Supplemental Digital Content is Available in the Text.
Key Words: antiretroviral therapy, HIV, decentralization, task shifting, community, urban
Abstract:
Facility-based antiretroviral therapy (ART) provision for stable patients with HIV congests health services in resource-limited countries. We assessed outcomes and risk factors for attrition after decentralization to community-based ART refill centers among 2603 patients with HIV in Kinshasa, Democratic Republic of Congo, using a multilevel Poisson regression model. Death, loss to follow-up, and transfer out were 0.3%, 9.0%, and 0.7%, respectively, at 24 months. Overall attrition was 5.66/100 person-years. Patients with >3 years on ART, >500 cluster of differentiation type-4 count, body mass index >18.5, and receiving nevirapine but not stavudine showed reduced attrition. ART refill centers are a promising task-shifting model in low-prevalence urban settings with high levels of stigma and poor ART coverage.
INTRODUCTION
Scale-up of antiretroviral therapy (ART) has increased the number of HIV-infected patients on treatment to 15 million in 2015, of which 11 million live in sub-Saharan Africa (SSA).1 The number of clinically stable patients accessing health services for ART collection without requiring medical care regularly has increased accordingly. This compounds existing problems in resource-low settings such as overcrowding and diverts scarce human resources needed to care for nonstable patients with HIV.2,3
In 2010, Médecins Sans Frontières (MSF) started a decentralization project to provide ART for clinically stable HIV patients through community-based drug refill centers in Kinshasa, Democratic Republic of Congo (DRC). No evaluation of such a task-shifting model exists to date.3–6 We assessed outcomes and risk factors for attrition after decentralization in this project.
METHODS
Setting
DRC's capital Kinshasa is a densely populated megacity of 11 million inhabitants.7 HIV prevalence is 1.2% countrywide and 1.6% in Kinshasa.8 Less than 25% of eligible patients are on treatment, which is substantially lower than the SSA average.9 HIV is still highly stigmatized in DRC.10
In 2002, MSF started an HIV project at Kabinda Hospital, a major referral hospital in the South-Western part of Kinshasa, in collaboration with the Ministry of Health. By 2010, more than 6500 patients were receiving ART at this facility. Its centralized provision of care led to overcrowding and long waiting hours became common, with patients traveling up to 3 hours to reach Kabinda Hospital due to traffic congestion and poor innercity public transport. Consequently, even simple ART collection could easily cost a patient an entire day.
In 2010, MSF started to decentralize ART provision for stable patients to community-based refill centers [“poste de distribution communautaire” (PODI)] in collaboration with the national organization “Réseau National des Organisations d'Assises Communautaires.” The main objective was to offer quick and demedicalized access to ART by separating medical care from drug supply. Within 1 year, 3 PODIs were opened across Kinshasa. PODIs are small entities and staffed with lay community workers, who are mostly HIV positive themselves. Appointments are 3 monthly, plus 1 yearly check-up, at Kabinda Hospital. The whole process of registration, adherence assessment, and ART dispensing usually require less than 15 minutes. No medical care is offered, but referral to Kabinda Hospital is organized if needed. Defaulting patients get traced by community volunteers. All services are free of charge. MSF provides medication, financial and technical support, and training for PODI staff.
Design and Study Population
This was a cohort analysis using previously collected routine data from HIV patients who were decentralized from Kabinda Hospital to PODI sites between June 2011 and September 2014. Decentralization criteria were: ≥18 years of age; being on first-line ART for at least 6 months; being clinically stable for the past 3 months; have a cluster of differentiation type-4 (CD4) cell count of >250 cells per microliter; and not be pregnant. The World Health Organization 2010 HIV guidelines11 were followed throughout the study period.
Data Management and Analysis
Patient characteristics comprised sex, age, marital status, educational level, nonnucleoside reverse transcriptase inhibitor (non-NRTI) used [efavirenz or nevirapine (NVP)], NRTI used [stavudine (D4T) or not D4T], time on ART, CD4 count, body mass index (BMI), travel distance to PODI, and decentralization site. Outcomes were defined as retained, died, transferred out, and loss to follow-up (LTFU). Attrition was defined as either death or LTFU. Data were regularly entered into a dedicated database as part of routine program activities.
