Abstract
The endocannabinoid system serves many physiological roles, including in the regulation of energy balance, food reward, and voluntary locomotion. Signaling at the cannabinoid type 1 receptor has been specifically implicated in motivation for rodent voluntary exercise on wheels. We studied four replicate lines of high runner (HR) mice that have been selectively bred for 81 generations based on average number of wheel revolutions on days five and six of a six-day period of wheel access. Four additional replicate lines are bred without regard to wheel running, and serve as controls (C) for random genetic effects that may cause divergence among lines. On average, mice from HR lines voluntarily run on wheels three times more than C mice on a daily basis. We tested the general hypothesis that circulating levels of endocannabinoids (i.e., 2-arachidonoylglycerol [2-AG] and anandamide [AEA]) differ between HR and C mice in a sex-specific manner. Fifty male and 50 female mice were allowed access to wheels for six days, while another 50 males and 50 females were kept without access to wheels (half HR, half C for all groups). Blood was collected by cardiac puncture during the time of peak running on the sixth night of wheel access or no wheel access, and later analyzed for 2-AG and AEA content by ultra-performance liquid chromatography coupled to tandem mass spectrometry. We observed a significant three-way interaction among sex, linetype, and wheel access for 2-AG concentrations, with females generally having lower levels than males and wheel access lowering 2-AG levels in some but not all subgroups. The number of wheel revolutions in the minutes or hours immediately prior to sampling did not quantitatively predict plasma 2-AG levels within groups. We also observed a trend for a linetype-by-wheel access interaction for AEA levels, with wheel access lowering plasma concentrations of AEA in HR mice, while raising them in C mice. In addition, females tended to have higher AEA concentrations than males. For mice housed with wheels, the amount of running during the 30 minutes before sampling was a significant positive predictor of plasma AEA within groups, and HR mice had significantly lower levels of AEA than C mice. Our results suggest that voluntary exercise alters circulating levels of endocannabinoids, and further demonstrate that selective breeding for voluntary exercise is associated with evolutionary changes in the endocannabinoid system.
Keywords: Artificial selection, Locomotor activity, Reward, Sex differences, Training effect, Voluntary exercise
1. Introduction
The endocannabinoid system is involved in a variety of physiological processes, including regulation of motor behavior (Dietrich and McDaniel 2004). Motor behavior is tremendously diverse, encompassing voluntary exercise, consummatory behaviors, spontaneous physical activity (SPA) of various types, including “fidgeting” (Garland, Jr. et al. 2011b), and performance during measures of forced-exercise capacity (Claghorn et al. 2016). Studies of cannabinoid effects on motor behavior have utilized a variety of approaches, involving variation in testing apparatus, length of observation period, time of day, etc., and, not surprisingly, this has led to conflicting results. Depending on the apparatus and length of test, these studies may be gauging multiple aspects of motor behavior, including, in some cases, reactivity to a novel environment. Generally, systemic administration of cannabinoids leads to a decrease in activity. In male rats, for example, doses of Δ-9-tetrahydrocannabinol higher than 1 mg/kg caused a decrease in ambulation and rearing as measured in a five-minute novel open-field test (Järbe et al. 2002). However, another study, also usingΔ-9-tetrahydrocannabinol in male rats, reported a triphasic effect, where very low and very high doses (0.2 mg/kg and 2.5 mg/kg, respectively) reduced the number of photobeam breaks produced in either a horizontal or vertical direction, and moderate doses (1–2 mg/kg) increased the number of beam breaks as measured over one hour in an activity chamber (Sañudo-Peña et al. 2000).
Cannabinoids activate the cannabinoid type-1 (CB1) and type-2 receptor (CB2), the former being found in high density in brain areas that control movement (basal ganglia, substantia nigra, etc., Tsou et al. 1998), including those involved in both spontaneous physical activity and voluntary exercise (Garland, Jr. et al. 2011b). Recent research suggests that the CB1 receptor is specifically involved in voluntary exercise. For example, male CB1 receptor knockout mice exhibit a 30–40% reduction in voluntary wheel running, but no change in number of horizontal squares crossed during five minutes in a large, dimly-illuminated, novel “activity cage” (Dubreucq et al. 2010). In a subsequent study, in which the CB1 receptor was deleted only from brain GABAergic neurons, mice showed a 25–30% decrease in wheel running and no difference in habituated locomotor activity measured over five days in a cage with infrared sensors to detect horizontal beam breaks (Dubreucq et al. 2013). Previous studies of CB1 knockout mice, however, have shown differences in other tests of locomotor activity. CB1 knockout mice spend more time immobile in a test of catalepsy, and showed fewer beam breaks during an open-field test (with undescribed parameters)(Zimmer et al. 1999). A separate experiment found that CB1 knockout mice had a significant decrease of ambulatory movements, as measured in a dimly-lit box with photocells placed to measure both horizontal and vertical movements (in this experiment, animals were measured for 15 minutes on several different days after being habituated to the cage) (Martin et al. 2000).
Limited evidence suggests that the endocannabinoid system may specifically affect motivation for voluntary exercise. The two primary endocannabinoids in humans and other mammals are 2-arachidonylglycerol (2-AG) and anandamide (AEA). Both 2-AG and AEA can cross the blood-brain barrier, bind to the CB1 receptor, and cause dopamine release in areas involved in reward signaling (such as the nucleus accumbens, reviewed in Gardner 2005). After rats were trained to press a door to unlock wheel access (as a reward), systemic administration of 2-AG reduced the number of times that obese rats would press the door to gain wheel access before giving up, and also reduced the revolutions run once the rats were in the wheel [both obese and lean individuals (Smith and Rasmussen 2010)]. In a similar study, rimonabant (a CB1 antagonist/inverse agonist) caused a similar reduction in number of door-presses prior to giving up, but did not affect number of revolutions run once rats did gain wheel access (Rasmussen and Hillman 2011). In addition, signaling through the CB1 receptor produces analgesia in both peripheral and central sites (reviewed in Dietrich and McDaniel 2004). Analgesia might interact with motivation per se by reducing pain that could occur during exercise (e.g., see Li et al. 2004; Fuss et al. 2015).
The endocannabinoid system is also activated by exercise. Several studies have examined how circulating levels of both 2-AG and AEA respond to exercise. In general, exercise increases levels of AEA in the blood, but does not seem to affect levels of 2-AG (Sparling et al. 2003; Feuerecker et al. 2012; Heyman et al. 2012; Raichlen et al. 2012, 2013). Most previous studies of circulating endocannabinoid levels have involved forced exercise, which may cause “stress” relative to voluntary exercise, or confound the effects of stress with exercise (Girard and Garland, Jr. 2002; Brown et al. 2007). Arguably, no (ethical) study of humans involves “forced” exercise, but an examination of the studies cited above shows that subjects were not allowed to choose the quantity, length of time, and/or the speed at which they exercised (but see Feuerecker et al. 2012), which may cause stress in either the psychological or physical sense. Voluntary exercise has been examined in two animal studies. One small-scale study (n = 3 per group; sex not reported) measured mice after they were allowed wheel access for 3 h/day for 8 days, with samples taken after 30 minutes of running. Plasma levels of both endocannabinoids tended to be lower in the running mice as compared with mice housed with locked wheels, although the differences were non-significant (Chaouloff et al. 2012). One additional study of rats used voluntary exercise, and although they did not measure circulating endocannabinoids, they did find an increase in AEA in the hippocampus after 8 days of wheel access (Hill et al. 2010).
The endocannabinoid system may also be altered in response to selective breeding for voluntary exercise behavior. An ongoing artificial selection experiment (currently in generation 81) has produced four replicate high-runner (HR) lines while also maintaining four non-selected control (C) lines (Swallow et al. 1998; Wallace and Garland, Jr. 2016; Garland, Jr. et al. 2017). In the routine breeding protocol, mice are allowed access to wheels for six days as young adults, and mice in the HR lines are selected to breed based on the average number of wheel revolutions on days five and six. Mice in the control lines are bred without regard to how much they run. Mice from the HR lines run about three times as much on a daily basis as compared with C mice, which they accomplish primarily by running faster (Girard et al. 2001; Garland, Jr. et al. 2011a; Careau et al. 2013). In addition, female mice run more than male mice, which they also accomplish by running faster (Koteja and Garland, Jr. 2001). When given a systemic injection of a CB1 receptor antagonist (SR141716; rimonabant), HR female mice decreased their running more than C female mice over the next hour, which was accomplished by a decrease in total revolutions, average speed and maximum speed, but not amount of time spent running (Keeney et al. 2008). Male HR and C mice did not show a differential response. In contrast, when given a systemic injection of a CB1 receptor agonist (WIN 55,212–2), both female and male HR mice showed a differential decrease in running (compared to C mice; (Keeney et al. 2012). For two hours after injection, female HR mice had a reduction in total revolutions, average speed, and maximum speed. In the second hour they also decreased their time spent running. Male HR mice showed decreased total revolutions, average speed and maximum speed during the entire two hours after injection. As a CB1 receptor agonist and antagonist both decreased running in HR mice in different ways in a sex-specific manner, it is difficult to infer from these studies alone precisely how the endocannabinoid system has changed in response to selective breeding.
