Abstract
The volatile organic compounds (VOCs) of four monofloral and one multifloral of Thai honeys produced by Apis cerana, Apis dorsata and Apis mellifera were analyzed by headspace solid-phase microextraction (HS-SPME) followed by gas chromatography and mass spectrometry (GC-MS). The floral sources were longan, sunflower, coffee, wild flowers (wild) and lychee. Honey originating from longan had more VOCs than all other floral sources. Sunflower honey had the least numbers of VOCs. cis-Linalool oxide, trans-linalool oxide, ho-trienol, and furan-2,5-dicarbaldehyde were present in all the honeys studied, independent of their floral origin. Interestingly, 2-phenylacetaldehyde was detected in all honey sample except longan honey produced by A. cerana. Thirty-two VOCs were identified as possible floral markers. After validating differences in honey volatiles from different floral sources and honeybee species, the results suggest that differences in quality and quantity of honey volatiles are influenced by both floral source and honeybee species. The group of honey volatiles detected from A. cerana was completely different from those of A. mellifera and A. dorsata. VOCs could therefore be applied as chemical markers of honeys and may reflect preferences of shared floral sources amongst different honeybee species.
Introduction
Honey is a well-known natural product derived from honeybees. Worker honeybees collect nectar from flower blossom and store them in a honey sack before they return to the hive. The nectar is then mixed with enzymes from bees resulting in the breakdown of complex sugars in the nectar to simple sugars such as glucose and fructose [1]. Besides sugar content, honey contains proteins, lipids and vitamins [2]. Honey may have aromatic compounds at very low concentrations in the form of volatile mixtures. Each volatile compound in the mixture may contribute a different aroma, taste and function leading to the uniqueness of honey. Volatile organic compounds (VOCs) contributing to the aroma of honey vary in quality and quantity because of different nectar sources, microbes in honey, transformation of plant compounds by bees, honey processing, and condition of storage [3, 4].
Numerous organic compounds have been detected and identified as characteristic compounds of honey [5–7]. Three well-known principal categories of honey volatiles are terpenes and their derivatives, norisoprenoids, and benzene derivatives [8]. Terpenes and their derivatives are known as important compounds that provide flavor, odor and biomedical properties in honey [9, 10]. Norisoprenoids have very low olfactory perception thresholds which strongly influence honey odor [11]. Aside the impact of norisoprenoids on honey odor, some of them are known to be anticarcinogenic [12]. Norisopreniods are products of Carotenoids [13, 14]. Furthermore, benzene derivatives can be used as pollution-monitor volatiles [15], although they have been found to be the main antibacterial volatile in New Zealand honeys [16]. All these categories of compounds have previously been detected in Thai honeys [17] in which terpenes were found to be the most abundant VOCs in both wild and coffee honeys. Although, there have been previous studies on honeys produced by Apis mellifera, information on VOCs from honey derived from other honeybee species are still lacking. In Northern Thailand, three species of honeybees (Apis mellfiera, Apis cerana and Apis dorsata) are known to produce honeys for human consumption. To bridge the information gap on honeys produced by other bee species, this present study aimed at determining the VOCs released by Thai honeys produced from different floral sources and three different honey species. Knowledge from this could inform the characteristics of honeys and subsequently used to distinguish honeys from different floral sources and honey bee species.
Materials and methods
Honey samples
Honeys produced from five different floral sources by three species of honey bees were obtained from retailers (processed honey) and beekeepers (unprocessed honey) in different provinces of Thailand (Chiang Mai, Chiang Rai, Lumphun, Nan, and Lopburi). The honey samples and their bee producers were Apis mellifera (longan honey, wild honey, lychee honey and sunflower honey), Apis cerana (longan honey, wild honey and coffee honey) and Apis dorsata (wild honey). All samples were kept in a dark room at 23–25°C until volatile samples were collected from them by solid-phase microextraction (SPME).
Analysis of volatile organic compounds
Sample preparation
For each of the honey samples from different floral sources, 2.5 g of honey was weighed into a 20 ml screw-top glass vial with PTFE/silicone lid. As internal standard, 5 μl of 1 mg/ml benzophenone (Sigma-Aldrich, Milan, Italy) in methanol (Sigma-Aldrich) was used, gently putting it on the inner wall of the vial.
Solid-phase microextraction of volatile samples
The volatile samples were analyzed by SPME where vials containing the honey samples and the internal standard were conditioned at 45°C in the agitator of the GC set-up for 15 minutes before SPME extraction. A 2 cm 50/30 μm divinylbenzene/carboxen/polymethylsiloxane (57299-U) SPME fiber was put into the vial to expose it to the headspace of the honey samples. The volatile samples were allowed 40 minutes to be adsorbed onto the SPME fiber at 45°C before GC injection. At injection into the GC, thermal desorption of volatile organic compounds from the SPME fiber lasted 5 minutes.
Gas chromatography—mass spectrometry
GC-MS analysis was done by using a GC (7890A, Agilent Technologies, Santa Clara, USA) coupled with an MS (5975C Network, Agilent Technologies). The GC had an HP 5MS column (non-polar column, Agilent Technologies), 30 m x 0.25 mm internal diameter (i.d.) and 0.25 μm film thickness. The carrier gas was helium flowing at a rate of 1.2 mL/ minute. The initial oven temperature was 40°C held for 3 minutes. It was raised to 200°C at 3°C/minute and then 16°C/minute until it reached 240°C and held at this temperature for 1.2 minutes. Mass spectra were recorded in a scanning range of 24–360 m/z.
Enhanced ChemStation (ver. E.02.02.1431, Agilent Technologies) was used for identification by comparing the mass spectra of VOCs with those in the database of NIST 11(Gaithersburg, MD, USA) and Wiley 7N (John Wiley, NY, USA). The linear retention indices (LRI) of the VOCs were calculated using the retention times of n-alkane series from C9 to C20 as reference compounds [18]. The calculated LRI were then compared to those in NIST webbook. Each peak area of characterized compound was compared to peak area of the internal standard (5 μL of 1 mg/mL benzophenone), the amounts of detected VOCs were expressed as relative amount (%) ± standard error.
Statistical analysis
The relative amounts of detected VOCs in the honey samples were analyzed for normality (Shapiro-Wilk test), Non-metric multidimensional scaling (NMDS) and grouping plot illustrations were generated using Paleontological statistics; PAST version 2. The normally distributed data were analyzed by one-way ANOVA (SPSS v.17.0; IBM, Chicago, IL, USA). Mann-Whitney tests (SPSS) were applied to data, which were not normally distributed. Significant levels were set at α = 0.05.
