Abstract
The two human herpesviruses that are causally associated with cancer are Epstein–Barr virus and Kaposi’s sarcoma-associated herpesvirus (KSHV). Both are lymphocryptoviruses that establish latency in B lymphocytes and persist for the lifetime of the host. EBV and KSHV are both linked to a variety of lymphomas. EBV is also a causative agent or cofactor in epithelial malignancies such as nasopharyngeal carcinoma whereas Kaposi’s sarcoma is of endothelial cell origin. Both viruses encode a limited number of proteins during latent replication that are important for growth transformation and evasion of the immune system. In addition, they express noncoding RNAs during both latent and lytic infection. Many of these RNAs have been highly conserved during evolution and are expressed in a wide variety of clinical settings, suggesting their fundamental importance in the viral life cycle. The function of some of these RNAs such as the nuclear EBV EBER RNAs remains elusive although they are some of the most abundant transcripts produced by each virus. Both EBV and KSHV also have recently been shown to encode and express microRNAs. The study of these viral microRNAs is just beginning although several of their cellular and viral gene targets have been established. Viral microRNAs appear to be involved in both modulation of the immune response as well as oncogenesis. Because each target gene may have many microRNAs acting on its mRNA, and each microRNA may have more than one target, there are likely to be many new discoveries regarding the complex interactions of viral microRNAs and host cell genes.
Gammaherpesviruses have long been of interest to molecular biologists because of their association with several human cancers. The discovery of Epstein–Barr virus (EBV) was in fact largely due to its association with Burkitt lymphoma. We now know that EBV contributes to the development of several epithelial and lymphoid malignancies including nasopharyngeal carcinoma, lymphoproliferative disorders in the immunosuppressed, Hodgkin’s lymphoma and gastric carcinoma. More recently, the second oncogenic human gammaherpesvirus, Kaposi’s sarcoma-associated herpesvirus (KSHV), also known as human herpesvirus 8, has been shown to cause primary effusion lymphoma (PEL) and Multicentric Castleman’s disease, a lymphoproliferative disorder. Both viruses establish lifelong latent infection in B lymphocytes and intermittently reactivate to undergo lytic replication, resulting in the production of infectious viral particles. Primate homologues of both viruses exist and particularly in the case of EBV, show a high degree of evolutionary conservation with their human counterpart, suggesting that these viruses have co-evolved with their host over millions of years. Thus it is not surprising that each virus expresses proteins that interact with host proteins and pathways in a highly specific manner. EBV, in addition to its association with malignancy, is a highly efficient transforming agent and immortalizes and growth transforms human B lymphocytes in vitro.
Although much has been learned about the proteins expressed by both EBV and KSHV that are important for mediating transformation, cell proliferation and immune evasion, these viruses also express noncoding RNAs about which much less is known. Both EBV and KSHV express noncoding nuclear RNAs in large amounts, indeed in amounts larger than any other viral transcript, yet we are still a long way from understanding their role in EBV and KSHV biology. EBV expresses small noncoding RNAs known as EBERs during latency and KSHV expresses a polyadenylated but noncoding nuclear transcript, PAN or nut-1 during lytic replication. There are tantalizing data that provide clues to EBER function from the work of several investigators over many years but very little information yet on PAN function. On the other hand, although the ability of both EBV and KSHV to express microRNAs has only been recently established, some very exciting studies have provided insight into the potential role of the viral microRNAs in oncogenesis. In this review, I will discuss what is known about EBER function and speculate about the role that they may play in the EBV life cycle and future directions for research, and in the second half, discuss the many interactions between EBV and KSHV microRNAs and host cell gene regulation (Fig. 1).
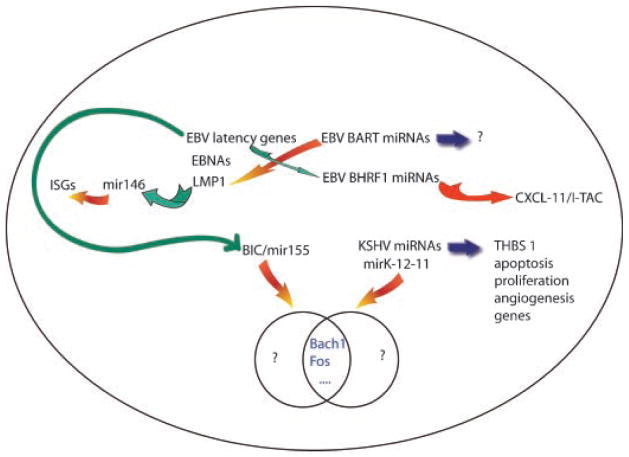
Fig. 1.
Interactions between gammaherpesvirus genes, cellular microRNAs, viral microRNAs and host cell genes. Both EBV and KSHV encode miRNAs that target cellular genes. Inhibitory or repressive effects are denoted by orange arrows and activating or inducing effects are shown by green arrows. EBV microRNA BHRF1 mir-3 targets the chemokine CXCL-11. EBV miRNAs may also downregulate EBV genes (LMP1 and DNA polymerase). EBV latent gene products such as LMP1 induce cell miRNAs which inhibit expression of interferon stimulated genes (ISGs) whereas introns from latent EBNA gene transcripts yield BHRF1 miRNAs. EBV latent gene products may also induce elevated levels of cellular mir155. Mir155 and KSHV mirK-12-11 have some cellular targets in common (BACH1 and Fos are shown as examples) but may also have other targets. The KSHV miRNA cluster likely targets arange of cellular genes important in oncogenesis, including thrombospondin 1 (TBHS 1). The full range of gammaherpesvirus microRNA targets remains to be defined as suggested by blue arrows and question marks.
