Abstract
Due to technical limitations, small, distal, and tortuous intracranial pathology is sometimes out of reach of the current armamentarium of microcatheters designed for intracranial coil embolization. The Marathon microcatheter (Medtronic, Minneapolis, Minnesota, USA), designed specifically for the delivery of Onyx, is longer and more flexible than most coil delivery catheters. We report on nine patients (three with arteriovenous fistula, three with arteriovenous malformation, two with intracranial aneurysm, and one with tumor) where Marathon was used to deliver commercially available platinum coils. We also conducted laboratory compatibility testing and conclude that the Marathon can be used as a coil delivery catheter for Barricade coils (Blockade Medical, Irvine, California, USA) with diameter less than 0.012 in.
Keywords: Coil embolization, endovascular, Marathon microcatheter
Introduction
Advancements in image processing, technique, and device technology have expanded the spectrum of treatable pathology for neuroendovascular therapy.1–3 As more remote pathology is addressed, one technical challenge remaining is microcatheter canalization of small and distal vessels for the delivery of embolic coils. The majority of microcatheters designed for deployment of detachable coils are typically 150 cm in length and at least 1.6 Fr in diameter. Recently, the delivery of platinum coil through the Marathon microcatheter (Medtronic, Minneapolis, Minnesota, USA), a 165 cm, 1.5 Fr catheter designed for Onyx delivery, was described.4–6 Herein, we outline our experience utilizing the Marathon catheter with commercially available coils, highlighting the benefits and pitfalls. Furthermore, we demonstrate the specific limitations utilizing a model system, which may help direct future decision-making.
Cases
Methods
A total of nine cases performed at University of California Los Angeles (UCLA) by the authors (GRD, ST, VS, RJ, NG, and FV) between September 2011–December 2015 were retrospectively investigated (Table 1). In each case, Barricade coils (Blockade Medical, Irvine, California, USA) were deployed through the Marathon microcatheter with or without Onyx or n-Butyl Cyanoacrylate (n-BCA). Pathology treated included arteriovenous fistula (AVF), arteriovenous malformation (AVM), aneurysm, and tumor. There was one case in which coils were unable to be placed secondary to resistance in the Marathon catheter in tortuous parent vessels.
Table 1.
The 10 patients who underwent coil embolization using through a Marathon catheter.
| Case | Age/sex | Disease | Location | Coil (diameter in mm/length in cm) | Liquid embolic agent | Outcome | Complication |
|---|---|---|---|---|---|---|---|
| 1 | 52F | PICA AN | PICA | B 2/2, 1/2 | None | Complete occlusion | None |
| 2 | 1F | AVF | Galen | B 2/4 (×2) | Onyx | Feeder occlusion | None |
| 3 | 29F | AVF | Posterior fossa | Failed B 6/16 | n-BCA | Complete occlusion | None |
| 4 | 45M | AVM | Neck | B 4/15, 2/8 (×2), 1.5/1, 4/10, 3/10 | Onyx | Feeder occlusion | None |
| 5 | 62F | AVF | Cavernous sinus | B 5/10, 4/10 | Onyx | Complete occlusion | None |
| 6 | 35F | AVM | Temporal | GDC 2/2 (5) | Onyx | Feeder occlusion | None |
| 7 | 65M | AVM | Temporal | B 1.5/3 | Onyx | Complete occlusion | None |
| 8 | 53M | 2 PICA AN | PICA | B 3/6, 1.5/2 (3) | None | Complete occlusion | None |
| 9 | 39F | Tumor | Frontal | B 1/2 | Onyx | Feeder occlusion | None |
AN: aneurysm; B: Barricade; AVF: arteriovenous fistula; n-BCA: n-Butyl Cyanoacrylate; AVM: arteriovenous malformation; GDC: Guglielmi detachable coil (version not currently offered); PICA posterior inferior cerebellar artery.
