Abstract
To prevent the intergenerational transfer of obesity and end the current epidemic, interventions are needed across the early life stages, from preconception to prenatal to infancy through the age of 2 years. The foundation for obesity is laid in early life by actions and interactions passed from parent to child that have long-lasting biologic and behavioral consequences. The purpose of this paper is to examine the best evidence about (a) factors in parents and offspring that promote obesity during the early life stages, (b) the social determinants and dimensions of obesity in early life, (c) promising and effective interventions for preventing obesity in early life, and (d) opportunities for future research into strategies to disrupt the intergenerational cycle of obesity that begins early in life. The pathway for halting the intergenerational obesity epidemic requires the discovery and development of evidence-based interventions that can act across multiple dimensions of influence on early life.
Keywords: intergenerational obesity, preconception, prenatal, intrauterine environment, social determinants
INTRODUCTION
The U.S. obesity epidemic saw its beginnings 30 years ago and has now yielded multiple generations of adults who are overweight or obese, and who now parent children who are also at risk for excess weight (48). As a result of multilevel factors contributing to an energy imbalance between calorie intake and expenditure, almost 69% of adults and 32% of children older than 2 years are overweight or obese (29, 90). Obesity is now prevalent even among our youngest children: During 2011--2012, 8.1% of infants and toddlers had weight-for-recumbent-length that was greater than the 95th percentile (100). This is particularly alarming because once obesity develops in these early years, it is likely to persist into adulthood, laying the foundation for the continued presence of obesity and related comorbid conditions, such as diabetes and cardiovascular disease, across future generations. Few reviews have described the risks associated with obesity in early life The purpose of this paper is to examine the best evidence about (a) factors in parents and offspring that promote obesity across early life stages (from preconception through the prenatal period to infancy through the age of 2 years), (b) the social determinants and dimensions of early life obesity, (c) promising and effective interventions for preventing early life obesity, and (d) opportunities for future research into strategies to disrupt the intergenerational cycle of obesity that begins in early life.
Conceptual Overview
Multiple reviews have focused on obesity and the risks of obesity in youth and preschool-aged children (33, 55, 70, 73, 76, 79). However, evidence suggests that the foundation for obesity is laid in early life by actions and interactions that can have long-lasting biologic and behavioral consequences. These influences on, and risks for, obesity are linked by and across generations, from parent to child.
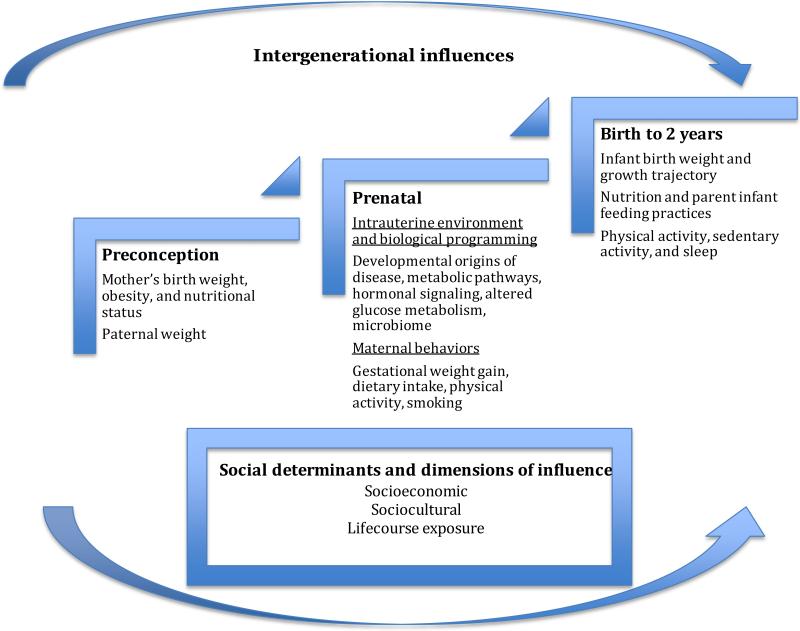
The intergenerational evolution of the risk of obesity can be described during the early life stages, from preconception through the prenatal period and into infancy through the age of 2 years. For example, the earliest risk for childhood obesity may be initiated due to excess maternal weight prior to conception (20, 22, 58, 144). Early life within the intrauterine environment may then shape the trajectory of weight gain and body composition throughout the life course. Eating and physical activity behaviors that are modeled and reinforced by parents and families further impact the weight trajectory of infants and toddlers (25, 95, 131). At a macro level, social determinants influence the interactions between infants and parents that may further impact the development of obesity in early life. These social determinants, defined by socioeconomic and sociocultural dimensions, influence the quality of the environments where a young child spends time (15, 16, 19, 79, 89, 132, 143); life-course exposure to poor-quality environments can further promote the onset of obesity in early life and increase its impact on health over time. The road map for halting the progression of obesity across generations should recognize these multiple dimensions of influence that occur at the earliest stages of human development, so that interventions can be effectively targeted. Figure 1 provides the organizational model that guides this review in addressing the multiple, intergenerational influences on early weight gain. Table 1 provides an overview of practice and research recommendations for preventing obesity in early life.
Figure 1.
Organizational model used in this review to address the intergenerational influences on the risk for obesity in early life.
Table 1.
Selected influences on, recommendations for, applications in practice for, and research gaps in preventing obesity in early lifea
| Recommendations | Application in practice | Research gaps |
|---|---|---|
| Preconception influences on early life obesity | ||
| Promote optimal weight prior to pregnancy Prior to conception, promote optimal health, weight, and dietary behaviors among men and women Prevent smoking prior to pregnancy |
Health care providers should include information about healthy weight, nutrition and activity as part of preconception care for women and men of childbearing age Encourage appropriate family planning options to optimize health during pregnancy, from conception to birth Assess smoking status of potential parents and encourage smoking cessation prior pregnancy |
The impact and effectiveness of preconception interventions on infant outcomes The effect of obesity-prevention and management interventions targeting men on their partner's pregnancy, and the infant's birth outcomes Effective methods for translating and disseminating health-promotion interventions to benefit all individuals of childbearing age |
| Prenatal influences on early life obesity | ||
| Promote early and regular prenatal care Encourage healthy gestational weight gain Promote a healthy diet and regular physical activity during pregnancy |
Health care providers should incorporate the most recent guidelines on gestational weight gain from the Institute of Medicine and the American College of Obstetricians and Gynecologists into prenatal and interconception care Incorporate counseling about nutrition, physical activity, and smoking cessation as standard components of prenatal care |
The impact of nutritional, hormonal, and microbiome environments on epigenetic modifications and how these affect trajectories of fetal and infant growth and the development of obesity in childhood The relationship between changes to the maternal diet during pregnancy and children's food preferences and dietary behaviors The impact of physical activity during pregnancy on immediate fetal outcomes, infant growth, and the development of obesity The moderating effects of developmental programming on the relationship between maternal behavior and long-term outcomes |
| Early life and obesity risk: birth to 2 years | ||
| Assess, monitor, and track infant's growth from birth to age 5 years | Health care providers should track an infant's growth from birth to 5 years using standardized approaches AND WHO or CDC growth charts AND assess parental BMI during routine assessment of a child's risk of obesity For children found to be at risk of obesity, promote healthy diet and activity behaviors for children and among caregivers who model these behaviors |
The effects of multilevel (biopsychosocial) and interactive influences o n patterns of weight gain among infants The immediate and long-term consequences of growth trajectories and obesity in early life The influence of routinely tracking infant growth on the practices of parents or caregivers, patterns of weight gain, and the development of obesity over time |
| Develop dietary guidelines for children for use from birth to 2 years Promote breast feeding during infancy Create a healthful eating environment responsive to children's hunger and fullness cues |
Health care providers should offer consistent recommendations regarding breastfeeding and infant nutrition during the prenatal and postnatal periods Counsel parents on responsive feeding practices and their role in infant growth Child care providers should receive training in, and practice, responsive feeding |
The role, timing, and type of exposures that predict the development of taste and food preferences, and their influence on infant growth Differences between breast- and formula feeding on nutrient composition and quality, and their relationship to patterns of infant growth and the long-term development of obesity Effective opportunities for modifying the feeding styles of the parent or caregiver and their impact on the infant's intake and eating patterns The impact of the timing of introducing, and the quality of, complementary feeding on an infant's growth and risk of developing obesity The role of the infant's temperament and its influence on feeding practices, intake, and growth |
| Increase physical activity and decrease sedentary behavior in young children | Train health care providers and professionals providing guidance to the parents of young children to counsel parents and caregivers on strategies to increase physical activity and decrease sedentary behavior | Methods for measuring physical activity and sedentary behavior in infants and young children Knowledge of which patterns of physical activity and sedentary behavior lead to healthy infant growth The role of television and other media exposures in shaping an infant's eating behavior The impact of interventions targeting parents, caregivers, and child care providers on increasing physical activity and decreasing sedentary behavior among infants and young children |
| Promote age-appropriate sleep patterns and duration among children | Health care providers should encourage parents to implement optimal sleep practices Child care agencies should adopt practices, behaviors, and environments that promote the age-appropriate duration of sleep |
The role of sleep duration, quality, timing, and consolidation on the development of obesity The development and validation of measures of sleep in infants and young children The impact of interventions targeting parents and caregivers designed to promote optimal sleep in infants and young children |
| Social determinants and dimension of early life obesity | ||
| Promote policies to ensure the availability and accessibility of healthy food, including in safety-net programs Promote high-quality childcare, education Disseminate evidence-based obesity-prevention information to diverse populations Promote urban planning, housing, land use, and transportation policies that create a built environment that prevents obesity |
States’ policies requiring physical activity in child care facilities should include the number of minutes of physical activity recommended per day or the length of time; they should also include limits on screen-time exposure Public health agencies should provide parents and caregivers with culturally sensitive materials that promote knowledge and practices consistent with optimal child growth and development Public health agencies should promote prepregnancy weight control and also prenatal care Local governments should foster optimal built environments in neighborhoods to promote access to healthy food, instead of fast food, and physical activity for all residents |
The impact of food policies on food security, intake, and obesity in infants and young children The roles of sociocultural beliefs and attitudes about childhood feeding and weight, and their influence on eating patterns and activity during early childhood The impact of policies requiring training in obesity prevention for child care providers and implementation of childcare nutrition and physical activity standards on the weight trajectories of infants and toddlers The impact of policies to ensure the quality and stability of housing and the built environment on nutrition, physical activity, and the weight trajectories of infants and toddlers |
PRECONCEPTION INFLUENCES ON EARLY LIFE OBESITY
A growing body of research suggests that the initiation of obesity in the next generation has its beginnings prior to conception. Although much of the work has focused on maternal influences, there is emerging evidence for the contributions made by both parents to their offspring's body weight prior to conception.