Results
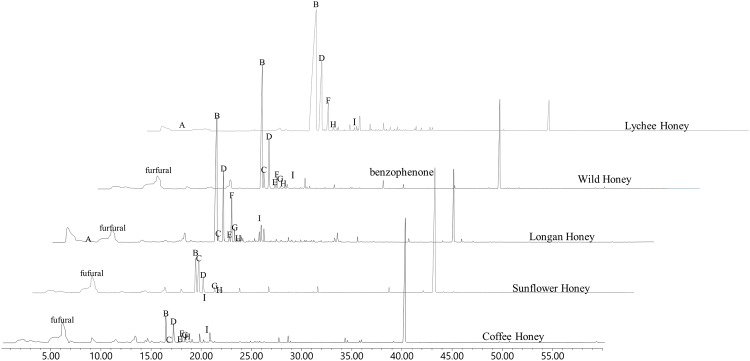
A total of 62 compounds were identified from the headspace volatiles of the honey samples studied. Of the total number of compounds, 48 were present in longan honey, 41 in wild honey, 21 in lychee honey, 23 in coffee honey and 8 in sunflower honey (Table 1). Moreover, the identified VOCs belonged to 7 chemical classes namely alcohols, aldehydes, ketones, esters, acids, hydrocarbons, and terpenes. Terpenes were the most abundant compounds among all the volatiles in all the samples. Three volatiles namely cis-linalool oxide (18.9–1,043.5%), furan-2,5-dicarbaldehyde (1.2–45.9%) and trans-linalool oxide (8.2–29.5%) were present in all honey samples (Fig 1). Some volatiles were absent in particular honeys, e.g. 2-phenylacetaldehyde, isophorone and methyl nonanoate were absent from longan honey from A. cerana while linalool, ho-trienol, benzyl ethanol, isophorone and epoxylinalool isomer II were absent from sunflower honey from A. mellifera. However, significantly high amounts of cis- and trans-linalool oxide were found in lychee (A. mellifera) and longan honeys (A. mellifera and A. cerana) than that found in the other honeys. Interestingly, 2-phenylacetaldehyde described as honey, sweet, and floral odor was detected in all honey sample except longan honey produced by A. cerana. When comparing the numbers of the identified volatile compounds, it was found that longan honey produced by A. mellifera had the highest amount of volatiles (48 VOCs) while, sunflower honey produced by A. mellifera has the least amount of volatiles (8 VOCs). The volatiles of longan honey produced by A. mellifera were double of ones of A. cerana, 48 and 22, respectively. In addition, the quantities of each volatile compound of longan honey produced by A. mellifera were generally more than the ones produced by A. cerana. When considering wild honeys, it was found that the ones produced by A. dorsata (31 VOCs) contained the highest number of volatile, followed by A. mellifera (26 VOCs) and A. cerana (14 VOCs), respectively.
Table 1. Relative amounts (%) ± standard error of Thai honeys volatile organic compounds as compared to the internal standard (benzophenone).
| No. | LRI | VOCs* | Longan | Wild | Lychee | Coffee | Sunflower | |||
|---|---|---|---|---|---|---|---|---|---|---|
| A. mellifera (n = 17) | A. cerana (n = 5) | A. dorsata (n = 8) | A. mellifera (n = 9) | A. cerana (n = 4) | A. mellifera (n = 4) | A. cerana (n = 5) | A. mellifera (n = 3) | |||
| 1 | <800 | 3-methylbutan-1-ol [isoamyl alcohol] | 24.39 ± 8.55 | 17.49 ± 5.90 | 30.79 ± 15.85 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 2 | 830 | furan-2-carbaldehyde [furfural] | 84.25 ± 21.24 | n.d. | 60.67 ± 30.93 | 60.27 ± 14.99 | n.d. | 24.59 ± 14.20 | 130.60 ± 55.91 | 109.92 ± 55.37 |
| 3 | 851 | 2-furanmethanol | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 12.32 ± 2.54 | n.d. |
| 4 | 910 | butyryl lactone | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 21.24 ± 7.76 | n.d. |
| 5 | 925 | methyl caproate | n.d. | n.d. | 1.34 ± 0.91 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 6 | 955 | benzaldehyde | n.d. | n.d. | 2.01 ± 1.32 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 7 | 961 | 5-methylfurfural | n.d. | n.d. | 4.62 ± 3.67 | n.d. | n.d. | n.d. | 12.02 ± 4.74 | n.d. |
| 8 | 1002 | 2-methyl-5-prop-1-en-2-ylcyclohexa-1,3-diene [1,5,8-p-menthatriene] | 3.46 ± 2.37 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 9 | 1019 | 1,8-nonadiyne | 4.64 ± 1.39 | n.d. | n.d. | 0.51 ± 0.34 | n.d. | n.d. | n.d. | n.d. |
| 10 | 1028 | ethyl heptanoate | 0.95 ± 0.53 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 11 | 1033 | phenylmethanol | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 10.82 ± 2.64 | n.d. |
| 12 | 1041 | 2-phenylacetaldehyde | 0.42 ± 0.28 | n.d. | 5.94 ± 3.06 | 2.91 ± 0.91 | 5.18 ± 1.03 | 1.26 ± 0.74 | 3.86 ± 1.65 | 3.51 ± 2.25 |
| 13 | 1070 | 2-(5-ethenyl-5-methyloxolan-2-yl)propan-2-ol [cis-linalool oxide] | 437.57 ± 87.28 ab | 377.40 ± 51.77 ab | 190.54 ± 58.04 b | 177.70 ± 64.95 b | 18.91 ± 6.14 b | 1043.49 ± 652.53 a | 54.90 ± 13.14 b | 32.29 ± 18.62 b |
| 14 | 1077 | furan-2,5-dicarbaldehyde | 24.11 ± 10.61 a | 1.22 ± 0.76 b | 45.92 ± 23.73 a | 23.26 ± 6.95 a | 1.62 ± 0.95 b | 7.16 ± 6.38 b | 3.09 ± 1.77 b | 33.50 ± 17.88 a |
| 15 | 1089 | 2-(5-ethenyl-5-methyloxolan-2-yl)propan-2-ol [trans-linalool oxide] | 112.71 ± 22.57 ab | 96.37 ± 11.96 ab | 80.30 ± 33.91 ab | 50.53 ± 17.28 b | 8.21 ± 2.32 b | 295.47 ± 170.70 a | 38.47 ± 8.45 b | 11.29 ± 6.52 b |
| 16 | 1100 | 3,7-dimethylocta-1,6-dien-3-ol [linalool] | 16.73 ± 3.17 a | 6.85 ± 1.27 b | 2.74 ± 1.40 b | 1.42 ± 0.65 c | 0.84 ± 0.50 c | 3.97 ± 1.95 b | 3.09 ± 1.19 b | n.d. |
| 17 | 1104 | (5E)-3,7-dimethylocta-1,5,7-trien-3-ol [ho-trienol] | 93.57 ± 13.37 a | 20.90 ± 6.98 b | 11.35 ± 5.65 c | 16.54 ± 4.69 c | 2.71 ± 1.07 c | 72.37 ± 43.08 b | 21.24 ± 10.17 b | n.d. |
| 18 | 1111 | benzyl ethanol | 18.32 ± 3.03 | 5.23 ± 0.87 | 18.62 ± 7.20 | 3.73 ± 1.08 | 4.29 ± 2.02 | 7.27 ± 6.14 | 10.65 ± 2.27 | n.d. |
| 19 | 1117 | 3,5,5-trimethyl-2-cyclohexene-1-one [isophorone] | 3.44 ± 0.76 | n.d. | 1.83 ± 0.98 | 6.17 ± 2.28 | 1.36 ± 0.81 | 10.42 ± 4.84 | 1.65 ± 1.07 | n.d. |
| 20 | 1126 | methyl octanoate | 1.22 ± 0.70 | n.d. | n.d. | 4.25 ± 1.72 | n.d. | n.d. | n.d. | n.d. |
| 21 | 1128 | (3E,5E)-2,6-dimethylocta-1,3,5,7-tetraene [cosmene] | 7.54 ± 1.10 | n.d. | n.d. | n.d. | n.d. | 7.66 ± 3.14 | n.d. | n.d. |
| 22 | 1142 | 4-oxoisophorone | 3.23 ± 1.36 b | n.d. | n.d. | 11.42 ± 4.37 a | 0.93 ± 0.55 c | 9.60 ± 2.68 a | 12.37 ± 3.66 a | n.d. |
