advantages, there are some downsides that one should remember when analysing CT scans. Firstly, the amount of bone defect of the anterior glenoid rim does not correlate in a linear way with the number of dislocations. The first two episodes of dislocations tend to have a greater impact on the thin glenoid rim. Conversely, re-dislocations have a relatively lesser impact on the increasingly thick glenoid rim [8]. Furthermore, the anterior glenoid rim may present a flattening in the stable shoulder. To diagnose the bony Bankart lesion, the anterior straight line needs to be associated with the shortening of the glenoid width [3].
Magnetic Resonance Imaging
Recently, new studies have investigated the use of 3D MR reconstructions to evaluate glenoid bone loss. The possibility of using 3D MR reconstruction instead of 3D CT may benefit the patients with soft Bankart who are suspected for extra glenoid bone loss, by sparing them the cost, time and radiation dose of an extra CT examination. So far, MR has been the gold standard for imaging soft tissue lesions but in several clinical studies it has also appeared to be an accurate modality for measuring glenoid bone loss [17–19]. The best-fit circle method is exactly the same when using CT or MR reconstructions (Figure 7). Gyftopoulos et al., after applying a circle in the inferior part of the glenoid, drew a line through the centre of the circle, which represents the maximum width of the glenoid. The line between the anterior margin of the circle and the anterior margin of an injured glenoid represents the size of the osseous loss. The size of the bone defect divided by the glenoid width and multiplied by 100 represents the percentage of glenoid bone loss [17]. 3D MR reconstructions are produced using water-based images from the Dixon sequence. Producing 3D MR osseous models of glenoid fossa takes comparable time to producing 3D CT reconstructions, and in addition, it spares the patient another radiation dose [17]. Because of radiation reasons, MR is favourable especially in young patients or when scanning both shoulders. In a recent publication, Owens et al. described a formula to estimate the expected width of the glenoid with a known height, which allows to calculate the amount of bone defect [20]. A significant disadvantage of this study was that the exact width of the bone loss does not precisely correspond to that estimated by the formula.
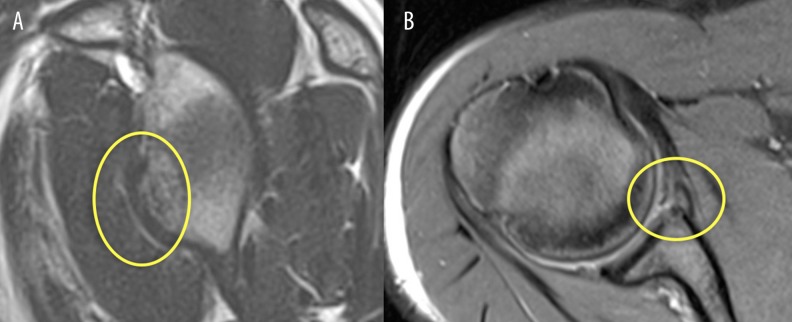
Figure 7.
Sagittal and axial T2 images of the shoulder. The bony Bankart lesion is visible.
Recently, also MR-arthro reliability in detecting and quantifying the glenoid bone loss has been evaluated with very good results [21,22]. MR and MR-arthro seem to be new, promising techniques for the detection and quantification of bone loss in patients with anterior shoulder instability.
Arthroscopic Measurements of Glenoid Bone Loss
In CT and MR reconstructions, a typical sign of severe bone deficiency is an inverted pear shape of the glenoid fossa. According to Burkhart at al., this inverted shape of the glenoid requires at least 25% defect of the entire width, measured in the inferior part of the glenoid [23]. The same authors described an arthroscopic measurement technique which is based on a bare spot landmark examination. Bare spot is a 3-mm area in the inferior part of the glenoid where cartilage presents local thinning. This area is being used as a reference spot of the glenoid centre, and the average length from this spot to the anterior, posterior and inferior rim of the glenoid is roughly 11 mm. The distance from the bare spot to the anterior rim of the glenoid becomes smaller when anterior bony deficiency is present. This deficiency allows for the quantification of bone defects of the glenoid [12,23,24]. The arthroscopic method has its limitations, however. Firstly, the bare spot has been questioned to be the centre of the glenoid which may lead to measurement overestimation [25]. Secondly, the bare spot may not be found in all glenoid fossas [26]. Also, the invasive aspect of this technique is concerning.
