Supplemental Digital Content is Available in the Text.
Key Words: cytokines, female genital tract, mucosa, plasma, chemokine gradient, HIV risk
Abstract
Background:
Mucosal and systemic immune mediators have been independently associated with HIV acquisition risk, but the relationship between compartments remains unclear.
Methods:
To address this, the concentrations of 12 cytokines were compared in matched plasma and cervicovaginal lavages (CVLs) from 57 HIV-positive women before their acquisition of HIV (cases) and 50 women who remained uninfected (controls) during the CAPRISA 004 trial.
Results:
Although genital IP-10 concentrations were significantly higher in cases, plasma IP-10 concentrations were inversely associated with HIV risk. Comparing differences in mucosal and systemic cytokine concentrations between cases and controls, mucosa-biased gradients indicating higher cervicovaginal lavage relative to plasma concentrations were observed for all 5 chemokines in the panel. Four were significantly associated with HIV acquisition, including IP-10 (odds ratio [OR] 1.73, 95% confidence interval [CI]: 1.27 to 2.36), macrophage inflammatory protein–1β (OR 1.72, 95% CI: 1.23 to 2.40), interleukin (IL)-8 (OR 1.50, 95% CI: 1.09 to 2.05), and monocyte chemotactic protein-1 (OR 1.36, 95% CI: 1.01 to 1.83). None of the other 7 cytokines tested predicted HIV risk. Decision tree analyses confirmed this association, with gradients of IP-10, IL-8, and granulocyte-macrophage colony-stimulating factor concentrations correctly classifying 77% of HIV outcomes.
Conclusions:
Our findings suggest that mucosa-biased gradients of IP-10, macrophage inflammatory protein–1β, IL-8, and monocyte chemotactic protein-1 are associated with an increased risk of HIV infection.
INTRODUCTION
Host immune factors that influence the probability of male-to-female HIV transmission remain poorly characterized. The immune barrier of the female genital tract generally provides effective protection against HIV, as indicated by low per-coital rates of HIV acquisition in the absence of transmission cofactors.1–3 However, in young South African women, studies have found extremely high per-partnership transmission probabilities of 0.74–1.0.4,5 A better understanding of the factors that may drive these high rates of HIV acquisition is of vital importance to HIV prevention efforts. Several investigators have hypothesized that availability of HIV target cells at the site of transmission may be determinants of HIV susceptibility.6 In the nonhuman primate model of simian immunodeficiency virus (SIV) infection, elevated concentrations of the chemokines macrophage inflammatory protein (MIP)-1α, MIP-1β, MIP-3α, and interleukin (IL)-8 were shown to be essential for establishment of a productive infection, and blocking of these chemokines prevented target cell recruitment and SIV infection.7 Although a relative paucity of HIV target cells in the reproductive tract of healthy women has previously been reported,8,9 various microbial and nonmicrobial cofactors have been shown to influence chemokine concentrations, which in turn has been associated with target cell recruitment.10–14
We have previously shown that elevated concentrations of genital tract chemokines, including IL-8, MIP-1α, MIP-1β, and interferon gamma-induced protein (IP-10), were associated with increased risk of HIV acquisition in young South African women participating in the CAPRISA 004 1% tenofovir gel microbicide trial.15 Of these, MIP-1α and MIP-1β specifically recruit CCR5+ target cells that are required to establish HIV infection.16 IP-10 is the ligand for CXCR3, which is expressed on a similar overlapping set of T-helper type 1 cells as CCR5.17 In another cohort, we similarly showed that increased concentrations of several cytokines and chemokines (IL-1β, IL-6, IL-8, and sCD40L) in cervicovaginal lavage (CVL) secretions were associated with HIV outcome.12 These findings are supported by recent work suggesting that elevated genital concentrations of the CCR5-binding chemokine, RANTES, were associated with an increased risk of HIV infection.18
Systemic immune mediators also predict risk for HIV seroconversion, although findings in these immunological studies have been somewhat inconsistent. Kahle et al19 showed that increased levels of IL-10 and IP-10 in blood were associated with HIV transmission when present in both the uninfected and infected sexual partners with HIV. Another prospective cohort study observed lower systemic IL-6 and IL-10 and higher IL-7 concentrations in those who eventually acquired HIV, but only in univariate analyses.20 Previously, our group also demonstrated that systemic tumor necrosis factor (TNF)-α, IL-2, IL-7, and IL-12p70 concentrations were elevated in women who later became HIV-infected compared with those who remained uninfected in the CAPRISA 004 trial.21
Although it has been shown separately that both female genital tract and systemic immune mediators can predict altered rates of HIV acquisition, the relationship between compartments remains unclear. In addition, most previous studies have focused on cytokines individually, considering them either univariately, or in a step-wise fashion with logistic regression. Here, we sought to reconcile the contribution of the genital environment and systemic compartments by directly comparing the relationship between HIV outcome and cytokines within compartments, and with genital and plasma cytokines in the same participants. These data suggest that the systemic–mucosal chemokine gradient may play a particularly important role in HIV acquisition, and lend further support to the hypothesis that increased trafficking of immune cells to the site of exposure might be a critical factor for establishment of HIV infection in humans.