| 23 | 1150 | 2-(5-ethenyl-5-methyloxolan-2-yl)propanal [lilac aldehyde C] | 2.40 ± 0.67 | n.d. | n.d. | 0.70 ± 0.54 | n.d. | 4.65 ± 3.08 | 2.11 ± 1.02 | n.d. |
| 24 | 1154 | 4-methyl-2-(2-methylprop-1-enyl)-3,6-dihydro-2H-pyran [nerol oxide] | 6.51 ± 1.88 a | n.d. | 0.42 ± 0.30 b | n.d. | n.d. | n.d. | n.d. | n.d. |
| 25 | 1167 | 2-(5-ethenyl-5-methyloxolan-2-yl)propan-2-ol isomer I [epoxylinalool 1] | 37.83 ± 4.86 a | n.d. | 1.26 ± 0.62 b | 6.00 ± 1.81 b | n.d. | 9.28 ± 4.50 b | n.d. | n.d. |
| 26 | 1173 | 2-(5-ethenyl-5-methyloxolan-2-yl)propan-2-ol isomer II [epoxylinalool 2] | 23.86 ± 3.47 b | 1.71 ± 0.52 b | 2.62 ± 0.91 b | 10.33 ± 3.03 b | 1.10 ± 0.41 b | 50.15 ± 20.35 a | 2.81 ± 0.88 b | n.d. |
| 27 | 1184 | butanedioic acid [succinic acid] | 4.91 ± 2.42 | 16.29 ± 7.76 | 8.39 ± 6.02 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 28 | 1188 | 2-(4-methyl-1-cyclohex-3-enyl)propan-2-ol [terpineol] | 2.07 ± 0.66 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 29 | 1196 | 2,6,6-trimethylcyclohexa-1,3-diene-1-carbaldehyde [safranal] | n.d. | n.d. | n.d. | 1.56 ± 0.75 | n.d. | 9.29 ± 7.06 | n.d. | n.d. |
| 30 | 1199 | tetrahydro-β,5-dimethyl-5-vinyl-2-furanethanol isomer I [lilac alcohol A] | 2.50 ± 0.71 | n.d. | 2.60 ± 1.38 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 31 | 1201 | tetrahydro-β,5-dimethyl-5-vinyl-2-furanethanol isomer III [lilac alcohol B] | 4.98 1.45 | 2.36 ± 0.75 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 32 | 1208 | tetrahydro-β,5-dimethyl-5-vinyl-2-furanethanol isomer II [lilac alcohol C] | 1.59 ± 0.46 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 33 | 1226 | methyl nonanoate | 9.65 ± 2.14 | n.d. | 2.09 ± 0.97 | 14.76 ± 5.07 | 1.80 ± 1.17 | 12.50 ± 9.31 | n.d. | 6.26 ± 3.30 |
| 34 | 1228 | 5-(hydroxymethyl)-2-furaldehyde [5-hydroxymethylfurfural] | n.d. | n.d. | 1.93 ± 0.97 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 35 | 1232 | 2,6,6-trimethyl-2-cyclohexen-1-ol | n.d. | n.d. | n.d. | 1.12 ± 0.57 | n.d. | n.d. | n.d. | n.d. |
| 36 | 1233 | 2-hydroxy-3,5,5-trimethyl-2-cyclohexen-1,4-dione | 5.81 ± 1.00 a | 1.86 ± 0.18 b | n.d. | n.d. | n.d. | 5.97 ± 2.85 a | n.d. | n.d. |
| 37 | 1244 | ethyl 2-phenylacetate | 1.22 ± 0.55 | 0.87 ± 0.39 | 1.67 ± 0.93 | n.d. | n.d. | n.d. | 1.07 ± 0.67 | n.d. |
| 38 | 1250 | 4-methoxybenzaldehyde [anisaldehyde] | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 1.90 ± 1.09 | n.d. |
| 39 | 1253 | tetrahydro-β,5-dimethyl-5-vinyl-2-furanethanol isomer IV [lilac alcohol D] | 1.61 ± 0.52 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 40 | 1257 | 2-phenylethyl acetate | 1.48 ± 1.24 | 3.79 ± 1.35 | 7.86 ± 7.07 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 41 | 1280 | (4-methoxyphenyl)methanol [anise alcohol] | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 1.31 ± 1.03 | n.d. |
| 42 | 1297 | ethyl nonanate | 3.33 ± 1.62 | 3.81 ± 1.19 | 2.91 ± 1.85 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 43 | 1314 | 3,4,5-trimethyl-phenol | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 5.69 ± 2.09 | n.d. |
| 44 | 1325 | methyl decanoate | 1.59 ± 0.47 | n.d. | 0.64 ± 0.33 | 1.94 ± 0.71 | n.d. | n.d. | 0.52 ± 0.33 | 1.08 ± 0.64 |
| 45 | 1329 | 2,2-dimethyl butanal | 3.94 ± 1.45 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 46 | 1382 | (E)-1-(2,6,6-trimethylcyclohexa-1,3-dien-1-yl)but-2-en-1-one [damascenone] | 6.96 ± 1.16 a | 1.49 ± 0.88 b | 2.15 ± 1.07 ab | 0.47 ± 0.36 b | n.d. | 0.63 ± 0.36 b | n.d. | n.d. |
| 47 | 1389 | diethyl hexanedioate | n.d. | 0.41 ± 0.25 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 48 | 1396 | ethyl decanoate | 0.40 ± 0.23 b | 1.28 ± 0.50 a | 1.57 ± 0.80 ab | n.d. | n.d. | n.d. | n.d. | n.d. |
| 49 | 1400 | tetradecane | 0.28 ± 0.20 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 50 | 1500 | pentadecane | 1.25 ± 0.77 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 51 | 1516 | (Z)-5,6-dimethyl-1,3-cyclohexadiene | n.d. | n.d. | n.d. | n.d. | n.d. | 2.18 ± 0.80 | n.d. | n.d. |
| 52 | 1537 | methyl 10-oxodecanoate | n.d. | n.d. | n.d. | 0.39 ± 0.26 | n.d. | n.d. | n.d. | n.d. |
| 53 | 1597 | ethyl dodecanoate | n.d. | 2.59 ± 1.26 | 1.60 ± 1.27 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 54 | 1600 | hexadecane | 0.46 ± 0.27 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 55 | 1646 | 1,4-dimethylindanyl acetate | 8.11 ± 1.62 a | n.d. | n.d. | 0.90 ± 0.61 b | n.d. | n.d. | n.d. | n.d. |
| 56 | 1652 | dimethyl decanedioate | 1.66 ± 0.48 ab | n.d. | 0.41 ± 0.27 b | 7.07 ± 1.85 a | 1.56 ± 0.82 ab | 1.53 ± 0.52 ab | n.d. | 4.09 ± 3.54 ab |
| 57 | 1727 | methyl tetradecanoate | n.d. | 0.61 ± 0.38 | n.d. | 0.36 ± 0.25 | 1.56 ± 0.71 | n.d. | n.d. | n.d. |
| 58 | 1790 | diethyl decanedioate | n.d. | 1.08 ± 0.81 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 59 | 1796 | ethyl tetradecanoate | n.d. | 4.04 ± 1.59 | 1.14 ± 0.66 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 60 | 1922 | metyl hexadecanoate | 0.66 ± 0.19 | 2.36 ± 0.73 | 2.22 ± 0.61 | 2.40 ± 1.23 | 3.59 ± 0.96 | 0.55 ± 0.32 | 5.60 ± 4.01 | n.d. |
| 61 | 1977 | ethyl hexadecanoate | n.d. | 9.30 ± 2.84 | 5.90 ± 4.23 | n.d. | n.d. | n.d. | 1.60 ± 1.03 | n.d. |
| 62 | 2051 | methyl oleate | 0.77 ± 0.21 | n.d. | n.d. | 0.36 ± 0.18 | n.d. | n.d. | n.d. | n.d. |
Values with different letters in the columns indicate statistical difference at P ≤ 0.05. nd: not detected
LRI: calculated linear retention index
*Common names of the VOCs are present in the square brackets. 10 dominant volatiles are present in bold.
