Abstract
Background
Forced oscillation technique (FOT) has been reported to be useful in the evaluation and management of obstructive lung disease, including COPD. To date, no data are available concerning long-term changes in respiratory system impedance measured by FOT. Additionally, although exacerbations have been reported to be associated with excessive lung function decline in COPD, the impact of exacerbations on the results of FOT has not been demonstrated. The aim of this study was to investigate the longitudinal changes in respiratory system impedance and the influence of exacerbations thereon.
Methods
Between March 2011 and March 2012, outpatients who attended Kobe City Medical Center West Hospital with a diagnosis of COPD were assessed for eligibility. Baseline patient characteristics (age, sex, body mass index, smoking history, current smoking status, COPD stage), lung function (post-bronchodilator forced expiratory volume in 1 second [FEV1]), blood tests (neutrophils and eosinophils), FOT, and COPD assessment test results were collected at enrollment. Lung function and FOT were examined every 6 months until March 2016. Annual changes in FEV1 and FOT parameters were obtained from the slope of the linear regression curve. The patients were divided into 2 groups based on exacerbation history.
Results
Fifty-one of 58 patients with COPD were enrolled in this study. The median follow-up period was 57 (52–59) months. Twenty-five (49%) patients experienced exacerbations. A significant annual decline in FEV1 and respiratory system impedance were shown. Additionally, annual changes in FEV1, respiratory system resistance at 5 Hz, respiratory system reactance at 5 Hz, and resonant frequency were greater in patients with exacerbations than in those without exacerbations.
Conclusion
Exacerbations of COPD lead not only to a decline in lung function but also to an increase in respiratory system impedance.
Keywords: chronic obstructive pulmonary disease, forced oscillation technique, lung function tests, respiratory system, impedance, resistance
Introduction
COPD is a major public health problem. It is the fourth leading cause of morbidity and mortality worldwide and is associated with increasing economic costs and social burdens.1 COPD is characterized by progressive airflow limitation that is not fully reversible.1 Forced expiratory volume in 1 second (FEV1) and the rate of annual decline in FEV1 are the most widely used outcome measures for clinical trials of pharmacotherapy or other interventions, such as smoking cessation, for COPD.2 Frequency of exacerbation3 has been shown to be one of the factors affecting annual decline in FEV1. The forced oscillation technique (FOT) is a noninvasive method used to measure lung mechanics.4 Recently, its usefulness in the evaluation or management of obstructive lung diseases, including COPD and asthma, has been reported.5–8 Although FOT is suitable for repeated measurements because of the noninvasive nature, to our knowledge, no data are available concerning long-term changes in respiratory system impedance in patients with COPD. Additionally, although exacerbations are important events in patients with COPD because they contribute to a further decline in lung function,3 impaired health-related quality of life,9,10 socioeconomic burden,11 and poor prognosis,12 there are no data on the impact of exacerbations on FOT parameters in patients with COPD. Therefore, we carried out a study of longitudinal changes in respiratory system impedance and the influence of exacerbations thereon.
Methods
The study design is a prospective observational study. Between March 2011 and March 2012, 83 outpatients with a diagnosis of COPD, who attended Kobe City Medical Center West Hospital (a 358-bed community teaching hospital in Kobe, Japan), were assessed for eligibility. All patients fulfilled the inclusion criteria of: 1) a smoking history of more than 20 pack-years; 2) a maximal FEV1/forced vital capacity ratio <0.7; 3) regular management and treatment at our outpatient clinic over 3 months; 4) no exacerbations in the previous 6 weeks; 5) no other lung diseases; 6) no uncontrolled comorbidities such as severe cardiovascular disease or malignancy; and 7) sufficient cognitive function to complete the questionnaire. The staging of COPD was assessed in accordance with the 2014 Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines.1 We defined an exacerbation as a symptomatic deterioration requiring treatment with antibiotics or systemic corticosteroids. Periodic assessment was postponed when patients were prescribed courses of prednisolone or treatment with antibiotics for exacerbations of COPD in the month prior to the visit.
The study was approved by the Institutional Review Board of Kobe City Medical Center West Hospital (reference number 14-023), and informed consent was obtained from all patients before entry into the study.
Data on patient characteristics (age, sex, body mass index, smoking history, current smoking status, and COPD stage), lung function (post-bronchodilator FEV1), blood tests (neutrophils and eosinophils), FOT (MostGraph®; CHEST MI, Tokyo, Japan), and COPD assessment test results13 were collected at enrollment. Lung function and FOT were examined every 6 months until March 2016. The same equipment and standardized methods were used in the examination at enrollment and the last follow-up.