METHODS
Study Participants
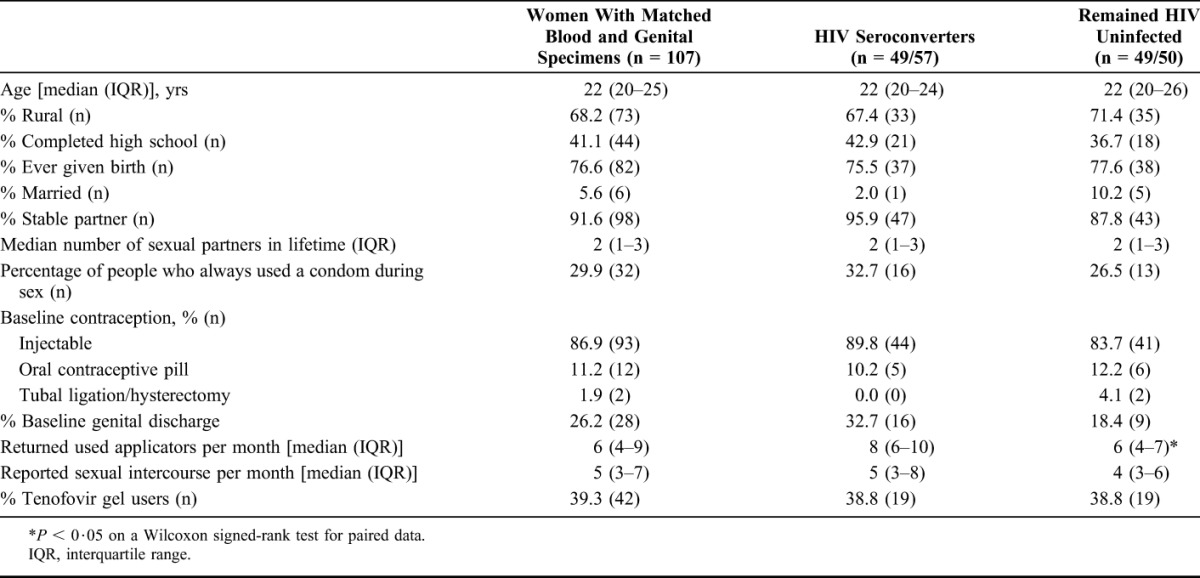
The CAPRISA 004 1% tenofovir gel microbicide trial included 889 HIV-uninfected South African women from both the rural Vulindlela and the urban eThekwini clinics.22 This investigation of systemic and genital cytokines was conducted in 57 women who acquired HIV during the 2.8 years of follow-up (cases), and 50 women who remained uninfected during the study (controls), all of whom had CVL and plasma samples collected from the same visits. Forty-nine cases and controls were matched on the basis of study arm and time of sampling. HIV testing was conducted monthly using 2 rapid HIV antibody tests and confirmed with 2 independent RNA–polymerase chain reaction assays at least 1 week apart.22 All samples from cases were analyzed for cytokine levels before HIV infection [median 4.5 months preinfection (interquartile range 2.4–6.9 months)]. Research Ethics Committees at the Universities of KwaZulu-Natal and Cape Town approved the study, and all participants provided written informed consent before participation.
Sample Collection
CVL samples were collected by repeatedly bathing the cervix with 3 mL sterile saline, with collection from the posterior fornix followed by aspiration into a plastic bulb pipette. Samples were centrifuged and the supernatant stored at −80°C. CVL samples were not collected from menstruating participants, in which case sampling was postponed to the following week. At the same visits, blood was collected by venipuncture into acetate citrate dextran vacutainer tubes and isolated blood plasma was stored at −80°C. The samples included in this study had not been previously thawed. Before cytokine measurements, samples were filtered by centrifugation using 0.2 μm cellulose acetate filters (Sigma, St Louie, MO).
