Abstract
Background:
Otilonium bromide (OB) is a spasmolytic agent acting as an L-type calcium channel antagonist in intestinal and colonic smooth muscle cells (SMCs). We analyzed three independent clinical trials with homogeneous design on patients with irritable bowel syndrome (IBS). After 2 weeks receiving placebo, patients were randomized to receive OB (3 × 40 mg daily) or placebo for 15 weeks. We aimed to perform a pooled analysis of the data from these homogeneous clinical trials to evaluate the efficacy of OB treatment on symptoms and global response of patients.
Methods:
A total of 883 patients with IBS (69.8% women, mean age 46.2 years, 43.8% mixed type) were included, 442 treated with OB and 441 with placebo. The efficacy results from the three studies at weeks 5, 10 and 15 were pooled in an intention-to-treat (ITT) strategy, analyzed with a logistic regression model and described by forest plots.
Results:
Despite a placebo effect in all efficacy variables, a significant therapeutic effect of OB was observed at weeks 10 and 15 with reference to: (a) intensity and frequency of abdominal pain; (b) rate of responders as evaluated by patients (71.8% at week 10 and 77.2% at week 15); (c) severity of bloating; (d) rate of responders as evaluated by physicians (55% at week 10 and 63.9% at week 15). No significant OB effect was observed in stool frequency and consistency.
Conclusions:
OB is more effective than placebo in IBS treatment. Therapeutic benefits are significant after 10 weeks and are maximal after 15 weeks of treatment.
Keywords: abdominal pain, bloating, distention, global response, intention-to-treat analysis, irritable bowel syndrome, otilonium bromide
Introduction
Irritable bowel syndrome (IBS) is a chronic, cyclic, and relapsing functional bowel disorder characterized by abdominal pain associated with changes in bowel habit or abdominal distention. It affects up to 11.5% of the general population in Europe and developing countries [Hungin et al. 2003]. In addition to the impact on patient’s health, IBS is also associated with impaired quality of life, diminished work productivity and increased health and economic costs [Agarwal and Spiegel, 2011]. Although there is consensus on the application of clinical Rome Criteria to diagnose IBS and its subtypes [Longstreth et al. 2006], up to 50% of patients still remain undiagnosed [Hungin et al. 2003]. In addition, there is a lack of consensus on IBS treatment in terms of optimal pharmacological treatment, most useful drugs, specific indications for spasmolytic agents, timing of clinical response and duration of treatment.
Otilonium Bromide (OB) is a musculotropic spasmolytic agent belonging to the family of quaternary ammonium derivatives and successfully used in the treatment of patients affected by IBS due to its specific pharmacokinetic and pharmacodynamic properties. The positive polarity of the head of the OB molecule determines the main pharmacokinetic property of this drug: a minimal systemic absorption and the consequently high safety profile. Studies on animal models revealed a specific OB accumulation in colonic circular muscle at therapeutic µm concentrations, while its plasma levels were 1000 times lower, together with a poor penetration of the drug in the central nervous system [Evangelista et al. 2000]. Consistently, after oral administration to healthy volunteers, the OB plasmatic concentration was very low, <1% of the drug was eliminated by urine, and 97% was eliminated by feces [Evangelista, 2004].
Recent clinical studies showed comparable safety and tolerability for OB and placebo [Clave et al. 2011]. OB was shown to inhibit the main patterns of human sigmoid motility in vitro, including: the tone of smooth muscle cells (SMCs); the rhythmic phasic contractions induced by the interstitial cells of Cajal; and the strong contractions induced by stimulation of enteric motor neurons mainly by blocking the calcium influx through L-type calcium channels on SMCs [Gallego et al. 2010]. Recent in vitro studies using cultured human colonic SMCs to further assess the musculotropic spasmolytic properties of OB confirmed that this drug causes smooth muscle relaxation through the inhibition of voltage-gated calcium channels (L-type > T-type) and the inhibition of muscarinic and tachykinergic effects [Martinez-Cutillas et al. 2013]. All the above described pharmacodynamic properties mediate the musculotropic spasmolytic effects of OB. In addition, its interaction with the NK1/NK2 tachykinergic receptors in sensory afferent nerves may also improve the visceral hypersensitivity that affects patients with IBS [Rychter et al. 2014].
We included in the present analysis three randomized, double-blind, placebo-controlled studies with a homogeneous treatment schedule of 3 × 40 mg OB daily for 15 weeks to measure the clinical efficacy of OB in patients with IBS. The first study was conducted in 23 Italian centers, and enrolled 325 patients with IBS [Battaglia et al. 1998]; the second study was conducted in 34 centers in eight European countries and enrolled 355 patients with IBS [Clave et al. 2011]; the third study was conducted in Greece and enrolled 203 patients with IBS [Menarini IFR, 2012]. In all these studies, OB improved the main symptoms of patients with IBS, mostly related to abdominal pain. Drawbacks included the difficult translation of results to clinical practice for both patients and physicians due to the long-term primary endpoints (15 weeks), the need to define a global treatment response on the basis of improvements in the most frequent symptoms reported by patients and a strict intention-to-treat (ITT) analysis, and the relevant placebo effect [Ford et al. 2008; Clave et al. 2011; Desborough and Ford, 2011; Hungin, 2011].
In the current analysis, we collected the individual data from the three homogeneous randomized controlled trials (RCTs) described above [Battaglia et al. 1998; Clave et al. 2011; Menarini IFR, 2012] and performed a pooled analysis, with the aim of evaluating the efficacy of OB treatment on the main IBS symptoms (including intensity and frequency of abdominal pain, severity of bloating, stool frequency and consistency), and the global treatment response assessed by patients and by their physicians. The results of the pooled analysis support the significant therapeutic effect of OB versus placebo, thus providing additional research-based data to guide clinical practice in IBS management.