One possible cause of differential responses to pharmacological manipulation could be differences in circulating levels of endocannabinoids (alternatively, tissue-specific receptor densities or receptor sensitivity might have evolved). In addition, circulating concentrations of endocannabinoids might show different quantitative responses to exercise, either between the sexes or potentially between HR and control lines of mice. The purpose of the present study was, therefore, to measure circulating endocannabinoid levels in mice given access to wheels, as compared with those housed in standard cages. We studied both male and female HR and C mice given access to wheels for six days (as used in the regular selection protocol), or housed without wheels for six days, and sampled during the time of peak running. We expected that circulating levels of endocannabinoids (2-AG and AEA) would differ between HR and C mice, possibly in a sex-specific manner. We also predicted that the amount of wheel running might be a quantitative predictor of circulating endocannabinoid levels. We also tested the relationship between the plasma concentrations of 2-AG and AEA at the level of individual variation within groups as well as among the average values for the eight subgroups (combinations of sex, linetype, and wheel access).
2. Materials & procedures
2.1. Ethical approval
All experimental procedures were approved by the UC Riverside Institutional Animal Care and Use Committee.
2.2. Experimental animals
Mice were taken from a long-term artificial selection experiment on voluntary wheel running that was started in 1993 (Swallow et al. 1998). Originally, outbred Hsd:ICR mice (Mus domesticus) were obtained from Harlan Sprague Dawley (Indianapolis, Indiana, USA). These mice were randomly split into eight closed lines, with four designated to become “high runner” (HR) and four designated as “control” (C) lines. All mice are allowed access to wheels for six days and HR mice are chosen to breed based on their average number of wheel revolutions on days five and six. Control mice are bred without regard to wheel running. HR mice now run on average three times as much as C mice do, primarily by running faster.
For the present study, 50 male and 50 female mice from generation 74 (half HR and half C) were allowed access to wheels for six days, while another 50 males and 50 females (also half HR and half C) were kept without access to wheels. Wheel revolutions for mice with wheels were recorded for 23 hours per day, and home-cage activity was recorded for all mice. On the sixth day of the study, animals were anesthetized with isoflurane and blood samples were taken by cardiac puncture. Animals were on a reversed photoperiod, with lights off from 7 am to 7 pm, so that sampling could occur during the time of peak wheel running, which starts approximately 2 hours after lights are turned off (Girard et al. 2001; Girard and Garland, Jr. 2002; Malisch et al. 2008, 2009). Sampling occurred from ~9 am to 1 pm (from 2 hours after lights off to 6 hours after lights off). The age range at the time of sampling was 71–91 days old.
Mini-muscle status was determined for each mouse by dissection and weighing of the triceps surae muscles at the end of the experiment. The “mini-muscle” phenotype is caused by a recessive allele that, when homozygous, reduces triceps surae and total hindlimb muscle mass by ~50% (Garland, Jr. et al. 2002). However, the mass-specific aerobic capacity of the muscles is approximately doubled (Houle-Leroy et al. 2003). Mini-muscle individuals tend to run faster, but for fewer minutes per day, as compared with unaffected individuals (Garland, Jr. et al. 2002; Houle-Leroy et al. 2003; Swallow et al. 2005; Syme et al. 2005; Kelly et al. 2006; Hannon et al. 2008). In addition, mini-muscle individuals have larger internal organs than normal-muscled mice (liver, kidneys, and heart ventricles: (Garland, Jr. et al. 2002; Swallow et al. 2005)). As the mini-muscle phenotype clearly has pleiotropic effects, mini-muscle status was used as a cofactor in all analyses.
2.3. Wheel running
Mice were housed individually during the experimental period and half were provided with cages with a hole for wheel access. The wheels used were the same as in the regular selection protocol (Swallow et al. 1998) – Wahman-type activity wheels with a circumference of 1.12 meters. Wheel revolutions are recorded automatically for 23 hours per day (one hour is used to download data and check mice), and a measure of wheel freeness was used as a covariate in all analyses of wheel running. We did not choose to provide locked wheels for the mice without wheel access, as we have shown that when HR mice are housed with locked wheels, they climb more than do mice from the non-selected Control lines (Koteja et al. 1999), so locked wheels, unfortunately, provide more than just “environmental enrichment.”
2.4. Home-cage activity
Home-cage activity (HCA) was measured using passive infrared sensors that detect motion. These sensors give readings 3 times per second – either 0 (no movement) or 1 (movement). Readings are then averaged over a one-minute interval and reported using arbitrary units. We used a similar setup and calibration procedure as previously reported (Acosta et al. 2015; Copes et al. 2015). HCA was measured for 23 hours per day and all analyses were performed using a measure of sensor sensitivity as a covariate (Acosta et al. 2015; Copes et al. 2015).
2.5. Measurement of plasma 2-AG and AEA
Isoflurane was used to anesthetize animals prior to tissue harvest. Blood was collected by cardiac puncture and stored in EDTA-lined tubes on ice, and then plasma was obtained by centrifugation (1500 g for 10 minutes, maintained at 4°C). All samples were stored at −80° C until processing. One hundred microliters (μL) of plasma was used for extraction of lipids in one milliliter (mL) of methanol containing the following internal standards: [2H5]-2-AG and [2H4]-AEA (Cayman Chemical, Ann Arbor, MI, USA). Lipids were extracted with chloroform (2.0 mL) and washed with 0.9% saline (0.9 mL). Organic phases were collected and fractionated by open-bed silica gel column chromatography as previously described (DiPatrizio et al. 2011). Eluted fractions were dried under N2 and reconstituted in 0.1 mL of methanol:chloroform (9:1) for liquid chromatography/tandem mass spectrometry (LC/MS/MS) analyses.
Lipids were analyzed using a Waters Acquity I-Class Ultra Performance Liquid Chromatography system coupled to a Waters TQS-micro Triple Quadrupole Mass Spectrometer. Lipids were separated using an Acquity UPLC BEH C18 column (50 × 2.1 mm; i.d. 1.7 μm), eluted by a gradient of methanol (0.25% acetic acid, 5 millimolar [mM] ammonium acetate) in water (0.25% acetic acid, 5mM ammonium acetate) (from 80 to 100% methanol in 2.5 minutes, 100% 2.5–3.0 minutes, 100–80% 3.0–3.1 minutes) at a flow rate of 0.4 mL/minute. Column temperature was kept at 40° C and samples were maintained in the sample manager at 10° C. Argon was used as collision gas. 2-AG, [2H5] 2-AG, AEA, and [2H4]-AEA were identified in the positive ionization mode, based on their retention times and MS2 properties, using authentic standards (Cayman Chemical) as references. Multiple reaction monitoring was used to acquire full-scan tandem MS spectra of selected ions. Extracted ion chromatograms were used to quantify 2-AG (m/z = 379.3>287.3), AEA (m/z = 348.3>62.04), and [2H5] 2-AG (m/z = 384.3 > 93.4) and [2H4]-AEA (m/z = 352.3>66.11), which were used as internal standards.
2.6. Statistical analyses
Following numerous previous studies of these eight lines of mice (Swallow et al. 1998; Rhodes et al. 2000), plasma endocannabinoid concentrations were analyzed by nested analysis of covariance (ANCOVA), with line nested within linetype (HR vs C) as a random effect, and with covariates of age and time of day that plasma sampling occurred (SAS Procedure Mixed).
For the individuals with wheel access, we repeated the foregoing analyses with amount of wheel running (revolutions/unit time) as a covariate. Although we expected a possible quantitative relationship with the amount of exercise prior to sampling, we could not predict the precise nature of this relationship. Therefore, we computed the number of wheel revolutions in each minute before plasma sampling, from 1 to 10 minutes before, and then in 10-minute bins from 10 to 120 minutes before sampling. We fitted models using each of these alternative covariates and examined the restricted maximum log-likelihood (REML) values from each analysis to determine which time period of summed running effort provided the best fit. For wheel running, the number of revolutions in the previous 30 minutes before plasma sampling provided the best fit and was used in all analyses with wheel running as a covariate. For consistency, we also used the amount of home-cage activity in the previous 30 minutes as a covariate in certain analyses.