Measurement of Cytokine Concentrations
We previously assessed genital inflammation in 58 control participants who remained HIV uninfected during the CAPRISA 004 trial, and in 58 matching cases before infection.15 For this purpose of comparable systemic cytokine assessment, the same panel of cytokines was extended to the plasma of all women with matching plasma specimens available (n = 107). In brief, the concentrations of IL-1α, IL-1β, IL-6, IL-7, IL-8, IL-10, granulocyte-macrophage colony-stimulating factor, IP-10, monocyte chemotactic protein-1, MIP-1α, MIP-1β, and TNF-α were measured preinfection (median of 20 weeks preinfection, range 2–71 weeks) using Milliplex High Sensitivity and Human Cytokine kits according to the manufacturer's protocol (Millipore, Billerica, MA). Data were collected using a Bio-Plex Suspension Array Reader (Bio-Rad Laboratories Inc., Hercules, CA) and a 5 PL regression formula was used to calculate sample concentrations from the standard curves. Data were analyzed using Bio-Plex manager software (version 4). Cytokine levels that were below the lower limit of detection of the assay were reported as the mid-point between zero and the lowest concentration measured for that given cytokine. Detection limits of the cytokines measured in both genital and blood specimens are listed in Supplemental Digital Content 1, http://links.lww.com/QAI/A939.
Statistical Analyses
Statistical analyses were performed using GraphPad Prism version 5 (GraphPad Software, San Diego, CA), STATA (StataCorp, College Station, TX), SAS version 9.3 (SAS Institute Inc., Cary, NC), and Matlab (Matlab 2014a; MathWorks, Natick, MA). Cytokine concentrations were log10-transformed. Conditional logistic regression was used with matched pairs as strata to determine the association between log10-transformed plasma cytokine concentrations and risk of HIV infection. Cytokine gradients were calculated as the ratio of log10-transformed, standardized genital over plasma cytokine concentrations. McNemar test was used to compare cases and controls in Table 1. P-values were adjusted for multiple comparisons using a false discovery rate step-down procedure.
TABLE 1.
Demographic and Behavioral Characteristics of Study Participants
We used decision tree analysis (DTA) to determine the hierarchical importance of plasma and CVL cytokine concentrations in classifying HIV infection outcome.23 A classification decision tree algorithm (Matlab 2014a; MathWorks) was used to predict infection status as a function of the CVL and systemic cytokine measurements made from each corresponding participant. Gini Diversity Index was chosen as the split criterion. Cross-validation was performed by generating decision trees after iteratively excluding samples in groups of ∼10, then testing excluded data using the generated model. Decision tree calibration and cross-validation error were determined by comparing the predicted and actual class for each individual. Reported cross-validation error for each model reflects the average of 10 simulations performed during the cross-validation. To prevent over-fitting, all trees were pruned to levels with lowest cross-validation and calibration error. For each model, the number of terminal nodes for the optimal pruned tree was selected by minimizing both calibration and cross-validation error (See Supplemental Digital Content 2, http://links.lww.com/QAI/A939, which illustrates the pruning of decision trees).
RESULTS
Plasma Cytokines and the Risk of HIV Acquisition
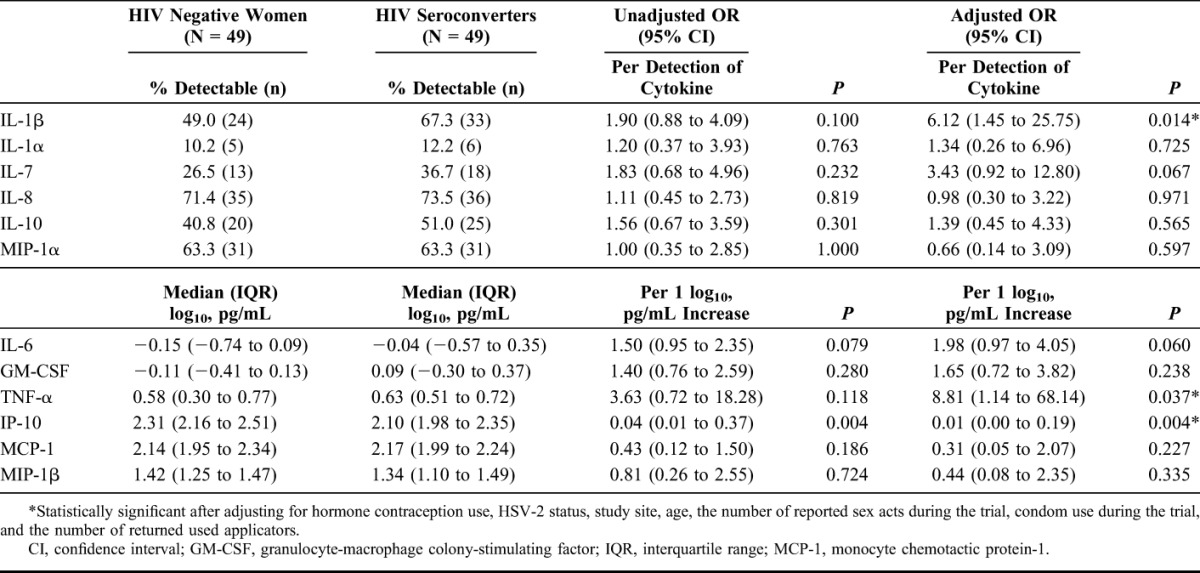
The concentrations of 12 cytokines were measured in plasma samples from 57 cases and 50 controls. Of the several demographic and behavioral characteristics assessed, only the number of returned used applicators per month differed significantly between cases and controls (Table 1). After adjusting for relevant confounders hormone contraception use, herpes simplex virus-2 status, study site (urban versus rural), age, the number of reported sex acts during the trial, condom use during the trial, and the number of returned used applicators in the multivariate analyses,15,22 3 cytokines (IP-10, TNF-α, and IL-1β) were associated with HIV as an outcome (Table 2). These data suggest that plasma cytokines are useful immunological measures for predicting differences in HIV risk.
