Abstract
Background and aim
The cysts may potentially affect any organ; adrenals cysts are rare. This is a review of the literature regarding adrenal cysts, focusing on children and young adults.
General data
Three major types have been described: pure cysts (endothelial, epithelial, and hemorrhagic or pseudocyst), parasitic (as hydatid) cysts and cystic part of a tumour (most frequent are neuroblastoma, ganglioneuroma, pheocromocytoma, and teratoma). The complications are: bleeding, local pressure effects; infection; rupture (including post-traumatic); arterial hypertension due to renal vessels compression. Adrenal hemorrhage represents a particular condition associating precipitating factors such as: coagulation defects as Factor IX or X deficiency, von Willebrand disease, thrombocytopenia; antiphospholipid syndrome; previous therapy with clopidogrel or corticosteroids; the rupture of a prior tumour. At birth, the most suggestive features are abdominal palpable mass, anemia, and persistent jaundice. Adrenal insufficiency may be found especially in premature delivery. The hemorrhage is mostly self-limiting. Antenatal ultrasound diagnosis of a cyst does not always predict the exact pathology result. The most important differential diagnosis of adrenal hemorrhage/hemorrhagic cyst is cystic neuroblastoma which is highly suggestive in the presence of distant metastases and abnormal catecholamine profile. The major clue to differentiate the two conditions is the fact that the tumor is stable or increases over time while the adrenal hemorrhage is expected to remit within one to two weeks.
Conclusion
Pediatric adrenal cysts vary from simple cysts with a benign behavior to neoplasia- related lesions displaying severe prognosis as seen in cystic neuroblastoma. A multidisciplinary team is required for their management which is conservative as close follow-up or it makes necessary different surgical procedures in cases with large masses or if a malignancy suspicion is presented. Recently, laparoscopic approach is regarded as a safe procedure by some authors but generally, open surgery is more frequent used compare to adults; in most cases the preservation of normal gland is advisable.
Keywords: adrenal cyst, adrenal hemorrhage, cystic neuroblastoma
Introduction
Adrenal lesions, either containing solid or liquid material, comprise a wide area of conditions which may be diagnosed during fetal life up to advanced ages [1,2,3]. Tumors of the adrenal cortex have a predominant endocrine behavior while the underlying pathological report typically involves adenoma, rarely nodular hyperplasia (often bilaterally), and exceptionally a carcinoma [4,5,6,7]. The adrenal medulla is the origin of tumors such as pheocromocytoma, ganglioneuroma, ganglioneuroblastoma [8,9,10]. The glands may be a secondary site of a prior cancer spreading, regardless unilateral or bilateral pattern [11,12,13]. The most frequent neoplasia with adrenal involvement is breast or lung cancer, and lymphomas [14,15,16]. Other rare adrenal lesions especially with tumor appearance are myelolipoma, neurofibroma, hamartoma, xanthomatosis, amyloidosis, granulomatosis [17,18,19]. The adrenal bed may locate masses of non-adrenal origin, like those arising from the pancreas, spleen or kidney and rarely imagery artefacts need to be differentiated [20,21,22]. Knowing this large panel of potential diagnosis related to an adrenal lesion, the cysts of this gland are rare conditions which may be recognized at any age, following the scenario of an incidentaloma or severe general disturbances [23,24]. A cystic incidentaloma does not necessarily associate a more severe prognosis since the term is currently accepted in endocrinology for pituitary and adrenal glands accidentally discovered tumors mostly with non-secretor profile and minimum risk of increasing the dimensions [25,26].
Objective
Our aim was to review the literature regarding the cystic lesions of the adrenal glands especially in children and young adults, based on a PubMed database guided research. The data are grouped based on the idea that an adrenal cyst may accidentally discovered during a routine abdominal ultrasound and the underlying diagnosis involves the general practitioners, pediatricians, endocrinologists, internists, surgeons, etc.
General data
The concept of cyst
The term of “cyst” is used in pathological conditions and it represents a closed structure surrounded by a capsule (a sac-like mass), either isolated or multiple-sited [27,28]. Anatomically, a cyst is a sac containing either liquid, gaseous or semi-solid substances, potentially involving any organ and varying in size from microscopic to extremely large (up to the level of displacing the host or neighboring organs ) [29,30].
Abdominal cyst & differential diagnosis
While most cysts are benign, some may be tumors and be formed inside of tumors (these have a higher malignancy potential), so their pathogenic environment is related to a solid tumor, genetic anomalies (with or without a solid tumor association), infections (including parasites), embryo-fetal defects, blockage of an otherwise normal duct (with secondary fluid collection) and trauma (impact injury that breaks a vessel) [31,32,33].
The abdominal cysts we mention especially related to pediatric population may be located at the liver, for instance hepatic hydatid cyst, mesenchymal hamartoma, traumatic cystic lesions [34,35,36]. Biliary ducts cysts such as choledochal cysts are rare, while the mesentery area sometimes involves lymphatic malformation, pure mesenteric cysts or traumatic pseudocysts [37,38,39,40,41]. Solid organs such as spleen may associate simple cysts or vascular malformations; pancreatic cysts aspects are mostly related to pseudocysts or cystadenoma [42,43,44]. Kidneys display cyst-like lesions such as cystic Wilms tumors or multicystic dysplastic kidney, etc. [45,46,47]. Simple renal cysts have also been described in pediatric population at routine computed tomography scans, for example 69 cases were diagnosed after 2991 scans in one study between 2007 and 2009 [48].
This complex picture of abdominal cystic lesions is fulfilled by adrenal cysts which are recognized in pediatric as well as adult population. The first lesion having this site was described in 1670 and subsequently more cases were published together as a series of seven by Henschen in 1906; and by Reimann and Guyton who published some observations in 1947 related to four papers that were found during a 10-year period in the American literature [49,50]. Their work as well as the data provided by Stock on the same year currently represents the first studies available on PubMed on this matter, followed by the 1950 article by Moore and Cermak regarding a fatal outcome in a child with both adrenal cysts and insufficiency [51,52]. An early series of cases (on 83 reports) and classification is from 1956 based on the data of Blumenthal and Probstein [53].