Mother's Birth Weight, Obesity, and Nutritional Status
A mother's birth weight, obesity, and nutritional status throughout her life can impact her offspring (21, 106, 111). In the United States, the Pregnancy Risk Assessment Monitoring System examined trends in prepregnancy obesity by maternal demographic and behavioral characteristics and found an increase over a 10-year period, from 13% during 1993--1994 to 22% during 2002--2003, across all categories of age, race, and education. The 2003--2009 National Health and Nutrition Examination Survey found more than one-half of pregnant women were overweight or obese (43). When compared with their lean counterparts, mothers who are overweight or obese when entering pregnancy are more likely to have children who are large for gestational age at birth (144) and as neonates (4), and who are more likely to develop obesity during childhood or adolescence (72, 106, 144) and in adulthood (20, 144). Whitaker et al. (142) found that maternal obesity during the first trimester of pregnancy doubled the risk of childhood obesity at 2 years of age. Other studies have suggested a dose--response association between the magnitude of maternal obesity and that of the child (58, 64). This relationship has genetic and epigenetic components. Epigenetic influences cause heritable alterations in gene expression, leading to changes in an organism's phenotype without changes to the DNA sequence (66, 106, 139).
Paternal Role
Fathers provide essential contributions to early embryonic development and fetal programming through genetic and epigenetic mechanisms (63, 125). Animal models have shown epigenetic changes in offspring resulting from modifications to the father's environment (125). Specifically, researchers have identified epigenetic changes connected to altered metabolism in offspring resulting from variations in the father's diet, indicating that paternal behaviors may put future children at risk of obesity and obesity-related outcomes (98, 103, 109). The paternal environment may also affect developing sperm, leading to the inheritance of epigenetic modifications (21).
Interventions
These studies have suggested that interventions to prevent obesity should target both males and females during their childbearing years. Interventions to improve nutritional behaviors and weight outcomes during the preconception period have focused primarily on women, particularly those intending to become pregnant (1, 133). These interventions have had positive effects on body mass index (BMI) and prenatal vitamin intake. Strategies for improving women's preconception health across their life span include ensuring that they have preventive visits (as an opportunity to provide pre-conception care), that interventions address identified risks, that they have interconception and prepregnancy care, and providing public health programs and interventions (e.g., increase awareness of the importance of preconception health, population surveillance, and through public programs that already serve women) (59, 64, 140). Further, policies and interventions focusing on encouraging males and females to achieve and maintain a healthy weight during adolescence and adulthood can be considered both health-promoting for those individuals, as well as preventative against obesity in the next generation. Given the finding of a recent survey that 77% of women favored engaging in a program to promote a healthy lifestyle before becoming pregnant, evidence-based interventions should be disseminated so they are broadly accessible (44). Table 1 highlights selected practice and research recommendations that can be implemented during the preconception stage to address early life obesity.
PRENATAL INFLUENCES ON EARLY LIFE OBESITY
Early life environments, and the quality and timing of exposure to factors within these environments, may produce interactions that become biologically embodied in the developing child (21, 66). The concordance that exists between maternal and early childhood obesity is the result of several shared biologic risk factors and conditions in the intrauterine environment (21, 66).
The Intrauterine Environment and Biologic Programming
Studies targeting the developmental origins of disease address questions about the plasticity and reversibility of the effects of gestational exposures, including the role of epigenetic influences. Although the gene sequence cannot be modified, the ability to alter the expression of genes in response to environmental cues (known as plasticity) is thought to be important in allowing an organism to maximize its chance of survival (33, 60, 139). During critical periods in development, an organism may use this plasticity to alter gene expression based on the environment (e.g., exposure to maternal nutritional status and hormones), and this expression is then much less plastic for the duration of the organism's life (37, 66, 82, 106, 139). For example, some have hypothesized that changes to the placenta, resulting from maternal stressors or nutritional status, or both, may act as a mediator between the developing child and the environment (60). In this way, the in utero experience acts as a preview to the fetus of the future environment, leading to morphological and epigenetic adaptations. These adaptations regulate eating behavior, obesity, and glucose tolerance (66). If, however, there is a mismatch between the intrauterine environment and the postnatal environment (e.g., intrauterine growth restriction or being small for gestational age during development coupled with nutritional excess after birth), the organism may suffer during later life (i.e., in childhood or adulthood) (52, 60, 66, 139). The effects of epigenetic changes can be observed in methylation studies, which have found associations between adiposity in childhood and greater methylation of specific genes prenatally (46). The extent to which these epigenetic modifications are heritable, such that an organism's in utero exposure can influence epigenetic gene expression in his or her offspring, is under investigation (60, 139).
Changes in certain metabolic pathways, hormonal signaling, and altered glucose metabolism during pregnancy increase an offspring's risk of being larger in size and having a higher percentage of body fat at birth (106). Developing offspring may experience maternal hormonal signals. For example, exposure to leptin during the prenatal period has been associated with a faster infant growth rate and with an increased BMI and adiposity in childhood. The patterns of association appear complex, and could possibly change direction as the child develops (12, 107, 136). Gestational diabetes has also been associated with the development of overweight, high blood pressure, and type 2 diabetes later in a child's life (14, 33). Among the mechanisms explaining the relationship between exposure to gestational diabetes and a child's outcomes are nutrient sensing, epigenetic modifications, and changes to the precursor stem cell of adipose cells and neurons related to appetite regulation (46, 66).
Emerging evidence is showing the importance of the composition of bacteria in the gut, the human microbiome. With regard to obesity, researchers have uncovered differences in the microbiomes of overweight individuals compared with normal-weight individuals (77). This difference is also seen among pregnant women, may be transferred from mother to child, and may, therefore, contribute to the intergenerational transfer of obesity (106). Further, because the infant's microbiome appears to be related to how he or she was delivered, the correlation between maternal prepregnancy BMI and the likelihood of cesarean delivery offers one mechanism for explaining differences in gut the microbiomes among babies of mothers with varying BMIs (6, 106).
Gestational Weight Gain and Maternal Lifestyle Behaviors
The intrauterine environment is influenced by maternal weight gain and lifestyle behaviors (eating, activity levels, and smoking) during pregnancy, and these are often modeled across generations; individually and together these factors influence the weight of offspring and the risk for developing obesity.
In addition to preconception obesity, excessive gestational weight gain ( GWG) increases the risk for alterations in maternal metabolism, including dyslipidemia, glucose intolerance or insulin resistance, hyperleptinemia, and inflammation, as well as increasing the incidence of gestational diabetes mellitus (13, 23, 58, 88). The offspring of mothers with increased GWG exhibit higher weight at birth, increased body fat during the neonatal period, and greater adiposity (e.g., BMI z-score, waist circumference) throughout early childhood and into adulthood (4, 33, 72, 83, 96, 118). They are also at greater risk for insulin resistance and increased plasma concentrations of leptin, insulin-like growth factor-1, and inflammatory markers (13, 58, 106, 136). These obesity-related outcomes may result from permanent changes in appetite, metabolism, and other functions set down during fetal development in response to greater nutrient transfer from overnourished mothers (111).
Dietary intake during pregnancy is associated with GWG, birth outcomes, and birth size (106). Pertinent dietary factors include under- and overnutrition, and micronutrient intake (e.g., folate and vitamin B12, iron, chromium) (14, 139). Dietary intervention studies inducing over- or undernutrition in pregnant women are impractical and unethical. Thus, much of the evidence for the effects of undernutrition comes from historical famine studies. These natural experiments, exploring associations between children born to mothers exposed to famine during pregnancy, have found consistent associations between prenatal famine and adult body size and diabetes (82). Other observational studies have found associations between dairy protein and birth weight, and with vitamin B12 status and insulin resistance in childhood (14, 102, 127). The food that a mother consumes and the experiences of taste and smell that function during fetal life, play key parts in a child's acquisition of food and flavor preferences, which have been documented at birth (87, 117, 126).
