Abstract
Poor executive functions (EFs) have been linked to substance use and abuse across multiple substances. However, it is unclear whether these associations are stronger for some EFs over others and/or some stages of substance use over others (e.g., ever using substances vs. dependence). It is also unknown whether such patterns change from adolescence to early adulthood, a transition that is characterized by changes to both EFs and substance use behaviors. In this longitudinal study of approximately 850 twins, we examined the relations between multiple EF abilities (including a Common EF factor predicting nine EF tasks) and measures of general substance use and dependence/abuse in late adolescence (mean age 17 years) and early adulthood (mean age 23 years). At the phenotypic level, Common EF in adolescence was negatively related to the number of substances ever used and to past six-month frequency of use, but to not dependence/abuse vulnerability (i.e., the number of dependence and abuse symptoms endorsed per substance that had been repeatedly used). However, in the same subjects in early adulthood, Common EF was only weakly related to the number of substances used, and not related to concurrent frequency of use nor dependence/abuse vulnerability. Twin analyses revealed that these associations were primarily genetic in origin, and that the genetic correlations were relatively stable over time. These results suggest that low Common EF is a genetic risk factor for increased polysubstance use in late adolescence, but that non-EF factors play a larger role in the progression to substance dependence/abuse.
Keywords: drug use, executive control, individual differences, heritability, twin study
Executive functions (EFs) are higher-level cognitive abilities that control and regulate goal-directed behavior (Miyake & Friedman, 2012). Poor EFs are associated with substance use disorder (Verdejo-Garcia, Bechara, Recknor, & Perez-Garcia, 2006) and risk for substance dependence (Giancola & Tarter, 1999), as well as externalizing and internalizing problems more generally (Snyder, Miyake, & Hankin, 2015). However, it remains unclear whether EFs are associated with some aspects of substance use more than others (e.g., trying substances vs. abusing them), and to what extent these relations change from late adolescence to adulthood, a time characterized by development of EFs and changes in substance use behaviors (Center for Behavioral Health Statistics and Quality, 2015; Friedman et al., 2016). We investigated these issues in a longitudinal study of twins who completed measures of EFs and substance use at two ages: late adolescence (mean age 17 years) and early adulthood (mean age 23 years).
We examined three aspects of polysubstance use — the lifetime number of substances ever used, the frequency of substance use in the past six months, and dependence/abuse symptoms. Our hypothesis was that genetic risk for lower general EF ability (specifically, a latent Common EF factor explaining variation across different types of EFs) is primarily associated with trying substances in adolescence, rather than with the development of abuse and dependence in adulthood.
EFs and Substance Use and Misuse
Prior research suggests an association between poor EF abilities and substance use problems. EFs are weaker in frequent users of illicit substances such as cocaine (Hester & Garavan, 2004), amphetamines (Ersche, Clark, London, Robbins, & Sahakian, 2006), cannabis (Solowij et al., 2002), and ecstasy (Roberts, Jones, & Montgomery, 2016), as well as legal substances such as tobacco and alcohol (Durazzo, Gazdzinscki, & Meyerhoff, 2007). Although these different substances likely affect the brain and body in different ways and may have their own unique relations with EFs, these findings suggest that there may be a general association between EFs and substance use behaviors. In fact, research indicates that there is a general genetic liability for the use/misuse of substances (Hopfer, Crowley, & Hewitt, 2003; Kendler, Jacobson, Prescott, & Neale, 2003; McGue, Elkins, & Iacono, 2000; Palmer et al., 2009; Rhee et al., 2003), suggesting that substance use behaviors and risk can be examined across substances, especially at the genetic level.
Although an association between poor EFs and substance use could arise because substance use impairs EFs, it could also arise if low EFs are a risk factor for substance use, or if substance use and EFs share genetic influences (these possibilities are not mutually exclusive). Consistent with the latter two possibilities, some evidence suggests that EF deficits are present before the onset of substance use. Across multiple studies, children of substance abusers have been shown to have lower EF abilities than children of non-abusers (Giancola & Tarter, 1999; Nigg et al., 2006), suggesting that individuals at familial risk for substance use also have lower EFs. In one prospective study, for example, lower EF ability predicted later alcohol-related problems and the number of illicit substances used, controlling for other factors such as IQ and parent alcoholism (Nigg et al., 2006). This body of work is most consistent with the view of low EFs as potential risk factors for substance use and dependence, rather than EFs being affected only after use.