Types of adrenal cysts
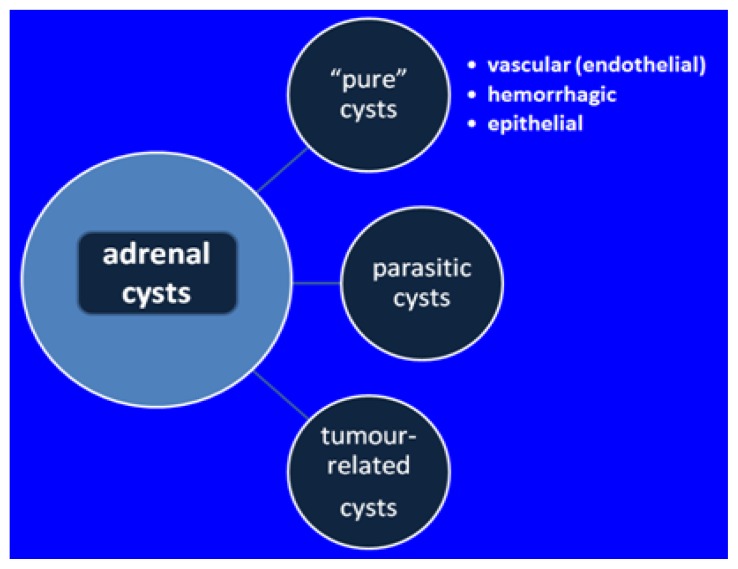
Cystic lesions of the adrenals, although exceptionally rare, may be grouped in three main types: “pure” cystic types, parasitic cysts and cystic part of an otherwise solid tumor usually related to a process of necrosis or hemorrhage [54,55].
Classical (or “pure”) type of cysts includes three different sub-types. The most frequent is vascular or endothelial cyst which has either lymphangiomatous or hemangiomatous origin [56]. Adrenal lymphangiomas have a benign behavior; a review published in 2015 found 53 cases reported in literature (including adults): females were more affected and one third had no symptoms before surgery [57]. Adrenal hemangiomas also have a good prognosis (that is why it is possible to be diagnosed during adult life) but surgical removal is considered in most situations with high diameters and in those where a hemorrhagic cyst is suspected with further potential of development [58].
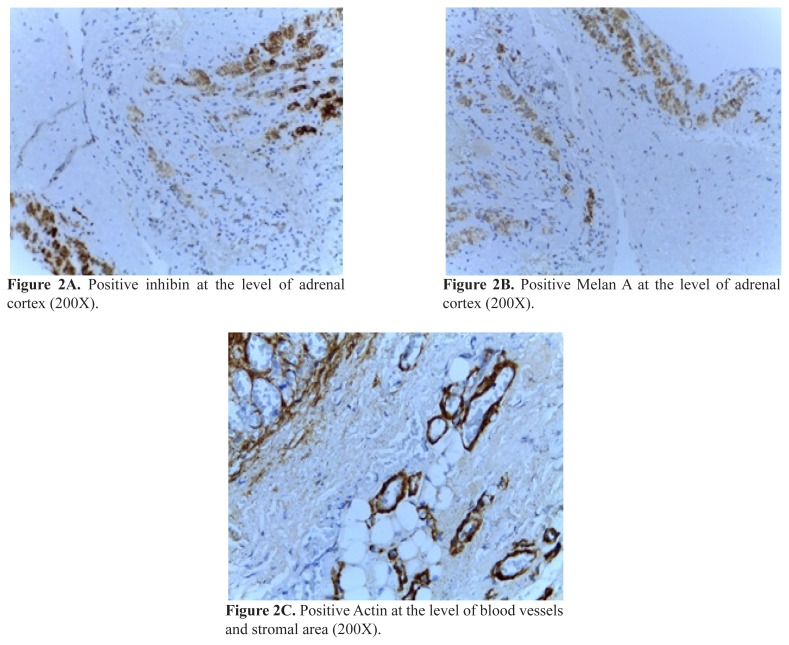
Coming second as prevalence is the hemorrhagic cyst, also called pseudocyst or hemorrhagic pseudocyst [59]. (Figure 1+2) Hemorrhagic cyst in children is associated with: pre / peri-natal stress; hypoxia; septicemia; hypotension; dehydration [60,61]. The vascular etiology is not accepted by all the authors (and an abdominal trauma may be enough to cause the lesion in some cases) thus the hormonal profile needs to be checked in all cases [62]. This type of cyst has walls of fibrous tissue; it characterized by the lack of epithelial & endothelial lining; and it is mostly the result of a prior hemorrhage [63]. The lesion is similar with other prenatally developed cysts as seen in lung, spleen, and the increased potential of regression has been found to be correlated with homogenous aspects opposite to multi-lobulated structures [64].
Figure 1.
Pathological exam: hematoxylin eosin stain.
Figure 2.
Immunohistochemistry examination
Adrenal hemorrhagic cyst associated with a local small blood vessel anomaly on a pre-pubertal boy without birth trauma or genetic/general conditions
The rarest sub-group is the epithelial cyst, named true cyst which is related to a congenital glandular sac and associated liquid retention [65]. (Figure 3)
Figure 3.
Main types of cysts involving the adrenal glands.
The adrenals are not a typical site for parasites, yet hydatid cyst formed by the larva of a parasite has been described [66,67].
The tumors of the adrenals described most frequently with a cystic component in children and young adults are: neuroblastoma, ganglioneuroma, pheocromocytoma, teratoma, and, exceptionally, adrenocortical carcinoma/adenoma [68,69,70]
Pheochromocytoma may suffer necrosis and the tumor become semicystic or semisolid [71]. The cystic aspect has been found in bilaterally, familial, genetic conditions as von Hippel-Lindau disease including hemangioblastomas, pancreatic cysts, renal cancer (autosomal dominant inheritance) [72]. In pediatric population, the adrenal tumor may be the only or the first manifestation of the syndrome [73].
