Abstract
Introduction/Purpose
To achieve aneurysm occlusion, flow diverters (FDs) must be accurately sized to maximize coverage over the neck and induce thrombosis. Catheterization for diagnostic angiography can cause vasospasm that may affect vessel measurements. This study evaluates impacts of intra-arterial infusion of a calcium channel blocker (CCB) on angiographic measurements in patients treated with FDs to determine effects on final diameter of the FD and subsequent occlusion.
Materials and methods
Pre-treatment measurements were recorded for diameter of the distal and proximal landing zones and maximum and minimum diameters between these segments. Post-treatment measurements of the stent following deployment were recorded at these locations. When CCB was infused, post-infusion pre-treatment measurements were recorded. Rates of occlusion were noted for all patients. T-tests were performed to assess for differences in pre- and post-treatment measurements and rates of occlusion between groups with and without CCB infusion.
Results
Twenty-eight FDs were deployed to treat 25 aneurysms in 24 patients. CCB infusion was performed prior to deployment of 12 (42.9%) devices. No significant difference was noted between groups for pre- and post-treatment measurement changes. Confirmed aneurysm occlusion was more likely to occur in the CCB infusion group (88.9% vs. 36.4%, p = 0.009).
Conclusion
Optimization of device sizing is important to increase FD density over the aneurysm neck and promote thrombosis. To improve measurement accuracy, CCB infusion can reduce effects of mild vasospasm. Subsequent aneurysm occlusion was more likely to occur following FD treatment when device size selection was based on measurements performed following CCB infusion.
Keywords: Aneurysm, Flow Diversion, Vasospasm, Technique, Calcium Channel Blocker
Introduction
To best achieve complete occlusion of aneurysms treated with flow diverters (FDs), devices must be accurately sized to precisely match the parent vessel diameter. Porosity is lowest for a Pipeline embolization device (PED) as the expanded diameter of the device approaches its labeled diameter.1 Appropriately sized PEDs will thus have maximal coverage over the aneurysm neck and will be more likely to induce aneurysm thrombosis. Catheterization for diagnostic angiography can cause vasospasm that may affect vessel measurements.2 Such catheter-induced vasospasm (CIV) can be ameliorated with intra-arterial infusion of a calcium channel blocker (CCB).3,4 This study evaluates the effects of CCB infusion on angiographic measurements in patients treated with PEDs, investigating the impact on the final diameter of the device following deployment and subsequent occlusion.
Materials and methods
According to an institutional review board (IRB)-approved protocol, retrospective analysis was performed of prospectively maintained procedure records to identify patients with aneurysms treated with PEDs. In all patients, pre-treatment measurements were recorded for vessel diameter of the future distal and proximal landing zones, as well as maximum and minimum diameters between these segments. Additionally, the size of the aneurysm and morphology were noted. Morphology was classified as saccular vs. fusiform, lobulated vs. not, and projecting in the direction of shear stress vs. not. Post-treatment measurements of the FD itself following deployment were recorded at the distal and proximal ends in addition to maximum and minimum diameters throughout the length of the device. When a CCB was infused prior to treatment, post-infusion pre-treatment measurements were recorded. All measurements were performed after calibrating to a catheter included on the image. Rates of occlusion and O’Kelly–Marotta (OKM) angiographic score were noted for all patients.5 A representative case is provided in Figure 1. For classification as an ordinal variable, OKM scores were converted from a hierarchical alphanumeric scale to a 10-point scale. Independent-sample two-tailed T-tests were performed to assess for differences in pre- and post-treatment measurements, rates of occlusion, and OKM score immediately after deployment and at first follow-up. Follow-up times were noted.
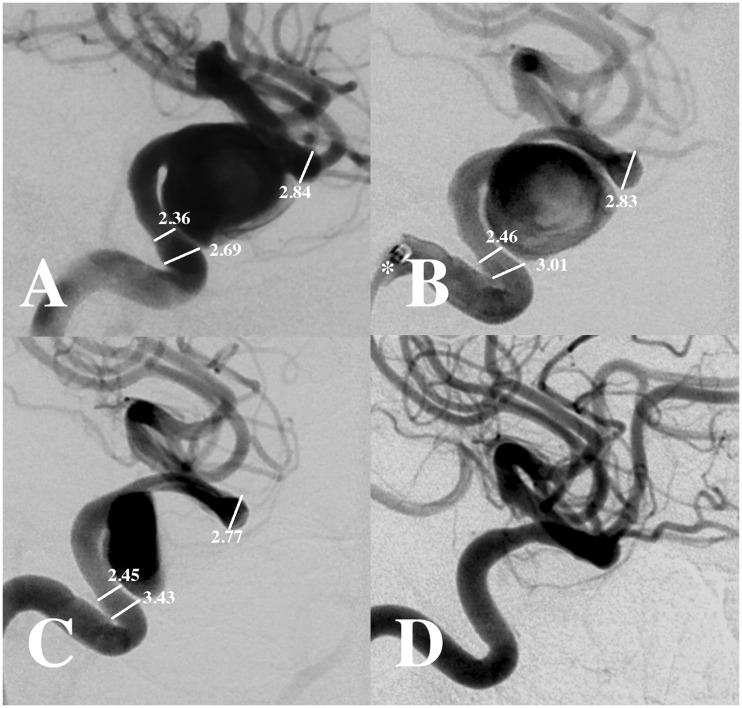
Figure 1.