Monthly cumulative incidence was calculated for each outcome. A multilevel Poisson regression model was built to identify risk factors for attrition using incidence rates and rate ratios with 95% confidence intervals, Lexis expansion for time after decentralization, and P-values from likelihood ratio tests.
Ethics
As only anonymized data were used and no intervention or patient contact was made for research purposes, the issue of informed consent did not apply. Exemption from ethics review was granted by the the DRC National Ethical Committee (Kinshasa, DRC). This study met the criteria for analysis of routinely collected program data of the MSF Ethics Review Board (Geneva, Switzerland) and of the Ethics Advisory Group of the International Union against Tuberculosis and Lung Disease (Paris, France).
RESULTS
From the total 2603 decentralized patients, 2259 (86.8%) were included in the analysis, contributing 4026.9 person-years with a median follow-up of 2.0 years (interquartile range 1.1–2.4) (see Figure, Supplemental Digital Content 1, http://links.lww.com/QAI/A937, displaying patient selection for analysis).
Most patients were female [1720 (76.1%)], on average 45 (SD 9.3) years old, married [917 (40.6%)], and had secondary-level education [1527 (67.6%)]. The majority received NVP as non-NRTI [1848 (81.8%)] and D4T as NRTI [1335 (59.1%)]. The mean time on ART at time of decentralization was 4.4 (SD 2.6) years; the median CD4 count was 545.5 cells per microliter (interquartile range 407–727); and the mean BMI was 23.4 kg/m2 (SD 4.4). The PODI in central Kinshasa was the biggest site with 1056 (46.8%) patients. Travel time to PODIs was less than 45 minutes for the majority of patients [1633 (72.3%)] (see Table, Supplemental Digital Content 2, http://links.lww.com/QAI/A937, displaying patient baseline characteristics).
Attrition increased steadily after decentralization, with LTFU and death reaching 50 (2.2%) and 2 (0.1%), 109 (4.8%) and 4 (0.2%), and 202 (9.0%) and 7 (0.3%), at 6, 12, and 24 months, respectively (Fig. 1). Crude attrition incidence was 5.66/100 person-years (95% confidence interval: 4.97 to 6.45) with little variation over time.
FIGURE 1.
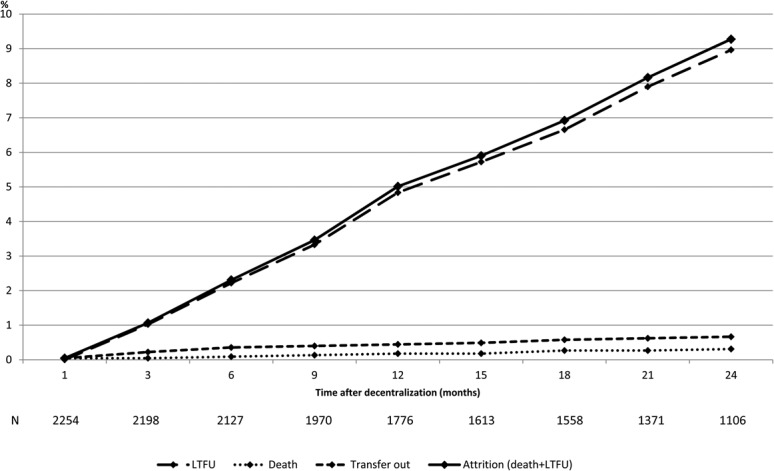
Cumulative treatment outcomes after decentralization. N, number of patients.
