Abstract
As a novel class of therapeutics, aptamers, or nucleic acid ligands, have garnered clinical interest due to the ease of isolating a highly specific aptamer against a wide range of targets, their chemical flexibility and synthesis, as well as their inherent ability to have their inhibitory ability reversed. The following review details the development and molecular mechanisms of aptamers targeting specific proteases in the coagulation cascade. The ability of these anticoagulant aptamers to bind to and inhibit exosite function rather than binding within the active site highlights the importance of exosites in blocking protein function. As both exosite inhibitors and reversible agents, the use of aptamers is a promising strategy for future therapeutics.
Keywords: aptamer, anticoagulation, coagulation cascade
Aptamers are small, single-stranded oligonucleotides whose linear sequences encode specific three-dimensional structures that allow them to bind to their target proteins or other molecules with high affinity and specificity. By screening large libraries of single stranded oligonucleotides in a combinatorial chemistry process termed SELEX, or systematic evolution of ligands by exponential enrichment, scientists can identify the oligonucleotide sequence or sequences responsible for binding to various molecules with high affinity1,2,3. This technology has been exploited to isolate aptamer sequences to a growing number of proteins, including growth factors, transcription factors, viral proteins and coagulation factors, a number of which are currently in clinical trials and which have been reviewed extensively elsewhere4–6.
Crystal structures of aptamers bound to proteins have shown that aptamers fold into a tertiary structure and present an extended conformational surface that is complementary to the surface of its target, thus binding and burying a large surface area (~1,000Å2) on the protein7, 8. Because of the numerous specific interactions along the extended binding interface, aptamers often bind their targets with high affinity (dissociation constants in the low nanomolar range) and specificity. Additionally, since a large part of the target protein is concealed by aptamer binding, aptamers tend to act as antagonists by blocking protein-protein interactions rather than inhibiting active site activity.
A unique benefit of aptamers as anticoagulants is their ability to be therapeutically regulated, either by controlling their circulating half-life or reversing their function with an antidote. Unmodified RNA aptamers are rapidly degraded in biological fluids by endogenous endonucleases, leading to extremely short half-lives. While DNA aptamers are inherently more stable, RNA is thought to be able to adopt more complex conformations which may allow for different binding patterns and higher affinity to a target protein. To increase stability of the RNA aptamers, chemically modified nucleotides are incorporated in the aptamer sequence during the selection process in order to prevent endonuclease cleavage9. Highly modified RNA aptamers are even more resistant to nuclease degradation than DNA aptamers in human blood. In addition, other strategies are used to further increase aptamer stability, such as capping the ends of the aptamer to protect it from exonuclease cleavage10. Both DNA aptamers and modified RNA aptamers are relatively small (8–15 kDa) and are therefore rapidly cleared by the renal system11. To further increase the circulating half-life of an aptamer, bulky, inert moieties, such polyethylene glycol (PEG) or lipophilic moieties such as cholesterol, can be conjugated to the 5′ end of an aptamer to increase the molecular weight and thereby decrease the renal clearance rate. In this way, the bioavailability of an aptamer can be manipulated depending on its eventual use, with cholesterol conjugation of an aptamer typically increasing the half-life to several hours12 and PEG conjugation extending the half-life to days or even weeks, depending on the mode of administration6, 13.
While the use of an unmodified or rapidly cleared aptamer would require a large amount of compound to produce an effect over a given amount of time, antidote administration to control a long-circulating aptamer formulation allows for the use of lower amounts of active compound. Two types of antidotes have been developed that can rapidly reverse the activity of the aptamer while leaving the target protein intact and functionally available. An oligonucleotide-based antidote recognizes and binds to the primary sequence of the aptamer by Watson-Crick base pairing, resulting in a disrupted aptamer structure that can no longer bind to its target protein14. A universal antidote can bind to the backbone of any aptamer in a sequence independent manner and thereby sequester any aptamer and subsequently reverse its effects14, 15. Both types of antidotes would allow clinicians to respond immediately to an urgent clinical need by titrating in the amount of antidote needed. For anticoagulation, this aptamer-antidote system has received significant attention for its ability to control anticoagulation, and therefore bleeding, to the degree required.
Current anticoagulation strategies have been hampered by nonselective agents, agents with narrow therapeutic windows, and agents with no dosing flexibility or the ability to be reversed. The application of the aptamer platform to anticoagulation has the ability to fill this void. Aptamers targeting several members of the coagulation pathway have been developed and progressed into clinical trials, and the first aptamer-antidote system, targeting factor IXa (FIXa), advanced to phase 3 clinical trials. Overall, this system exhibited a rapid and reproducible onset of anticoagulation in a readily measurable manner, with the ability to be predictably controlled with antidote administration16, 17. While this aptamer-antidote system has paved the way for the translation of other aptamer-based antithrombotics, this review will detail the development of several aptamer-based inhibitors against members of the coagulation cascade (Table 1 and Figure 1).
Table 1.
Characteristics of aptamers targeting the coagulation cascade.
| Name | Target | Oligonucleotide | Binding Affinity | Mechanism of Action |
|---|---|---|---|---|
| ARC183/HD1 | FIIa | DNA | 7.1nM | Inhibits pro/exosite I31 |
| HD22 | FIIa | DNA | 2.4nM | Inhibits exosite II31 |
| HD1-22 | FIIa | DNA | 0.65nM | Inhibits pro/exosite I and exosite II31 |
| Tog25 | FII | RNA | 3nM | Inhibits exosite II32 |
| R9d14t | FII/FIIa | RNA | 10nM/1nM | Inhibits pro/exosite I34 |
| 11F7t | FXa | RNA | 1.1nM | Blocks FXa/FVa assembly36 |
| 16.3 | FVIIa | RNA | 11.3nM | Inhibits TF/FVIIa assembly37 |
| 7S-1/7S-2 | FVII | RNA | 65.7nM/59.2nM | Unknown38 |
| 9.3t | FIXa | RNA | 0.58nM | Inhibit FIXa-mediated activation of FX without affecting FIXa/FVIIIa complex assembly14, 39 |
| R4cXII-1 | FXII/FXIIa | RNA | 8.9nM/0.5nM | Inhibits autoactivation of FXIIa and FXIIa-mediated activation of FXI54 |
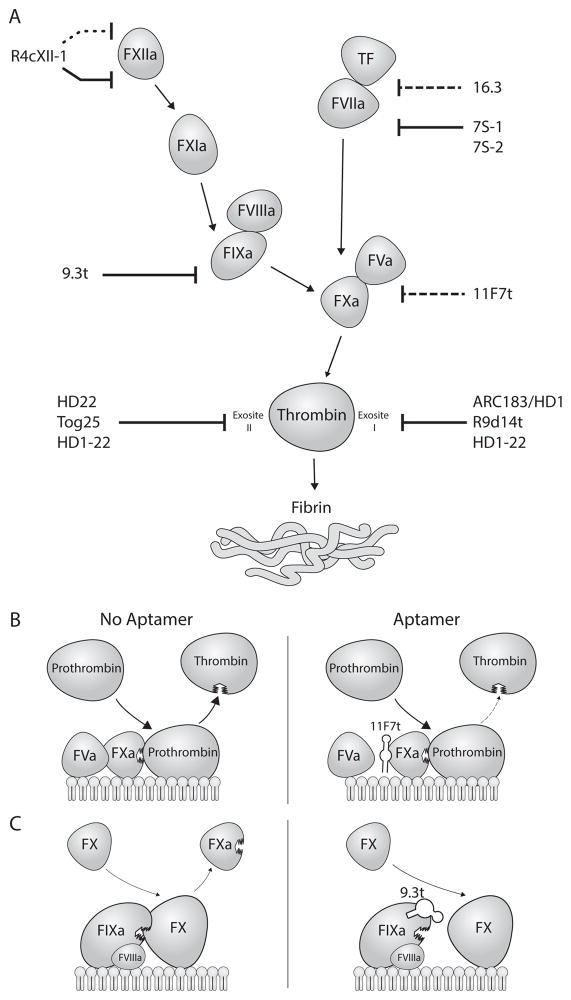
Figure 1. Mechanisms of aptamer inhibition of the coagulation cascade.