Lateral projection digital subtraction angiography (a) demonstrates a cavernous internal carotid artery aneurysm. Measurements are noted (a) before and (b) after intra-arterial administration of 5 mg verapamil. Note inclusion of the Navien catheter (asterisk) in the image (b), which was used as an internal reference to calibrate measurements. Angiography in the same projection (c) following deployment of a Pipeline embolization device (PED) demonstrates stasis with the aneurysm (O’Kelly-Marotta (OKM) score A3). Six-month follow-up angiography (d) demonstrates occlusion of the aneurysm. Calibrated measurements are shown in mm ((a)–(c)).
Results
Twenty-eight PEDs were deployed to treat 25 aneurysms in 24 patients. Patient demographics and FD sizes for the two groups are summarized in Table 1. CCB infusion was performed prior to deployment of 12 (42.9%) devices. The changes in measurements noted after CCB infusion are summarized in Table 2. No significant difference was noted between groups for pre- and post-treatment measurement changes, which are listed in Table 3. Follow-up data were available for 20 devices. Mean time of first follow-up was 209 days (interquartile range (IQR) 186–235 days). There was no significant difference (p = 0.115) in days to first angiographic follow-up between the CCB group (199.7 ± 56.2 days) and non-CCB group (217.6 ± 41.5 days). No difference was found for OKM score immediately following deployment, although FDs sized after CCB infusion had higher OKM scores at first follow-up (C1 vs. C3, p = 0.024). No significant difference was identified between groups for overall angiographic follow-up time (192.0 vs. 246.3 days, p = 0.294). No differences were noted in outcomes based on aneurysm size or morphology. Confirmed aneurysm occlusion was more likely to occur in the CCB infusion group (88.9% vs. 36.4%, p = 0.009). OKM score and occlusion data are summarized in Table 4.
Table 1.
Patient demographics and FD sizes.
| Parameter | No CCB (n = 16) | CCB (n = 12) | p value |
|---|---|---|---|
| Mean age (years) | 56.8 | 56.4 | 0.436 |
| Female | 15 (93.8%) | 12 (100.0%) | 0.076 |
| FD diameter (mm) | 4.44 ± .461 | 4.29 ± 3.82 | 0.379 |
| FD length (mm) | 19.2 ± 3.75 | 24.2 ± 8.50 | <0.001 |
| Mean last angiographic follow-up (days) | 246.4 ± 316 | 192.0 ± 183 | 0.294 |
FD: flow diverter; CCB: calcium channel blocker.
Table 2.
Diameter change after CCB infusion.
| Measurement | Change (mm) |
|---|---|
| Distal LZ | 0.343 |
| Proximal LZ | 0.478 |
| Maximum Diameter | 0.453 |
| Minimum Diameter | 0.228 |
CCB: calcium channel blocker; LZ: landing zone.
Table 3.
Pre-Post diameter change.
| Measurement | No CCB | CCB | p value |
|---|---|---|---|
| Distal LZ | 2.50% | 0.80% | 0.285 |
| Proximal LZ | 4.40% | –3.30% | 0.612 |
| Maximum diameter | 2.40% | 5.00% | 0.592 |
| Minimum diameter | 2.80% | 14.10% | 0.244 |
CCB: calcium channel blocker; LZ: landing zone.
Table 4.
OKM scorea and confirmed occlusion.
| Measurement | No CCB (n = 11) | CCB (n = 9) | p value |
|---|---|---|---|
| Immediate score | A3 (3.06) | A3 (3.33) | 0.686 |
| First follow-up score | C1 (7.20) | C3 (9.20) | 0.024 |
| Confirmed Occlusion | 4 (36.4%) | 8 (88.9%) | 0.009 |
Ordinal mean converted to nearest corresponding alphanumeric OKM value.
OKM: O’Kelly-Marotta; CCB: calcium channel blocker.
