Abstract
Purpose
To determine if propoxyphene withdrawal from the U.S. market was associated with opioid continuation, continued chronic opioid use and secondary propoxyphene related adverse events (emergency department visits, opioid-related events, and acetaminophen toxicity).
Methods
Medical service use and pharmacy data from 11/19/08 to 11/19/11 were collected from the national Veterans Healthcare Administration (VHA) healthcare databases. A quasi-experimental pre-post retrospective cohort design utilizing a historical comparison group provided the study framework. Logistic regression controlling for baseline covariates was used to estimate the effect of propoxyphene withdrawal.
Results
There were 24,328 subjects (policy-affected n=10,747; comparison n=13,581) meeting inclusion criteria. In the policy-affected cohort, 10.6% of users ceased using opioids and 26.6% stopped chronic opioid use compared to 3.8% and 13.5% in the historical comparison cohort respectively. Those in the policy-affected cohort were 2.7 (95%CI: 2.5–2.8) and 3.2 (95%CI: 2.9–3.6) times more likely than those in the historical comparison cohort to discontinue chronic opioid and any opioid use respectively. Changes in adverse events and ED visits were not different between policy affected and historical comparison cohorts (p>0.05).
Conclusions
The withdrawal of propoxyphene containing products resulted in rapid and virtually complete elimination in propoxyphene prescribing in the Veterans population; however, nearly 90% of regular users of propoxyphene switched to an alternate opioid and three quarters continued to use opioids chronically.
Keywords: Propoxyphene withdrawal, opioid discontinuation
Introduction
Propoxyphene is a short-acting, Drug Enforcement Agency Schedule IV, synthetic opioid introduced in 1957 that was indicated for mild to moderate pain. Propoxyphene was formulated either by itself (Darvon 65mg) or in combination with other analgesics, most commonly with acetaminophen. When compared to other analgesics in acute and chronic pain, propoxyphene with acetaminophen was consistently shown to be equivalent or inferior to other commonly used analgesics. In postoperative pain alone, propoxyphene with acetaminophen was found less effective in pain relief than ibuprofen 400 milligrams and equally or less effective than codeine with acetaminophen or tramadol.1–4 A meta-analysis performed on 26 randomized controlled trials showed the addition of propoxyphene to acetaminophen in managing postoperative, arthritis and musculoskeletal pain was no more effective than acetaminophen alone.5
Propoxyphene posed serious safety concerns. The mostly widely known serious side effect of propoxyphene is cardiotoxicity leading to potentially fatal arrhythmias.6 Propoxyphene has also been associated with dependency, abuse, and overdoses leading to hospitalization and death.7–14 Per 100,000 prescriptions, propoxyphene was linked to more drug-related deaths than either hydrocodone or tramadol and more emergency department visits than codeine.15
Despite the potential risks of propoxyphene and non-superior analgesic properties relative to common alternatives, propoxyphene with acetaminophen was a commonly used drug. It ranked 79th in prescription volume in the United States in 2009.16 Because of accumulating data regarding cardio-toxicity and following previous action by European regulatory agencies to withdraw propoxyphene from their markets, FDA requested that propoxyphene be removed from the U.S. market.17–19 On November 19th, 2010, Xanodyne Pharmaceuticals agreed to remove propoxyphene from the U.S. market.19 This withdrawal occurred in the context when prescription opioid abuse was the fastest growing form of drug abuse20–21 with a parallel rise in fatal opioid overdoses.22 Clinicians and policy makers have tried to address opioid misuse and abuse through a variety of mechanisms including expansion of prescription drug monitoring programs, encouraging development of abuse deterrent opioid formulations, and pain contracts.23 To date, we are not aware of data on the effect of withdrawing a popular opioid in the U.S. such as propoxyphene on opioid use and chronic use.
The withdrawal of propoxyphene from the U.S. market created a natural experiment to understand how withdrawal of this popular medication would impact use of opioids in a cohort of regular users. Using data from a Veterans Affairs data repository, our study sought to determine if propoxyphene withdrawal from the U.S. market was associated with opioid discontinuation, continued chronic opioid use, changes in daily opioid dose expressed in morphine equivalent dose (MED), and opioid days supplied among chronic propoxyphene users. For regular users of propoxyphene, the withdrawal essentially forced clinicians and their patients to re-evaluate their analgesic strategy and our primary interest was to determine the extent patients switched to another opioid and continued opioids chronically. Secondary analyses sought to determine the influence of the propoxyphene withdrawal on potential propoxyphene-related adverse events (emergency department visits, opioid-related events, and acetaminophen toxicity).
Methods
Study Design and Data Sources
This study used a quasi-experimental pre-post historical comparison cohort study design, exploiting the natural experiment created when the FDA removed propoxyphene from the market. We created two cohorts (policy-affected cohort and historical comparison cohort) with a pre- and post-period for each cohort (Figure 1).24 For the policy-affected cohort, the pre-period was defined as the 365-day period before DPP removal (11/19/2009 – 11/18/2010) and the post-period was defined as the 365-day period after DPP removal (11/19/2010 – 11/19/2011). The historical comparison group was created using data one year prior to the policy-affected cohort with 365 day pre- and post-periods constructed around November 19, 2009 to control for any seasonal influences. For the historical comparison cohort the pre-period was defined as 11/19/2008 – 11/18/2009 and the post-period was defined as 11/19/2009 – 11/19/2010.