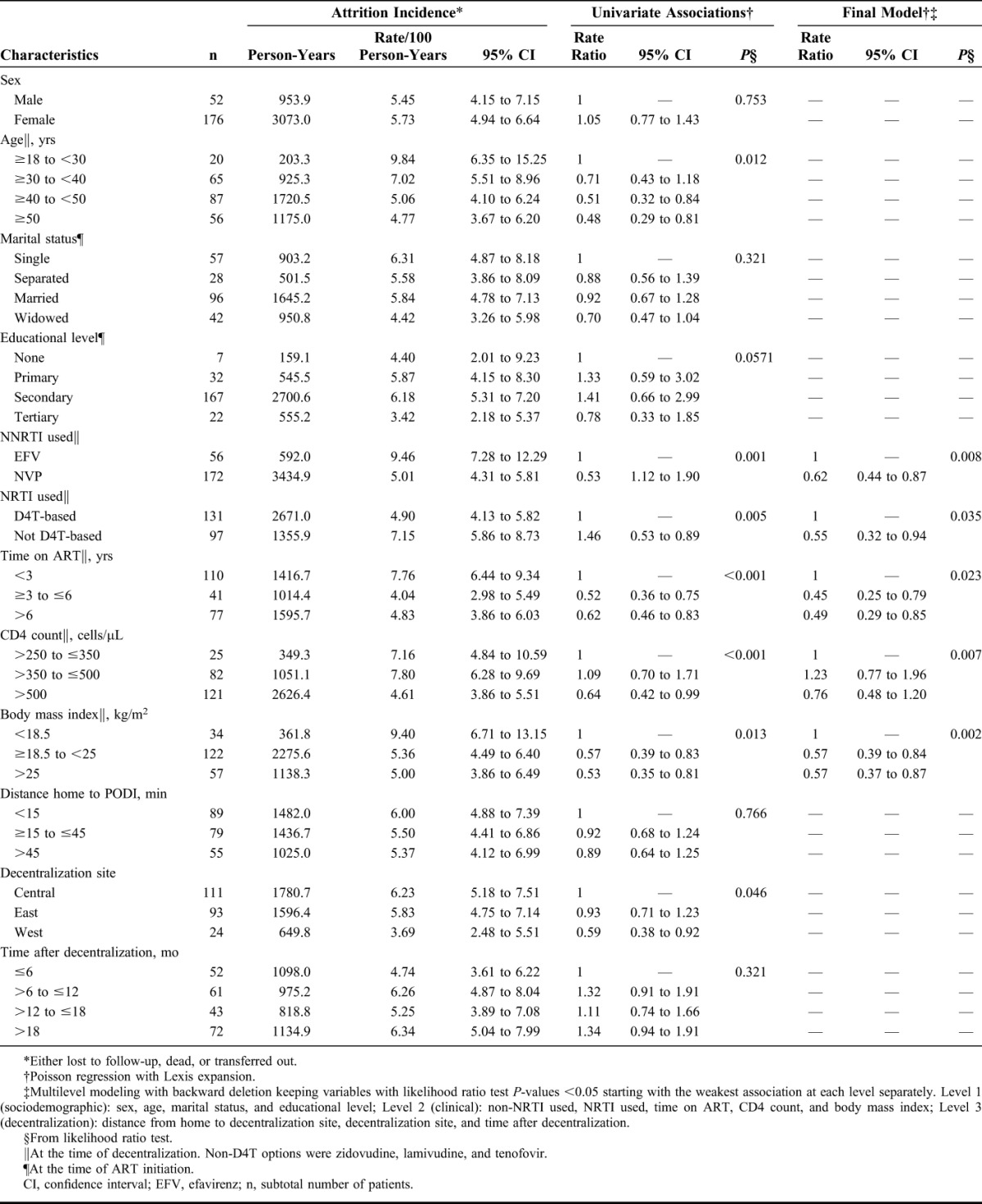
Neither demographic or decentralization-related variables but all clinical variables were associated with attrition in the final model. The risk of attrition was reduced for patients receiving NVP (P = 0.008), those not receiving D4T (P = 0.035), those with a CD4 count >500 cells per microliter (P = 0.007), those with a BMI of ≥18.5 kg/m2 (P = 0.002), and those who were already on ART for more than 3 years (P = 0.023) (Table 1).
TABLE 1.
Incidence of and Factors Associated With Attrition
DISCUSSION
This is the first evaluation of a decentralization model of HIV care based on community-run ART refill centers. We found low attrition throughout follow-up. Patients with beneficial clinical characteristics had a particularly low risk of attrition.
Most community decentralization initiatives do not task-shift ART provision.12–20 Some programs use community health workers (CHWs) for home visits for ART supply21–29 or run community ART groups (CAGs) for collective drug collection.30–33 Dispensing ART through community-based drug refill centers is a unique feature of this project. Also, most decentralization activities in SSA happen in rural areas,34 making this study a rare example of task shifting in an urban setting.