The cystic (mature) adrenal teratoma represents a rare germ cell neoplasia with an atypical site at the level of the gland; usually the first recognition is based on imaging findings not on symptoms, and more frequently the diagnosis is established in children than adults, including a prenatal identification of an adrenal lesion, which might improve the outcome since an early diagnosis and total resection with histological confirmation is essential [74,75].
Evaluation of Adrenal Cysts
The evaluation of a cystic lesion includes imaging scans such as ultrasound, computed tomography or magnetic resonance. Biopsy is not recommended in most of cases. A complete endocrine panel is necessary for the adrenal profile.
Cysts complications
Generally, the cyst early discovery and good prognosis is related to the tumor/lesion size; patients’ age and pubertal development; the presence of abdominal pain, the palpable mass or lump and potentially hormonal dysfunction (with or without genetic background) [76]. The complications are: bleeding into the cyst; local pressure effects; cyst infection; rupture of the cyst (including post-traumatic cyst rupture); arterial hypertension due to renal vessels compression [77,78].
Particular conditions of adrenal pediatric cysts
Adrenal hemorrhage may be spontaneous, but a number of precipitating factors have been involved such as: anticoagulation; pre-existing benign adrenal cyst; coagulation defects like Factor IX or X deficiency, von Willebrand disease, thrombocytopenia; antiphospholipid syndrome; previous therapy with clopidogrel or corticosteroids; the rupture of a prior tumor [79,80,81]. The hemorrhage is mostly self-limiting and the spontaneous resolution is the usual outcome; yet some cases have been reported in correlation with gastrointestinal diseases, therefore close surveillance in the peri-natal period is necessary [82]. A prenatal diagnosis of the adrenal hemorrhage is possible as generally it is for adrenal and thoracic cysts of non-hemorrhagic type; antenatal ultrasound does not always predict the exact pathological report but it is extremely important for further management of the newborn, and eventually for informing and counseling the future parents [83,84]. The right adrenal is more often affected than the left one, and left site may associate renal vein thrombosis (or thrombus at other levels if a prior pro-thrombotic condition is presented) [85]. The clinical neonatal findings in severe cases are abdominal palpable mass, anemia, and persistent jaundice [86,87]. Adrenal insufficiency may be found especially in premature delivery [88]. Also, the bilateral hemorrhagic event may be seen in correlation with Beckwith-Wiedemann syndrome (complete or incomplete forms) [89]. The syndrome associates macroglossia, abdominal wall defects, visceromegaly, gigantism, hemi-hypertrophy, hypoglycemia, and high risk of malignancy (including neuroblastoma), which requires close follow-up or even resection in case of a persistent cystic lesion of the adrenals [90,91].
During the neonatal period the most important differential diagnosis of adrenal hemorrhage is cystic neuroblastoma which is highly suggestive in the presence of distant metastases and abnormal catecholamine profile [92]. The major clue to differentiate the two conditions is the fact that the cystic tumor is stable or increases over time while the adrenal hemorrhage is expected to remit within one to two weeks [93]. The hemorrhagic structure remission has different stages: acute (while the adrenal cystic mass has variable echogenicity), sub-acute with hypoechoic ultrasound pattern due to liquefaction and later on a hyperechoic structure due to clot retraction and potential calcification is registered [94]. The cystic neuroblastoma associates an aggressive profile (including metastasis at the moment of first diagnosis) and requires a multi-disciplinary team [95]. The tumor arises from adrenal or any neural crest element; the ultrasound pattern is anechoic or complex echogenic, and half of cases have calcifications [96].
Adrenal cysts management
There is no standard management; small cysts may be followed up by ultrasound, computed tomography, magnetic resonance imaging; glucocorticoid replacement is necessary if adrenal insufficiency is caused by bilateral adrenal hemorrhage; since the pre-operatory assessment does not definitively distinguish the malignant lesions, surgery may represent on option [97,98]. In cases with lesions larger than 3 centimeters, associating endocrine disturbances, local symptoms or rapid growth, surgical removal should be taken into consideration or, alternatively, cyst un-roofing, exploratory laparotomy [99,100,101]. There is no general consensus if laparoscopy or open surgery should be recommended but, opposite to adults, the procedures with adrenal parenchyma preservation are recommended [102,103]. Minimally invasive surgery in children may be used in adrenal neuroblastoma with good results regarding the safety and effectiveness [104]. Imaging investigations allowing antenatal diagnosis of a cystic mass should be performed, followed if necessary by early surgery after birth [105]. Conservative therapy is recommended for adrenal hemorrhage but close check-up is necessary; generally a tumor identified before birth or immediately after without a precise diagnosis may be followed for one month without worsening the prognosis [106].
Conclusion
Adrenal cysts are rare lesions in the pediatric population, varying from pure cysts with a benign behavior to tumor-related types displaying severe prognosis, like those in cystic neuroblastoma. A multidisciplinary team is required for their management, which is conservative as close follow-up (including the cases with ante- or peri- natal diagnosis of an adrenal hemorrhage); or it makes necessary different surgical procedures in cases with large masses or suspected malignancy. Recently, laparoscopic approach is regarded as a safe procedure by some authors but generally, open surgery is more frequently used than in adults; in most cases the preservation of the normal gland is advisable.