Figure 1.
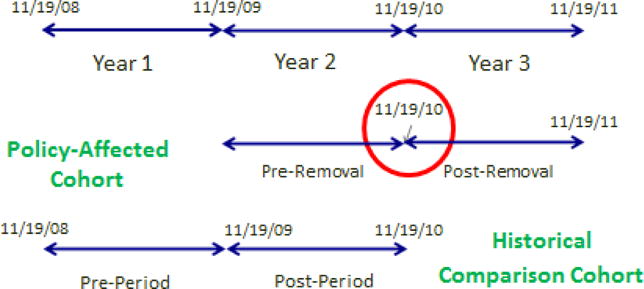
Visual Demonstration of the Quasi-Experimental Pre-Post Policy-Affected/Historical Comparison Study Design
Data for this study were obtained from three sources: VA Pharmacy Benefits Management Service (PBM), VA National Patient Care Database (NPCD), and VA Vital Status File. The PBM data included records of outpatient opioid prescriptions dispensed by VA for all Veterans who met the inclusion criteria (see study sample) for each cohort. Each record included medication name, strength, number dispensed, date dispensed and days supplied. The NPCD data extract included demographic data (e.g. marital status, gender, etc) as well as diagnosis codes for painful conditions, mental health and substance use disorders. The Vital status file was used to identify Veterans who died during the follow up period. Approval was obtained from the Institutional Review Boards at both Central Arkansas Veterans Healthcare System and the University of Arkansas for Medical Sciences.
Study Sample
In order to focus the analysis on persons whom the withdrawal would require a re-evaluation of analgesia and not treatment patterns for acute pain, the policy-affected and historical comparison cohorts each included Veterans who used opioids chronically in the respective pre-periods. To be included, subjects were required to have: (1) received a minimum 90 days’ supply of propoxyphene or a propoxyphene combination product within the 365 day pre-period25; (2) had at least two outpatient visits during the pre-period to ensure persons accessed VA services; (3) had at least two clinic visits during the post-period to ensure persons continued to access VA care; (4) had at least one propoxyphene prescription in the 60 day period immediately before the end of the pre-period to exclude persons that discontinued propoxyphene prior to the policy. They were required to be alive at the end of the post-periods.
Outcome Variables
We defined six key opioid use outcome variables based on utilization in the post-periods: 1) Any opioid use 2) Chronic opioid use 3) Opioid discontinuation 4) Chronic opioid discontinuation 5) Average Opioid Daily Dose 6) Months of opioids supplied. Any opioid use was defined as the receipt of any opioid containing analgesic prescription on or after the respective cohorts date defining pre- and post-periods. Chronic opioid use was defined as at least 90 days’ supply of any opioid or propoxyphene in the 365 day post-periods. Opioid discontinuation was defined as no fills for any opioid containing analgesic prescription within the post-periods. Chronic opioid discontinuation was defined as opioid prescriptions for less than 90 days’ supply, including zero use, of an opioid containing analgesic within the post-period. Each of these opioid use measures were further categorized based on exclusive propoxyphene use (propoxyphene only opioid recorded), propoxyphene and other opioids (propoxyphene recorded with at least one non-propoxyphene opioid), other opioids only (at least one non-propoxyphene opioid recorded and no propoxyphene), and no opioid use (opioid discontinuation definition) in both the pre- and post-periods. Opioid use measures were also categorized according to the DEA classification of opioids, (Schedule III/IV vs. Schedule II) and Schedule II opioids were further categorized into short-acting and long-acting opioids. MED was calculated by multiplying the opioid strength by the quantity dispensed and multiplied by a morphine conversion factor.26 The total MED was divided by the total days’ supply to obtain average daily dose. Months’ supplied was calculated by summing the days’ supplied and dividing by 30. Average opioid daily dose and months of opioid supply were defined in both post-periods and pre-periods. To prevent prescription fills counting in both pre- and post-periods, those fills that occurred in the pre-period were truncated the day before the index date. Therefore, the dose and days’ supply on or after the index date weren’t additive in the average daily dose and months’ (days’) supply calculations.
Secondary outcome variables were created to examine whether adverse events were less likely to occur after propoxyphene was withdrawn from the market. For each cohort, these included all cause emergency department visits and specific events diagnosed in the inpatient, emergency department, or outpatient settings; opioid-related events (see Appendix)27; and acetaminophen toxicity (see Appendix).28
Sociodemographic and Clinical Variables
For both cohorts, pre-period mental health, pain, and substance use disorder diagnoses were assigned using ICD-9 codes from inpatient and outpatient VA service (see Appendix).27, 29–30 Mental health diagnoses were defined as affective disorder, anxiety, delirium, dementia, major depressive disorder, other acute reaction disorder, post-traumatic stress disorder, schizophrenia, other psychosis, and other mental health disorder. Pain diagnoses were defined as arthritis, back pain, headaches, fractures, musculoskeletal pain, neuropathy, reproductive system pain, visceral pain, wound/injury, and other pain.27, 30 Substance use disorders were defined as alcohol, opioid and non-opioid use disorders.30 Demographic data included age (18–25, 26–35, 36–45, 46–55, 56–65, 66–75, 76+), sex, race (white/Caucasian, African American, multiracial, unknown/declined and other), and marital status (married, not married, and unknown).