Fig 1. Representative chromatograms of Thai honeys on HP-5MS column.
A: isoamyl alcohol. B: cis-linalool oxide. C: 2,5-furandicarboxaldehyde. D: trans-linalool oxide. E: linalool. F: ho-trienol. G: benzyl ethanol. H: isophorone. I: epoxylinalool.
Lilac alcohol B was only found in longan honey (both A. mellifera and A. cerana) (Table 2). Furthermore, 1,5,8-p-menthatriene, ethyl heptanoate, terpineol, lilac alcohol C, lilac alcohol D, 2,2-dimethyl butanal, tetradecane, pentadecane and hexadecane were only found in longan honey from A. mellifera (Table 2). Diethyl hexanedioate and diethyl decanedioate were only found in longan honey from A. cerana. Methyl caproate, benzaldehyde and 5-hydroxymethylfurfural were only found in wild honey from A. dorsata while methyl 10-oxodecanoate, 2,6,6-trimethyl-2-cyclohexen-1-ol and methyl oleate were only found in wild honey from A. mellifera. (Z)-5,6-dimethyl-1,3-cyclohexadiene and were only found in lychee honey. 2-Furanmethanol, butyryl lactone, phenylmethanol, anisaldehyde, anise alcohol and 3,4,5-trimethyl-phenol were only found in coffee honey from A. cerana (Table 2). Interestingly, 2-hydroxy-3,5,5-trimethyl-2-cyclohexen-1,4-dione was only found in lychee from A. mellifera and longan honey from both A.mellifera and A. cerana.
Table 2. List of VOCs that could serve as possible floral markers and their odor descriptors.
| Honey | Compound | Odor descriptor | ||
|---|---|---|---|---|
| Present | longan honey (A. mellifera) | 1 | 2-methyl-5-prop-1-en-2-ylcyclohexa-1,3-diene [1,5,8-p-menthatriene] | roasted |
| 2 | ethyl heptanoate | fruity | ||
| 3 | 2-(4-methyl-1-cyclohex-3-enyl)propan-2-ol [terpineol] | odorless | ||
| 4 | tetrahydro-β,5-dimethyl-5-vinyl-2-furanethanol isomer II [lilac alcohol C] | green, grassy and fresh | ||
| 5 | tetrahydro-β,5-dimethyl-5-vinyl-2-furanethanol isomer IV [lilac alcohol D] | green, grassy and fresh | ||
| 6 | 2,2-dimethyl butanal | - | ||
| 7 | hexadecane | - | ||
| 8 | tetradecane | waxy | ||
| 9 | pentadecane | waxy | ||
| longan honey (A. mellifera & A. cerana) | 10 | tetrahydro-β,5-dimethyl-5-vinyl-2-furanethanol isomer III [lilac alcohol B] | green, grassy and fresh | |
| longan honey (A. cerana) | 11 | diethyl hexanedioate | - | |
| 12 | diethyl decanedioate | fruity | ||
| wild honey (A. dorsata) | 13 | methyl caproate | fruity | |
| 14 | benzaldehyde | almond, fruity, powdery, nutty and benzaldehyde-like | ||
| 15 | 5-(hydroxymethyl)-2-furaldehyde [5-hydroxymethylfurfural] | fatty, buttery, musty, waxy and caramellic | ||
| wild honey (A. mellifera) | 16 | methyl 10-oxodecanoate | - | |
| 17 | methyl oleate | fatty | ||
| 18 | 2,6,6-trimethyl-2-cyclohexen-1-ol | - | ||
| lychee honey (A. mellifera) | 19 | (Z)-5,6-dimethyl-1,3-cyclohexadiene | - | |
| coffee honey (A. cerana) | 20 | 2-furanmethanol | brown caramellic, bready and coffee | |
| 21 | butyryl lactone | Creamy, fatty and dairy-like | ||
| 22 | phenylmethanol | floral | ||
| 23 | 4-methoxybenzaldehyde [anisaldehyde] | sweet, powdery, vanilla, anise and woody | ||
| 24 | (4-methoxyphenyl)methanol [anise alcohol] | weet, powdery creamy, balsamic and coumarin | ||
| 25 | 3,4,5-trimethyl-phenol | green | ||
| Absent | longan honey (A. cerana) | 26 | 2-phenylacetaldehyde | honey and floral rose |
| Sunflower (A.mellifera) | 27 | 3,7-dimethylocta-1,6-dien-3-ol (linalool) | citrus, orange, floral, terpy, waxy and rose | |
| 28 | (5E)-3,7-dimethylocta-1,5,7-trien-3-ol [ho-trienol] | sweet, tropica and ginger | ||
| 29 | benzyl ethanol | Sweet, floral and fruity | ||
| 30 | 2-(5-ethenyl-5-methyloxolan-2-yl)propan-2-ol isomer II [epoxylinalool 2] | - | ||
| 31 | metyl hexadecanoate | Waxy |
Common names of the VOCs are present in the square brackets.
