Abstract
Background:
Recently, there has been an increase in clinical success rates using nonsurgical methods to resolve anastomotic biliary strictures (ABSs) that develop after liver transplantation (LT). However, some strictures are particularly refractory and cannot be completely resolved by an endoscopic or percutaneous procedure. Consequently, the aim of this study was to examine the feasibility and efficacy of using a newly designed fully covered self-expandable metal stent (FCSEMS) to resolve refractory ABS.
Methods:
A total of 35 patients with an ABS that developed after LT, but could not be resolved by an endoscopic or percutaneous procedure, were included in this study. FCSEMSs were positioned endoscopically and removed after 2–3 months. After stent removal, the patients were followed to assess complications, including re-stenosis.
Results:
The mean period from LT to stricture was 13.7 months, and the mean duration of the stricture was 31.8 months. The type and mean number of procedures previously attempted were endoscopic retrograde cholangiopancreatography (ERCP) (9.1 ± 5.1) in 19 patients and percutaneous transhepatic biliary drainage (9.2 ± 4.8) in 16 patients. All patients had successful FCSEMS insertions and removals; the mean stent indwelling time was 3.2 months. The mean follow-up period was 18.7 months (range: 6.4–37.8 months). Stricture recurrence was observed in 6 of 29 patients (recurrence rate: 20.7%). The anastomotic stricture resolved with the FCSEMS insertion in 29 of 35 patients (clinical success rate: 82.9%).
Conclusions:
The newly designed FCSEMS is a potentially feasible and effective treatment for anastomotic strictures that develop after LT but are not amenable to treatment by conventional procedures.
Keywords: anastomosis, complication, liver transplantation, stent, stricture
Introduction
Although complications after liver transplantation (LT) have generally become less of a problem because of advances in surgical techniques, organ preservation, and immunosuppressive management, biliary complications remain common [Thuluvath et al. 2005; Sharma et al. 2008; Zimmerman et al. 2013]. Of these, biliary strictures and leakage occur most frequently. They are influenced by various factors, including the type of graft, reconstruction technique, use of biliary stents, and other recipient and donor characteristics. In particular, anastomotic biliary strictures (ABSs) can result from ischemia at the end of the bile duct, causing a fibro-proliferative response and small bile leaks that primarily produce peri-anastomotic fibro-inflammatory responses [Verdonk et al. 2006b; Sharma et al. 2008; Akamatsu et al. 2011].
The rate of post-LT ABS is 5–10% in deceased-donor liver transplantation (DDLT) and 15–30% in living-donor liver transplantation (LDLT) [Thuluvath et al. 2005; Graziadei et al. 2006; Sharma et al. 2008]. Post-LT ABS is often regarded as the ‘Achilles’ heel’ of LT because the problem is not resolved completely in many cases, despite the use of a variety of treatments [Koneru et al. 2006]. Treatment strategies for ABS include both surgical and nonsurgical approaches, such as endoscopic and percutaneous procedures. Nonsurgical management has recently become more popular. Although the optimal strategy for treating ABS remains to be determined, multiple sessions of balloon dilatation (BD) followed by endoscopic placement of multiple side-by-side plastic stents (MPSs) is the most common approach [Hsieh et al. 2013; Kao et al. 2013]. The success rate of BD with MPSs is approximately 70–91% in patients with DDLT and 60–100% in patients with LDLT [Thuluvath et al. 2005; Zoepf et al. 2006; Pasha et al. 2007; Sharma et al. 2008; Seo et al. 2009; Hsieh et al. 2013; Kao et al. 2013; Chok et al. 2014]. Moreover, percutaneous methods may be used for complex strictures or for strictures that cannot be treated by an endoscopic approach [Kim et al. 2009]. The success rate of these methods is reportedly as high as 80% [Roumilhac et al. 2003; Sharma et al. 2008].
With recent technological advances and the introduction of the rendezvous method, the clinical success rate for ABS resolution has increased for both endoscopic and percutaneous treatments, but these methods are not suitable for all patients. In such cases, patients may require lifelong indwelling percutaneous drainage catheters or, alternatively, reassessment for surgery. The aim of this study was to evaluate the feasibility and efficacy of a newly developed fully covered self-expandable metal stent (FCSEMS) as treatment for post-LT ABS recalcitrant to conventional endoscopic and percutaneous methods.
Patients and methods
Patients
Between August 2014 and July 2015, 35 patients with one or more post-LT anastomotic strictures received the newly modified FCSEMS by endoscopic insertion. The patients were referred to our institution from three university hospitals. Previously, endoscopic management was initially performed to resolve strictures. The percutaneous approach was performed if endoscopic stent insertion failed. As many plastic stents as possible were inserted for maximal dilation. Plastic stents or percutaneous transhepatic biliary drainage (PTBD) catheters were inserted for 2–3 months. Refractory strictures were defined as unresolved strictures after repeated plastic stent or PTBD catheter replacement, regardless of the number of procedures performed over 1 year, and the FCSEMSs were used in these patients. The inclusion criteria for enrollment were an age of 18 years or older, one or more bilio-biliary ABSs after LT, and one or more refractory ABSs amendable to conventional endoscopic and percutaneous methods. The exclusion criteria were a contraindication to an endoscopic procedure and a suspected malignant stricture. The data gathered following FCSEMS insertion were evaluated retrospectively. The Institutional Review Board at Gangnam Severance Hospital, South Korea, approved this study. All participants provided written informed consent.
Stents
The FCSEMS (Kaffes®, Taewoong Medical, Seoul, Korea) used in this study has a central ‘waist’. The stent is 10 mm in diameter at each end with gradual tapering to a central diameter of 8 mm (Figure 1). This design was developed to prevent stent migration. It has three radiopaque markers for precise positioning of the stent at the stricture site. The stents used in this study were 4, 5, or 6 cm long, depending on the stricture length. The stents had a diameter of 6, 8, or 10 mm, and the delivery catheter had a diameter of 8.5 Fr. A retrievable radiopaque string, 10 cm in length, was located at the end of the stent to facilitate its removal by standard endoscopic biopsy forceps.