Many trials evaluating CHW interventions for home-based ART provision enroll patients at time of ART initiation.21–29 As mortality and LTFU are the highest during the early post-initiation phase,35,36 their results are hardly comparable with those of our PODI patients. One trial from rural Kenya enrolling only clinically stable HIV patients with monthly ART delivery by CHWs to patients' homes had LTFU of 5% at 12 months,23,24 which is similar to our findings. CAG-based ART distribution models aim at reducing the travel time for patients to health facilities by sending one patient per group to collect drugs for up to 10 patients at a time.37 Retention from such a project in Mozambique with similar inclusion criteria as in our project was 98%, 96%, and 93% at 12, 24, and 36 months, respectively, with the overall attrition rate being 2.1/100 person years.20,31 Another CAG project in Lesotho reported 98% retention at 12 months.32 Although these outcomes are slightly better than in our PODI project, it is worth noting that only 50% of patients in Mozambique and only 33% of patients in Lesotho opted to join CAGs, which might bias these results. Another similar project in an urban slum in South Africa had an attrition rate of 3/100 person years,33 which is about half of what we observed in Kinshasa. Evidence on community-based ART interventions from DRC does not exist. One analysis of physician-based ambulatory ART clinics restricted to patients receiving long-term ART showed retention of 81%, 75%, 65%, and 57% at 6, 12, 24, and 36 months, respectively,38 which is considerably worse than in the PODI project and resembles conventional models of care from other SSA countries.36,39
In our analysis, no sociodemographic or decentralization-related factor was associated with attrition. This suggests that PODIs are suitable for heterogeneous patient groups. Low CD4 cell count and low BMI were also identified as risk factors for attrition elsewhere.31,33,38 D4T was still widely used in DRC during our study period.40 The reduced risk of attrition found among PODI patients who did not receive D4T as NRTI could be explained by its well-known problematic side-effect profile.41,42
Each context needs its own tailored task-shifting model.43,44 Home-based ART supply by CHWs is a heavy intervention demanding lots of staff, financial investments, and intensive supervision.45 This was not considered sustainable for Kinshasa. Also, patients have to be at home for CHW visits and their homes need to be locatable easily, which is challenging in a highly mobile population mostly living in informal housing. The CAG model is suitable for high-prevalence rural settings with thin-spread health facilities where group members live close by to each other (eg, in the same village). In an urban low-prevalence setting, CAGs lose their comparative advantage compared with conventional care because patients still need to meet regularly to obtain their medication. Scheduling CAG meetings in a congested megacity such as Kinshasa where patients with HIV live scattered across the city would be time consuming and inconvenient for patients, and logistically difficult to organize. Also, CAG-related stigma has been reported,32 and receiving CHW visits or attending group meetings exposes people as HIV positive. The high stigmatization levels would have compromised either CHW or CAG models in Kinshasa. PODIs assured the necessary level of anonymity for this context. Tellingly, the PODI in central Kinshasa was the most frequented of all sites, as its location in the commercial downtown area allowed patients to pick up their medication anonymously during their work day.
Health systems alone will not manage the patient load that comes with further ART scale-up.2,46 Also, the World Health Organization is now endorsing ART maintenance by local communities.47 The PODI model seems to be a promising approach in a context such as Kinshasa and as such has been included in the national strategic plan against HIV 2014–2017 by the Ministry of Health.48
The strengths of our study include the big sample size and the long follow-up duration, which covers both short- and long-term decentralization effects. Our data derive from routine program implementation, thereby better reflecting real-life conditions of HIV care in SSA than artificial trial environments.
Our research was subject to all limitations inherent to retrospective data analyses. Most importantly, we did not have a comparison group. A concurrent group of nondecentralized patients with similar characteristics from the same setting or alternative outcome data from the same patients before decentralization would have enabled us to clearly attribute and quantify the effects of decentralizing patients to PODIs on attrition. Second, we had no data available about patients who met the decentralization criteria but were not decentralized. This might have biased our results. Similarly, our findings should not be extrapolated to patient groups with substantially different demographic characteristics. Third, ascertainment of death relied on information provided by patient relatives to defaulter tracers. Also, patients who reported health problems were sent to Kabinda Hospital for care. Deaths among those patients were not captured. Although this did not affect our overall attrition estimates, it might have led to inflated LTFU and deflated death estimates.49,50 Also, the steady trajectory of the attrition levels requires long-term evaluation. Fourth, we did not have clinical or laboratory markers available to monitor adherence, treatment failure, or disease progression after decentralization.