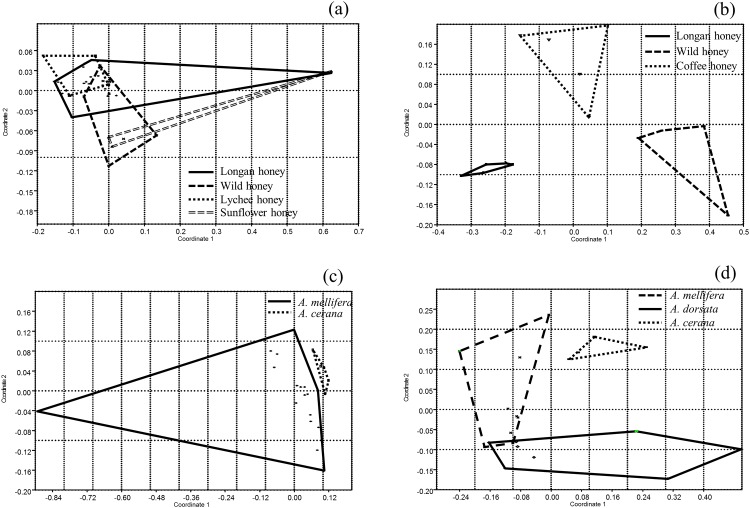
Non-metric multidimensional scaling and plot grouping analysis revealed that differences in floral sources and honeybee species lead to differences in VOC groupings. Among volatiles from honey produced by the same honeybee species, A. mellifera, longan honey was the largest group with numerous volatile compounds (Fig 2a). Volatiles in longan honey slightly overlapped with volatiles from three different groups of floral sources namely, lychee, wild and sunflower (Fig 2a). Likewise, the volatile group of wild honey overlapped with the longan, lychee and sunflower groups. However, volatiles belonging to lychee honey were completely separated from volatiles belonging to sunflower honey (Fig 2a). There were clear differences among volatiles of honey produced by A. cerana from three different floral sources (longan, wild and coffee) (Fig 2b). Volatiles from longan honey produced by A. mellifera and A. cerana were distinctly different (Fig 2c). Moreover, volatiles from wild honey produced by A. cerana was distinctly different from those produced by A. mellifera and A. dorsata (Fig 2d) but wild honey from A. mellifera and A. dorsata slightly overlapped (Fig 2d). These results reflect the differences among VOC groups due to floral sources and honeybee species.
Fig 2. Groupings among volatile organic compounds of (a) longan honey, wild honey, lychee honey and sunflower honey from A. mellifera (b) longan honey, wild honey, coffee honey from A. cerana showing how VOCs from same honeybee species are clustered or separated from VOCs from different floral sources; (c) longan honey from A. mellifera and A. cerana (d) wild honey from A. mellifera, A. dorsata and A. cerana showing how VOCs from same floral sources are clustered or separated from VOCs from different honeybee species.
Discussion
This study is the first to investigate the volatile organic compounds of Thai honeys from several floral sources and different honeybee species. In Thailand, honey is often marketed as unifloral honey. The floral origin of the honey gives it a unique flavor and fragrance. In fact, it is not only the botanical origin of flora that influence the quality of honey but also some extended factors such as honeybee species and storage condition. This present study focused on the influence of floral source and honeybee species on aroma of honey.
To the best of our knowledge, butyryl lactone, 1,8-nonadiyne, ethyl heptanoate, 2,6,6-trimethyl-2-cyclohexen-1-ol, 2-hydroxy-3,5,5-trimethyl-2-cyclohexen-1,4-dione, anise alcohol, 2,2-dimethyl butanal and (Z)-5,6-dimethyl-1,3-cyclohexadiene, which were found in Thai honeys in this study have never been reported in other honeys. Moreover, some particular volatiles in the headspace of specific honey types have been considered to be floral markers for those particular honeys. Thirty-one of such potential markers were observed. For instance, 1,5,8-p-menthatriene, ethyl heptanoate, terpineol, lilac alcohol C, lilac alcohol D, 2,2-dimethyl butanal, pentadecane and hexadecane were classified as markers for longan honey from A. mellifera while diethyl hexanedioate and diethyl decanedioate were classified as markers for longan honey from A. cerana. Furthermore, lilac alcohol B was classified as a marker for longan honey from both A. mellifera and A. cerana. Methyl caproate, benzaldehyde and 5-hydroxymethylfurfural were classified as markers for wild honey from A. dorsata while methyl 10-oxodecanoate, 2,6,6-trimethyl-2-cyclohexen-1-ol and methyl oleate were classified as markers for wild honey from A. mellifera. (Z)-5,6-dimethyl-1,3-cyclohexadiene could be characteristic volatiles for lychee honey from A. mellifera whiles 2-furanmethanol, butyryl lactone, phenylmethanol, anisaldehyde, anise alcohol and 3,4,5-trimethyl-phenol could be considered as markers for coffee honey from A. cerana. Although, these identified volatiles were detected in small quantities they could be considered as marker VOCs because of their specificity and/or uniqueness to those honeys. On the contrary, although the amounts of cis- and trans-linalool oxide were notably high, they could not be considered specific to a particular honey because they have been found in both lychee honey (A. mellifera) and longan honeys (A.mellifera and A. cerana). However, 2-hydroxy-3,5,5-trimethyl-2-cyclohexen-1,4-dione was found in longan and lychee honeys which could be because of these two honey originated from floral sources of the same family (Sapindaceae) [19].
An important finding of this study is that volatiles emitted by honeys are dependent on both the floral source and honeybee species that produced the honey. Honeybees feeding on different floral sources produce honeys with different quality and quantity of volatiles. The highest number of volatiles detected were from longan honey followed by wild honey, lychee honey, coffee honey and sunflower honey, which had the least number of volatiles. This finding supports the evidence that longan honey is the most fragrant honey, while sunflower honey is the least fragrant honey among monofloral honey as highlighted by Thai beekeepers. In addition, this is in agreement with many previous studies that found floral sources to be a major factor for differentiating honey aroma [4, 7, 20, 21]. It is well known that wild honey is multifloral honey derived from a variety of nectar sources. It is therefore reasonable to suggest that VOCs of wild honey mimic a combination of VOCs from various monofloral honeys. This is in agreement with our results, which showed that the group of VOCs from wild honey overlapped with every other group of monofloral VOCs. In particular, plants from same family tended to have similar quality and quantity of volatile compounds. It was therefore not surprising that longan and lychee both of which belong to the family Sapindaceae [19] had groups of volatiles that overlapped as seen in Fig 2a. As expected, the VOCs of sunflower (family: Asteraceae) [22], barely overlapped with other monofloral groups.
