Abstract
Plasma membrane monoamine transporter (PMAT) is a new polyspecific organic cation transporter that transports a variety of biogenic amines and xenobiotic cations. Highly expressed in the brain, PMAT represents a major uptake2 transporter for monoamine neurotransmitters. At the blood–cerebrospinal fluid (CSF) barrier, PMAT is the principal organic cation transporter for removing neurotoxins and drugs from the CSF. Here I summarize our latest understanding of PMAT and its roles in monoamine uptake and xenobiotic disposition.
ORGANIC CATION TRANSPORT AND THE DISCOVERY OF PMAT
Numerous endogenous compounds (e.g., biogenic amines) and xenobiotics (e.g., drugs, environmental toxins) carry a primary, secondary, or tertiary amine moiety that is protonated at physiological pH. Compounds containing a quaternary ammonium entity are permanently charged cations. These molecules are collectively termed organic cations (OCs). OCs are diverse in chemical structures and display a wide spectrum of physiological, pharmacological, and toxicological activities in vitro and in vivo. Due to their charge and hydrophilicity, many OCs cannot readily cross cell membranes by passive diffusion. To facilitate OC entry to or exit from cells, mammalian cells have evolved complex OC transport systems, including the classic organic cation transporters 1–3 (OCT1-3) from the solute carrier 22 (SLC22) family and the multidrug and toxin extrusion proteins 1 and 2-K (MATE1/2-K) encoded by the SLC47 family.1,2 OCT1-3 mediate Na+-independent, electrogenic OC uptake, and utilize the inside-negative membrane potential as a driving force. MATE1/2-K, on the other hand, function as proton/OC exchangers that couple OC efflux with a physiologic inwardly directed proton gradient. In excretory organs such as the kidney and liver, OCTs and MATEs are respectively expressed at the basolateral and apical membranes of the secretory epithelium to sequentially mediate transepithelial secretion of OCs into the urine or bile.1,2 OCTs and MATEs are considered “polyspecific” or “multispecific,” as they interact with a wide array of cationic compounds with diverse chemical structures. A few neutral compounds (e.g., steroid hormones) also interact with the OCTs.1
In 2004 we reported the cloning and functional characterization of a novel brain monoamine transporter—PMAT.3 PMAT (SLC29A4) was first identified from public genomic databases as the fourth member in the mammalian SLC29 gene family, which encodes equilibrative nucleoside transporters (ENTs) that are molecularly and functionally distinct from the OCTs.4 Except PMAT, all other members of the SLC29 family, namely, ENT1-3, function as nucleoside transporters that specifically transport purine and pyrimidine nucleosides (e.g., uridine, adenosine, cytidine) and their structural analogs (Table 1). ENT1 and 2 are classic nucleoside transporters that play important roles in cellular uptake of physiologic nucleosides and therapeutic nucleoside analogs (e.g., cytarabine, fludarabine).5,6 ENT3 is an intracellular transporter crucial for lysosomal and mitochondrial nucleoside transport.7,8 Alternatively named ENT4, PMAT was initially hypothesized to transport nucleosides or related compounds. However, a series of studies carried out in our laboratory demonstrated that other than a moderate activity for adenosine, PMAT does not interact with nucleosides, nucleobases, or nucleotides.3,9,10 Instead, it robustly transports serotonin (or 5-hydroxytrptamine, 5-HT), dopamine (DA), and other monoamine neurotransmitters. We thus named the transporter the “plasma membrane monoamine transporter (PMAT)” to reflect its cellular localization and physiologic substrate profile.3 We subsequently found that, besides monoamine neurotransmitters, PMAT also transports a variety of structurally diverse OCs and shares a striking functional similarity to the OCTs.9 The discovery of PMAT as a previously unknown monoamine and OC transporter has since introduced a new player to the arenas of monoamine physiology as well as disposition of cationic drugs and toxins.
Table 1.
Members of the human SLC29 family
| SLC29A1 | SLC29A2 | SLC29A3 | SLC29A4 | |
|---|---|---|---|---|
| Gene locus | 6p21.1 | 11q13 | 10q22.1 | 7p22.1 |
| Encoded protein (a.a. length) | hENT1 (456 a.a.) | hENT2 (456 a.a) | hENT3 (475 a.a.) | PMAT or ENT4 (530 a.a) |
| Subcellular location | plasma membrane | plasma membrane | intracellular (lysosomes, mitochondria) | plasma membrane |
| Transport mode | facilitated carrier electroneutral | facilitated carrier electroneutral | not determined pH-sensitive | electrogenic pH-sensitive |
| Substrate | nucleosides and related analogs | nucleosides and related analogs | nucleosides and related analogs | monoamines, organic cations (e.g., MPP+, metformin), adenosine |
MOLECULAR AND FUNCTIONAL CHARACTERISTICS OF PMAT
Molecular features
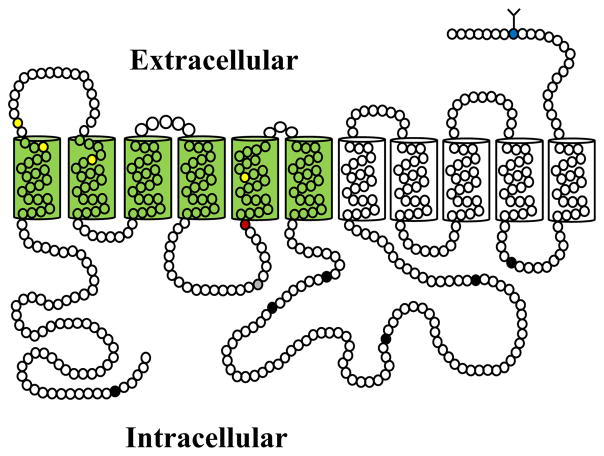
The full-length human PMAT cDNA was first cloned from a human kidney cDNA library.3 Subsequently, cDNAs encoding the rodent homologs were also isolated from the mouse and rat.11,12 The human PMAT cDNA encodes a membrane protein of 530 amino acid residues with a predicted molecular mass of 58 kDa. Human PMAT protein is predicted to possess 11 membrane-spanning domains with a long intracellular N-terminus and a short extracellular C-terminus (Figure 1). There is one potential N-linked glycosylation site (Asn-523) and several putative protein kinase C and cAMP-dependent kinase phosphorylation sites. Mouse and rat Pmat proteins share 86–87% sequence identity with human PMAT and are predicted to possess a similar membrane topology.
Figure 1.
Proposed topology of human PMAT depicting 11 transmembrane domains. Circles represent individual amino acid residues. Blue circle with branched lines indicates the potential N-linked glycosylation site. PMAT has six consensus sites for protein kinase C phosphorylation (filled black circles) and one cAMP-dependent protein kinase phosphorylation site (filled gray circle). TM1-6 important for substrate recognition are shaded in green. Residues involved in charge recognition (E206, red) and substrate interaction (Y85, Y112, I89, T220; yellow) are indicated.