References
- 1.Słapa RZ, Jakubowski WS, Dobruch-Sobczak K, Kasperlik-Załuska AA. Standards of ultrasound imaging of the adrenal glands. J Ultrason. 2015;15(63):377–387. doi: 10.15557/JoU.2015.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quinn E, McGee R, Nuccio R, Pappo AS, Nichols KE. Genetic Predisposition to Neonatal Tumors. Curr Pediatr Rev. 2015;11(3):164–178. doi: 10.2174/1573396311666150714110229. [DOI] [PubMed] [Google Scholar]
- 3.Baudin E Endocrine Tumor Board of Gustave Roussy. Adrenocortical carcinoma. Endocrinol Metab Clin North Am. 2015;44(2):411–434. doi: 10.1016/j.ecl.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Basham KJ, Hung HA, Lerario AM, Hammer GD. Mouse models of adrenocortical tumors. Mol Cell Endocrinol. 2016;421:82–97. doi: 10.1016/j.mce.2015.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elfiky A. Adrenocortical Carcinoma: A Clinician’s Perspective. Surg Pathol Clin. 2015;8(4):751–754. doi: 10.1016/j.path.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Duregon E, Volante M, Bollito E, Goia M, Buttigliero C, Zaggia B, et al. Pitfalls in the diagnosis of adrenocortical tumors: a lesson from 300 consultation cases. Hum Pathol. 2015;46(12):1799–807. doi: 10.1016/j.humpath.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Carsote M, Ghemigian A, Valea A, Dumitrascu A, Chirita C, Poiana C. Sublinical Cushing’s syndrome with bilateral adrenal tumours in a patient with gallbladder multiple stone: therapeutical options. ARS Medica Tomitana. 2015;3(21):124–127. [Google Scholar]
- 8.Plouin PF, Amar L, Dekkers OM, Fassnacht M, Gimenez-Roqueplo AP, Lenders JW, et al. European Society of Endocrinology Clinical Practice Guideline for long-term follow-up of patients operated on for a pheochromocytoma or a paraganglioma. Eur J Endocrinol. 2016;174(5):G1–G10. doi: 10.1530/EJE-16-0033. [DOI] [PubMed] [Google Scholar]
- 9.Ilias I, Meristoudis G, Notopoulos A. A probabilistic assessment of the diagnosis of paraganglioma/pheochromocytoma based on clinical criteria and biochemical/imaging findings. Hell J Nucl Med. 2015;18(1):63–65. [PubMed] [Google Scholar]
- 10.Lenders JW, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SK, Murad MH, et al. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(6):1915–1942. doi: 10.1210/jc.2014-1498. [DOI] [PubMed] [Google Scholar]
- 11.Glenn JA, Kiernan CM, Yen TW, Solorzano CC, Carr AA, Evans DB, et al. Management of suspected adrenal metastases at 2 academic medical centers. Am J Surg. 2016;211(4):664–670. doi: 10.1016/j.amjsurg.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 12.Kawaguchi T. Organ Preference of Cancer Metastasis and Metastasis-Related Cell Adhesion Molecules Including Carbohydrates. Cardiovasc Hematol Disord Drug Targets. 2016;15(3):164–186. doi: 10.2174/1871529x15666151102102551. [DOI] [PubMed] [Google Scholar]
- 13.Yoo JY, McCoy KL, Carty SE, Stang MT, Armstrong MJ, Howell GM, et al. Adrenal Imaging Features Predict Malignancy Better than Tumor Size. Ann Surg Oncol. 2015;22(Suppl 3):S721–S727. doi: 10.1245/s10434-015-4684-z. [DOI] [PubMed] [Google Scholar]
- 14.Paunovic I, Zivaljevic V, Diklic A, Tausanovic K, Stojanic R, Sipetic S. Prognostic parameters after surgery for adrenal metastases: a single institution experience. Acta Chir Belg. 2014;114(3):198–202. doi: 10.1080/00015458.2014.11681008. [DOI] [PubMed] [Google Scholar]
- 15.Wagnerova H, Lazurova I, Felsoci M. Adrenal metastases. Bratisl Lek Listy. 2013;114(4):237–240. doi: 10.4149/bll_2013_049. [DOI] [PubMed] [Google Scholar]
- 16.Akhtar K, Sherwani R, Kahkhashan E. Carcinoma breast metastasis to the suprarenal gland: an unusual presentation. Pol J Pathol. 2012;63(4):284–285. doi: 10.5114/pjp.2012.32777. [DOI] [PubMed] [Google Scholar]
- 17.Baisakh MR, Chattoraj A, Narayanan R, Mohanty R, Mishra M. Adrenal myelolipoma: A rare lesion of adrenal gland. Indian J Cancer. 2015;52(4):597–8. doi: 10.4103/0019-509X.178402. [DOI] [PubMed] [Google Scholar]
- 18.Gupta P, Aggarwal R, Sarangi R. Solitary neurofibroma of the adrenal gland not associated with type-1 neurofibromatosis. Urol Ann. 2015;7(1):124–126. doi: 10.4103/0974-7796.148661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozdemir D, Dagdelen S, Erbas T. Endocrine involvement in systemic amyloidosis. Endocr Pract. 2010;16(6):1056–1063. doi: 10.4158/EP10095.RA. [DOI] [PubMed] [Google Scholar]