Statistical Analysis
Descriptive analysis using chi-squares and t-tests were used to describe unadjusted results and to compare policy-affected and historical comparison cohorts. A difference in difference approach was used to assess the effect of the policy, and regression models were estimated with a GROUP indicator variable to reflect policy-affected and historical comparison cohort membership, a PERIOD indicator variable to reflect pre- and post-periods and a PERIOD*GROUP interaction variable to estimate the effect of the withdrawal policy. For the models describing any opioid use and chronic opioid use, the PERIOD and PERIOD*GROUP interactions were not included because 100% of subjects were chronic opioid users by design in the pre-periods and the GROUP variable would reflect the influence of the withdrawal policy. Because a portion of the subjects in the historical comparison cohort also contributed to the policy-affected cohort, a repeated measures approach was used in each of the models. Logistic regression models were used to estimate the influence of the withdrawal policy on any opioid use and chronic opioid use in the post period. Emergency department visits were modeled using ordinary least squares linear regression while opioid-related events were modeled using Poisson regression. All models were adjusted for the demographic and clinical variables previously described after eliminating potential noise variables in the final models using the Stepwise function in SAS. Variables were retained in the final models if p<=0.05. All analyses were completed using SAS Enterprise 5.1.
Results
A total of 24,328 Veterans met inclusion criteria across both cohorts. Of the 10,747 persons in the policy-affected cohort, the average age was 66 years, 92% were male, 7.5% were black, and on average used 7.72 months’ supply of opioids (Table 1). Arthritis followed by back pain was the most common pain diagnoses and more than half had one or more mental health disorders. The socio-demographic and clinical characteristics of the 13,581 persons in the historical comparison cohort were largely comparable to the policy-affected cohort, but they used a slightly higher months’ supply of opioids (7.79, p<0.05) and had slightly more persons that classified themselves as black (8.5%, p<0.05).
Table 1.
Clinical and Demographic Characteristics of the Policy-Affected and Historical Comparisons Cohorts
| Variables | Categories | Policy-Affected Cohort | Historical Comparison Control | P values | ||
|---|---|---|---|---|---|---|
| N=10,747 | N=13,581 | |||||
| N | % | N | % | |||
| Age | 18–25 | 10 | 0.09 | 8 | 0.06 | 0.66 |
| 26–35 | 106 | 0.99 | 134 | 0.99 | ||
| 36–45 | 395 | 3.68 | 463 | 3.41 | ||
| 46–55 | 1242 | 11.56 | 1532 | 11.28 | ||
| 56–65 | 3833 | 35.67 | 4785 | 35.23 | ||
| 66–75 | 2507 | 23.33 | 3231 | 23.79 | ||
| 76+ | 2654 | 24.70 | 3428 | 25.24 | ||
| Race | White | 8,615 | 79.62 | 10,824 | 79.21 | 0.003 |
| Black | 813 | 7.51 | 1,190 | 8.71 | ||
| Other | 193 | 1.78 | 217 | 1.59 | ||
| Missing | 1,199 | 11.08 | 1,434 | 10.49 | ||
| Gender | Male | 9,909 | 92.20 | 12,585 | 92.67 | 0.17 |
| Marital Status | Married | 6,891 | 64.12 | 8,670 | 63.84 | 0.59 |
| Not Married | 3,850 | 35.82 | 4,899 | 36.07 | ||
| Unknown | 6 | 0.06 | 12 | 0.09 | ||
| Pain Diagnosis | Arthritis | 6,019 | 56.01 | 7,666 | 56.45 | 0.49 |
| Back Pain | 4,620 | 42.99 | 5,882 | 43.31 | 0.62 | |
| Headache | 838 | 7.74 | 1,060 | 7.76 | 0.98 | |
| Fractures | 302 | 2.79 | 407 | 2.98 | 0.40 | |
| Neuropathy | 1,356 | 12.62 | 1,756 | 12.93 | 0.47 | |