Measurements
Lung function tests
After the patients had inhaled bronchodilator (salbutamol 400 µg) spirometry, subdivisions of lung volume were measured using a Chestac-65V (Chest M.I.). The predicted pulmonary function test values were calculated based on the Japanese Respiratory Society guidelines.14 In this paper, FEV1 means post-bronchodilator FEV1.
Forced oscillation system
After the use of the inhaled bronchodilator (salbutamol 400 µg), respiratory impedance was measured using the commercially available FOT device (MostGraph®). During the measurements, the subjects were placed in the seated position, wore a nose clip, and breathed at tidal volume through a mouthpiece. In addition, their cheeks were supported to reduce upper airway shunting. A noise signal was applied to the airway, and the respiratory system impedance was automatically calculated using the MostGraph-01 software (version 1.31, Chest M.I.). Recordings were performed for 60 seconds after steady breathing was confirmed. The mean respiratory system resistance (Rrs) at 5 Hz (R5), mean Rrs at 20 Hz (R20), their difference (R5–R20), respiratory system reactance (Xrs) at 5 Hz (X5), and resonant frequency (Fres) were calculated after manually excluding artifacts (whole-breath analysis). Differences between mean Rrs, Xrs, and Fres in the expiratory phase and inspiratory phase were also examined (expressed as ⊿). Whole-breath analysis and within-breath analysis were performed automatically by the software. The average levels of at least 3 acceptable measurements were used.
Measurement of FeNO
Fractional exhaled nitric oxide (FeNO) levels were measured using a nitric oxide monitor (NIOX MINO®, Aerocrine, Solna, Sweden) according to the 2005 American Thoracic Society/European Respiratory Society guidelines.15 The subjects were asked about current medications or food intake that could interfere with the FeNO measurement results, and were instructed to avoid smoking, exercise, and ingestion of food, water, or caffeine for at least 1 hour before testing.
While seated, the subjects exhaled fully, inhaled for 2–3 seconds to total lung capacity through an NIOX filter, and then exhaled with an upper airway pressure of 5–20 cm H2O. FeNO measurements were taken from a 3-second stable plateau of the exhaled NO concentration. The lowest detection limit of the NIOX MINO® is 5 parts per billion (ppb), and values <5 ppb were regarded as 2.5 ppb. The average values of at least 2 acceptable measurements were used.
Statistical analysis
All statistical analyses were performed using JMP 9 software (SAS Institute, Cary, NC, USA). Categorical data were summarized as counts, and quantitative data were summarized as the median. We divided the patients into two groups, ie, patients with exacerbations during the observation period and those without. The frequency of exacerbations was obtained by dividing the times of exacerbations during the observation period by the years of observation. The annual changes were obtained from the slope of the linear regression curve. Baseline variables and annual changes between the groups were compared using the chi-squared test for categorical variables and unpaired t-tests or Mann-Whitney U tests as appropriate for continuous variables.
Results
Patient characteristics
The characteristics of the study subjects as well as the baseline respiratory system impedance and FEV1 are shown in Tables 1 and 2. Fifty-eight patients with COPD were enrolled in this study and the final study population comprised 51 patients. Seven patients were excluded for the following reasons: death (1 patient), serious condition (1 patient), new lesions of the lung (3 patients), and loss of follow-up (2 patients). The median duration of follow-up was 57 months. All the subjects were in a stable condition and had not had exacerbations for 3 months prior to their examination. The median CAT score was 6, indicating that COPD in the study population was controlled.
Table 1.
Characteristics of subjects at enrollment
| N=51 | |
|---|---|
| Observation period (months) | 57 (52–59) |
| Age (years) | 72 (64–76) |
| Sex (male/female) | 43/8 |
| Body mass index (kg/m2) | 20.9 (18.8–23.0) |
| Smoking state (current/ex) | 11/40 |
| Smoking (pack-year) | 45 (30–70) |
| GOLD stage (1/2/3/4) | 2/30/18/1 |
| Medication for COPD | |
| Any medications | 47 |
| LAMA | 47 |
| ICS + LABA | 19 |
| COPD assessment test score | 6 (3–10) |
| Neutrophils (/µL) | 3,602 (3,130–4,295) |
| Eosinophils (/µL) | 156 (98.3–231) |
| IgE (IU/mL) | 78 (18–173) |
| FeNO (ppb) | 15 (11–20) |
Notes: Values are shown as the median (25%–75%). Neutrophils, eosinophils, and IgE were measured in blood.