Figure 1.
Structure of a FCSEMS. The stent has a central ‘waist’ at the mid-portion and three radiopaque markers to allow the radial force of the metallic stent to be directed maximally to the center of the stricture, hence inhibiting migration. The fully covered short stent has a long, platinum, radiopaque-marked retrieval string. The stent imparts pressure over a large area of the normal duct and reduces the risk of necrosis and fibrosis. The long string with vivid platinum markers facilitates removal of the stent from its location high in the common bile duct.
Procedure
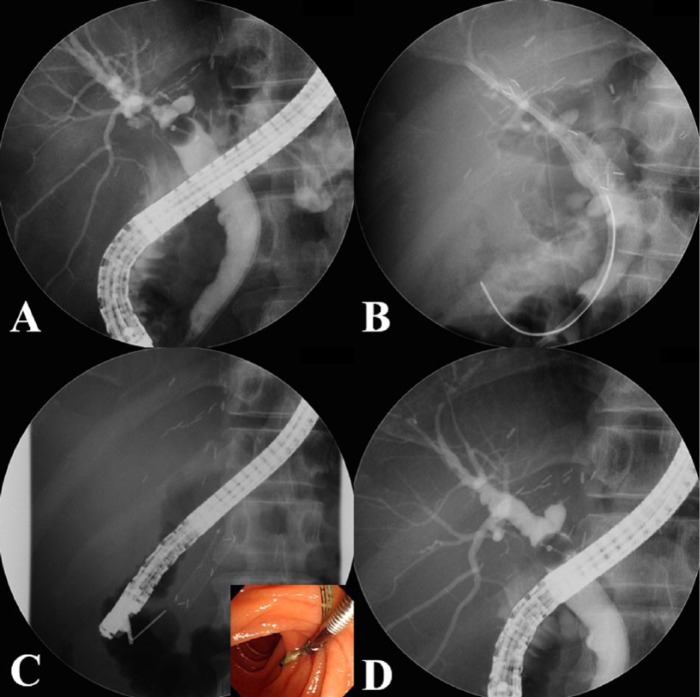
Endoscopic retrograde cholangiopancreatography (ERCP) was performed on all patients using a video duodenoscope (JF240 or TJF240; Olympus Optical, Tokyo, Japan; Figure 2). The previously inserted PTBD catheter or plastic stent was removed. Patients who had previously undergone endoscopic sphincterotomy (EST) were not treated again, but patients who only had a PTBD catheter did undergo EST. The guidewire was inserted through the stricture site after selective cannulation. The position and length of the stricture were measured using a cholangiogram, and the stent size required to cover the stricture was determined. After moving the metal stent to the stricture site, the central label consisting of three radiopaque markers was located adjacent to the center of the stricture, and the stent was then deployed by means of a conventional self-expandable metal stent (SEMS). The stent covered the stricture sufficiently, as confirmed by visualization of the two radiopaque markers on either side of the stent.
Figure 2.
Insertion procedure for a FCSEMS. (A) After removing the patient’s drainage catheter or plastic stent, a cholangiogram is performed to visualize the shape and length of the stricture site. (B) The FCSEMS is inserted at the stricture site, and the insertion site of the plastic stent (used to prevent cholangitis) is determined based on the presence of a bile duct stone or sludge. (C) After an indwelling time of 2–3 months, the FCSEMS is removed by grasping the retrieval string using biopsy forceps (color figure). (D) The cholangiogram demonstrates resolution of the stricture.
If the stricture was too tight to introduce the catheter, it was dilated using a balloon dilator (6 mm, Hurricane™, Boston Scientific, Natick, MA, USA) or a tapered tip bougie dilator (Soehendra biliary dilation catheter™, Cook Medical, Bloomington, IN, USA). A plastic stent was also inserted if obstruction of a branch of the bile duct by the FCSEMS was suspected. If multiple strictures were present, multiple FCSEMSs were inserted at each stricture site. Antibiotics (1 g cefoperazone-sulbactam intravenously, or 200 mg ciprofloxacin intravenously for patients with allergies) were administered routinely before and after each procedure.
Patient follow up
After stent insertion, patients were monitored in our outpatient department for clinical symptoms, abnormal laboratory findings, and signs of complications, including cholangitis. An abdominal X-ray was taken 1 month after stent insertion to detect any stent migration. The FCSEMSs were maintained for 2–3 months and then removed using endoscopic biopsy forceps. Resolution of the ABS was evaluated at stent removal, and a new FCSEMS was reinserted if the stricture was not completely resolved. Clinical symptoms and laboratory test results were recorded at 3-month intervals after stent removal to monitor for re-stenosis. If re-stenosis was suspected, one or more of the following tests were performed: computed tomography, magnetic resonance cholangiopancreatography, or a diisopropyl iminodiacetic acid scan. A new FCSEMS was reinserted if re-stenosis was confirmed.
Statistical analysis
The continuous variables were expressed as means, standard deviations, and ranges. Categorical variables were summarized as frequencies and percentages. Outcomes, success, and recurrence rate were expressed as binary data.
Results
The FCSEMS was inserted endoscopically in 35 patients with ABS after LDLT. Basic patient characteristics are shown in Table 1. The most common indication was hepatocellular carcinoma secondary to hepatitis B virus. The mean period between LT and stricture appearance was 13.7 months, and the mean duration of the stricture was 31.8 months. A total of 7 patients (20%) had complex ABSs and each had a total of 2 strictures. Overall, two FCSEMSs were inserted into these patients, one into each stricture (Figure 3). The type and mean (± standard deviation) number of interventions previously attempted in the patients were as follows: 19 patients underwent previous ERCPs (9.1 ± 5.1 interventions), and 16 underwent previous PTBDs (9.2 ± 4.8 interventions). Among the latter group of patients, the 5 who underwent PTBD alone had a mean of 9.4 interventions, and the 11 who received both PTBD and ERCP underwent a mean of 8.8 interventions.
Table 1.