A likely mechanism by which low EFs may increase risk is through their association with more general impulsive risk-taking and externalizing behavior, which is also associated with substance use (Coolidge, Thede, & Young, 2000; Young et al., 2009). Several studies have estimated externalizing factors that include substance use, novelty seeking, conduct disorder, and attention-deficit/hyperactivity disorder. For example, Caspi et al. (2014) found that a latent externalizing factor was associated with lower EFs in a large longitudinal sample including measures from age 3–38 years. Moreover, Young et al. (2009), using the same longitudinal sample as the current study, found that a latent externalizing factor at ages 12 and 17 years (called Behavioral Disinhibition) was associated with poorer prepotent response inhibition measured at age 17 (latent variable rs= −.47 and −.39, respectively). This relationship was largely due to shared genetic influences (rg= −.60 and −.61 for Behavioral Disinhibition at ages 12 and 17, respectively).
Subsequent reanalyses of the Young et al. (2009) data with an alternative bifactor parameterization of the EF model (Herd et al., 2014; Miyake & Friedman, 2012) found that Behavioral Disinhibition was primarily related to a Common EF factor (predicting nine tasks assessing three separable EFs). As discussed by Miyake and Friedman (2012), at the level of individual differences, this Common EF factor is isomorphic with the response inhibition factor used in earlier work. Given these findings regarding EFs and externalizing behaviors, it is likely that the association between EFs and substance use is driven by the Common EF variance underlying many EF tasks, and through a tendency to engage in externalizing behaviors.
In the current study, we tested the hypothesis that low EFs Common EF is a genetic risk factor for polysubstance use. In doing so, we examine the relations of EFs with multiple aspects of the substance use to dependence trajectory: ever using multiple substances, using them repeatedly, and eventually abusing them and/or becoming dependent. Measures capturing these stages may show different relations to EFs. Hines, Morley, Mackie, and Lynskey (2015) hypothesized that genes associated with novelty seeking and risk taking would be associated with early aspects of use, whereas the progression to regular/heavy use and abuse and dependence would be related to genes influencing subjective effects and drug metabolism. Similarly, others have highlighted substance-specific neurobiological and environmental changes (e.g., withdrawal-based negative affect, sensitization to drug cues) that may play a larger role once dependence has developed (Koob & Le Moal, 1997; Robinson & Berridge, 1993).
To the extent that Common EF relates to substance use through a genetic association with novelty seeking and risk taking, a stage-sequential model would predict that Common EF should be most related to the early aspects of substance use (e.g., trying multiple substances). Moreover, to the extent that dependence and abuse is related to dysregulation of the brain's reward system (Koob & Le Moal, 1997), the type of cognitive control captured by Common EF may have limited impact on behavior once drug sensitization and counteradaptation processes become abnormal. That is, Common EF may be more related to trying substances than to variance unique to substance dependence and abuse.
To better understand how EFs relate to stages of substance use and misuse, we examined these relations in the same sample at two ages: late adolescence (mean age 17 years) and early adulthood (mean age 23 years). These ages are critical ones for both the development of EFs and the progression of substance use. The neural substrates related to EFs (particularly the prefrontal cortex) continue to develop from late adolescence into adulthood (Giedd et al., 1999), as does performance on EF tasks (Luciana, Conklin, Hooper, & Yarger, 2005). This developmental course has been invoked to partially explain increased risk taking in adolescence (Pharo, Sim, Graham, Gross, & Hayne, 2011). Thus, risky behaviors linked to poor EFs may be more pronounced in adolescence.