Dependent variables were transformed when needed to improve the normality of residuals. Residuals that were >3 standard deviations above or below the mean were excluded from analyses. Main effects were considered statistically significant when p < 0.05. Interactions of main effects were considered significant when p < 0.10 because the power to detect interactions is generally substantially lower than for detecting main effects in ANOVAs (Wahlsten 1990, 1991). Least squares means and associated standard errors from SAS Procedure Mixed are presented in figures and were inspected to determine the directions of main effects and interactions. In addition, for some pairwise comparisons of subgroup means, we refer to differences of least squares means from Proc Mixed, unadjusted for multiple comparisons.
3. Results
3.1. Wheel running
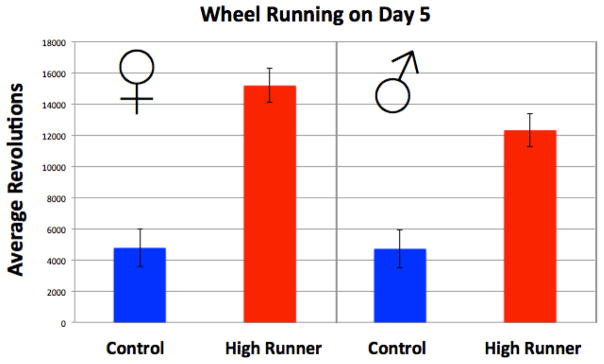
Day five of the wheel access period was analyzed, as mice were removed and blood collected in the middle of night six (Fig. 1). As expected, HR mice ran more than C mice (p = 0.0004). Neither the effect of sex (p = 0.1431) nor the sex by linetype interaction (p = 0.1576) was significant. Wheel freeness, mini-muscle status, and age were not statistically significant predictors of wheel running (results not shown).
Figure 1.
Wheel running on day five of experiment. Values are LS means +/− standard error from SAS Proc Mixed. N = 90.
3.2. Home-cage activity
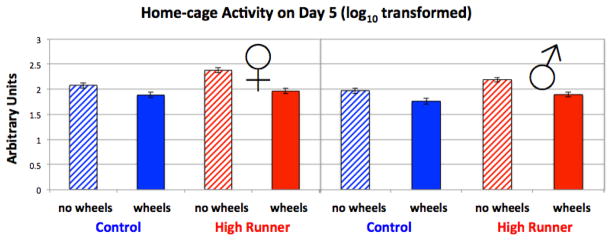
Data from day five of the experiment were analyzed, as day six was interrupted when mice were removed from cages for collection of blood. Values from each minute were summed over 23 hours, then log10 transformed to improve normality of the residuals. In the three-way analysis of covariance (Fig. 2, Table 1), mice with wheel access always had reduced HCA (p = 0.0001), this reduction was greater in HR than in C mice (wheel access by linetype interaction, p = 0.0558), females always had higher HCA than males (p = 0.0047), and HR mice always had higher HCA than C mice (p = 0.007). In addition, mice with the mini-muscle trait had lower HCA than those that did not (p = 0.018). Age and a measure of sensor sensitivity were not significant predictors of home-cage activity. We also tested body mass as an additional covariate, but it had no statistical effect (p > 0.50) and caused little change in the significance levels of the other factors and covariates (results not shown).
Figure 2.
HCA on day 5 of experiment. See Table 1 for statistical results. Mice with wheel access always had reduced HCA (p = 0.0001), this reduction was greater in HR than in C mice (linetype by wheel access interaction, p = 0.0558), females always had higher HCA than males (p = 0.0047), and HR mice always had higher HCA than C mice (p = 0.007). Values are LS means (log10 transformed) +/− standard error from SAS Proc Mixed. N = 190.
Table 1.
Three-way analysis of covariance of home-cage activity on day 5 (log10 transformed) (N = 190). See Figure 2 for graphical representation of adjusted group means.
| Effect | d.f. | F | P |
|---|---|---|---|
| Sex | 1,6 | 19.22 | 0.0047 |
| Linetype | 1,6 | 16.08 | 0.0070 |
| Wheel Access | 1,6 | 73.52 | 0.0001 |
| Sex*Linetype | 1,6 | 0.07 | 0.8043 |
| Sex*Wheel Access | 1,6 | 0.81 | 0.4029 |
| Linetype*Wheel Access | 1,6 | 5.60 | 0.0558 |
| Sex*Linetype*Wheel Access | 1,6 | 1.49 | 0.2681 |
| Mini-muscle | 1,155 | 5.72 | 0.0180 |
| Age | 1,155 | 0.53 | 0.4695 |
| HCA sensor sensitivity | 1,155 | 0.62 | 0.4306 |
3.3. Plasma 2-AG concentrations
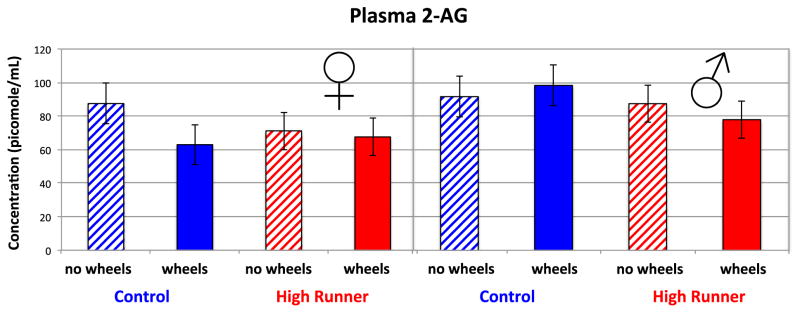
Females had lower levels of 2-AG than males in all four experimental groups, and the main effect of sex was significant (p = 0.0265). Figure 3 illustrates the significant three-way interaction among sex, linetype, and wheel access (p = 0.0408). Levels of 2-AG are lower in mice with wheel access, except for C males. However, when examining the pairwise comparisons, 2-AG levels were significantly lower for mice with wheel access only for the C female group (p = 0.0301). The main effect of linetype was not significant (p = 0.4604).
Figure 3.
Levels of 2-AG in mouse plasma collected during peak activity on the 6th night of wheel running. See Table 2 for statistical results. The three-way interaction among sex, linetype, and wheel access was statistically significant (p = 0.0408), with females also having lower levels than males (p = 0.0265). Values are LS means +/− standard error from SAS Proc Mixed. N = 189.
Separate analyses of mice with (N = 92) and without (N = 94) wheels (not including amount of physical activity as covariates) indicated that females had significantly lower levels of 2-AG only when they had wheel access (p = 0.0169 for mice with wheels, p = 0.1235 for mice without wheels).
Mini-muscle status was not a significant factor for any analyses, but both time of day that plasma sampling occurred and age at time of plasma sampling were significant in the overall analysis and the analysis of mice without wheel access. Only time of day was significant for the analysis of mice with wheels. Plasma 2-AG concentrations tended to be higher later in the sampling period, which ranged from ~9 am to 1 pm. Age was positively correlated with 2-AG levels (age range was 71–91 days old).
3.4. Plasma 2-AG concentrations with wheel running as a covariate
The first analysis was restricted to mice housed with wheel access. The amount of wheel running (transformed to the 0.4 power to reduce positive skew) in the previous 30 minutes (before the cardiac puncture and plasma sample) was added as a covariate to the statistical models indicated above. Amount of prior running was not a significant predictor of 2-AG levels, nor was the amount of home-cage activity during this period (also transformed to the 0.4 power), but females still had significantly lower 2-AG concentrations than males (p = 0.0274), with no difference between HR and Control lines (Table 3). Age was still a significant positive predictor, but time and mini-muscle status were not.
Table 3.
Plasma concentration of 2-AG with wheel running as a covariate (N = 92).
| Effect | d.f. | F | P |
|---|---|---|---|
| Sex | 1,6 | 8.39 | 0.0274 |
| Linetype | 1,6 | 0.24 | 0.6446 |
| Sex*Linetype | 1,6 | 2.41 | 0.1713 |
| Mini-muscle | 1,64 | 3.77 | 0.0567 |
| Age | 1,64 | 19.12 | <.0001 |
| Time of Day | 1,64 | 3.30 | 0.0742 |
| Running in previous 30 min | 1,64 | 0.57 | 0.4518 |
| HCA in previous 30 min | 1,64 | 1.40 | 0.2405 |
To test whether acute physical activity had a different effect than the 5 days of wheel access, we reran the analysis with the amount of wheel running and home-cage activity in the previous 30 minutes as covariates, but this time also including the mice housed without wheels and assigning values of zero for their wheel running (as in Copes et al. 2015). In this analysis (N = 186), neither measure of physical activity was a significant predictor of 2-AG concentrations (both p > 0.5), and the effects of sex (p = 0.0252) and the 3-way interaction (p = 0.0544) remained similar to those reported in Table 2.