Coil embolization was performed in each case in the typical fashion under general anesthesia with biplane angiography, 3D reconstruction, and roadmapping. The Marathon catheter was navigated into parent vessels utilizing a coaxial technique and each patient was systemically heparinized with activated clotting times routinely checked. In each case it was necessary to remove the rotating hemostatic valve to fully deploy the coil.
Illustrative cases
Case 1
A 52-year-old woman with a history of hypertension presented with acute onset of worst headache of life to the emergency room. CT brain demonstrated dense subarachnoid hemorrhage with intraventricular hemorrhage. Digital subtraction angiography revealed a distal left posterior inferior cerebellar artery (PICA) branch aneurysm measuring approximately 1.6 × 2 × 1.9 mm. A Navien catheter (Medtronic, Minneapolis, Minnesota, USA) was advanced to the left V2 vertebral segment and initially a Xpedion-10 microguidewire (Medtronic, Minneapolis, MN, USA) was advanced into an Echelon-10 (Medtronic, Minneapolis, Minnesota, USA); however, the sharply angled distal PICA branch take-off prevented safe passage even of angled Echelon catheters. Thus, a Mirage microguidewire (Medtronic, Minneapolis, Minnesota, USA) was inserted into a Marathon microcatheter and navigated to the aneurysm with roadmapping technique (Figure 1). A Barricade 2 mm × 2 cm helical finish and 1 mm × 2 cm complex finish were used to fully occlude the aneurysm. The patient was discharged home from the hospital three weeks later without gross neurologic deficit. Patient had a follow-up angiogram at six weeks demonstrating continued embolization of the aneurysm.
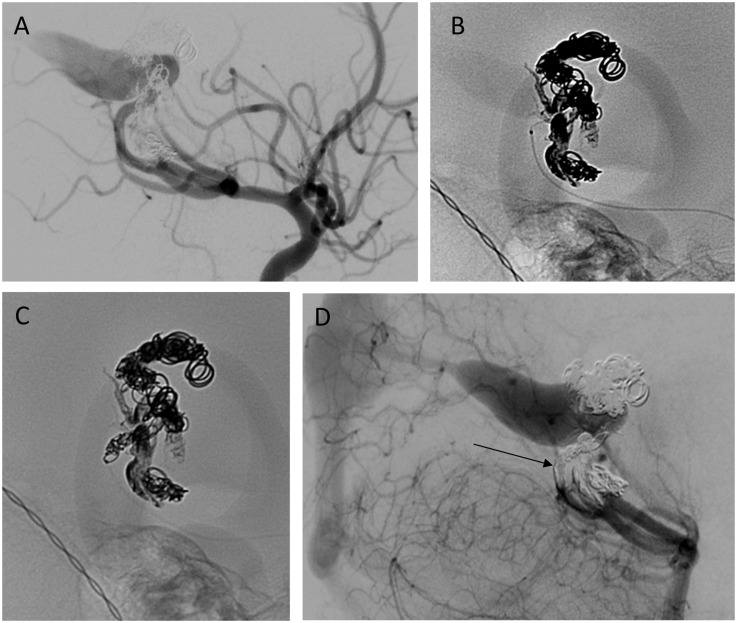
Figure 1.
Patient 52F with left distal posterior inferior cerebellar artery (PICA) branch aneurysm. (a) Magnified superselective angiogram of left PICA from Echelon-10 demonstrating aneurysm. Arrow indicates 90° turn into aneurysm. (b) Coil deployment via Marathon – note the lack of proximal marker. (c) Magnified superselective angiogram of aneurysm (arrow) post-coil embolization. (d) Six-week follow-up angiogram demonstrating continued aneurysm occlusion and patency of PICA.