Discussion
Further dislocations are strongly depended on the age of the patient=the younger the patient, the higher the risk of future re-dislocation [27]. Rowe et al. were the first authors to describe the relationship between the amount of bone loss and the risk of future re-dislocation after surgical treatment. Since then, there have been many reports suggesting different cut-off values of the glenoid defects that should be treated with open bone grafting [28,29]. Typically, major glenoid bone loss requires open bone grafting, but recent studies suggest good outcomes after arthroscopic stabilisation in patients with a significant bone loss [30–32]. Nevertheless, the detection and precise quantification of the glenoid bone loss is crucial for the therapeutic desicion-making [33]. Burkhart and DeBeer report that 67% of the patients with an osseous loss involving at least 25% of the glenoid width demonstrated re-dilocation after arthroscopic Bankart repair [34]. The authors of this publication, similarly to Bigliani et al., reported an osseous loss that equals 25% of glenoid width as a significant glenoid bone loss, which should be considered as a cut-off value for an open surgery [4,34]. In a post-mortem study, Itoi et al. suggested that a glenoid defect in which the width is at least 21% of the glenoid length may result in shoulder instability after Bankart repair [35]. Although we know that a major glenoid bone loss requires surgical intervention, none of the studies performed so far answered the question what size of the defect should be an indication for open surgery procedures. [35,36]. Moreover, there is still no consensus on the exact percentage glenoid loss that results in a higher risk of re-dislocations and what imaging technique should be used. [36]. Moreover, in the opinion of some authors, the evaluation of precise threshold values is unnecessary, especially when a bone fragment is available for surgical glenoid repair [30]. Huijsmans et al. reported that the amount of bone defect is commonly overestimated due to the lack of precise measurements in everyday orthopaedic practice. A general estimation, without detailed measuring techniques, may lead to wrong decisions and unfavourable clinical outcomes [3,34]. In our opinion, as well as in the opinion of other authors, there is a strong need for a consensus on universally accepted measuring techniques of the glenoid defect. Similarly, it is crucial to evaluate algorithms with validated glenoid bone loss threshold values for therapeutic decision-making [37].
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest regarding the publication of this paper.
References
- 1.Ji JH, Kwak DS, Yang PS, et al. Comparisons of glenoid bony defects between normal cadaveric specimens and patients with recurrent shoulder dislocation: an anatomic study. J Shoulder Elbow Surg. 2012;21:822–27. doi: 10.1016/j.jse.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 2.Magarelli N, Milano G, Sergio P, et al. Intra-observer and interobserver reliability of the ‘Pico’ computed tomography method for quantification of glenoid bone defect in anterior shoulder instability. Skeletal Radiol. 2009;38:1071–75. doi: 10.1007/s00256-009-0719-5. [DOI] [PubMed] [Google Scholar]
- 3.Griffith JF, Antonio GE, Yung PS, et al. Prevalence, pattern, and spectrum of glenoid bone loss in anterior shoulder dislocation: CT analysis of 218 patients. Am J Roentgenol. 2008;190:1247–54. doi: 10.2214/AJR.07.3009. [DOI] [PubMed] [Google Scholar]
- 4.Bigliani LU, Newton PM, Steinmann SP, et al. Glenoid rim lesions associated with recurrent anterior dislocation of the shoulder. Am J Sports Med. 1998;26:41–45. doi: 10.1177/03635465980260012301. [DOI] [PubMed] [Google Scholar]