Tissue distribution and subcellular localization
Northern blot, real-time reverse-transcription polymerase chain reaction (RT-PCR), and multiple tissue expression array analyses showed that PMAT mRNA is expressed in multiple human tissues including brain, heart, small intestine, pancreas, kidney, skeletal muscle, and liver.3,13,14 Among these analyzed tissues, the brain consistently showed the highest expression of PMAT. In the human brain, PMAT transcripts are broadly distributed in many brain regions, with high levels of expression found in cerebral cortex, hippocampus, substantia nigra, medulla oblongata, cerebellum, and choroid plexus.3,14,15 Immunoblotting and immunostaining studies with specific antibodies have confirmed the expression of PMAT protein in human choroid plexus, cerebellum, intestine, kidney, and heart.11,13,15–17 Similar to human PMAT, mouse and rat Pmats were also found in multiple tissues, with brain being a major site of expression.11,18 In the mouse brain, Pmat mRNA and protein are widely distributed in numerous anatomical areas, with high levels of expression found in fore-brain cortex, olfactory tubercle, dentate gyrus of the hippocampus, cerebellum, and epithelial cells of the choroid plexus.11 There may be some difference in PMAT expression between species. For instance, PMAT/Pmat mRNA and protein are expressed in human and rat kidneys16; but mouse kidney appears to lack significant expression of Pmat.11,16 Interestingly, in human and rat kidneys PMAT/Pmat proteins were not found in renal tubular cells but were predominantly localized to glomerular podocytes, which are highly specialized visceral epithelial cells crucial for maintaining the integrity of the glomerular filtration barrier.16
When heterologously expressed in Madin-Darby Canine Kidney (MDCK) and Human Embronic Kidney (HEK) cells, PMAT protein is localized to the cell surface membrane, gaining its name as a plasma membrane transporter.3,14 In polarized MDCK monolayer culture, PMAT is specifically targeted to the apical membrane.19 Consistent with these findings, endogenous PMAT/Pmat proteins have been localized to the apical membranes of human and mouse choroid plexus epithelial cells.15 In human intestine, PMAT protein was found to concentrate on the apical surface of the mucosal epithelial layer, although punctate intracellular staining was also observed.17 Similarly, in rat primary cardiomyocytes both cell surface and punctate intracellular staining were overserved.13 The nature and functional significance of the intracellularly expressed PMAT in these cells are currently unknown.
Substrate and inhibitor specificity
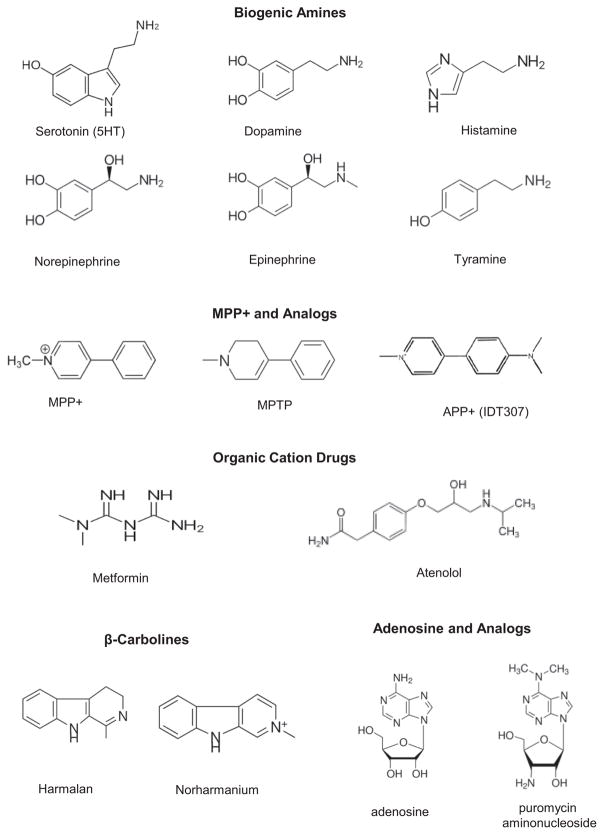
Numerous OCs with diverse chemical structures interact with PMAT either as substrates or as inhibitors.9,20 Most PMAT substrates identified to date are small and polar OCs (i.e., type I cations), which include biogenic amines, cationic drugs, and neurotoxins (Figure 2). While many PMAT OC substrates contain one or more aromatic rings, aliphatic OCs, such as tetraethylammonium (TEA) and metformin, are also transported by PMAT.9,17 Most PMAT substrates identified to date are also known substrates of the OCTs. For example, 1-methyl-4-phenylpyridinium (MPP+) and metformin, two prototype OCT substrates, are efficiently transported by PMAT with apparent binding affinities (Km) similar to those of the OCTs.3,17 The monoamine neurotransmitters, which are endogenous PMAT substrates, are all known to be transported by OCT1-3.21 Nevertheless, there are notable differences in substrate specificity and transport kinetics among these transporters. For instance, TEA is a very good substrate for OCT1 and OCT2, but only a moderate substrate for OCT3 and PMAT.9,21 In Flp-in HEK293 cells expressing human PMAT and OCT3 at comparable levels, PMAT and OCT3 showed substantial kinetic differences towards the monoamine neurotransmitters.14 PMAT showed a strong kinetic preference for 5-HT and DA over other mono-amines (i.e., histamine, norepinephrine, and epinephrine), whereas a seemingly opposite preference was observed for OCT3. Another interesting feature is that while PMAT does not typically interact with nucleosides or nucleoside analogs, it does exhibit a moderate transport activity towards the purine nucleoside adenosine.10,13 Nevertheless, under both physiologic (pH 7.4) and acidic (pH 6.6) conditions, PMAT demonstrated a strong preference for OC substrates over adenosine, only transporting adenosine at an efficiency (defined as Vmax/Km) less than one-tenth of that of MPP+ or 5-HT.10
Figure 2.
Structures of selected substrates of PMAT.
Many bulkier and more hydrophobic cations (i.e., type II cations) interact with PMAT as inhibitors. A range of known OCT inhibitors have been tested for their interactions with PMAT, and most of them cross-inhibited PMAT.9 Several compounds, including decynium-22 (D22), quinidine, and rhodamine 123 potently inhibited PMAT, but none of them are selective toward PMAT. For example, D22, a cation derivative of quinoline, inhibits PMAT and OCT3 with equal potency (Ki ~0.1 μM); and D22 also inhibits OCT1 and 2 at low micromolar concentrations (Table 2).9,22 Among the tested OCT inhibitors, corticosterone, an uncharged steroid hormone, showed more selectivity toward the OCTs over PMAT. While corticosterone inhibits human OCT3 with greater potency than OCT1 and OCT2, it is much less potent for PMAT than for OCT1-3 (Table 2).9,22 Due to the lack of a PMAT-specific inhibitor, sensitivity to D22 but not to corticosterone has been used as an indirect approach in many cell and tissue studies to discern PMAT activity from those of the OCTs.14,23–26 Recently, we identified the fluorescent MPP+ analog, IDT307, as a transportable substrate for PMAT and developed a fluorescence assay for rapid identification and characterization of PMAT inhibitors.27 Using this assay, we found that human PMAT and OCTs exhibit distinct sensitivity toward HIV protease inhibitors. PMAT is highly sensitive to HIV protease inhibitors, whereas OCT2 and 3 are resistant. OCT1 showed an intermediate sensitivity and a distinct inhibition profile from PMAT. Furthermore, we found that lopinavir is a potent and selective PMAT inhibitor, exhibiting more than 120-fold selectivity toward PMAT over OCT1, with no significant interaction with OCT2 and 3 (Table 2). Although lopinavir also interacts with other drug transporters (e.g., P-gp) and has limited permeability across the blood–brain barrier,28 it may represent a useful chemical tool to differentiate PMAT activity from those of OCTs in in vitro and ex vivo studies.
Table 2.