TABLE 2.
Relationship Between Systemic Cytokines and Risk of HIV Infection in Women From the CAPRISA 004 Trial
Mucosa-Biased Chemokine Gradients Are Independent Predictors of HIV Acquisition
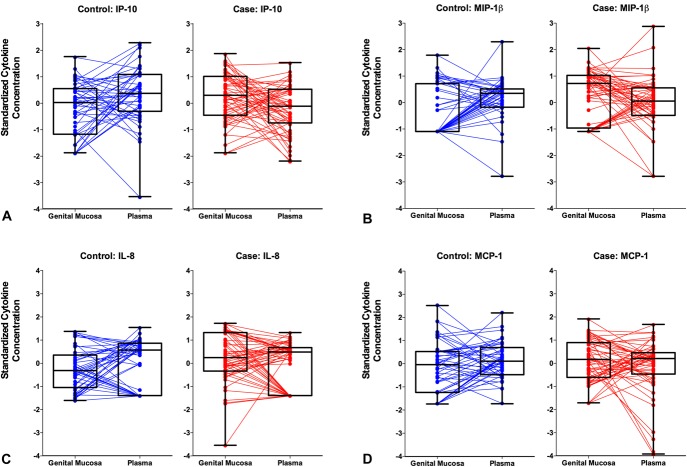
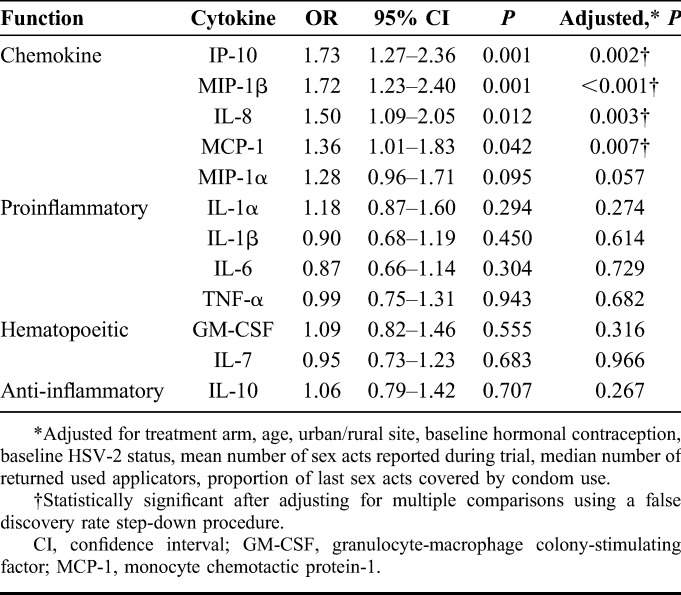
Based on our previous finding that genital tract IP-10 concentrations were significantly higher in women who subsequently seroconverted compared with those who remained uninfected,15 and the current observation of lower plasma IP-10 concentrations associated with HIV risk in matched participants, we investigated the impact of mucosal–systemic chemokine gradients on HIV acquisition risk. Cytokine gradients were determined by quantifying the difference between standardized cytokine concentrations of the genital mucosa and plasma on a per-individual basis. In this analysis a “positive gradient” was indicative of higher relative levels in the genital tract compared with the plasma. Logistic regression analyses demonstrated that a positive gradient was associated with HIV outcome for 4 of the 5 chemokines represented in the panel (IP-10, MIP-1β, IL-8, and monocyte chemotactic protein-1), and no other cytokine (Fig. 1; Table 3). The MIP-1α gradient trended toward a significant association with increased HIV acquisition risk. The data suggest that increased chemotaxis of immune cells to the mucosal tissue is an important factor determining risk, rather than a generalized inflammatory effect.
FIGURE 1.