Discussion
The decreased porosity produced by the increased metallic coverage of FDs compared to conventional stents allow for stagnation within and eventual occlusion of aneurysms. The true porosity of an FD when deployed in a vessel depends on the labeled diameter of the device and the diameter of the vessel into which it expands. As the diameter of expansion for a PED increases, the device foreshortens, and the metallic coverage per area increases (Figure 2).1 Additionally, the complex structure of intracranial vasculature leads to variability in the extent of expansion.
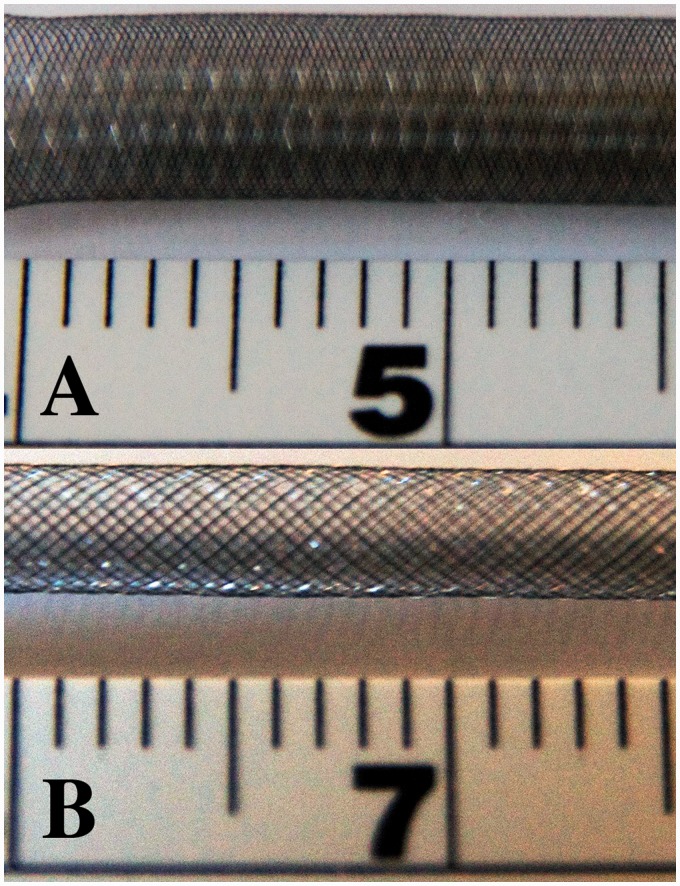
Figure 2.
Photographs of the same 4.0 mm diameter Pipeline embolization device (PED) (a) unconstrained and (b) constrained to 2.9 mm width demonstrates increased porosity of the device when not fully expanded compared to its unconstrained configuration.
Two considerations are most important for selecting the appropriate FD size. First, the device should be selected so the device will appose the vessel wall at both ends to ensure stability within the vessel and prevent type 1 endoleak. Second, within the limits imposed by appropriately sizing the ends at their landing zones, the FD size should be sized to optimize coverage over the aneurysm neck to better achieve thrombosis. To optimize device sizing, accurate measurements of the vessel to be treated are obligatory.
Precise measurement of intracranial vasculature can be challenging. As intracranial vessels course through the head, their distances from the X-ray source and detector change. This decreases accuracy and precision of angiographic measurements.6–8 To mitigate these effects, standardized references with known dimensions, such as coins, washers, or fiducials, can be placed on patients’ heads to allow more accurate calibration.6–8 However, such techniques are still prone to inaccuracy because of differential distances between markers and the vessel of interest, although these distortions can be reduced with multiple markers.6–8 To further address these limitations and achieve more accurate measurements, diagnostic angiography prior to FD treatment now routinely includes measurements obtained with the catheter visualized on the image so that the catheter can be used as a measurement standard to best measure the vessel to be treated.9 However, the most accurately calibrated measurements will be unsatisfactory if the vessels are imaged while they are not in their native state.
CIV is commonly encountered during neurointerventional techniques.2 While it is often mild and of little clinical consequence, minor changes in measurements can have profound effects on FD performance.2 Such CIV is routinely addressed with CCB infusion.3,4 In our practice, CIV is treated with 5 mg verapamil diluted in 10 ml saline and infused over three to five minutes. In addition to the benefit of selecting the most appropriately sized FD, CCB infusion can also reduce and prevent CIV incurred during FD deployment. If CIV occurs upstream to the device, it would reduce perfusion of the downstream parenchyma. CIV encountered at the site of treatment would limit expansion of the FD, reducing metal coverage over the aneurysm neck (Figure 2).
During the treatment of patients with FDs in our department, the effects of CIV on vessel measurements were noted anecdotally during several procedures. At times this was treated with CCB infusion, and repeat measurements were obtained. To evaluate the effect of CCB infusion on measurements and outcomes, we compared those with and without CCB infusion. Vessel measurements (proximal landing zone, distal landing zone, minimum diameter, maximum diameter) did vary between the two groups, but there was no statistical significance to these changes. However, there was an effect on subsequent OKM score on initial follow-up and aneurysm occlusion, with FDs deployed after measurements based on post-CCB infusion images performing more favorably. This suggests CCB infusion prior to angiography for measurements on which a device will be selected can promote the desired outcome of aneurysm occlusion.