EBV-Encoded Small RNAs
EBER RNAs, the small noncoding RNAs encoded by EBV, have been both mysterious and controversial since their original description (Lerner et al., 1981; Rosa et al., 1981). EBER1 and EBER2 are two small (167 and 172 nucleotide) RNAs produced in large numbers in EBV-infected cells. In fact, they are the most abundant RNAs in most EBV-infected cells, at greater than 5 × 106 copies per cell (Lerner et al., 1981). The EBERs are similar in size and gene organization to two small adenovirus RNAs, VAI and VAII. These and other similarities between the two sets of RNAs such as transcription by polymerase III and binding to the cellular La protein, led to the hypothesis that they may perform analogous functions in infected cells. The VA RNAs have been shown to be critical for adenovirus replication by rescuing cells from the shutdown of protein translation mediated by the cellular kinase PKR, which is induced by interferon and activated by double-stranded RNAs produced during replication of many viruses (Hovanessian, 1989; Ghadge et al., 1994). Construction of adenovirus mutants in which VA RNAs were replaced by EBERs showed that EBERs could indeed functionally substitute for the VA RNAs (Bhat and Thimmappaya, 1983, 1985). Although rescue of adenovirus replication by EBERs was only partial, it was nevertheless considered strong evidence that EBERs may play a role in counteracting the antiviral effects of interferon and PKR activation in infected cells. Consistent with this model in vitro studies demonstrated that EBERs could directly bind PKR and inhibit its activity (Clarke et al., 1990). Although EBERs clearly have the potential to interact with PKR and inhibit its activation, whether they actually play such a role during EBV infection remains controversial. One of the main objections to EBERs acting as a barrier to translational inhibition is their nuclear location. In situ hybridization studies have demonstrated a nuclear localization for EBERs (Howe and Steitz, 1986). One subsequent paper reported finding EBERs in perinuclear areas, consistent with endoplasmic reticulum and Golgi localization, by confocal microscopy, but this is the only such report (Schwemmle et al., 1992). Furthermore, a recent carefully performed study from Joan Steitz’s laboratory demonstrates that under conditions where UI small nuclear RNAs undergo nucleocytoplasmic shuttling in heterokaryons, the EBERs do not shuttle (Fok et al., 2006a). In addition, EBERs injected into Xenopus oocyte nuclei remained confined to the nucleus whereas tRNAs underwent rapid cytoplasmic export. EBER1 was shown to have a half-life of 25–30 h, and was more stable than RNAs that did undergo shuttling, indicating that rapid cytoplasmic degradation was not responsible for the inability to detect shuttling. A nuclear location for the EBERs is clearly difficult to reconcile with a role in modulating the cytoplasmic function of PKR in blocking translation initiation.
EBERs, apoptosis, and lymphomagenesis
This controversy over whether EBERs actually inhibit PKR in vivo is also related to a potential role of EBERs in oncogenesis and cell survival. EBV-infected Burkitt lymphoma cells examined shortly after biopsy usually express a limited number of EBV genes, primarily EBNA1, a nuclear protein required for episomal EBV maintenance, and EBER RNAs. Several cell lines derived from Burkitt lymphoma cells maintain this restricted pattern of EBV gene expression. One such cell line, Akata, spontaneously loses EBV genomes in culture (Shimizu et al., 1994). The resulting EBV-negative cells are noticeably less robust, undergo spontaneous apoptosis, are more serum-dependent, and less able to form colonies in soft agar or tumors in nude mice (Komano et al., 1998; Ruf et al., 1999). Reinfection of the EBV-negative clones with EBV restores the parental phenotype, indicating that EBV gene expression contributes to these correlates of oncogenicity (Komano et al., 1998; Ruf et al., 1999). Transfection of EBERs also at least partially restores resistance to both spontaneous and interferon-induced apoptosis (Komano et al., 1999; Ruf et al., 2000). How do EBERs protect against apoptosis? PKR activation can induce apoptosis by multiple mechanisms including eIF2-phosphorylation and therefore PKR inhibition was again implicated as the mediator of the EBER protective effect against apoptosis. In order to test this hypothesis, PKR kinase activity, acting on either itself or eIF2-α as a substrate, has been measured in EBER-negative and positive cells treated with interferon (Nanbo et al., 2002). When EBV-negative Akata cells transfected with EBERs were analyzed, PKR autophosphorylation in vitro was inhibited. However, these assays were performed with PKR immunoprecipitated from cell lysate in which non-physiological mixing of EBERs with PKR could have occurred. Endogenous cellular eIF-2α phosphorylation was also decreased in EBER positive, EBV-negative Akata clones compared to EBER-negative transfectants, but these comparisons were based on isolated stably transfected clones and thus intrinsically subject to the problem of individual clonal variation. In direct contrast, experiments from Ruf et al. (2005) while confirming a modest protective effect of EBERs against interferon-induced apoptosis, found that there was no effect of EBERs on either PKR phosphorylation or eIF-2α phosphorylation in comparisons of EBV-infected, EBV-negative and EBER-transfected cells. What then is the most likely mechanism of the EBER protective effect in Burkitt lymphoma cells? Other studies also indirectly argue against PKR being an important target of EBERs. B cell lines transformed by EBV in which the EBER genes were specifically deleted are no more sensitive to the antiviral effects of interferon as measured by their ability to support VSV replication (Swaminathan et al., 1992). Similarly, when EBER-positive cells were infected with a VAI-deleted adenovirus, they were able to support replication of the defective adenovirus but PKR phosphorylation was not significantly altered, suggesting that EBERs may actually rescue VAI-deleted adenovirus by a PKR-independent mechanism (Wang et al., 2005). When considered in combination with the data on nuclear EBER location, it appears that while EBERs do inhibit apoptosis, it is unlikely that inhibition of PKR is the primary mechanism for this effect.
EBERS in cell transformation
EBER RNAs are expressed 36 h after infection of primary B lymphocytes in vitro, well after other latent gene products (Alfieri et al., 1991). Because they are expressed in large amounts in latently infected cells and virtually all EBV-associated tumors, it had long been speculated that they might play an important role in initiation or maintenance of transformation. The generation of EBER-deleted EBV recombinants in the early 1990s demonstrated that they were not essential for transformation of B lymphocytes in vitro (Swaminathan et al., 1991). However, transformation assays performed with high titers of recombinant EBV generated in lymphoma cells indicated that EBERs may in fact provide an advantage in transforming ability, although these results were obtained from comparison of single selected clones of EBV which can display considerable variation in growth transformation phenotype (Yajima et al., 2005). Regardless, immortalized cell lines derived from EBV deleted for EBERs maintain latent infection and a growth phenotype typical of EBER-positive LCLs, indicating that in vitro EBERs are not essential for maintenance of the transformed phenotype (Swaminathan et al., 1991).