Methods
Studies and patients
A total of 889 patients with IBS were enrolled in three independent RCTs [Battaglia et al. 1998; Clave et al. 2011; Menarini IFR, 2012]. In more detail, the study by Battaglia and colleagues evaluated the efficacy of OB by comparing 158 patients treated with the study drug versus 158 patients receiving a placebo (study code in the pooled analysis: Battaglia) [Battaglia et al. 1998]. The primary objective of the OBIS study was to confirm the efficacy of OB for symptom control in patients with IBS in a superiority trial versus placebo by comparing 169 patients receiving the study drug versus 170 patients receiving a placebo (study code in the pooled analysis: OBIS) [Clave et al. 2011]. The third study was conducted in Greece and evaluated the efficacy of OB by comparing 88 patients with IBS treated with the study drug versus 96 patients receiving a placebo (study code in the pooled analysis: Greek) [Menarini IFR, 2012]. The inclusion/exclusion criteria and the design (RCT, OB versus placebo) of the three studies were homogeneous and included: (a) age > 18 years; (b) exclusion of major organic disease in patients with age >50 years; (c) a positive diagnosis of IBS established using similar clinical criteria (Rome I in the Battaglia and the Greek studies and Rome II in the OBIS study) [Thompson et al. 1999]; (d) after a run-in period of 2 weeks of single blind placebo treatment, mild-to-severe symptomatic IBS patients were randomized to receive treatment in a double-blinded manner: OB, 3 tablets (3 × 40 mg) daily before meals (120 mg OB/day) or matching placebo, 3 tablets daily before meals as well, for 15 weeks. Patient symptoms were monitored by means of diaries. Follow-up visits were scheduled after 5, 10 and 15 weeks of treatment.
Efficacy variables
The following efficacy parameters included in this analysis were considered as primary efficacy variables in the three selected studies [Battaglia et al. 1998; Clave et al. 2011; Menarini IFR, 2012]: (1) intensity of abdominal pain as reported by patients through a score; (2) intensity of abdominal pain evaluated by visual analogue scale (VAS) as reported by patients; (3) number of episodes of abdominal pain as reported by patients; (4) intensity and frequency of abdominal pain by type of IBS; (5) responder patients by global evaluation of pain as composite parameter of intensity and frequency scores; (6) stool frequency and consistency; (7) severity of bloating; and (8) responder patients as evaluated by the physician.
Intensity of abdominal pain was assessed by adapting the 4-level rating scale, where 0 = absent, 1 = mild/moderate, 2 = severe, and 3 = extremely severe, to the three RCTs selected for this analysis.
Weekly frequency of episodes of abdominal pain was assessed by adapting the 4-level rating scale where score 0 = none, 1 = 1–3 episodes, 2 = 4–7 episodes and 3 = 8 or more episodes during the week to the three RCTs selected for this analysis.
As a global measure of treatment response, a composite pain score (global evaluation of pain as composite parameter of intensity of pain and episodes of pain as reported by patients) was calculated for this study as: abdominal pain score × score for episodes of pain.
Severity of bloating was scored using a scale where 0 = absent; 1 = mild-to-moderate; 2 = severe; and 3 = extremely severe.
Intensity, frequency and global evaluation of abdominal pain and severity of bloating were recorded in a diary for 2 weeks before randomization. The worst score recorded during the 2 weeks was used as the baseline score for further analysis. During the follow up, the scores were recorded in the diary every week. The score recorded in the last week before the follow-up visit was used for the analysis.
Follow-up data were analyzed in an ITT analysis. Responders with regards to intensity, frequency, global evaluation of pain as a composite index of intensity and frequency, or severity of distention, were defined as patients having a reduction of score ⩾1 after 5, 10, and 15 weeks of treatment. The intensity of abdominal pain was also reported by patients using a VAS at baseline and at the end of treatment.
The stool frequency was measured by the number of days without stools and the average number of stools in the remaining days of the week reported by patients. The latter was evaluated by a four-level scale where: 0 = 1 per day; 1 = 2 per day; 2 = 3 or 4 per day; and 3 = 5 or more per day. Results on stool consistency reported by patients were transformed into a dichotomous variable, where: 0 = normal consistency and 1 = abnormal consistency (either loose or hard).
Responders were also examined according to the global efficacy assessment evaluated by the physician using a 4-point score at each visit after randomization: 0 = no efficacy; 1 = moderate efficacy; 2 = good efficacy; and 3 = excellent efficacy. Responders were defined as patients showing good or excellent improvement in the global evaluation by the physician.
The efficacy analysis was based on an ITT population analysis. The full analysis set (FAS, ITT population) included all randomized patients who had taken at least one dose of the study drug and had undergone at least one efficacy assessment for the primary efficacy endpoint (evaluation of the diaries) after baseline.
Methods for the pooled analysis
All the efficacy data derived from the three studies were reported individually per study and as weighted averages taking into account the number of patients enrolled in each study. First, an analysis of variance (ANOVA) was used for the direct comparison between treatment groups, and then a general linear model (GLM) was used for the comparisons in which an impact of covariates was expected. Data were statistically analyzed using the analysis of covariance, including baseline intensity, sex, age, body mass index (BMI) as covariates and treatment as factor. The GLM was used to compare the efficacy parameters between the treatment groups considering the model baseline values of each parameter, age, sex and BMI as covariates. The GLM procedure provides regression analysis and ANOVA for one dependent variable (efficacy parameter) by more factors (treatment group and sex) and covariates (baseline values, age and BMI).
The same method used for the analysis of the end-of-treatment data (week 15) was applied to the data obtained at the intermediate visits (weeks 5 and 10).
Severity of pain assessed by VAS was analyzed using ANOVA performed on changes from baseline to the end of treatment.
Frequency tables were completed at each relevant time point for the rate of responders. Treatment responders were analyzed using a binary logistic regression model including factors for treatment. For an exploratory subgroup analysis, the following three subgroups were defined according to the IBS type: predominant diarrhea (IBS-D), predominant constipation (IBS-C), mixed type (IBS-M) [Thompson et al. 1999; Longstreth et al. 2006]. The same descriptive statistics on change from baseline in the frequency of abdominal pain for the whole population were provided in the FAS, stratifying by subgroup category.