While these findings suggest CCB infusion has a beneficial impact on sizing and subsequent performance of FDs, there are several limitations in this analysis. Patients evaluated were analyzed retrospectively, and the decision to employ CCB infusion was not made based on a standardized protocol. Additionally, while there were no differences in follow-up between the groups, post-treatment surveillance was not standardized. This analysis did not control for patients undergoing concomitant aneurysm coiling. This analysis did not find differences in aneurysm size or morphology, although aneurysms treated in our practice may differ from those treated elsewhere. Additionally, it is our practice to not treat with FD those aneurysms that have a major end artery arising from its dome or base. Also, it is our practice to perform percutaneous transluminal balloon angioplasty (PTA) or place another FD if apposition is inadequate or if there is inadequate neck coverage (>5 mm of stent deployed in the proximal and distal arterial segments). These results should not be applied to practices that employ different techniques. Device-related factors like bending and twisting or patient-specific factors like hemodynamics or fluid dynamics could affect deployment and subsequent occlusion; evaluation of such factors is beyond the scope of this analysis. Finally, aneurysm occlusion can be affected by changes in dual-antiplatelet therapy (DAPT). It is our practice to not alter DAPT until after the first follow-up angiogram, but it is possible that such changes could affect results. Therapeutic response to DAPT was not available at our center during the study period. To mitigate this potential bias, we examined both the OKM score at first follow-up, when DAPT should still be uniform, and overall occlusion, which could be affected by DAPT changes not reflected in the current analysis. The effect identified for both measures helps confirm the benefit of CCB infusion.
Conclusion
Optimization of device sizing is important to increase FD density over the aneurysm neck and promote thrombosis. To improve accuracy of measurements of parent vessels prior to device selection, CCB infusion can reduce the effects of mild vasospasm. In this study, subsequent aneurysm occlusion was more likely to occur following FD treatment when device size selection was based on measurements performed following CCB infusion. Further investigation is warranted to evaluate effects of CCB infusion and other factors affecting FD treatment of aneurysms.
Acknowledgments
MDA authored and edited the manuscript. All authors participated in patient care, edited images, and edited the manuscript. All authors contributed substantially to the conception or design of the work, or the acquisition, analysis, or interpretation of data for the work. All authors participated in drafting the work or revising it critically for important intellectual content. All authors gave final approval of the version to be published. Authors are in agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Shapiro M, Raz E, Becske T, et al. Variable porosity of the Pipeline embolization device in straight and curved vessels: A guide for optimal deployment strategy. AJNR Am J Neuroradiol 2014; 35: 727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ishihara H, Ishihara S, Niimi J, et al. Risk factors and prevention of guiding catheter-induced vasospasm in neuroendovascular treatment. Neurol Med Chir (Tokyo) 2015; 55: 261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coon AL, Colby GP, Mack WJ, et al. Treatment of mechanically-induced vasospasm of the carotid artery in a primate using intra-arterial verapamil: A technical case report. BMC Cardiovasc Disord 2004; 4: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eckard DA, Purdy PD, Girson MS, et al. Intraarterial papaverine for relief of catheter-induced intracranial vasospasm. AJR Am J Roentgenol 1992; 158: 883–884. [DOI] [PubMed] [Google Scholar]
- 5.O’Kelly CJ, Krings T, Fiorella D, et al. A novel grading scale for the angiographic assessment of intracranial aneurysms treated using flow diverting stents. Interv Neuroradiol 2010; 16: 133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox AJ, Millar J, Raymond J, et al. Dangerous advances in measurements from digital subtraction angiography: When is a millimeter not a millimeter? AJNR Am J Neuroradiol 2009; 30: 459–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horton JA. Sizing rings: A simple technique for measuring intracranial lesions. AJNR Am J Neuroradiol 1995; 16: 1449–1451. [PMC free article] [PubMed] [Google Scholar]
- 8.Malek AM, Higashida RT, Halbach VV, et al. Patient presentation, angiographic features, and treatment of strangulation-induced bilateral dissection of the cervical internal carotid artery. Report of three cases. J Neurosurg 2000; 92: 481–487. [DOI] [PubMed] [Google Scholar]
- 9.Saatci I, Yavuz K, Ozer C, et al. Treatment of intracranial aneurysms using the Pipeline flow-diverter embolization device: A single-center experience with long-term follow-up results. AJNR Am J Neuroradiol 2012; 33: 1436–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]