EBERS and cellular gene expression
What might be the basis of the complex effects that EBERs have on protection from apoptosis and enhancement of B cell survival? Takada and colleagues have described a variety of cytokines and growth factors that are upregulated in several types of EBER-expressing cells. Using either EBV-negative B lymphoma, T cell lymphoma, or gastric carcinoma cell lines, they have found that stable expression of EBERs is associated with IL-10, IL-9, or IGF1 induction, respectively (Kitagawa et al., 2000; Iwakiri et al., 2003, 2005; Yang et al., 2004). The increase in the levels of these cytokines was determined to be at the level of mRNA, thus implicating induction of transcription or transcript stabilization by the EBERs. While these effects are intriguing, it is difficult to know whether these are truly the primary mechanism of EBER function or due to secondary effects. In the absence of evidence for direct or indirect interaction with promoter sequences or mRNAs, a plausible mechanism for the action of EBERs to induce highly gene-specific effects is still lacking. EBERs are known to interact with two cellular proteins, the aforementioned La protein, and L22, a component of the large ribosomal subunit (Toczyski et al., 1994; Fok et al., 2006b). La is known to be important in the biogenesis and maturation of polIII transcripts, and is possibly involved in enhancing the stability or translation of some mRNAs (Wolin and Cedervall, 2002). Although EBERs are present at high copy numbers, the levels of La in the cell are high enough that sequestration of La does not seem to be a likely mechanism for EBER effects (Wolin and Cedervall, 2002). L22 is an RNA binding protein and each EBER1 RNA binds three L22 molecules (Fok et al., 2006b). EBER binding causes relocalization of L22 to the nucleoplasm from the nucleolus and cytoplasm (Toczyski et al., 1994). Thus a significant amount of L22 may be sequestered by EBERs although depletion of L22 from ribosomes has not been observed to occur. Recently L22 knockout mice have been shown to suffer a depletion of αβ T cells due to p53 induction but have overtly normal B cell function (Anderson et al., 2007). Thus our current knowledge regarding L22 function does not yield further insight into possible EBER functions.
A hypothesis regarding EBER function
The evolutionary conservation of EBERs among primate homologs of EBV and their ubiquitous expression suggests that they play an important role in EBV biology. Cellular noncoding RNAs (ncRNAs) have increasingly come to be recognized as having important roles in the nucleus as transcriptional regulators. A recent report describes how PIP7S, a nuclear La-related protein, binds and stabilizes 7SK, a small ncRNA involved in regulating transcriptional elongation by polII (He et al., 2008). 7SK acts by binding and sequestering P-TEFb, a kinase that facilitates elongation by phosphorylating the CTD domain of pol II (Nguyen et al., 2001). The complex dissociates in response to various external stressors, releasing P-TEFb, thereby enhancing RNA elongation. Interestingly, there are particularly important roles for several noncoding RNAs in the cellular heat shock response. HSR-1 RNA enhances trimerization and promoter binding of heat-shock factor protein-1 (HSF-1) during periods of stress, leading to activation of several heat shock genes (Shamovsky et al., 2006). Perhaps even more relevant to the possible role of EBERs in transcriptional regulation is the recent identification of Alu RNAs as the human equivalent of murine B2 RNAs, noncoding RNAs which repress the majority of gene transcription during heat shock (Anderson et al., 2007). Mariner et al. (2008) have now demonstrated that human Alu RNAs are found at the promoters of heat shock-repressed genes and inhibit polII activity at the preinitiation phase of transcription. The activity of Alu RNAs appears to be modular, with a polII interaction domain, and an unstructured region required for the repressive function. Expression of Alu RNAs is increased during various types of stress and it is tempting to speculate that EBER RNAs may be involved in rescuing EBV from cytoprotective transcriptional repression under particular stress conditions in vivo. Alternatively, EBERs may be involved in selectively activating genes by interacting with specific cellular proteins that are stress-induced. It is becoming increasingly clear that ncRNAs participate in selective transcriptional regulation by a variety of mechanisms, and it is highly likely that EBER RNAs are viral counterparts of cellular ncRNAs. Ultimately, the solution to the persistent questions regarding biologically relevant EBER function may depend on the generation of EBER mutants in the rhesus lymphocryptovirus homolog of EBV (cercopithecine virus 15, CeHV15) (Rivailler et al., 2002). Such EBER-deleted mutants would allow the performance of in vivo experiments in macaques to study the biological role of EBERs in latent infection and persistence (Moghaddam et al., 1997).
Gammaherpes Virus Micro RNAs
Just as herpesviruses express larger noncoding RNAs, they are now known to also express microRNAs. MicroRNAs (miRNAs) are 22–24 nt noncoding RNAs that perform their gene regulatory role as a component of the RISC complex, which targets and either inhibits translation or promotes degradation of target mRNAs (for review, see Ambros, 2004; Bartel, 2004). Virtually every combination of regulatory interaction between cellular and viral genes and miRNAs has now been described in gammaherpesvirus infections. Viral miRNAs regulate both cellular and viral genes, viral genes affect expression of cellular miRNAs, and the transcriptional milieu of the host cell determines the pattern of viral miRNA expression.
MicroRNAs are processed from a primary transcript, or pri-miRNA, usually encoded in introns or other noncoding transcripts. Pri-miRNAs are cleaved in the nucleus by the endonuclease Drosha to yield hairpin pre-miRNA molecules that are transported to the cytoplasm by an exportin 5/RAN-GTP complex. In the cytoplasm, after cleavage by Dicer to yield double stranded products, one strand of the pre-miRNA, which is complementary to the target mRNA, is incorporated into a RISC complex with Argonaute and other cellular proteins. The RISC complex interacts with mRNA sequences in the 3′ UTR of the target mRNA that are either fully or partially complementary to a 6 nt “seed” sequence of the miRNA. Proteins in the RISC complex interact with translation initiation factors or polyadenylation factors to exert their effects. Although the exact mechanisms and determinants of translational repression versus mRNA destabilization are still debated, in most instances, miRNAs repress target gene expression. However, in at least one instance, they have been shown to activate translation of specific genes in growth-arrested cells where RISC proteins inhibit cellular proteins that bind ARE elements in the 3′UTR and promote decay of short-lived transcripts (Vasudevan et al., 2007).