Results were also represented through forest plots, designed to illustrate the relative strength of treatment effects in multiple quantitative studies addressing the same question. The vertical line in a forest plot corresponds to lack of effect. If the confidence intervals (CIs) for individual studies do not overlap with this line, it demonstrates that, at the given level of confidence (95%), their effect sizes differ from no effect. The same applies for the pooled measure of effect (pooled dataset): if the CI for the pooled dataset does not overlap with the vertical line corresponding to lack of effect, the overall result of pooled analysis differs from no effect at the 95% level of confidence. The analysis was performed in SAS (version 9.02, SAS Institute Inc, Raleigh, NC, USA).
Results
Demographic data of patients included in the pooled analysis
The patient population recruited in the three RCTs selected for the pooled analysis of the data consisted of 883 patients of both sexes, with a prevalence of women (69.8%), and a median age of 46.2 years, as summarized in Table 1. No significant differences were observed between the two treatment groups with respect to demographic data in the three studies. Table 1 also includes the baseline clinical features of enrolled patients suffering mild-to-severe IBS, with >3 episodes of abdominal pain per week, with moderate-to-severe pain intensity, moderate-to-severe bloating, and abnormal stool consistency. IBS subtypes could only be derived from studies by Battaglia and OBIS, and included 25.2% patients with IBS-D, 31% with IBS-C and 43.8% with IBS-M. No differences in baseline clinical features were observed between OB and placebo groups (Table 1). Mean treatment compliance was high and comparable in both treatment groups at all visits, ranging from 95% to 97%, and the dropout rates were 16.1% in both the treatment groups.
Table 1.
Demographic and baseline features of the patients included in the pooled analysis (Safety population).
| Parameter | OB (n = 442) |
Placebo
(n = 441) |
p-value |
|---|---|---|---|
| Age (mean ± SD) | 46.9 ± 14.8 | 46.3 ± 15.6 | 0.575 |
| Sex [n (%)] | |||
| Females | 310 (70.3) | 304 (69.2) | 0.735 |
| Males | 131 (29.7) | 135 (30.8) | |
| BMI (mean ± SD) | 24.4 ± 4.1 | 24.1 ± 4.1 | 0.690 |
| Smoker status [n (%)] | |||
| No + ex | 338 (76.5) | 334 (75.7) | 0.798 |
| Smoker | 104 (23.5) | 107 (24.3) | |
| Alcohol [n (%)] | |||
| No | 343 (78.0) | 328 (74.5) | 0.235 |
| Average | 97 (22.0) | 112 (25.5) | |
| Number of subjects in the ITT population (% of safety population) | 415 (93.9) | 424 (96.1) | |
| Dropout rates | 71 (16.1) | 71 (16.1) | ns |
| Baseline features | |||
| Intensity of abdominal pain (score, mean ± SD) | 1.36 ± 0.58 | 1.37 ± 0.62 | 0.844 |
| Intensity of abdominal pain (VAS, mean ± SD) | 5.00 ± 2.14 | 4.92 ± 2.06 | 0.593 |
| Frequency of abdominal pain (mean ± SD) | 1.81 ± 0.69 | 1.72 ± 0.72 | 0.066 |
| Global pain index (mean ± SD) | 2.71 ± 1.82 | 2.49 ± 1.68 | 0.087 |
| IBS subtype (%) | |||
| IBS-D | 25.7 | 24.7 | |
| IBS-C | 28.1 | 33.8 | ns |
| IBS-M | 46.2 | 41.5 | |
| Stool frequency (mean ± SD) | 0.63 ± 0.54 | 0.60 ± 0.54 | 0.472 |
| Normal stool consistency (%) | 26.1 | 30 | 0.25 |
| Severity of bloating (mean ± SD) | 1.37 ± 0.73 | 1.31 ± 0.705 | 0.284 |
BMI, body mass index; IBS, irritable bowel syndrome; IBS-C, IBS with predominant constipation; IBS-D, IBS with predominant diarrhea; IBS-M, mixed type IBS; ITT, intention-to-treat; ns, not significant; OB, otilonium bromide; SD, standard deviation; VAS, visual analogue scale.
OB effect on intensity of abdominal pain
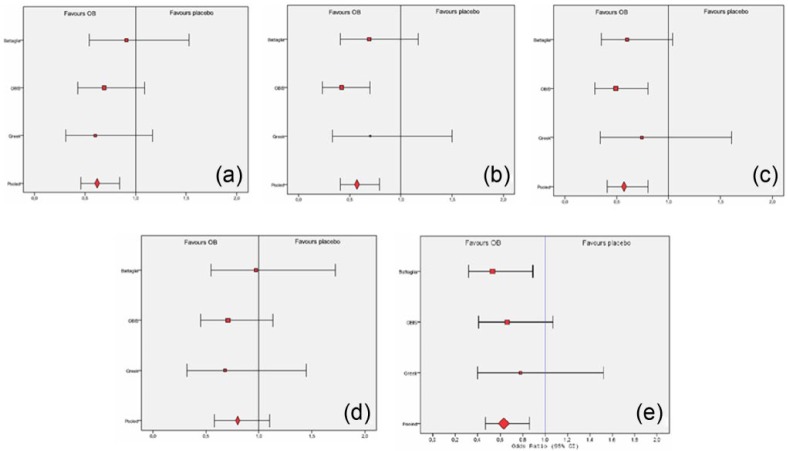
A significant placebo effect was observed from week 5 (p < 0.001), as abdominal pain score decreased significantly both in the OB group and the placebo group at the end of the treatment (Figure 1). However, the comparison between treatment groups of the abdominal pain score reduction from baseline showed a greater effect in the OB group in comparison with placebo at all visits with significant differences at week 10 and week 15 for this primary endpoint (Figure 1). The GLM model, providing regression analysis and ANOVA for abdominal pain score reduction by more factors (treatment group and sex) and covariates (baseline value of abdominal pain score, age and BMI), also revealed a significant therapeutic effect of OB versus placebo at week 10 (p = 0.009) and 15 (p = 0.006) (Figure 1). The proportion (%) of OB/placebo responders with regards to intensity of pain was 42.5/36.2 at week 5; 52.7/44.5 at week 10 and 67.1/50.0 at week 15. Figure 2a depicts the forest plot of responders according to the intensity of pain evaluated by the patients, showing a strong therapeutic effect of OB versus placebo after 10 and 15 weeks of treatment. In addition, the analysis of VAS on abdominal pain at the end of treatment showed significant improvement of VAS scores for OB, as well as for placebo treatment, 2.03 ± 1.74 for OB and 2.44 ± 2.23 for placebo (p = 0.009). Regarding IBS subtype analysis, we observed a tendency towards a greater reduction of abdominal pain intensity with OB in comparison with placebo in all groups at all visits, reaching a significance at week 15 if all groups were pooled together (p = 0.020). The reduction of pain intensity by OB was statistically significant also in the group of patients with predominant IBS-M (week 15, p = 0.015).