Basic patient characteristics.
| Characteristic | Value |
|---|---|
| Number of patients | 35 |
| Age (year, mean ± SD) | 56.6 ± 8.9 |
| Male : female | 27 : 8 |
| Diagnosis | |
| HCC : LC : LC + HCC | 20 : 9 : 6 |
| Cause | |
| HBV : alcohol : HCV | 29 : 5 : 1 |
| Duration between LT and stricture [months, mean ± SD (range)] | 13.7 ± 25.9 (10.2–110.1) |
| Duration of stricture [months, mean ± SD (range)] | 31.8 ± 22.1 (12.8–92.4) |
| No. of strictures | |
| Single : multiple | 28 : 7 |
| Previous procedures [no. of patients (no. of interventions, mean ± SD)] | |
| ERCP | 19 (9.1 ± 5.1) |
| PTBD | 16 (9.2 ± 4.8) |
| PTBD only | 5 (9.4 ± 7.6) |
| ERCP + PTBD | 11 (8.8 ± 3.5) |
DDLT, deceased-donor liver transplantation; ERCP, endoscopic retrograde cholangiopancreatography; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; LC, liver cirrhosis; LDLT, living-donor liver transplantation; LT, liver transplantation; No., number; PTBD, percutaneous transhepatic biliary drainage; SD, standard deviation.
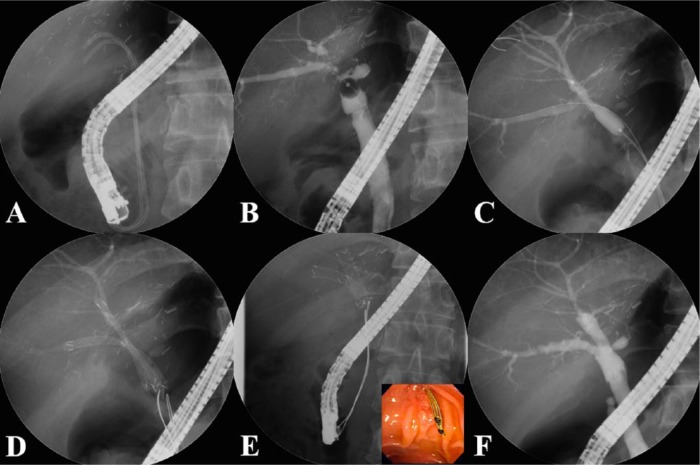
Figure 3.
Insertion procedure for multiple FCSEMSs. (A) Previously inserted plastic stents are removed endoscopically. (B) The cholangiogram shows multiple anastomotic strictures at the posterior and inferior intrahepatic ducts. (C) The strictures are dilated using a balloon dilator to allow passage of the FCSEMSs. (D) The FCSEMSs are inserted sequentially into the stricture sites. (E) After an indwelling time of 2–3 months, the FCSEMSs are removed by grasping the retrieval strings using biopsy forceps. (F) The cholangiogram demonstrates resolution of the multiple strictures.
The mean follow-up period was 18.7 months, and the mean FCSEMS indwelling duration was 3.2 months (Table 2). The only early (within 1 month of insertion) complication was proximal stent migration. Late (>1 month after insertion) complications were stent-related jaundice, cholangitis, and an intrahepatic bile duct (IHD) stone. These complications resolved with stent removal and reinsertion of the FCSEMS. Sludge was found in 11 patients (31.4%) at stent removal and a common bile duct (CBD) or IHD stone in 12 patients (34.3%) (Figure 4). A plastic stent was additionally inserted in 14 patients (40%) to help prevent obstruction of bile duct branch due to the presence of the FCSEMS. Balloon dilation, dilation with a tapered tip bougie, or both were performed in 8 patients (22.9%) in whom the FCSEMS was not easily deployed because of a particularly narrow stricture. Insertion and removal of the FCSEMS were technically possible in all enrolled patients (Table 3). Re-stenosis occurred in 6 patients (17.1%) at a mean duration of 5.8 months after stent removal. The short-term clinical success rate of FCSEMS was thus 82.9%. In those patients with re-stenosis, an additional FCSEMS was inserted and removed after a 3-month indwelling period, which resolved the stricture recurrence. No statistically significant factors were found to be associated with the response to FCSEMS in univariate and multivariate analyses. However, there was a tendency for patients with single strictures to have a higher response rate compared with patients with multiple strictures in the multivariate analysis (Table 4, p = 0.054).
Table 2.
Clinical outcomes of FCSEMS procedures.
| Clinical outcome | Value |
|---|---|
| Balloon dilatation before stent insertion [no. of patients (%)] | 8/35 (22.9) |
| Single SEMS versus double SEMS (no. of patients) | 28/7 |
| SEMS only: SEMS + PS (no. of patients) | 21/14 |
| FCSEMS stent | |
| Length (cm, mean ± SD) | 4.2 ± 0.6 |
| Width (mm, mean ± SD) | 7.4 ± 1.5 |
| Duration of stent indwelling [months, mean ± SD (range)] | 3.2 ± 1.4 (1.0–6.7) |
| Sludge formation at stent removal [no. of patients (%)] | 11/35 (31.4) |
| CBD or IHD stone formation at stent removal [no. of patients (%)] | 12/35 (34.3) |
| Follow-up period [months, mean ± SD (range)] | 18.7 ± 8.3 (6.4–37.8) |
| Complications | 14.3 (5/35) |
| Early complications (⩽1 month) | 1 |
| – stent migration | 1 |
| Late complications (>1 month) | 4 |
| – jaundice | 1 |
| – stent migration | 1 |
| – cholangitis | 2 |
CBD, common bile duct; FCSEMS, fully covered self-expandable metal stent; IHD, intrahepatic bile duct; No., number; SD, standard deviation; SEMS, self-expandable metal stent.
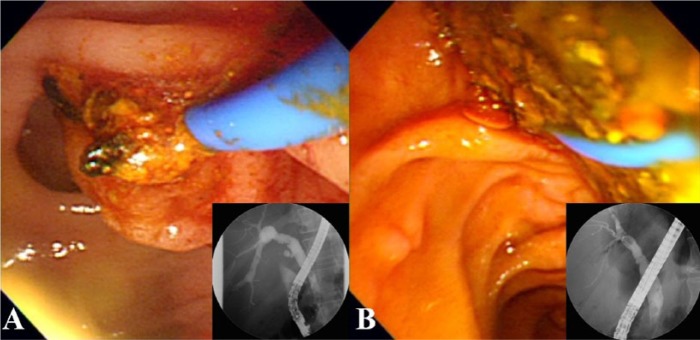
Figure 4.