EBV microRNAs
EBV was initially reported to express five miRNAs from two separate regions of the EBV genome (Pfeffer et al., 2004). Cells used in this original study were infected with the prototype EBV strain B95-8, which carries a 12 kb deletion in the BamHI A region of the EBV genome. Subsequent analyses using additional EBV infected cell lines revealed that EBV is capable of expressing up to 22 miRNAs and most of these are encoded in the DNA missing from the B95-8 genome (Cai et al., 2006; Grundhoff et al., 2006). The expression patterns of the various EBV miRNAs appear to be fairly complex and depend on both the cell type and on the overall pattern of EBV gene expression. The most striking differences in tissue specific expression are found in a comparison of epithelial cell lines derived from nasopharyngeal carcinoma (NPC) and B lymphocyte cell lines derived from Burkitt lymphoma (BL) or primary B cells transformed in vitro. In NPC cell lines, the cluster of miRNAs from BamHI A (BART miRNAs) is robustly expressed, whereas they are less abundant in the majority of B lymphocyte-derived lines (Cai et al., 2006).
Conversely the other cluster of miRNAs, encoded in the BamHI H region of the genome, referred to as BHRF1 miRNAs, is not detected in NPC cells, but is expressed in B cells (Cai et al., 2006; Xing and Kieff, 2007). Here the pattern of BHRF1 miRNA expression is dependent on the type of EBV replication that is taking place. During latent infection, the EBV genome replicates as a plasmid and several patterns of gene expression may occur. In B lymphocyte lines transformed by EBV in vitro, and in lymphoproliferative disorders arising in the presence of immunosuppression, the full repertoire of latency genes is expressed. This pattern of gene expression, referred to as type III latency, in which all the EBV nuclear antigens (EBNAs) are transcribed from one of two major latency promoters, Cp or Wp, is associated with cell proliferation driven by EBV gene expression. In such cells, BHRF1 miRNAs most likely derive from an intron generated by splicing of the Cp and Wp transcripts (Xing and Kieff, 2007). In contrast, in BL and NPC cells, the only latency associated gene expressed is EBNA1, transcribed from a separate promoter, and BHRF1 miRNAs have not been detected at high levels. Thus it appears that BHRF1 miRNAs may play an important role in cells undergoing EBV-driven proliferation and type III latent infection. Correspondingly, the BART transcripts and BART miRNAs are most highly expressed in NPC cells (Hitt et al., 1989; Cai et al., 2006). To further add to the complexity, induction of lytic EBV replication induces expression of many but not all the miRNAs (Cai et al., 2006; Xing and Kieff, 2007). Presumably, each of these miRNAs plays an important and specific role in the cell phenotype of each type of infection. The importance of miRNAs in EBV infection is also supported by the high degree of evolutionary conservation of most of the miRNAs between rhesus lymphocryptovirus and EBV (Cai et al., 2006).
Targets of EBV microRNAs
What then, are the cellular targets of gammaherpesvirus miRNAs? Since a given miRNA may have many targets and each gene may have many miRNAs acting upon it, the answers are only beginning to emerge. In the case of the BHRF1-3 miRNA, one target that was predicted from bioinformatic analysis and then verified by analysis of EBV-related lymphoma biopsies, is the IFN-inducible T-cell attracting chemokine, CXCL-11/I-TAC (Xia et al., 2008). Thus BHRF1-3 miRNA may inhibit the host interferon response by suppressing CXCL-11/I-TAC production during EBV-driven cell proliferation or lytic replication in vivo. It also appears that at least in two cases, an EBV miRNA may target an EBV gene. The gene for EBV DNA polymerase, BALF5, is transcribed from the opposite strand as the one encoding mirBART2, and BALF5 transcripts decrease in abundance when mirBART2 is overexpressed (Barth et al., 2008). The other EBV gene whose expression appears to be modulated by EBV miRNAs is LMP1. Although LMP1 has transforming properties and is thought to be important in the pathogenesis of NPC, over expression of LMP1 may inhibit proliferation and increase susceptibility to apoptotic stress (Eliopoulos et al., 1996; Kaykas and Sugden, 2000). The LMP1 3′ UTR has several partly complementary matches to BART miRNAs and BART miRNA expression was found to suppress LMP production and protect against apoptotic stimuli (Lo et al., 2007). These findings are particularly significant since LMP1 protein levels in NPC tissues often do not correlate with transcript levels suggesting a role for miRNA induced translational inhibition (Tsao et al., 2002; Lo et al., 2007).
EBV induces cell micro RNAs with effects on immune responses and oncogenesis
EBV may also use cellular miRNAs to regulate gene expression. There are at least two cellular miRNAs with pleiotropic cellular effects that may be induced by EBV proteins. LMP1 activates the promoter for mir-146a, a cellular miRNA which downregulates a large number of interferon-responsive genes (Lo et al., 2007). Thus EBV may co-opt a cellular miRNA pathway involved in modulating the interferon response in order to enhance EBV replication in vivo. Mir-155 is a cellular miRNA derived from BIC, a noncoding RNA whose expression is upregulated in a variety of B cell malignancies including diffuse large B cell lymphomas, CLL and Hodgkin’s lymphoma (Eis et al., 2005; Kluiver et al., 2005; Fulci et al., 2007). Consistent with an important role for mir-155 in B cell malignancy, transgenic mice carrying a miR155 transgene develop B cell lymphomas (Costinean et al., 2006). Most Burkitt lymphomas however do not express elevated levels of mir155 (van den Berg et al., 2003). As described above, EBV protein expression in BL cells is limited to EBNA1. However, when grown in vitro, BL cells often change to a type III pattern of gene expression and express the full complement of latent EBV proteins. When such type III BL cells and cells from post-transplant lymphomas, which also display a type III pattern of gene expression, were examined, mir-155 and/or BIC expression were found to be increased (Kluiver et al., 2006). These findings raise the intriguing possibility that one or more latent EBV proteins induce mir-155 expression.