Abbreviations: GOLD, Global Initiative for Chronic Obstructive Lung Disease; LAMA, long-acting muscarinic antagonist; ICS, inhaled corticosteroid; LABA, long-acting beta agonist; IgE, immunoglobulin E; FeNO, fractional exhaled nitric oxide; ppb, parts per billion.
Table 2.
Respiratory system impedance and FEV1 at enrollment
| N=51 | |
|---|---|
| R5 (cmH2O/L/s) | |
| Whole breath | 3.52 (2.51–4.02) |
| Inspiratory | 3.20 (2.29–3.67) |
| Expiratory | 3.62 (2.68–4.23) |
| ⊿R5 | 0.42 (0.18–0.8) |
| R20 (cmH2O/L/s) | |
| Whole breath | 2.54 (2.14–2.98) |
| Inspiratory | 2.28 (1.80–2.65) |
| Expiratory | 2.62 (2.25–3.23) |
| ⊿R20 | 0.22 (0.11–0.44) |
| R5–R20 (cmH2O/L/s) | |
| Whole breath | 1.14 (0.51–1.51) |
| Inspiratory | 0.98 (0.27–1.56) |
| Expiratory | 0.91 (0.22–1.49) |
| ⊿R5–R20 | 0.30 (0.10–0.65) |
| X5 (cmH2O/L/s) | |
| Whole breath | −1.14 (−2.02 to −0.70) |
| Inspiratory | −0.82 (−1.60 to −0.56) |
| Expiratory | −1.27 (−2.10 to −0.59) |
| ⊿X5 | −0.36 (−1.00 to −0.11) |
| Fres (Hz) | |
| Whole breath | 12.35 (8.51–15.13) |
| Inspiratory | 11.13 (8.14–14.52) |
| Expiratory | 12.99 (8.95–16.62) |
| ⊿Fres | 1.00 (−0.05–2.79) |
| FEV1 (L) | 1.38 (0.95–1.80) |
Notes: Values are shown as the median (25%–75%). ⊿, difference between inspiratory and expiratory phases.
Abbreviations: FEV1, forced expiratory volume in 1 second; R5, respiratory system resistance at 5 Hz; R20, respiratory system resistance at 20 Hz; X5, respiratory system reactance at 5 Hz; Fres, resonant frequency.
Medication
Four patients (8%) were not taking any medication for COPD, and the remaining patients (47 patients) were using a long-acting muscarinic antagonist; 19 patients were using a combination of an inhaled corticosteroid (ICS) and a long-acting beta agonist (LABA). There was a tendency for patients with severe COPD to be prescribed a combination of ICS and LABA.
Annual changes in respiratory system impedance and FEV1
The annual changes in respiratory system impedance and FEV1 are shown in Table 3. FEV1 decreased and almost all of the absolute values for respiratory system impedance increased.
Table 3.
Annual changes of respiratory system impedance and FEV1
| Annual change | |
|---|---|
| R5 (cmH2O/L/s) | |
| Whole breath | 0.055 (−0.144–0.227) |
| Inspiratory | 0.039 (−0.101–0.190) |
| Expiratory | 0.024 (−0.169–0.251) |
| ⊿R5 | 0.007 (−0.078–0.056) |
| R20 (cmH2O/L/s) | |
| Whole breath | 0.058 (−0.030–0.143) |
| Inspiratory | −0.006 (−0.179–0.139) |
| Expiratory | 0.039 (−0.213–0.196) |
| ⊿R20 | −0.005 (−0.049–0.053) |
| R5–R20 (cmH2O/L/s) | |
| Whole breath | 0.013 (−0.162–0.191) |
| Inspiratory | −0.036 (−0.172–0.216) |
| Expiratory | 0.014 (−0.171–0.138) |
| ⊿R5–R20 | 0.024 (−0.068–0.075) |
| X5 (cmH2O/L/s) | |
| Whole breath | −0.087 (−0.231 to −0.005) |
| Inspiratory | −0.075 (−0.172 to −0.017) |
| Expiratory | −0.110 (−0.309–0.011) |
| ⊿X5 | −0.050 (−0.156–0.068) |
| Fres (Hz) | |
| Whole breath | 0.733 (0.340–1.140) |
| Inspiratory | 0.510 (0.317–1.045) |
| Expiratory | 0.747 (0.222–1.259) |
| ⊿Fres | 0.105 (−0.233–0.558) |
| FEV1(mL) | −24.6 (−55.2–4.4) |
Notes: Values are shown as the median (25%–75%). ⊿, difference between inspiratory and expiratory phases.