PODIs seem to be perform considerably better than conventional models of care, similar to CHW home visit interventions, and slightly worse than CAGs. PODIs are a promising decentralization approach in urban contexts with high levels of stigma, low HIV prevalence, and low ART coverage. Replications of this model in other settings are needed to appraise its potential to relieve resource-constraint health systems from the burden of stable patients with HIV in the wake of continued ART scale-up.
Supplementary Material
Footnotes
The authors have no funding or conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
REFERENCES
- 1.Joint United Nations Programme on HIV/AIDS. How AIDS Changed Everything. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS; 2015. [Google Scholar]
- 2.Price J, Binagwaho A. From medical rationing to rationalizing the use of human resources for AIDS care and treatment in Africa: a case for task shifting. Dev World Bioeth. 2010;10:99–103. [DOI] [PubMed] [Google Scholar]
- 3.Young T, Busgeeth K. Home-based care for reducing morbidity and mortality in people infected with HIV/AIDS. Cochrane database Syst Rev. 2010:CD005417. [DOI] [PubMed] [Google Scholar]
- 4.Decroo T, Rasschaert F, Telfer B, et al. Community-based antiretroviral therapy programs can overcome barriers to retention of patients and decongest health services in sub-Saharan Africa: a systematic review. Int Health. 2013;5:169–179. [DOI] [PubMed] [Google Scholar]
- 5.Mwai GW, Mburu G, Torpey K, et al. Role and outcomes of community health workers in HIV care in sub-Saharan Africa: a systematic review. J Int AIDS Soc. 2013;16:18586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wouters E, Van Damme W, van Rensburg D, et al. Impact of community-based support services on antiretroviral treatment programme delivery and outcomes in resource-limited countries: a synthetic review. BMC Health Serv Res. 2012;12:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demographia. Demographia World Urban Areas. 11th Annual ed 2015. Available at: http://www.demographia.com/db-worldua.pdf. Accessed November 10, 2016. [Google Scholar]
- 8.Ministère du Plan et Suivi de la Mise en oeuvre de la Révolution de la Modernité, Ministère de la Santé Publique, ICF International. Enquête Démographique et de Santé en République Démocratique du Congo 2013-2014. Rockville, MD: ICF International; 2014. [Google Scholar]
- 9.Joint United Nations Programme on HIV/AIDS. The Gap Report. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS; 2014. [Google Scholar]
- 10.L'Union Congolaise des Organisations des Personnes vivant avec le VIH. Index de stigmatisation et de discrimination des personnes vinant avec le VIH en RDC. Kinshasa, Democratic Republic of the Congo: L'Union Congolaise des Organisations des Personnes vivant avec le VIH; 2012. [Google Scholar]
- 11.World Health Organization. Antiretroviral Therapy for HIV Infection in Adults and Adolescents: Recommendations for a Public Health Approach—2010 Revision. Geneva, Switzerland: World Health Organization; 2010. [PubMed] [Google Scholar]
- 12.Cohen R, Lynch S, Bygrave H, et al. Antiretroviral treatment outcomes from a nurse-driven, community-supported HIV/AIDS treatment programme in rural Lesotho: observational cohort assessment at two years. J Int AIDS Soc. 2009;12:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abaasa AM, Todd J, Ekoru K, et al. Good adherence to HAART and improved survival in a community HIV/AIDS treatment and care programme: the experience of the AIDS Support Organization (TASO), Kampala, Uganda. BMC Health Serv Res. 2008;8:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bedelu M, Ford N, Hilderbrand K, et al. Implementing antiretroviral therapy in rural communities: the Lusikisiki model of decentralized HIV/AIDS care. J Infect Dis. 2007;196:S464–S468. [DOI] [PubMed] [Google Scholar]