So far, relatively few studies have focused on the honeybee species as a factor that causes differences in honey aroma [23, 24]. As it is depicted in Fig 2, showing volatile organic compounds plot grouping of honey produced from different floral sources and bee species. The volatile compounds of honey produced by A. mellifera were overlapped (Fig 2a). On the contrary, as shown in Fig 2b, volatile compounds of honey produced by A. cerana were clearly grouped. Additionally, Fig 2c and 2d confirm that bee species affect the volatiles of honey even if they feed on the same floral source even though a slight overlap of VOCs was observed between wild honey from A. mellifera and A. dorsata. This study put the spotlight on the honeybee as an important factor that could influence the aroma of honey. The differences in the volatile profiles of the different monofloral honeys are likely influenced by the different honeybee species. For instance, we found that 2-phenylacetaldehyde was absent only in longan honey produced by A. cerana. Meanwhile longan honey from A. mellifera produced more volatiles than any other honey. The foraging behavior of bees that keeps nectar in their honey stomach for some time during their flight back to hives before transferring them into honey combs contribute to these observed differences. During the period that nectar is in the honey stomach, they may be transformed and that likely influence the VOCs. A recent study (18) in which a unique lactic acid bacteria (LAB) symbiont was discovered gives credence to this assertion. In that study, it was established that symbionts from the honey stomach of honeybees influence the production of metabolites including volatile compounds. Most importantly, the LAB symbionts in the honeybees’ honey stomach have also been found to influence antimicrobial properties of tested honeys. Furthermore, the lactic acid bacteria vary in different honeybee species [25]. Therefore, it may be possible that the metabolites produced due to LAB symbionts from different honeybee species would have impact not only on antibacterial properties but could also vary the volatile compounds in honeys. Foraging distance in each honeybee species could also be a factor that contributed to varied volatile compounds in tested honeys. It was found that in wild honey collected from A. dorsata provided more volatiles than ones from A. mellifera and A. cerana. It is known that A. dorsata can forage beyond 5 km [26], A. mellifera may forage up to 3 km [27] while A. cerana around 1–1.5 km [27]. The longer foraging distance may increase the chance to collect nectar from various sources leading to more detected volatile compounds in tested honeys. However, more investigation is required.
Many of the dominant volatiles in this study have been reported as common honey volatiles e.g. cis-linalool oxide [28–30], trans-linalool oxide [28, 30–35], ho-trienol [28, 30, 36, 37], epoxylinalool [38], 2-phenylacetaldehyde [4, 8, 39–41], furan-2,5-dicarbaldehyde [42], benzyl ethanol [7, 42, 43], isophorone [21, 44, 45] and methyl nonanoate [46, 47]. Above all, the identified compounds appeared to be typical constituents of Thai honey at various proportions and therefore provide the uniqueness of their aroma. Aside influencing the aroma of honey, the type and proportion of volatiles in the honey is known to influence the therapeutic properties including antioxidant, antibacterial and even immune enhancement. For instance, linalool and nerol oxide have been described as antibiotic volatiles which show broad-spectrum antimicrobial activity [48–57]. Moreover, linalool had previously been identified as an antioxidant volatile [58, 59], likewise safranal [60, 61], 4-oxoisophorone [62], and damascenone [62]. The antioxidant capacity of 4-oxoisophorone and damascenone have been found to have the potential to enhance the vertebrate immune system [63]. Based on the concentration of these compounds in the headspace of the honey samples, their biomedical ability could be slightly low compared to four salient factors of honey (hydrogen peroxide, high osmotic pressure, acidity and non-peroxide factors). It would be interesting to conduct further experiments to find out whether there are differences among volatiles of honey produced by the same bee species feeding on the same floral source in different geographical regions.
Conclusion
There were a total of 63 compounds detected in the headspace of the honey samples. The highest (48) and the least (8) numbers of volatiles were identified in longan honey and sunflower honey, respectively. Each of the Thai honey samples studied had a complex and unique volatile organic compound profile which varied in quality and quantity from other honey samples. The variation in volatile compounds in the honey samples lead to uniqueness of fragrances, tastes and potential biomedical properties. These characteristic depend not only on the nectar-providing plant species, but also on honeybee species. Therefore, volatile compound profiles may be used as chemical markers for tracing the origin of honey. Additionally, NMDS and grouping plot can be used for controlling honey produced by different floral sources and bee species.
Acknowledgments
The authors thank the Faculty of Science and Technology, Free University of Bozen-Bolzano, Italy for lab facilities, Chiang Mai Healthy Product Co, Ltd., for supplying Thai honey samples, the Royal Golden Jubilee Program and Graduate School of Chiang Mai University, Thailand for supporting this research.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by The Royal Golden Jubilee (RGJ) Ph.D. Programme Tel: 02-278-8200 ext. 1 (http://rgj.trf.or.th/main/en/) to PP and PC. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Elsheikh ATA. Physicochemical Properties of Bee Honey from Different Floral Origins as Compared to Those of Sugarcane Honey: UOFK; 2015. [Google Scholar]
- 2.Al-Qassemi R, Robinson R. Some special nutritional properties of honey-a brief review. Nutrition & Food Science. 2003;33(6):254–60. [Google Scholar]
- 3.Jerković I, Marijanović Z. Screening of Volatile Composition of Lavandula hybridaReverchon II Honey Using Headspace Solid‐Phase Microextraction and Ultrasonic Solvent Extraction. Chemistry & biodiversity. 2009;6(3):421–30. [DOI] [PubMed] [Google Scholar]
- 4.Alissandrakis E, Tarantilis PA, Harizanis PC, Polissiou M. Evaluation of four isolation techniques for honey aroma compounds. Journal of the Science of Food and Agriculture. 2005;85(1):91–7. [Google Scholar]
- 5.Alissandrakis E, Tarantilis PA, Harizanis PC, Polissiou M. Comparison of the volatile composition in thyme honeys from several origins in Greece. Journal of agricultural and food chemistry. 2007;55(20):8152–7. 10.1021/jf071442y [DOI] [PubMed] [Google Scholar]
- 6.Odeh I, Abu-Lafi S, Dewik H, Al-Najjar I, Imam A, Dembitsky VM, et al. A variety of volatile compounds as markers in Palestinian honey from Thymus capitatus, Thymelaea hirsuta, and Tolpis virgata. Food Chemistry. 2007;101(4):1393–7. [Google Scholar]