A. Aptamers inhibit the serine proteases of the coagulation cascade by binding to exosites and either disrupting cofactor binding (long dashed line), downstream zymogen binding (solid line), or activation of itself (short dashed line) leading to inhibited or reduced activation of the downstream zymogen, and thus reducing fibrin formation. B. An example of an aptamer (11F7t) disrupting cofactor binding by inhibiting FVa to bind to FXa, leading to a reduction in the amount of thrombin formed. C. An example of an aptamer (9.3t) disrupting zymogen binding by inhibiting FX binding to FIXa, leading to an inhibition of FX activation. FIXa binding to its cofactor FVIIIa is still intact.
Prothrombin/Thrombin Aptamers
Thrombin is the final step in the blood coagulation pathway, and mediates many important features of blood coagulation, including cleaving fibrinogen into fibrin, activating platelets, and amplifying the coagulation cascade18. Thrombin has three functionally important binding sites: the catalytic active site, and two anionic binding sites, exosite I and II, which are located on opposite sides of the protein. Pro-exosite I is present on both prothrombin and thrombin, and binds to fibrinogen, factor V (FV), factor VIII (FVIII), thrombomodulin, and platelet PAR receptors18. In this way, pro-exosite I mediates fibrinogen cleavage, feedback activation of clotting cofactors, and platelet activation. The other anion binding site, exosite II, is only present on thrombin and binds to heparin, FV, FVIII, and the platelet receptor GPIbα18. Therefore, exosite II also mediates platelet activation, as well as inhibition by heparin. Thus, inhibitors targeting different sites on thrombin have different modes of action, and combining inhibitors to both exosites can result in a very potent inhibition of thrombin function19.
Because of thrombin’s central role in coagulation, this well-characterized protein has been the target for some of the first in vitro aptamer selections. The first aptamer to be isolated to thrombin was also the first aptamer isolated to a non-nucleic acid binding protein. This aptamer, termed HD1 (also known as ARC183) is an unmodified 15 nucleotide single-stranded DNA aptamer that forms a stable G-quadruplex and binds to pro-exosite I on both prothrombin and thrombin20–22. This aptamer inhibits pro-exosite I functions, including fibrinogen and FV cleavage, and competes with FVa for prothrombin binding, thus inhibiting prothrombinase function20, 23. In addition, HD1 blocks platelet PAR interactions with pro-exosite I to inhibit thrombin mediated platelet activation and aggregation24. Unlike heparin, which inhibited only 35% of clot bound thrombin at clinically relevant doses, HD1 inhibited more than 80% of clot-bound thrombin in ex vivo studies25. Due to its anticoagulant activity in human plasma, this aptamer was explored in several animal models as a cardiopulmonary bypass agent. In cynomolgous monkeys, HD1 has a very short half-life (108 seconds) and rapid clearance by the body (2–4 minutes); therefore, a continuous infusion was needed to achieve efficacious anticoagulation as monitored by an increase in a prothrombin time assay26. Once administration was halted, coagulation values returned to baseline in approximately 10 minutes, abolishing the need for a reversal antidote. Similar studies in canines determined that the aptamer compound was well tolerated, and exhibited similar anticoagulant profiles as in monkeys with continual infusion of compound27. While preliminary human studies were commenced, the large quantities of aptamer required for continual administration for anticoagulation resulted in a suboptimal dosing profile, and the human clinical trials were ended28. Moreover, the variation in renal function of patients undergoing cardiopulmonary bypass makes dosing and monitoring of such rapidly clearing anticoagulant agents challenging.
Another DNA aptamer, Nu172, was developed by Archemix/Nuvelo to be a more potent, short-acting thrombin aptamer than ARC183. Modeled after ARC183, this 26-nucleotide aptamer produced a dose dependent increase of clinical coagulation assays during phase I testing in healthy male volunteers. Since this aptamer has a short half-life, after terminating infusion of Nu172, coagulation values quickly returned to baseline without requiring the use of an antidote29.
HD22 is another thrombin-binding unmodified DNA aptamer that is 29 nucleotides long and also forms a G-quadruplex. This aptamer, however, binds to exosite II on thrombin to inhibit thrombin-mediated activation of platelets and FV/FVIII activation, but has minimal effects on fibrinogen cleavage30. A very potent DNA aptamer was made by designing a hybrid aptamer joining both HD1 and HD22 with a poly-dA linker. HD1–22 thus binds to both exosites and exhibits tighter binding and more efficacious anticoagulation than either of the two aptamers alone31.
While many of the first thrombin aptamers were DNA, several RNA aptamers targeting thrombin have since been developed. Whereas unmodified DNA has a very short half-life in the body, RNA can be modified to be resistant to endonuclease cleavage and greatly increase an aptamer’s plasma stability. A 25 nucleotide 2′fluoropyrimidine RNA aptamer, Tog25, was selected against thrombin using a “toggle” SELEX method where the selection was toggled back and forth each round with human and porcine thrombin in order to promote species cross-reactivity32. Tog25 binds to exosite II of thrombin, thus inhibiting thrombin-mediated platelet activation but having a minimal effect on fibrinogen cleavage33. In contrast to the G-quadruplex architecture of the DNA aptamers, Tog25 has a traditional stem-loop structure with an internal bulge. A crystal structure of Tog25 in complex with human thrombin shows that the RNA forms an elaborate three-dimensional structure to present an extended molecular surface complementary to the protein. A number of key interactions, including an “A-Arg zipper”, which involves a number of adenine-arginine stacking interactions, as well as hydrogen bonds and van der Walls interactions, contribute to the intricate folding that allows Tog25 to tightly interact with thrombin7. While Tog25 is not a potent anticoagulant, dual administration with the pro-exosite I binding DNA aptamer, HD-1, results in synergistic anticoagulation, similar to the bivalent DNA aptamer described above19.
Recently, another 2’fluoropyrimidine modified RNA aptamer that binds to thrombin was described34. R9d14t is 58 nucleotides and binds to both prothrombin and thrombin at pro-exosite I, thus inhibiting fibrin clot formation, FV feedback activity, and platelet activation mediated by the PAR receptors. In addition, because the aptamer binds to pro-exosite I on prothrombin, it inhibits thrombin generation by the prothrombinase complex of FXa/FVa. This aptamer is a dose-dependent, potent inhibitor of coagulation in clinical clotting assays and an oligonucleotide antidote was developed to rapidly and stably reverse this anticoagulation. While this aptamer binds to the same exosite as the DNA aptamer HD-1, R9d14t is a more potent inhibitor of thrombin due to its increased stability and higher binding affinity34.