Table 2.
Three-way analysis of covariance of plasma concentration of 2-AG (N = 189). See Figure 3 for graphical representation of adjusted group means.
| Effect | d.f. | F | P |
|---|---|---|---|
| Sex | 1,6 | 8.56 | 0.0265 |
| Linetype | 1,6 | 0.62 | 0.4604 |
| Wheel Access | 1,6 | 2.42 | 0.1711 |
| Sex*Wheel Access | 1,6 | 3.04 | 0.1316 |
| Sex*Linetype | 1,6 | 0.33 | 0.5883 |
| Linetype*Wheel Access | 1,6 | 0.05 | 0.8244 |
| Sex*Linetype*Wheel Access | 1,6 | 6.75 | 0.0408 |
| Mini-muscle | 1,147 | 0.71 | 0.4021 |
| Age | 1,147 | 11.34 | 0.0010 |
| Time of Day | 1,147 | 8.23 | 0.004 |
3.5. Plasma AEA concentrations
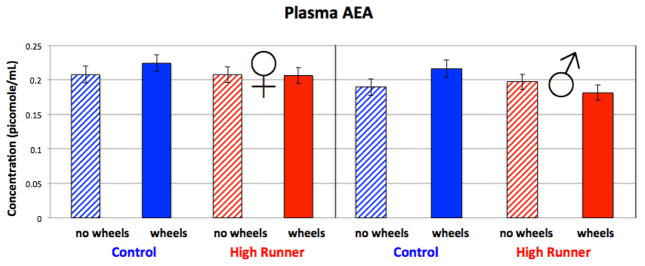
Females tended to have higher levels of AEA than males (p = 0.0599, Figure 4, Table 4). A linetype by wheel access interaction (p = 0.0628) indicated that wheel access tended to increase levels of AEA in C mice (and decrease levels in HR males).
Figure 4.
Levels of AEA in mouse plasma collected during peak activity on the 6th night of wheel running. See Table 4 for statistical results, which indicated a linetype by wheel access interaction (p = 0.0628). Values are LS means +/− standard error from SAS Proc Mixed. N = 185.
Table 4.
Three-way analysis of covariance of plasma concentration of AEA (N = 185). See Figure 4 for graphical representation of adjusted group means.
| Effect | d.f. | F | P |
|---|---|---|---|
| Sex | 1,6 | 5.36 | 0.0599 |
| Linetype | 1,6 | 2.29 | 0.1809 |
| Wheel Access | 1,6 | 0.92 | 0.3741 |
| Sex*Wheel Access | 1,6 | 0.04 | 0.8550 |
| Sex*Linetype | 1,6 | 0.12 | 0.7451 |
| Linetype*Wheel Access | 1,6 | 5.20 | 0.0628 |
| Sex*Linetype*Wheel Access | 1,6 | 0.86 | 0.3886 |
| Mini-muscle | 1,143 | 1.35 | 0.2476 |
| Age | 1,143 | 0.00 | 0.9484 |
| Time of Day | 1,143 | 82.97 | <.0001 |
Separate analyses of mice with and without wheels revealed a trend for females to have higher levels only when they were housed without wheels (p = 0.0683 without wheels, p = 0.1637 with wheels). In addition, there was a trend for HR mice to have lower levels when housed with wheels (p = 0.0883 with wheels, p = 0.8950 without wheels).
Mini-muscle status and age at time of plasma sampling were not significant factors for any analyses. Time of day that plasma sampling occurred was significant for all three analyses (overall, mice with wheels, mice without wheels), indicating reduced values later in the sampling period.
3.6. Plasma AEA concentrations with wheel running as a covariate
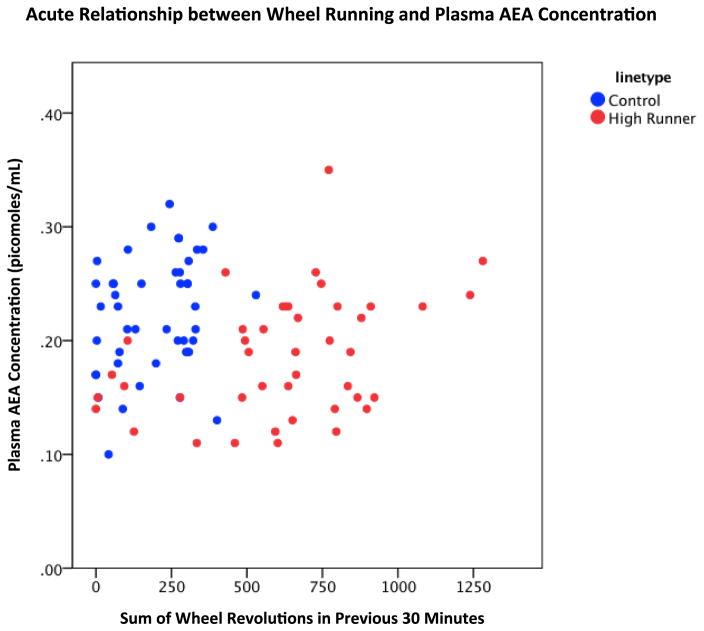
The amount of wheel running (raised to the 0.4 power) was a highly significant positive predictor (p = 0.0043) of the amount of plasma AEA (Table 5, Fig. 5), home-cage activity was a negative predictor (also raised to the 0.4 power, p = 0.0383), and HR mice had significantly lower plasma AEA concentrations than C mice (p = 0.0235), with no difference between the sexes. Both age (positive) at the time of plasma sampling and time of day that sampling occurred (negative) were also significant predictors of plasma AEA.
Table 5. Plasma concentration of AEA with wheel running as a covariate.
(N = 89; see also Fig. 5).
| Effect | d.f. | F | P |
|---|---|---|---|
| Sex | 1,6 | 2.97 | 0.1354 |
| Linetype | 1,6 | 9.11 | 0.0235 |
| Sex*Linetype | 1,6 | 0.33 | 0.5859 |
| Mini | 1,61 | 0.02 | 0.9029 |
| Age | 1,61 | 4.89 | 0.0307 |
| Time of Day | 1,61 | 33.95 | <.0001 |
| Running in previous 30 min | 1,61 | 8.79 | 0.0043 |
| HCA in previous 30 min | 1,61 | 4.49 | 0.0383 |
Figure 5.
Plasma AEA concentration from mice with wheel access as a function of the number of wheel revolutions in the 30 minutes prior to plasma sampling (N = 89). The number of wheel revolutions (transformed to the 0.4 power for statistical analyses [Table 5], but shown here as raw values) was a significant positive predictor of AEA values (p = 0.0043), home-cage was a negative predictor (p = 0.0383), and HR mice had lower levels than C mice after adjusting for these relationships (Table 5). The interactions between linetype and amount of wheel running or home-cage activity were not statistically significant (results not shown and these terms not included in final statistical model).
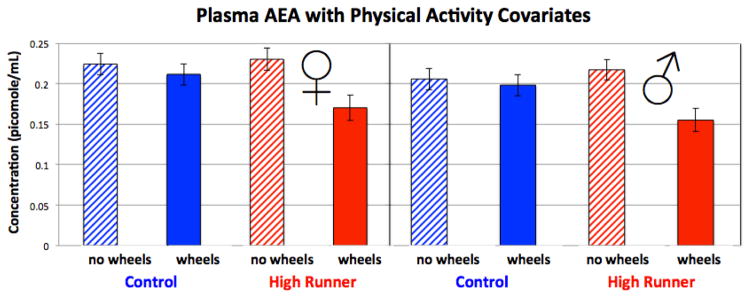
For the analysis including mice housed without wheel access (assigning them values of zero revolutions, as also done for plasma 2-AG concentrations - see Section 3.4 above), the amount of wheel running was still a significant positive predictor of AEA concentrations (p = 0.0044), home-cage activity became non-significant (p = 0.1954, negative effect), the effects of sex (p = 0.0548) and wheel access (p = 0.0503) were marginally non-significant, and the wheel access by linetype interaction was significant (p = 0.0110). In this analysis, wheel access lowered AEA levels in all mice, but much more so in HR lines than in Control lines (Table 6, Figure 6).