The KSHV miRNA cluster may target multiple cellular genes important for KSHV pathogenesis
Kaposi’s sarcoma-associated herpes virus, the other human lymphocryptovirus, is also associated with the development of primary effusion lymphoma (PEL) and multicentric Castleman’s disease, a lymphoproliferative disorder. Three groups reported discovery of a cluster of 12 microRNAs expressed during latent infection in PEL cells in 2005 (Cai et al., 2005; Pfeffer et al., 2005; Samols et al., 2005). Since that time it has been shown that these miRNAs are remarkably conserved among isolates from many geographically diverse populations and clinical sources (Marshall et al., 2007). Remarkable progress has been made in elucidating the targets of these miRNAs by a combination of bioinformatic and experimental approaches. One such study identified several potential cellular target genes whose mRNA levels are specifically decreased by expression of the microRNA cluster (Samols et al., 2007). These genes were predicted to be KSHV miRNA targets by the identification of seed sequence matches in their 3′ UTRs. Five of the genes most highly downregulated are involved in proliferation, immune modulation, angiogenesis, and apoptosis pathways. One of these genes, thrombospondin 1, has antiproliferative, anti-angiogenic and immune modulatory activity and is known to be downregulated in several cancers, including KS. Thus KSHV miRNAs may target cellular genes that are important in the pathogenesis of KS and other KSHV-associated malignancies.
A KSHV microRNA targets the same genes as a cellular microRNA important in oncogenesis
One of the KSHV miRNAs, mirK12-11 contains the identical seed sequence as the previously mentioned mir-155. The Cullen and Renne laboratories have now shown that exogenous expression of mirK12-11 in epithelial cells or in B cells reduces expression of a group of cellular genes also targeted by mir-155 (Gottwein et al., 2007; Skalsky et al., 2007). The Renne laboratory found that 20 genes downregulated by both mir-155 and mirK12-11 contained seed match sites. Many of these genes were members of cell signaling, cell division, apoptosis, and T-cell activation pathways. In the study from the Cullen laboratory, 14 genes were directly validated as being common targets of mirK12-11 and mir155. Among this group were several signal transduction pathway kinases and transcription factors. Interestingly, only one gene was identified by both groups, BACH-1 a transcriptional repressor involved in hypoxia-induced signaling and in DNA damage repair (Sun et al., 2002; Peng et al., 2006). The lack of concordance between the findings of the two groups may be due to the different cell types used in the two studies.
Conclusion
Viruses have long provided insights into cellular physiology as they serve as natural experiments that have taken place over millions of years of evolution. Viral strategies that manipulate cellular gene expression to enhance viral persistence, viral replication, and survival of infected cells have provided numerous clues to mechanisms of gene expression and their dysregulation during tumor development. The fact that viral noncoding RNAs have been highly conserved and are expressed in virtually every infected cell suggests that they perform essential roles in gammaherpesvirus biology. With the recent explosion in our knowledge of the mechanisms of microRNAs and other noncoding RNAs, many new discoveries about the complex relationship between transforming herpesviruses and oncogenesis are imminent. Because of the combinatorial nature of their effects, it is unlikely that gammaherpesvirus miRNAs will have only a few functions, but rather will be found to participate in many cellular processes, both as activators and repressors, in a manner similar to transcription factors. Given the evidence currently available, it is likely that viral microRNAs will become increasingly established as important in viral oncogenesis and in preventing elimination of the virus by the host immune response. If the hypothesis advanced in this mini-review is correct, many of the other highly conserved noncoding RNAs expressed by gammaherpesviruses such as EBERs will be shown to play an important role in modulating gene expression during the host cell stress response. Considering the complex array of possible interactions between noncoding RNAs and cellular genes, both virologists and cell biologists are likely to be productively occupied for a long time.
Literature Cited
- Alfieri C, Birkenbach M, Kieff E. Early events in Epstein-Barr virus infection of human B lymphocytes. Virology. 1991;181:595–608. doi: 10.1016/0042-6822(91)90893-g. [DOI] [PubMed] [Google Scholar]
- Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- Anderson SJ, Lauritsen JP, Hartman MG, Foushee AM, Lefebvre JM, Shinton SA, Gerhardt B, Hardy RR, Oravecz T, Wiest DL. Ablation of ribosomal protein L22 selectively impairs alphabeta T cell development by activation of a p53-dependent checkpoint. Immunity. 2007;26:759–772. doi: 10.1016/j.immuni.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Barth S, Pfuhl T, Mamiani A, Ehses C, Roemer K, Kremmer E, Jaker C, Hock J, Meister G, Grasser FA. Epstein-Barr virus-encoded microRNA miR-BART2 down-regulates the viral DNA polymerase BA LF5. Nucleic Acids Res. 2008;36:666–675. doi: 10.1093/nar/gkm1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat RA, Thimmappaya B. Two small RNAs encoded by Epstein-Barr virus can functionally substitute for the virus-associated RNAs in the lytic growth of adenovirus 5. Proc Natl Acad Sci USA. 1983;80:4789–4793. doi: 10.1073/pnas.80.15.4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat RA, Thimmappaya B. Construction and analysis of additional adenovirus substitution mutants confirm the complementation of VAI RNA function by two small RNAs encoded by Epstein-Barr virus. J Virol. 1985;56:750–756. doi: 10.1128/jvi.56.3.750-756.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Lu S, Zhang Z, Gonzalez CM, Damania B, Cullen BR. Kaposi’s sarcoma-associated herpesvirus expresses an array of viral microRNAs in latently infected cells. Proc Natl Acad Sci USA. 2005;102:5570–5575. doi: 10.1073/pnas.0408192102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Schafer A, Lu S, Bilello JP, Desrosiers RC, Edwards R, Raab-Traub N, Cullen BR. Epstein-Barr virus microRNAs are evolutionarily conserved and differentially expressed. PLoS Pathog. 2006;2:e23. doi: 10.1371/journal.ppat.0020023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PA, Sharp NA, Clemens MJ. Translational control by the Epstein-Barr virus small RNA EBER-1. Reversal of the double-stranded RNA-induced inhibition of protein synthesis in reticulocyte lysates. Eur J Biochem. 1990;193:635–641. doi: 10.1111/j.1432-1033.1990.tb19381.x. [DOI] [PubMed] [Google Scholar]
- Costinean S, Zanesi N, Pekarsky Y, Tili E, Volinia S, Heerema N, Croce CM. Pre-B cell proliferation and lymphoblastic leukemia/high-grade lymphoma in E{micro}-miR155 transgenic mice. Proc Natl Acad Sci. 2006;103:7024–7029. doi: 10.1073/pnas.0602266103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eis PS, Tam W, Sun L, Chadburn A, Li Z, Gomez MF, Lund E, Dahlberg JE. Accumulation of miR-155 and BIC RNA in human B cell lymphomas. Proc Natl Acad Sci. 2005;102:3627–3632. doi: 10.1073/pnas.0500613102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliopoulos AG, Dawson CW, Mosialos G, Floettmann JE, Rowe M, Armitage RJ, Dawson J, Zapata JM, Kerr DJ, Wakelam MJ, Reed JC, Kieff E, Young LS. CD40-induced growth inhibition in epithelial cells is mimicked by Epstein-Barr Virus-encoded LM P1: Involvement of TRAF3 as a common mediator. Oncogene. 1996;13:2243–2254. [PubMed] [Google Scholar]
- Fok V, Friend K, Steitz JA. Epstein-Barr virus noncoding RNAs are confined to the nucleus, whereas their partner, the human La protein, undergoes nucleocytoplasmic shuttling. J Cell Biol. 2006a;173:319–325. doi: 10.1083/jcb.200601026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fok V, Mitton-Fry RM, Grech A, Steitz JA. Multiple domains of EBER 1, an Epstein-Barr virus noncoding RNA, recruit human ribosomal protein L22. RNA. 2006b;12:872–882. doi: 10.1261/rna.2339606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulci V, Chiaretti S, Goldoni M, Azzalin G, Carucci N, Tavolaro S, Castellano L, Magrelli A, Citarella F, Messina M, Maggio R, Peragine N, Santangelo S, Mauro FR, Landgraf P, Tuschl T, Weir DB, Chien M, Russo JJ, Ju J, Sheridan R, Sander C, Zavolan M, Guarini A, Foa R, Macino G. Quantitative technologies establish a novel microRNA profile of chronic lymphocytic leukemia. Blood. 2007;109:4944–4951. doi: 10.1182/blood-2006-12-062398. [DOI] [PubMed] [Google Scholar]
- Ghadge GD, Malhotra P, Furtado MR, Dhar R, Thimmapaya B. In vitro analysis of virus-associated RNA I (VAI RNA): Inhibition of the double-stranded RNA-activated protein kinase PKR by VAI RNA mutants correlates with the in vivo phenotype and the structural integrity of the central domain. J Virol. 1994;68:4137–4151. doi: 10.1128/jvi.68.7.4137-4151.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottwein E, Mukherjee N, Sachse C, Frenzel C, Majoros WH, Chi JT, Braich R, Manoharan M, Soutschek J, Ohler U, Cullen BR. A viral microRNA functions as an orthologue of cellular miR-155. Nature. 2007;450:1096–1099. doi: 10.1038/nature05992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundhoff A, Sullivan CS, Ganem D. A combined computational and microarray-based approach identifies novel microRNAs encoded by human gamma-herpesviruses. RNA. 2006;12:733–750. doi: 10.1261/rna.2326106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He N, Jahchan NS, Hong E, Li Q, Bayfield MA, Maraia RJ, Luo K, Zhou Q. A La-related protein modulates 7SK snRNP integrity to suppress P-TEFb-dependent transcriptional elongation and tumorigenesis. Mol Cell. 2008;29:588–599. doi: 10.1016/j.molcel.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitt MM, Allday MJ, Hara T, Karran L, Jones MD, Busson P, Tursz T, Ernberg I, Griffin BE. EBV gene expression in an NPC-related tumour. EMBO J. 1989;8:2639–2651. doi: 10.1002/j.1460-2075.1989.tb08404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovanessian AG. The double stranded RNA-activated protein kinase induced by interferon: dsRNA-PK. J Interferon Res. 1989;9:641–647. doi: 10.1089/jir.1989.9.641. [DOI] [PubMed] [Google Scholar]
- Howe JG, Steitz JA. Localization of Epstein-Barr virus-encoded small RNAs by in situ hybridization. Proc Natl Acad Sci USA. 1986;83:9006–9010. doi: 10.1073/pnas.83.23.9006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakiri D, Eizuru Y, Tokunaga M, Takada K. Autocrine growth of Epstein-Barr virus-positive gastric carcinoma cells mediated by an Epstein-Barr virus-encoded small RNA. Cancer Res. 2003;63:7062–7067. [PubMed] [Google Scholar]