Case 2
This is a 14-month-old girl with history of high output cardiac failure at birth secondary to a Vein of Galen malformation, who received a partial coil embolization at 10 days of age and returned for further embolization. Via a 4F sheath, a 4-Fr pediatric Glidecath (Terumo, Somerset, New Jersey, USA) was exchanged for a DAC 044 intermediate catheter (Stryker, Kalamazoo, Michigan, USA) in the right vertebral artery and the primary arterial supply, multiple right-sided posterior choroidal, and thalamoperforating branches were identified. The soft Marathon microcatheter was then chosen due to the presumed fragility of the neonatal feeders serving the malformation. A Marathon microcatheter was used to deliver Onyx 34 to two small posterior choroidal pedicles with minimal reflux. A third, larger posterior choroidal artery pedicle was then embolized first with a Barricade 2 mm × 4 cm complex finishing coil in order to assist deposition without distal embolization during the injection of Onyx 34 (Figure 2). Finally, an additional left thalamoperforating artery pedicle was embolized with a single Barricade 2 mm × 4 cm complex finishing coil. The post-embolization angiogram demonstrated residual supply from two small thalamic perforating branches with a reduced arteriovenous transit time. There was no distal venous or lung embolization noted in the procedure. The patient tolerated the procedure well and a five-month follow-up magnetic resonance angiogram demonstrated progressive, near-complete thrombosis.
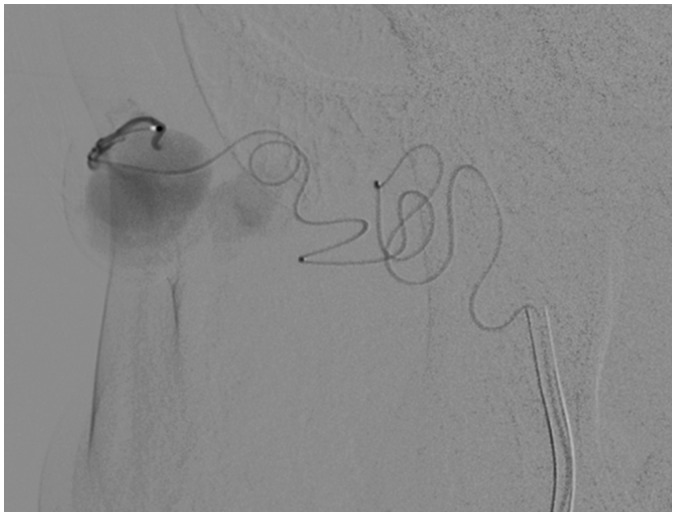
Figure 2.
Patient 1F with Vein of Galen malformation. Marathon was selected due to the fragility of the neonatal feeders. (a) Magnified right internal carotid artery injection demonstrating previous coil masses and arteriovenous shunting. (b) Superselective catheterization of posterior choroidal artery with Marathon. (c) Coil deployment (followed by Onyx 34 delivery). (d) Post-treatment angiogram with improved shunting (arrow demonstrates coil and Onyx embolized posterior choroidal). There was no distal venous or lung embolization.
Case 3
A 29-year-old woman presented with a pulsatile posterior-auricular swelling since the age of 15 years. Digital subtraction angiography demonstrated an arteriovenous malformation fed primarily by an enlarged and tortuous posterior auricular artery (Figure 3). The Marathon microcatheter, selected due to extreme vessel tortuosity, was successfully navigated through the very torturous and enlarged posterior auricular artery to the venous pouch. During passage of a 6 mm × 16 cm Barricade framing coil, it detached within the catheter secondary to the extreme tortuosity. The microsystem was withdrawn, and embolization was then completed utilizing the Codman Trufill (Neurovascular, Raynham, Massachusetts, USA) liquid embolic system transarterially as well as transvenous coiling with Axium coils (Medtronic, Minneapolis, Minnesota, USA) through an Echelon-10 microcatheter. The patient tolerated the procedure well without angiographic evidence of residual AVM and was discharged home neurologically intact to return later for cosmetic surgery.
Figure 3.
Patient 29F with auricular arteriovenous malformation. Superselective angiogram of posterior auricular artery showing shunting to large venous varix. Note the arterial tortuosity.