Mucosal–plasma chemokine gradients in women who remained uninfected (controls; n = 50) and in HIV- women who subsequently acquired HIV (cases; n = 57) during the CAPRISA 004 trial. Intercompartmental cytokine gradients were determined by quantifying the difference between standardized cytokine concentrations of the female genital tract and blood plasma for each participant. Four of the 5 gradients associated with HIV outcome in multivariate analysis are depicted here: (A) IP-10, (B) MIP-1β, (C) IL-8, and (D) monocyte chemotactic protein-1 (MCP-1). “Positive” gradients are indicative of higher relative levels in the genital tract compared with the blood.
TABLE 3.
Relationship Between Mucosa–Plasma Cytokine Gradients and Risk of HIV Infection in Women From the CAPRISA 004 Trial
Hierarchical Relationships Between Systemic and Mucosal Cytokines and HIV Outcome
Colinearity is a common drawback of using logistic regression for multiplex cytokine analyses, because cytokines are often highly correlated within the same sample, precluding their entry in multivariate step-wise analyses because of violations of key assumptions of these models. To overcome this, we used DTA, a multivariate computational technique that emphasizes contingent rather than independent contributions of cytokines and chemokines to classifying women based on HIV outcome.23,24 DTA was applied to (1) the complete data set of plasma and CVL cytokine measurements (Fig. 2A) and (2) the complete set of gradient measurements (Fig. 2B), and used to identify the most influential combination of cytokine measurements associated with HIV outcome (Fig. 2). In the tree generated from combined CVL and plasma data, genital tract MIP-1β was identified as the most influential cytokine node, with 41/57 (71.9%) cases having genital MIP-1β concentrations greater than 1.8 pg/mL (Fig. 2A). Other key nodes selected by the decision tree algorithm were plasma IL-1β and plasma IP-10, both of which were higher in women who became infected. Overall, the optimally pruned tree was able to differentiate women based on infection status with 80% calibration accuracy (Fig. 2E) and 67% cross-validation accuracy (Fig. 2F).
FIGURE 2.
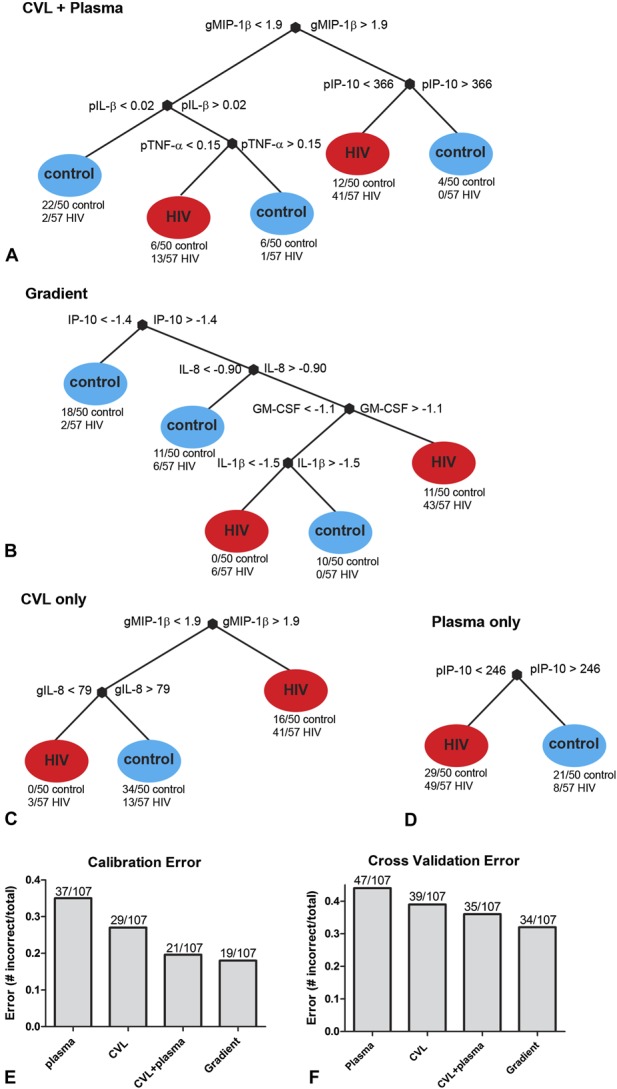
Decision tree analyses to identify the most influential combinations of genital and plasma, cytokine measurements associated with HIV outcome. Cytokine concentrations determined in cases (n = 57) and controls (n = 50) were modeled to include (A) the concentrations of all 12 cytokines in genital and plasma specimens, (B) mucosal-Plasma gradients, (C) all 12 genital cytokines alone, or (D) all 12 plasma cytokines. Comparison of the (E) calibration and (F) cross-Validation errors for decision trees generated using either plasma, CVL, combined CVL and plasma, or cytokine gradients, respectively.