Photographs showing sludge or stone formation at stent removal. After the stent is removed, sludge (A) or small stones (B) are found and removed using a balloon catheter.
Table 3.
Success and recurrence outcomes of FCSEMS procedures.
| Outcome | Value |
|---|---|
| Technical success rate [no. of patients (%)] | 35/35 (100) |
| Clinical success rate [no. of patients (%)] | 29/35 (82.9) |
| Recurrence rate [no. of patients (%)] | 6/29 (20.7) |
| Duration between recurrence and stent removal [months, mean ± SD (range)] | 5.8 ± 2.4 (3.1–10.2) |
| Treatment after recurrence of stricture | |
| Second FCSEMS insertion | 6/6 (100) |
FCSEMS, fully covered self-expandable metal stent; no., number; SD, standard deviation.
Table 4.
Univariate and multivariate analyses assessing factors associated with the response to FCSEMSs in refractory strictures after LDLT.
| Total patients, n | Clinical success, n (%) |
Univariate p-value |
Multivariate p-value |
|
|---|---|---|---|---|
| Sex | 0.694 | 0.733 | ||
| Male | 27 | 22 (81.4) | ||
| Female | 8 | 7 (87.5) | ||
| Age, years | 0.973 | 0.244 | ||
| ⩾50 | 29 | 24 (82.7) | ||
| <50 | 6 | 5 (83.3) | ||
| Duration of stricture | 0.166 | 0.077 | ||
| ⩾12 months | 30 | 26 (86.6) | ||
| <12 months | 5 | 3 (60.0) | ||
| Previous number of procedures (n) | 0.912 | 0.998 | ||
| ⩾6 | 24 | 20 (83.3) | ||
| <6 | 11 | 9 (81.8) | ||
| Multiple strictures | 0.061 | 0.054 | ||
| Yes | 7 | 4 (57.1) | ||
| No | 28 | 25 (89.2) | ||
| Indwelling duration | 0.817 | 0.208 | ||
| <3 months | 16 | 13 (81.3) | ||
| ⩾3 months | 19 | 16 (84.2) | ||
| Stone or sludge† | 0.336 | 0.181 | ||
| Yes | 23 | 18 (78.3) | ||
| No | 12 | 11 (91.7) | ||
| Plastic stent insertion | 0.585 | 0.675 | ||
| No | 21 | 18 (85.7) | ||
| Yes | 14 | 11 (78.6) | ||
| Balloon dilatation | 0.100 | 0.546 | ||
| No | 27 | 24 (88.9) | ||
| Yes | 8 | 5 (62.5) |
Stone or sludge was found at removal of metal stent.
FCSEMS, fully covered self-expandable metal stent; LDLT, living-donor liver transplantation.
Discussion
The clinical success rate of the newly modified FCSEMS was 82.9%, which was high compared with that of conventional methods, because this study was performed in recalcitrant strictures as opposed to typical strictures in previous studies (Tables 5 and 6). The recurrence rate was similar to that of conventional self-expandable metal stents (SEMs) [Traina et al. 2009; Chaput et al. 2010; Sauer et al. 2012; Cerecedo-Rodriguez et al. 2013]. Endoscopic treatments for ABS show high success rates (70–100%) in DDLT patients and somewhat lower rates (60–91%) in LDLT patients [Graziadei et al. 2006; Pasha et al. 2007; Sharma et al. 2008; Akamatsu et al. 2011; Albert et al. 2013; Hsieh et al. 2013; Chok et al. 2014]. Reported recurrence rates are approximately 0–20%, and recurrences are usually managed by repeat endoscopic stent placement [Graziadei et al. 2006; Gomez et al. 2009; Kato et al. 2009; Seo et al. 2009; Chok et al. 2014]. Generally, post-LT ABS that occurs 1–2 months after LT results from transient narrowing caused by postoperative edema and inflammation [Verdonk et al. 2006a]. This type of early ABS shows a good response to BD with temporary stent placement [Thuluvath et al. 2005; Sharma et al. 2008]. Conversely, treatment is more difficult for late strictures, which occur in the majority of patients. These strictures are generally caused by fibrotic scarring from ischemia near the bile duct anastomosis site [Verdonk et al. 2006b], which makes them more difficult to dilate. Therefore, late ABS is managed more aggressively, with periodic ERCP sessions every 2–3 months and a longer duration of stent insertion, sometimes involving the use of stents suitable for a prolonged period of time (12–24 months) [Kao et al. 2013]. The strictures treated in this study were refractory ABSs because they occurred at least 6 months after LT and had failed to resolve after more than 9 attempts using conventional methods. As these were clearly difficult situations, treatment using the FCSEMS can be considered to have a relatively high success rate.
Table 5.
Endoscopic treatment of anastomotic biliary strictures with plastic stents following orthotopic liver transplantation.