Case 4
This was a 45-year-old man without significant past medical history who presented with a symptomatic left facial AVM. The parotid region AVM/AVF was fed predominately through the left internal maxillary artery via the left vertebral artery and left thyrocervical trunk with a large varix emptying to the left jugular vein. The left external carotid artery (ECA) was occluded at the level of the bifurcation with reconstitution via anastomosis from the vidian artery, ascending cervical, and vertebra-occipital collaterals. These feeders retrogradely filled the middle meningeal and distal internal maxillary arteries, which then retrogradely filled the ECA to the level of the fistula. Again the Marathon microcatheter was selected based on extreme vessel tortuosity. Given that the ECA was occluded, we utilized a combination of Mirage and X-pedion 10 microwires to advance a Marathon microcatheter with roadmapping technique through the left vertebral artery, via occipital collaterals to the ECA and then to the main pedicles from the internal maxillary artery and the middle meningeal artery which were then occluded with several Barricade coils (Table 1, Case 4) (Figure 4). After feeder occlusion the Marathon was drawn back into the distal internal maxillary artery and with subtraction fluoroscopy Onyx 34 was slowly infused into the ECA to the level of the fistula. Post-treatment angiogram demonstrated approximately 70% reduction in flow to the AVM. The patient tolerated the procedure well without complication.
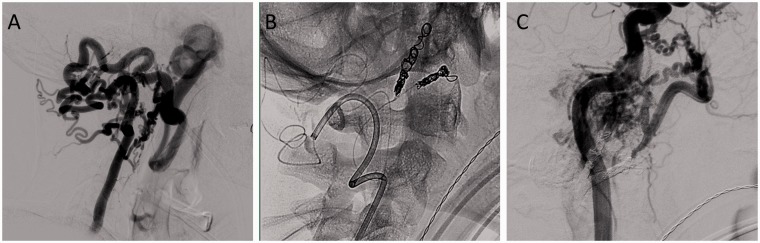
Figure 4.
Patient 45M with large left facial arteriovenous malformation. (a) Left vertebral injection demonstrating arterio-venous shunting to the left jugular vein from the external carotid artery (ECA). Note the extreme tortuosity of the collateralized feeding vessels to the ECA fistula. (b) Catheterization of the ECA via the left vertebral artery with Marathon and deployment of coils into the internal maxillary artery and middle meningeal artery. (c) Post-embolization angiogram of the left internal carotid artery demonstrating reduction of the nidus.
Laboratory testing
Based on the findings in Case 3, coil compatibility testing was conducted in conjunction with Blockade Medical. Both Target coils (Stryker, Kalamazoo, Michigan, USA) and Axium coils (Medtronic, Minneapolis, Minnesota, USA) utilize a slightly larger pusher wire (0.014 in) compared to the Blockade pusher of 0.0125 in. The Marathon microcatheter inner diameter (ID) is 0.015 in proximally and 0.013 in distally. This tapered ID prevents complete deployment of Target and Axium coils and, if used, they must be detached within the catheter and expelled. Previous versions of the Guglielmi detachable coil (GDC) (Stryker, Kalamazoo, Michigan, USA) were compatible with the Marathon. However, currently available Stryker coils now have a larger pusher wire and cannot be fully deployed.
We investigated the tracking of several Barricade coils through the Marathon microcatheter in a straight configuration and when inserted into a silicon-based tortuous vessel model (Figure 5). When the Marathon catheter is in a straight configuration it accommodates Barricade coils up to 0.013 in; however, in tortuosity, only coils of less than 0.012 in were compatible past 146 cm (Table 2).
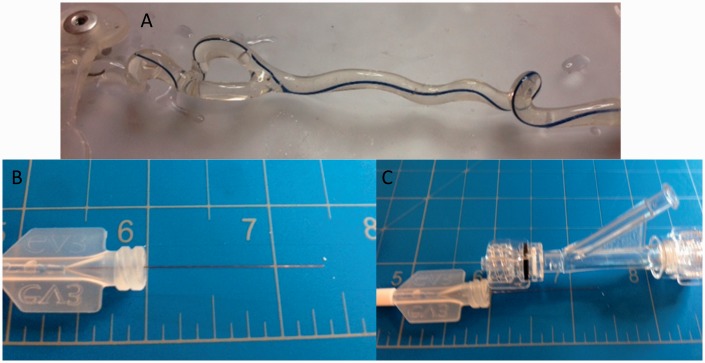
Figure 5.