Figure 1.
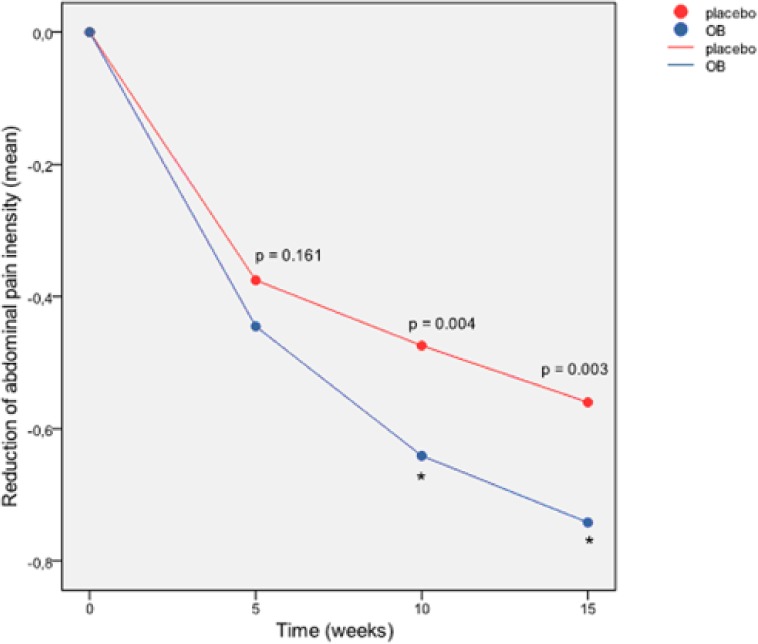
Reduction (difference versus baseline) of abdominal pain intensity score (ITT pooled dataset). p = ANOVA p-value. *p < 0.05 in the GLM (both significant at week 10 and 15).
ANOVA, analysis of variance; GLM, general linear model; ITT, intention-to-treat; OB, otilonium bromide.
Figure 2.
Forest plots including results from the pooled analysis on the primary efficacy variables from Battaglia and colleagues [Battaglia et al. 1998], OBIS [Clave et al. 2011], and Greek [Menarini IFR, 2012] studies. (a) Responder patients according to intensity of pain evaluated by the patient (ITT pooled dataset, week 15); (b) Responder patients according to number of episodes of abdominal pain evaluated by the patient (ITT pooled dataset, week 15); (c) Responder patients according to global evaluation of pain evaluated by the patient (ITT pooled dataset, week 15); (d) Responder patients according to severity of bloating score evaluated by the patient (ITT pooled dataset, week 15); (e) Responder patients according to global efficacy assessment evaluated by the physician.
ITT, intention-to-treat; OB, otilonium bromide.
OB effect on the frequency of abdominal pain as reported by patients
A significant placebo effect was observed from week 5 (p < 0.001) and at the end of the treatment in the scores of weekly number of episodes of abdominal pain. They decreased significantly both in the OB group and in the placebo group (Figure 3). However, the comparison between groups showed a favorable trend in the OB group in comparison with placebo, with significant differences at week 10 and week 15 by ANOVA and at week 15 by GLM (p = 0.002) (Figure 3). The proportion (%) of OB/placebo responders with regards to the reduction of number of episodes of abdominal pain was 54.5/47.4 at week 5; 67.4/58.6 at week 10 and 73.0/60.6 at week 15. Figure 2b depicts the forest plot of frequency of abdominal pain evaluated by the patient, showing a strong therapeutic effect of OB versus placebo after 10 and 15 weeks of treatment. The comparison for IBS subtypes found significant differences (OB versus placebo) in the group of patients with predominant IBS-C (week 10, p = 0.031 and week 15, p = 0.032), IBS-M (week 15, p < 0.001) and in all groups combined (week 5, p = 0.044, week 10, p = 0.011 and week 15, p < 0.001).
Figure 3.
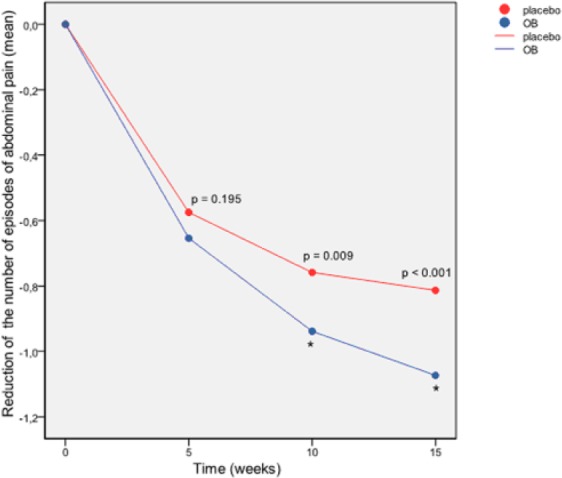
Reduction of the number of episodes of abdominal pain score (ITT pooled dataset). p = ANOVA p-value. *p < 0.05 in the GLM at week 15.
ANOVA, analysis of variance; GLM, general linear model; ITT, intention-to-treat; OB, otilonium bromide.
OB effect on global evaluation of pain index and treatment response by patients
A significant placebo effect was observed at week 5 (p < 0.001) and at the end of treatment for the global evaluation of pain index considering intensity and frequency of abdominal pain which decreased significantly both in the OB and in the placebo group (Figure 4). However, the comparison between treatment groups of this global index of response showed a greater reduction in the OB group versus the placebo group, with significant differences at week 10 and week 15 (by ANOVA), and at week 15 (p = 0.003) following the regression analysis in the GLM (Figure 4). The proportion (%) of OB/placebo responders with regards to the global evaluation of pain was 60.5/54.9 at week 5; 71.8/62.4 at week 10 and 77.2/65.8 at week 15. Figure 2c depicts the forest plot of weekly global evaluation of treatment response evaluated by patients, also showing a strong therapeutic effect of OB versus placebo after 10 and 15 weeks of treatment.