Ki or IC50 values (μM) of selected inhibitors of human PMAT and OCT1-3
| hPMAT | hOCT1 | hOCT2 | hOCT3 | |
|---|---|---|---|---|
| Decynium 22 (D22) | 0.10 ± 0.03a | 0.98 ± 0.31b | 1.13 ± 0.19b | 0.09 ± 0.01b |
| Corticosterone | 450.5 ± 76.5a | 21.7 ± 2.44b | 34.2 ± 6.47b | 0.29 ± 0.04b |
| Lopinavir | 1.4 ± 0.2c | 174 ± 40.1c | N.I.c | N.I.c |
Two studies have explored the structure–activity relationship of PMAT in interacting with OC substrates and inhibitors.9,20 A positively charged nitrogen atom and a hydrophobic mass are the two common features of PMAT substrates and inhibitors. A planar aromatic mass is associated with high-affinity interaction with PMAT.9 Using a series of phenylalkylamine analogs, the optimal distance between the positively charged nitrogen atom and the aromatic ring was determined to be in the range of 5.2–7.7 Å, which corresponds to a spacer chain length of 2–3 carbons.20 Using molecular modeling, several 3D pharmacophore models have been developed based on the analysis of known PMAT substrates and inhibitors.20 These models are characterized by a hydrogen bond donor, representing the positively charged nitrogen atom, and 2–3 hydrophobic features. The distance between the hydrogen bond donor and the hydrophobic features ranges between 5.20 and 7.02 Å, which is in good agreement with results obtained with the phenylalkylamine analogs. Not surprisingly, these PMAT pharmacophore models share similar features with those previously developed for the OCTs. The 2–3 hydrophobic features and their defined distance to the positively charged nitrogen center are molecular descriptors known to be important for high affinity interactions with the OCTs.29,30
Mechanism of transport
Effect of membrane potential
In virtually all animal cells, there is a baseline electric potential difference across the plasma membrane, which typically ranges from −40 to −90 mV. This inside-negative membrane potential, rising largely from the diffusional property of the K+ ion across the cell membrane, influences multiple cellular and physiological processes. It is used by the OCTs as a driving force to power cellular uptake of OCs, allowing these transporters to accumulate OCs at intracellular concentrations 10–15 times their extracellular concentrations.31 Similar to the OCTs, radiotracer uptake studies in PMAT-expressing MDCK cells or Xenopus laevis oocytes showed that PMAT-mediated OC transport is Na+- and Cl−-independent, but is sensitive to membrane potential.3,17,32 Depolarization of cell membranes, either by increasing extracellular K+ concentrations or pharmacological blockade of K+ channels, significantly reduced PMAT-mediated uptake of MPP+ and metformin.3,17 On the other hand, membrane hyperpolarization increased PMAT uptake activities. Two-microelectrode voltage-clamp studies in oocytes further showed that PMAT-mediated histamine uptake is associated with substrate-evoked, inwardly directed currents under voltage-clamp conditions.32 Substrate-induced currents were independent of Na+ but increased proportionally as the membrane potential became more negative. Detailed kinetic analysis revealed that this energizing effect of negative membrane potential on PMAT was due to an increase in maximal transport velocity, with little effect on apparent binding affinity.32 Together, these studies established that PMAT is an electrogenic transporter that utilizes the physiologic inside-negative membrane potential as a driving force to facilitate cellular uptake of OCs.
Effect of pH
Extracellular pH may influence carrier-mediated transport through multiple mechanisms. Protons may be directly used as coupling ions to drive substrate transport, as in the case of H+-coupled oligopeptide cotransporters (e.g., PepTs) or the H+/OC antiporters MATEs. Alternatively, protons may exert an effect on the folding or ionization state of the transporter protein, leading to a change in transport activity. Protons may also uniquely influence OC transport either by changing the degree of ionization of the substrate or by modulation of membrane potential. The activities of OCTs have been reported to be sensitive to extracellular pH.33–35 However, further analyses suggest that the observed pH effects were nonspecific and likely due to indirect effects of protons on substrate ionization or alteration of membrane potential.33,35
Different from the OCTs, protons appear to exert a specific stimulatory effect on PMAT-mediated transport. Barnes et al. first reported that PMAT-mediated adenosine uptake was simulated by an acidic extracellular pH, whereas 5-HT transport by PMAT was relatively insensitive to pH changes.13 We subsequently demonstrated that the stimulatory effect of acidic pH is not specific to adenosine, but occurs with all other PMAT substrates, such as MPP+, 5-HT, metformin, and histamine.10,17,19,32 Lowering extracellular pH from 7.4 to 6.6 stimulated PMAT-mediated uptake of by 3–4-fold, whereas elevating extracellular pH to 8.2 abolished transport activity. For PMAT-mediated MPP+ uptake, the Vmax increased by 4-fold at pH 6.6, whereas there was no change in Km.19 Because MPP+ is a permanently charged cation, this stimulatory effect of protons is unlikely due to an increase in substrate ionization. Similar effects of protons were observed in two-microelectrode voltage-clamp analysis.32 Histamine-induced currents exhibited great sensitivity to the pH of the perfusate. Consistent with results from radiotracer uptake studies, an acidic condition (pH 6.0) increased the maximal rate of histamine-induced current by 3–4-fold without affecting the apparent binding affinity.32 Because these studies were conducted under voltage-clamp conditions with fixed membrane holding potentials, the effect of protons is unlikely due to an effect on membrane potential. Instead, these data support a more specific effect of protons on PMAT, which may occur via a direct H+/OC coupling mechanism or through a proton-induced change in the intrinsic transport activity of PMAT. More studies are needed to definitively establish the role of protons in PMAT-mediated transport.
Structure–function relationship
The unique substrate selectivity of PMAT in the SLC29 family has brought up some intriguing questions: why does PMAT behave so differently from the ENTs? Is it structurally more related to the ENTs or to the OCTs? At the protein level, the sequence similarity of PMAT to the OCTs is insignificant. While human and rodent PMATs only exhibit an overall low sequence identity (~20%) to the ENTs, sequence identity between PMAT and ENTs increases significantly up to 35–40% in the transmembrane (TM) region. Both PMAT and ENTs are predicted to possess an 11-TM topology, whereas the OCTs are predicted to have 12 membrane-spanning domains.1,3,5 Moreover, PMAT appears to retain several features reminiscent of the ENTs. It transports the purine nucleoside adenosine and preserves low-affinity binding to the classic ENT inhibitors NBMPR, dipyridamole, and dilazep.10,36 Based on these observations, we hypothesized that PMAT maintains the overall protein architecture of the ENTs but has diverged from the ENT lineage to handle structurally diverse OCs via changes of key amino acid residues in its substrate binding pocket. Indeed, a chimeric transporter consisting of the N-terminal (TM1-6) half of PMAT and the C-terminal half (TM7-11) of ENT1 is functional and behaved like PMAT, transporting MPP+ but not uridine.37 These data suggest that, although the C-terminal half of PMAT may participate in the formation of the substrate permeation pathway, its major OC recognition sites are located within the N-terminal half (Figure 1), a region also known to be important for the ENTs in their interaction with nucleoside substrates. Within TM1-6, site-directed mutagenesis analyses further identified a number of key residues involved in substrate recognition and interaction.36–38 A negatively charged glutamate residue (E206) on TM5 is critical for the cation selectivity of PMAT.37 Replacement of this residue by charge reversal (E206R) or neutralization (E206Q) abolished OC transport activity, whereas conserving the negative charge (E206D) restored transporter function. Interestingly, mutant E206Q, which possessed the equivalent residue in ENT1, gained a transport activity for uridine,37 further supporting an intrinsic relationship between PMAT and the ENTs. Another residue in TM5, T220, also showed a direct influence on PMAT transport activity towards OCs. Helical wheel analysis revealed a distinct amphipathic pattern of residue distribution on TM5 with E206 and T220 clustered in the center of a hydrophilic face, suggesting a critical role of TM5 in forming part of the substrate permeation pathway.37 Several additional residues (Y85, Y112, I89) on TM1 and TM2 were also identified to be important for PMAT to interact with its substrates or inhibitors.36,38
ROLE IN MONOAMINE NEUROTRANSMITTER REGULATION
The main physiological substrates of PMAT are the bioactive amines including 5-HT, DA, norepinephrine, histamine, and epinephrine (Figure 2). Synthesized and released by specific mono-aminergic neurons and adrenal glands, these neurotransmitters and neurohormones regulate a myriad of physiological, endocrine, behavioral, and cognitive processes.39,40 5-HT is a key mediator of mood, vascular function, and gastrointestinal motility. DA plays an important role in higher-order brain functions such as cognitive control, arousal, reward, and motivation. Norepinephrine, also known as noradrenaline, is a main neurotransmitter of the sympathetic nervous system and a major mediator of the “fight-or-flight” response.41 Numerous drugs act on these monoamine signaling systems. Norepinephrine, DA, and epinephrine themselves are used as injectable drugs for the treatment of several clinical conditions such as critically low blood pressure, cardiac arrest, and anaphylaxis.