- 20.Schieda N, Al Dandan O, Kielar AZ, Flood TA, McInnes MD, Siegelman ES. Pitfalls of adrenal imaging with chemical shift MRI. Clin Radiol. 2014;69(11):1186–1197. doi: 10.1016/j.crad.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 21.Davarpanah AH, Isrel GM. MR imaging of the kidneys and adrenal glands. Radiol Clin North Am. 2014;52(4):779–798. doi: 10.1016/j.rcl.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Ciçekçi M, Onur MR, Aydin AM, Gül Y, Ozkan Y, Akpolat N, et al. The role of apparent diffusion coefficient values in differentiation between adrenal masses. Clin Imaging. 2014;38(2):148–153. doi: 10.1016/j.clinimag.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Nieman LK. Approach to the patient with an adrenal incidentaloma. J Clin Endocrinol Metab. 2010;95(9):4106–13. doi: 10.1210/jc.2010-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reginelli A, Di Grezia G, Izzo A, D’andrea A, Gatta G, Cappabianca S, et al. Imaging of adrenal incidentaloma: our experience. Int J Surg. 2014;12(Suppl 1):S126–S131. doi: 10.1016/j.ijsu.2014.05.029. [DOI] [PubMed] [Google Scholar]
- 25.Papierska L, Cichocki A, Sankowski AJ, Cwikła JB. Adrenal incidentaloma imaging - the first steps in therapeutic management. Pol J Radiol. 2013;78(4):47–55. doi: 10.12659/PJR.889541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carsote M, Chirita C, Dumitrascu A, Hortopan D, Fica S, Poiana C. Pituitary incidentalomas-how often is too often? J Med Life. 2009;2(1):92–97. [PMC free article] [PubMed] [Google Scholar]
- 27.Oughterson AW, Taffel M. Pulmonary Cysts: A Review of the Subject, with a Case Report. Yale J Biol Med. 1936;9(1):77–100. [PMC free article] [PubMed] [Google Scholar]
- 28.Robertson FP, Tsironis D, Davidson BR. A diaphragmatic retroperitoneal cyst. Ann R Coll Surg Engl. 2015;97(5):e77–e78. doi: 10.1308/003588415X14181254790329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cianci P, Tartaglia N, Altamura A, Fersini A, Vovola F, Sanguedolce F, Ambrosi A, et al. A recurrent epidermoid cyst of the spleen: report of a case and literature review. World J Surg Oncol. 2016 Apr 1;14(1):98. doi: 10.1186/s12957-016-0857-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carsote M, Dumitrascu A, Mihai A, Geleriu A, Procopiuc L, Radoi V, et al. Incidental endocrine cysts. Current Health Sciences Journal. 2014;40(Suppl):71–74. [Google Scholar]
- 31.Elluru RG, Balakrishnan K, Padua HM. Lymphatic malformations: diagnosis and management. Semin Pediatr Surg. 2014;23(4):178–185. doi: 10.1053/j.sempedsurg.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Dubey JP, Newell TK, Verma SK, Calero-Bernal R, Stevens EL. Toxoplasma gondii infection in llama (Llama glama): acute visceral disseminated lesions, diagnosis, and development of tissue cysts. J Parasitol. 2014;100(3):288–294. doi: 10.1645/13-427.1. [DOI] [PubMed] [Google Scholar]
- 33.Singh KK, Nizarudeen A, Sulfikar MS, Maheshwaran A, George D. Post-traumatic (hemorrhagic) liver cyst. Indian J Surg. 2013 Jun;75(Suppl 1):425–427. doi: 10.1007/s12262-012-0753-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lardière-Deguelte S, Ragot E, Amroun K, Piardi T, Dokmak S, Bruno O, et al. Hepatic abscess: Diagnosis and management. J Visc Surg. 2015;152(4):231–243. doi: 10.1016/j.jviscsurg.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 35.Biava MF, Dao A, Fortier B. Laboratory diagnosis of cystic hydatic disease. World J Surg. 2001;25(1):10–14. doi: 10.1007/s002680020002. [DOI] [PubMed] [Google Scholar]
- 36.Rosado E, Cabral P, Campo M, Tavares A. Mesenchymal hamartoma of the liver--a case report and literature review. J Radiol Case Rep. 2013;7(5):35–43. doi: 10.3941/jrcr.v7i5.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Micković S, Bezmarević M, Nikolić-Micković I, Mitrović M, Tufegdzić I, Mirković D, et al. Traumatic mesenteric pseudocyst. Vojnosanit Pregl. 2014;71(7):685–688. doi: 10.2298/vsp121116029m. [DOI] [PubMed] [Google Scholar]
- 38.Martin RF. Biliary cysts: a review and simplified classification scheme. Surg Clin North Am. 2014;94(2):219–232. doi: 10.1016/j.suc.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 39.Atkinson JJ, Davenport M. Controversies in choledochal malformation. S Afr Med J. 2014;104(11 Pt 2):816–819. doi: 10.7196/samj.8633. [DOI] [PubMed] [Google Scholar]
- 40.Gezer HÖ, Oğuzkurt P, Temiz A, İnce E, Ezer SS, Hiçsönmez A. Choledochal cysts in children: Intrahepatic ductal dilatation does not indicate true intrahepatic biliary duct disease. Turk J Gastroenterol. 2016;27(1):23–29. doi: 10.5152/tjg.2015.150211. [DOI] [PubMed] [Google Scholar]
- 41.Niramis R, Narumitsuthon R, Watanatittan S, Anuntkosol M, Buranakitjaroen V, Tongsin A, et al. Clinical differences between choledochal cysts in infancy and childhood: an analysis of 160 patients. J Med Assoc Thai. 2014;97(Suppl 11):S122–S128. [PubMed] [Google Scholar]