Figure 4.
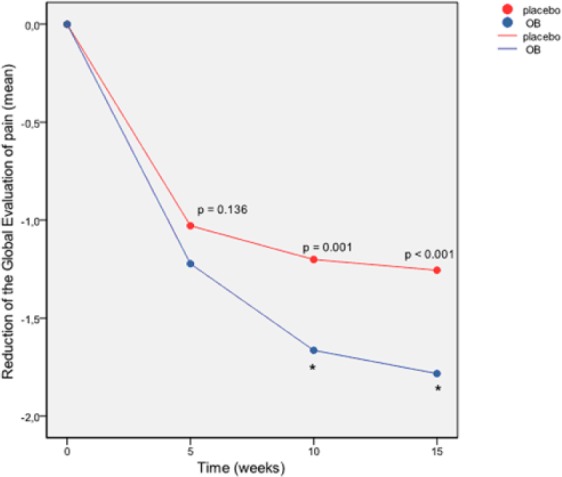
Reduction of the global evaluation of pain index (ITT pooled dataset).
p = ANOVA p-value. *p < 0.05 in the GLM at week 15.
ANOVA, analysis of variance; GLM, general linear model; ITT, intention-to-treat; OB, otilonium bromide.
OB effect on stool frequency and consistency
Baseline data on stool frequency and consistency are summarized in Table 1. During the treatment, the stool frequency score decreased significantly both in the OB group and in the placebo group (Figure 5). The analysis included patients with all evaluations recorded at scheduled visits (baseline, week 5, week 10, and week 15). The comparison between treatment groups showed a trend for a greater reduction of stool frequency in the OB group in comparison with placebo, but without significant differences at any time point (Figure 5). Final scores for stool frequency at week 15 were 0.35 ± 0.50 for patients treated with OB and 0.43 ± 0.64 for patients receiving the placebo (p = 0.76, ns). Regarding stool consistency, both groups of patients showed an improvement during the treatment period. However, OB administration increased the prevalence of patients with normal stools during treatment visits versus patients receiving the placebo, with significant differences at week 5, with 61.6% patients with normal stools in the OB group and 53.9% in the placebo group (p = 0.043). At the end of treatment, after 15 weeks, 74.8% patients in the OB group and 68.5% patients in the placebo group presented normal stool consistency (p = 0.084, ns).
Figure 5.

Reduction of stool frequency (ITT pooled dataset). p = ANOVA p-value.
ANOVA, analysis of variance; ITT, intention-to-treat; OB, otilonium bromide.
OB effect on severity of bloating/distension
A relevant placebo effect was observed from week 5 (p < 0.001) in reference to evaluation of bloating/distension. At the end of the treatment, the severity of bloating score decreased significantly both in the OB group and in the placebo group. However, the pooled analysis revealed a greater reduction of bloating in the OB group versus the placebo at all visits, with significant differences at week 10 and week 15 (Figure 6). The GLM model, providing a regression analysis for the severity of bloating score reduction by more factors and covariate, also revealed a significant therapeutic effect of OB versus placebo at week 10 (p = 0.016) and 15 (p = 0.037). The proportion (%) of OB/placebo responders with regards to the severity of bloating was 43.8/40.8 at week 5; 57.7/49.1 at week 10 and 59.2/53.7 at week 15. Figure 2d depicts the forest plot of responders according to severity of bloating evaluated by patients, showing a significant effect of OB after 15 weeks of treatment.
Figure 6.

Reduction (difference versus baseline) of severity of bloating-meteorism score (ITT dataset). p = ANOVA p-value. *p < 0.05 in the GLM at week 10 and 15.
ANOVA, analysis of variance; GLM, general linear model; ITT, intention-to-treat; OB, otilonium bromide.
OB effect on responders evaluated by physicians
The proportion (%) of OB/placebo responders according to global evaluation of IBS symptoms assessed by physicians was 62.6/46.5 at week 5; 74.6/59.7 at week 10 and 79.1/67.3 at week 15. The physicians considered the global efficacy as good or excellent for 38.5% (week 5), 55.0% (week 10) and 63.9% (week 15) of patients treated with OB. Figure 2e depicts the forest plot of responder patients according to the global evaluation of pain score, highlighting an overall relevant and significant therapeutic effect of OB versus placebo.
Discussion
The pooled analysis we performed reveals that OB is more effective than placebo in the treatment of the most relevant symptoms of IBS and in the global response to treatment assessed both by physicians and by patients. The second finding of the pooled analysis is related to the timing of the therapeutic benefit of OB compared with placebo: for most of the efficacy variables, the specific improvement that can be attributed to OB started after 10 weeks of treatment and was maximal and stable until the end of treatment (at week 15).
The results from this pooled analysis are supported by the strength of its design, with three independent but homogeneous studies, an ITT analysis, a rigorous assessment of the placebo effect, equal dropout rates, global efficacy outcomes evaluated by patients and physicians, and the statistical approach, which included a binary logistic regression model and forest plots. The rationale for the specific inclusion of the Battaglia, the OBIS and the Greek studies in the pooled analysis is that they are RCTs with the same experimental design, including a total of 883 patients, thus providing a large study population to evaluate the efficacy of OB treatment versus placebo on the main symptoms and global response of patients with IBS.
This analysis includes some unpublished data, originating from a RCT conducted in Greece and filed by a local pharmaceutical company that produced the drug. The results of the unpublished (Greek) study are similar to those obtained in the other studies (Battaglia and OBIS) included in this analysis (Figure 2a and 2b) and, in some cases, worse than those reported in the OBIS study in reference to the frequency of abdominal pain, the global composite score of pain, and the responder rates. Overall, our results show OB treatment is globally effective in a representative population of patients with mild-to-severe IBS of different subtypes, further supporting its introduction to clinical practice.