- 7.Piasenzotto L, Gracco L, Conte L. Solid phase microextraction (SPME) applied to honey quality control. Journal of the Science of Food and Agriculture. 2003;83(10):1037–44. [Google Scholar]
- 8.Castro-Vázquez L, Díaz-Maroto MC, Pérez-Coello MS. Volatile composition and contribution to the aroma of Spanish honeydew honeys. Identification of a new chemical marker. Journal of agricultural and food chemistry. 2006;54(13):4809–13. 10.1021/jf0604384 [DOI] [PubMed] [Google Scholar]
- 9.Vázquez LC, Díaz-Maroto MC, Guchu E, Pérez-Coello MS. Analysis of volatile compounds of eucalyptus honey by solid phase extraction followed by gas chromatography coupled to mass spectrometry. European Food Research and Technology. 2006;224(1):27–31. [Google Scholar]
- 10.Peña RM, Barciela J, Herrero C, García‐Martín S. Solid‐phase microextraction gas chromatography‐mass spectrometry determination of monoterpenes in honey. Journal of separation science. 2004;27(17‐18):1540–4. [DOI] [PubMed] [Google Scholar]
- 11.Ferreira ACS, de Pinho PG. Nor-isoprenoids profile during port wine ageing—influence of some technological parameters. Analytica Chimica Acta. 2004;513(1):169–76. [Google Scholar]
- 12.Yue X-D, Qu G-W, Li B-F, Xue C-H, Li G-S, Dai S-J. Two new C13-norisoprenoids from Solanum lyratum. Journal of Asian natural products research. 2012;14(5):486–90. 10.1080/10286020.2012.678331 [DOI] [PubMed] [Google Scholar]
- 13.Wang S-L, Jiao L-X, Li Y-H, Fan M-T. Degradation of β-carotene to volatile compounds in an aqueous model system to simulate the production of sea buckthorn wine. International Journal of Food Properties. 2012;15(6):1381–93. [Google Scholar]
- 14.Ningrum A, Minh NN, Schreiner M. Carotenoids and Norisoprenoids as Carotenoid Degradation Products in Pandan Leaves (Pandanus amaryllifolius Roxb.). International Journal of Food Properties. 2015;18(9):1905–14. [Google Scholar]
- 15.Bentivenga G, D'Auria M, Fedeli P, Mauriello G, Racioppi R. SPME‐GC‐MS analysis of volatile organic compounds in honey from Basilicata. Evidence for the presence of pollutants from anthropogenic activities. International journal of food science & technology. 2004;39(10):1079–86. [Google Scholar]
- 16.Tan ST, Holland PT, Wilkins AL, Molan PC. Extractives from New Zealand honeys. 1. White clover, manuka and kanuka unifloral honeys. J Agricult Food Chem. 1988;36(3):453–60. [Google Scholar]
- 17.Pattamayutanon P, Angeli S, Thakeow P, Abraham J, Disayathanoowat T, Chantawannakul P. Biomedical Activity and Related Volatile Compounds of Thai Honeys from 3 Different Honeybee Species. Journal of food science. 2015;80(10):M2228–M40. 10.1111/1750-3841.12993 [DOI] [PubMed] [Google Scholar]
- 18.Van den Dool H, Kratz PD. A generalization of the retention index system including linear temperature programmed gas—liquid partition chromatography. J Chromatogr A. 1963;11:463–71. [DOI] [PubMed] [Google Scholar]
- 19.Wall MM. Ascorbic acid and mineral composition of longan (Dimocarpus longan), lychee (Litchi chinensis) and rambutan (Nephelium lappaceum) cultivars grown in Hawaii. Journal of Food Composition and Analysis. 2006;19(6):655–63. [Google Scholar]
- 20.Stanimirova I, Üstün B, Cajka T, Riddelova K, Hajslova J, Buydens L, et al. Tracing the geographical origin of honeys based on volatile compounds profiles assessment using pattern recognition techniques. Food Chemistry. 2010;118(1):171–6. [Google Scholar]
- 21.de la Fuente E, Martínez‐Castro I, Sanz J. Characterization of Spanish unifloral honeys by solid phase microextraction and gas chromatography‐mass spectrometry. Journal of separation science. 2005;28(9‐10):1093–100. [DOI] [PubMed] [Google Scholar]
- 22.Brickell C. RHS AZ. Encyclopedia of Garden Plants, UK. Dorling Kindersley. 2008;1136.
- 23.White JW. Honey. Advances in food research. 1978;24:287–374. [DOI] [PubMed] [Google Scholar]
- 24.Molan P, Russell K. Non-peroxide antibacterial activity in some New Zealand honeys. J Apicult Res. 1988. [Google Scholar]
- 25.Disayathanoowat T, Young JPW, Helgason T, Chantawannakul P. T-RFLP analysis of bacterial communities in the midguts of Apis mellifera and Apis cerana honey bees in Thailand. FEMS microbiology ecology. 2012;79(2):273–81. 10.1111/j.1574-6941.2011.01216.x [DOI] [PubMed] [Google Scholar]
- 26.Koeniger N, Vorwohl G. Competition for food among four sympatric species of Apini in Sri Lanka (Apis dorsata, Apis cerana, Apis florea and Trigona iridipennis). Journal of Apicultural Research. 1979;18(2):95–109. [Google Scholar]
- 27.Abrol D. Foraging range of subtropical bees, Megachile flavipes, Megachile nana (Hymenoptera: Megachilidae) and Apis florea (Hymenoptera: Apidae). Journal of the Indian Institute of Science. 2013;68(1&2):43. [Google Scholar]
- 28.Aronne G, Giovanetti M, Sacchi R, De Micco V. From Flower to Honey Bouquet: Possible Markers for the Botanical Origin of Robinia Honey. The Scientific World Journal. 2014;2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radovic BS, Careri M, Mangia A, Musci M, Gerboles M, Anklam E. Contribution of dynamic headspace GC–MS analysis of aroma compounds to authenticity testing of honey. Food Chemistry. 2001;72(4):511–20. 10.1016/S0308-8146(00)00263-6. [DOI] [Google Scholar]
- 30.Jerković I, Hegić G, Marijanović Z, Bubalo D. Organic extractives from Mentha spp. honey and the bee-stomach: methyl syringate, vomifoliol, terpenediol I, hotrienol and other compounds. Molecules. 2010;15(4):2911–24. 10.3390/molecules15042911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jerković I, Marijanović Z, Malenica-Staver M, Lušić D. Volatiles from a rare acer spp. honey sample from Croatia. Molecules. 2010;15(7):4572–82. 10.3390/molecules15074572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jerković I, Obradović M, Kuś PM, Šarolić M. Bioorganic Diversity of Rare Coriandrum sativum L. Honey: Unusual Chromatographic Profiles Containing Derivatives of Linalool/Oxygenated Methoxybenzene. Chem Biodivers. 2013;10(8):1549–58. 10.1002/cbdv.201300074 [DOI] [PubMed] [Google Scholar]
- 33.Castro-Vázquez L, Pérez-Coello M, Cabezudo M. Analysis of volatile compounds of rosemary honey. Comparison of different extraction techniques. Chromatographia. 2003;57(3–4):227–33. [Google Scholar]
- 34.Soria AC, Martínez‐Castro I, Sanz J. Analysis of volatile composition of honey by solid phase microextraction and gas chromatography‐mass spectrometry. Journal of Separation Science. 2003;26(9‐10):793–801. [DOI] [PubMed] [Google Scholar]
- 35.Stanimirova I, Üstün B, Cajka T, Riddelova K, Hajslova J, Buydens L, et al. Tracing the geographical origin of honeys based on volatile compounds profiles assessment using pattern recognition techniques. Food Chem. 2010;118(1):171–6. [Google Scholar]
- 36.Soria AC, Sanz J, Martínez-Castro I. SPME followed by GC–MS: a powerful technique for qualitative analysis of honey volatiles. European Food Research and Technology. 2009;228(4):579–90. [Google Scholar]
- 37.Jerković I, Marijanović Z, Jelić M, editors. Headspace volatiles from unifloral honeys of Satureja montana L. and Salvia officinalis L. of Croatian origin isolated by solid phase microextraction (SPME). 55th Int Congr Ann Meet Soc Med Plant Res; 2007.