- 15.Nachega JB, Chaisson RE, Goliath R, et al. Randomized controlled trial of trained patient-nominated treatment supporters providing partial directly observed antiretroviral therapy. AIDS. 2010;24:1273–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Igumbor JO, Scheepers E, Ebrahim R, et al. An evaluation of the impact of a community-based adherence support programme on ART outcomes in selected government HIV treatment sites in South Africa. AIDS Care. 2011;23:231–236. [DOI] [PubMed] [Google Scholar]
- 17.Rich ML, Miller AC, Niyigena P, et al. Excellent clinical outcomes and high retention in care among adults in a community-based HIV treatment program in rural Rwanda. J Acquir Immune Defic Syndr. 2012;59:e35–e42. [DOI] [PubMed] [Google Scholar]
- 18.Zachariah R, Teck R, Buhendwa L, et al. Community support is associated with better antiretroviral treatment outcomes in a resource-limited rural district in Malawi. Trans R Soc Trop Med Hyg. 2007;101:79–84. [DOI] [PubMed] [Google Scholar]
- 19.Chang LW, Kagaayi J, Nakigozi G, et al. Effect of peer health workers on AIDS care in Rakai, Uganda: a cluster-randomized trial. PLoS One. 2010;5:e10923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torpey KE, Kabaso ME, Mutale LN, et al. Adherence support workers: a way to address human resource constraints in antiretroviral treatment programs in the public health setting in Zambia. PLoS One. 2008;3:e2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaffar S, Amuron B, Foster S, et al. Rates of virological failure in patients treated in a home-based versus a facility-based HIV-care model in Jinja, southeast Uganda: a cluster-randomised equivalence trial. Lancet. 2009;374:2080–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amuron B, Levin J, Birunghi J, et al. Mortality in an antiretroviral therapy programme in Jinja, south-east Uganda: a prospective cohort study. AIDS Res Ther. 2011;8:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wools-Kaloustian KK, Sidle JE, Selke HM, et al. A model for extending antiretroviral care beyond the rural health centre. J Int AIDS Soc. 2009;12:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selke HM, Kimaiyo S, Sidle JE, et al. Task-shifting of antiretroviral delivery from health care workers to persons living with HIV/AIDS: clinical outcomes of a community-based program in Kenya. J Acquir Immune Defic Syndr. 2010;55:483–490. [DOI] [PubMed] [Google Scholar]
- 25.Kipp W, Konde-Lule J, Saunders LD, et al. Results of a community-based antiretroviral treatment program for HIV-1 infection in Western Uganda. Curr HIV Res. 2010;8:179–185. [DOI] [PubMed] [Google Scholar]
- 26.Kipp W, Konde-Lule J, Saunders LD, et al. Antiretroviral treatment for HIV in rural Uganda: two-year treatment outcomes of a prospective health centre/community-based and hospital-based cohort. PLoS One. 2012;7:e40902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weidle PJ, Wamai N, Solberg P, et al. Adherence to antiretroviral therapy in a home-based AIDS care programme in rural Uganda. Lancet. 2006;368:1587–1594. [DOI] [PubMed] [Google Scholar]
- 28.Mermin J, Were W, Ekwaru JP, et al. Mortality in HIV-infected Ugandan adults receiving antiretroviral treatment and survival of their HIV-uninfected children: a prospective cohort study. Lancet. 2008;371:752–759. [DOI] [PubMed] [Google Scholar]
- 29.Moore DM, Yiannoutsos CT, Musick BS, et al. Determinants of early and late mortality among HIV-infected individuals receiving home-based antiretroviral therapy in rural Uganda. J Acquir Immune Defic Syndr. 2011;58:289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Decroo T, Telfer B, Biot M, et al. Distribution of antiretroviral treatment through self-forming groups of patients in Tete Province, Mozambique. J Acquir Immune Defic Syndr. 2011;56:e39–e44. [DOI] [PubMed] [Google Scholar]
- 31.Decroo T, Koole O, Remartinez D, et al. Four-year retention and risk factors for attrition among members of community ART groups in Tete, Mozambique. Trop Med Int Health. 2014;19:514–521. [DOI] [PubMed] [Google Scholar]
- 32.Vandendyck M, Motsamai M, Mubanga M. Community-based ART resulted in excellent retention and can leverage community em-powerment in rural Lesotho, a mixed method study. HIV/AIDS Res Treat. 2015;2:44–50. [Google Scholar]