Table 6.
Analysis of covariance of plasma concentration of AEA with physical activity covariates (N = 183). See Figure 6 for graph of adjusted group means.
| Effect | d.f. | F | P |
|---|---|---|---|
| Sex | 1,6 | 5.66 | 0.0548 |
| Linetype | 1,6 | 3.93 | 0.0946 |
| Wheel Access | 1,6 | 5.97 | 0.0503 |
| Sex*Wheel Access | 1,6 | 0.01 | 0.9080 |
| Sex*Linetype | 1,6 | 0.02 | 0.8906 |
| Linetype*Wheel Access | 1,6 | 13.17 | 0.0110 |
| Sex*Linetype*Wheel Access | 1,6 | 0.09 | 0.7796 |
| Mini-muscle | 1,139 | 1.08 | 0.3011 |
| Age | 1,139 | 1.01 | 0.3175 |
| Time of Day | 1,139 | 72.65 | <.0001 |
| Running in previous 30 min | 1,139 | 8.38 | 0.0044 |
| HCA in previous 30 min | 1,139 | 1.69 | 0.1954 |
Figure 6.
Levels of AEA in mouse plasma collected during peak activity on the 6th night of wheel running, with amount of wheel running and home-cage activity used as covariates (see text), and including mice without wheels in the analysis by assigning them zero for wheel revolutions run. Values are LS means +/− standard error from SAS Proc Mixed, based on analyses presented in Table 6. N = 183.
3.7. Relationship between plasma 2-AG and AEA concentrations
We examined the relationship between circulating levels of these two endocannabinoids in several ways, as also shown in the Online Supplemental Material. Overall, these analyses indicate that 2-AG and AEA concentrations tend to be positively related.
First, considering the raw values for all 191 mice, the Pearson correlation was 0.175, 2-tailed p = 0.0155. Second, we analyzed the correlation within each of the eight subgroups (N = 23–24 per group), and found values ranging from −0.07 to + 0.54, with only one of the eight correlations differing significantly from zero by a 2-tailed test (Control females housed with wheel access r = 0.535, N = 24, p = 0.007). The mean value of the eight correlations was 0.207 with a standard error of 0.0815 (Supplemental material), suggesting an overall positive correlation, on average. Third, we computed simple mean values for each of the eight subgroups (linetype by sex by wheel access) and found no statistically significant correlation (r = −0.199, p = 0.636).
Fourth, we used the model presented in Table 2 for 2-AG, and added concentrations of AEA as an additional covariate. In this model, AEA is a highly significant positive predictor of 2-AG levels (p < 0.0001). We then performed the reciprocal analysis, adding 2-AG levels as an additional independent variable for the model presented in Table 4, and found the same thing: 2-AG is a highly significant positive predictor of AEA levels (p < 0.0001).
Fifth, we repeated this procedure for the model shown in Table 3 for 2-AG, and found that AEA levels were again a highly significant positive predictor of 2-AG (p < 0.0001). We did the same for the model in Table 5 for AEA, and again found that 2-AG levels were a positive predictor (p = 0.0003).
Sixth, we compared residuals from the models shown in Tables 2 and 4, and found the following correlation: r = 0.347, N = 184, p = 0.000001. Seventh, we compared residuals from Tables 3 and 5, and found r = 0.430, N = 89, p = 0.000026.
4. Discussion
Much remains to be understood regarding the role of the endocannabinoid system in exercise behavior and physiology. In the present study, we used selectively bred lines of mice to test four specific hypotheses regarding circulating levels of endocannabinoids, i.e., that they would (i) differ between the sexes, (ii) be affected by selective breeding for high levels of voluntary exercise on wheels, (iii) change following six days of wheel access, and (iv) be affected by the acute amount of wheel running or home-cage activity immediately prior to sampling. We measured plasma concentrations of two endocannabinoids, 2-AG and AEA, during the time of normal peak wheel running. Our results indicate that circulating levels of 2-AG and AEA differ (i) between sexes, (ii) between selectively bred HR and non-selected C lines of mice, (iii) are affected by six days of wheel access (training effect), and (iv) are affected acutely by physical activity. Furthermore, some of these effects differ between 2-AG and AEA. Finally, we tested the relationship between the plasma concentrations of 2-AG and AEA at the level of individual variation within groups as well as among the average values for the eight subgroups, and found evidence that the two endocannabinoids tend to covary positively.
4.1. Physical activity
As expected from numerous previous studies (e.g., Rhodes et al. 2000, 2003; Girard et al. 2001; Malisch et al. 2009; Copes et al. 2015), HR mice of both sexes ran much more than Control mice on day five of wheel access (Fig. 1). Also, as reported previously for HR and Control mice (Acosta et al. 2015; Copes et al. 2015), housing with wheel access reduced the amount of home-cage activity measured simultaneously (Fig. 2 and Table 1). Irrespective of housing condition, HR mice had higher HCA than did Control mice, again consistent with previous reports that studied mice only when housed without wheel access (Rhodes et al. 2001b; Malisch et al. 2008, 2009). In addition, irrespective of housing condition, females of both linetypes had higher HCA than males, a difference not reported previously when mice were housed and tested without wheels, using a different apparatus (Malisch et al. 2009). Finally, mice with the mini-muscle phenotype had lower HCA than those that did not (Table 1), an effect not observed previously (Copes et al. 2015).
4.2. Training effects on plasma endocannabinoid levels
To our knowledge, the effects of several days of training (physical conditioning) on plasma endocannabinoid levels have not previously been reported for both male and female mice. Our combined analyses of all groups indicated a three-way interaction among sex, linetype, and wheel access for 2-AG levels (Table 2). However, it is important to note that our sampling design included 2–4 hours of acute exercise prior to blood sampling, in addition to the five prior days of wheel access, which could confound training effects with acute exercise effects. Hence, we also conducted analyses of 2-AG levels that included the acute amounts of wheel running and home-cage activity as covariates, and found that they showed the same three-way interaction (see Results Section 3.4). Thus, our results show that, with respect to circulating 2-AG concentrations, even when taking differences in acute exercise into account, the endocannabinoid system responds to exercise differently based on sex and genetic background, and these differences may be a result of the exercise training undergone by the animal.
For plasma AEA levels, we found an interaction between linetype and wheel access, with wheel access lowering AEA levels for HR mice but raising them for C mice (Fig. 4, Table 4). Analyses that included the acute amounts of wheel running and home-cage activity as covariates indicated a stronger two-way interaction and a trend for an overall decrease in AEA levels for mice housed with wheel access (Table 6, Fig. 6). Interestingly, however, the effect of acute wheel running was highly significant and positive within groups (Fig. 6). Thus, our results show that, for AEA, the effects of voluntary exercise on the endocannabinoid system differ acutely versus chronically.
Physical conditioning in response to aerobic exercise has been studied extensively in both rodents and humans, although typically over the course of weeks rather than days (Harpur 1980; Saltin and Gollnick 1983; Scribbans et al. 2016; Stanford and Goodyear 2016). Only two previous studies of rodents have examined endocannabinoids in relation to voluntary exercise that lasts for days, and these support our general finding that the endocannabinoid system can “train” in response to physical activity. Eight days of wheel access for male rats was associated with increased AEA levels in the hippocampus, but not the prefrontal cortex, and no effects on 2-AG levels in either brain region (Hill et al. 2010). In addition, CB1 receptor density was increased in the hippocampus. However, the authors did not examine plasma levels of endocannabinoids. An additional small study measured endocannabinoid levels in mice after they were allowed to run for three hours per day for eight days, and compared them to mice who were in locked wheels for the same amount of time (Chaouloff et al. 2012). They did not find any significant differences in plasma endocannabinoid levels, although AEA was decreased in the hippocampus of running mice. However, since these animals also experienced acute exercise right before measurements were done, there is no way to tell if these differences are due to the acute or chronic effects of exercise.