- Iwakiri D, Sheen TS, Chen JY, Huang DP, Takada K. Epstein-Barr virus-encoded small RNA induces insulin-like growth factor 1 and supports growth of nasopharyngeal carcinoma-derived cell lines. Oncogene. 2005;24:1767–1773. doi: 10.1038/sj.onc.1208357. [DOI] [PubMed] [Google Scholar]
- Kaykas A, Sugden B. The amino-terminus and membrane-spanning domains of LMP-1 inhibit cell proliferation. Oncogene. 2000;19:1400–1410. doi: 10.1038/sj.onc.1203365. [DOI] [PubMed] [Google Scholar]
- Kitagawa N, Goto M, Kurozumi K, Maruo S, Fukayama M, Naoe T, Yasukawa M, Hino K, Suzuki T, Todo S, Takada K. Epstein-Barr virus-encoded poly(A)(−) RNA supports Burkitt’s lymphoma growth through interleukin-10 induction. EMBO J. 2000;19:6742–6750. doi: 10.1093/emboj/19.24.6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluiver J, Poppema S, de Jong D, Blokzijl T, Harms G, Jacobs S, Kroesen BJ, van den Berg A. BIC and miR-155 are highly expressed in Hodgkin, primary mediastinal and diffuse large B cell lymphomas. J Pathol. 2005;207:243–249. doi: 10.1002/path.1825. [DOI] [PubMed] [Google Scholar]
- Kluiver J, Haralambieva E, de Jong D, Blokzijl T, Jacobs S, Kroesen BJ, Poppema S, van den Berg A. Lack of BIC and microRNA miR-155 expression in primary cases of Burkitt lymphoma. Genes Chromosomes Cancer. 2006;45:147–153. doi: 10.1002/gcc.20273. [DOI] [PubMed] [Google Scholar]
- Komano J, Sugiura M, Takada K. Epstein-Barr virus contributes to the malignant phenotype and to apoptosis resistance in Burkitt’s lymphoma cell line Akata. J Virol. 1998;72:9150–9156. doi: 10.1128/jvi.72.11.9150-9156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komano J, Maruo S, Kurozumi K, Oda T, Takada K. Oncogenic role of Epstein-Barr virus-encoded RNAs in Burkitt’s lymphoma cell line Akata. J Virol. 1999;73:9827–9831. doi: 10.1128/jvi.73.12.9827-9831.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner MR, Andrews NC, Miller G, Steitz JA. Two small RNAs encoded by Epstein-Barr virus and complexed with protein are precipitated by antibodies from patients with systemic lupus erythematosus. Proc Natl Acad Sci USA. 1981;78:805–809. doi: 10.1073/pnas.78.2.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo AK, To KF, Lo KW, Lung RW, Hui JW, Liao G, Hayward SD. Modulation of LMP1 protein expression by EBV-encoded microRNAs. Proc Natl Acad Sci USA. 2007;104:16164–16169. doi: 10.1073/pnas.0702896104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariner PD, Walters RD, Espinoza CA, Drullinger LF, Wagner SD, Kugel JF, Goodrich JA. Human Alu RNA is a modular transacting repressor of mRNA transcription during heat shock. Mol Cell. 2008;29:499–509. doi: 10.1016/j.molcel.2007.12.013. [DOI] [PubMed] [Google Scholar]
- Marshall V, Parks T, Bagni R, Wang CD, Samols MA, Hu J, Wyvil KM, Aleman K, Little RF, Yarchoan R, Renne R, Whitby D. Conservation of virally encoded microRNAs in Kaposi sarcoma—Associated herpesvirus in primary effusion lymphoma cell lines and in patients with Kaposi sarcoma or multicentric Castleman disease. J Infect Dis. 2007;195:645–659. doi: 10.1086/511434. [DOI] [PubMed] [Google Scholar]
- Moghaddam A, Rosenzweig M, Lee-Parritz D, Annis B, Johnson RP, Wang F. An animal model for acute and persistent Epstein-Barr virus infection. Science. 1997;276:2030–2033. doi: 10.1126/science.276.5321.2030. [DOI] [PubMed] [Google Scholar]
- Nanbo A, Inoue K, Adachi-Takasawa K, Takada K. Epstein-Barr virus RNA confers resistance to interferon-alpha-induced apoptosis in Burkitt’s lymphoma. EMBO J. 2002;21:954–965. doi: 10.1093/emboj/21.5.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen VT, Kiss T, Michels AA, Bensaude O. 7SK small nuclear RNA binds to and inhibits the activity of CDK9/cyclin T complexes. Nature. 2001;414:322–325. doi: 10.1038/35104581. [DOI] [PubMed] [Google Scholar]
- Peng M, Litman R, Jin Z, Fong G, Cantor SB. BACH1 is a DNA repair protein supporting BRCA1 damage response. Oncogene. 2006;25:2245–2253. doi: 10.1038/sj.onc.1209257. [DOI] [PubMed] [Google Scholar]
- Pfeffer S, Zavolan M, Grasser FA, Chien M, Russo JJ, Ju J, John B, Enright AJ, Marks D, Sander C, Tuschl T. Identification of virus-encoded microRNAs. Science. 2004;304:734–736. doi: 10.1126/science.1096781. [DOI] [PubMed] [Google Scholar]
- Pfeffer S, Sewer A, Lagos-Quintana M, Sheridan R, Sander C, Grasser FA, van Dyk LF, Ho CK, Shuman S, Chien M, Russo JJ, Ju J, Randall G, Lindenbach BD, Rice CM, Simon V, Ho DD, Zavolan M, Tuschl T. Identification of microRNAs of the herpesvirus family. Nat Methods. 2005;2:269–276. doi: 10.1038/nmeth746. [DOI] [PubMed] [Google Scholar]
- Rivailler P, Jiang H, Cho YG, Quink C, Wang F. Complete nucleotide sequence of the rhesus lymphocryptovirus: Genetic validation for an Epstein-Barr virus animal model. J Virol. 2002;76:421–426. doi: 10.1128/JVI.76.1.421-426.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa MD, Gottlieb E, Lerner MR, Steitz JA. Striking similarities are exhibited by two small Epstein-Barr virus-encoded ribonucleic acids and the adenovirus-associated ribonucleic acids VAI and VAII. Mol Cell Biol. 1981;1:785–796. doi: 10.1128/mcb.1.9.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruf IK, Rhyne PW, Yang H, Borza CM, Hutt-Fletcher LM, Cleveland JL, Sample JT. Epstein-barr virus regulates c-MYC, apoptosis, and tumorigenicity in Burkitt lymphoma. Mol Cell Biol. 1999;19:1651–1660. doi: 10.1128/mcb.19.3.