Animal and human studies have suggested that fetal epigenetic changes may be a mediator for the effects of dietary factors (37, 66, 82). Studies are exploring the links between over- and undernutrition during pregnancy and epigenetic imprinting in offspring, linking diet to altered DNA modification and these alterations to obesity in children (106). Further work has linked disparities in environmental influences, which can lead to epigenetic modifications, to disparities in chronic conditions, such as hypertension and diabetes in adulthood (71).
Physical activity (PA) during pregnancy may influence obesity development by altering GWG and birth weight. Several studies and review articles over the past 10-15 years (129, 130) have suggested that women who routinely participate in exercise during pregnancy have better glucose control (among women with gestational diabetes mellitus) (31), have less GWG (91, 120), have less postpartum weight retention (91), and give birth to infants that are smaller (but within the normal weight range) than those who do not exercise (61). Hawkins et al. (56) have demonstrated the benefits of a clinically feasible exercise intervention in diverse populations. Mourtakos et al. (92) found that moderate PA during pregnancy, as well as GWG and smoking status, were significantly associated with offspring obesity at age 8 years. In contrast, other reviews have been more mixed or inconclusive (34, 114). A review by Catalano (22) found only modest success for diet and PA interventions in decreasing excessive GWG to within recommended guidelines and found there was little impact on excessive fetal growth; Catalano concluded that preconception interventions are needed to address early alterations in maternal and placental function during the first trimester of pregnancy. These findings suggest that engaging in PA prior to and during pregnancy may improve glucose metabolism and the quality of the intrauterine environment, thus reducing the risk for obesity development in offspring.
Finally, smoking during the prenatal period is strongly associated with obesity development in offspring during childhood and adulthood (20, 33). Durmuş et al. (38) reported associations between both maternal and paternal smoking during pregnancy and risks of overweight and obesity in offspring at the ages of 1, 2, 3, and 4 years. A review by Oken et al. (101) found that maternal smoking during pregnancy was associated with a 50% higher risk of childhood obesity by ages 3--7 years. Interventions to prevent smoking during the prenatal period have had limited success due to stress and other related factors (110). Additional research that focuses on individual-level, as well as policy-level interventions, is needed to prevent smoking and promote cessation prior to and during pregnancy.
In summary, intergenerational influences and maternal biology and behavior affect the intrauterine environment and the developing fetus, enhancing or reducing the risk for developing obesity early in life. There is limited research in this area. However, it appears that modifying maternal lifestyle behaviors has added health benefits for mother and child, as well as holding the promise of reducing the risk for obesity across generations. Table 1 summarizes the best evidence on practice recommendations for the prenatal period and suggests future questions for investigation.
EARLY LIFE AND OBESITY RISK: BIRTH TO 2 YEARS
Research on indicators of obesity among young children is generally limited in scope, sample size, and design. Additionally, there are variations in thresholds for defining excess weight across studies, which use either the US Centers for Disease Control and Prevention's or the World Health Organization's growth charts for children younger than 2 years of age, and this can confuse the interpretation of research findings (100). Despite these limitations, several physiologic, behavioral, and parental indicators have been studied during the early life of offspring that may inform the risk for obesity development. The following section addresses the most influential of these associations: an infant's birth weight and growth trajectory, nutrition and feeding patterns, and physical activity, sedentary behavior, and sleep.
Infant Birth Weight and Growth Trajectory
An infant's birth weight appears to be an early indicator for the development of obesity or its sequelae, or both (20). Studies of extreme variations in birth weight, defined as children born either small for gestational age or large for gestational age, have shown that by adulthood these children have increased rates of overweight and obesity, metabolic disorders, and cardiovascular diseases (33, 49, 66). As described above, maternal weight at conception and GWG appear to have direct associations with an infant's weight. Babies born large for gestational age to obese women are more likely to develop obesity and early chronic disease (83, 118).
Rapid infant weight gain is also an important indicator of early childhood obesity (123). Accelerated weight gain occurring during the first 4 months of life has been associated with obesity at 7 years (104) and with a 60% increase in risk if the weight gain occurs within the first 2 years of life (116). Rapid infant weight gain likely results from several factors, including shared genetic influences and epigenetic processes, as well as differences in placental leptin levels and an infant's microbiota (24, 35, 39, 78). Catch-up growth among small-for-gestational-age infants may also be related to their adult BMI, particularly if the rapid growth occurs after the age of 12 months (20, 41). Additionally, there is a significant association between excess weight at ages 6 months, 1 year, and 2 years and adult obesity. Studies have suggested that parents, particularly those who are overweight or from underserved communities, are unlikely to recognize rapid weight gain or overweight in their young child (76, 113). Regular monitoring of infants’ and children's weight by health care providers affords an opportunity for recognizing excessive weight gain and providing early lifestyle interventions (26).
Nutrition and Infant-Feeding Practices
Parents’ practices for infant feeding may have a direct impact on the quality and quantity of food intake, weight gain in early life, and the development of obesity in later life. Consistent evidence-based guidance is lacking for parents and caregivers who are responsible for feeding infants and toddlers. This is due, in part, to the absence of recommended dietary guidelines for children who are younger than 2 years of age. The lack of guidance may influence the extent to which parents and caregivers transmit their own experiences to feeding the young children in their care, patterns that have immediate and ongoing implications for a child's weight. The following section reviews the research on nutrition and infant-feeding practices as related to obesity in early life.
Infants self-regulate their energy intake from birth; eating is initiated in response to hunger and terminated in response to satiation signals (10). Ultimately, infants are born knowing how much to eat, when to eat, and when they are full. Infants respond to differences in energy density early in life, particularly when breastfeeding, and are able to self-regulate energy intake to accommodate their nutritional needs for growth and development (10, 89, 105, 115, 117). Many studies have found that formula-fed infants weigh more and are longer in length than breastfed infants by the end of the first year of life, possibly due to differences associated with the amount and pattern of feeding by the parent (69). A review by Thompson (134) has suggested that infants weaned earlier gain weight more rapidly (5), possibly due to higher energy intakes from formula (57), impaired self-regulation (7), and earlier complementary feeding (62). However, a recent large-scale trial reported no direct effect of breastfeeding on the development of childhood obesity (84). This is consistent with other reviews that have found no association between breastfeeding or formula feeding and obesity (20). This evidence suggests that breastfeeding remains the gold standard for infant nutrition due to its substantial health benefits, but other nutritional strategies for preventing early obesity should be studied (45).
Infants and toddlers depend upon parents or caregivers for feeding; ultimately, the infant's ability to self-regulate is influenced or eliminated by parental or caregiver decisions about what, how, and when to feed a child (10). Data from the 2008 Feeding Infants and Toddler Study showed that infants and toddlers consistently exceed their daily calorie needs (8). Research has suggested that beliefs about infant feeding may be reflected in a parent's feeding style, which have been defined as laissez-faire, pressuring and controlling, restrictive and controlling, responsive, or indulgent (135). These styles or practices may promote or compromise an infant's or toddler's ability to self-regulate intake. For example, controlling or restrictive child-feeding practices are associated with the development of behaviors that promote overeating and obesity (27). In contrast, responsive feeding practices, in which the parent interprets signals of hunger and fullness from the infant and responds quickly and appropriately to those signals (e.g., offering types of soothing other than giving food), support self-regulatory eating behaviors. There is limited evidence linking feeding styles to early life obesity. However, Birch & Doub (8) have described feeding practices as influencing gene--environment interactions; genes are expressed in the context of family environments defined by the availability and intake of foods, routines around meals, and early feeding practices supported by family culture. The quality of these interactions can increase the risk of an obese phenotype (8).
A parent's feeding practices may also influence, or be influenced by, an infant's temperament and food preferences. An infant's temperament (e.g., negative emotionality) has been reported to be associated with more rapid weight gain in infancy, but this association has received mixed support in findings from large cohort studies and may be explained by parents using feeding to soothe the infant (128). There has also been extensive research examining the development of an infant's or toddler's flavor or food preferences as primary predictors of intake and eating patterns (9, 124). Early preferences for sweet versus sour tastes are influenced by prenatal exposure to the mother's diet, are detectable at birth, and are modifiable through exposure (86, 126). However, an infant's food preferences, selections, likes, and dislikes are reinforced by the feeding practices of the parent or caregiver, by what they are provided to eat, and by whom they observe as the model of food intake (122).
Studies have also examined the introduction and quality of complementary solid foods as related to the development of obesity (50, 62). The American Academy of Pediatrics (67) recommends the introduction of complementary solid foods between the ages of 4 months and 6 months. However, several studies have reported on the early introduction of foods for infants for different reasons, including infant soothing and parental beliefs or cultural differences (42, 138). Little is known about the quality of the nutrient composition of complementary foods and the impact on infant growth or growth trajectories. There is also inconsistent evidence for the role and timing of introducing solid food in the development of early obesity (50, 62).