Abbreviations: FEV1, forced expiratory volume in 1 second; R5, respiratory system resistance at 5 Hz; R20, respiratory system resistance at 20 Hz; X5, respiratory system reactance at 5 Hz; Fres, resonant frequency.
The impact of exacerbations
Patient characteristics
The characteristics of the study subjects and the baseline respiratory system impedance and FEV1 subdivided by episodes of exacerbation are shown in Tables 4 and 5. Twenty-five subjects experienced exacerbations, and the median rate of exacerbations in this group was 0.44 times per year. The median times of exacerbations experienced in the year before recruitment were significantly higher in the group with exacerbation. There was no significant difference in FOT parameters or lung function tests.
Table 4.
Characteristics of subjects with and without exacerbations at enrollment
| Exacerbation | Nonexacerbation | P-value | |
|---|---|---|---|
| Subjects (n) | 25 | 26 | |
| Sex (male/female) | 24/1 | 19/7 | |
| Age (years) | 72.0 (66.5–74.5) | 71.5 (61.8–78.5) | 0.92 |
| Smoking history (pack-year) | 45 (25–65) | 47.5 (30–65) | 0.29 |
| Smoking of recruitment | 15 (60%) | 12 (46%) | |
| BMI (kg/m2) | 23.5 (20.4–25.8) | 21.8 (20.4–23.8) | 0.18 |
| COPD assessment test score | 6 (3–10) | 6 (4–10) | 0.69 |
| GOLD stage (1/2/3/4) | 1/14/10/0 | 1/16/8/1 | |
| Exacerbation in last year | *1 (1–1) | 0 (0–1) | <0.01 |
| Exacerbation during observation period | 0.44 (0.23–0.63) | 0 (0–0) | |
| Neutrophils (/µL) | 3,495 (2,960–3,907) | 3,708 (3,264–4,873) | 0.06 |
| Eosinophils (/µL) | 148 (58–223) | 176 (50–218) | 0.75 |
| IgE (IU/mL) | 54 (13–108) | 95 (23–123) | 0.52 |
| FeNO (ppb) | 15 (11–19) | 15 (12–23) | 0.47 |
Notes: Values are shown as the median (25%–75%).
P<0.05. Neutrophils, eosinophils, and IgE were measured in blood.
Abbreviations: BMI, body mass index; GOLD, Global Initiative for Chronic Obstructive Lung Disease; IgE, immunoglobulin E; FeNO, fractional exhaled nitric oxide; ppb, parts per billion.
Table 5.
Respiratory system impedance and FEV1 of subjects with and without exacerbations at enrollment
| Exacerbation | Nonexacerbation | P-value | |
|---|---|---|---|
| Subjects (n) | 25 | 26 | |
| R5 (cmH2O/L/s) | |||
| Whole breath | 3.29 (2.09–3.78) | 3.66 (3.11–4.20) | 0.06 |
| Inspiratory | 3.02 (1.95–3.57) | 3.35 (2.88–4.12) | 0.06 |
| Expiratory | 3.56 (2.13–4.26) | 3.74 (3.29–4.33) | 0.21 |
| ⊿R5 | 0.40 (0.20–0.80) | 0.44 (0.17–1.00) | 0.95 |
| R20 (cmH2O/L/s) | |||
| Whole breath | 2.54 (2.15–2.99) | 2.54 (2.09–2.98) | 0.44 |
| Inspiratory | 2.44 (2.09–2.79) | 2.10 (1.71–2.54) | 0.16 |
| Expiratory | 2.64 (2.28–3.30) | 2.60 (2.25–3.22) | 0.87 |
| ⊿R20 | 0.18 (0.06–0.34) | 0.23 (0.11–0.44) | 0.28 |
| R5–R20 (cmH2O/L/s) | |||
| Whole breath | 0.93 (0.43–1.58) | 1.15 (0.68–1.47) | 0.37 |
| Inspiratory | 0.78 (0.21–1.76) | 1.29 (0.48–1.56) | 0.06 |
| Expiratory | 0.86 (0.17–1.51) | 1.06 (0.42–1.31) | 0.74 |
| ⊿R5–R20 | 0.37 (0.16–0.96) | 0.20 (0.04–0.51) | 0.25 |
| X5 (cmH2O/L/s) | |||
| Whole breath | −0.96 (−1.62 to −0.57) | −1.20 (−2.58 to −0.80) | 0.08 |
| Inspiratory | −0.69 (−1.33 to −0.43) | −0.80 (−1.76 to −0.65) | 0.14 |
| Expiratory | −0.97 (−2.05 to −0.50) | −1.35 (−2.38 to −0.79) | 0.09 |
| ⊿X5 | −0.24 (−0.62 to −0.08) | −0.52 (−1.68–0.10) | 0.30 |
| Fres (Hz) | |||
| Whole breath | 12.02 (8.86–14.75) | 12.51 (10.45–16.30) | 0.22 |
| Inspiratory | 11.04 (8.59–14.06) | 11.70 (8.82–13.89) | 0.74 |
| Expiratory | 12.51 (8.87–16.41) | 13.36 (10.74–16.61) | 0.29 |
| ⊿Fres | 1.03 (0.21–2.43) | 0.99 (−0.46–3.70) | 0.81 |
| FEV1(L) | 1.40 (0.98–1.84) | 1.31 (0.95–1.70) | 0.60 |
Note: Values are shown as the median (25%–75%). ⊿, difference between inspiratory and expiratory phases.