OB has specific pharmacodynamic characteristics, allowing it to counteract the increased pain sensitivity and the impaired small bowel and colon motility that characterize IBS [Drossman et al. 2002]. Visceral hypersensitivity is associated with the intensity of abdominal pain, while impaired colonic motility (hypercontractility, hyperreactivity and increased tone) is associated with frequency of pain episodes and with impaired bowel habits [Kanazawa et al. 2008]. Patients with IBS are thought to present abdominal pain following stimulation of sensitive nerve endings located in the intestinal wall by strong tonic or phasic intestinal contractions in a context of low threshold of stimulation due to visceral hypersensitivity [Clave, 2011]. The mechanisms of action of OB in colonic muscle cells, colonic motility patterns and primary afferent neurons have been recently revised [Rychter et al. 2014]. Studies demonstrated a reduction in visceral sensitivity to rectal balloon distention in IBS patients following treatment with OB [Baldi et al. 1992; Czimmer et al. 2001]. The combined action of OB on normalization of exaggerated motility patterns and inhibition of primary afferent nociceptors probably underlies the strong therapeutic effects of this drug on frequency and intensity of abdominal pain in IBS patients.
The first observation resulting from this pooled analysis is the relevant placebo effect observed in all efficacy variables, increasing from the beginning of treatment with a proportion of placebo responders with regards to the global evaluation of pain index of 54.9–65.8% patients from week 5 to week 15. This effect is well known in IBS studies. For example, it was measured previously in a meta-analysis of 45 placebo-controlled studies and found to be highly variable, ranging from 16–71%, with an average of >40% [Patel et al. 2005]. Slightly higher rates of placebo response were also observed in the OBIS study [Clave et al. 2011] and were attributed to the entry criteria of the study, which included patients with severe IBS symptoms and hence with a greater chance for spontaneous improvement, considering the cyclic nature of IBS.
The second relevant result from our pooled analysis is the strong therapeutic effect of OB, greater than the placebo response in almost all the primary efficacy variables (intensity and frequency of pain, bloating and patient response outcomes, such as global evaluation of pain considering both intensity and frequency of episodes and VAS, and global efficacy evaluated by physicians). A previous meta-analysis by Poynard and colleagues, including four ancillary clinical studies with OB, also found a significant effect of this drug on the proportion of patients showing an improved global assessment (odds ratio of 2.33; 95% CI: 1.60 ± 3.40), and an improvement in pain and distention at the end of treatment [Poynard et al. 2001]. A meta-analysis by Lesbros-Pantoflickova found that when excluding the low-quality trials, an improvement of global IBS symptoms with all antispasmodics was maintained only for OB, but on the basis of only two studies [Lesbros-Pantoflickova et al. 2004]. Finally, a meta-analysis by Ford and colleagues, including the same four studies selected by Poynard and colleagues, also suggested a beneficial effect for OB, as symptoms of IBS persisted in 51% patients treated with OB and in 71% patients treated with placebo [Ford et al. 2008]. However, these last authors underlined a significant heterogeneity among designs and endpoint of studies, as the sample size in three studies was small, the duration of treatment varied, and the largest trial [Glende et al. 2002] showed only a modest benefit of OB in the rate of monthly responders, corresponding to 11–14% [Desborough and Ford, 2011]. The OBIS study included in this pooled analysis found an effect of OB in the reduction of episodes of abdominal pain, reduction of abdominal bloating, global efficacy by patient assessment and protection from IBS symptom relapse significantly greater than placebo at the end of the 15-week treatment period (primary endpoint) [Clave et al. 2011]. In addition, safety and tolerability of OB was very high and similar to placebo, a result consistent with Poynard and colleagues’ meta-analysis [Poynard et al. 2001; Clave et al. 2011]. A study conducted on Asian patients confirmed the effectiveness of OB in alleviating IBS symptoms [Chang et al. 2011]. A recent literature review of the efficacy and tolerability of OB in the long-term management of patients with IBS confirmed that this drug reduces abdominal pain and discomfort in patients with IBS, exhibiting greater efficacy and tolerability than placebo or other similar drugs [Triantafillidis and Malgarinos, 2014]. Finally, a very recent study aiming at evaluating the dose-response relationship of 20 mg, 40 mg, 80 mg of OB or placebo administered three times daily for 4 weeks on functional or clinical efficacy IBS variables demonstrated that 40 mg and 80 mg of OB can improve individual and global clinical symptoms of IBS compared with placebo over a 4-week period [Chmielewska-Wilkon et al. 2014]. Another recent literature review found OB was effective also in comparison with other drugs, such as pinaverium bromide and mebeverine, with a favorable tolerability profile [Forte et al. 2012].
Overall, all these studies clearly prove that OB is well tolerated and is able to improve the symptoms of IBS.
Recently, the recommendation on primary endpoints to be used in trials for IBS has been changed from a co-primary endpoint of global assessment and pain to the separate evaluation of efficacy on stool-related abnormalities and on pain [European Medicines Agency, 2014]. In our pooled analysis, we found that OB improved both the intensity and the frequency of abdominal pain (Figure 2). The weak effect of OB administration on stool frequency and consistency we revealed could be attributed to an over-simplistic dichotomic analysis (normal/abnormal consistency instead of the specific Bristol scale) as well as to the relevant placebo effect, as the OBIS study showed a slight but significant reduction of stool frequency at the end of the OB treatment [Clave et al. 2011].
IBS is a chronic and relapsing disease with cyclic periods of symptoms and frequent relapses to short [Jones et al. 1999] and long-term treatments [Chey et al. 2004]. Antispasmodics are recommended as first line treatment for IBS patients with pain and bloating as dominant symptoms, but there is no agreement on the type of protocol to be selected for treatment (continuous, cyclic or intermittent, or ‘on demand’). Recently, an initial approach of 2–3 months’ treatment has been shown to be effective in most patients [Evangelista, 2012; Boeckxstaens et al. 2013], as also supported by the relevant therapeutic effect observed at 10 and 15 weeks in this pooled analysis. However, symptom relapse occurs in up to 50% of IBS patients, making cyclic treatment necessary. Consequently, only well-tolerated drugs with proved effects on the specific relapsing symptoms should be used [Boeckxstaens et al. 2013]. The OBIS study revealed that the therapeutic effect of OB during the 10-week follow-up period subsequent to the 15-week treatment period remained greater than the effect of placebo in terms of relapse-free probability and withdrawal rate due to symptom relapse [Clave et al. 2011].