Cellular uptake of released monoamine neurotransmitters by membrane transporters is a major mechanism to inactivate these chemical messengers. By removing the transmitter from its receptor-binding site, transporter-mediated uptake determines the intensity and duration of the signaling process. Two distinct systems, termed uptake1 and uptake2, are responsible for cellular uptake of monoamine neurotransmitters.42,43 Uptake1 consists of highly specific neurotransmitter transporters from the SLC6 family and includes the serotonin transporter (SERT), the dopamine transporter (DAT), and the norepinephrine transporter (NET).44,45 Predominantly expressed on serotonergic, dopaminergic, or adrenergic neurons, SERT, DAT, and NET mediate Na+- and Cl−-dependent, high-affinity and low-capacity uptake of released neurotransmitters. These uptake1 transporters play a major role in clearing released monoamines from the synaptic cleft and are the targets for numerous clinically used antidepressants (e.g., selective serotonin reuptake inhibitors or SSRIs), psychostimulants (e.g., amphetamines, cocaine), and neurotoxins (e.g., MPP+).44,45 Different from uptake1, monoamine transport by uptake2 is Na+- and Cl−-independent, broadly selective, and of low-affinity and high-capacity.42,43 Originally found in sympathetically innervated tissues such as heart and smooth muscle cells, uptake2 was initially thought to facilitate monoamine uptake for their metabolism by intracellular monoamine oxidases and catechol-O-methyltransferase.46,47 Uptake2 activities are also present in the brain and these transporters are thought to play a backup role for uptake1 in brain monoamine clearance.3,35,42,43,48
The molecular identity of uptake2 monoamine transporters was unclear for a long time. In 1998, two groups reported the cloning of OCT3 as the extraneuronal monoamine transporter or uptake2.35,48 With the identification and functional characterization of PMAT, it is now increasingly being recognized that uptake2 consists of multiple OC transporters with broad mono-amine selectivity. While OCT1-3 and PMAT are all capable of mediating Na+- and Cl−-independent, low-affinity and high-capacity monoamine uptake, PMAT and OCT3 are likely to represent the most prominent uptake2 transporters due to their marked expression in the central nervous system (CNS) and sympathetically innervated tissues. In the brain, PMAT is broadly expressed in many areas that may or may not express uptake1. PMAT is predominantly found in neurons,11,18 although some studies also reported expression in astrocytes.49 OCT3 is also broadly expressed in multiple brain regions in both neural and astroglial cells.50,51 In human and rodent brains, the expression of PMAT in most tested brain regions is much higher than that of OCT3, suggesting a critical role of PMAT in monoamine regulation in the CNS.14 In contrast, OCT3 is highly expressed in adrenal glands and skeletal muscle, where norepinephrine and epinephrine release regulates several physiological responses. Interestingly, the tissue distribution of these two transporters appears to coincide with their preference for individual mono-amines.14 While PMAT and OCT3 both broadly transport biogenic amines, they exhibit significant kinetic differences in their transport efficiency towards the monoamine neurotransmitters. PMAT shows a strong preference for 5-HT and DA, two major centrally acting neurotransmitters. In contrast, OCT3 favors histamine, norepinephrine, and epinephrine, which act on both central and peripheral systems. Therefore, PMAT may represent the primary uptake2 transporter for 5-HT and DA in the CNS, whereas OCT3 is likely to be the major uptake2 transporter in histaminergic and adrenergic systems.
Analyses of PMAT interaction with a number of clinically used uptake1 inhibitors, including SSRIs and tricyclic antidepressants, showed that PMAT is generally resistant to uptake1 inhibitors at clinically used concentrations.3,52,53 Based on its strong brain expression and robust in vitro transport activity for mono-amines, we hypothesized that PMAT is responsible for clearing released neurotransmitters that have escaped neuronal reuptake by the uptake1 transporters.3,53 Furthermore, the transporter may play an active role in monoamine uptake in brain areas lacking significant expression of uptake1 or when uptake1 function is compromised by pharmacological inhibition (e.g., chronic use of SSRIs) or by genetic polymorphisms. To date, evidence supporting this hypothesis primarily came from pharmacological studies taking the advantage that PMAT is highly sensitive to D22, but resistant to uptake1 inhibitors (e.g., SSRIs) and the OCT inhibitor corticosterone. Using this approach, we found that PMAT may contribute up to 20–35% of total uptake of 5-HT and DA in mouse brain synaptosomes.14 In the nucleus tractus solitarii of anaesthetized rats, fast-cyclic voltammetry studies suggested that 5-HT clearance is under the regulation of the PMAT, but not SERT or OCT3.54 In cultured human astrocytes, which coexpress PMAT and OCT3 but not uptake1 transporters, pharmacological inhibition and gene knockdown studies suggested a major contribution of PMAT to monoamine transport.26,49 Despite these encouraging results, a direct link of PMAT with monoamine neurotransmission in vivo is still missing. With the recent development of a mouse knockout model of Pmat,15 detailed neurochemistry studies combined with comprehensive behavioral analysis in these animals may hold the key for further understanding the physiological and neurological function of PMAT in vivo.
In the CNS, dysregulation of monoamine neurotransmission is critically involved in a number of brain disorders, such as depression, autism, schizophrenia, Parkinson’s disease, and drug addiction. Although a recent study in patients with autism spectrum disorder with altered CSF levels of 5-HT suggested a potential association with rare mutations in the PMAT (SLC29A4) gene,55 little is currently known regarding the involvement of PMAT in monoamine-related brain disorders. If PMAT is proven to indeed regulate monoamine neurotransmission in vivo, there could be enormous clinical and therapeutic implications for this transporter. Up to 30–40% patients do not satisfactorily respond to currently marketed SSRIs, which act by specifically inhibiting SERT-mediated 5-HT uptake.56 Compensatory 5-HT uptake by PMAT may buffer the effects of these frontline antidepressants, contributing to clinical resistance to these drugs.42,53 If proven true, PMAT may represent a promising drug target for the development of more effective treatment for depression.
ROLE IN XENOBIOTIC CATION DISPOSITION
Besides endogenous OCs (e.g., biogenic amines), PMAT transports a broad spectrum of structurally diverse xenobiotic cations including clinically used drugs and environmental toxins (Figure 2).). To date, limited studies have been performed to comprehensively characterize the substrate profile of PMAT towards therapeutic drugs. However, given its large substrate overlap with the OCTs, it is reasonable to suspect that many of the OCT substrate drugs are likely to be PMAT substrates. Indeed, metformin, an oral antihyperglycemic and a prototype OCT substrate, is an excellent PMAT substrate.17 Unpublished data from our laboratory also showed that atenolol, a widely used β-blocker and a substrate of OCT1/2,57 is also a transportable substrate of PMAT. Besides therapeutic drugs, a number of MPP+-like neurotoxins, including certain β-carbolines and isoquinolines, have been shown to be transported by PMATs.20,58 Thus, PMAT may also play a role in the disposition of cationic drugs and toxins in epithelial barrier tissues such as the blood–CSF barrier and the intestinal barrier, where significant expression of PMAT has been demonstrated.