- 38.Čajka T, Hajšlová J, Cochran J, Holadová K, Klimánková E. Solid phase microextraction–comprehensive two‐dimensional gas chromatography–time‐of‐flight mass spectrometry for the analysis of honey volatiles. Journal of Separation Science. 2007;30(4):534–46. [DOI] [PubMed] [Google Scholar]
- 39.Baroni MV, Nores ML, Díaz MDP, Chiabrando GA, Fassano JP, Costa C, et al. Determination of volatile organic compound patterns characteristic of five unifloral honey by solid-phase microextraction-gas chromatography-mass spectrometry coupled to chemometrics. Journal of agricultural and food chemistry. 2006;54(19):7235–41. 10.1021/jf061080e [DOI] [PubMed] [Google Scholar]
- 40.Serra Bonvehí J, Ventura Coll F. Flavour index and aroma profiles of fresh and processed honeys. Journal of the Science of Food and Agriculture. 2003;83(4):275–82. [Google Scholar]
- 41.Shimoda M, Wu Y, Osajima Y. Aroma compounds from aqueous solution of haze (Rhus succedanea) honey determined by adsorptive column chromatography. Journal of Agricultural and Food Chemistry. 1996;44(12):3913–8. [Google Scholar]
- 42.Wolski T, Tambor K, Rybak-Chmielewska H, Kedzia B. Identification of honey volatile components by solid phase microextraction (SPME) and gas chromatography/mass spectrometry (GC/MS). Journal of Apicultural Science. 2006;50(2):115–26. [Google Scholar]
- 43.Viuda‐Martos M, Ruiz‐Navajas Y, Zaldivar‐Cruz JM, Kuri V, Fernández‐López J, Carbonell‐Barrachina ÁA, et al. Aroma profile and physico‐chemical properties of artisanal honey from Tabasco, Mexico. International journal of food science & technology. 2010;45(6):1111–8. [Google Scholar]
- 44.Montenegro G, Gómez M, Casaubon G, Belancic A, Mujica A, Peña R. Analysis of volatile compounds in three unifloral native Chilean honeys. Phyton (Buenos Aires). 2009;78:61–5. [Google Scholar]
- 45.De la Fuente E, Sanz M, Martínez-Castro I, Sanz J, Ruiz-Matute A. Volatile and carbohydrate composition of rare unifloral honeys from Spain. Food Chemistry. 2007;105(1):84–93. [Google Scholar]
- 46.Jerković I, Tuberoso CI, Marijanović Z, Jelić M, Kasum A. Headspace, volatile and semi-volatile patterns of Paliurus spina-christi unifloral honey as markers of botanical origin. Food Chem. 2009;112(1):239–45. [Google Scholar]
- 47.Alissandrakis E, Tarantilis PA, Pappas C, Harizanis PC, Polissiou M. Investigation of organic extractives from unifloral chestnut (Castanea sativa L.) and eucalyptus (Eucalyptus globulus Labill.) honeys and flowers to identification of botanical marker compounds. LWT-Food Science and Technology. 2011;44(4):1042–51. [Google Scholar]
- 48.Soković M, Glamočlija J, Marin PD, Brkić D, van Griensven LJ. Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules. 2010;15(11):7532–46. 10.3390/molecules15117532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park S-N, Lim YK, Freire MO, Cho E, Jin D, Kook J-K. Antimicrobial effect of linalool and α-terpineol against periodontopathic and cariogenic bacteria. Anaerobe. 2012;18(3):369–72. 10.1016/j.anaerobe.2012.04.001 [DOI] [PubMed] [Google Scholar]
- 50.Alviano W, Mendonça‐Filho R, Alviano D, Bizzo H, Souto‐Padrón T, Rodrigues M, et al. Antimicrobial activity of Croton cajucara Benth linalool‐rich essential oil on artificial biofilms and planktonic microorganisms. Oral Microbiol Immunol. 2005;20(2):101–5. 10.1111/j.1399-302X.2004.00201.x [DOI] [PubMed] [Google Scholar]
- 51.Cha J-D, Jung E-K, Kil B-S, Lee K-Y. Chemical composition and antibacterial activity of essential oil from Artemisia feddei. J Microbiol Biotechnol. 2007;17(12):2061–5. [PubMed] [Google Scholar]
- 52.Gopanraj G, Dan M, Shiburaj S, Sethuraman MG, George V. Chemical composition and antibacterial activity of the rhizome oil of Hedychium larsenii. Acta Pharm. 2005;(55):315–20. [PubMed] [Google Scholar]
- 53.Park M, Gwak K, Yang I, Kim K, Jeung E, Chang J, et al. Effect of citral, eugenol, nerolidol and α-terpineol on the ultrastructural changes of Trichophyton mentagrophytes. Fitoterapia. 2009;80(5):290–6. 10.1016/j.fitote.2009.03.007 [DOI] [PubMed] [Google Scholar]
- 54.Kotan R, Kordali S, Cakir A. Screening of antibacterial activities of twenty-one oxygenated monoterpenes. Z Naturforsch C. 2007;62(7/8):507. [DOI] [PubMed] [Google Scholar]
- 55.Bougatsos C, Ngassapa O, Runyoro DK, Chinou IB. Chemical composition and in vitro antimicrobial activity of the essential oils of two Helichrysum species from Tanzania. Zeitschrift fur Naturforschung C-Journal of Biosciences. 2004;59(5–6):368–72. [DOI] [PubMed] [Google Scholar]
- 56.El-Moaty HIA. Essential oil and iridoide glycosides of Nepeta septemcrenata Erenb. J Nat Prod. 2010;3:103. [Google Scholar]
- 57.Inouye S, Takizawa T, Yamaguchi H. Antibacterial activity of essential oils and their major constituents against respiratory tract pathogens by gaseous contact. J Antimicrob Chemother. 2001;47(5):565–73. [DOI] [PubMed] [Google Scholar]
- 58.Lee S-J, Umano K, Shibamoto T, Lee K-G. Identification of volatile components in basil (Ocimum basilicum L.) and thyme leaves (Thymus vulgaris L.) and their antioxidant properties. Food Chem. 2005;91(1):131–7. [Google Scholar]
- 59.Pripdeevech P, Chumpolsri W, Suttiarporn P, Wongpornchai S. The chemical composition and antioxidant activities of basil from Thailand using retention indices and comprehensive two-dimensional gas chromatography. J Serb Chem Soc. 2010;75(11):1503–13. [Google Scholar]
- 60.Samarghandian S, Borji A, Delkhosh MB, Samini F. Safranal treatment improves hyperglycemia, hyperlipidemia and oxidative stress in streptozotocin-induced diabetic rats. Int J Pharm Pharm Sci. 2013;16(2):352–62. [DOI] [PubMed] [Google Scholar]
- 61.Farahmand SK, Samini F, Samini M, Samarghandian S. Safranal ameliorates antioxidant enzymes and suppresses lipid peroxidation and nitric oxide formation in aged male rat liver. Biogerontology. 2013;14(1):63–71. 10.1007/s10522-012-9409-0 [DOI] [PubMed] [Google Scholar]
- 62.Bianchi F, Careri M, Musci M. Volatile norisoprenoids as markers of botanical origin of Sardinian strawberry-tree (Arbutus unedo L.) honey: Characterisation of aroma compounds by dynamic headspace extraction and gas chromatography–mass spectrometry. Food Chem. 2005;89(4):527–32. [Google Scholar]
- 63.Gómez-Caravaca A, Gómez-Romero M, Arráez-Román D, Segura-Carretero A, Fernández-Gutiérrez A. Advances in the analysis of phenolic compounds in products derived from bees. J Pharm Biomed Anal. 2006;41(4):1220–34. 10.1016/j.jpba.2006.03.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.