To identify the hierarchical importance of cytokine gradients associated with HIV risk, a second decision tree was modeled to include the 12 cytokine gradients described. Interestingly, in this analysis the decision tree algorithm identified IP-10, IL-8, and granulocyte-macrophage colony-stimulating factor as key cytokine gradients and a pruned tree using these nodes was able to classify individuals with 82% calibration accuracy and 68% cross-validation accuracy (Fig. 2B). Separate CVL (Fig. 2C) and plasma (Fig. 2D) cytokine decision trees demonstrated that, of the cytokines measured, genital cytokine concentrations predicted HIV infection outcome more accurately than plasma cytokine concentrations. However, of all the means of investigating immune mediators of HIV risk assessed here, the models of gradient contributions and combined CVL and plasma cytokine contributions were most accurate by virtue of their reduced calibration (Fig. 2E) and cross-validation errors (Fig. 2F).
DISCUSSION
Understanding host predictors of HIV risk has important implications for risk profiling and design of better HIV prevention methods. Immune associations of HIV transmission have been proposed at both mucosal and systemic levels; however, findings from systemic samples in particular have not been consistent across studies.19–21 In light of these differences, the primary goal of this investigation was to understand how relative cytokine levels in systemic and mucosal compartments at the same preinfection time point impact the risk of HIV seroconversion. We previously described separately the genital15 and systemic21 cytokine associations with HIV risk in 2 different subsets of women within the same trial. A major strength of this study was the ability to evaluate paired mucosal and systemic specimens from the same cases and controls at the same visits using a single cytokine panel. The term gradient refers here not to the classical indication of a standard change in magnitude for example, of cytokines in one compartment relative to the other, but rather to the difference in standardized concentrations of cytokines between compartments, where positive differences or slopes were indicative of mucosa-biased or elevated genital concentrations relative to blood. Our data demonstrate that the relative intercompartmental differences in concentrations of all of the chemokines assessed, in particular, were independent predictors of HIV acquisition. The definitive association with chemokines suggests a mechanism focused on immune cell recruitment, because this is the primary physiological function of chemokines. Indeed, we have recently demonstrated that increased concentrations of several chemokines in the female genital tract correlate with increased frequencies of endocervical CD4+ T cells.25 A study of highly exposed but seronegative (HESN) sex workers reported similar results with respect to reduced levels of IP-10, MIP-1α, and MIP-1β in the genital tract relative to plasma.26,27 These data are also in line with data from the nonhuman primate model of SIV transmission,7 where chemokine production preceded the recruitment of essential CD4+ T-cell target cells to the female genital tract, and highlighted the potential role of local MIP-1α and MIP-1β concentrations in the establishment of productive SIV infection. Further studies to link this hypothesis empirically to HIV acquisition in human cohorts will be important to validate these findings.
Despite the differences between compartments, several systemic cytokines were associated with HIV acquisition, including those that support and extend a previous study of plasma cytokines in the same cohort.21 Our study corroborates a trend of increased IL-7 in cases compared with controls as previously reported.21 However, the association between lower IP-10 in blood and increased HIV acquisition risk is contrary to the findings of Kahle et al, who found increased IP-10 in plasma associated with increased HIV risk in discordant couples.19 Lehman et al20 did not report any significant differences between cases and controls with respect to systemic cytokines, but their panel did not include chemokines other than IL-8.
That several genital tract chemokines were positively associated with HIV outcome while plasma IP-10 was inversely associated suggests that mucosal stimuli might drive the chemokine gradient by interacting with epithelial cells or resident immune cells (CD8 T cells, dendritic cells, macrophages, and natural killer cells) via pattern recognition or other innate immune receptors.28,29 This hypothesis is supported by the finding that most plasma chemokines did not correlate between cases and controls,15 indicating that cytokines are likely produced in the female genital tract in response to localized stimuli, and reinforces the importance of assessing mucosal specimens. Indeed, of the decision tree models, the mucosa-only analysis was superior to the plasma-only tree in terms of classifying cases and controls. However, the combined or gradient decision tree algorithms were the most consistent and were the preferred models for classifying the cases and controls in comparison. These data support a model whereby the plasma analysis might detect more “global” immunological differences between cases and controls, whereas mucosal chemokine concentrations reflect processes and/or cofactors that operate locally as mediators of HIV risk.