Role at the blood–CSF barrier
The mammalian brain is protected from circulating drugs, toxins, and blood-borne pathogens by the blood–brain barrier formed by the tightly sealed brain capillaries. However, in the lateral, third and fourth brain ventricles, the capillaries are fenestrated, shifting the barrier function to the blood–CSF barrier formed by the tightly jointed choroid plexus epithelial cells.59 Besides CSF production, the choroid plexus epithelial cells express numerous membrane transporters to actively remove metabolic waste, foreign substances, and excess neurotransmitters from the CSF.60,61 Earlier studies showed that choroid plexus is able to accumulate monoamine neurotransmitters and possesses uptake activities for xenobiotic OCs.62,63 This OC transport system may play a significant role in clearing monoamine neurotransmitters, cationic neurotoxins, drugs, and drug metabolites from the brain. Although OCTs have been postulated to play a role in OC transport at the blood–CSF barrier, there is essentially no expression or functional data to support their roles in OC transport in choroid plexus.
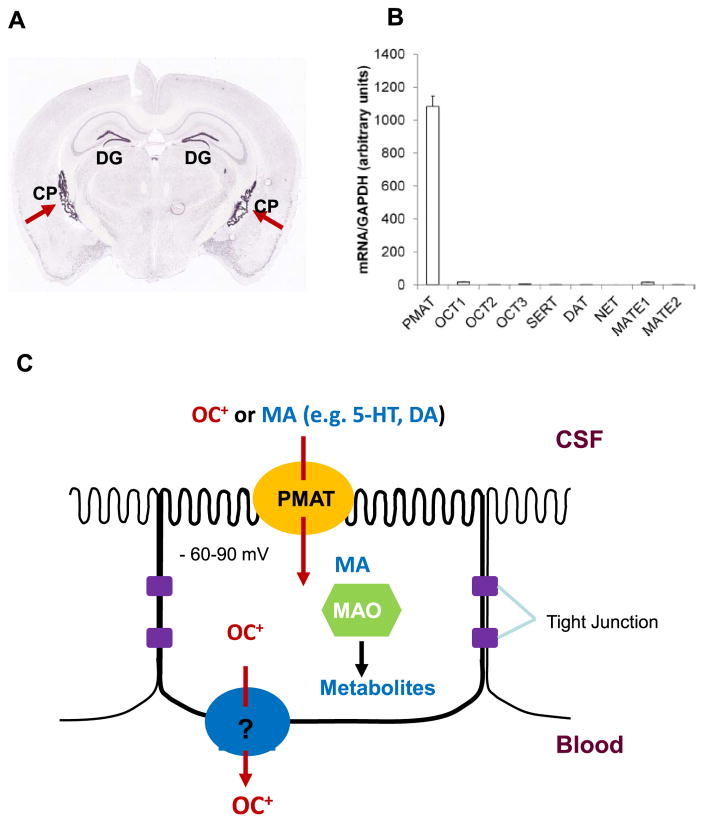
In situ hybridization and immunohistochemical studies in rodent brain revealed choroid plexus as one of the brain regions with highest Pmat mRNA and protein expression (Figure 3).11,18 Expression profiling analysis of in situ hybridization data from the Allen Brain Atlas further revealed that Pmat is among the top 10% highly expressed Slc genes in the mouse choroid plexus.60,64 Real-time PCR, western blot, and proteomic analyses also confirmed the abundant expression of PMAT mRNA and protein in human choroid plexus.15 In contrast, other OC and monoamine transporters, including OCT1-3, MATE1/2-K, and the uptake1 monoamine transporters (SERT, NET, DAT), are minimally expressed in human and mouse choroid plexuses (Figure 3).15 Immunolocalization studies further demonstrated that PMAT protein is localized to the apical CSF-facing membrane of the choroid plexus epithelium.15 These data strongly suggest a role of PMAT in transporting endogenous monoamines as well as xenobiotic OCs from the CSF into choroid plexus epithelial cells (Figure 3). Once inside the cells, monoamine neurotransmitters (e.g., 5-HT, DA) can be metabolized by monoamine oxidases or catechol-O-methyltransferase that are known to be expressed in the choroid plexus.65,66 Nonmetabolizable OCs may be further exported into the blood at the basolateral membrane by a mechanism yet to be clarified.
Figure 3.
(a) Distribution of Pmat mRNA in a coronal cryosection of mouse brain analyzed by in situ hybridization. Image was from the Allen Mouse Brain Atlas (http://mouse.brain-map.org/). Red arrows indicate choroid plexus (CP) in lateral ventricles. DG denotes dentate gyrus of the hippocampal formation. (b) Expression of PMAT and functionally related monoamine and organic cation transporters in human choroid plexus quantified by real-time PCR (data taken from Ref. 15). A similar expression pattern was observed in mouse choroid plexus.15 (c) A cellular model for monoamine and organic cation transport at the blood–CSF barrier. MA, monoamine; OC+, organic cation; MAO, monoamine oxidase; 5-HT, serotonin; DA, dopamine.
By conducting CSF clearance studies in anesthetized rats, Okura et al. first showed that [3H]MPP+ administered to the left ventricle was quickly eliminated from the CSF, likely through PMAT-mediated CSF-to-blood transport.12 However, nonselective inhibitors were used in that study. To further evaluate the role of PMAT in vivo, we generated a mouse model with targeted deletion of exons 3–7 of the murine Slc29a4 gene.15 This resulted in a defective mRNA transcript encoding a short peptide with no transport activity. While the Pmat knockout mice are viable, fertile with no overt physiological abnormalities, the uptake of MPP+, 5-HT, and DA was severely impaired in choroid plexus from the knockout mice.15 Furthermore, D22 had no effect on choroid plexus OC uptake in Pmat null mice but reduced the uptake in wildtype mice to the same level seen in knockout mice. In contrast, corticosterone, which strongly inhibits OCT1-3 but not PMAT (Table 2), had no effect on OC uptake in choroid plexus from both wildtype and knockout mice. Similarly, RTI-55, a potent inhibitor of uptake1 monoamine transporters, also showed no effect on 5-HT or DA uptake in choroid plexus.15 Together, these data demonstrated that PMAT is the principal OC uptake transporter at the blood–CSF barrier responsible for transporting bioactive amines and xenobiotic OCs from the CSF into choroid plexus. OCTs and uptake1 transporters, on the other hand, do not significantly contribute to OC and monoamine transport at the blood–CSF barrier.
Derived from the protoxin MPTP, the PMAT substrate MPP+ is a dopaminergic neurotoxin that produces Parkinson’s syndrome in humans and animal models. CNS exposure to environmental or endogenously produced MPP+-like toxins, such as paraquat, certain β-carboline and tetrahydroisoquinoline metabolites, has long been implicated in the etiology of Parkinson’s disease.67,68 Using cytotoxicity assays, we showed that two β-carbolines, harmalan and norharmanium, are transportable substrates of PMAT.20 HEK293 cells expressing PMAT are 14–15-fold more sensitive to the toxicity of harmalan and norharmanium. 1-Benzyl-1,2,3,4-tetrahydroisoquinoline, an endogenously produced neurotoxin with reportedly higher CSF levels in patients with Parkinson’s disease,67 was also reported to be a PMAT substrate.58 By removing MPP+-like cationic neurotoxins from the CSF and preventing brain accumulation of cationic neurotoxins, PMAT may play a protective role against Parkinson’s disease.