- 5.Lazarus MD, Sidles JA, Harryman DT, Matsen FA. Effect of a chondral-labral defect on glenoid concavity and glenohumeral stability: A cadaveric model. J Bone Joint Surg Am. 1996;78:94–102. doi: 10.2106/00004623-199601000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Jankauskas L, Rüdiger HA, Pfirrmann CW, et al. Loss of the sclerotic line of the glenoid on anteroposterior radiographs of the shoulder: A diagnostic sign for an osseous defect of the anterior glenoid rim. J Shoulder Elbow Surg. 2010;19:151–56. doi: 10.1016/j.jse.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 7.Edwards TB, Boulahia A, Walch G. Radiographic analysis of bone defects in chronic anterior shoulder instability. Arthroscopy. 2003;19:732–39. doi: 10.1016/s0749-8063(03)00684-4. [DOI] [PubMed] [Google Scholar]
- 8.Griffith JF, Antonio GE, Tong CW, Ming CK. Anterior shoulder dislocation: Quantification of glenoid bone loss with CT. Am J Roentgenol. 2003;180:1423–30. doi: 10.2214/ajr.180.5.1801423. [DOI] [PubMed] [Google Scholar]
- 9.Sonin A, Manaster BJ, Andrews CL, et al. Diagnostic imaging: Musculoskeletal: Trauma. 1st ed. Amyrsis; 2011. [Google Scholar]
- 10.Baudi P, Righi P, Bolognesi D, et al. How to identify and calculate glenoid bone deficit. Chir Organi Mov. 2005;90:145–52. [PubMed] [Google Scholar]
- 11.Sugaya H, Moriishi J, Dohi M, et al. Glenoid rim morphology in recurrent anterior glenohumeral instability. J Bone Joint Surg Am. 2003;85:878–84. doi: 10.2106/00004623-200305000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Griffith JF, Yung PS, Antonio GE, et al. CT compared with arthroscopy in quantifying glenoid bone loss. Am J Roentgenol. 2007;189:1490–93. doi: 10.2214/AJR.07.2473. [DOI] [PubMed] [Google Scholar]
- 13.Chuang TY, Adams CR, Burkhart SS. Use of preoperative three dimensional computed tomography to quantify glenoid bone loss in shoulder instability. Arthroscopy. 2008;24:376–82. doi: 10.1016/j.arthro.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Barchilon VS, Kotz E, Barchilon Ben-Av M, et al. A simple method for quantitative evaluation of the missing area of the anterior glenoid in anterior instability of the glenohumeral joint. Skeletal Radiol. 2008;37:731–36. doi: 10.1007/s00256-008-0506-8. [DOI] [PubMed] [Google Scholar]
- 15.Shi L, Griffith JF, Huang J, Wang D. Excellent side-to-side symmetry in glenoid size and shape. Skeletal Radiol. 2013;42:1711–15. doi: 10.1007/s00256-013-1728-y. [DOI] [PubMed] [Google Scholar]
- 16.Roger B, Skaf A, Hooper AW, et al. Imaging findings in the dominant shoulder of throwing athletes: comparison of radiography, arthrography, CT arthrography, and MR arthrography with arthroscopic correlation. Am J Roentgenol. 1999;172:1371–80. doi: 10.2214/ajr.172.5.10227520. [DOI] [PubMed] [Google Scholar]
- 17.Gyftopoulos S, Beltran LS, Yemin A, et al. Use of 3DMR reconstructions in the evaluation of glenoid bone loss: A clinical study. Skeletal Radiol. 2014;43:213–18. doi: 10.1007/s00256-013-1774-5. [DOI] [PubMed] [Google Scholar]
- 18.Gyftopoulos S, Yemin A, Mulholland T, et al. 3DMR osseous reconstructions of the shoulder using a gradient-echo based two-point Dixon reconstruction: A feasibility study. Skeletal Radiol. 2013;42:347–52. doi: 10.1007/s00256-012-1489-z. [DOI] [PubMed] [Google Scholar]
- 19.Souza PM, Brandão BL, Brown E, et al. Recurrent anterior glenohumeral instability: The quantification of glenoid bone loss using magnetic resonance imaging. Skeletal Radiol. 2014;43:1085–92. doi: 10.1007/s00256-014-1894-6. [DOI] [PubMed] [Google Scholar]
- 20.Owens BD, Burns TC, Campbell SE, et al. Simple method of glenoid bone loss calculation using ipsilateral magnetic resonance imaging. Am J Sports Med. 2013;41:622–24. doi: 10.1177/0363546512472325. [DOI] [PubMed] [Google Scholar]
- 21.Lee RK, Griffith JF, Tong MM, et al. Glenoid bone loss: Assessment with MR imaging. Radiology. 2013;267:496–502. doi: 10.1148/radiol.12121681. [DOI] [PubMed] [Google Scholar]