We acknowledge several limitations of this study. Although every effort was made to include all relevant specimens in this study, our sample size was limited by the number of seroconversions in the CAPRISA 004 study, the number of participants who consented to sample storage, and on the sample availability pre-HIV infection. Although the selected panel of 12 cytokines includes those known to play important roles in inflammatory conditions,11,12,15 it is likely that additional cytokine associations could be observed if a larger cytokine panel was measured. However, because multiple chemokines can bind the same receptors, the inclusion of 5 key chemokines covers a broad range of immunological effects. It is interesting to note that associations with HIV risk were not limited to CCR5 ligands. IP-10 is the ligand for CXCR3, a Th1 marker,30 and a role for CXCR3 ligands has been implicated in various animal models of mucosal viral infection.31,32 CCR5 is also highly expressed on Th1 cells, so some overlap between these chemokines/chemokine receptors is expected. Another limitation was that the dilution factors of CVL specimens were not known, whereas plasma was undiluted. We addressed the sampling differences by first standardizing the genital and plasma cytokine measurements to allow their equal contribution to the scale before calculating mucosa-plasma ratios. The differences between genital and plasma cytokine concentrations observed for each woman was therefore relative to the other study participants. Moreover, the specificity of the association with chemokine rather than cytokine gradients is strongly suggestive that the chemokine gradient might be a useful measure and is linked to a biologically plausible hypothesis around mucosal target cell recruitment and increased HIV risk. Further studies are required to address the contribution of sexually transmitted infection, hormonal contraception, other proinflammatory agents to the chemokine gradient direction, and HIV risk.
In summary, this study compared parallel cytokine levels in blood and mucosa and linked these hierarchically and prospectively to HIV as an outcome. These data further underscore the importance of chemokines as determinants of HIV acquisition. Further studies to validate these findings could provide critical biomarkers for HIV risk profiling, allowing accurate classification of risk to implement more targeted HIV prevention strategies or conduct more rapid efficacy assessments of HIV prevention candidates. Translation of these findings through safe and effective manipulation of chemokine gradients, or by limiting their production or effects, could represent a novel and targeted host-directed HIV prevention modality.
Supplementary Material
ACKNOWLEDGMENTS
The authors acknowledge the CAPRISA 004 and Tenofovir gel Research for AIDS Prevention Science study teams for coordinating the collection, processing, storage, and shipping of the samples.
Footnotes
The following establishments provided research and salary support: the DST-NRF Centre of Excellence in HIV Prevention (L.J.P.L., D.A., S.S.A.K., Q.A.K., J.-A.S.P., and L. M.), the South African Medical Research Council (D.A. and L.M.), the Carnegie Corporation (L.M.), University of Cape Town Clinical Infectious Diseases Research Initiative (CIDRI) (L.M.), and the National Research Foundation of South Africa (L.J.P.L., D.A., and L.M.). This study is part of the CAPRISA TRAPS Program, which is funded by CONRAD, Eastern Virginia Medical School (USAID cooperative grant GP00-08-00005-00, subproject agreement PPA-09-046), and the South African Department of Science & Technology. Cytokine assays were funded by a grant from the Poliomyelitis Research Foundation of South Africa (J.-A.S.P.).
The authors have no conflicts of interest to disclose.
L.J.P.L., L.M., K.B.A., and L.R.M. contributed equally.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
REFERENCES
- 1.Powers KA, Poole C, Pettifor AE, et al. Rethinking the heterosexual infectivity of HIV-1: a systematic review and meta-analysis. Lancet Infect Dis. 2008;8:553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boily MC, Baggaley RF, Wang L, et al. Heterosexual risk of HIV-1 infection per sexual act: systematic review and meta-analysis of observational studies. Lancet Infect Dis. 2009;9:118–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel P, Borkowf CB, Brooks JT, et al. Estimating per-act HIV transmission risk: a systematic review. AIDS. 2014;28:1509–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Auvert B, Ballard R, Campbell C, et al. HIV infection among youth in a South African mining town is associated with herpes simplex virus-2 seropositivity and sexual behaviour. AIDS. 2001;15:885–898. [DOI] [PubMed] [Google Scholar]
- 5.Pettifor AE, Hudgens MG, Levandowski BA, et al. Highly efficient HIV transmission to young women in South Africa. AIDS. 2007;21:861–865. [DOI] [PubMed] [Google Scholar]
- 6.McKinnon LR, Kaul R. Quality and quantity. Curr Opin HIV AIDS. 2012;7:195–202. [DOI] [PubMed] [Google Scholar]