(a) Tortuous vessel model with Marathon catheter; (b) remaining pusher-wire of Barricade coil when fully deployed in Marathon catheter; (c) comparison with size of rotating hemostatic valve.
Table 2.
Testing results of Barricade coils tracking through Marathon catheter in straight and tortuous configurations.
| Primary wind coil (in) | Straight configuration tracking | Tortuous configuration tracking |
|---|---|---|
| 0.010 × 0.00125 0.010 × 0.0015 | Excellent | Increased resistance |
| 0.011 × 0.0015 | Excellent | Increased resistance |
| 0.012 × 0.002 0.012 × 0.00225 | Increased resistance | Unable to track past 146 cm |
| 0.013 × 0.00225 | Unable to track past 146 cm | Unable to track past 146 cm |
| 0.014 × 0.003 | Unable to track past 146 cm | Unable to track past 146 cm |
Discussion
The Marathon microcatheter is a flow-directed 165 cm catheter with a distal inner diameter of 0.013 in designed for the delivery of Onyx. Compared to the majority of catheters designed for coil delivery, the Marathon is longer, thinner, and more flexible. These characteristics are favorable for improved access through tortuous and distal vessels. However, Onyx and n-BCA are not always preferred as liquid agents can be difficult to control in high-flow fistulae, and may infer too great a risk of distal embolism.7–10 This series highlights the “cross-compatibility” of the Marathon system as both a detachable coil and embolic liquid delivery catheter.
Utilizing Marathon as both a coil delivery and Onyx delivery catheter has a number of advantages. First, a single Marathon catheter can be used to deliver coils for occlusion or protective embolization, or as scaffolding coils prior to injection of liquid agents, making it a powerful tool in the embolization of arteriovenous fistula and malformations (as shown in Case 2). Ultimately, this technique allows for decreased catheter exchanges, improving risk, cost, and time of procedure. Second, the improved length of the catheter enables delivery of coils to distal pathology otherwise not accessible. Finally, the narrower diameter allows for improved tracking through small tortuous vessels in which larger diameter catheters cannot safely transverse.
Coaxial deployment of detachable coils through the Marathon catheter has a few technical considerations and challenges. First and foremost is coil compatibility. After the inadvertent intracatheter coil deployment in Case 3 we conducted compatibility testing with Blockade (Figure 5, Table 2). In our straight model, coils equal to the minimum ID of the marathon were successfully passed and detached; however, in our tortuous vessel model, larger Barricade coils (D2 > 0.011 in) could not be advanced past 146 cm secondary to increased resistance. Thus, the commercially available coils in the USA compatible with the Marathon catheter are the Barricade finishing coil (1.5–2.5 mm and 3–6 mm) as well as the Framing coil (2–4 mm) (Table 3). Coil delivery from Marathon has also been performed when GDCs were available in the market (Table 2, Case 6). The delivery wire of the GDC served as a mono-polar current pathway for the electrolytic coil detachment mechanism. This structurally simple delivery wire could fit in a small caliber microcatheter such as the Marathon. However, the more recent, technologically advanced detachable coils utilize bipolar current pathway, complex mechanical detachment systems in the delivery wire, or a larger diameter pusher to assist in delivery, each of which make the delivery wire thicker and incompatible with Marathon’s ID. Currently Barricade is the only coil that utilizes a 0.0125 in pusher and is therefore compatible with the Marathon. An additional consideration is the mismatch between microcatheter length and the length of the coil pusher wire. As others have found,4,6 it is necessary to remove the rotating hemostatic valve (RHV) to utilize the detachment tool (Figure 5). Without the RHV, it is not possible to maintain continuous flush, which may increase the chance of clot formation.4 Lastly, the Marathon microcatheter lacks the more proximal detachment marker (Figure 1) and this can make coil advancement treacherous if the catheter tip is not well visualized (i.e. hidden within the coil mass). To more safely place coils, the position of the proximal coil marker must be memorized at deployment before the next coil positioning. We recommend careful stepwise checking of position and detachment.