Physical Activity, Sedentary Behavior, and Sleep
There is a paucity of research defining the impact of an infant's or toddler's activity level on short- or long-term energy balance. Conducting these studies is difficult because our knowledge of the nature of physical activity in relation to infants and toddlers is lacking, as are reliable or valid measures of this movement. Based on the best evidence, a report by the Institute of Medicine (26) suggested that exposure to television and media, and the use of car seats and strollers (although important for safety) may be encouraging sedentary behavior in young children. Studies of caregivers have revealed that children as young as 3 months are exposed to television or other forms of media entertainment, suggesting that patterns of sedentary behavior are being ingrained early (137, 145). Recommendations for infants and young children aim to encourage movement and play to prevent excessive weight gain and to maximize development, to provide infants with access to “tummy time”, placing a baby on his or her stomach while supervised, and to ensure that toddlers are active at least 15 minutes per hour (26, 73, 79). Further work is needed to understand the role of physical activity or sedentary behavior in relation to energy balance and obesity in infants and toddlers.
Shorter sleep duration appears to be a risk factor for obesity and related diseases that may track into adulthood (2, 53, 97, 119). Insufficient sleep in childhood is associated with metabolic and other physical dysfunctions (68, 112, 141). Sleep deprivation or irregular sleeping habits are associated with an elevated BMI among children as young as 4 years (3). Further, irregular sleep may be associated with distractions in the environment, including having a television in the bedroom (65, 70, 89, 94). The use of food as a strategy to soothe infants is associated with poor sleep habits and may also be a factor in weight gain (27). There is a dearth of studies addressing strategies for promoting healthy sleep habits in infants and children and their impact on weight.
In summary, an infant's birth weight and rapid weight gain during the first months of life are important indicators of future obesity risk. The practices of the parent or caregiver in feeding the infant or in promoting activity may have a role in establishing long-term lifestyle patterns associated with weight gain. There is a lack of clear guidance on nutrition or activity for young children, which could be used by parents to avoid the development of obesogenic patterns in their children. Table 1 suggests that rigorous research is needed to identify and interrupt lifestyle patterns that are becoming socially ingrained in early life and transmitted across families and generations.
SOCIAL DETERMINANTS AND DIMENSIONS OF INFLUENCE ON EARLY LIFE OBESITY
The risk for early childhood obesity is influenced not only by biology and behavior, but by macro-level, upstream social determinants that impact the quality of the environments in which eating and activity behaviors are learned by young children and reinforced by their parents and families (19, 143). These determinants, often defined by dimensions of socioeconomic, sociocultural, and life-course exposure, converge with biomedical processes and are important in understanding the development and intergenerational nature of childhood obesity in early life (11, 16).
Offspring are most vulnerable to the harmful effects of socioeconomic deprivation during the prenatal period and early childhood. Women who live in poverty have a higher prevalence of obesity and are more likely to enter pregnancy obese, laying the foundation for early obesity for their offspring (33). Several studies have reported that socioeconomic deprivation may explain some of the racial and ethnic disparities in the prevalence of adult and childhood obesity (33). African Americans, Hispanics, and Native Americans have high prevalences of obesity; they are also twice as likely as other racial and ethnic groups to be living in economic deprivation (17, 40, 93). These socioeconomic differences may influence birth outcomes across racial and ethnic groups, and place young children, also exposed to and living in poverty, at high risk for obesity and related chronic diseases (17--19, 85, 121).
There are multiple pathways by which socioeconomic status might impact early life obesity. Poverty, as one dimension of socioeconomic status, is associated with food insecurity, which is described as an environment in which there is not access to a sufficient quantity of food or to food of a sufficient quality, and in which periods of hunger are common (36). Food insecurity has been associated with pregravid maternal obesity and is associated with poor pregnancy outcomes (32, 74, 75). Infants and children living in poverty are at risk for exposure to poor quality or unstable neighborhoods, housing conditions, or childcare services, or some combination of these, and are unlikely to have access to built environments that promote safe activity during the formative early years (29, 54). Ensuring that children have appropriate and stable housing, safe living conditions, and access to healthy food can have a direct impact on eating behaviors associated with the prevention and control of childhood obesity (54). As importantly, a recent longitudinal study by Oddo & Jones-Smith (99) assessed childhood anthropometrics and family income at 2 years and again from 4 to 6 years, and found that improvements in the family's poverty-to-income ratio promoted healthy weight outcomes among young girls. This suggests that the early life risk for obesity associated with poverty might be sensitive to, and mitigated by, improvements in economic status.
Sociocultural dimensions may also influence early life obesity through a number of pathways that impact how a parent feeds a child. These include, but are not limited to, structural and community factors that shape beliefs around child-feeding practices, and social networks that communicate and share cultural beliefs around breastfeeding and children's weight. For example, the devaluation of breastfeeding and the preference for formula feeding has been influenced by historical contexts (e.g., perceptions of wet nurses), beliefs (e.g., that breast milk transmits harmful substances from the maternal diet), easy access to formula through Women, Infants, and Children programs and other programs, and structural limitations on where it is possible to breastfeed (135). Additionally, cultural beliefs that define a larger infant as representing a healthy and active child may encourage parental feeding practices that promote excessive formula feeding and the addition of cereal in bottles to ensure that the child eats enough to look healthy (132). Experiences in the first hours and days after birth influence breastfeeding outcomes. Maternity care policies, such as the Baby-Friendly Hospital Initiative, define evidence-based hospital policies for implementing 10 steps to support breastfeeding practices, and these have been shown to improve early breastfeeding rates nationally (108). In 2013, 53.9% of hospitals nationally had implemented more than half of the 10 steps, suggesting there is a continuing need for improvement in implementing this policy.
Studies have shown there are ethnic differences among African American and Hispanic parents who perceive a thin child as unhealthy, which may then encourage the early introduction of complementary foods and a child's access to sugar-sweetened beverages and fast food (33). High rates of age-inappropriate feeding among African American mothers have also been reported, with more than 75% of their infants receiving solids or juice by 3 months of age, thus increasing the daily energy intake to levels higher than necessary for optimal growth (134). A review by Grote & Theurich (51) concluded that it was not specific types of complementary foods that were associated with childhood obesity, but rather a combination of parental attitudes and beliefs, family dynamics, and socioeconomic factors that influence complementary feeding practices and partially explain the early obesity risk. Ultimately, the development, progression, and sustainability of adverse feeding behaviors that are culturally supported can become socially patterned, putting generations at risk for the early development of obesity.
Finally, multiple dimensions of life-course exposure to early childhood disadvantage have been associated with an increased risk of obesity persisting into adulthood; those with the longest exposure to disadvantaged circumstances are at the highest risk for negative health outcomes (28). The persistence of stress across the life course, associated with markers of socioeconomic disadvantage, influences physiologic outcomes (e.g., neuroendocrine, inflammatory, immune), leading to obesity-related chronic diseases (47). In infants and young children, there may be a dose--response relationship to exposure to poor-quality environments. Alternatively, the cumulative burden of exposure to negative environments may significantly impact long-term health and contribute to the development of obesity (30, 80). Braveman et al. (16) have suggested that the intergenerational transmission of social disadvantage and health outcomes, such as obesity, may be partially explained by epigenetic changes in gene expression that are passed across generations.
Policy initiatives, as noted in Table 1, may provide a way to equalize opportunities for disadvantaged populations by improving access to high-quality nutrition and PA environments in the places where young children and their parents spend time. Making improvements across the multiple dimensions that define the social context within which children grow and develop may benefit and support caregivers and their very young children as they pursue and maintain obesity-prevention behaviors. Making such improvements holds promise for ensuring immediate and long-standing intergenerational impacts on obesity prevention and long-term health (26).
CONCLUSIONS
Obesity is intergenerational. Preventing obesity in early life holds the promise of delaying or halting the current epidemic. There is limited research, but more is emerging, about the influences on the development of obesity during the early stages of life. It is critical to develop an understanding of the roles of both parents as biologic transmitters and behavioral models of obesity risk. More evidence is needed to inform interventions designed to reset entrenched obesity-promoting lifestyle practices that parents pass on to their offspring. An understanding is also needed of the context within which young children live and the interactions of economic and cultural determinants on the behaviors of parents and children across generations. Interventions should be tested at a macro- or policy-level to determine how to interrupt the development of obesity by eliminating early life exposure to poor-quality environments. The pathway for halting the intergenerational obesity epidemic requires additional discovery and the development of evidence-based interventions that can act across multiple dimensions of influence on early life.
ACKNOWLEDGMENTS
This research was supported by grant number number P30DK092950 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). All statements, conclusions, and findings are solely the responsibility of the authors and do not necessarily represent the official views of the NIDDK.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Debra Haire-Joshu, Public Health and Medicine, Brown School, Washington University in St. Louis, St. Louis, Missouri 63130; djoshu@wustl.edu.
Rachel Tabak, Prevention Research Center, Brown School, Washington University in St. Louis, St. Louis, Missouri 63130; rtabak@wustl.edu.