Factor Xa (FXa) Aptamer
FXa combines with its cofactor, FVa, on the surface of a platelet to cleave prothrombin to thrombin. While FXa has some protease activity itself, the formation of the prothrombinase complex yields a marked increase in thrombin generation. Since prothrombinase cleavage of prothrombin is the only reaction not duplicated in the coagulation pathway, inhibition of this complex would yield a potent anticoagulant. Several new oral anticoagulants specifically targeting FXa have recently shown remarkable efficacy in preventing stroke and managing venous thromboembolism, although none of these currently have an effective antidote approved for use35. An aptamer, 11F7t, was isolated that binds tightly to FX and FXa with an apparent dissociation constant of approximately 1nM36. This aptamer works as a potent anticoagulant in clinical coagulation assays by blocking the assembly of the prothrombinase complex. Biochemically, 11F7t binds to FXa and competes for binding with FVa. Therefore, in the presence of aptamer, the prothrombinase complex cannot form, resulting in a decreased amount of thrombin able to be produced (Figure 1B). Albeit its potent anticoagulant effect, the aptamer does not affect the ability of FX to bind to a phospholipid membrane surface, its catalytic site activity, or influence substrate binding. In addition, the aptamer 11F7t blocks the ability of FX to be activated by the FVIIIa/FIXa complex, but does not have an effect on FX activation by the FVIIa/TF complex. On the other hand, the aptamer blocks tissue factor pathway inhibitor (TFPI) from inhibiting FXa. Thus, the aptamer biochemically exhibits both procoagulant and anticoagulant effects, and this emphasizes the importance of the net effect of the interactions with which the aptamer interferes to exhibit its anticoagulant effect36.
Factor VII (FVIIa) Aptamer
The principal activator of the extrinsic pathway, and also the major activator in vivo, is the TF/FVIIa complex. Upon TF exposure, FVIIa binds to TF, allowing FVIIa to then cleave FIX and FX to their active proteases, and thus initiate thrombin generation at the site of injury. An inhibitor against FVIIa would therefore shut down the initiation of thrombin generation. A 2′aminopyrimidine RNA aptamer, termed 16.3, was generated that binds to FVII/FVIIa with a disassociation constant of 10nM. This aptamer inhibits the TF/FVIIa mediated activation of FX, at least in part by preventing this complex to form37. In a prothrombin time assay, this aptamer could dose dependently prolong the clotting time. Unfortunately, the folding of aptamer 16.3 is temperature sensitive, and at 37°C, has decreased binding affinity for FVIIa. Therefore, this aptamer was not further developed clinically. Subsequently, FVII binding aptamers were isolated using a convergent SELEX approach, where five rounds of selection against the GLA proteome was performed followed by two rounds of selection against purified FVII. Two 2′fluoropyrimidine RNA aptamers were identified that bound to FVII at 37°C with similar disassociation constants of ~60nM, but differed in their anticoagulant potency in a prothrombin time assay38.
Factor IXa (FIXa) Aptamer
Although a member of the intrinsic pathway, FIX can be activated by both FXIa and the TF/FVIIa complex and is thus involved in both pathways. FIXa then forms a complex with its cofactor, FVIIIa, to activate a large amount of FX on the platelet surface, leading to stable fibrin clot formation. A 35 nucleotide, 2′fluoropyrimidine modified RNA aptamer termed 9.3t was isolated that binds to both FIX and FIXa. Biochemical studies indicate that 9.3t blocks an extended substrate binding site so as to inhibit FIX-mediated activation of FX, but it does not inhibit FVIIIa/FIXa complex formation (Figure 1C)39. Saturating doses of this aptamer can inhibit greater than 99% of FIXa activity and results in prolonged in vitro plasma clotting times. In addition, a number of short 2′O-methyl modified RNA antidote oligonucleotides complementary to different portions of the aptamer sequence were identified that could bind to the FIXa aptamer and rapidly reverse all aptamer activity14. The most potent of these antidotes was able to reverse anticoagulation within ten minutes in human plasma, and this effect was stable over five hours14.
The FIXa binding aptamer was also effective in several animal models. In a murine ferric chloride injury model, a cholesterol modified version of 9.3t protected mice from occlusive thrombus formation12. Additionally, administration of the aptamer-antidote pair in swine exhibited reproducible anticoagulation and subsequent reversal, with the antidote neutralizing greater than 95% of anticoagulant effects within 10 minutes12. Use of the aptamer-antidote system in a one-hour, neonatal porcine cardiopulmonary bypass surgery model was as effective as using heparin and protamine for reversible anticoagulation40. Overall, these preclinical studies established the FIXa aptamer-antidote pair to be a predictable and nontoxic reversible anticoagulation system, and the two compounds (aptamer and antidote) were optimized for clinical studies as the REG1 Anticoagulation System (Regado Biosciences, Durham, NC)29.
Phase I clinical studies established REG1 as a safe, effective, and reproducible anticoagulation system in healthy patients or patients with stable coronary artery disease on antiplatelet therapy (Table 2)13, 16, 41. Bolus or weight-adjusted administration of RB006, the optimized version of the FIXa aptamer 9.3t conjugated to a 40kDa PEG carrier, resulted in a dose-dependent increase in clinical clotting assays over a 24 hour period13. Subsequent administration of RB007, the 2′OMe modified 15-nucleotide RNA antidote, led to reversal of anticoagulant activity within one to two minutes in both patient populations16. No rebound anticoagulation was seen over 72 hours, indicating that the aptamer/antidote complex was stable with irreversible binding of aptamer to antidote. While clearance of the free RB006 aptamer involves mainly intravascular and some renal mechanisms, clearance of the antidote RB007, which is not formulated with a PEG, is achieved by rapid renal filtration, allowing for the re-administration of aptamer with immediate restoration of anticoagulant effects16, 29. Across these phase I studies, no major bleeding events or serious adverse side effects were seen with REG1 use. Importantly, drug reversal could be closely controlled by titrating in antidote as needed to either fully or partially neutralize the anticoagulant effects of the aptamer to the necessary degree intended41. These initial studies were highly significant because they were the first reported clinical studies on any aptamer administered systemically.
Table 2. Clinical trials of aptamers targeting proteins involved in coagulation.