Considering the results from this pooled analysis together with the outcomes from previous studies, we can conclude that OB is well tolerated, has a strong therapeutic effect on abdominal pain, bloating and rate of responders as assessed by patients and by physicians, and prevents symptom relapse. OB has been on the market for over 30 years in over 40 countries in Europe, Central America, Asia (China, India, Pakistan, Korea and Philippines), the Middle East, and Africa (Egypt, French African countries). Our findings further support the therapeutic utility of OB in clinical practice.
Acknowledgments
Authors contribution: Pere Clavé and Jan Tack designed the study, acquired and analyzed and interpreted the data and drafted the manuscript.
We are grateful to Giorgio Reggiardo, Valentina Mirisola and Mateu Serra-Prat for statistical analysis, Jane Lewis for reviewing the manuscript and Drs Stefano Evangelista and Massimo Notari from Menarini International (Florence, Italy) for critical review of the manuscript.
Footnotes
Funding: P. Clavé has served as a speaker for Menarini International and has received research funding from Laboratorios Menarini SA-Menarini Group, Badalona, Spain and Menarini International, Florence, Italy.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Pere Clavé, Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd), Hospital de Mataró, Universitat Autònoma de Barcelona, C/ Cirera s/n, 08304, Mataró, Spain.
Jan Tack, Translational Research Center for Gastrointestinal Disorders (TARGID), University of Leuven, Belgium Department of Gastroenterology, University Hospital Gasthuisberg, Leuven, Belgium.
References
- Agarwal N., Spiegel B. (2011) The effect of irritable bowel syndrome on health-related quality of life and health care expenditures. Gastroenterol Clin North Am 40: 11–19. [DOI] [PubMed] [Google Scholar]
- Auli M., Martinez E., Gallego D., Opazo A., Espin F., Marti-Gallostra M., et al. (2008) Effects of excitatory and inhibitory neurotransmission on motor patterns of human sigmoid colon in vitro. Br J Pharmacol 155: 1043–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldi F., Longanesi A., Blasi A., Monello S., Cestari R., Missale G., et al. (1992) Octylonium bromide in the treatment of the irritable bowel syndrome: a clinical-functional study. Hepatogastroenterology 39: 392–395. [PubMed] [Google Scholar]
- Battaglia G., Morselli-Labate A., Camarri E., Francavilla A., De Marco F., Mastropaolo G., et al. (1998) Otilonium bromide in irritable bowel syndrome: a double-blind, placebo-controlled, 15-week study. Aliment Pharmacol Ther 12: 1003–1010. [DOI] [PubMed] [Google Scholar]
- Boeckxstaens G., Corazziari E., Mearin F., Tack J. (2013) IBS and the role of otilonium bromide. Int J Colorectal Dis 28: 295–304. [DOI] [PubMed] [Google Scholar]
- Chang F., Lu C., Luo J., Chen T., Chen M., Chang H. (2011) The evaluation of otilonium bromide treatment in asian patients with irritable bowel syndrome. J Neurogastroenterol Motil 17: 402–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chey W., Chey W., Heath A., Dukes G., Carter E., Northcutt A., et al. (2004) Long-term safety and efficacy of alosetron in women with severe diarrhea-predominant irritable bowel syndrome. Am J Gastroenterol 99: 2195–2203. [DOI] [PubMed] [Google Scholar]
- Chmielewska-Wilkon D., Reggiardo G., Egan C. (2014) Otilonium bromide in irritable bowel syndrome: a dose-ranging randomized double-blind placebo-controlled trial. World J Gastroenterol 20: 12283–12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavé P. (2011) Treatment of IBS-D with 5-HT3 receptor antagonists vs spasmolytic agents: similar therapeutical effects from heterogeneous pharmacological targets. Neurogastroenterol Motil 23: 1051–1055. [DOI] [PubMed] [Google Scholar]
- Clavé P., Acalovschi M., Triantafillidis J., Uspensky Y., Kalayci C., Shee V., et al. (2011) Randomised clinical trial: otilonium bromide improves frequency of abdominal pain, severity of distention and time to relapse in patients with irritable bowel syndrome. Aliment Pharmacol Ther 34: 432–442. [DOI] [PubMed] [Google Scholar]
- Czimmer J., Suto G., Kiraly A., Mozsik G. (2001) Otilonium bromide enhances sensory thresholds of volume and pressure in patients with irritable bowel syndrome. J Physiol Paris 95: 153–156. [DOI] [PubMed] [Google Scholar]
- Desborough M., Ford A. (2011) Is otilonium bromide globally effective in irritable bowel syndrome? Aliment Pharmacol Ther 34: 1034–1036. [DOI] [PubMed] [Google Scholar]
- Drossman D., Camilleri M., Mayer E., Whitehead W. (2002) Aga technical review on irritable bowel syndrome. Gastroenterology 123: 2108–2131. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency (2014) Guideline on the evaluation of medicinal products for the treatment of irritable bowel syndrome. CPMP/EWP/785/97 Rev. 1. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/09/WC500173457.pdf.