| Studies | Study design | Pts (n) | No. of procedures per patient (mean) | No. of stents (mean) | Interval of stenting, (mean or median, months) | Stenting period, (mean or median, months) | Stent–free follow up (mean or median, months) | Technical success rate (%) | Clinical success rate (%) | Recurrence rate (%) | Complication rate (%) | Recurrence treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rerknimitr et al. [2002] | R | 43 | 3.8 | 2.8 | 3.6 | 15.8 | 39.6 | 100 | 100 | 0 | 6.6 | NA |
| Morelli et al. [2003] | R | 25 | 3.1 | 2 | 3.3 | 6 | 54 | 88 | 80 | 9.1 | 3.7 | ERCP |
| Zoepf et al. [2006] | R | 15 | 4 | 2.8 | 2.5 | 4 | 4 | 100 | 88 | 30 | 8 | Surgery |
| Verdonk et al. [2006b] | R | 27 | 3 | 2 | NA | 3 | NA | 75 | 69 | 19 | 37 | ERCP, surgery |
| Alazmi et al. [2006] | R | 107 | 3.1 | 1–2 | 2–3 | 5 | 32 | 96.6 | 100 | 18 | NA | ERCP |
| Graziadei et al. [2006] | P | 65 | 3 | 1–2 | 3 | 3 | 42.2 | 89.2 | 77 | 18.5 | 1.2 | PTBD, surgery |
| Holt et al. [2007] | P | 53 | 3 | 4 | NA | 11.3 | 18 | 92 | 74 | 3 | 20.7 | Surgery |
| Pasha et al. [2007] | R | 25 | 3.5 | 2 | 2–3 | 5.6 | 21.5 | 88 | 91 | 18.1 | 5 | ERCP, surgery |
| Morelli et al. [2008] | P | 38 | 3.4 | 2.5 | 2 weeks | 3.6 | 12 | 100 | 87 | 15 | 5.2 | ERCP, surgery |
| Tabibian et al. [2010] | R | 69 | 3 | 2.4 | 3 | 15 | 11 | 83.1 | 94 | 3 | 5.7 | ERCP |
| Poley et al. [2013] | P | 31 | 5 | 4 | 3 | NA | 28 | 100 | 80.6 | 0 | 67.7 | None |
| Tringali et al. [2016] | R | 51 | 4 | 4 | 3–4 | 11.5 | 69.6 | 98 | 98 | 6 | 5.3 | ERCP |
ERCP, endoscopic retrograde cholangiopancreatogram; NA, not available; no, number; P, prospective; PTBD, percutaneous transhepatic biliary drainage; Pts, patients; R, retrospective.
Table 6.
Endoscopic treatment of anastomotic biliary strictures with covered SEMSs following orthotopic liver transplantation.
| Studies | Study design | Pts (n) | SEMS type | Stenting period, (mean or median, months) | Stent–free follow up (mean or median, months) | Clinical success rate (%) | Recurrence rate (%) | Stent migration (%) | Complication rate (%) | Recurrence treatment |
|---|---|---|---|---|---|---|---|---|---|---|
| Kahaleh et al. [2008] | R | 16 | Partial | 4 | 12 | 94 | 0 | 14† | 11.4† | NA |
| Mahajan et al. [2009] | R | 9 | Fully | 3.3 | 3.8 | 100 | 0 | 4† | 28† | NA |
| Traina et al. [2009] | P | 16 | Fully | 2 | 10.12 | 87.5 | 6.3 | 37.5 | 37.5 | PTBD |
| Chaput et al. [2010] | P | 22 | Partial | 2 | 12 | 86.4 | 47.3 | 27.2 | 23 | BD and plastic stenting SEMS reinsertion |
| Garcia-Pajares et al. [2010] | R | 22 | NA | NA | 12.5 | 95.5 | 5.2 | 23.8 | 41† | SEMS reinsertion |
| Hu et al. [2011] | R | 13 | Fully | 5.4 | 12.1 | 92.3 | 7.7 | 0 | 7.7 | SEMS reinsertion |
| Haapamaki et al. [2012] | R | 16 | Fully | 6.8 | 21.7 | 100 | 11.7 | 23.5 | 15.5 | SEMS reinsertion |
| Tarantino et al. [2012] | R | 15 | Fully | 2 | 14.4 | 53.3 | 25 | 46.7 | NA | NA |
| Kahaleh et al. [2013] | R | 35 | Fully | 3.2 | >18 | 61.3 | 38.7 | 35.7 | 12.5† | SEMS reinsertion |
| Cerecedo-Rodriguez et al. [2013] | R | 19 | Partial | 4.3 | 38.9 | 74 | NA | 10.5 | 16 | Surgery |
| 21 | Fully, with fin | 4.1 | 24.3 | 71 | NA | 4.7 | 29 | |||
| 15 | Fully, with flared end | 4.5 | 4.6 | 60 | NA | 6.6 | 7 | |||
| Deviere et al. [2014] | P | 42 | Fully | 4–6 | 20 | 68 | 27.9 | 18 | 38.1 | SEMS reinsertion |
| Kaffes et al. [2014] | P | 10 | Fully | 3 | 24.5 | 100 | 30 | 0 | 10 | SEMS reinsertion |
Authors reported results without specifying the groups.
BD, balloon dilatation; NA, not available; no, number; P, prospective; PTBD, percutaneous transhepatic biliary drainage; Pts, patients; R, retrospective; SEMS, self-expandable metal stent.
Although endoscopic BD with MPS management is less invasive and shows a high success rate, its primary disadvantage is that it requires repeat ERCPs every 3–4 months for 1–2 years [Kao et al. 2013; Fernandez-Simon et al. 2014]. The FCSEMS may reduce the overall number of procedures (including ERCP, PTBD, or both) required to resolve ABS. In this study, the previous mean number of ERCP or PTBD interventions was >9, whereas the mean number of FCSEMS interventions was approximately 1.3. Furthermore, post-LT complex ABSs may have characteristics that are distinct from those of benign biliary strictures. The ABS angle is acute and the IHD is narrow, such that insertion of multiple plastic stents may be difficult, and it may be possible to insert only one or two plastic stents. These limitations may hinder sufficient dilation of the stricture. Moreover, the inserted plastic stent may easily migrate to a proximal or distal portion because the center of the plastic stent cannot be placed in the center of the ABS stricture.