| Other Pain | 377 | 3.51 | 470 | 3.46 | 0.84 | |
| Reproductive | 26 | 0.24 | 39 | 0.29 | 0.50 | |
| Wound/Injury | 264 | 2.46 | 322 | 2.37 | 0.67 | |
| Visceral Pain | 522 | 4.86 | 645 | 4.75 | 0.70 | |
| Musculoskeletal Pain | 2,341 | 21.78 | 2,903 | 21.38 | 0.44 | |
| No Pre-Defined Pain Diagnosis | 1,878 | 17.47 | 2,275 | 16.75 | 0.13 | |
| Mental Health Disorders | Affective Disorder | 283 | 2.63 | 369 | 2.72 | 0.69 |
| Anxiety | 1,618 | 15.06 | 2,023 | 14.90 | 0.73 | |
| Delirium | 43 | 0.40 | 45 | 0.33 | 0.38 | |
| Dementia | 306 | 2.85 | 343 | 2.53 | 0.13 | |
| Major Depressive Disorder | 2575 | 23.96 | 3301 | 24.31 | 0.53 | |
| Other Acute Reaction Disorder | 321 | 2.99 | 440 | 3.24 | 0.26 | |
| Other Psychosis | 102 | 0.95 | 160 | 1.18 | 0.08 | |
| PTSD | 1,539 | 14.32 | 1,883 | 13.86 | 0.31 | |
| Schizophrenia | 172 | 1.60 | 211 | 1.55 | 0.77 | |
| Other Mental Health Disorder | 2935 | 27.31 | 3806 | 28.02 | 0.22 | |
| No Pre-Defined Mental Health Disorder | 5,144 | 47.87 | 6,530 | 48.08 | 0.74 | |
| Substance Use Disorders | Alcohol | 609 | 5.67 | 782 | 5.76 | 0.76 |
| Opioids | 88 | 0.82 | 121 | 0.89 | 0.54 | |
| Non Opioids | 289 | 2.69 | 402 | 2.96 | 0.21 | |
| Mean | Mean | |||||
| Months of Opioid Use | Pre-Period | 7.72 | 7.79 | 0.03 | ||
| Average Daily Dose (per 100 mg MED) | Pre-Period | 79.50 | 79.72 | 0.63 | ||
In the policy-affected cohort, 10.6% of Veterans discontinued all opioid use in the post-period as compared to 3.8% of Veterans in the historical comparison cohort (Figure 2). Use of propoxyphene in the policy-affected cohort dropped significantly after propoxyphene was withdrawn. Those who used propoxyphene as their exclusive opioid dropped from 71.2% in the pre-period to 2.1% in the policy-affected cohort compared to a drop from 69.7% to 54.3% in the historical comparison cohort. Use of other opioids alone increased in the post-periods in both groups; however, the policy-affected cohort experienced the greatest increase with 69.6% in the policy-affected cohort using other opioids exclusively in the post-period compared to 10.6% in the historical comparison cohort. Most of the increase observed for other opioids in the policy-affected cohort were for schedule III-IV short-acting non-propoxyphene containing opioids; 87% of the cohort had one or more prescription fills for a schedule III–IV non-propoxyphene containing opioid in the post-period compared to a rate of 40% for the historical comparison cohort. Less than 15% of the cohort had one or more prescription fills for a long-acting or short-acting schedule II opioid in the post-period (Figure 3).
Figure 2.
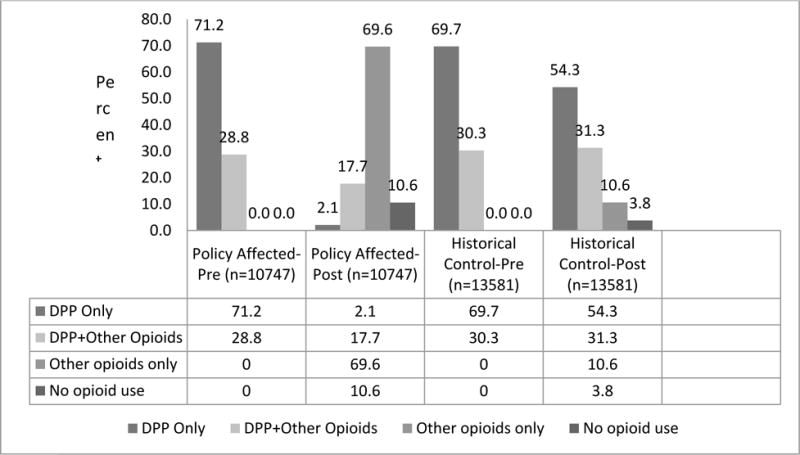
Pre and Post Propoxyphene and Opioid Utilization for Policy-Affected Cohort and Historical Comparison Cohort
Figure 3.
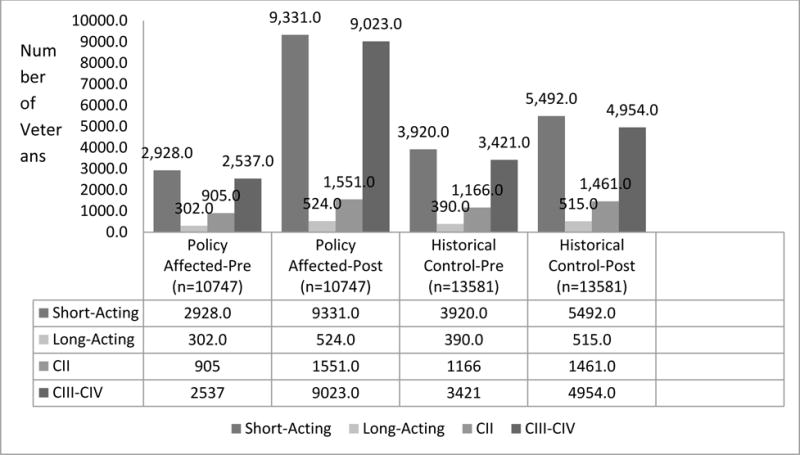
Pre and Post Opioid Utilization based on Duration of Action and Controlled Substances Schedule for Policy Affected Cohort and Historical Comparison Cohort
The proportion of those discontinuing chronic opioid use was 25.6% in the policy-affected cohort compared to 13.5% in the historical comparison cohort (Figure 4). In both cohorts, approximately 70% of subjects used propoxyphene exclusively as their chronic opioid in the pre-periods and after the policy virtually none of the policy-affected cohort used propoxyphene chronically (0.1%) compared to nearly half (48.4%) in the historical comparison cohort in the post-period. In the policy-affected and historical comparison cohorts, 56.9% and 8.3% respectively had chronic use of other opioids only in the post-periods.
Figure 4.