LITERATURE CITED
- 1.Agha M, Agha RA, Sandell J. Interventions to reduce and prevent obesity in pre-conceptual and pregnant women: a systematic review and meta-analysis. PLOS ONE. 2014;9:e95132. doi: 10.1371/journal.pone.0095132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al Mamun A, Lawlor DA, Cramb S, O'Callaghan M, Williams G, Najman J. Do childhood sleeping problems predict obesity in young adulthood? Evidence from a prospective birth cohort study. Am. J. Epidemiol. 2007;166:1368–73. doi: 10.1093/aje/kwm224. [DOI] [PubMed] [Google Scholar]
- 3.Anderson SE, Whitaker RC. Household routines and obesity in US preschool-aged children. Pediatrics. 2010;125:420–28. doi: 10.1542/peds.2009-0417. [DOI] [PubMed] [Google Scholar]
- 4.Au CP, Raynes-Greenow CH, Turner RM, Carberry AE, Jeffery H. Fetal and maternal factors associated with neonatal adiposity as measured by air displacement plethysmography: a large cross-sectional study. Early Hum. Dev. 2013;89:839–43. doi: 10.1016/j.earlhumdev.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 5.Baker JL, Michaelsen KF, Rasmussen KM, Sørensen TI. Maternal prepregnant body mass index, duration of breastfeeding, and timing of complementary food introduction are associated with infant weight gain. Am. J. Clin. Nutr. 2004;80:1579–88. doi: 10.1093/ajcn/80.6.1579. [DOI] [PubMed] [Google Scholar]
- 6.Barau G, Robillard PY, Hulsey T, Dedecker F, Laffite A, et al. Linear association between maternal pre-pregnancy body mass index and risk of caesarean section in term deliveries. BJOG. 2006;113:1173–77. doi: 10.1111/j.1471-0528.2006.01038.x. [DOI] [PubMed] [Google Scholar]
- 7.Bartok CJ, Ventura AK. Mechanisms underlying the association between breastfeeding and obesity. Int. J. Pediatr. Obes. 2009;4:196–204. doi: 10.3109/17477160902763309. [DOI] [PubMed] [Google Scholar]
- 8.Birch LL, Doub AE. Learning to eat: birth to age 2 y. Am. J. Clin. Nutr. 2014;99:723S–28S. doi: 10.3945/ajcn.113.069047. [DOI] [PubMed] [Google Scholar]
- 9.Birch LL, Fisher JO. Development of eating behaviors among children and adolescents. Pediatrics. 1998;101:539–49. [PubMed] [Google Scholar]
- 10.Birch LL, Ventura AK. Preventing childhood obesity: what works? Int. J. Obes. 2009;33(Suppl. 1):S74–81. doi: 10.1038/ijo.2009.22. [DOI] [PubMed] [Google Scholar]
- 11.Blumenshine P, Egerter S, Barclay CJ, Cubbin C, Braveman PA. Socioeconomic disparities in adverse birth outcomes: a systematic review. Am. J. Prev. Med. 2010;39:263–72. doi: 10.1016/j.amepre.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 12.Boeke CE, Mantzoros CS, Hughes MD, L Rifas-Shiman S, Villamor E, et al. Differential associations of leptin with adiposity across early childhood. Obesity. 2013;21:1430–37. doi: 10.1002/oby.20314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115:e290–96. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- 14.Bouret S, Levin BE, Ozanne SE. Gene-environment interactions controlling energy and glucose homeostasis and the developmental origins of obesity. Physiol. Rev. 2015;95:47–82. doi: 10.1152/physrev.00007.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braveman P. A health disparities perspective on obesity research. Prev. Chronic Dis. 2009;6:A91. [PMC free article] [PubMed] [Google Scholar]
- 16.Braveman P, Egerter S, Williams DR. The social determinants of health: coming of age. Annu. Rev. Public Health. 2011;32:381–98. doi: 10.1146/annurev-publhealth-031210-101218. [DOI] [PubMed] [Google Scholar]
- 17.Braveman P, Krieger N, Lynch J. Health inequalities and social inequalities in health. Bull. World Health Organ. 2000;78:232–34. discussion 234-35. [PMC free article] [PubMed] [Google Scholar]
- 18.Braveman PA. Monitoring equity in health and healthcare: a conceptual framework. J. Health Popul. Nutr. 2003;21:181–92. [PubMed] [Google Scholar]
- 19.Braveman PA, Kumanyika S, Fielding J, Laveist T, Borrell LN, et al. Health disparities and health equity: the issue is justice. Am. J. Public Health. 2011;101(Suppl. 1):S149–55. doi: 10.2105/AJPH.2010.300062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brisbois T, Farmer A, McCargar L. Early markers of adult obesity: a review. Obes. Rev. 2012;13:347–67. doi: 10.1111/j.1467-789X.2011.00965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bygren LO. Intergenerational health responses to adverse and enriched environments. Annu. Rev. Public Health. 2013;34:49–60. doi: 10.1146/annurev-publhealth-031912-114419. [DOI] [PubMed] [Google Scholar]
- 22.Catalano P. Maternal obesity and metabolic risk to the offspring: why lifestyle interventions may have not achieved the desired outcomes. Int. J. Obes. 2015 doi: 10.1038/ijo.2015.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiavaroli V, Giannini C, D'Adamo E, de Giorgis T, Chiarelli F, Mohn A. Insulin resistance and oxidative stress in children born small and large for gestational age. Pediatrics. 2009;124:695–702. doi: 10.1542/peds.2008-3056. [DOI] [PubMed] [Google Scholar]
- 24.Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat. Rev. Genet. 2012;13:260–70. doi: 10.1038/nrg3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cole TJ, Power C, Moore GE. Intergenerational obesity involves both the father and the mother. Am. J. Clin. Nutr. 2008;87:1535–36. doi: 10.1093/ajcn/87.5.1535. author reply 36-37. [DOI] [PubMed] [Google Scholar]
- 27.Comm. Evaluating Prog. Obes. Prev. Efforts . Evaluating Obesity Prevention Efforts: A Plan for Measuring Progress. IOM (Inst. Med.), Natl. Acad. Press; Washington, DC: 2013. [Google Scholar]
- 26.Comm. Obes. Prev. Policies Young Child . Early Childhood Obesity Prevention Policies. IOM (Inst. Med.), Natl. Acad. Press; Washington, DC: 2011. [Google Scholar]
- 28.Coogan PE, Wise LA, Cozier YC, Palmer JR, Rosenberg L. Lifecourse educational status in relation to weight gain in African American women. Ethn. Dis. 2012;22:198–206. [PMC free article] [PubMed] [Google Scholar]
- 29.Dalenius K, Borland E, Smith B, Polhamus B, Grummer-Strawn L. Pediatric Nutrition Surveillance 2010 Report. US Depart. Health Hum. Serv., Cent. Dis. Control Prev.; Atlanta: 2012. [Google Scholar]
- 30.Das A. How does race get “under the skin”? Inflammation, weathering, and metabolic problems in late life. Soc. Sci. Med. 2013;77:75–83. doi: 10.1016/j.socscimed.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Barros MC, Lopes MA, Francisco RP, Sapienza AD, Zugaib M. Resistance exercise and glycemic control in women with gestational diabetes mellitus. Am. J. Obstet. Gynecol. 2010;203:556, e1–e6. doi: 10.1016/j.ajog.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 32.Dehlendorf C, Marchi K, Vittinghoff E, Braveman P. Sociocultural determinants of teenage childbearing among Latinas in California. Matern. Child Health J. 2010;14:194–201. doi: 10.1007/s10995-009-0443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dixon B, Peña M-M, Taveras EM. Lifecourse approach to racial/ethnic disparities in childhood obesity. Adv. Nutr. 2012;3:73–82. doi: 10.3945/an.111.000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dodd JM, Crowther CA, Robinson JS. Dietary and lifestyle interventions to limit weight gain during pregnancy for obese or overweight women: a systematic review. Acta Obstet. Gynecol. Scand. 2008;87:702–6. doi: 10.1080/00016340802061111. [DOI] [PubMed] [Google Scholar]
- 35.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. PNAS. 2010;107:11971–75. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Downs SM, Fraser SN, Storey KE, Forbes LE, Spence JC, et al. Geography influences dietary intake, physical activity and weight status of adolescents. J. Nutr. Metab. 2012;2012:816834. doi: 10.1155/2012/816834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drake AJ, McPherson RC, Godfrey KM, Cooper C, Lillycrop KA, et al. An unbalanced maternal diet in pregnancy associates with offspring epigenetic changes in genes controlling glucocorticoid action and foetal growth. Clin. Endocrinol. 2012;77:808–15. doi: 10.1111/j.1365-2265.2012.04453.x. [DOI] [PubMed] [Google Scholar]
- 38.Durmuş B, Kruithof CJ, Gillman MH, Willemsen SP, Hofman A, et al. Parental smoking during pregnancy, early growth, and risk of obesity in preschool children: the Generation R Study. Am. J. Clin. Nutr. 2011;94:164–71. doi: 10.3945/ajcn.110.009225. [DOI] [PubMed] [Google Scholar]
- 39.Elks CE, Loos RJ, Sharp SJ, Langenberg C, Ring SM, et al. Genetic markers of adult obesity risk are associated with greater early infancy weight gain and growth. PLOS Med. 2010;7:e1000284. doi: 10.1371/journal.pmed.1000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evans-Campbell T. Historical trauma in American Indian/Native Alaska communities: a multilevel framework for exploring impacts on individuals, families, and communities. J. Interpers. Violence. 2008;23:316–38. doi: 10.1177/0886260507312290. [DOI] [PubMed] [Google Scholar]