- 22.Markenstein JE, Jaspars KC, van der Hulst VP, Willems WJ. The quantification of glenoid bone loss in anterior shoulder instability; MR-arthro compared to 3D-CT. Skeletal Radiol. 2014;43:475–83. doi: 10.1007/s00256-013-1780-7. [DOI] [PubMed] [Google Scholar]
- 23.Burkhart SS, Debeer JF, Tehrany AM, Parten PM. Quantifying glenoid bone loss arthroscopically in shoulder instability. Arthroscopy. 2002;18:488–91. doi: 10.1053/jars.2002.32212. [DOI] [PubMed] [Google Scholar]
- 24.Burkhart SS, De Beer JF, Barth JR, et al. Results of modified Latarjet reconstruction in patients with anteroinferior instability and significant bone loss. Arthroscopy. 2007;23:1033–41. doi: 10.1016/j.arthro.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 25.Kralinger F, Aigner F, Longato S, et al. Is the bare spot a consistent landmark for shoulder arthroscopy? A study of 20 embalmed glenoids with 3-dimensional computed tomographic reconstruction. Arthroscopy. 2006;22:428–32. doi: 10.1016/j.arthro.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 26.Huysmans PE, Haen PS, Kidd M, et al. The shape of the inferior part of the glenoid: A cadaveric study. J Shoulder Elbow Surg. 2006;15:759–63. doi: 10.1016/j.jse.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Rowe CR, Patel D, Southmayd WW. The Bankart procedure: A long-term end-result study. J Bone Joint Surg Am. 1978;60:1–16. [PubMed] [Google Scholar]
- 28.Rockwood CA, Matsen FA, editors. The Shoulder. 2nd ed. Philadelphia: Saunders; 1998. [Google Scholar]
- 29.Gill TJ, Micheli LJ, Gebhard F, Binder C. Bankart repair for anterior instability of the shoulder. Long-term outcome. J Bone Joint Surg Am. 1997;79:850–57. doi: 10.2106/00004623-199706000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Mologne TS, Provencher MT, Menzel KA, et al. Arthroscopic stabilization in patients with an inverted pear gleanoid: Results in patients with bone loss of the anterior glenoid. Am J Sports Med. 2007;35:1276–83. doi: 10.1177/0363546507300262. [DOI] [PubMed] [Google Scholar]
- 31.Sugaya H, Kon Y, Tsuchiya A. Arthroscopic repair of glenoid fractures using suture anchors. Arthroscopy. 2005;21:635. doi: 10.1016/j.arthro.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 32.Sugaya H, Moriishi J, Kanisawa I, Tsuchiya A. Arthroscopic osseous Bankart repair for chronic recurrent traumatic anterior glenohumeral instability. Surgical technique. J Bone Joint Surg Am. 2006;882:159–69. doi: 10.2106/JBJS.F.00319. [DOI] [PubMed] [Google Scholar]
- 33.Huijsmans PE, de Witte PB, de Villiers RV, et al. Recurrent anterior shoulder instability: accuracy of estimations of glenoid bone loss with computed tomography is insufficient for therapeutic decision-making. Skeletal Radiol. 2011;40:1329–34. doi: 10.1007/s00256-011-1184-5. [DOI] [PubMed] [Google Scholar]
- 34.Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: Significance of the inverted-pear glenoid and the humeral engaging Hill-Sachs lesion. Arthroscopy. 2000;16:677–94. doi: 10.1053/jars.2000.17715. [DOI] [PubMed] [Google Scholar]
- 35.Itoi E, Lee SB, Berglund LJ, et al. The effect of a glenoid defect on anteroinferior stability of the shoulder after Bankart repair: A cadaveric study. J Bone Joint Surg Am. 2000;82:35–46. doi: 10.2106/00004623-200001000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Longo UG, Loppini M, Rizzello G, et al. Glenoid and humeral head bone loss in traumatic anterior glenohumeral instability: A systematic review. Knee Surg Sports Traumatol Arthrosc. 2014;22:392–414. doi: 10.1007/s00167-013-2403-5. [DOI] [PubMed] [Google Scholar]
- 37.Huijsmans PE, Haen PS, Kidd M, et al. Quantification of a glenoid defect with three-dimensional computed tomography and magnetic resonance imaging: A cadaveric study. J Shoulder Elbow Surg. 2007;16:803–9. doi: 10.1016/j.jse.2007.02.115. [DOI] [PubMed] [Google Scholar]