Abbreviations: FEV1, forced expiratory volume in 1 second; R5, respiratory system resistance at 5 Hz; R20, respiratory system resistance at 20 Hz; X5, respiratory system reactance at 5 Hz; Fres, resonant frequency.
Annual changes in respiratory system impedance and FEV1
The annual changes in measurements in the subjects subdivided by episodes of exacerbation are shown in Table 6. The changes in FEV1, R5, X5, and Fres were significantly larger in the group with exacerbations. Annual changes in the difference between the mean X5 in the expiratory phase and that in the inspiratory phase were significantly larger in the group with exacerbations. There was no significant difference in annual changes for parameters other than FEV1 and FOT between the groups.
Table 6.
Annual changes of respiratory system impedance and FEV1 of subjects with and without exacerbations at enrollment
| Exacerbation | Nonexacerbation | P-value | |
|---|---|---|---|
| Subjects (n) | 25 | 26 | |
| R5 (cmH2O/L/s) | |||
| Whole breath | *0.188 (0.056–0.310) | −0.037 (−0.170–0.103) | <0.01 |
| Inspiratory | *0.146 (0.052–0.268) | −0.039 (−0.145–0.081) | <0.01 |
| Expiratory | *0.187 (0.019–0.360) | −0.021 (−0.128–0.124) | <0.01 |
| ⊿R5 | 0.010 (−0.048–0.095) | −0.003 (−0.085–0.053) | 0.40 |
| R20 (cmH2O/L/s) | |||
| Whole breath | 0.048 (−0.041–0.159) | −0.065 (−0.025–0.124) | 0.52 |
| Inspiratory | 0.047 (−0.114–0.163) | −0.105 (−0.216–0.151) | 0.20 |
| Expiratory | 0.044 (−0.117–0.155) | −0.073 (−0.278–0.215) | 0.34 |
| ⊿R20 | 0.001 (−0.065–0.067) | −0.018 (−0.049–0.038) | 0.45 |
| R5–R20 (cmH2O/L/s) | |||
| Whole breath | 0.041 (−0.076–0.232) | −0.093 (−0.229–0.114) | 0.07 |
| Inspiratory | *0.050 (−0.101–0.294) | −0.152 (−0.183–0.142) | 0.02 |
| Expiratory | 0.060 (−0.146–0.234) | −0.053 (−0.211–0.096) | 0.10 |
| ⊿R5–R20 | −0.006 (−0.134–0.076) | 0.028 (−0.055–0.070) | 0.56 |
| X5 (cmH2O/L/s) | |||
| Whole breath | *−0.183 (−0.258 to −0.099) | −0.017 (−0.092–0.016) | <0.01 |
| Inspiratory | *−0.103 (−0.212 to −0.056) | −0.049 (−0.127 to −0.023) | 0.03 |
| Expiratory | *−0.257 (−0.309 to −0.067) | 0.006 (−0.140–0.092) | <0.01 |
| ⊿X5 | *−0.121 (−0.228 to −0.012) | −0.004 (−0.073–0.148) | <0.01 |
| Fres (Hz) | |||
| Whole breath | *1.044 (0.669–1.434) | 0.266 (−0.022–0.767) | <0.01 |
| Inspiratory | *0.891 (0.392–1.185) | 0.410 (0.142–0.556) | <0.01 |
| Expiratory | *1.013 (0.632–1.384) | 0.293 (−0.029–0.839) | <0.01 |
| ⊿Fres | 0.239 (−0.289–0.662) | 0.014 (−0.198–0.553) | 0.26 |
| FEV1 (mL) | *−38.6 (−79.3–5.8) | −14.2 (−31.7–12.2) | <0.01 |
Notes: Values are shown as the median (25%–75%).