With regard to PMAT expression at the blood–brain barrier, conflicting data have been reported. While we and another group found no significant expression of Pmat in mouse brain endothelial cells,11,69 two other groups reported its expression in rat and mouse brain capillaries.12,58 The reason for this discrepancy is unclear, but likely due to the use of more sensitive PCR analysis, potential contamination of brain microvessels by other types of brain cells, or the use of nonspecific antibodies. Nevertheless, the expression of PMAT in brain endothelial cells is unlikely to be high, consistent with a low, nonsaturable transport of MPP+ at the blood–brain barrier as revealed by in situ carotid perfusion studies in mice.69
Role in metformin absorption
We previously showed that the 58 kDa PMAT protein is expressed in human small intestine and concentrated on the tips of the mucosal epithelial layer.17 PMAT robustly transports metformin and thus may be involved in intestinal absorption of this widely used antidiabetic drug.17 PMAT-mediated metformin transport can be further stimulated by an acidic pH, making it an attractive candidate for metformin absorption in the acidic environment of the gut lumen. However, currently there is no in vivo data to suggest an impact of PMAT on metformin pharmacokinetics. Two pharmacogenetics studies have examined the impact of single nucleotide polymorphisms (SNPs) of metformin transporters (OCT1, OCT2, OCT3, MATE1, and PMAT) on metformin pharmacokinetics in healthy subjects and diabetic patients treated with metformin.70,71 No significant association was found between PMAT SNPs and metformin pharmacokinetics and pharmacodynamics. However, these two studies used small sample size and also failed to detect an association with OCT2 and MATE1, which are known to be important determinants of metformin pharmacokinetics. Furthermore, the functional consequence of the studied PMAT SNPs is unknown, as no study has systematically characterized the PMAT SNPs and their allele frequencies in healthy and diabetic populations. Lastly, multiple metformin transporters, including OCT1 and OCT3, are expressed in the intestinal enterocytes, which may compensate and diminish the impact of PMAT.72 Clearly, more studies are necessary to clarify the role of PMAT in the disposition and clinical response of metformin and other OC drugs.
CONCLUSION
Molecular and biochemical work performed over the past decade has greatly advanced our understanding of PMAT from a previously unknown transporter to an important player in membrane transport of monoamine neurotransmitters and xenobiotic cations. We now know that PMAT is highly and broadly expressed in the brain, and represents a major brain uptake2 transporter for centrally acting monoamine neurotransmitters. By serving as a complementary clearance mechanism for released monoamine neuro-transmitters, PMAT may play a regulatory role in monoamine neurotransmission, and may thus likely be involved in a number of monoamine-related brain disorders. We now also know that PMAT is abundantly expressed at the blood–CSF barrier, specifically localized to the CSF-facing apical membrane, and facilitates OC uptake from the CSF. By doing so, PMAT may be an important determinant of brain exposure to cationic neurotoxins of both endogenous and environmental origins. Nevertheless, despite this progress, we are still at the beginning of appreciating the in vivo function and significance of this transporter in health and disease. Elucidating the roles of PMAT in monoamine neurotransmission and exploring its potential as a novel drug target are exciting research directions for this recently identified transporter. Understanding the clinical significance of PMAT in brain and tissue-specific disposition of OC drugs and toxins and exploring its linkage as a risk factor for Parkinson’s disease are equally important research areas for the next decades to come.
Acknowledgments
I thank all past and present members of the Wang laboratory for their hard work and contribution to our knowledge on PMAT. This work is supported by National Institutes of Health grant GM066233.
Footnotes
CONFLICT OF INTEREST
I have no conflicts of interest to declare.
References
- 1.Koepsell H, Lips K, Volk C. Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm Res. 2007;24:1227–1251. doi: 10.1007/s11095-007-9254-z. [DOI] [PubMed] [Google Scholar]
- 2.Morrissey KM, Stocker SL, Wittwer MB, Xu L, Giacomini KM. Renal transporters in drug development. Annu Rev Pharmacol Toxicol. 2013;53:503–529. doi: 10.1146/annurev-pharmtox-011112-140317. [DOI] [PubMed] [Google Scholar]
- 3.Engel K, Zhou M, Wang J. Identification and characterization of a novel monoamine transporter in the human brain. J Biol Chem. 2004;279:50042–50049. doi: 10.1074/jbc.M407913200. [DOI] [PubMed] [Google Scholar]
- 4.Acimovic Y, Coe IR. Molecular evolution of the equilibrative nucleoside transporter family: identification of novel family members in prokaryotes and eukaryotes. Mol Biol Evol. 2002;19:2199–2210. doi: 10.1093/oxfordjournals.molbev.a004044. [DOI] [PubMed] [Google Scholar]
- 5.Baldwin SA, et al. The equilibrative nucleoside transporter family, SLC29. Pflugers Arch. 2004;447:735–743. doi: 10.1007/s00424-003-1103-2. [DOI] [PubMed] [Google Scholar]
- 6.Ho HT, Wang J. The nucleoside transporters CNT and ENT. In: You G, Morris ME, editors. Drug Transporters: Molecular Characterization and Role in Drug Disposition. John Wiley & Sons; Hoboken, NJ: 2014. pp. 107–126. [Google Scholar]
- 7.Govindarajan R, et al. Facilitated mitochondrial import of antiviral and anticancer nucleoside drugs by human equilibrative nucleoside transporter-3. Am J Physiol Gastrointest Liver Physiol. 2009;296:G910–922. doi: 10.1152/ajpgi.90672.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu CL, et al. Equilibrative nucleoside transporter 3 deficiency perturbs lysosome function and macrophage homeostasis. Science. 2012;335:89–92. doi: 10.1126/science.1213682. [DOI] [PubMed] [Google Scholar]
- 9.Engel K, Wang J. Interaction of organic cations with a newly identified plasma membrane monoamine transporter. Mol Pharmacol. 2005;68:1397–1407. doi: 10.1124/mol.105.016832. [DOI] [PubMed] [Google Scholar]
- 10.Zhou M, Duan H, Engel K, Xia L, Wang J. Adenosine transport by plasma membrane monoamine transporter: reinvestigation and comparison with organic cations. Drug Metab Dispos. 2010;38:1798–1805. doi: 10.1124/dmd.110.032987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dahlin A, Xia L, Kong W, Hevner R, Wang J. Expression and immunolocalization of the plasma membrane monoamine transporter in the brain. Neuroscience. 2007;146:1193–1211. doi: 10.1016/j.neuroscience.2007.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okura T, et al. Functional characterization of rat plasma membrane monoamine transporter in the blood-brain and blood-cerebrospinal fluid barriers. J Pharm Sci. 2011;100:3924–3938. doi: 10.1002/jps.22594. [DOI] [PubMed] [Google Scholar]
- 13.Barnes K, et al. Distribution and functional characterization of equilibrative nucleoside transporter-4, a novel cardiac adenosine transporter activated at acidic pH. Circ Res. 2006;99:510–519. doi: 10.1161/01.RES.0000238359.18495.42. [DOI] [PubMed] [Google Scholar]