There have been attempts to overcome the limitations of periodic plastic stent replacements using temporary single-session SEMS placement as an alternative [Traina et al. 2009; Sauer et al. 2012; Tarantino et al. 2012; Cerecedo-Rodriguez et al. 2013; Kao et al. 2013]. However, since SEMSs exhibit higher migration rates and variable results, they have been unable to achieve consistently superior resolution of ABS compared with maximal plastic stent therapy [Kahaleh et al. 2013; Kao et al. 2013]. The ABS resolution rate is 80–95% when SEMS patency is maintained for at least 3 months and 94–100% when dilation and plastic stent treatments persist for 12 months [Kao et al. 2013]. Although the clinical success rate of biliary stricture treatment using SEMS was high at 86.4–100%, the migration rate (4–37%) and complication rate (0–41%) were also relatively high [Kim et al. 2010; Tarantino et al. 2012; Kao et al. 2013]. The main concern in using covered SEMSs is migration and the risk of occluding secondary branch ducts or the pancreatic duct, which could cause cholangitis or pancreatitis. Mucosal hyperplasia-induced strictures can occur in the proximal uncovered end of partially covered SEMSs [Kahaleh et al. 2008]. Moreover, SEMS removal is labor-intensive and can occasionally cause mucosal ulceration and bleeding from damage caused by anti-migration systems (e.g. anchor fins) [Mahajan et al. 2009]. Therefore, a new type of fully covered FCSEMS without a potentially damaging anti-migration system is required for benign biliary strictures.
The specific modified FCSEMS used in this study has a central waist to prevent migration and a long string to facilitate removal using standard endoscopic biopsy forceps. The FCSEMS has a diameter of 10 mm at each end and gradually tapers to a center that is 8 mm in diameter. In addition, radiopaque markers in the center of the stent allow the center of the stricture to match the center of the stent, so the radial force of the stent can be directed maximally to the center. The purpose of these modifications is to help prevent migration, which is the primary problem with FCSEMSs. Using the shortest possible stent length to optimize the stent length along the stricture length imparts pressure over a large area of the duct and reduces the potential risk of necrosis and fibrosis, as well as obstruction of a separate branch. The stent used in this study was a short fully covered metal stent restricted to the stricture site, whereas the distal end of the traditional long stent overlaps into the duodenum. As a result, longer stents can cause pancreatitis, migration, and gastrointestinal content reflux, whereas the modified stent reduces the risk of these adverse effects. The long string of the short stent, with vivid platinum markers, facilitates its removal. These structural characteristics of the modified FCSEMS can be expected to improve the clinical resolution of post-LDLT ABS and reduce the number of complications. However, sludge or stone formation, with a resulting risk of cholangitis occurred relatively frequently in this study using the modified FCSEMS. Although the definite cause or mechanism could not be ascertained, we assumed that the FCSEMS could promote obstruction of branches of the bile duct and cholestasis. Although the stent is as short as possible, it might still block a side branch duct. Later in our study, we inserted an additional plastic stent in an attempt to resolve these sludge or stone formation issues and to prevent cholangitis following obstruction of the side branch duct.
It has been reported that SEMSs are effective in patients with post-LT ABS, and there are arguments for employing this as the initial treatment method [Traina et al. 2009; Tarantino et al. 2012; Kao et al. 2013]. In previous studies [Kahaleh et al. 2008; Traina et al. 2009; Sauer et al. 2012; Tarantino et al. 2012; Cerecedo-Rodriguez et al. 2013; Kao et al. 2013; Kaffes et al. 2014]. MPSs showed a stricture resolution rate very similar to that for SEMSs. Moreover BD with MPS treatment has a good clinical success rate and low recurrence rate in ABS occurring within 1 month of LT [Thuluvath et al. 2005; Hsieh et al. 2013; Kao et al. 2013]. Therefore, FCSEMSs may be a salvage treatment if plastic stenting treatment fails [Traina et al. 2009; Curcio et al. 2012; Tarantino et al. 2012], and the results of this study show that FCSEMSs are effective in treating refractory post-LT ABS that has not resolved with more conventional methods. Further studies are required to investigate the feasibility and effectiveness of using FCSEMSs as the initial treatment for post-LT ABS.
The limitations of this study include its retrospective design, as well as the additional plastic stent insertion and BD procedures that were performed in some patients. The plastic stent insertions were necessary to prevent sludge or stone formation and possible blocking of other intrahepatic duct biliary drainage by the covering membrane. A further limitation is that the follow-up data are too short-term to fully evaluate the rate of re-stenosis. Furthermore, our indwelling time for the FCSEMS was 2–3 months, which may have increased the recurrence rate if this was not long enough to resolve the stricture. Further studies with long-term follow up of FCSEMS in post-LT ABS are needed, and large-scale prospective studies will be necessary to establish the most effective indwelling duration of FCSEMSs to overcome elastic recoil at the stricture site and reduce the recurrence rate of post-LT ABS.
Treatment with endoscopic methods, percutaneous procedures, or both for ABSs after LT have been highly successful. The success rate has further increased because of the rendezvous method combined with an endoscopic approach, along with advancements in cutting balloons, catheter development, and guidewire techniques. However, if both approaches fail, the FCSEMS has emerged as a new salvage treatment option, with reasonable efficacy and feasibility. The clinical success rate for treating post-LT ABS, regarded as an ‘Achilles’ heel’, can be improved through the refinement and interplay of various treatment options, including the newly developed FCSEMS.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Sung Ill Jang, Department of Internal Medicine, Kangnam Sacred Heart Hospital, Hallym University College of Medicine, Seoul, South Korea Department of Medicine, The graduate school of Yonsei University, Seoul, South Korea.
Se Yong Sung, Department of Internal Medicine, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
Hyunsung Park, Department of Internal Medicine, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
Kwang-Hun Lee, Department of Radiology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, South Korea.
Seung-Moon Joo, Department of Radiology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, South Korea.