- 42.Ingle SB, Hinge Ingle CR, Patrike S. Epithelial cysts of the spleen: a minireview. World J Gastroenterol. 2014;20(38):13899–13903. doi: 10.3748/wjg.v20.i38.13899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dudeja V, Allen PJ. Premalignant cystic neoplasms of the pancreas. Semin Oncol. 2015;42(1):70–85. doi: 10.1053/j.seminoncol.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 44.Al Efishat M, Allen PJ. Therapeutic Approach to Cystic Neoplasms of the Pancreas. Surg Oncol Clin N Am. 2016;25(2):351–361. doi: 10.1016/j.soc.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ranganath SH, Lee EY, Eisenberg RL. Focal cystic abdominal masses in pediatric patients. Am J Roentgenol. 2012 Jul;199(1):W1–16. doi: 10.2214/AJR.11.6642. [DOI] [PubMed] [Google Scholar]
- 46.Wedmid A, Palese M. Diagnosis and treatment of the adrenal cyst. Curr Urol Rep. 2010 Feb;11(1):44–50. doi: 10.1007/s11934-009-0080-1. [DOI] [PubMed] [Google Scholar]
- 47.Karmazyn B, Tawadros A, Delaney LR, Marine MB, Cain MP, Rink RC, et al. Ultrasound classification of solitary renal cysts in children. J Pediatr Urol. 2015 Jun;11(3):149.e1–e6. doi: 10.1016/j.jpurol.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 48.Marietti S, Woldrich J, Durbin J, Sparks S, Kaplan G, Chiang G. Urologic findings on computed tomography of the abdomen and pelvis in a pediatric population. J Pediatr Urol. 2013;9(5):609–612. doi: 10.1016/j.jpurol.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 49.Reimann DL, Guyton WL. Cysts of the Adrenal Gland with Case Report. Am J Pathol. 1947;23(3):479–483. [PMC free article] [PubMed] [Google Scholar]
- 50.Armitage HV. Benign cyst of the adrenal gland. AMA Arch Surg. 1959;79(1):148–151. doi: 10.1001/archsurg.1959.04320070152026. [DOI] [PubMed] [Google Scholar]
- 51.Stock FE. Cysts of the adrenal gland. Postgrad Med J. 1947;23(265):530–533. doi: 10.1136/pgmj.23.265.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moore FP, 2nd, Cermak EG. Adrenal cysts and adrenal insufficiency in an infant with fatal termination. J Pediatr. 1950;36(1):91–95. doi: 10.1016/s0022-3476(50)80184-1. [DOI] [PubMed] [Google Scholar]
- 53.Blumenthal HT, Probstein JG. Adrenal cyst with extensive intracystic hemorrhage; observations on criteria for clinical and pathological diagnosis. AMA Arch Surg. 1956;73(6):1026–1030. doi: 10.1001/archsurg.1956.01280060126028. [DOI] [PubMed] [Google Scholar]
- 54.Ricci Z, Chernyak V, Hsu K, Mazzariol FS, Flusberg M, Oh S, et al. Adrenal cysts: natural history by long-term imaging follow-up. Am J Roentgenol. 2013;201(5):1009–1016. doi: 10.2214/AJR.12.9202. [DOI] [PubMed] [Google Scholar]
- 55.Kirks DR, Merten DF, Grossman H, Bowie JD. Diagnostic imaging of pediatric abdominal masses: an overview. Radiol Clin North Am. 1981;19(3):527–545. [PubMed] [Google Scholar]
- 56.Khan MR, Ajmal S, Saleem T. Giant adrenal endothelial cyst associated with acute and chronic morbidity in a young female: a case report. Cases J. 2009 Sep 9;2:8841. doi: 10.4076/1757-1626-2-8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Michalopoulos N, Laskou S, Karayannopoulou G, Pavlidis L, Kanellos I. Adrenal Gland Lymphangiomas. Indian J Surg. 2015 Dec;77(Suppl 3):1334–42. doi: 10.1007/s12262-015-1206-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lykoudis PM, Nastos C, Dellaportas D, Kairi-Vassilatou E, Dastamani C, Kondi-Pafiti A. Uncommon benign lesions of the adrenal glands mimicking sinister pathologies: report of 8 cases. J BUON. 2015;20(6):1630–1634. [PubMed] [Google Scholar]
- 59.Khilnani GC, Kumar A, Bammigatti C, Sharma R, Gupta SD. Hemorrhagic pseudocyst of the adrenal gland causing acute abdominal pain. J Assoc Physicians India. 2008;56:379–380. [PubMed] [Google Scholar]
- 60.Habeb AM, Zulali MA, Yamani AS, Yassine SM. Neonatal adrenal hematoma with urinary tract infection: risk factor or a chance association? Saudi J Kidney Dis Transpl. 2014;25(2):376–380. doi: 10.4103/1319-2442.128552. [DOI] [PubMed] [Google Scholar]
- 61.Kawashima A, Sandler CM, Ernst RD, Takahashi N, Roubidoux MA, Goldman SM, et al. Imaging of nontraumatic hemorrhage of the adrenal gland. Radiographics. 1999;19(4):949–963. doi: 10.1148/radiographics.19.4.g99jl13949. [DOI] [PubMed] [Google Scholar]
- 62.Roupakias S, Papoutsakis M, Mitsakou P. Blunt adrenal gland trauma in the pediatric population. Asian J Surg. 2011;34(3):103–110. doi: 10.1016/j.asjsur.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 63.Poiana C, Carsote M, Chirita C, Terzea D, Paun S, Beuran M. Giant adrenal cyst: case study. J Med Life. 2010 Jul-Sep;3(3):308–313. [PMC free article] [PubMed] [Google Scholar]
- 64.Lecompte JF, Hery G, Potier A, Gorincour G, Giudicelli B, Philip N, et al. Putative criteria for predicting spontaneous regression of prenatally diagnosed thoracoabdominal cystic lesions. Eur J Pediatr Surg. 2014;24(5):426–429. doi: 10.1055/s-0033-1353492. [DOI] [PubMed] [Google Scholar]