Data from www.clinicaltrials.gov. TTP = thrombotic thrombocytopenic purpura, VWD = von Willebrand Disease
| Aptamer (Sponsor) Target | Condition | Trial Phase | ClinicalTrials.gov Identifier and References |
|---|---|---|---|
| NU172 (ARCA Biopharma, Inc.) Thrombin |
Heart Disease | Phase 2 Recruitment Status Unknown (June 2011) |
NCT00808964 |
|
| |||
| REG1 (Regado Biosciences, Inc.) FIX/FIXa aptamer/antidote system |
Healthy | Phase 1 Completed (Oct 2005) |
NCT0011399713 |
| Coronary Artery Disease | Phase 2 (REVERSAL-PCI) Completed (Oct 2008) |
NCT0071545517 | |
| Acute Coronary Syndrome | Phase 2 (RADAR) Completed (Jan 2011) |
NCT0093210043, 44 | |
| Coronary Artery Disease | Phase 3 (REGULATE-PCI) Terminated (Oct 2014) |
NCT01848106 | |
|
| |||
| REG2 (Regado Biosciences, Inc.) FIX/FIXa aptamer/antidote system |
Healthy (Subcutaneous Delivery) | Phase 1 Completed (Dec 2009) |
NCT0187257251 |
|
| |||
| ARC1779 (Archemix Corp.) Von Willebrand Factor |
Thrombosis | Phase 1 Completed (March 2007) |
NCT0043277064 |
| TTP, VWD-Type 2B | Phase 2 Completed (Dec 2008) |
NCT0063224267, 68 | |
| Acute Myocardial Infarction | Phase 2 Terminated (Jan 2009) |
NCT00507338 | |
| VWD | Phase 2 Withdrawn (Aug 2009) |
NCT00694785 | |
| Thrombotic Microangiopathy, TTP | Phase 2 Terminated (Nov 2009) |
NCT0072654466 | |
| Intracranial Embolism, Cerebral Thromboembolism, Carotid Stenosis | Phase 2 Terminated (Jan 2010) |
NCT0074261273 | |
|
| |||
|
ARC19499 (Baxalta US Inc.) TFPI |
Hemophilia | Phase 1 Terminated (Dec 2011) |
NCT0119137262 |
Because of the success of the phase I studies, REG1 was next compared to unfractionated heparin as the anticoagulant for elective percutaneous coronary intervention (PCI) in patients with stable coronary artery disease in the phase 2a pilot study Reversal-PCI (NCT00715455)(Table 2)42. Patients received the antiplatelet drugs aspirin and clopidogrel, as well as an appropriate dose of the aptamer RB006 to inhibit greater than 99% of FIXa activity. Anticoagulation could be predictably controlled with either full or partial aptamer reversal with the antidote RB007, and overall the REG1 system exhibited low patient-to-patient variability with stable anticoagulation and reversal17. A larger phase2B study termed RADAR (A Randomized, Partially Blinded, Multicenter, Active-Controlled, Dose-Ranging Study Assessing the Safety, Efficacy, and Pharmacodynamics of the REG1 Anticoagulation System in Patients with acute coronary syndrome) (NCT00932100) was designed to assess the degree of reversal required to prevent thrombosis and control bleeding in planned cardiac catheterization43. A total of 640 patients with acute coronary syndrome were randomly assigned to one of four REG1 treatment arms, all receiving a weight adjusted dose of the FIX aptamer RB006 (now termed pegnivacogin) with four increasing levels of antidote RB007 (now termed anivamersen) to achieve 25%–100% reversal44. Overall, the REG1 system effectively anticoagulated patients undergoing invasive cardiac procedures while preventing thrombotic and bleeding events in patients receiving at least 50% reversal. Moreover, a trend toward reduced ischemic events and bleeding was observed in patients receiving REG144. While the incidence of adverse events was similar with REG1 and heparin, enrollment in RADAR was halted after three allergic events, albeit with sufficient clinical data from a total of 640 patients enrolled44. Despite the three adverse events, a phase 3, 13,000 patient clinical trial, REGULATE-PCI, was initiated to test the REG1 system compared in PCI compared to bivalirudin (A Study to Determine the Efficacy and Safety of REG1 Compared to Bivalirudin in Patients Undergoing PCI) (NCT01848106). However, the trial was terminated early after enrolling 3,232 patients due to a recommendation from the trial’s data and safety monitoring board, purportedly concerning rare, severe allergic reactions, although data from the trial has yet to be published (Regado Biosciences, Inc., now Tobira Therapeutics). It has not been reported to date the frequency or what elicited such allergic reactions but since the trial passed through its 1,000 patient safety assessment it is likely that the events are rare. Moreover, since the event occurred prior to antidote administration, it has been speculated that the allergic reactions must be due to recognition of either the aptamer or PEG portion (the high molecular weight modification that was appended to the end of the aptamer to increase circulating half-life) of pegnivacogin. To this end, no immune reactions or complement activation due to any aptamer administration have been reported in pre-clinical or clinical trials. However, antibodies to PEG have been detected both in animal studies45, 46 and in humans 47, 48. In addition, there is some evidence that PEGylated compounds can induce complement activation leading to hypersensitivity reactions and anaphylactic shock, although the mechanism involved is not determined49, 50. It is not clear if a response to PEG or pre-existing PEG antibodies could have caused the adverse reactions seen in the clinical trials, and we await the publication of the REGULATE-PCI data to shed light on this question.
Overall, REG1 is the first aptamer-antidote pair to be tested in humans, and aside from rare, adverse reactions in later clinical trials, this system has been shown to be a successful anticoagulant strategy with extreme and rapid control over drug activity in many hundreds of patients. While the REG1 system was administered intravenously in these studies, a phase I clinical trial of a subcutaneous administration of the system (termed REG2) was able to increase the duration of anticoagulation to a half-life of approximately six days(Table 2)51. Subsequent antidote administration successfully reversed anticoagulation, but did not inhibit further absorption of aptamer into the bloodstream. No adverse reactions were reported during the trial51. As the first subcutaneously administered aptamer, it is unclear whether this mode of administration will affect the adverse reactions seen during intravenous application in the REGULATE-PCI trail, but could offer an additional strategy for long-term anticoagulation.
Factor XII (FXII) Aptamer
FXII is the initiating member of the contact pathway, and is activated when exposed to a negatively charged surface, including substances, such as collagen, extracellular nucleic acids, or polyphosphates, that are present in the bloodstream at the site of a growing thrombus. Once activated, FXIIa then cleaves FXI to initiate the coagulation cascade, leading to thrombin generation and fibrin clot formation. FXIIa also activates kallikrein, leading to bradykinin-induced proinflammatory signaling. Activation of the contact pathway has been implicated in thrombus formation either on the surface of a foreign material, such as a catheter, or during inflammatory events, such as plaque rupture in atherosclerosis52, 53. However, members of the contact pathway are not considered essential in hemostasis, as patients deficient in FXII or kallikrein do not present with a bleeding phenotype, and some patients deficient in FXI may have a mild hemophilia. Inhibition or deficiency of FXII has been effective at limiting thrombosis without interrupting hemostasis in several animal models.
Recently, a 2′fluoropyrimidine modified RNA aptamer inhibitor of FXII has been isolated54. This aptamer, R4cXII-1, binds to both FXII and FXIIa, but has no effect on active site activity. While the aptamer itself does not induce the activation of FXII, it does block the ability of other negatively charged reagents, such as kaolin or ellagic acid, from activating FXII. In addition, R4cXII-1 blocks the ability of FXII to activate FXI to decrease thrombin generation and thus increase the clotting time in plasma-based assays activated through the intrinsic pathway. This aptamer, however, is specific for inhibition of the coagulation cascade, as it does not block the ability of FXIIa to activate kallikrein54.