Aside from training effects per se, several studies have now shown that AEA levels are raised after acute exercise (Sparling et al. 2003; Feuerecker et al. 2012; Heyman et al. 2012; Raichlen et al. 2012, 2013). The same studies generally suggest that 2-AG levels are not raised after acute exercise (Heyman et al. 2012), although three studies have reported a non-significant trend for raised 2-AG levels in humans and dogs (Sparling et al. 2003; Feuerecker et al. 2012; Raichlen et al. 2012). Finally, dogs and ferrets did not show significant increases in plasma 2-AG levels following treadmill exercise (Raichlen et al. 2012). Thus, it appears that species of mammals may differ in the extent to which exercise acutely alters circulating 2-AG levels. Alternatively, some of the apparent species differences could be explained by methodological differences related to the exercise intensity imposed on, or chosen by, the running subjects. For example, Raichlen and colleagues (2013) measured four different levels of exercise intensity in humans and found that AEA levels only increased for the two middle intensities. In addition, the amount of training experienced by the individual may affect how the endocannabinoid system reacts to acute exercise. Although plasma AEA was positively correlated with the amount of wheel running for both C and HR mice, there was a significant effect of linetype, with HR mice having lower levels of AEA even though they run more. This finding may also indicate that selective breeding for voluntary exercise has caused associated evolutionary changes in how the endocannabinoid system responds to exercise.
4.3. Sex differences in plasma endocannabinoid levels
We found that female mice have lower levels of 2-AG than males, especially when exercising, and tend to have higher levels of AEA (Figs. 3–5). Sex differences are not unexpected, given previous studies on these mice that show sex differences in the response of wheel running to CB1 agonists and antagonists (Keeney et al. 2008, 2012). Aside from those studies on the HR and C lines of mice, most previous studies of plasma endocannabinoids have used only males (Sparling et al. 2003; Feuerecker et al. 2012; Heyman et al. 2012). Two studies of humans included both males and females, but did not test for sex differences (Raichlen et al. 2012, 2013). Studies in rats show that females, especially adolescents, are more vulnerable than males to disruption of CB1 signaling by repeated exposure to THC (Burston et al. 2010), and studies of humans indicate that females are more sensitive than males to the effects of cannabis (Craft et al. 2013).
4.4. Time-of-day effects
Plasma 2-AG concentrations tended to be higher later in the sampling period, which ranged over approximately four hours during the early part of the dark phase, the time when mice are normally most active. This pattern is similar to one seen in a study of human subjects, where their levels of 2-AG also increased during the morning and peaked in midafternoon (Hanlon et al. 2014). For plasma AEA, time of day was significant for all three analyses (overall, mice with wheels, mice without wheels), indicating reduced values later in the sampling period. This pattern is similar to a study of AEA in the cerebrospinal fluid of rats, which found that it decreased during the dark phase (Murillo-Rodriguez et al. 2006). Thus, although our study was not intended to sample over a time period long enough to address circadian patterns per se, the time-related variation that we observed is consistent with previous reports for other species.
4.5. Conclusions & Future Directions
Overall, our results demonstrate that voluntary physical exercise affects circulating endocannabinoid levels differently, depending on sex, recent activity, and genetic background. More specifically, we found that acute voluntary exercise was associated with plasma AEA concentrations in a way similar to the effects of forced exercise reported in previous studies, in that the amount of wheel running done by a rodent before plasma sampling was a positive predictor of the level of AEA found in the blood (Raichlen et al. 2012, 2013). We also found differences in circulating 2-AG and AEA levels between the sexes and between lines of mice bred for high levels of voluntary exercise, when compared to their non-selected control lines. Furthermore, the effects of five days of wheel access on endocannabinoid levels varied between the sexes and/or between HR and C mice.
Although not considered in the present study, receptor density or sensitivity may also have evolved in our mice. Broad-scale (macroevolutionary) patterns of endocannabinoid receptor evolution have been discussed elsewhere (Elphick and Egertová 2009), but we do not know of studies that have considered microevolutionary variation in CB receptors (e.g., among closely related species). Although microevolutionary studies are lacking, laboratory mice and rats can show changes in CB receptor gene expression and sensitivity over the course of several days. For example, female mice given wheel access for 10 days had increased CB1 receptor gene expression in the hippocampus as compared with sedentary controls (Wolf et al. 2010). In another study, male rats that had wheel access for eight days had an increase in CB1 receptor binding site density in the hippocampus (Hill et al. 2010).
In future studies, we will further examine acute and chronic effects of voluntary exercise on the endocannabinoid system, including possible changes in receptor densities in various target organs (brain, muscle, gut). Interestingly, although HR mice run much more than C mice, they had lower circulating levels of AEA when the acute effect of wheel running was taken into account (Fig. 6). This finding suggests that the HR endocannabinoid system is differentially regulated, although it is unknown at what level changes have occurred (receptor density, number of converting or recycling enzymes, etc.). Therefore, future studies will also explore the mechanisms and genetics underlying the evolved endocannabinoid system of these unique, high-activity lines of mice, and how this system may interact with neurotransmitter and endocrine systems that are also known to have evolved in the HR mice (Rhodes et al. 2001a; Girard and Garland, Jr. 2002; Malisch et al. 2008).
Supplementary Material
HIGHLIGHTS.
The endocannabinoid system is involved in regulation of voluntary locomotion
We studied 4 replicate lines of high runner (HR) mice bred for wheel exercise
Blood was collected during peak running on night 6 of wheel access
2-AG and/or AEA levels differed between the sexes and between HR and control lines
Levels were also affected by wheel access (training effect) and acutely by activity
Acknowledgments
This work was supported by NSF grant IOS-1121273 to Theodore Garland, Jr. and NIH grant DA034009 to Nicholas DiPatrizio. We thank Jaspreet Kaur for technical assistance. We thank Layla Hiramatsu and three anonymous reviewers for their helpful comments.
Footnotes
Conflict of Interest Statement
The authors declare there is no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References in Zotero
- Acosta W, Meek TH, Schutz H, Dlugosz EM, Vu KT, Garland T., Jr Effects of early-onset voluntary exercise on adult physical activity and associated phenotypes in mice. Physiology & Behavior. 2015;149:279–286. doi: 10.1016/j.physbeh.2015.06.020. [DOI] [PubMed] [Google Scholar]
- Brown DA, Johnson MS, Armstrong CJ, Lynch JM, Caruso NM, Ehlers LB, Fleshner M, Spencer RL, Moore RL. Short-term treadmill running in the rat: what kind of stressor is it? Journal of Applied Physiology. 2007;103:1979–1985. doi: 10.1152/japplphysiol.00706.2007. [DOI] [PubMed] [Google Scholar]
- Burston JJ, Wiley JL, Craig AA, Selley DE, Sim-Selley LJ. Regional enhancement of cannabinoid CB1 receptor desensitization in female adolescent rats following repeated Δ9-tetrahydrocannabinol exposure. British Journal of Pharmacology. 2010;161:103–112. doi: 10.1111/j.1476-5381.2010.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Careau V, Wolak ME, Carter PA, Garland T., Jr Limits to behavioral evolution: the quantitative genetics of a complex trait under directional selection. Evolution. 2013;67:3102–3119. doi: 10.1111/evo.12200. [DOI] [PubMed] [Google Scholar]
- Chaouloff F, Dubreucq S, Matias I, Marsicano G. Routledge Handbook of Physical Activity and Mental Health. Routledge; London: 2012. Physical activity feel-good effect; pp. 71–87. [Google Scholar]