- 65.Saadai P, Arora S, Greenstein AJ, Lewis M, Divino CM, Prinz RA, et al. The pathological features of surgically managed adrenal cysts: a 15-year retrospective review. Am Surg. 2013 Nov;79(11):1159–1162. [PubMed] [Google Scholar]
- 66.Mishra A, Ehtuish EF. Hydatid cyst--an unusual presentation. Saudi Med J. 2006;27(6):892–893. [PubMed] [Google Scholar]
- 67.Di Cataldo A, Trombatore G, Greco R, Lanteri R, Li Destri G, Licata A. Hydatid disease in a very unusual location: the adrenal gland. A case report. Chir Ital. 2003;55(2):275–278. [PubMed] [Google Scholar]
- 68.Hammond NA, Lostumbo A, Adam SZ, Remer EM, Nikolaidis P, Yaghmai V, et al. Imaging of adrenal and renal hemorrhage. Abdom Imaging. 2015;40(7):2747–2760. doi: 10.1007/s00261-015-0453-5. [DOI] [PubMed] [Google Scholar]
- 69.Paterson A. Adrenal pathology in childhood: a spectrum of disease. Eur Radiol. 2002;12(10):2491–2508. doi: 10.1007/s00330-002-1311-8. [DOI] [PubMed] [Google Scholar]
- 70.Li Y, Zhong Z, Zhao X. Primary mature teratoma presenting as an adrenal tumor in a child. Urology. 2011;78(3):689–691. doi: 10.1016/j.urology.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 71.Andreoni C, Krebs RK, Bruna PC, Goldman SM, Kater CE, Alves MT, et al. Cystic pheochromocytoma is a distinctive subgroup with special clinical, imaging and histological features that might mislead the diagnosis. BJU Int. 2008;101(3):345–350. doi: 10.1111/j.1464-410X.2007.07370.x. [DOI] [PubMed] [Google Scholar]
- 72.Catli G, Abaci A, Neumann HC, Altincik A, Demir K, Böber E. Bilateral pheochromocytoma as first manifestation of von Hippel-Lindau disease: a case report. Turk J Pediatr. 2012;54(5):532–535. [PubMed] [Google Scholar]
- 73.Richard S, Beigelman C, Duclos JM, Fendler JP, Plauchu H, Plouin PF, et al. Pheochromocytoma as the first manifestation of von Hippel-Lindau disease. Surgery. 1994;116(6):1076–1081. [PubMed] [Google Scholar]
- 74.Oguzkurt P, Ince E, Temiz A, Demir S, Akabolat F, Hicsonmez A. Prenatal diagnosis of a mass in the adrenal region that proved to be a teratoma. J Pediatr Hematol Oncol. 2009;31(5):350–351. doi: 10.1097/MPH.0b013e318190d765. [DOI] [PubMed] [Google Scholar]
- 75.Nadeem M, Ather MH, Sulaiman MN, Pervez S. “Looks Can Be Deceiving”: Adrenal Teratoma Causing Diagnostic Difficulty. Case Rep Urol”. 2015;2015:232591. doi: 10.1155/2015/232591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Holschbach V, Lattrich CR, Ortmann O, Müller AM, Germer U. Upper abdominal cysts in first trimester fetuses. Ultraschall Med. 2012;33(7):E372–E373. doi: 10.1055/s-0031-1299316. [DOI] [PubMed] [Google Scholar]
- 77.Chodisetti S, Boddepalli Y, Kota M. Giant adrenal cyst displacing the right kidney. Indian J Urol. 2016 Jan-Mar;32(1):81–82. doi: 10.4103/0970-1591.173119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arena F, Romeo C, Manganaro A, Centorrino A, Basile M, Arena S, et al. Bilateral neonatal adrenal abscess. Report of two cases and review of the literature. Pediatr Med Chir. 2003;25(3):185–189. [PubMed] [Google Scholar]
- 79.Zhou F, Xu Y, Zhang Z, Wu X, Jin R. Severe Hemorrhage in Chinese Children With Immune Thrombocytopenia. J Pediatr Hematol Oncol. 2015;37(3):e158–e161. doi: 10.1097/MPH.0000000000000292. [DOI] [PubMed] [Google Scholar]
- 80.Medeiros D, Buchanan GR. Major hemorrhage in children with idiopathic thrombocytopenic purpura: immediate response to therapy and long-term outcome. J Pediatr. 1998;133(3):334–339. doi: 10.1016/s0022-3476(98)70265-3. [DOI] [PubMed] [Google Scholar]
- 81.Ramon I, Mathian A, Bachelot A, Hervier B, Haroche J, Boutin-Le Thi Huong D, et al. Primary adrenal insufficiency due to bilateral adrenal hemorrhage-adrenal infarction in the antiphospholipid syndrome: long-term outcome of 16 patients. J Clin Endocrinol Metab. 2013;98(8):3179–3189. doi: 10.1210/jc.2012-4300. [DOI] [PubMed] [Google Scholar]
- 82.Sepulveda W, Dickens K, Casasbuenas A, Gutierrez J, Dezerega V. Fetal abdominal cysts in the first trimester: prenatal detection and clinical significance. Ultrasound Obstet Gynecol. 2008;32(7):860–864. doi: 10.1002/uog.6142. [DOI] [PubMed] [Google Scholar]
- 83.Sherwood W, Boyd P, Lakhoo K. Postnatal outcome of antenatally diagnosed intra-abdominal cysts. Pediatr Surg Int. 2008;24(7):763–765. doi: 10.1007/s00383-008-2148-2. [DOI] [PubMed] [Google Scholar]
- 84.Schrauder MG, Hammersen G, Siemer J, Goecke TW, Meurer B, Hart N, et al. Fetal adrenal hemorrhage--two-dimensional and three-dimensional imaging. Fetal Diagn Ther. 2008;23(1):72–75. doi: 10.1159/000109230. [DOI] [PubMed] [Google Scholar]
- 85.Improda N, Capalbo D, Di Mase R, De Martino L, Coppola A, Salerno M. Acute adrenal insufficiency in a neonate with bilateral adrenal hemorrhage and combined prothrombotic risk factors. J Endocrinol Invest. 2012 Apr;35(4):449. doi: 10.1007/BF03345432. [DOI] [PubMed] [Google Scholar]