P<0.05. ⊿, difference between inspiratory and expiratory phases.
Abbreviations: FEV1, forced expiratory volume in 1 second; R5, respiratory system resistance at 5 Hz; R20, respiratory system resistance at 20 Hz; X5, respiratory system reactance at 5 Hz; Fres, resonant frequency.
Discussion
This prospective observational study demonstrated that the absolute values for annual changes in respiratory system impedance measured by FOT increase and that the annual changes in respiratory system impedance were significantly greater in patients with exacerbations than those in without. To our knowledge, this is the first longitudinal study to demonstrate the annual changes in respiratory system impedance and its relationship in patients with COPD.
In our study, the annual decline in FEV1 was significantly larger in the exacerbation group. The GOLD guidelines emphasize that COPD exacerbations accelerate the rate of decline in lung function.1 Kanner et al reported that lower respiratory tract illness promoted a decline in FEV1 in current smokers with mild COPD, but not in ex-smokers.16 Furthermore, they showed a faster annual decline in FEV1 in patients with frequent exacerbations defined by symptom-based criteria (more than 2.92 events per person per year) when compared with patients suffering infrequent exacerbations (less than 2.92 events per person per year).3 In the present study, 60% of the subjects were current smokers. Furthermore, the criteria for exacerbation in our study were more stringent than in the study reported by Donaldson et al, because exacerbations in our study required not only a symptomatic deterioration but also treatment with antibiotics and/or systemic corticosteroids.3 Thus, it is reasonable that the effect of exacerbation events on the annual decline in FEV1 in our study was significantly large.
In this study, not only a significant annual decline in FEV1 but also a significant annual increase in respiratory system impedance was observed. Moreover, it is evident that the annual changes in R5, X5, and Fres were significantly greater in subjects with exacerbation than in those without. There have been cross-sectional studies about the association between lung function tests and FOT parameters that have shown that the strongest correlation is generally found between Fres and Xrs and FEV1 in both asthma and COPD.8,17 To date, however, it is not clear why Fres or X5 has such significant relationships with markers of airway obstruction. In this study, the ratio of annual decline of FEV1 to the baseline value was 1.8%, while the ratio of the changes in respiratory system impedance to baseline value was 1.6% for R5, 2.3% for R20, 1.1% for R5–R20, 7.6% for X5, and 5.9% for Fres (Figure 1). The ratio of annual changes in respiratory system impedance, especially in X5 and Fres, was more apparent than that of FEV1. Moreover, in the group of subjects with and without exacerbations, the ratios of annual changes to the baseline value were 3.0% and 0.8% for FEV1, 5.7% and −1.0% for R5, 1.5% and −2.6% for R20, 4.4% and −8.1% for R5–R20, 19.1% and 1.4% for X5, and 8.7% and 2.1% for Fres, respectively (Figure 2). The ratio of X5 and Fres was remarkably greater in subjects with exacerbations than in those without. Therefore, X5 and Fres could reflect the time course of changes in the airway and the influence of exacerbations on the airway more clearly than FEV1. Johnson et al reported on the recovery of respiratory system impedance from a single episode of COPD exacerbation.18 Comparing respiratory system impedance within 48 hours from an episode of exacerbation with that 6 weeks after the first assessment, although there were no changes in Rrs, Xrs recovered by 27.4% at inspiration and 37.1% at expiration.18 This recovery of Xrs was correlated with an improvement in arterial partial pressure of oxygen, symptoms, and health-related quality of life.18 In their study, baseline respiratory system impedance before the episode of exacerbation was not evaluated, so the irreversible influence of exacerbation on respiratory system impedance was unknown. However, our study suggests an influence of exacerbation on respiratory system impedance, especially on Xrs. Studies demonstrating the impact of a single episode of exacerbation on respiratory system impedance will be needed.
Figure 1.
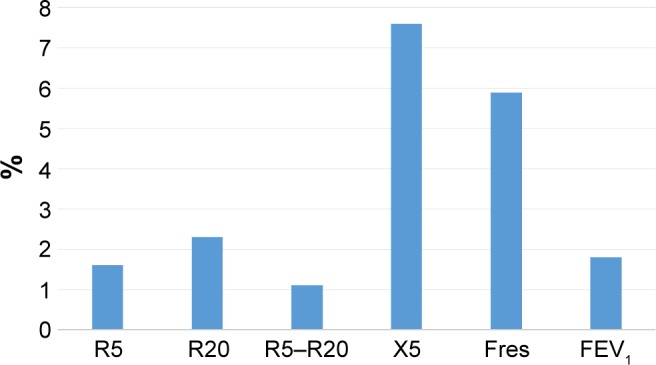
The percentage of the annual change to baseline.