- 14.Duan H, Wang J. Selective transport of monoamine neurotransmitters by human plasma membrane monoamine transporter and organic cation transporter 3. J Pharmacol Exp Ther. 2010;335:743–753. doi: 10.1124/jpet.110.170142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duan H, Wang J. Impaired monoamine and organic cation uptake in choroid plexus in mice with targeted disruption of the plasma membrane monoamine transporter (Slc29a4) gene. J Biol Chem. 2013;288:3535–3544. doi: 10.1074/jbc.M112.436972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xia L, Zhou M, Kalhorn TF, Ho HT, Wang J. Podocyte-specific expression of organic cation transporter PMAT: implication in puromycin aminonucleoside nephrotoxicity. Am J Physiol Renal Physiol. 2009;296:F1307–1313. doi: 10.1152/ajprenal.00046.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou M, Xia L, Wang J. Metformin transport by a newly cloned proton-stimulated organic cation transporter (plasma membrane monoamine transporter) expressed in human intestine. Drug Metab Dispos. 2007;35:1956–1962. doi: 10.1124/dmd.107.015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vialou V, Balasse L, Dumas S, Giros B, Gautron S. Neurochemical characterization of pathways expressing plasma membrane monoamine transporter in the rat brain. Neuroscience. 2007;144:616–622. doi: 10.1016/j.neuroscience.2006.09.058. [DOI] [PubMed] [Google Scholar]
- 19.Xia L, Engel K, Zhou M, Wang J. Membrane localization and pH-dependent transport of a newly cloned organic cation transporter (PMAT) in kidney cells. Am J Physiol Renal Physiol. 2007;292:F682–690. doi: 10.1152/ajprenal.00302.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho HT, et al. Molecular analysis and structure-activity relationship modeling of the substrate/inhibitor interaction site of plasma membrane monoamine transporter. J Pharmacol Exp Ther. 2011;339:376–385. doi: 10.1124/jpet.111.184036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grundemann D, Liebich G, Kiefer N, Koster S, Schomig E. Selective substrates for non-neuronal monoamine transporters. Mol Pharmacol. 1999;56:1–10. doi: 10.1124/mol.56.1.1. [DOI] [PubMed] [Google Scholar]
- 22.Hayer-Zillgen M, Bruss M, Bonisch H. Expression and pharmacological profile of the human organic cation transporters hOCT1, hOCT2 and hOCT3. Br J Pharmacol. 2002;136:829–836. doi: 10.1038/sj.bjp.0704785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baganz NL, et al. Organic cation transporter 3: Keeping the brake on extracellular serotonin in serotonin-transporter-deficient mice. Proc Natl Acad Sci U S A. 2008;105:18976–18981. doi: 10.1073/pnas.0800466105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hosford PS, Mifflin SW, Ramage AG. 5-hydroxytryptamine-mediated neurotransmission modulates spontaneous and vagal-evoked glutamate release in the nucleus of the solitary tract effect of uptake blockade. J Pharmacol Exp Ther. 2014;349:288–296. doi: 10.1124/jpet.113.211334. [DOI] [PubMed] [Google Scholar]
- 25.Matthaeus F, Schloss P, Lau T. Differential uptake mechanisms of fluorescent substrates into stem-cell-derived serotonergic neurons. ACS Chem Neurosci. 2015;6:1906–1912. doi: 10.1021/acschemneuro.5b00219. [DOI] [PubMed] [Google Scholar]
- 26.Naganuma F, et al. Predominant role of plasma membrane monoamine transporters in monoamine transport in 1321N1, a human astrocytoma-derived cell line. J Neurochem. 2014;129:591–601. doi: 10.1111/jnc.12665. [DOI] [PubMed] [Google Scholar]
- 27.Duan H, et al. Potent and selective inhibition of plasma membrane monoamine transporter by HIV protease inhibitors. Drug Metab Dispos. 2015;43:1773–1780. doi: 10.1124/dmd.115.064824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Waterschoot RA, et al. Effects of cytochrome P450 3A (CYP3A) and the drug transporters P-glycoprotein (MDR1/ABCB1) and MRP2 (ABCC2) on the pharmacokinetics of lopinavir. Br J Pharmacol. 2010;160:1224–1233. doi: 10.1111/j.1476-5381.2010.00759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bednarczyk D, Ekins S, Wikel JH, Wright SH. Influence of molecular structure on substrate binding to the human organic cation transporter, hOCT1. Mol Pharmacol. 2003;63:489–498. doi: 10.1124/mol.63.3.489. [DOI] [PubMed] [Google Scholar]
- 30.Zolk O, Solbach TF, Konig J, Fromm MF. Structural determinants of inhibitor interaction with the human organic cation transporter OCT2 (SLC22A2) Naunyn Schmiedebergs Arch Pharmacol. 2009;379:337–348. doi: 10.1007/s00210-008-0369-5. [DOI] [PubMed] [Google Scholar]
- 31.Wright SH, Dantzler WH. Molecular and cellular physiology of renal organic cation and anion transport. Physiol Rev. 2004;84:987–1049. doi: 10.1152/physrev.00040.2003. [DOI] [PubMed] [Google Scholar]
- 32.Itagaki S, et al. Electrophysiological characterization of the polyspecific organic cation transporter plasma membrane monoamine transporter. Drug Metab Dispos. 2012;40:1138–1143. doi: 10.1124/dmd.111.042432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barendt WM, Wright SH. The human organic cation transporter (hOCT2) recognizes the degree of substrate ionization. J Biol Chem. 2002;277:22491–22496. doi: 10.1074/jbc.M203114200. [DOI] [PubMed] [Google Scholar]
- 34.Sweet DH, Pritchard JB. rOCT2 is a basolateral potential-driven carrier, not an organic cation/proton exchanger. Am J Physiol. 1999;277:F890–898. doi: 10.1152/ajprenal.1999.277.6.F890. [DOI] [PubMed] [Google Scholar]
- 35.Wu X, et al. Identity of the organic cation transporter OCT3 as the extraneuronal monoamine transporter (uptake2) and evidence for the expression of the transporter in the brain. J Biol Chem. 1998;273:32776–32786. doi: 10.1074/jbc.273.49.32776. [DOI] [PubMed] [Google Scholar]
- 36.Ho HT, Xia L, Wang J. Residue Ile89 in human plasma membrane monoamine transporter influences its organic cation transport activity and sensitivity to inhibition by dilazep. Biochem Pharmacol. 2012;84:383–390. doi: 10.1016/j.bcp.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou M, Xia L, Engel K, Wang J. Molecular determinants of substrate selectivity of a novel organic cation transporter (PMAT) in the SLC29 family. J Biol Chem. 2007;282:3188–3195. doi: 10.1074/jbc.M609421200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ho HT, Wang J. Tyrosine 112 is essential for organic cation transport by the plasma membrane monoamine transporter. Biochemistry. 2010;49:7839–7846. doi: 10.1021/bi100560q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carlsson A. Perspectives on the discovery of central monoaminergic neurotransmission. Annu Rev Neurosci. 1987;10:19–40. doi: 10.1146/annurev.ne.10.030187.000315. [DOI] [PubMed] [Google Scholar]
- 40.Hensler JG, et al. Catecholamine/serotonin interactions: systems thinking for brain function and disease. Adv Pharmacol. 2013;68:167–197. doi: 10.1016/B978-0-12-411512-5.00009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tank AW, Lee Wong D. Peripheral and central effects of circulating catecholamines. Compr Physiol. 2015;5:1–15. doi: 10.1002/cphy.c140007. [DOI] [PubMed] [Google Scholar]
- 42.Daws LC. Unfaithful neurotransmitter transporters: focus on serotonin uptake and implications for antidepressant efficacy. Pharmacol Ther. 2009;121:89–99. doi: 10.1016/j.pharmthera.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eisenhofer G. The role of neuronal and extraneuronal plasma membrane transporters in the inactivation of peripheral catecholamines. Pharmacol Ther. 2001;91:35–62. doi: 10.1016/s0163-7258(01)00144-9. [DOI] [PubMed] [Google Scholar]