- 33.Luque-Fernandez MA, Van Cutsem G, Goemaere E, et al. Effectiveness of patient adherence groups as a model of care for stable patients on antiretroviral therapy in Khayelitsha, Cape Town, South Africa. PLoS One. 2013;8:e56088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Callaghan M, Ford N, Schneider H. A systematic review of task- shifting for HIV treatment and care in Africa. Hum Resour Health. 2010;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fox MP, Rosen S. Retention of adult patients on antiretroviral therapy in low- and middle-income countries: systematic review and meta-analysis 2008-2013. J Acquir Immune Defic Syndr. 2015;69:98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS Med. 2011;8:e1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joint United Nations Programme on HIV/AIDS, Médecins Sans Frontières. Community-based Antiretroviral Therapy Delivery. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS; 2015. [Google Scholar]
- 38.Koole O, Kalenga L, Kiumbu M, et al. Retention in a NGO supported antiretroviral program in the Democratic Republic of Congo. PLoS One. 2012;7:e40971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tassie JM, Baijal P, Vitoria MA, et al. Trends in retention on antiretroviral therapy in national programs in low-income and middle-income countries. J Acquir Immune Defic Syndr. 2010;54:437–441. [DOI] [PubMed] [Google Scholar]
- 40.World Health Organization. Towards Universal Access—Scaling up Priority HIV/AIDS Interventions in the Health Sector. Geneva, Switzerland: World Health Organization; 2009. [Google Scholar]
- 41.Van Griensven J, Zachariah R, Rasschaert F, et al. Stavudine- and nevirapine-related drug toxicity while on generic fixed-dose antiretroviral treatment: incidence, timing and risk factors in a three-year cohort in Kigali, Rwanda. Trans R Soc Trop Med Hyg. 2010;104:148–153. [DOI] [PubMed] [Google Scholar]
- 42.Subbaraman R, Chaguturu SK, Mayer KH, et al. Adverse effects of highly active antiretroviral therapy in developing countries. Clin Infect Dis. 2007;45:1093–1101. [DOI] [PubMed] [Google Scholar]
- 43.Mdege ND, Chindove S, Ali S. The effectiveness and cost implications of task-shifting in the delivery of antiretroviral therapy to HIV-infected patients: a systematic review. Health Policy Plan. 2013;28:223–236. [DOI] [PubMed] [Google Scholar]
- 44.Lazarus JV, Safreed-Harmon K, Nicholson J, et al. Health service delivery models for the provision of antiretroviral therapy in sub-Saharan Africa: a systematic review. Trop Med Int Health. 2014;19:1198–1215. [DOI] [PubMed] [Google Scholar]
- 45.Wringe A, Cataldo F, Stevenson N, et al. Delivering comprehensive home-based care programmes for HIV: a review of lessons learned and challenges ahead in the era of antiretroviral therapy. Health Policy Plan. 2010;25:352–362. [DOI] [PubMed] [Google Scholar]
- 46.Rabkin M, El-Sadr WM, De Cock KM. The impact of HIV scale-up on health systems: a priority research agenda. J Acquir Immune Defic Syndr. 2009;52(suppl 1):S6–S11. [DOI] [PubMed] [Google Scholar]
- 47.World Health Organization. March 2014 Supplement to the 2013 Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. Geneva, Switzerland: World Health Organization; 2014. [Google Scholar]
- 48.Programme National Multisectoriel de Lutte contre le Sida République Démocratique du Congo. Plan Stratégique National de lutte contre le VIH et le sida 2014–2017. Kinshasa, Democratic Republic of the Congo: Programme National Multisectoriel de Lutte contre le Sida République Démocratique du Congo; 2014. [Google Scholar]
- 49.Yu JK, Chen SC, Wang KY, et al. True outcomes for patients on antiretroviral therapy who are “lost to follow-up” in Malawi. Bull World Health Organ. 2007;85:550–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bisson GP, Gaolathe T, Gross R, et al. Overestimates of survival after HAART: implications for global scale-up efforts. PLoS One. 2008;3:e1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