- 41.Ezzahir N, Alberti C, Deghmoun S, Zaccaria I, Czernichow P, et al. Time course of catch-up in adiposity influences adult anthropometry in individuals who were born small for gestational age. Pediatr. Res. 2005;58:243–47. doi: 10.1203/01.PDR.0000169980.35179.89. [DOI] [PubMed] [Google Scholar]
- 42.Fein SB, Labiner-Wolfe J, Scanlon KS, Grummer-Strawn LM. Selected complementary feeding practices and their association with maternal education. Pediatrics. 2008;122:S91–97. doi: 10.1542/peds.2008-1315l. [DOI] [PubMed] [Google Scholar]
- 43.Fisher S, Kim S, Sharma A, Rochat R, Morrow B. Is obesity still increasing among pregnant women? Prepregnancy obesity trends in 20 states, 2003-2009. Prev. Med. 2013;56:372–78. doi: 10.1016/j.ypmed.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Funk K, LeBlanc E, Vesco K, Stevens V. Women's attitudes towards a preconception healthy lifestyle programme. Clin. Obes. 2015;5:67–71. doi: 10.1111/cob.12088. [DOI] [PubMed] [Google Scholar]
- 45.Gillman MW. Commentary: Breastfeeding and obesity--the 2011 scorecard. Int. J. Epidemiol. 2011;40:681–84. doi: 10.1093/ije/dyr085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Godfrey KM, Sheppard A, Gluckman PD, Lillycrop KA, Burdge GC, et al. Epigenetic gene promoter methylation at birth is associated with child's later adiposity. Diabetes. 2011;60:1528–34. doi: 10.2337/db10-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Golden SH, Brown A, Cauley JA, Chin MH, Gary-Webb TL, et al. Health disparities in endocrine disorders: biological, clinical, and nonclinical factors--an Endocrine Society scientific statement. J. Clin. Endocrinol. Metab. 2012;97:E1579–639. doi: 10.1210/jc.2012-2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gortmaker SL, Dietz WH, Sobol AM, Wehler CA. Increasing pediatric obesity in the United States. Am. J. Dis. Child. 1987;141:535–40. doi: 10.1001/archpedi.1987.04460050077035. [DOI] [PubMed] [Google Scholar]
- 49.Grissom NM, Reyes TM. Gestational overgrowth and undergrowth affect neurodevelopment: similarities and differences from behavior to epigenetics. Int. J. Dev. Neurosci. 2013;31:406–14. doi: 10.1016/j.ijdevneu.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grote V, Schiess SA, Closa-Monasterolo R, Escribano J, Giovannini M, et al. The introduction of solid food and growth in the first 2 y of life in formula-fed children: analysis of data from a European cohort study. Am. J. Clin. Nutr. 2011;94:14. doi: 10.3945/ajcn.110.000810. [DOI] [PubMed] [Google Scholar]
- 51.Grote V, Theurich M. Complementary feeding and obesity risk. Curr. Opin. Clin. Nutr. Metab. Care. 2014;17:273–77. doi: 10.1097/MCO.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 52.Guénard F, Deshaies Y, Cianflone K, Kral JG, Marceau P, Vohl M-C. Differential methylation in glucoregulatory genes of offspring born before versus after maternal gastrointestinal bypass surgery. PNAS. 2013;110:11439–44. doi: 10.1073/pnas.1216959110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haines J, McDonald J, O'Brien A, Sherry B, Bottino CJ, et al. Healthy Habits, Happy Homes: Randomized Trial to Improve Household Routines for Obesity Prevention Among Preschool-Aged Children. JAMA Pediatr. 2013;167:1072–79. doi: 10.1001/jamapediatrics.2013.2356. [DOI] [PubMed] [Google Scholar]
- 54.Haire-Joshu D. Cancer prevention through policy interventions that alter childhood disparities in energy balance. In: Bowen DJ, Denis GV, Berger NA, editors. Impact of Energy Balance on Cancer Disparities. Springer Int.; Basel: 2014. pp. 283–303. [Google Scholar]
- 55.Haire-Joshu D, Yount BW, Budd EL, Schwarz C, Schermbeck R, et al. The quality of school wellness policies and energy-balance behaviors of adolescent mothers. Prev. Chronic Dis. 2011;8:A34. [PMC free article] [PubMed] [Google Scholar]
- 56.Hawkins M, Hosker M, Marcus B, Rosal M, Braun B, et al. A pregnancy lifestyle intervention to prevent gestational diabetes risk factors in overweight Hispanic women: a feasibility randomized controlled trial. Diabet. Med. 2015;32:108–15. doi: 10.1111/dme.12601. [DOI] [PubMed] [Google Scholar]
- 57.Heinig MJ, Nommsen LA, Peerson JM, Lonnerdal B, Dewey KG. Intake and growth of breast-fed and formula-fed infants in relation to the timing of introduction of complementary foods: the DARLING study. Acta Paediatr. 1993;82:999–1006. doi: 10.1111/j.1651-2227.1993.tb12798.x. [DOI] [PubMed] [Google Scholar]
- 58.Herring SJ, Oken E. Obesity and diabetes in mothers and their children: Can we stop the intergenerational cycle? Curr. Diabetes Rep. 2011;11:20–27. doi: 10.1007/s11892-010-0156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hillemeier MM, Downs DS, Feinberg ME, Weisman CS, Chuang CH, et al. Improving women's preconceptional health: findings from a randomized trial of the Strong Healthy Women intervention in the Central Pennsylvania women's health study. Women's Health Issues. 2008;18:S87–96. doi: 10.1016/j.whi.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hochberg Ze, Feil R, Constancia M, Fraga M, Junien C, et al. Child health, developmental plasticity, and epigenetic programming. Endocr. Rev. 2010;32:159–224. doi: 10.1210/er.2009-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hopkins SA, Baldi JC, Cutfield WS, McCowan L, Hofman PL. Exercise training in pregnancy reduces offspring size without changes in maternal insulin sensitivity. J. Clin. Endocrinol. Metab. 2010;95:2080–88. doi: 10.1210/jc.2009-2255. [DOI] [PubMed] [Google Scholar]
- 62.Huh SY, Rifas-Shiman SL, Taveras EM, Oken E, Gillman MW. Timing of solid food introduction and risk of obesity in preschool-aged children. Pediatrics. 2011;127:e544–51. doi: 10.1542/peds.2010-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jodar M, Selvaraju S, Sendler E, Diamond MP, Krawetz SA. The presence, role and clinical use of spermatozoal RNAs. Hum. Reprod. Update. 2013;19:604–24. doi: 10.1093/humupd/dmt031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johnson K, Posner SF, Biermann J, Cordero JF, Atrash HK, et al. Recommendations to improve preconception health and health care--United States. A report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care. MMWR Recomm. Rep. 2006;55:1–23. [PubMed] [Google Scholar]
- 65.Jolin EM, Weller RA. Television viewing and its impact on childhood behaviors. Curr. Psychiatry Rep. 2011;13:122–28. doi: 10.1007/s11920-011-0175-5. [DOI] [PubMed] [Google Scholar]
- 66.Kelishadi R, Poursafa P. A review on the genetic, environmental, and lifestyle aspects of the early-life origins of cardiovascular disease. Curr. Probl. Pediatr. Adolesc. Health Care. 2014;44:54–72. doi: 10.1016/j.cppeds.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 67.Kleinman RE, Greer FR. Pediatric Nutrition. Am. Acad. Pediatr.; Elk Grove Village, IL: 2013. [Google Scholar]
- 68.Knutson KL. Sociodemographic and cultural determinants of sleep deficiency: implications for cardiometabolic disease risk. Soc. Sci. Med. 2013;79:7–15. doi: 10.1016/j.socscimed.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kramer MS, Guo T, Platt RW, Vanilovich I, Sevkovskaya Z, et al. Feeding effects on growth during infancy. J. Pediatr. 2004;145:600–5. doi: 10.1016/j.jpeds.2004.06.069. [DOI] [PubMed] [Google Scholar]
- 70.Kuhl ES, Clifford LM, Stark LJ. Obesity in preschoolers: behavioral correlates and directions for treatment. Obesity. 2012;20:3–29. doi: 10.1038/oby.2011.201. [DOI] [PubMed] [Google Scholar]
- 71.Kuzawa CW, Sweet E. Epigenetics and the embodiment of race: developmental origins of US racial disparities in cardiovascular health. Am. J. Hum. Biol. 2009;21:2–15. doi: 10.1002/ajhb.20822. [DOI] [PubMed] [Google Scholar]
- 72.Laitinen J, Jääskeläinen A, Hartikainen AL, Sovio U, Vääräsmäki M, et al. Maternal weight gain during the first half of pregnancy and offspring obesity at 16 years: a prospective cohort study. BJOG. 2012;119:716–23. doi: 10.1111/j.1471-0528.2012.03319.x. [DOI] [PubMed] [Google Scholar]
- 73.Lambourne K, Donnelly JE. The role of physical activity in pediatric obesity. Pediatr. Clin. North Am. 2011;58:1481–91. doi: 10.1016/j.pcl.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 74.Laraia BA, Siega-Riz AM, Gundersen C. Household food insecurity is associated with self-reported pregravid weight status, gestational weight gain, and pregnancy complications. J. Am. Diet. Assoc. 2010;110:692–701. doi: 10.1016/j.jada.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Laraia BA, Siega-Riz AM, Gundersen C, Dole N. Psychosocial factors and socioeconomic indicators are associated with household food insecurity among pregnant women. J. Nutr. 2006;136:177–82. doi: 10.1093/jn/136.1.177. [DOI] [PubMed] [Google Scholar]