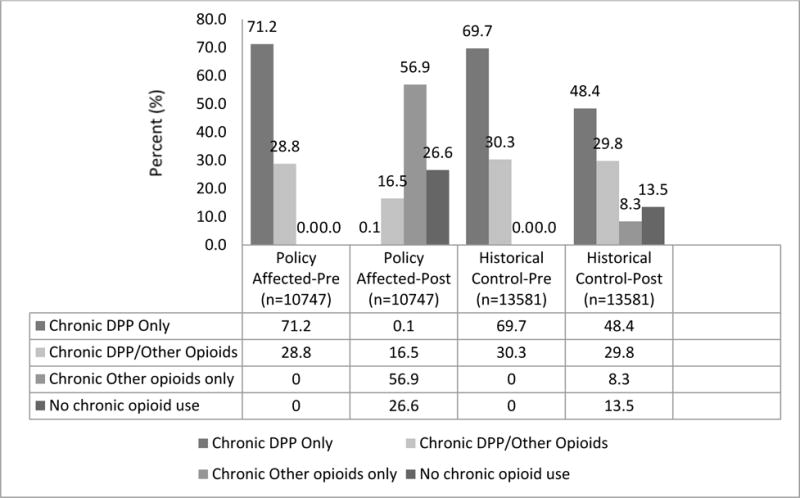
Pre and Post Chronic Opioid Utilization for Policy Affected Cohort and Historical Comparison Cohort
Table 2 displays the final logistic regression model estimating the influence of the policy on opioid discontinuation. The withdrawal policy increased the odds of discontinuing opioids entirely, and the magnitude of the effect was large (OR=3.2; 95%CI: 2.9 – 3.6) (Table 2). Higher doses and longer durations of opioid therapy in the pre-periods were associated with lower odds of discontinuation. Being diagnosed with dementia and schizophrenia increased the odds of opioid discontinuation and having a musculoskeletal or neuropathy diagnosis decreased the odds of opioid discontinuation.
Table 2.
Odds Ratios and 95% Confidence Intervals of Logistic Regression Model of Any Opioid Discontinuation
| Parameter* | OR | 95% CI |
|---|---|---|
| Group (Policy Affected vs. Comparison) | 3.20 | (2.87, 3.57) |
|
| ||
| Months of Opioid Use | 0.80 | (0.78, 0.82) |
|
| ||
| Average Daily Dose (per 100 mg MED) | 0.45 | (0.38, 0.54) |
|
| ||
| Mental Health Diagnosis:† | ||
| Dementia | 1.68 | (1.31, 2.15) |
| Schizophrenia | 1.72 | (1.18, 2.51) |
| Other Psychosis | 1.72 | (1.11, 2.67) |
|
| ||
| Pain Diagnosis:† | ||
| Musculoskeletal | 0.83 | (0.72, 0.95) |
| Neuropathy | 0.78 | (0.66, 0.93) |
|
| ||
| Age: | ||
| 18–25 | 2.62 | (0.79, 8.73) |
| 26–35 | 1.29 | (0.78, 2.13) |
| 36–45 | 0.80 | (0.58, 1.11) |
| 46–55 | 0.97 | (0.80, 1.18) |
| 66–75 | 1.12 | (0.97, 1.29) |
| 76+ | 1.56 | (1.37, 1.78) |
| Referent: Age: 56–65 | ||
significant parameters (p<0.05) after stepwise regression
For each diagnosis (mental health or pain), the null serves as the referent
The logistic models for chronic opioid discontinuation found similar results. The odds of discontinuing chronic opioid use was 2.7 (95%CI: 2.5 – 2.8) times higher for the policy affected veterans compared to those in the historical comparison cohort (Table 3). Higher doses and longer durations of opioid therapy in the pre-periods were also associated with lower odds of discontinuing chronic opioid therapy. Being diagnosed with dementia, major depressive disorder, substance use disorders, and schizophrenia were all associated with a higher likelihood of chronic opioid discontinuation; however, none of the pain conditions were associated with chronic opioid discontinuation.
Table 3.
Odds Ratios and 95% Confidence Intervals of Logistic Regression Model of Chronic Opioid Discontinuation
| Parameter* | OR | 95% CI |
|---|---|---|
| Group (Policy Affected vs. Comparison) | 2.65 | (2.48, 2.84) |
|
| ||
| Months of Opioid Use | 0.74 | (0.73, 0.76) |
|
| ||
| Average Daily Dose (per 100 mg MED) | 0.46 | (0.41, 0.52) |
|
| ||
| Mental Health Diagnosis:† | ||
| Dementia | 1.43 | (1.19, 1.73) |
| MDD | 1.16 | (1.07, 1.27) |
| Schizophrenia | 1.40 | (1.05, 1.86) |
|
| ||
| Substance Use Disorders† | ||
| Non Opioid | 1.41 | (1.13, 1.77) |
|
| ||
| Race: | ||
| Black | 1.15 | (1.01, 1.30) |
| Other | 1.14 | (1.02, 1.27) |
| Missing | 1.02 | (0.77, 1.34) |
| Referent: Race: White | ||
|
| ||
| Age: | ||
| 18–25 | 1.64 | (0.51, 5.24) |
| 26–35 | 1.62 | (1.16, 2.26) |
| 36–45 | 0.86 | (0.71, 1.05) |
| 46–55 | 0.97 | (0.85, 1.10) |
| 66–75 | 1.24 | (1.12, 1.36) |
| 76+ | 1.51 | (1.38, 1.65) |
| Referent: Age: 56–65 | ||
significant parameters (p<0.05) after stepwise regression
For each diagnosis (mental health, substance use disorder, or pain), the null serves as the referent
In the pre-periods, average daily dose in milligrams of morphine equivalent dose for the policy-affected and historical comparison cohorts were similar (79.7mg/day and 79.5 mg/day respectively). Average daily dose in the post-period of the historical comparison cohort was similar to that of its pre-period (70.7mg/day). However, average daily dose in the post-period of the policy-affected cohort was drastically lower as compared to its pre-period (26.7 mg/day).