Dong Ki Lee, Department of Internal Medicine, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
References
- Akamatsu N., Sugawara Y., Hashimoto D. (2011) Biliary reconstruction, its complications and management of biliary complications after adult liver transplantation: a systematic review of the incidence, risk factors and outcome. Transpl Int 24: 379–392. [DOI] [PubMed] [Google Scholar]
- Alazmi W., Fogel E., Watkins J., Mchenry L., Tector J., Fridell J., et al. (2006) Recurrence rate of anastomotic biliary strictures in patients who have had previous successful endoscopic therapy for anastomotic narrowing after orthotopic liver transplantation. Endoscopy 38: 571–574. [DOI] [PubMed] [Google Scholar]
- Albert J., Filmann N., Elsner J., Moench C., Trojan J., Bojunga J., et al. (2013) Long-term follow-up of endoscopic therapy for stenosis of the biliobiliary anastomosis associated with orthotopic liver transplantation. Liver Transpl 19: 586–593. [DOI] [PubMed] [Google Scholar]
- Cerecedo-Rodriguez J., Phillips M., Figueroa-Barojas P., Kumer S., Gaidhane M., Schmitt T., et al. (2013) Self-expandable metal stents for anastomotic stricture following liver transplant. Dig Dis Sci 58: 2661–2666. [DOI] [PubMed] [Google Scholar]
- Chaput U., Scatton O., Bichard P., Ponchon T., Chryssostalis A., Gaudric M., et al. (2010) Temporary placement of partially covered self-expandable metal stents for anastomotic biliary strictures after liver transplantation: a prospective, multicenter study. Gastrointest Endosc 72: 1167–1174. [DOI] [PubMed] [Google Scholar]
- Chok K., Chan S., Cheung T., Sharr W., Chan A., Fan S., et al. (2014) A retrospective study on risk factors associated with failed endoscopic treatment of biliary anastomotic stricture after right-lobe living donor liver transplantation with duct-to-duct anastomosis. Ann Surg 259: 767–772. [DOI] [PubMed] [Google Scholar]
- Curcio G., Traina M., Miraglia R., Tarantino I., Barresi L., Granata A. (2012) Treatment of a refractory biliary stricture after living donor liver transplantation, with a short fully covered metal stent with a long string. Endoscopy 44(Suppl. 2)UCTN: E74–E75. [DOI] [PubMed] [Google Scholar]
- Deviere J., Nageshwar Reddy D., Puspok A., Ponchon T., Bruno M., Bourke M., et al. (2014) Successful management of benign biliary strictures with fully covered self-expanding metal stents. Gastroenterology 147: 385–395; quiz e315. [DOI] [PubMed] [Google Scholar]
- Fernandez-Simon A., Diaz-Gonzalez A., Thuluvath P., Cardenas A. (2014) Endoscopic retrograde cholangiography for biliary anastomotic strictures after liver transplantation. Clin Liver Dis 18: 913–926. [DOI] [PubMed] [Google Scholar]
- Garcia-Pajares F., Sanchez-Antolin G., Pelayo S., Gomez De La Cuesta S., Herranz Bachiller M., Perez-Miranda M., et al. (2010) Covered metal stents for the treatment of biliary complications after orthotopic liver transplantation. Transplant Proc 42: 2966–2969. [DOI] [PubMed] [Google Scholar]
- Gomez C., Dumonceau J., Marcolongo M., De Santibanes E., Ciardullo M., Pekolj J., et al. (2009) Endoscopic management of biliary complications after adult living-donor versus deceased-donor liver transplantation. Transplantation 88: 1280–1285. [DOI] [PubMed] [Google Scholar]
- Graziadei I., Schwaighofer H., Koch R., Nachbaur K., Koenigsrainer A., Margreiter R., et al. (2006) Long-term outcome of endoscopic treatment of biliary strictures after liver transplantation. Liver Transpl 12: 718–725. [DOI] [PubMed] [Google Scholar]
- Haapamaki C., Udd M., Halttunen J., Lindstrom O., Makisalo H., Kylanpaa L. (2012) Endoscopic treatment of anastomotic biliary complications after liver transplantation using removable, covered, self-expandable metallic stents. Scand J Gastroenterol 47: 116–121. [DOI] [PubMed] [Google Scholar]
- Holt A., Thorburn D., Mirza D., Gunson B., Wong T., Haydon G. (2007) A prospective study of standardized nonsurgical therapy in the management of biliary anastomotic strictures complicating liver transplantation. Transplantation 84: 857–863. [DOI] [PubMed] [Google Scholar]
- Hsieh T., Mekeel K., Crowell M., Nguyen C., Das A., Aqel B., et al. (2013) Endoscopic treatment of anastomotic biliary strictures after living donor liver transplantation: outcomes after maximal stent therapy. Gastrointest Endosc 77: 47–54. [DOI] [PubMed] [Google Scholar]
- Hu B., Gao D., Yu F., Wang T., Pan Y., Yang X. (2011) Endoscopic stenting for post-transplant biliary stricture: usefulness of a novel removable covered metal stent. J Hepatobiliary Pancreat Sci 18: 640–645. [DOI] [PubMed] [Google Scholar]
- Kaffes A., Griffin S., Vaughan R., James M., Chua T., Tee H., et al. (2014) A randomized trial of a fully covered self-expandable metallic stent versus plastic stents in anastomotic biliary strictures after liver transplantation. Therap Adv Gastroenterol 7: 64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahaleh M., Behm B., Clarke B., Brock A., Shami V., De La, Rue S., et al. (2008) Temporary placement of covered self-expandable metal stents in benign biliary strictures: a new paradigm? (with video). Gastrointest Endosc 67: 446–454. [DOI] [PubMed] [Google Scholar]
- Kahaleh M., Brijbassie A., Sethi A., Degaetani M., Poneros J., Loren D., et al. (2013) Multicenter trial evaluating the use of covered self-expanding metal stents in benign biliary strictures: time to revisit our therapeutic options? J Clin Gastroenterol 47: 695–699. [DOI] [PubMed] [Google Scholar]
- Kao D., Zepeda-Gomez S., Tandon P., Bain V. (2013) Managing the post-liver transplantation anastomotic biliary stricture: multiple plastic versus metal stents: a systematic review. Gastrointest Endosc 77: 679–691. [DOI] [PubMed] [Google Scholar]