- 76.Larson N, Ward DS, Neelon SB, Story M. What role can child-care settings play in obesity prevention? A review of the evidence and call for research efforts. J. Am. Diet. Assoc. 2011;111:1343–62. doi: 10.1016/j.jada.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 77.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–23. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 78.Llewellyn CH, van Jaarsveld CH, Plomin R, Fisher A, Wardle J. Inherited behavioral susceptibility to adiposity in infancy: a multivariate genetic analysis of appetite and weight in the Gemini birth cohort. Am. J. Clin. Nutr. 2012;95:633–39. doi: 10.3945/ajcn.111.023671. [DOI] [PubMed] [Google Scholar]
- 79.Loprinzi PD, Cardinal BJ, Loprinzi KL, Lee H. Benefits and environmental determinants of physical activity in children and adolescents. Obes. Facts. 2012;5:597–610. doi: 10.1159/000342684. [DOI] [PubMed] [Google Scholar]
- 80.Love C, David RJ, Rankin KM, Collins JW., Jr Exploring weathering: effects of lifelong economic environment and maternal age on low birth weight, small for gestational age, and preterm birth in African-American and white women. Am. J. Epidemiol. 2010;172:127–34. doi: 10.1093/aje/kwq109. [DOI] [PubMed] [Google Scholar]
- 81.Lumeng JC, Taveras EM, Birch L, Yanovski SZ. Prevention of obesity in infancy and early childhood: a National Institutes of Health workshop. JAMA Pediatr. 2015;169:484–90. doi: 10.1001/jamapediatrics.2014.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lumey LH, Stein AD, Susser E. Prenatal famine and adult health. Annu. Rev. Public Health. 2011;32:237–62. doi: 10.1146/annurev-publhealth-031210-101230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mamun AA, O'Callaghan M, Callaway L, Williams G, Najman J, Lawlor DA. Associations of gestational weight gain with offspring body mass index and blood pressure at 21 years of age: evidence from a birth cohort study. Circulation. 2009;119:1720–27. doi: 10.1161/CIRCULATIONAHA.108.813436. [DOI] [PubMed] [Google Scholar]
- 84.Martin RM, Patel R, Kramer MS, Guthrie L, Vilchuck K, et al. Effects of promoting longer-term and exclusive breastfeeding on adiposity and insulin-like growth factor-I at age 11.5 years: a randomized trial. JAMA. 2013;309:1005–13. doi: 10.1001/jama.2013.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.May AL, Freedman D, Sherry B, Blanck HM. Obesity: United States, 1999-2010. MMWR Surveill. Summ. 2013;62(Suppl. 3):120–28. [PubMed] [Google Scholar]
- 86.Mennella JA. Ontogeny of taste preferences: basic biology and implications for health. Am. J. Clin. Nutr. 2014;99:704S–11S. doi: 10.3945/ajcn.113.067694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mennella JA, Jagnow CP, Beauchamp GK. Prenatal and postnatal flavor learning by human infants. Pediatrics. 2001;107:E88. doi: 10.1542/peds.107.6.e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Merzouk H, Madani S, Korso N, Bouchenak M, Prost J, Belleville J. Maternal and fetal serum lipid and lipoprotein concentrations and compositions in type 1 diabetic pregnancy: relationship with maternal glycemic control. J. Lab. Clin. Med. 2000;136:441–48. doi: 10.1067/mlc.2000.111004. [DOI] [PubMed] [Google Scholar]
- 89.Monasta L, Batty GD, Cattaneo A, Lutje V, Ronfani L, et al. Early-life determinants of overweight and obesity: a review of systematic reviews. Obes. Rev. 2010;11:695–708. doi: 10.1111/j.1467-789X.2010.00735.x. [DOI] [PubMed] [Google Scholar]
- 90.Moss BG, Yeaton WH. Young children's weight trajectories and associated risk factors: results from the Early Childhood Longitudinal Study-Birth Cohort. Am. J. Health Promot. 2011;25:190–98. doi: 10.4278/ajhp.090123-QUAN-29. [DOI] [PubMed] [Google Scholar]
- 91.Mottola MF, Giroux I, Gratton R, Hammond J-A, Hanley A, et al. Nutrition and exercise prevent excess weight gain in overweight pregnant women. Med. Sci. Sports Exerc. 2010;42:265. doi: 10.1249/MSS.0b013e3181b5419a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mourtakos SP, Tambalis KD, Panagiotakos DB, Antonogeorgos G, Arnaoutis G, et al. Maternal lifestyle characteristics during pregnancy, and the risk of obesity in the offspring: a study of 5,125 children. BMC Pregnancy Childbirth. 2015;15:66. doi: 10.1186/s12884-015-0498-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Muhlhausler BS, Gugusheff JR, Ong ZY, Vithayathil MA. Nutritional approaches to breaking the intergenerational cycle of obesity. Can. J. Physiol. Pharmacol. 2013;91:421–28. doi: 10.1139/cjpp-2012-0353. [DOI] [PubMed] [Google Scholar]
- 94.Must A, Parisi SM. Sedentary behavior and sleep: paradoxical effects in association with childhood obesity. Int. J. Obes. 2009;33(Suppl. 1):S82–86. doi: 10.1038/ijo.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nader PR, Huang TT, Gahagan S, Kumanyika S, Hammond RA, Christoffel KK. Next steps in obesity prevention: altering early life systems to support healthy parents, infants, and toddlers. Child. Obes. 2012;8:195–204. doi: 10.1089/chi.2012.0004. [DOI] [PubMed] [Google Scholar]
- 96.Nehring I, Lehmann S, Kries R. Gestational weight gain in accordance to the IOM/NRC criteria and the risk for childhood overweight: a meta-analysis. Pediatr. Obes. 2013;8:218–24. doi: 10.1111/j.2047-6310.2012.00110.x. [DOI] [PubMed] [Google Scholar]
- 97.Nevarez MD, Rifas-Shiman SL, Kleinman KP, Gillman MW, Taveras EM. Associations of early life risk factors with infant sleep duration. Acad. Pediatr. 2010;10:187–93. doi: 10.1016/j.acap.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ng S-F, Lin RC, Laybutt DR, Barres R, Owens JA, Morris MJ. Chronic high-fat diet in fathers programs β-cell dysfunction in female rat offspring. Nature. 2010;467:963–66. doi: 10.1038/nature09491. [DOI] [PubMed] [Google Scholar]
- 99.Oddo VM, Jones-Smith JC. Gains in income during early childhood are associated with decreases in BMI z scores among children in the United States. Am. J. Clin. Nutr. 2015;101:1225–31. doi: 10.3945/ajcn.114.096693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311:806–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Oken E, Levitan E, Gillman M. Maternal smoking during pregnancy and child overweight: systematic review and meta-analysis. Int. J. Obes. 2008;32:201–10. doi: 10.1038/sj.ijo.0803760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Olsen SF, Halldorsson TI, Willett WC, Knudsen VK, Gillman MW, et al. Milk consumption during pregnancy is associated with increased infant size at birth: prospective cohort study. Am. J. Clin. Nutr. 2007;86:1104–10. doi: 10.1093/ajcn/86.4.1104. [DOI] [PubMed] [Google Scholar]
- 103.Öst A, Lempradl A, Casas E, Weigert M, Tiko T, et al. Paternal diet defines offspring chromatin state and intergenerational obesity. Cell. 2014;159:1352–64. doi: 10.1016/j.cell.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 104.Ounsted M, Sleigh G. The infant's self-regulation of food intake and weight gain: difference in metabolic balance after growth constraint or acceleration in utero. Lancet. 1975;305:1393–97. doi: 10.1016/s0140-6736(75)92605-7. [DOI] [PubMed] [Google Scholar]
- 105.Owen CG, Martin RM, Whincup PH, Smith GD, Cook DG. Effect of infant feeding on the risk of obesity across the life course: a quantitative review of published evidence. Pediatrics. 2005;115:1367–77. doi: 10.1542/peds.2004-1176. [DOI] [PubMed] [Google Scholar]
- 106.Paliy O, Piyathilake CJ, Kozyrskyj A, Celep G, Marotta F, Rastmanesh R. Excess body weight during pregnancy and offspring obesity: potential mechanisms. Nutrition. 2014;30:245–51. doi: 10.1016/j.nut.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 107.Parker M, Rifas-Shiman SL, Belfort MB, Taveras EM, Oken E, et al. Gestational glucose tolerance and cord blood leptin levels predict slower weight gain in early infancy. J. Pediatr. 2011;158:227–33. doi: 10.1016/j.jpeds.2010.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Perrine CG, Galuska DA, Dohack JL, Shealy KR, Murphy PE, et al. Vital signs: improvements in maternity care policies and practices that support breastfeeding--United States, 2007-2013. MMWR. 2014;64:1112–17. doi: 10.15585/mmwr.mm6439a5. [DOI] [PubMed] [Google Scholar]
- 109.Phimister EG, Ozanne SE. Epigenetic signatures of obesity. N. Engl. J. Med. 2015;372:973–74. doi: 10.1056/NEJMcibr1414707. [DOI] [PubMed] [Google Scholar]
- 110.Pledger AB. Exploring the experiences of pregnant women using an NHS stop smoking service: a qualitative study. Perspect. Public Health. 2015;135:138–44. doi: 10.1177/1757913915577156. [DOI] [PubMed] [Google Scholar]