Opioid-related events and ED visits significantly increased in the post-periods as compared to the pre-periods for both groups (ED visits: B=0.049, 95%CI: 0.014, 0.084; Opioid-related events: B=1.056, 95%CI: 0.252, 1.861); however, there was not a significant association between the policy groups and the group*period interaction reflecting no association between the policy and these measures (ED visits: B=0.037, 95%CI: −0.016, 0.090; Opioid-related events: B= −0.363, 95%CI: −1.5755, 0.8497). There were only 5 acetaminophen adverse events across both groups and time periods and hence models were not estimated given the low event rates for this outcome (data not shown).
Discussion
Our data show that among those who were regular users of the weak opioid propoxyphene, withdrawing the drug from the market modestly reduced any opioid use and chronic opioid use. Only 10% of subjects discontinued opioids entirely and nearly three out of four individuals continued using opioids on a chronic basis. These data clearly demonstrate that withdrawing an opioid product is much more likely to result in prescribing an alternative opioid rather than trying a non-opioid alternative to manage pain. This has important implications for policy makers and persons managing drug formularies. Restricting access to a specific opioid product through mechanisms such as prior authorization or making a product non-formulary may reduce overall opioid use and chronic opioid use, however, the reductions are likely to be modest as the vast majority of persons taking a restricted opioid are likely to switch to another opioid.
This is the first U.S. study to examine the impact of the propoxyphene withdrawal policy on opioid use. A French cohort study that followed 103 elderly patients who used propoxyphene for chronic pain management found that 40.8% switched to Step 1 analgesics (defined by the World Health Organization analgesic ladder) such as acetaminophen and ibuprofen.31 At least in this study, the French were 4 times as successful in discontinuing opioid use. This study is also novel in its use of a historical control in a pre-post difference in difference framework. Historical controls have been used in the literature; however, this is the first study to integrate a pre- and post-period within a historical control methodology. This design has clear advantages over a simple pre-post design in that it can more clearly attribute changes in our outcome measures to the withdrawal and be less influence by traditional biases such as history or maturation. Indeed, our historical controls exhibited declines in our outcome measures, particularly for chronic opioid use.
Adverse events, defined as opioid-related events and emergency department visits were significantly more frequent in the post-period as compared to the pre-period for both the policy-affected and historical comparison cohorts which may suggest that adverse events increase for chronic propoxyphene users as time progresses; however, since the frequency of events did not differ by group, the removal of propoxyphene from the market does not appear to contribute to a decrease or increase in adverse events.
There are several limitations to this study. First, prescription fills and medical record data alone are not sufficient to differentiate appropriate and inappropriate opioid use and it is unknown if withdrawing propoxyphene reduced opioid misuse or abuse. Given the modest reduction of opioid use and chronic use after the withdrawal, any effect of the policy on opioid misuse and abuse is likely to be negligible or modest. Second, records of medications obtained outside of VA were not available for this study. Therefore, some veterans could have filled opioids outside of VA and still be counted as having discontinued opioid use. Third, VA data can be difficult to generalize to non-VA patients, especially in generalizing to women and non-white populations since both populations are still a small portion of patients in VA care. Future studies should address these minority groups. Next, persons were required to survive the one year post-period and our secondary analyses of propoxyphene-related adverse events may be influenced by survival bias. Moreover, converting doses of propoxyphene to morphine equivalents is variable and imprecise. We chose a conversion where 1mg of oral morphine was considered equianalgesic with 4.35mg of propoxyphene napsylate to be consistent with the TROUP study and other studies utilizing this data; however, some opioid conversion tables equate 1mg morphine to as much as 20mg of propoxyphene.32 The use of the 1 to 4.35mg equianalgesic factor converted doses of propoxyphene to relatively high MED in most of our groups and time periods and is also the most likely explanation of why we observed a pronounced decline in MED in the policy-affected cohort in the post-period in which there was virtually no use of propoxyphene. Additionally, prescription records for propoxyphene dispensed in the pre-period of either group where the days’ supply spanned one period to the next were not counted in the post-period for days’ supply, dose, nor used in the post-period. Truncating the fills in this fashion underestimates both months’ covered and average daily dose. Lastly, in May 2010, VHA guidelines were released for the management of opioid therapy for chronic pain, along with new patient/provider tools, including a sample opioid pain care agreement, and a table on urine drug screens.33 The release of this guideline occurred in the post-period of our historical comparison and in the pre-period of our policy affected cohort. This would primarily influence our observed pre – post differences of our historical comparison group such that the observed differences (decreases) would be greater than those that would be expected absent the release of the May 2010 guidelines. In turn, our difference in difference estimates might be understating the true effect of the propoxyphene withdrawal policy on our opioid use measures. Given that only 3.8% of persons discontinued opioids entirely in the historical comparison group, the magnitude of this bias is likely to be small for that opioid measure but may be larger for other measures, such as discontinuation of chronic opioid therapy in which 13.5% discontinued chronic use in the historical comparison group.