- Claghorn GC, I, Fonseca AT, Thompson Z, Barber C, Garland T., Jr Serotonin-mediated central fatigue underlies increased endurance capacity in mice from lines selectively bred for high voluntary wheel running. Physiology & Behavior. 2016;161:145–154. doi: 10.1016/j.physbeh.2016.04.033. [DOI] [PubMed] [Google Scholar]
- Copes LE, Schutz H, Dlugosz EM, Acosta W, Chappell MA, Garland T., Jr Effects of voluntary exercise on spontaneous physical activity and food consumption in mice: results from an artificial selection experiment. Physiology & Behavior. 2015;149:86–94. doi: 10.1016/j.physbeh.2015.05.025. [DOI] [PubMed] [Google Scholar]
- Craft RM, Marusich JA, Wiley JL. Sex differences in cannabinoid pharmacology: a reflection of differences in the endocannabinoid system? Life Sciences. 2013;92:476–481. doi: 10.1016/j.lfs.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewsbury DA. Wheel-running behavior in 12 species of muroid rodents. Behavioural Processes. 1980;5:271–280. doi: 10.1016/0376-6357(80)90007-8. [DOI] [PubMed] [Google Scholar]
- Dietrich A, McDaniel WF. Endocannabinoids and exercise. British Journal of Sports Medicine. 2004;38:536–541. doi: 10.1136/bjsm.2004.011718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPatrizio NV, Astarita G, Schwartz G, Li X, Piomelli D. Endocannabinoid signal in the gut controls dietary fat intake. Proceedings of the National Academy of Sciences. 2011;108:12904–12908. doi: 10.1073/pnas.1104675108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreucq S, Durand A, Matias I, Bénard G, Richard E, Soria-Gomez E, Glangetas C, Groc L, Wadleigh A, Massa F, et al. Ventral tegmental area cannabinoid type-1 receptors control voluntary exercise performance. Biological Psychiatry. 2013;73:895–903. doi: 10.1016/j.biopsych.2012.10.025. [DOI] [PubMed] [Google Scholar]
- Dubreucq S, Koehl M, Abrous DN, Marsicano G, Chaouloff F. CB1 receptor deficiency decreases wheel-running activity: consequences on emotional behaviours and hippocampal neurogenesis. Experimental Neurology. 2010;224:106–113. doi: 10.1016/j.expneurol.2010.01.017. [DOI] [PubMed] [Google Scholar]
- Elphick MR, Egertová M. Cannabinoid receptor genetics and evolution. In: Reggio PH, editor. The Cannabinoid Receptors. Humana Press; Totowa, NJ: 2009. pp. 123–149. [Google Scholar]
- Feuerecker M, Hauer D, Toth R, Demetz F, Hölzl J, Thiel M, Kaufmann I, Schelling G, Chouker A. Effects of exercise stress on the endocannabinoid system in humans under field conditions. European Journal of Applied Physiology. 2012;112:2777–2781. doi: 10.1007/s00421-011-2237-0. [DOI] [PubMed] [Google Scholar]
- Fuss J, Gass P. Endocannabinoids and voluntary activity in mice: runner’s high and long-term consequences in emotional behaviors. Experimental Neurology. 2010;224:103–105. doi: 10.1016/j.expneurol.2010.03.016. [DOI] [PubMed] [Google Scholar]
- Fuss J, Steinle J, Bindila L, Auer MK, Kirchherr H, Lutz B, Gass P. A runner’s high depends on cannabinoid receptors in mice. Proceedings of the National Academy of Sciences. 2015;112:13105–13108. doi: 10.1073/pnas.1514996112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galdino G, Romero TR, Silva JFP, Aguiar DC, de Paula AM, Cruz JS, Parrella C, Piscitelli F, Duarte ID, Di Marzo V, et al. The endocannabinoid system mediates aerobic exercise-induced antinociception in rats. Neuropharmacology. 2014;77:313–324. doi: 10.1016/j.neuropharm.2013.09.022. [DOI] [PubMed] [Google Scholar]
- Gardner EL. Endocannabinoid signaling system and brain reward: emphasis on dopamine. Pharmacology Biochemistry and Behavior. 2005;81:263–284. doi: 10.1016/j.pbb.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Garland T, Jr, Kelly SA, Malisch JL, Kolb EM, Hannon RM, Keeney BK, Van Cleave SL, Middleton KM. How to run far: multiple solutions and sex-specific responses to selective breeding for high voluntary activity levels. Proceedings of the Royal Society B: Biological Sciences. 2011a;278:574–581. doi: 10.1098/rspb.2010.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland T, Jr, Morgan MT, Swallow JG, Rhodes JS, Girard I, Belter JG, Carter PA. Evolution of a small-muscle polymorphism in lines of house mice selected for high activity levels. Evolution. 2002;56:1267–1275. doi: 10.1111/j.0014-3820.2002.tb01437.x. [DOI] [PubMed] [Google Scholar]
- Garland T, Jr, Schutz H, Chappell MA, Keeney BK, Meek TH, Copes LE, Acosta W, Drenowatz C, Maciel RC, Van Dijk G, et al. The biological control of voluntary exercise, spontaneous physical activity and daily energy expenditure in relation to obesity: human and rodent perspectives. Journal of Experimental Biology. 2011b;214:206–229. doi: 10.1242/jeb.048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland T, Jr, Zhao M, Saltzman W. Hormones and the evolution of complex traits: insights from artificial selection on behavior. Integrative and Comparative Biology. 2017;57 doi: 10.1093/icb/icw040. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard I, Garland T., Jr Plasma corticosterone response to acute and chronic voluntary exercise in female house mice. Journal of Applied Physiology. 2002;92:1553–1561. doi: 10.1152/japplphysiol.00465.2001. [DOI] [PubMed] [Google Scholar]
- Girard I, McAleer MW, Rhodes JS, Garland T., Jr Selection for high voluntary wheel-running increases speed and intermittency in house mice (Mus domesticus) Journal of Experimental Biology. 2001;204:4311–4320. doi: 10.1242/jeb.204.24.4311. [DOI] [PubMed] [Google Scholar]
- Hanlon EC, Tasali E, Leproult R, Stuhr KL, Doncheck E, De Wit H, Hillard CJ, Van Cauter E. Circadian rhythm of circulating levels of the endocannabinoid 2-arachidonoylglycerol. The Journal of Clinical Endocrinology & Metabolism. 2014;100:220–226. doi: 10.1210/jc.2014-3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon RM, Kelly SA, Middleton KM, Kolb EM, Pomp D, Garland T., Jr Phenotypic effects of the “mini-muscle” allele in a large HR x C57BL/6J mouse backcross. Journal of Heredity. 2008;99:349–354. doi: 10.1093/jhered/esn011. [DOI] [PubMed] [Google Scholar]
- Harpur RP. The rat as a model for physical fitness studies. Comparative Biochemistry and Physiology Part A: Physiology. 1980;66:553–574. [Google Scholar]
- Heyman E, Gamelin FX, Goekint M, Piscitelli F, Roelands B, Leclair E, Di Marzo V, Meeusen R. Intense exercise increases circulating endocannabinoid and BDNF levels in humans—possible implications for reward and depression. Psychoneuroendocrinology. 2012;37:844–851. doi: 10.1016/j.psyneuen.2011.09.017. [DOI] [PubMed] [Google Scholar]
- Hill MN, Titterness AK, Morrish AC, Carrier EJ, Lee TTY, Gil-Mohapel J, Gorzalka BB, Hillard CJ, Christie BR. Endogenous cannabinoid signaling is required for voluntary exercise-induced enhancement of progenitor cell proliferation in the hippocampus. Hippocampus. 2010;20:513–523. doi: 10.1002/hipo.20647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houle-Leroy P, Guderley H, Swallow JG, Garland T., Jr Artificial selection for high activity favors mighty mini-muscles in house mice. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2003;284:R433–R443. doi: 10.1152/ajpregu.00179.2002. [DOI] [PubMed] [Google Scholar]
- Jacob W, Yassouridis A, Marsicano G, Monory K, Lutz B, Wotjak CT. Endocannabinoids render exploratory behaviour largely independent of the test aversiveness: role of glutamatergic transmission. Genes, Brain and Behavior. 2009;8:685–698. doi: 10.1111/j.1601-183X.2009.00512.x. [DOI] [PubMed] [Google Scholar]
- Järbe TU, Andrzejewski ME, DiPatrizio NV. Interactions between the CB1 receptor agonist Δ 9-THC and the CB1 receptor antagonist SR-141716 in rats: open-field revisited. Pharmacology Biochemistry and Behavior. 2002;73:911–919. doi: 10.1016/s0091-3057(02)00938-3. [DOI] [PubMed] [Google Scholar]
- Keeney BK, Meek TH, Middleton KM, Holness LF, Garland T., Jr Sex differences in cannabinoid receptor-1 (CB1) pharmacology in mice selectively bred for high voluntary wheel-running behavior. Pharmacology Biochemistry and Behavior. 2012;101:528–537. doi: 10.1016/j.pbb.2012.02.017. [DOI] [PubMed] [Google Scholar]
- Keeney BK, Raichlen DA, Meek TH, Wijeratne RS, Middleton KM, Gerdeman GL, Garland T., Jr Differential response to a selective cannabinoid receptor antagonist (SR141716: rimonabant) in female mice from lines selectively bred for high voluntary wheel-running behaviour. Behavioural Pharmacology. 2008;19:812–820. doi: 10.1097/FBP.0b013e32831c3b6b. [DOI] [PubMed] [Google Scholar]