- Kato H., Kawamoto H., Tsutsumi K., Harada R., Fujii M., Hirao K., et al. (2009) Long-term outcomes of endoscopic management for biliary strictures after living donor liver transplantation with duct-to-duct reconstruction. Transpl Int 22: 914–921. [DOI] [PubMed] [Google Scholar]
- Kim E., Lee B., Won J., Choi J., Lee D. (2009) Percutaneous transhepatic biliary drainage may serve as a successful rescue procedure in failed cases of endoscopic therapy for a post-living donor liver transplantation biliary stricture. Gastrointest Endosc 69: 38–46. [DOI] [PubMed] [Google Scholar]
- Kim J., Ko G., Sung K., Gwon D., Lee S., Kim K., et al. (2010) Percutaneously placed covered retrievable stents for the treatment of biliary anastomotic strictures following living donor liver transplantation. Liver Transpl 16: 1410–1420. [DOI] [PubMed] [Google Scholar]
- Koneru B., Sterling M., Bahramipour P. (2006) Bile duct strictures after liver transplantation: a changing landscape of the achilles’ heel. Liver Transpl 12: 702–704. [DOI] [PubMed] [Google Scholar]
- Mahajan A., Ho H., Sauer B., Phillips M., Shami V., Ellen K., et al. (2009) Temporary placement of fully covered self-expandable metal stents in benign biliary strictures: midterm evaluation (with video). Gastrointest Endosc 70: 303–309. [DOI] [PubMed] [Google Scholar]
- Morelli G., Fazel A., Judah J., Pan J., Forsmark C., Draganov P. (2008) Rapid-sequence endoscopic management of posttransplant anastomotic biliary strictures. Gastrointest Endosc 67: 879–885. [DOI] [PubMed] [Google Scholar]
- Morelli J., Mulcahy H., Willner I., Cunningham J., Draganov P. (2003) Long-term outcomes for patients with post-liver transplant anastomotic biliary strictures treated by endoscopic stent placement. Gastrointest Endosc 58: 374–379. [DOI] [PubMed] [Google Scholar]
- Pasha S., Harrison M., Das A., Nguyen C., Vargas H., Balan V., et al. (2007) Endoscopic treatment of anastomotic biliary strictures after deceased donor liver transplantation: outcomes after maximal stent therapy. Gastrointest Endosc 66: 44–51. [DOI] [PubMed] [Google Scholar]
- Poley J., Lekkerkerker M., Metselaar H., Kuipers E., Bruno M. (2013) Clinical outcome of progressive stenting in patients with anastomotic strictures after orthotopic liver transplantation. Endoscopy 45: 567–570. [DOI] [PubMed] [Google Scholar]
- Rerknimitr R., Sherman S., Fogel E., Kalayci C., Lumeng L., Chalasani N., et al. (2002) Biliary tract complications after orthotopic liver transplantation with choledochocholedochostomy anastomosis: endoscopic findings and results of therapy. Gastrointest Endosc 55: 224–231. [DOI] [PubMed] [Google Scholar]
- Roumilhac D., Poyet G., Sergent G., Declerck N., Karoui M., Mathurin P., et al. (2003) Long-term results of percutaneous management for anastomotic biliary stricture after orthotopic liver transplantation. Liver Transpl 9: 394–400. [DOI] [PubMed] [Google Scholar]
- Sauer P., Chahoud F., Gotthardt D., Stremmel W., Weiss K., Buchler M., et al. (2012) Temporary placement of fully covered self-expandable metal stents in biliary complications after liver transplantation. Endoscopy 44: 536–538. [DOI] [PubMed] [Google Scholar]
- Seo J., Ryu J., Lee S., Park J., Yang K., Kim Y., et al. (2009) Endoscopic treatment for biliary stricture after adult living donor liver transplantation. Liver Transpl 15: 369–380. [DOI] [PubMed] [Google Scholar]
- Sharma S., Gurakar A., Jabbour N. (2008) Biliary strictures following liver transplantation: past, present and preventive strategies. Liver Transpl 14: 759–769. [DOI] [PubMed] [Google Scholar]
- Tabibian J., Asham E., Han S., Saab S., Tong M., Goldstein L., et al. (2010) Endoscopic treatment of postorthotopic liver transplantation anastomotic biliary strictures with maximal stent therapy (with video). Gastrointest Endosc 71: 505–512. [DOI] [PubMed] [Google Scholar]
- Tarantino I., Traina M., Mocciaro F., Barresi L., Curcio G., Di Pisa M., et al. (2012) Fully covered metallic stents in biliary stenosis after orthotopic liver transplantation. Endoscopy 44: 246–250. [DOI] [PubMed] [Google Scholar]
- Thuluvath P., Pfau P., Kimmey M., Ginsberg G. (2005) Biliary complications after liver transplantation: the role of endoscopy. Endoscopy 37: 857–863. [DOI] [PubMed] [Google Scholar]
- Traina M., Tarantino I., Barresi L., Volpes R., Gruttadauria S., Petridis I., et al. (2009) Efficacy and safety of fully covered self-expandable metallic stents in biliary complications after liver transplantation: a preliminary study. Liver Transpl 15: 1493–1498. [DOI] [PubMed] [Google Scholar]
- Tringali A., Barbaro F., Pizzicannella M., Boskoski I., Familiari P., Perri V., et al. (2016) Endoscopic management with multiple plastic stents of anastomotic biliary stricture following liver transplantation: long-term results. Endoscopy 48: 546–551. [DOI] [PubMed] [Google Scholar]
- Verdonk R., Buis C., Porte R., Haagsma E. (2006a) Biliary complications after liver transplantation: a review. Scand J Gastroenterol Suppl: 89–101. [DOI] [PubMed] [Google Scholar]
- Verdonk R., Buis C., Porte R., Van Der Jagt E., Limburg A., Van Den Berg A., et al. (2006b) Anastomotic biliary strictures after liver transplantation: causes and consequences. Liver Transpl 12: 726–735. [DOI] [PubMed] [Google Scholar]
- Zimmerman M., Baker T., Goodrich N., Freise C., Hong J., Kumer S., et al. (2013) Development, management, and resolution of biliary complications after living and deceased donor liver transplantation: a report from the adult-to-adult living donor liver transplantation cohort study consortium. Liver Transpl 19: 259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoepf T., Maldonado-Lopez E., Hilgard P., Malago M., Broelsch C., Treichel U., et al. (2006) Balloon dilatation vs balloon dilatation plus bile duct endoprostheses for treatment of anastomotic biliary strictures after liver transplantation. Liver Transpl 12: 88–94. [DOI] [PubMed] [Google Scholar]