Note: The ratio of annual changes in respiratory system impedance, especially in X5 and Fres, was more apparent than that of FEV1.
Abbreviations: R5, respiratory system resistance at 5 Hz; R20, respiratory system resistance at 20 Hz; X5, respiratory system reactance at 5 Hz; Fres, resonant frequency; FEV1, forced expiratory volume in 1 second.
Figure 2.
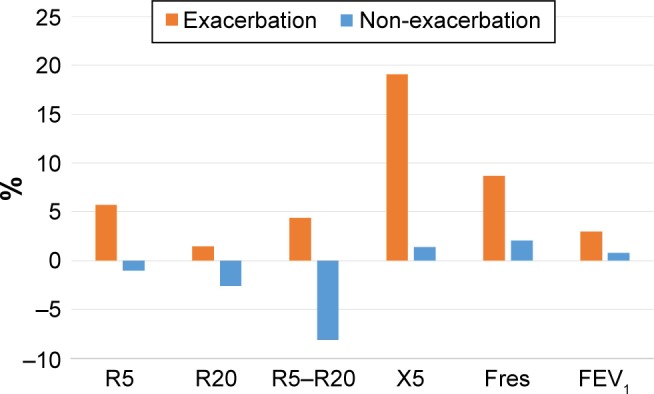
The percentage of the annual change to baseline in subjects with and without exacerbations.
Note: The ratio of X5 and Fres was remarkably greater in subjects with exacerbations than in those without.
Abbreviations: R5, respiratory system resistance at 5 Hz; R20, respiratory system resistance at 20 Hz; X5, respiratory system reactance at 5 Hz; Fres, resonant frequency; FEV1, forced expiratory volume in 1 second.
Although the relationship between lung function tests and FOT has often been mentioned, the information provided by lung function tests is obtained by forced respiration, whereas the information provided by FOT is obtained by resting respiration, so FOT and lung function tests are not of the same modality. FOT can be useful for long-term assessment of COPD as a representative chronic lung disease.
In our study, annual changes in the difference between the expiratory phase and the inspiratory phase of only whole-breath X5 were significantly larger in the group with exacerbation. Dellacà et al reported that within-breath variations in Xrs (⊿X5) measured by FOT allowed detection of expiratory flow limitation (EFL).19–21 This phenomenon is common in patients with severe COPD and a major determinant of dynamic hyperinflation and exercise limitation. EFL is caused by a loss of lung elastic recoil. It is supposed that reactance normally reflects the elastic and inertial properties of the respiratory system but, with flow limitation, oscillatory signals cannot pass through the choke points and reach the alveoli, producing a marked reduction in apparent compliance and a fall in reactance.19–21 Dynamic airway narrowing during quiet breathing at rest has been visually detected in a patient with severe COPD by high-speed electron-beam computed tomography.22 Our results indicate that exacerbations in COPD subjects might result in deterioration of EFL, suggesting the utility of ⊿X5 as a surrogate marker of EFL.
Limitations
The present study has some limitations. First, it was a single-center investigation, so some degree of selection bias might have affected the findings. Second, it was based on a small number of patients, and a larger study population would be required to validate the results. Third, although a previous study showed that exacerbations in COPD significantly affected progression of emphysema detected by computed tomography, emphysema was not evaluated in this study. Longitudinal studies in consideration of emphysema will be needed. Fourth, there might be an influence of changes and/or addition of treatment during the observation period on the results in our study.