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruf IK, Rhyne PW, Yang C, Cleveland JL, Sample JT. Epstein-Barr virus small RNAs potentiate tumorigenicity of Burkitt lymphoma cells independently of an effect on apoptosis. J Virol. 2000;74:10223–10228. doi: 10.1128/jvi.74.21.10223-10228.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruf IK, Lackey KA, Warudkar S, Sample JT. Protection from interferon-induced apoptosis by Epstein-Barr virus small RNAs is not mediated by inhibition of PKR. J Virol. 2005;79:14562–14569. doi: 10.1128/JVI.79.23.14562-14569.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samols MA, Hu J, Skalsky RL, Renne R. Cloning and identification of a microRNA cluster within the latency-associated region of Kaposi’s sarcoma-associated herpesvirus. J Virol. 2005;79:9301–9305. doi: 10.1128/JVI.79.14.9301-9305.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samols MA, Skalsky RL, Maldonado AM, Riva A, Lopez MC, Baker HV, Renne R. Identification of cellular genes targeted by KSHV-encoded microRNAs. PLoS Pathog. 2007;3:e65. doi: 10.1371/journal.ppat.0030065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwemmle M, Clemens MJ, Hilse K, Pfeifer K, Troster H, Muller WE, Bachmann M. Localization of Epstein-Barr virus-encoded RNAs EBER-1 and EBER-2 in interphase and mitotic Burkitt lymphoma cells. Proc Natl Acad Sci USA. 1992;89:10292–10296. doi: 10.1073/pnas.89.21.10292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamovsky I, Ivannikov M, Kandel ES, Gershon D, Nudler E. RNA-mediated response to heat shock in mammalian cells. Nature. 2006;440:556–560. doi: 10.1038/nature04518. [DOI] [PubMed] [Google Scholar]
- Shimizu N, Tanabe-Tochikura A, Kuroiwa Y, Takada K. Isolation of Epstein-Barr virus (EBV)-negative cell clones from the EBV-positive Burkitt’s lymphoma (BL) line Akata: Malignant phenotypes of BL cells are dependent on EBV. J Virol. 1994;68:6069–6073. doi: 10.1128/jvi.68.9.6069-6073.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalsky RL, Samols MA, Plaisance KB, Boss IW, Riva A, Lopez MC, Baker HV, Renne R. Kaposi’s sarcoma-associated herpesvirus encodes an ortholog of miR-155. J Virol. 2007;81:12836–12845. doi: 10.1128/JVI.01804-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Hoshino H, Takaku K, Nakajima O, Muto A, Suzuki H, Tashiro S, Takahashi S, Shibahara S, Alam J, Taketo MM, Yamamoto M, Igarashi K. Hemoprotein Bach1 regulates enhancer availability of heme oxygenase-1 gene. EMBO J. 2002;21:5216–5224. doi: 10.1093/emboj/cdf516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan S, Tomkinson B, Kieff E. Recombinant Epstein-Barr virus with small RNA (EBER) genes deleted transforms lymphocytes and replicates in vitro. Proc Natl Acad Sci USA. 1991;88:1546–1550. doi: 10.1073/pnas.88.4.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan S, Huneycutt BS, Reiss CS, Kieff E. Epstein-Barr virus-encoded small RNAs (EBERs) do not modulate interferon effects in infected lymphocytes. J Virol. 1992;66:5133–5136. doi: 10.1128/jvi.66.8.5133-5136.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toczyski DP, Matera AG, Ward DC, Steitz JA. The Epstein-Barr virus (EBV) small RNA EBER1 binds and relocalizes ribosomal protein L22 in EBV-infected human B lymphocytes. Proc Natl Acad Sci USA. 1994;91:3463–3467. doi: 10.1073/pnas.91.8.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao SW, Tramoutanis G, Dawson CW, Lo AK, Huang DP. The significance of LMP1 expression in nasopharyngeal carcinoma. Semin Cancer Biol. 2002;12:473–487. doi: 10.1016/s1044579x02000901. [DOI] [PubMed] [Google Scholar]
- van den Berg A, Kroesen BJ, Kooistra K, de Jong D, Briggs J, Blokzijl T, Jacobs S, Kluiver J, Diepstra A, Maggio E, Poppema S. High expression of B-cell receptor inducible gene BIC in all subtypes of Hodgkin lymphoma. Genes Chromosomes Cancer. 2003;37:20–28. doi: 10.1002/gcc.10186. [DOI] [PubMed] [Google Scholar]
- Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science. 2007;318:1931–1934. doi: 10.1126/science.1149460. [DOI] [PubMed] [Google Scholar]
- Wang Y, Xue SA, Hallden G, Francis J, Yuan M, Griffin BE, Lemoine NR. Virus-associated RNA I-deleted adenovirus, a potential oncolytic agent targeting EBV-associated tumors. Cancer Res. 2005;65:1523–1531. doi: 10.1158/0008-5472.CAN-04-3113. [DOI] [PubMed] [Google Scholar]
- Wolin SL, Cedervall T. The La protein. Annu Rev Biochem. 2002;71:375–403. doi: 10.1146/annurev.biochem.71.090501.150003. [DOI] [PubMed] [Google Scholar]
- Xia T, O’Hara A, Araujo I, Barreto J, Carvalho E, Sapucaia JB, Ramos JC, Luz E, Pedroso C, Manrique M, Toomey NL, Brites C, Dittmer DP, Harrington WJ., Jr EBV microRNAs in primary lymphomas and targeting of CXCL-11 by ebv-mir-BHR F1-3. Cancer Res. 2008;68:1436–1442. doi: 10.1158/0008-5472.CAN-07-5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing L, Kieff E. Epstein-Barr virus BHRF1 micro- and stable RNAs during latency III and after induction of replication. J Virol. 2007;81:9967–9975. doi: 10.1128/JVI.02244-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima M, Kanda T, Takada K. Critical role of Epstein-Barr Virus (EBV)-encoded RNA in efficient EBV-induced B-lymphocyte growth transformation. J Virol. 2005;79:4298–4307. doi: 10.1128/JVI.79.7.4298-4307.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Aozasa K, Oshimi K, Takada K. Epstein-Barr virus (EBV)-encoded RNA promotes growth of EBV-infected T cells through interleukin-9 induction. Cancer Res. 2004;64:5332–5337. doi: 10.1158/0008-5472.CAN-04-0733. [DOI] [PubMed] [Google Scholar]