Conclusions
The withdrawal of propoxyphene containing products results in rapid and virtually complete elimination in propoxyphene prescribing in the Veterans population and the policy was associated with decreases in opioid use and chronic opioid use; however, nearly 90% of regular users of propoxyphene switched to alternate opioids and three quarters continued to use opioids chronically. The use of an alternative opioid likely reduces the risk of propoxyphene-related cardiac conduction disorders; nevertheless, the impact of the withdrawal on the risks of opioid-related misuse, dependence, abuse, and death is likely to be more modest.
Supplementary Material
Key Points.
Ninety percent of chronic propoxyphene users continued opioid use after the removal of propoxyphene from the US market.
Three quarters of chronic propoxyphene users continued chronic opioid use after propoxyphene was removed from the US market.
The removal of propoxyphene does not appear to contribute to a decrease or increase in adverse events as defined as opioid-related events, emergency department visits, and acetaminophen toxicity.
Acknowledgments
The data extraction was supported by funding to Drs. Hudson and Edlund from the National Institute of Drug Abuse (RO1 DA030300-01) At the time of this study, a portion of Dr. Martin’s and Dr. Hudson’s effort was supported by the UAMS Translational Research Institute (1UL-1RR029884). Dr. Sullivan and Dr. Fortney were also Co-investigators of the NIDA-funded grant and contributed to the overall concept of this study as well as the design of study measures for this project.
Sponsors:
This research was supported through a grant from:
(Hudson, Edlund, Sullivan, Martin, Fortney, Austen, Williams)
NIDA R01 DA030300-01
Footnotes
Conflicts of Interest:
None of the authors have conflicts of interest to declare.
Prior Presentation:
The results of this study were presented by Dr. Bradley C Martin as a Podium Presentation on October 25, 2013 for the 2013 Addiction Health Services Research Conference in Portland, Oregon.
References
- 1.Collins SL, Edwards JE, Moore RA, et al. Single dose dextropropoxyphene, alone and with paracetamol (acetaminophen), for postoperative pain. Cochrane Database Syst Rev. 2000;(2):CD001440. doi: 10.1002/14651858.CD001440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crighton IM, Hobbs GJ, Wrench IJ. Analgesia after day case laparoscopic sterilisation. A comparison of tramadol with paracetamol/dextropropoxyphene and paracetamol/codeine combinations. Anaesthesia. 1997;52:649–52. doi: 10.1111/j.1365-2044.1997.142-az0146.x. [DOI] [PubMed] [Google Scholar]
- 3.Sagne S, Henrikson PA, Kahnberg KE, et al. Analgesic efficacy and side-effect profile of paracetamol/codeine and paracetamol/dextropropoxyphene after surgical removal of a lower wisdom tooth. Journal of International Medical Research. 1987;15:83–8. doi: 10.1177/030006058701500204. [DOI] [PubMed] [Google Scholar]
- 4.Edmondson HD, Bradshaw AJ. Analgesia following oral surgery: a comparative study of Solpadeine and a soluble form of dextropropoxyphene napsylate and paracetamol. Journal of International Medical Research. 1983;11:228–31. doi: 10.1177/030006058301100406. [DOI] [PubMed] [Google Scholar]
- 5.Li Wan Po A, Zhang WY. Systematic overview of co-proxamol to assess analgesic effects of addition of dextropropoxyphene to paracetamol. BMJ. 1997;315:1565–71. doi: 10.1136/bmj.315.7122.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barkin RL, Barkin SJ, Barkin DS. Propoxyphene (dextropropoxyphene): a critical review of a weak opioid analgesic that should remain in antiquity. American Journal of Therapeutics. 2006;13(6):534–542. doi: 10.1097/01.mjt.0000253850.86480.fb. [DOI] [PubMed] [Google Scholar]
- 7.Lader M. Abuse of weak opioid analgesics. Human Toxicology. 1984;3(Suppl):229S–236S. doi: 10.1177/096032718400300120. [DOI] [PubMed] [Google Scholar]
- 8.Garfield T, Kissin W, Ball J. Year-end 1999 emergency department data from the Drug Abuse Warning Network. Rockville: 2000. (DAWN Series D-15, DHHS Publication No. (SMA) 00-3462). [Google Scholar]
- 9.Whittington R. Dextropropoxyphene deaths: coroner’s report. Human Toxicology. 1984;3(Suppl):175S–185S. doi: 10.1177/096032718400300116. [DOI] [PubMed] [Google Scholar]
- 10.Hawton K, Simkin S, Deeks J. Co-proxamol and suicide: a study of national mortality statistics and local nonfatal self poisonings. British Medical Journal. 2003;326:1006–1008. doi: 10.1136/bmj.326.7397.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah R, Uren Z, Baker A, et al. Trends in suicide from drug overdose in the elderly in England and Wales, 1993–1999. International Journal of Geriatric Psychiatry. 2002;17:416–421. doi: 10.1002/gps.625. [DOI] [PubMed] [Google Scholar]
- 12.Steentoft A, Teige B, Ceder G, et al. Fatal poisoning in drug addicts in the Nordic countries. Forensic Science International. 2001;123:63–69. doi: 10.1016/s0379-0738(01)00524-2. [DOI] [PubMed] [Google Scholar]