Other aptamers targeting coagulation proteins
While this review focuses on aptamers targeting the proteases of the coagulation cascade, several aptamers have been identified against other proteins involved in other aspects of coagulation, such as anticoagulant proteins, platelet receptors, and von willebrand factor (VWF), that will be briefly mentioned. A panel of single stranded DNA aptamers was isolated to the anticoagulant protein activated protein C, and these aptamers bind to the main exosite on APC to inhibit cleavage of FVa and FVIIa and thus inhibit anticoagulant function55. In addition, an aptamer targeting TFPI was developed to treat hemophilia, and this aptamer, ARC19499, interacts with multiple domains on the protein to inhibit its function both in vitro and in vivo56–61. Because of its efficacy as a procoagulant, this aptamer was tested in a Phase I clinical trial in hemophilia patients (Table 2). However, the study was prematurely terminated due to increased bleeding events. Mechanistically, this side effect was reported to be due to several reasons, namely that aptamer binding decreased TFPI clearance and thus increased its circulatory half-life, leading to an actual increase in the amount of TFPI, resulting in the increased anticoagulant activity seen in patients62. Aptamers have also been developed as antiplatelet agents, with an aptamer-antidote system preliminarily described against the collagen platelet receptor glycoprotein VI63. Additionally, several aptamers targeting VWF have been developed64, 65, and the aptamer ARC1779 advanced in clinical trials for acute coronary syndromes, von Willebrand disease (VWD), and thrombotic thrombocytopenic purpura, among other indications (Table 2)66–68. The development, mechanisms, and clinical trials of the VWF aptamer have been reviewed extensively elsewhere6, 29, 69, 70, and we refer the reader to the cited literature for additional discussion on these aptamers.
Conclusions
As shown in Table 1 and Figure 1A, anticoagulant aptamers have now been generated against most of the coagulation proteases. These aptamers have been shown to be selective, bind tightly to their target proteins, and act as anticoagulants by interfering with the protein-protein interactions that are central to the coagulation cascade. Thus, aptamers may prove to be valuable tools and analytical reagents for identifying and defining functionally important regions of the coagulation factors. In this regard, aptamers have several advantages over antibodies, including being chemically synthesizable with minimal batch-to-batch variability, having an unlimited shelf life and able to withstand temperature insult, utilizing a relatively quick in vitro system for their isolation, being able to be manipulated with ease, and exhibiting binding affinities comparable to antibodies. Unfortunately, to date, the only crystal structures reported in the coagulation field are for aptamers bound to thrombin7, 23, 71 and VWF8, and aptamers as a whole have yet to be universally adopted as reagents72. To this end, additional structural and biochemical information is greatly needed to further elucidate the mechanisms aptamers employ to bind their target proteins, and this information could yield insight into exosite functions for the entire coagulation system.
As therapeutics, aptamers have received significant attention as rapid onset anticoagulants, with aptamers to thrombin, FIX/FIXa, and VWF evaluated in clinical studies (Table 2)13, 16, 17, 41, 44. Overall, coagulation aptamers make up four out of the ten aptamers assessed in clinical trials to date6. While the disease states of these other aptamers range from cancer to diabetes, one aptamer, Macugen (pegaptanib), targeting vascular endothelial growth factor, has been approved for use in age related macular degeneration since 2004 and another aptamer, Fovista (E10030), targeting platelet derived growth factor, is rapidly progressing through definitive phase 3 studies for the same indication(NCT01944839). The majority of the other clinically tested aptamers are in phase 2 or awaiting phase 3 clinical trials, highlighting that the field has increasingly excelled at overcoming challenges inherent in defining a new class of drugs. The recent results from the clinical studies of anticoagulant aptamers are encouraging from the perspective of defining a novel class of rapidly reversible anticoagulant agents. While gains have been made, the recent issues with the unexpected effects of the TFPI aptamer, as well as the rare allergic responses to the FIX/FIXa aptamer pegnivacogin, highlight the need for continued effort so that additional translation of these promising agents can proceed.
Significance.
Aptamers are a novel, emerging class of therapeutics that distinguish themselves from other classes of drugs for several reasons: their ability to specifically target a wide range of proteins, their ease of isolation, their chemical flexibility and synthesis, and their inherent ability to have their activities reversed with an antidote. This latter property lends itself well to anticoagulation, as maintaining a balance between bleeding and thrombosis is critical for patient care. Aptamers have been developed that target many proteases of the coagulation cascade, and studies into the mechanisms of aptamer inhibition determine that this class of drugs mainly mediates anticoagulation by binding to exosites and inhibiting protein-protein interactions. While these aptamers have been used to probe protein function, several of these anticoagulant aptamers have also been tested in clinical trials.
Acknowledgments
The authors thank D. Monroe for helpful discussion. This study was supported by a National Institutes of Health grants to B. Sullenger (R01HL65222 and U54HL112307).
Footnotes
Disclosures
BAS is a scientific founder of Regado Biosciences, Inc.
References
- 1.Ellington AD, Szostak JW. In vitro selection of rna molecules that bind specific ligands. Nature. 1990;346:818–822. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- 2.Gold L, Polisky B, Uhlenbeck O, Yarus M. Diversity of oligonucleotide functions. Annual review of biochemistry. 1995;64:763–797. doi: 10.1146/annurev.bi.64.070195.003555. [DOI] [PubMed] [Google Scholar]