- 44.Kristensen AS, et al. SLC6 neurotransmitter transporters: structure, function, and regulation. Pharmacol Rev. 2011;63:585–640. doi: 10.1124/pr.108.000869. [DOI] [PubMed] [Google Scholar]
- 45.Torres GE, Gainetdinov RR, Caron MG. Plasma membrane monoamine transporters: structure, regulation and function. Nat Rev Neurosci. 2003;4:13–25. doi: 10.1038/nrn1008. [DOI] [PubMed] [Google Scholar]
- 46.Lightman SL, Iversen LL. The role of uptake2 in the extraneuronal metabolism of catecholamines in the isolated rat heart. Br J Pharmacol. 1969;37:638–649. doi: 10.1111/j.1476-5381.1969.tb08502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trendelenburg U, Cassis L, Grohmann M, Langeloh A. The functional coupling of neuronal and extraneuronal transport with intracellular monoamine oxidase. J Neural Transm Suppl. 1987;23:91–101. doi: 10.1007/978-3-7091-8901-6_6. [DOI] [PubMed] [Google Scholar]
- 48.Grundemann D, Schechinger B, Rappold GA, Schomig E. Molecular identification of the corticosterone-sensitive extraneuronal catecholamine transporter. Nat Neurosci. 1998;1:349–351. doi: 10.1038/1557. [DOI] [PubMed] [Google Scholar]
- 49.Yoshikawa T, et al. Molecular mechanism of histamine clearance by primary human astrocytes. Glia. 2013;61:905–916. doi: 10.1002/glia.22484. [DOI] [PubMed] [Google Scholar]
- 50.Cui M, et al. The organic cation transporter-3 is a pivotal modulator of neurodegeneration in the nigrostriatal dopaminergic pathway. Proc Natl Acad Sci U S A. 2009;106:8043–8048. doi: 10.1073/pnas.0900358106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gasser PJ, Orchinik M, Raju I, Lowry CA. Distribution of organic cation transporter 3, a corticosterone-sensitive monoamine transporter, in the rat brain. J Comp Neurol. 2009;512:529–555. doi: 10.1002/cne.21921. [DOI] [PubMed] [Google Scholar]
- 52.Haenisch B, Bonisch H. Interaction of the human plasma membrane monoamine transporter (hPMAT) with antidepressants and antipsychotics. Naunyn Schmiedebergs Arch Pharmacol. 2010;381:33–39. doi: 10.1007/s00210-009-0479-8. [DOI] [PubMed] [Google Scholar]
- 53.Zhou M, Engel K, Wang J. Evidence for significant contribution of a newly identified monoamine transporter (PMAT) to serotonin uptake in the human brain. Biochem Pharmacol. 2007;73:147–154. doi: 10.1016/j.bcp.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hosford PS, Millar J, Ramage AG. Cardiovascular afferents cause the release of 5-HT in the nucleus tractus solitarii; this release is regulated by the low- (PMAT) not the high-affinity transporter (SERT) J Physiol. 2015;593:1715–1729. doi: 10.1113/jphysiol.2014.285312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Adamsen D, et al. Autism spectrum disorder associated with low serotonin in CSF and mutations in the SLC29A4 plasma membrane monoamine transporter (PMAT) gene. Mol Autism. 2014;5:43. doi: 10.1186/2040-2392-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steffens DC, Krishnan KR, Helms MJ. Are SSRIs better than TCAs? Comparison of SSRIs and TCAs: a meta-analysis. Depress Anxiety. 1997;6:10–18. doi: 10.1002/(sici)1520-6394(1997)6:1<10::aid-da2>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 57.Yin J, Duan H, Shirasaka Y, Prasad B, Wang J. Atenolol renal secretion is mediated by human organic cation transporter 2 and multidrug and toxin extrusion proteins. Drug Metab Dispos. 2015;43:1872–1881. doi: 10.1124/dmd.115.066175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu KC, Lu YH, Peng YH, Hsu LC, Lin CJ. Effects of lipopolysaccharide on the expression of plasma membrane monoamine transporter (PMAT) at the blood-brain barrier and its implications to the transport of neurotoxins. J Neurochem. 2015;135:1178–1188. doi: 10.1111/jnc.13363. [DOI] [PubMed] [Google Scholar]
- 59.Johanson CE, Stopa EG, McMillan PN. The blood-cerebrospinal fluid barrier: structure and functional significance. Methods Mol Biol. 2011;686:101–131. doi: 10.1007/978-1-60761-938-3_4. [DOI] [PubMed] [Google Scholar]
- 60.Ho HT, Dahlin A, Wang J. Expression profiling of solute carrier gene families at the blood-CSF barrier. Front Pharmacol. 2012;3:154. doi: 10.3389/fphar.2012.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Keep RF, Smith DE. Choroid plexus transport: gene deletion studies. Fluids Barriers CNS. 2011;8:26. doi: 10.1186/2045-8118-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lindvall M, Hardebo JE, Owman C. Barrier mechanisms for neutrotransmitter monoamines in the choroid plexus. Acta Physiol Scand. 1980;108:215–221. doi: 10.1111/j.1748-1716.1980.tb06525.x. [DOI] [PubMed] [Google Scholar]
- 63.Miller DS, Villalobos AR, Pritchard JB. Organic cation transport in rat choroid plexus cells studied by fluorescence microscopy. Am J Physiol. 1999;276:C955–968. doi: 10.1152/ajpcell.1999.276.4.C955. [DOI] [PubMed] [Google Scholar]
- 64.Dahlin A, Royall J, Hohmann JG, Wang J. Expression profiling of the solute carrier gene family in the mouse brain. J Pharmacol Exp Ther. 2009;329:558–570. doi: 10.1124/jpet.108.149831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kaplan GP, Hartman BK, Creveling CR. Localization of catechol-O-methyltransferase in the leptomeninges, choroid plexus and ciliary epithelium: implications for the separation of central and peripheral catechols. Brain Res. 1981;204:353–360. doi: 10.1016/0006-8993(81)90594-1. [DOI] [PubMed] [Google Scholar]
- 66.Vitalis T, et al. Developmental expression of monoamine oxidases A and B in the central and peripheral nervous systems of the mouse. J Comp Neurol. 2002;442:331–347. doi: 10.1002/cne.10093. [DOI] [PubMed] [Google Scholar]
- 67.Kotake Y, Tasaki Y, Makino Y, Ohta S, Hirobe M. 1-Benzyl-1,2,3,4-tetrahydroisoquinoline as a parkinsonism-inducing agent: a novel endogenous amine in mouse brain and parkinsonian CSF. J Neurochem. 1995;65:2633–2638. doi: 10.1046/j.1471-4159.1995.65062633.x. [DOI] [PubMed] [Google Scholar]
- 68.Nagatsu T. Isoquinoline neurotoxins in the brain and Parkinson’s disease. Neurosci Res. 1997;29:99–111. doi: 10.1016/s0168-0102(97)00083-7. [DOI] [PubMed] [Google Scholar]
- 69.Andre P, et al. Transport of biogenic amine neurotransmitters at the mouse blood-retina and blood-brain barriers by uptake1 and uptake2. J Cereb Blood Flow Metab. 2012;32:1989–2001. doi: 10.1038/jcbfm.2012.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Christensen MM, et al. The pharmacogenetics of metformin and its impact on plasma metformin steady-state levels and glycosylated hemoglobin A1c. Pharmacogenet Genomics. 2011;21:837–850. doi: 10.1097/FPC.0b013e32834c0010. [DOI] [PubMed] [Google Scholar]
- 71.Duong JK, et al. Population pharmacokinetics of metformin in healthy subjects and patients with type 2 diabetes mellitus: simulation of doses according to renal function. Clin Pharmacokinet. 2013;52:373–384. doi: 10.1007/s40262-013-0046-9. [DOI] [PubMed] [Google Scholar]
- 72.Han TK, et al. Four cation-selective transporters contribute to apical uptake and accumulation of metformin in Caco-2 cell monolayers. J Pharmacol Exp Ther. 2015;352:519–528. doi: 10.1124/jpet.114.220350. [DOI] [PMC free article] [PubMed] [Google Scholar]