- 86.Khuri FJ, Alton DJ, Hardy BE, Cook GT, Churchill BM. Adrenal hemorrhage in neonates: report of 5 cases and review of the literature. J Urol. 1980;124(5):684–687. doi: 10.1016/S0022-5347(17)55609-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mutlu M, Karagüzel G, Aslan Y, Cansu A, Okten A. Adrenal hemorrhage in newborns: a retrospective study. World J Pediatr. 2011;7(4):355–357. doi: 10.1007/s12519-011-0259-7. [DOI] [PubMed] [Google Scholar]
- 88.Demirel N, Baş AY, Zenciroğlu A, Taşci-Yildiz Y. Adrenal bleeding in neonates: report of 37 cases. Turk J Pediatr. 2011;53(1):43–47. [PubMed] [Google Scholar]
- 89.Gocmen R, Basaran C, Karcaaltincaba M, Cinar A, Yurdakok M, Akata D, et al. Bilateral hemorrhagic adrenal cysts in an incomplete form of Beckwith-Wiedemann syndrome: MRI and prenatal US findings. Abdom Imaging. 2005;30(6):786–789. doi: 10.1007/s00261-005-0337-1. [DOI] [PubMed] [Google Scholar]
- 90.Taide DV, Bendre PS, Redkar R, Hambarde S. Adrenal masses associated with Beckwith Wiedemann syndrome in the newborn. Afr J Pediatr Surg. 2010;7(3):209–210. doi: 10.4103/0189-6725.70431. [DOI] [PubMed] [Google Scholar]
- 91.Ogundiran TO, Aghahowa ME, Brown BJ, Irabor DO. Beckwith-Wiedemann Syndrome (BWS): a case report and literature review. West Afr J Med. 2003;22(1):101–102. [PubMed] [Google Scholar]
- 92.Wright JE, Bear JW. Adrenal hemorrhage presenting as an abdominal mass in the newborn. Aust Pediatr J. 1987;23(5):305–307. doi: 10.1111/j.1440-1754.1987.tb00278.x. [DOI] [PubMed] [Google Scholar]
- 93.Gali S, Anat I. Purely cystic adrenal lesion in a newborn evolving into a solid neuroblastoma. J Clin Ultrasound. 2015;43(2):126–128. doi: 10.1002/jcu.22193. [DOI] [PubMed] [Google Scholar]
- 94.Wang CH, Chen SJ, Yang LY, Tang RB. Neonatal adrenal hemorrhage presenting as a multiloculated cystic mass. J Chin Med Assoc. 2008;71(9):481–484. doi: 10.1016/S1726-4901(08)70153-9. [DOI] [PubMed] [Google Scholar]
- 95.Avanzini S, Conte M, Granata C, Zamorani EM, Sementa AR, Garaventa A, et al. Life-threatening bilateral adrenal cystic neuroblastoma in an infant. J Pediatr Hematol Oncol. 2009;31(12):963–964. doi: 10.1097/MPH.0b013e3181b79641. [DOI] [PubMed] [Google Scholar]
- 96.Kenney PJ, Stanley RJ. Calcified adrenal masses. Urol Radiol. 1987;9(1):9–15. doi: 10.1007/BF02932620. [DOI] [PubMed] [Google Scholar]
- 97.Masiakos PT, Gerstle JT, Cheang T, Viero S, Kim PC, Wales P. Is surgery necessary for incidentally discovered adrenal masses in children? J Pediatr Surg. 2004;39(5):754–758. doi: 10.1016/j.jpedsurg.2004.01.039. [DOI] [PubMed] [Google Scholar]
- 98.Deeb SA, Rosenberg RB, Wilkerson RJ, Griswold JA. Adrenal hemorrhage in a pediatric burn patient. Burns. 2001;27(6):658–661. doi: 10.1016/s0305-4179(00)00163-7. [DOI] [PubMed] [Google Scholar]
- 99.Hubertus J, Pohl A, Schmid I, von Schweinitz D. Laparoscopic Adrenalectomy is Feasible for Suspected Adrenal Tumors in Children Younger than 24 Months of Age - But is it Always Justified? Klin Padiatr. 228(3):135–138. doi: 10.1055/s-0042-101030. 201. [DOI] [PubMed] [Google Scholar]
- 100.Kadamba P, Habib Z, Rossi L. Experience with laparoscopic adrenalectomy in children. J Pediatr Surg. 2004;39(5):764–767. doi: 10.1016/j.jpedsurg.2004.01.043. [DOI] [PubMed] [Google Scholar]
- 101.El-Hefnawy AS, El Garba M, Osman Y, Eraky I, El Mekresh M, Ibrahim el-H. Surgical management of adrenal cysts: single-institution experience. BJU Int. 2009;104(6):847–850. doi: 10.1111/j.1464-410X.2009.08537.x. [DOI] [PubMed] [Google Scholar]
- 102.da Silva EC, Viamontez F, Silva VS, Andrade A, Júlio Neto G, Gomes Cde P, et al. Hemorrhagic adrenal cyst. Einstein (Sao Paulo) 2012;10(1):96–99. doi: 10.1590/s1679-45082012000100020. [DOI] [PubMed] [Google Scholar]
- 103.Nerli RB, Reddy MN, Guntaka A, Patil S, Hiremath M. Laparoscopic adrenalectomy for adrenal masses in children. J Pediatr Urol. 2011;7(2):182–186. doi: 10.1016/j.jpurol.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 104.Mattioli G, Avanzini S, Pini Prato A, Pio L, Granata C, Garaventa A, et al. Laparoscopic resection of adrenal neuroblastoma without image-defined risk factors: a prospective study on 21 consecutive pediatric patients. Pediatr Surg Int. 2014;30(4):387–394. doi: 10.1007/s00383-014-3476-z. [DOI] [PubMed] [Google Scholar]
- 105.Moon SB, Shin HB, Seo JM, Lee SK. Clinical features and surgical outcome of a suprarenal mass detected before birth. Pediatr Surg Int. 2010;26(3):241–246. doi: 10.1007/s00383-009-2531-7. [DOI] [PubMed] [Google Scholar]
- 106.Yao W, Li K, Xiao X, Zheng S, Chen L. Neonatal suprarenal mass: differential diagnosis and treatment. J Cancer Res Clin Oncol. 2013;139(2):281–286. doi: 10.1007/s00432-012-1316-x. [DOI] [PubMed] [Google Scholar]