- 13.Tennant FS. Complications of propoxyphene abuse. Archives of Internal Medicine. 1973;132:191–194. [PubMed] [Google Scholar]
- 14.Hawton K, Simkin S, Gunnell D, et al. A multicentre study of coproxamol poisoning suicides based on coroners’ records in England. British Journal of Clinical Pharmacology. 2005;59:207–212. doi: 10.1111/j.1365-2125.2004.02252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Office of Surveillance and Epidemiology. Updated Epidemiological Review of Propoxyphene Safety. Washington, DC: Food and Drug Administration Center for Drug Evaluation and Research; 2010. [Google Scholar]
- 16.Bartholow M. Top 200 Prescription Drugs of 2009. Pharmacy Times; May, 2010. www.pharmaytimes.com/publications/issue/2010/May2010/RxFocusTopDrugs-0510#sthash.Cqg2lybd.dpuf (accessed 7 June 2014) [Google Scholar]
- 17.Adler A, Viskin S, Bhuiyan ZA, et al. Propoxyphene-induced torsades de pointes. Heart Rhythm. 2011;8(12):1952. doi: 10.1016/j.hrthm.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 18.Food And Drug Administration Public Health Service U S Department Of Health And Human Services. Food and Drug Administration recommends against the continued use of propoxyphene. Journal of Pain and Palliative Care Pharmacotherapy. 2011;25(1):80–82. doi: 10.3109/15360288.2010.549553. [DOI] [PubMed] [Google Scholar]
- 19.U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA recommends against the continued use of propoxyphene. Department of Health and Human Services; Nov, 2010. http://www.fda.gov/Drugs/DrugSafety/ucm234338.htm (accessed 19 September 2012) [Google Scholar]
- 20.Gilson AM, Ryan KM, Joranson DE, et al. A reassessment of trends in the medical use and abuse of opioid analgesics and implications for diversion control: 1997–2002. Journal of Pain Symptom Management. 2004;28(2):176–188. doi: 10.1016/j.jpainsymman.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and stategies. Drug Alcohol Dependence. 2006;81(2):103–107. doi: 10.1016/j.drugalcdep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Paulozzi LJ, Ryan GW. Opioid analgesics and rates of fatal drug poisoning in the United States. American Journal of Preventive Medicine. 2006;31(6):506–511. doi: 10.1016/j.amepre.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 23.Cobaugh DJ, Gainor C, Gaston CL, et al. The opioid abuse and misuse epidemic: implications for pharmacists in hospitals and health systems. American Journal of Health-System Pharmacy. 2014 Sep 15;71(18):1539–54. doi: 10.2146/ajhp140157. [DOI] [PubMed] [Google Scholar]
- 24.Martin BC, Fan MY, Edlund MJ, et al. Long-term chronic opioid therapy discontinuation rates from the TROUP study. Journal of General Internal Medicine. 2011;26(12):1450–7. doi: 10.1007/s11606-011-1771-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Launey Y, Nesseler N, Le Cousin A, et al. Effect of a fever control protocol-based strategy on ventilator-associated pneumonia in severely brain-injured patients. Critical Care. 2014;18(6):689. doi: 10.1186/s13054-014-0689-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sullivan MD, Edlund MJ, Fan MY, et al. Trends in use of opioids for non-cancer pain conditions 2000–2005 in commercial and Medicaid insurance plans: the TROUP study. Pain. 2008;138(2):440–449. doi: 10.1016/j.pain.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seal KH, Shi Y, Cohen G, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307(9):940–947. doi: 10.1001/jama.2012.234. [DOI] [PubMed] [Google Scholar]
- 28.Prior MJ, Cooper K, Cummins P, et al. Acetaminophen availability increases in Canada with no increase in the incidence of reports of inpatient hospitalizations with acetaminophen overdose and acute liver toxicity. American Journal of Therapeutics. 2004;11(6):443–52. doi: 10.1097/01.mjt.0000140217.48324.e3. [DOI] [PubMed] [Google Scholar]
- 29.Owen R. Management of Metabolic Side Effects of Antipsychotics in Six VISNS. 2012 [Google Scholar]
- 30.Edlund MJ, Martin BC, Devries A, et al. Trends in use of opioids for chronic noncancer pain among individuals with mental health and substance use disorders: the TROUP study. The Clinical Journal of Pain. 2010;26(1):1–8. doi: 10.1097/AJP.0b013e3181b99f35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Becquemont L, Delespierre T, Bauduceau B, et al. Consequences of dextropropoxyphene market withdrawal in elderly patients with chronic pain. European Jouranl of Clinical Pharmacology. 2014;70(10):1237–42. doi: 10.1007/s00228-014-1722-x. [DOI] [PubMed] [Google Scholar]
- 32.Equianalgesic (Narcotic) conversion chart. Global RPh. http://www.globalrph.com/narcotic.htm (accessed 22 July 2014)
- 33.Veterans Health Administration. Va/DoD Clinical Practice Guideline for the Management of Opioid Therapy for Chronic Pain, v2.0. Washington, DC: Department of Defense; 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