Conclusion
In conclusion, this study showed an annual increase in respiratory system impedance and those exacerbations could accelerate this increase in patients with COPD. Respiratory system impedance might reflect the clinical course of COPD and the impact of exacerbations on the airways more clearly than pulmonary function tests, and there is the possibility that FOT could be useful for longtime assessment of COPD.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.global Initiative for Chronic Obstructive Lung Disease (GOLD) The Global Strategy for the Diagnosis, Management, and Prevention of COPD. 2014. 2016. [Accessed January 10, 2016]. Available from: http://goldcopd.org/global-strategy-diagnosis-management-prevention-copd-2016/
- 2.Wise RA. The value of forced expiratory volume in 1 second decline in the assessment of chronic obstructive pulmonary disease progression. Am J Med. 2006;119(10 Suppl 1):4–11. doi: 10.1016/j.amjmed.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847–852. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oostveen E, MacLeod D, Lorino H, et al. ERS Task Force on Respiratory Impedance Measurements The forced oscillation technique in clinical practice: methodology, recommendations and future developments. Eur Respir J. 2003;22(6):1026–1041. doi: 10.1183/09031936.03.00089403. [DOI] [PubMed] [Google Scholar]
- 5.Paredi P, Goldman M, Alamen A, et al. Comparison of inspiratory and expiratory resistance and reactance in patients with asthma and chronic obstructive pulmonary disease. Thorax. 2010;65(3):263–267. doi: 10.1136/thx.2009.120790. [DOI] [PubMed] [Google Scholar]
- 6.Kanda S, Fujimoto K, Komatsu Y, Yasuo M, Hanaoka M, Kubo K. Evaluation of respiratory impedance in asthma and COPD by an impulse oscillation system. Intern Med. 2010;49(1):23–30. doi: 10.2169/internalmedicine.49.2191. [DOI] [PubMed] [Google Scholar]
- 7.Yamaguchi M, Niimi A, Ueda T, et al. Effect of inhaled corticosteroids on small airways in asthma: investigation using impulse oscillometry. Pulm Pharmacol Ther. 2009;22(4):326–332. doi: 10.1016/j.pupt.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Mori K, Shirai T, Mikamo M, et al. Colored 3-dimensional analyses of respiratory resistance and reactance in COPD and asthma. COPD. 2011;8(6):456–463. doi: 10.3109/15412555.2011.626818. [DOI] [PubMed] [Google Scholar]
- 9.Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1418–1422. doi: 10.1164/ajrccm.157.5.9709032. [DOI] [PubMed] [Google Scholar]
- 10.Miravitlles M, Ferrer M, Pont A, et al. Effect of exacerbations on quality of life in patients with chronic obstructive pulmonary disease: a 2 year follow up study. Thorax. 2004;59(5):387–395. doi: 10.1136/thx.2003.008730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wouters EF. The burden of COPD in The Netherlands: results from the Confronting COPD survey. Respir Med. 2003;97(Suppl C):S51–S59. doi: 10.1016/s0954-6111(03)80025-2. [DOI] [PubMed] [Google Scholar]
- 12.Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–931. doi: 10.1136/thx.2005.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648–654. doi: 10.1183/09031936.00102509. [DOI] [PubMed] [Google Scholar]
- 14.Japanese Society of Chest Disease Standards of pulmonary function tests for Japanese. Jpn J Respir Soc. 1993;31:421–427. [Google Scholar]
- 15.American Thoracic Society. European Respiratory Society ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171(8):912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 16.Kanner RE, Anthonisen NR, Connett JE, Lung Health Study Research Group Lower respiratory illnesses promote FEV1 decline in current smokers but not ex-smokers with mild chronic obstructive pulmonary disease: results from the lung health study. Am J Respir Crit Care Med. 2001;164(3):358–364. doi: 10.1164/ajrccm.164.3.2010017. [DOI] [PubMed] [Google Scholar]
- 17.Shirai T, Mori K, Mikamo M, et al. Respiratory mechanics and peripheral airway inflammation and dysfunction in asthma. Clin Exp Allergy. 2013;43(5):521–526. doi: 10.1111/cea.12083. [DOI] [PubMed] [Google Scholar]
- 18.Johnson MK, Birch M, Carter R, Kinsella J, Stevenson RD. Measurement of physiological recovery from exacerbation of chronic obstructive pulmonary disease using within-breath forced oscillometry. Thorax. 2007;62(4):299–306. doi: 10.1136/thx.2006.061044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dellacà RL, Pompilio PP, Walker PP, Duffy N, Pedotti A, Calverley PM. Effect of bronchodilatation on expiratory flow limitation and resting lung mechanics in COPD. Eur Respir J. 2009;33:1329–1337. doi: 10.1183/09031936.00139608. [DOI] [PubMed] [Google Scholar]
- 20.Dellacà RL, Santus P, Aliverti A, et al. Detection of expiratory flow limitation in COPD using the forced oscillation technique. Eur Respir J. 2004;23(2):232–240. doi: 10.1183/09031936.04.00046804. [DOI] [PubMed] [Google Scholar]
- 21.Dellacà RL, Duffy N, Pompilio PP, et al. Expiratory flow limitation detected by forced oscillation and negative expiratory pressure. Eur Respir J. 2007;29(2):363–374. doi: 10.1183/09031936.00038006. [DOI] [PubMed] [Google Scholar]
- 22.Kurosawa H, Kohzuki M. Images in clinical medicine. Dynamic airway narrowing. N Engl J Med. 2004;350(10):1036. doi: 10.1056/NEJMicm030626. [DOI] [PubMed] [Google Scholar]