- 3.Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: Rna ligands to bacteriophage t4 DNA polymerase. Science. 1990;249:505–510. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- 4.Keefe AD, Pai S, Ellington A. Aptamers as therapeutics. Nature reviews Drug discovery. 2010;9:537–550. doi: 10.1038/nrd3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nimjee SM, Rusconi CP, Harrington RA, Sullenger BA. The potential of aptamers as anticoagulants. Trends Cardiovasc Med. 2005;15:41–45. doi: 10.1016/j.tcm.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Sundaram P, Kurniawan H, Byrne ME, Wower J. Therapeutic rna aptamers in clinical trials. European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences. 2013;48:259–271. doi: 10.1016/j.ejps.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 7.Long SB, Long MB, White RR, Sullenger BA. Crystal structure of an rna aptamer bound to thrombin. RNA. 2008;14:2504–2512. doi: 10.1261/rna.1239308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang RH, Fremont DH, Diener JL, Schaub RG, Sadler JE. A structural explanation for the antithrombotic activity of arc1172, a DNA aptamer that binds von willebrand factor domain a1. Structure. 2009;17:1476–1484. doi: 10.1016/j.str.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keefe AD, Cload ST. Selex with modified nucleotides. Current opinion in chemical biology. 2008;12:448–456. doi: 10.1016/j.cbpa.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 10.Beigelman L, Matulic-Adamic J, Haeberli P, Usman N, Dong B, Silverman RH, Khamnei S, Torrence PF. Synthesis and biological activities of a phosphorodithioate analog of 2′,5′-oligoadenylate. Nucleic acids research. 1995;23:3989–3994. doi: 10.1093/nar/23.19.3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Healy JM, Lewis SD, Kurz M, Boomer RM, Thompson KM, Wilson C, McCauley TG. Pharmacokinetics and biodistribution of novel aptamer compositions. Pharmaceutical research. 2004;21:2234–2246. doi: 10.1007/s11095-004-7676-4. [DOI] [PubMed] [Google Scholar]
- 12.Rusconi CP, Roberts JD, Pitoc GA, Nimjee SM, White RR, Quick G, Jr, Scardino E, Fay WP, Sullenger BA. Antidote-mediated control of an anticoagulant aptamer in vivo. Nature biotechnology. 2004;22:1423–1428. doi: 10.1038/nbt1023. [DOI] [PubMed] [Google Scholar]
- 13.Dyke CK, Steinhubl SR, Kleiman NS, Cannon RO, Aberle LG, Lin M, Myles SK, Melloni C, Harrington RA, Alexander JH, Becker RC, Rusconi CP. First-in-human experience of an antidote-controlled anticoagulant using rna aptamer technology: A phase 1a pharmacodynamic evaluation of a drug-antidote pair for the controlled regulation of factor ixa activity. Circulation. 2006;114:2490–2497. doi: 10.1161/CIRCULATIONAHA.106.668434. [DOI] [PubMed] [Google Scholar]
- 14.Rusconi CP, Scardino E, Layzer J, Pitoc GA, Ortel TL, Monroe D, Sullenger BA. Rna aptamers as reversible antagonists of coagulation factor ixa. Nature. 2002;419:90–94. doi: 10.1038/nature00963. [DOI] [PubMed] [Google Scholar]
- 15.Oney S, Lam RT, Bompiani KM, Blake CM, Quick G, Heidel JD, Liu JY, Mack BC, Davis ME, Leong KW, Sullenger BA. Development of universal antidotes to control aptamer activity. Nat Med. 2009;15:1224–1228. doi: 10.1038/nm.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan MY, Cohen MG, Dyke CK, Myles SK, Aberle LG, Lin M, Walder J, Steinhubl SR, Gilchrist IC, Kleiman NS, Vorchheimer DA, Chronos N, Melloni C, Alexander JH, Harrington RA, Tonkens RM, Becker RC, Rusconi CP. Phase 1b randomized study of antidote-controlled modulation of factor ixa activity in patients with stable coronary artery disease. Circulation. 2008;117:2865–2874. doi: 10.1161/CIRCULATIONAHA.107.745687. [DOI] [PubMed] [Google Scholar]
- 17.Cohen MG, Purdy DA, Rossi JS, Grinfeld LR, Myles SK, Aberle LH, Greenbaum AB, Fry E, Chan MY, Tonkens RM, Zelenkofske S, Alexander JH, Harrington RA, Rusconi CP, Becker RC. First clinical application of an actively reversible direct factor ixa inhibitor as an anticoagulation strategy in patients undergoing percutaneous coronary intervention. Circulation. 2010;122:614–622. doi: 10.1161/CIRCULATIONAHA.109.927756. [DOI] [PubMed] [Google Scholar]
- 18.Adams TE, Huntington JA. Thrombin-cofactor interactions: Structural insights into regulatory mechanisms. Arterioscler Thromb Vasc Biol. 2006;26:1738–1745. doi: 10.1161/01.ATV.0000228844.65168.d1. [DOI] [PubMed] [Google Scholar]
- 19.Nimjee SM, Oney S, Volovyk Z, Bompiani KM, Long SB, Hoffman M, Sullenger BA. Synergistic effect of aptamers that inhibit exosites 1 and 2 on thrombin. RNA. 2009;15:2105–2111. doi: 10.1261/rna.1240109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bock LC, Griffin LC, Latham JA, Vermaas EH, Toole JJ. Selection of single-stranded DNA molecules that bind and inhibit human thrombin. Nature. 1992;355:564–566. doi: 10.1038/355564a0. [DOI] [PubMed] [Google Scholar]
- 21.Kretz CA, Stafford AR, Fredenburgh JC, Weitz JI. Hd1, a thrombin-directed aptamer, binds exosite 1 on prothrombin with high affinity and inhibits its activation by prothrombinase. The Journal of biological chemistry. 2006;281:37477–37485. doi: 10.1074/jbc.M607359200. [DOI] [PubMed] [Google Scholar]
- 22.Kretz CA, Cuddy KK, Stafford AR, Fredenburgh JC, Roberts R, Weitz JI. Hd1, a thrombin- and prothrombin-binding DNA aptamer, inhibits thrombin generation by attenuating prothrombin activation and thrombin feedback reactions. Thrombosis and haemostasis. 2010;103:83–93. doi: 10.1160/TH09-04-0237. [DOI] [PubMed] [Google Scholar]
- 23.Padmanabhan K, Padmanabhan KP, Ferrara JD, Sadler JE, Tulinsky A. The structure of alpha-thrombin inhibited by a 15-mer single-stranded DNA aptamer. The Journal of biological chemistry. 1993;268:17651–17654. doi: 10.2210/pdb1hut/pdb. [DOI] [PubMed] [Google Scholar]
- 24.Boncler MA, Koziolkiewicz M, Watala C. Aptamer inhibits degradation of platelet proteolytically activatable receptor, par-1, by thrombin. Thrombosis research. 2001;104:215–222. doi: 10.1016/s0049-3848(01)00357-7. [DOI] [PubMed] [Google Scholar]
- 25.Li WX, Kaplan AV, Grant GW, Toole JJ, Leung LL. A novel nucleotide-based thrombin inhibitor inhibits clot-bound thrombin and reduces arterial platelet thrombus formation. Blood. 1994;83:677–682. [PubMed] [Google Scholar]
- 26.Griffin LC, Tidmarsh GF, Bock LC, Toole JJ, Leung LL. In vivo anticoagulant properties of a novel nucleotide-based thrombin inhibitor and demonstration of regional anticoagulation in extracorporeal circuits. Blood. 1993;81:3271–3276. [PubMed] [Google Scholar]
- 27.DeAnda A, Jr, Coutre SE, Moon MR, Vial CM, Griffin LC, Law VS, Komeda M, Leung LL, Miller DC. Pilot study of the efficacy of a thrombin inhibitor for use during cardiopulmonary bypass. The Annals of thoracic surgery. 1994;58:344–350. doi: 10.1016/0003-4975(94)92206-3. [DOI] [PubMed] [Google Scholar]
- 28.Mayer G, Rohrbach F, Potzsch B, Muller J. Aptamer-based modulation of blood coagulation. Hamostaseologie. 2011;31:258–263. doi: 10.5482/ha-1156. [DOI] [PubMed] [Google Scholar]
- 29.Becker RC, Povsic T, Cohen MG, Rusconi CP, Sullenger B. Nucleic acid aptamers as antithrombotic agents: Opportunities in extracellular therapeutics. Thrombosis and haemostasis. 2010;103:586–595. doi: 10.1160/TH09-10-0716. [DOI] [PubMed] [Google Scholar]