- 111.Power C, Kuh D, Morton S. From developmental origins of adult disease to life course research on adult disease and aging: insights from birth cohort studies. Annu. Rev. Public Health. 2013;34:7–28. doi: 10.1146/annurev-publhealth-031912-114423. [DOI] [PubMed] [Google Scholar]
- 112.Reiter RJ, Tan DX, Korkmaz A, Ma S. Obesity and metabolic syndrome: association with chronic disruption, sleep deprivation, and melatonin suppression. Ann. Med. 2012;44:564–77. doi: 10.3109/07853890.2011.586365. [DOI] [PubMed] [Google Scholar]
- 113.Rietmeijer-Mentink M, Paulis WD, van Middelkoop M, Bindels PJ, van der Wouden JC. Difference between parental perception and actual weight status of children: a systematic review. Matern. Child Nutr. 2013;9:3–22. doi: 10.1111/j.1740-8709.2012.00462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ronnberg A, Nilsson K. Interventions during pregnancy to reduce excessive gestational weight gain: a systematic review assessing current clinical evidence using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system. BJOG. 2010;117:1327–34. doi: 10.1111/j.1471-0528.2010.02619.x. [DOI] [PubMed] [Google Scholar]
- 115.Saavedra JM, Deming D, Dattilo A, Reidy K. Lessons from the feeding infants and toddlers study in North America: what children eat, and implications for obesity prevention. Ann. Nutr. Metab. 2013;62(Suppl. 3):27–36. doi: 10.1159/000351538. [DOI] [PubMed] [Google Scholar]
- 116.Samuel J, Anderson TA, Nelson SE. Influence of formula concentration on caloric intake and growth of normal infants. Acta Paediatr. 1975;64:172–81. doi: 10.1111/j.1651-2227.1975.tb03818.x. [DOI] [PubMed] [Google Scholar]
- 117.Savage JS, Fisher JO, Birch LL. Parental influence on eating behavior: conception to adolescence. J. Law Med. Ethics. 2007;35:22–34. doi: 10.1111/j.1748-720X.2007.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schack-Nielsen L, Michaelsen KF, Gamborg M, Mortensen EL, Sorensen TI. Gestational weight gain in relation to offspring body mass index and obesity from infancy through adulthood. Int. J. Obes. 2010;34:67–74. doi: 10.1038/ijo.2009.206. [DOI] [PubMed] [Google Scholar]
- 119.Schmidt ME, Rich M, Rifas-Shiman SL, Oken E, Taveras EM. Television viewing in infancy and child cognition at 3 years of age in a US cohort. Pediatrics. 2009;123:e370–75. doi: 10.1542/peds.2008-3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shirazian T, Monteith S, Friedman F, Rebarber A. Lifestyle modification program decreases pregnancy weight gain in obese women. Am. J. Perinatol. 2010;27:411–14. doi: 10.1055/s-0029-1243368. [DOI] [PubMed] [Google Scholar]
- 121.Shrewsbury V, Wardle J. Socioeconomic status and adiposity in childhood: a systematic review of cross-sectional studies 1990-2005. Obesity. 2008;16:275–84. doi: 10.1038/oby.2007.35. [DOI] [PubMed] [Google Scholar]
- 122.Shutts K, Kinzler KD, DeJesus JM. Understanding infants’ and children's social learning about foods: previous research and new prospects. Dev. Psychol. 2013;49:419–25. doi: 10.1037/a0027551. [DOI] [PubMed] [Google Scholar]
- 123.Singhal A. Does early growth affect long-term risk factors for cardiovascular disease? Presented at Nestle Nutr. Workshop Ser. Pediatr. Program. 2010 doi: 10.1159/000281145. [DOI] [PubMed] [Google Scholar]
- 124.Skinner JD, Carruth BR, Wendy B, Ziegler PJ. Children's food preferences: a longitudinal analysis. J. Am. Diet. Assoc. 2002;102:1638–47. doi: 10.1016/s0002-8223(02)90349-4. [DOI] [PubMed] [Google Scholar]
- 125.Soubry A, Hoyo C, Jirtle RL, Murphy SK. A paternal environmental legacy: evidence for epigenetic inheritance through the male germ line. BioEssays. 2014;36:359–71. doi: 10.1002/bies.201300113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Steiner JE, Glaser D, Hawilo ME, Berridge KC. Comparative expression of hedonic impact: affective reactions to taste by human infants and other primates. Neurosci. Biobehav. Rev. 2001;25:53–74. doi: 10.1016/s0149-7634(00)00051-8. [DOI] [PubMed] [Google Scholar]
- 127.Stewart CP, Christian P, Schulze KJ, Arguello M, LeClerq SC, et al. Low maternal vitamin B-12 status is associated with offspring insulin resistance regardless of antenatal micronutrient supplementation in rural Nepal. J. Nutr. 2011;141:1912–17. doi: 10.3945/jn.111.144717. [DOI] [PubMed] [Google Scholar]
- 128.Stifter CA, Anzman-Frasca S, Birch LL, Voegtline K. Parent use of food to soothe infant/toddler distress and child weight status: an exploratory study. Appetite. 2011;57:693–99. doi: 10.1016/j.appet.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 129.Streuling I, Beyerlein A, Rosenfeld E, Hofmann H, Schulz T, Von Kries R. Physical activity and gestational weight gain: a meta-analysis of intervention trials. BJOG. 2011;118:278–84. doi: 10.1111/j.1471-0528.2010.02801.x. [DOI] [PubMed] [Google Scholar]
- 130.Streuling I, Beyerlein A, von Kries R. Can gestational weight gain be modified by increasing physical activity and diet counseling? A meta-analysis of interventional trials. Am. J. Clin. Nutr. 2010;92:678–87. doi: 10.3945/ajcn.2010.29363. [DOI] [PubMed] [Google Scholar]
- 131.Sutherland G, Brown S, Yelland J. Applying a social disparities lens to obesity in pregnancy to inform efforts to intervene. Midwifery. 2013;29:338–43. doi: 10.1016/j.midw.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 132.Taveras EM, Gillman MW, Kleinman K, Rich-Edwards JW, Rifas-Shiman SL. Racial/ethnic differences in early-life risk factors for childhood obesity. Pediatrics. 2010;125:686–95. doi: 10.1542/peds.2009-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Temel S, van Voorst SF, Jack BW, Denktaş S, Steegers EA. Evidence-based preconceptional lifestyle interventions. Epidemiol. Rev. 2014;36:19–30. doi: 10.1093/epirev/mxt003. [DOI] [PubMed] [Google Scholar]
- 134.Thompson AL. Intergenerational impact of maternal obesity and postnatal feeding practices on pediatric obesity. Nutr. Rev. 2013;71:S55–61. doi: 10.1111/nure.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Thompson AL, Mendez MA, Borja JB, Adair LS, Zimmer CR, Bentley ME. Development and validation of the infant feeding style questionnaire. Appetite. 2009;53:210–21. doi: 10.1016/j.appet.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tsai PJ, Yu CH, Hsu SP, Lee YH, Chiou CH, et al. Cord plasma concentrations of adiponectin and leptin in healthy term neonates: positive correlation with birthweight and neonatal adiposity. Clin. Endocrinol. 2004;61:88–93. doi: 10.1111/j.1365-2265.2004.02057.x. [DOI] [PubMed] [Google Scholar]
- 137.Vandewater EA, Rideout VJ, Wartella EA, Huang X, Lee JH, Shim M-S. Digital childhood: electronic media and technology use among infants, toddlers, and preschoolers. Pediatrics. 2007;119:e1006–15. doi: 10.1542/peds.2006-1804. [DOI] [PubMed] [Google Scholar]
- 138.Wasser H, Bentley M, Borja J, Goldman BD, Thompson A, et al. Infants perceived as “fussy” are more likely to receive complementary foods before 4 months. Pediatrics. 2011;127:229–37. doi: 10.1542/peds.2010-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Waterland RA. Epigenetic mechanisms affecting regulation of energy balance: many questions, few answers. Annu. Rev. Nutr. 2014;34:337–55. doi: 10.1146/annurev-nutr-071813-105315. [DOI] [PubMed] [Google Scholar]
- 140.Weisman CS, Hillemeier MM, Downs DS, Feinberg ME, Chuang CH, et al. Improving women's preconceptional health: long-term effects of the Strong Healthy Women behavior change intervention in the Central Pennsylvania Women's Health Study. Women's Health Issues. 2011;21:265–71. doi: 10.1016/j.whi.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Weiss R, Bremer AA, Lustig RH. What is metabolic syndrome, and why are children getting it? Ann. N. Y. Acad. Sci. 2013;1281:123–40. doi: 10.1111/nyas.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N. Engl. J. Med. 1997;337:869–73. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- 143.Woolf SH, Braveman P. Where health disparities begin: the role of social and economic determinants--and why current policies may make matters worse. Health Aff. 2011;30:1852–59. doi: 10.1377/hlthaff.2011.0685. [DOI] [PubMed] [Google Scholar]
- 144.Yu Z, Han S, Zhu J, Sun X, Ji C, Guo X. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PLOS ONE. 2013;8:e61627. doi: 10.1371/journal.pone.0061627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zimmerman FJ, Christakis DA, Meltzoff AN. Television and DVD/video viewing in children younger than 2 years. Arch. Pediatr. Adolesc. Med. 2007;161:473–79. doi: 10.1001/archpedi.161.5.473. [DOI] [PubMed] [Google Scholar]