- Evangelista S. (2004) Quaternary ammonium derivatives as spasmolytics for irritable bowel syndrome. Curr Pharm Des 10: 3561–3568. [DOI] [PubMed] [Google Scholar]
- Evangelista S., Cochet P., Bromet N., Criscuoli M., Maggi C. (2000) A distribution study with (14)C-otilonium bromide in the rat: evidence for selective tropism for large intestine after oral administration. Drug Metab Dispos 28: 643–647. [PubMed] [Google Scholar]
- Evangelista S. (2012) Benefits from long-term treatment in irritable bowel syndrome. Gastroenterol Res Pract 2012: 936960: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford A., Talley N., Spiegel B., Foxx-Orenstein A., Schiller L., Quigley E., et al. (2008) Effect of fibre, antispasmodics, and peppermint oil in the treatment of irritable bowel syndrome: systematic review and meta-analysis. BMJ 337: a2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forte E., Pizzoferrato M., Lopetuso L., Scaldaferri F. (2012) The use of anti-spasmodics in the treatment of irritable bowel syndrome: focus on otilonium bromide. Eur Rev Med Pharmacol Sci 16: 25–37. [PubMed] [Google Scholar]
- Gallego D., Auli M., Aleu J., Martinez E., Rofes L., Marti-Rague J., et al. (2010) Effect of otilonium bromide on contractile patterns in the human sigmoid colon. Neurogastroenterol Motil 22: e180–e191. [DOI] [PubMed] [Google Scholar]
- Glende M., Morselli-Labate A., Battaglia G., Evangelista S. (2002) Extended analysis of a double-blind, placebo-controlled, 15-week study with otilonium bromide in irritable bowel syndrome. Eur J Gastroenterol Hepatol 14: 1331–1338. [DOI] [PubMed] [Google Scholar]
- Holzer P., Holzer-Petsche U. (2001) Tachykinin receptors in the gut: physiological and pathological implications. Curr Opin Pharmacol 1: 583–590. [DOI] [PubMed] [Google Scholar]
- Hungin A., Whorwell P., Tack J., Mearin F. (2003) The prevalence, patterns and impact of irritable bowel syndrome: an international survey of 40,000 subjects. Aliment Pharmacol Ther 17: 643–650. [DOI] [PubMed] [Google Scholar]
- Hungin A. (2011) Otilonium bromide - challenges of putting trial data into practice. Aliment Pharmacol Ther 34: 1030–1031. [DOI] [PubMed] [Google Scholar]
- Jones R., Holtmann G., Rodrigo L., Ehsanullah R., Crompton P., Jacques L., et al. (1999) Alosetron relieves pain and improves bowel function compared with mebeverine in female nonconstipated irritable bowel syndrome patients. Aliment Pharmacol Ther 13: 1419–1427. [DOI] [PubMed] [Google Scholar]
- Kanazawa M., Palsson O., Thiwan S., Turner M., Van Tilburg M., Gangarosa L., et al. (2008) Contributions of pain sensitivity and colonic motility to IBS symptom severity and predominant bowel habits. Am J Gastroenterol 103: 2550–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesbros-Pantoflickova D., Michetti P., Fried M., Beglinger C., Blum A. (2004) Meta-analysis: the treatment of irritable bowel syndrome. Aliment Pharmacol Ther 20: 1253–1269. [DOI] [PubMed] [Google Scholar]
- Longstreth G., Thompson W., Chey W., Houghton L., Mearin F., Spiller R. (2006) Functional bowel disorders. Gastroenterology 130: 1480–1491. [DOI] [PubMed] [Google Scholar]
- Marger F., Gelot A., Alloui A., Matricon J., Ferrer J., Barrere C., et al. (2011) T-Type calcium channels contribute to colonic hypersensitivity in a rat model of irritable bowel syndrome. Proc Natl Acad Sci USA 108: 11268–11273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Cutillas M., Gil V., Gallego D., Mane N., Martin M., Jimenez M. (2013) Mechanisms of action of otilonium bromide (OB) in human cultured smooth muscle cells and rat colonic strips. Neurogastroenterol Motil 25: e803–e812. [DOI] [PubMed] [Google Scholar]
- Menarini IFR (2012) Controlled double-blind vs placebo clinical study to evaluate efficacy and tolerability of otilonium bromide on quality of life of patients with irritable bowel syndrome. Study Spcim/Gr on file at Menarini IFR, Firenze Italy. [Google Scholar]
- Narducci F., Bassotti G., Granata M., Pelli M., Gaburri M., Palumbo R., et al. (1986) Colonic motility and gastric emptying in patients with irritable bowel syndrome. Effect of pretreatment with octylonium bromide. Dig Dis Sci 31: 241–246. [DOI] [PubMed] [Google Scholar]
- Patel S., Stason W., Legedza A., Ock S., Kaptchuk T., Conboy L., et al. (2005) The placebo effect in irritable bowel syndrome trials: a meta-analysis. Neurogastroenterol Motil 17: 332–340. [DOI] [PubMed] [Google Scholar]
- Poynard T., Regimbeau C., Benhamou Y. (2001) Meta-analysis of smooth muscle relaxants in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther 15: 355–361. [DOI] [PubMed] [Google Scholar]
- Qian A., Song D., Li Y., Liu X., Tang D., Yao W., et al. (2013) Role of voltage gated CA2+ channels in rat visceral hypersensitivity change induced by 2,4,6-Trinitrobenzene sulfonic acid. Mol Pain 9: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S., Sadeghi P., Beaty J., Kavlock R., Ackerson K. (2001) Ambulatory 24-H colonic manometry in healthy humans. Am J Physiol Gastrointest Liver Physiol 280: G629–G639. [DOI] [PubMed] [Google Scholar]
- Rychter J., Espin F., Gallego D., Vergara P., Jimenez M., Clavé P. (2014) Colonic smooth muscle cells and colonic motility patterns as a target for irritable bowel syndrome therapy: mechanisms of action of otilonium bromide. Therap Adv Gastroenterol 7: 156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strege P., Evangelista S., Lyford G., Sarr M., Farrugia G. (2004) Otilonium bromide inhibits calcium entry through L-Type calcium channels in human intestinal smooth muscle. Neurogastroenterol Motil 16: 167–173. [DOI] [PubMed] [Google Scholar]
- Thompson W., Longstreth G., Drossman D., Heaton K., Irvine E., Müller-Lissner S. (1999) Functional bowel disorders and functional abdominal pain. Gut 45(Suppl 2): II43–II47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulouse M., Coelho A., Fioramonti J., Lecci A., Maggi C., Bueno L. (2000) Role of tachykinin NK2 receptors in normal and altered rectal sensitivity in rats. Br J Pharmacol 129: 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triantafillidis J., Malgarinos G. (2014) Long-term efficacy and safety of otilonium bromide in the management of irritable bowel syndrome: a literature review. Clin Exp Gastroenterol 7: 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]