- 30.Tasset DM, Kubik MF, Steiner W. Oligonucleotide inhibitors of human thrombin that bind distinct epitopes. Journal of molecular biology. 1997;272:688–698. doi: 10.1006/jmbi.1997.1275. [DOI] [PubMed] [Google Scholar]
- 31.Muller J, Freitag D, Mayer G, Potzsch B. Anticoagulant characteristics of hd1-22, a bivalent aptamer that specifically inhibits thrombin and prothrombinase. Journal of thrombosis and haemostasis : JTH. 2008;6:2105–2112. doi: 10.1111/j.1538-7836.2008.03162.x. [DOI] [PubMed] [Google Scholar]
- 32.White R, Rusconi C, Scardino E, Wolberg A, Lawson J, Hoffman M, Sullenger B. Generation of species cross-reactive aptamers using “toggle” selex. Molecular therapy : the journal of the American Society of Gene Therapy. 2001;4:567–573. doi: 10.1006/mthe.2001.0495. [DOI] [PubMed] [Google Scholar]
- 33.Jeter ML, Ly LV, Fortenberry YM, Whinna HC, White RR, Rusconi CP, Sullenger BA, Church FC. Rna aptamer to thrombin binds anion-binding exosite-2 and alters protease inhibition by heparin-binding serpins. FEBS letters. 2004;568:10–14. doi: 10.1016/j.febslet.2004.04.087. [DOI] [PubMed] [Google Scholar]
- 34.Bompiani KM, Monroe DM, Church FC, Sullenger BA. A high affinity, antidote-controllable prothrombin and thrombin-binding rna aptamer inhibits thrombin generation and thrombin activity. Journal of thrombosis and haemostasis : JTH. 2012;10:870–880. doi: 10.1111/j.1538-7836.2012.04679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weitz JI. Anticoagulation therapy in 2015: Where we are and where we are going. Journal of thrombosis and thrombolysis. 2015;39:264–272. doi: 10.1007/s11239-015-1194-6. [DOI] [PubMed] [Google Scholar]
- 36.Buddai SK, Layzer JM, Lu G, Rusconi CP, Sullenger BA, Monroe DM, Krishnaswamy S. An anticoagulant rna aptamer that inhibits proteinase-cofactor interactions within prothrombinase. The Journal of biological chemistry. 2010;285:5212–5223. doi: 10.1074/jbc.M109.049833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rusconi CP, Yeh A, Lyerly HK, Lawson JH, Sullenger BA. Blocking the initiation of coagulation by rna aptamers to factor viia. Thrombosis and haemostasis. 2000;84:841–848. [PubMed] [Google Scholar]
- 38.Layzer JM, Sullenger BA. Simultaneous generation of aptamers to multiple gamma-carboxyglutamic acid proteins from a focused aptamer library using deselex and convergent selection. Oligonucleotides. 2007;17:1–11. doi: 10.1089/oli.2006.0059. [DOI] [PubMed] [Google Scholar]
- 39.Sullenger B, Woodruff R, Monroe DM. Potent anticoagulant aptamer directed against factor ixa blocks macromolecular substrate interaction. The Journal of biological chemistry. 2012;287:12779–12786. doi: 10.1074/jbc.M111.300772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nimjee SM, Keys JR, Pitoc GA, Quick G, Rusconi CP, Sullenger BA. A novel antidote-controlled anticoagulant reduces thrombin generation and inflammation and improves cardiac function in cardiopulmonary bypass surgery. Molecular therapy : the journal of the American Society of Gene Therapy. 2006;14:408–415. doi: 10.1016/j.ymthe.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 41.Chan MY, Rusconi CP, Alexander JH, Tonkens RM, Harrington RA, Becker RC. A randomized, repeat-dose, pharmacodynamic and safety study of an antidote-controlled factor ixa inhibitor. Journal of thrombosis and haemostasis : JTH. 2008;6:789–796. doi: 10.1111/j.1538-7836.2008.02932.x. [DOI] [PubMed] [Google Scholar]
- 42.Cohen MG, Purdy DA, Rossi JS, Grinfeld LR, Aberle LH, Greenbaum AB, Fry ET, Alexander JH, Rusconie CP, Becker RC. First in clinical application of an actively reversible direct factor ixa inhibitor in elective percutaneous coronary intervention. European heart journal. 2009:30. [Google Scholar]
- 43.Povsic TJ, Cohen MG, Chan MY, Zelenkofske SL, Wargin WA, Harrington RA, Alexander JH, Rusconi CP, Becker RC. Dose selection for a direct and selective factor ixa inhibitor and its complementary reversal agent: Translating pharmacokinetic and pharmacodynamic properties of the reg1 system to clinical trial design. Journal of thrombosis and thrombolysis. 2011;32:21–31. doi: 10.1007/s11239-011-0588-3. [DOI] [PubMed] [Google Scholar]
- 44.Povsic TJ, Vavalle JP, Aberle LH, Kasprzak JD, Cohen MG, Mehran R, Bode C, Buller CE, Montalescot G, Cornel JH, Rynkiewicz A, Ring ME, Zeymer U, Natarajan M, Delarche N, Zelenkofske SL, Becker RC, Alexander JH on behalf of the RI. A phase 2, randomized, partially blinded, active-controlled study assessing the efficacy and safety of variable anticoagulation reversal using the reg1 system in patients with acute coronary syndromes: Results of the radar trial. European heart journal. 2012 doi: 10.1093/eurheartj/ehs232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richter AW, Akerblom E. Antibodies against polyethylene glycol produced in animals by immunization with monomethoxy polyethylene glycol modified proteins. International archives of allergy and applied immunology. 1983;70:124–131. doi: 10.1159/000233309. [DOI] [PubMed] [Google Scholar]
- 46.Sroda K, Rydlewski J, Langner M, Kozubek A, Grzybek M, Sikorski AF. Repeated injections of peg-pe liposomes generate anti-peg antibodies. Cellular & molecular biology letters. 2005;10:37–47. [PubMed] [Google Scholar]
- 47.Armstrong JK, Hempel G, Koling S, Chan LS, Fisher T, Meiselman HJ, Garratty G. Antibody against poly(ethylene glycol) adversely affects peg-asparaginase therapy in acute lymphoblastic leukemia patients. Cancer. 2007;110:103–111. doi: 10.1002/cncr.22739. [DOI] [PubMed] [Google Scholar]
- 48.Hershfield MS, Ganson NJ, Kelly SJ, Scarlett EL, Jaggers DA, Sundy JS. Induced and pre-existing anti-polyethylene glycol antibody in a trial of every 3-week dosing of pegloticase for refractory gout, including in organ transplant recipients. Arthritis research & therapy. 2014;16:R63. doi: 10.1186/ar4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chanan-Khan A, Szebeni J, Savay S, Liebes L, Rafique NM, Alving CR, Muggia FM. Complement activation following first exposure to pegylated liposomal doxorubicin (doxil): Possible role in hypersensitivity reactions. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2003;14:1430–1437. doi: 10.1093/annonc/mdg374. [DOI] [PubMed] [Google Scholar]
- 50.Szebeni J. Complement activation-related pseudoallergy: A new class of drug-induced acute immune toxicity. Toxicology. 2005;216:106–121. doi: 10.1016/j.tox.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 51.Vavalle JP, Rusconi CP, Zelenkofske S, Wargin WA, Alexander JH, Becker RC. A phase 1 ascending dose study of a subcutaneously administered factor ixa inhibitor and its active control agent. Journal of thrombosis and haemostasis : JTH. 2012;10:1303–1311. doi: 10.1111/j.1538-7836.2012.04742.x. [DOI] [PubMed] [Google Scholar]