Table 3.
Table of Barricade coil compatibility with Marathon catheter.
| Primary wind coil (in) | Product | Marathon catheter compatible |
|---|---|---|
| 0.010 × 0.00125 | Finish coil (1.5–2.5 mm) | Yes |
| 0.010 × 0.0015 | Finish coil (3–6 mm) | Yes |
| 0.011 × 0.0015 | Frame coil (2–4 mm) | Yes |
| 0.012 × 0.002 | Frame coil (5–10 mm) Fill coil (3–6 mm) | No |
| 0.012 × 0.00225 | Fill coil (7–10 mm) | No |
| 0.013 × 0.00225 | Frame coil 18s (6–10 mm) | No |
| 0.014 × 0.003 | Frame coil 18s (11–15 mm) | No |
Conclusion
The Marathon microcatheter is a viable delivery catheter for both liquid embolic material and detachable coils. Its superior length and flexibility allow for safer navigation to distal intracranial pathology. The main limitations for coil deployment though the Marathon are coil and pusher-wire diameter, thus, Blockade Medical produces the only compatible US-commercially available coils. The main technical considerations include loss of continuous flush and absence of proximal detachment marker.
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Gary Duckwiler is a stock holder at Blockade Medical.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Blackham KA, Meyers PM, Abruzzo TA, et al. Endovascular therapy of acute ischemic stroke: Report of the Standards of Practice Committee of the Society of NeuroInterventional Surgery. J Neurointerv Surg 2012; 4: 87–93. [DOI] [PubMed] [Google Scholar]
- 2.Duffis EJ, Tank V, Gandhi CD, et al. Recent advances in neuroendovascular therapy. Clin Neurol Neurosurg 2013; 115: 853–858. [DOI] [PubMed] [Google Scholar]
- 3.Yamaki VN, Brinjikji W, Murad MH, et al. Endovascular treatment of very small intracranial aneurysms: Meta-analysis. AJNR Am J Neuroradiol 2016; 37: 862–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stidd DA, Lopes DK, Chen M. Aneurysm coil embolization using a 1.5-Fr distal outer diameter microcatheter. Neurointervention 2014; 9: 39–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horie N, Hayashi K, Morikawa M, et al. Selective coil embolization through flow-directed microcatheter for intracranial arteriovenous malformations. Acta Neurochir 2012; 154: 989–991. [DOI] [PubMed] [Google Scholar]
- 6.Horie N, Hayashi K, Morikawa M, et al. A novel method for super-selective coil embolization using an extremely soft bare coil through a liquid embolic delivery microcatheter. Neurol Med Chir (Tokyo) 2014; 55: 605–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lv X, Wu Z, Jiang C, et al. Complication risk of endovascular embolization for cerebral arteriovenous malformation. Eur J Radiol 2011; 80: 776–779. [DOI] [PubMed] [Google Scholar]
- 8.Lv X, Jiang C, Zhang J, et al. Complications related to percutaneous transarterial embolization of intracranial dural arteriovenous fistulas in 40 patients. AJNR Am J Neuroradiol 2009; 30: 462–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geibprasert S, Pongpech S, Armstrong D, et al. Dangerous extracranial-intracranial anastomoses and supply to the cranial nerves: Vessels the neurointerventionalist needs to know. AJNR Am J Neuroradiol 2009; 30: 1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuki I, Kim RH, Duckwiler G, et al. Treatment of brain arteriovenous malformations with high-flow arteriovenous fistulas: Risk and complications associated with endovascular embolization in multimodality treatment. Clinical article. J Neurosurg 2010; 113: 715–722. [DOI] [PubMed] [Google Scholar]