- 7.Li Q, Estes JD, Schlievert PM, et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458:1034–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coombs RW, Reichelderfer PS, Landay AL. Recent observations on HIV type-1 infection in the genital tract of men and women. AIDS. 2003;17:455–480. [DOI] [PubMed] [Google Scholar]
- 9.Haase AT. Perils at mucosal front lines for HIV and SIV and their hosts. Nat Rev Immunol. 2005;5:783–792. [DOI] [PubMed] [Google Scholar]
- 10.Kyongo JK, Crucitti T, Menten J, et al. Cross-sectional analysis of selected genital tract immunological markers and molecular vaginal microbiota in Sub-Saharan African women, with relevance to HIV risk and prevention. Clin Vaccin Immunol. 2015;22:526–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masson L, Mlisana K, Little F, et al. Defining genital tract cytokine signatures of sexually transmitted infections and bacterial vaginosis in women at high risk of HIV infection: a cross-sectional study. Sex Transm Infect. 2014;90:580–587. [DOI] [PubMed] [Google Scholar]
- 12.Mlisana K, Naicker N, Werner L, et al. Symptomatic vaginal discharge is a poor predictor of sexually transmitted infections and genital tract inflammation in high-risk women in South Africa. J Infect Dis. 2012;206:6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharkey DJ, Tremellen KP, Jasper MJ, et al. Seminal fluid induces leukocyte recruitment and cytokine and chemokine mRNA expression in the human cervix after coitus. J Immunol. 2012;188:2445–2454. [DOI] [PubMed] [Google Scholar]
- 14.Deese J, Masson L, Miller W, et al. Injectable progestin-only contraception is associated with increased levels of pro-inflammatory cytokines in the female genital tract. Am J Reprod Immunol. 2015;74:357–367. [DOI] [PubMed] [Google Scholar]
- 15.Masson L, Passmore JAS, Liebenberg LJ, et al. Genital inflammation and the risk of HIV acquisition in women. Clin Infect Dis. 2015;61:260–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grivel J-C, Shattock RJ, Margolis LB. Selective transmission of R5 HIV-1 variants: where is the gatekeeper? J Transl Med. 2011;9(suppl 1):S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Groom JR, Luster AD. CXCR3 in T cell function. Exp Cell Res. 2011;317:620–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrison C, Fichorova R, Mauck C, et al. Cervical inflammation and immunity associated with hormonal contraception, pregnancy and HIV-1 seroconversion. JAIDS J Acquir Immune Defic Syndr. 2014;66:1. [DOI] [PubMed] [Google Scholar]
- 19.Kahle EM, Bolton M, Hughes JP, et al. Plasma cytokine levels and risk of HIV type 1 (HIV-1) transmission and acquisition: a nested case-control study among HIV-1-serodiscordant couples. J Infect Dis. 2015;211:1451–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lehman DA, Ronen K, Blish CA, et al. Systemic cytokine levels Show limited correlation with risk of HIV-1 acquisition. JAIDS J Acquir Immune Defic Syndr. 2014;66:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naranbhai V, Abdool Karim SS, Altfeld M, et al. Innate immune activation enhances HIV acquisition in women, diminishing the effectiveness of tenofovir microbicide gel. J Infect Dis. 2012;206:993–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geurts P, Irrthum A, Wehenkel L. Supervised learning with decision tree-based methods in computational and systems biology. Mol Biosyst. 2009;5:1593–1605. [DOI] [PubMed] [Google Scholar]
- 24.Arnold KB, Szeto GL, Alter G, et al. CD4+ T cell-dependent and CD4+ T cell-independent cytokine-chemokine network changes in the immune responses of HIV-infected individuals. Sci Signal. 2015;8:ra104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnold KB, Burgener A, Birse K, et al. Increased levels of inflammatory cytokines in the female reproductive tract are associated with altered expression of proteases, mucosal barrier proteins, and an influx of HIV-susceptible target cells. Mucosal Immunol. 2015;9:194–205. [DOI] [PubMed] [Google Scholar]
- 26.Lajoie J, Juno J, Burgener A, et al. A distinct cytokine and chemokine profile at the genital mucosa is associated with HIV-1 protection among HIV-exposed seronegative commercial sex workers. Mucosal Immunol. 2012;5:277–287. [DOI] [PubMed] [Google Scholar]
- 27.Lajoie J, Kimani M, Plummer FA, et al. Association of sex work with reduced activation of the mucosal immune system. J Infect Dis. 2014;210:319–329. [DOI] [PubMed] [Google Scholar]
- 28.Reis Machado J, da Silva MV, Cavellani CL, et al. Mucosal immunity in the female genital tract, HIV/AIDS. Biomed Res Int. 2014;2014:350195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rancez M, Couëdel-Courteille A, Cheynier R, et al. Chemokines at mucosal barriers and their impact on HIV infection. Cytokine Growth Factor Rev. 2011;23:233–243. [DOI] [PubMed] [Google Scholar]
- 30.Acosta-Rodriguez EV, Rivino L, Geginat J, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. [DOI] [PubMed] [Google Scholar]
- 31.Schenkel JM, Fraser KA, Beura LK, et al. T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science. 2014;346:98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shin H, Iwasaki A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature. 2012;491:463–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.