- Kelly SA, Czech PP, Wight JT, Blank KM, Garland T., Jr Experimental evolution and phenotypic plasticity of hindlimb bones in high-activity house mice. Journal of Morphology. 2006;267:360–374. doi: 10.1002/jmor.10407. [DOI] [PubMed] [Google Scholar]
- Kolb EM, Rezende EL, Holness L, Radtke A, Lee SK, Obenaus A, Garland T. Mice selectively bred for high voluntary wheel running have larger midbrains: support for the mosaic model of brain evolution. The Journal of experimental biology. 2013;216:515–523. doi: 10.1242/jeb.076000. [DOI] [PubMed] [Google Scholar]
- Koteja P, Garland T, Jr, Sax JK, Swallow JG, Carter PA. Behaviour of house mice artificially selected for high levels of voluntary wheel running. Animal Behaviour. 1999;58:1307–1318. doi: 10.1006/anbe.1999.1270. [DOI] [PubMed] [Google Scholar]
- Koteja P, Garland T., Jr Response to R. Eikelboom Animal Behaviour. 2001;61:F25–F26. [Google Scholar]
- Li G, Rhodes JS, Girard I, Gammie SC, Garland T., Jr Opioid-mediated pain sensitivity in mice bred for high voluntary wheel running. Physiology & Behavior. 2004;83:515–524. doi: 10.1016/j.physbeh.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Malisch JL, Breuner CW, Gomes FR, Chappell MA, Garland T., Jr Circadian pattern of total and free corticosterone concentrations, corticosteroid-binding globulin, and physical activity in mice selectively bred for high voluntary wheel-running behavior. General and Comparative Endocrinology. 2008;156:210–217. doi: 10.1016/j.ygcen.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Malisch JL, Breuner CW, Kolb EM, Wada H, Hannon RM, Chappell MA, Middleton KM, Garland T., Jr Behavioral despair and home-cage activity in mice with chronically elevated baseline corticosterone concentrations. Behavior Genetics. 2009;39:192–201. doi: 10.1007/s10519-008-9246-8. [DOI] [PubMed] [Google Scholar]
- Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O. Cocaine, but not morphine, induces conditioned place preference and sensitization to locomotor responses in CB1 knockout mice. European Journal of Neuroscience. 2000;12:4038–4046. doi: 10.1046/j.1460-9568.2000.00287.x. [DOI] [PubMed] [Google Scholar]
- Meng ID, Manning BH, Martin WJ, Fields HL. An analgesia circuit activated by cannabinoids. Nature. 1998;395:381–383. doi: 10.1038/26481. [DOI] [PubMed] [Google Scholar]
- Murillo-Rodriguez E, Désarnaud F, Prospéro-García O. Diurnal variation of arachidonoylethanolamine, palmitoylethanolamide and oleoylethanolamide in the brain of the rat. Life Sciences. 2006;79:30–37. doi: 10.1016/j.lfs.2005.12.028. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nature Reviews Neuroscience. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Raichlen DA, Foster AD, Gerdeman GL, Seillier A, Giuffrida A. Wired to run: exercise-induced endocannabinoid signaling in humans and cursorial mammals with implications for the “runner’s high”. Journal of Experimental Biology. 2012;215:1331–1336. doi: 10.1242/jeb.063677. [DOI] [PubMed] [Google Scholar]
- Raichlen DA, Foster AD, Seillier A, Giuffrida A, Gerdeman GL. Exercise-induced endocannabinoid signaling is modulated by intensity. European Journal of Applied Physiology. 2013;113:869–875. doi: 10.1007/s00421-012-2495-5. [DOI] [PubMed] [Google Scholar]
- Rasmussen EB, Hillman C. Naloxone and rimonabant reduce the reinforcing properties of exercise in rats. Experimental and Clinical Psychopharmacology. 2011;19:389. doi: 10.1037/a0024142. [DOI] [PubMed] [Google Scholar]
- Rhodes J, Hosack G, Girard I, Kelley A, Mitchell G, Garland T. Differential sensitivity to acute administration of cocaine, GBR 12909, and fluoxetine in mice selectively bred for hyperactive wheel-running behavior. Psychopharmacology. 2001a;158:120–131. doi: 10.1007/s002130100857. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Gammie SC, Garland T., Jr Neurobiology of mice selected for high voluntary wheel-running activity. Integrative and Comparative Biology. 2005;45:438–455. doi: 10.1093/icb/45.3.438. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Garland T, Jr, Gammie SC. Patterns of brain activity associated with variation in voluntary wheel-running behavior. Behavioral Neuroscience. 2003;117:1243. doi: 10.1037/0735-7044.117.6.1243. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Hosack GR, Girard I, Kelley AE, Mitchell GS, Garland T., Jr Differential sensitivity to acute administration of cocaine, GBR 12909, and fluoxetine in mice selectively bred for hyperactive wheel-running behavior. Psychopharmacology. 2001b;158:120–131. doi: 10.1007/s002130100857. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Koteja P, Swallow JG, Carter PA, Garland T., Jr Body temperatures of house mice artificially selected for high voluntary wheel-running behavior: repeatability and effect of genetic selection. Journal of Thermal Biology. 2000;25:391–400. doi: 10.1016/s0306-4565(99)00112-6. [DOI] [PubMed] [Google Scholar]
- Saltin B, Gollnick PD. Skeletal muscle adaptability: significance for metabolism and performance. Comprehensive Physiology. 1983:555–631. [Google Scholar]
- Sañudo-Peña MC, Romero J, Seale GE, Fernandez-Ruiz JJ, Walker JM. Activational role of cannabinoids on movement. European Journal of Pharmacology. 2000;391:269–274. doi: 10.1016/s0014-2999(00)00044-3. [DOI] [PubMed] [Google Scholar]
- Scribbans TD, Vecsey S, Hankinson PB, Foster WS, Gurd BJ. The effect of training intensity on VO2max in young healthy adults: a meta-regression and meta-analysis. International Journal of Exercise Science. 2016;9:230. doi: 10.70252/HHBR9374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestri C, Di Marzo V. The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metabolism. 2013;17:475–490. doi: 10.1016/j.cmet.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Smith SL, Rasmussen EB. Effects of 2-AG on the reinforcing properties of wheel activity in obese and lean Zucker rats. Behavioural Pharmacology. 2010;21:292–300. doi: 10.1097/FBP.0b013e32833aec4d. [DOI] [PubMed] [Google Scholar]
- Sparling PB, Giuffrida A, Piomelli D, Rosskopf L, Dietrich A. Exercise activates the endocannabinoid system. Neuroreport. 2003;14:2209–2211. doi: 10.1097/00001756-200312020-00015. [DOI] [PubMed] [Google Scholar]
- Stanford KI, Goodyear LJ. Exercise regulation of adipose tissue. Adipocyte. 2016;5:153–162. doi: 10.1080/21623945.2016.1191307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swallow JG, Carter PA, Garland T., Jr Artificial selection for increased wheel-running behavior in house mice. Behavior Genetics. 1998;28:227–237. doi: 10.1023/a:1021479331779. [DOI] [PubMed] [Google Scholar]
- Swallow JG, Rhodes JS, Garland T., Jr Phenotypic and evolutionary plasticity of organ masses in response to voluntary exercise in house mice. Integrative and Comparative Biology. 2005;45:426–437. doi: 10.1093/icb/45.3.426. [DOI] [PubMed] [Google Scholar]
- Syme DA, Evashuk K, Grintuch B, Rezende EL, Garland T., Jr Contractile abilities of normal and “mini” triceps surae muscles from mice (Mus domesticus) selectively bred for high voluntary wheel running. Journal of Applied Physiology. 2005;99:1308–1316. doi: 10.1152/japplphysiol.00369.2005. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Wahlsten D. Insensitivity of the analysis of variance to heredity-environment interaction. Behavioral and Brain Sciences. 1990;13:109–120. [Google Scholar]
- Wahlsten D. Sample size to detect a planned contrast and a one degree-of-freedom interaction effect. Psychological Bulletin. 1991;110:587–595. [Google Scholar]
- Wallace IJ, Garland T., Jr Mobility as an emergent property of biological organization: insights from experimental evolution. Evolutionary Anthropology. 2016;25:98–104. doi: 10.1002/evan.21481. [DOI] [PubMed] [Google Scholar]
- Wolf SA, Bick-Sander A, Fabel K, Leal-Galicia P, Tauber S, Ramirez-Rodriguez G, Müller A, Melnik A, Waltinger TP, Ullrich O, et al. Cannabinoid receptor CB1 mediates baseline and activity-induced survival of new neurons in adult hippocampal neurogenesis. Cell Communication and Signaling. 2010:8. doi: 10.1186/1478-811X-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Shearman LP. Voluntary exercise augments acute effects of CB1-receptor inverse agonist on body weight loss in obese and lean mice. Pharmacology Biochemistry and Behavior. 2004;77:117–125. doi: 10.1016/j.pbb.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proceedings of the National Academy of Sciences. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.