- 52.Yau JW, Stafford AR, Liao P, Fredenburgh JC, Roberts R, Weitz JI. Mechanism of catheter thrombosis: Comparison of the antithrombotic activities of fondaparinux, enoxaparin, and heparin in vitro and in vivo. Blood. 2011;118:6667–6674. doi: 10.1182/blood-2011-07-364141. [DOI] [PubMed] [Google Scholar]
- 53.Kuijpers MJ, van der Meijden PE, Feijge MA, Mattheij NJ, May F, Govers-Riemslag J, Meijers JC, Heemskerk JW, Renne T, Cosemans JM. Factor xii regulates the pathological process of thrombus formation on ruptured plaques. Arterioscler Thromb Vasc Biol. 2014;34:1674–1680. doi: 10.1161/ATVBAHA.114.303315. [DOI] [PubMed] [Google Scholar]
- 54.Woodruff RS, Xu Y, Layzer J, Wu W, Ogletree ML, Sullenger BA. Inhibiting the intrinsic pathway of coagulation with a factor xii-targeting rna aptamer. Journal of thrombosis and haemostasis : JTH. 2013;11:1364–1373. doi: 10.1111/jth.12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muller J, Isermann B, Ducker C, Salehi M, Meyer M, Friedrich M, Madhusudhan T, Oldenburg J, Mayer G, Potzsch B. An exosite-specific ssdna aptamer inhibits the anticoagulant functions of activated protein c and enhances inhibition by protein c inhibitor. Chemistry & biology. 2009;16:442–451. doi: 10.1016/j.chembiol.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 56.Waters EK, Genga RM, Schwartz MC, Nelson JA, Schaub RG, Olson KA, Kurz JC, McGinness KE. Aptamer arc19499 mediates a procoagulant hemostatic effect by inhibiting tissue factor pathway inhibitor. Blood. 2011;117:5514–5522. doi: 10.1182/blood-2010-10-311936. [DOI] [PubMed] [Google Scholar]
- 57.Parunov LA, Fadeeva OA, Balandina AN, Soshitova NP, Kopylov KG, Kumskova MA, Gilbert JC, Schaub RG, McGinness KE, Ataullakhanov FI, Panteleev MA. Improvement of spatial fibrin formation by the anti-tfpi aptamer bax499: Changing clot size by targeting extrinsic pathway initiation. Journal of thrombosis and haemostasis : JTH. 2011;9:1825–1834. doi: 10.1111/j.1538-7836.2011.04412.x. [DOI] [PubMed] [Google Scholar]
- 58.Gorczyca ME, Nair SC, Jilma B, Priya S, Male C, Reitter S, Knoebl P, Gilbert JC, Schaub RG, Dockal M, McGinness KE, Pabinger I, Srivastava A. Inhibition of tissue factor pathway inhibitor by the aptamer bax499 improves clotting of hemophilic blood and plasma. Journal of thrombosis and haemostasis : JTH. 2012;10:1581–1590. doi: 10.1111/j.1538-7836.2012.04790.x. [DOI] [PubMed] [Google Scholar]
- 59.Chang JY, Chantrathammachart P, Monroe DM, Key NS. Studies on the mechanism of action of the aptamer bax499, an inhibitor of tissue factor pathway inhibitor. Thrombosis research. 2012;130:e151–157. doi: 10.1016/j.thromres.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 60.Gissel M, Orfeo T, Foley JH, Butenas S. Effect of bax499 aptamer on tissue factor pathway inhibitor function and thrombin generation in models of hemophilia. Thrombosis research. 2012;130:948–955. doi: 10.1016/j.thromres.2012.08.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Waters EK, Genga RM, Thomson HA, Kurz JC, Schaub RG, Scheiflinger F, McGinness KE. Aptamer bax 499 mediates inhibition of tissue factor pathway inhibitor via interaction with multiple domains of the protein. Journal of thrombosis and haemostasis : JTH. 2013;11:1137–1145. doi: 10.1111/jth.12201. [DOI] [PubMed] [Google Scholar]
- 62.Dockal MPR, Hartmann R, Knappe S, Sorensen B, Wong WY, Conlan M, Cecerle M, Ewenstein BM, Ehrlich HJ, Scheiflinger F. Biological explanation of clinically observed elevation of tfpi plasma levels after treatment with tfpi-antagonistic aptamer bax 499. 54th ASH Annual Meeting and Exposition; 2012; p. 120. [Google Scholar]
- 63.Rusconi CP, LJ, Stell BG, Mahanty SK, Quick RJ, Wyrzykiewicz TK, Brooks DG, Zelenkofske SL. Discovery of a novel aptamer-based inhibitor of gpvi and its matched active control agent. American Heart Association Meeting; 2010. [Google Scholar]
- 64.Gilbert JC, DeFeo-Fraulini T, Hutabarat RM, Horvath CJ, Merlino PG, Marsh HN, Healy JM, Boufakhreddine S, Holohan TV, Schaub RG. First-in-human evaluation of anti von willebrand factor therapeutic aptamer arc1779 in healthy volunteers. Circulation. 2007;116:2678–2686. doi: 10.1161/CIRCULATIONAHA.107.724864. [DOI] [PubMed] [Google Scholar]
- 65.Oney S, Nimjee SM, Layzer J, Que-Gewirth N, Ginsburg D, Becker RC, Arepally G, Sullenger BA. Antidote-controlled platelet inhibition targeting von willebrand factor with aptamers. Oligonucleotides. 2007;17:265–274. doi: 10.1089/oli.2007.0089. [DOI] [PubMed] [Google Scholar]
- 66.Cataland SR, Peyvandi F, Mannucci PM, Lammle B, Kremer Hovinga JA, Machin SJ, Scully M, Rock G, Gilbert JC, Yang S, Wu H, Jilma B, Knoebl P. Initial experience from a double-blind, placebo-controlled, clinical outcome study of arc1779 in patients with thrombotic thrombocytopenic purpura. American journal of hematology. 2012;87:430–432. doi: 10.1002/ajh.23106. [DOI] [PubMed] [Google Scholar]
- 67.Jilma B, Paulinska P, Jilma-Stohlawetz P, Gilbert JC, Hutabarat R, Knobl P. A randomised pilot trial of the anti-von willebrand factor aptamer arc1779 in patients with type 2b von willebrand disease. Thrombosis and haemostasis. 2010;104:563–570. doi: 10.1160/TH10-01-0027. [DOI] [PubMed] [Google Scholar]
- 68.Jilma-Stohlawetz P, Gorczyca ME, Jilma B, Siller-Matula J, Gilbert JC, Knobl P. Inhibition of von willebrand factor by arc1779 in patients with acute thrombotic thrombocytopenic purpura. Thrombosis and haemostasis. 2011;105:545–552. doi: 10.1160/TH10-08-0520. [DOI] [PubMed] [Google Scholar]
- 69.Firbas C, Siller-Matula JM, Jilma B. Targeting von willebrand factor and platelet glycoprotein ib receptor. Expert review of cardiovascular therapy. 2010;8:1689–1701. doi: 10.1586/erc.10.154. [DOI] [PubMed] [Google Scholar]
- 70.Povsic TJ, Sullenger BA, Zelenkofske SL, Rusconi CP, Becker RC. Translating nucleic acid aptamers to antithrombotic drugs in cardiovascular medicine. Journal of cardiovascular translational research. 2010;3:704–716. doi: 10.1007/s12265-010-9230-6. [DOI] [PubMed] [Google Scholar]
- 71.Padmanabhan K, Tulinsky A. An ambiguous structure of a DNA 15-mer thrombin complex. Acta crystallographica Section D, Biological crystallography. 1996;52:272–282. doi: 10.1107/S0907444995013977. [DOI] [PubMed] [Google Scholar]
- 72.Baird GS. Where are all the aptamers? American journal of clinical pathology. 2010;134:529–531. doi: 10.1309/AJCPFU4CG2WGJJKS. [DOI] [PubMed] [Google Scholar]
- 73.Markus HS, McCollum C, Imray C, Goulder MA, Gilbert J, King A. The von willebrand inhibitor arc1779 reduces cerebral embolization after carotid endarterectomy: A randomized trial. Stroke; a journal of cerebral circulation. 2011;42:2149–2153. doi: 10.1161/STROKEAHA.111.616649. [DOI] [PubMed] [Google Scholar]