Somewhat paradoxically, the continued improvement of EFs in early adulthood is accompanied by an increase in substance use. Adolescents are typically just beginning to try substances, and few report many dependence symptoms (Center for Behavioral Health Statistics and Quality, 2015). In contrast, many individuals approaching their mid-20s are at their peak level of use (Center for Behavioral Health Statistics and Quality, 2015), and most have tried at least one substance (Moss, Chen, & Yi, 2014; Palmer et al., 2009). Such age-related changes in substance use behaviors at the population level likely reflect cultural norms rather than EF-related processes, but it remains an open question how changes to the contexts surrounding substance use in adulthood vs. adolescence affect associations with EFs (Day, Kahler, Ahern, & Clark. 2015). One possibility is that in adulthood, EF associations with ever using substances weaken, once substance use becomes more normative. However, to the extent that the genetic influences on EFs and substance use are stable across this transition, the associations may persist despite environmental changes.
EF Framework Used in the Current Study
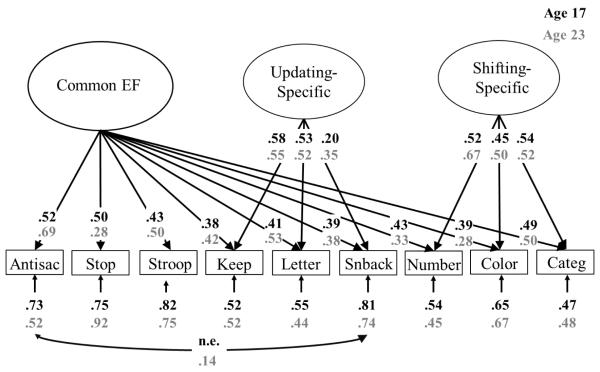
In this study, we used a bifactor EF model (Friedman & Miyake, 2016; Miyake & Friedman, 2012), shown for both time points we examine in Figure 1, as the guiding framework. It includes three orthogonal factors: (1) Common EF, which predicts nine laboratory EF tasks assessing prepotent response inhibition, working memory updating, or mental set shifting; (2) Updating-Specific, which predicts performance on the three updating tasks above and beyond the common factor; and (3) Shifting-Specific, which predicts performance on the three shifting tasks above and beyond the common factor.1 There is no Inhibiting-Specific factor, because once the Common EF factor is included, there are no remaining correlations among the inhibiting tasks (i.e., the Common EF factor explains all the variance in the Inhibiting factor; a finding replicated across independent datasets). This model has been recently replicated in an independent sample (Ito et al., 2015), and shows high stability across waves of assessment in the current sample (Friedman et al., 2016).
Figure 1.
Bifactor model of EFs, shown for late adolescence (black font) and early adulthood (grey font) in the current sample. All EF tasks were residualized on age and sex before analysis. Numbers on arrows are standardized factor loadings, those under the smaller arrows are residual variances, and the number on the curved double-headed arrow is a residual correlation. In this model, there is a Common EF latent variable on which all nine EF tasks load, as well as two latent variables on which the updating and shifting tasks, respectively, also load. The Common EF variance is isomorphic with an Inhibiting latent variable, so there was no Inhibiting-Specific factor at either time point. Because the Common EF factor captures the variance common to all three EFs, the Updating-Specific and Shifting-Specific factors capture the variance that is unique to Updating and Shifting, respectively. Hence, they are uncorrelated with the Common EF factor and with each other. All parameters were statistically significant (p < .05). Antisac = antisaccade, Stop = stop-signal, Keep = keep track, Letter = letter memory, Snback = spatial n-back, Number = number–letter, Color = color–shape, Categ = category-switch; n.e. = not estimated at wave 1.
We hypothesized that Common EF is the primary aspect of EF associated with adolescent substance use, and that lower Common EF is associated with higher levels of substance use in adolescence. This Common EF factor is hypothesized to tap the ability to actively maintain and effectively manage goals and use them to bias lower-level processing (Friedman & Miyake, 2016; Miyake & Friedman, 2012). Strong goal maintenance and management is important in all EF tasks, particularly response inhibition tasks that require keeping a goal strongly active to prevent dominant responses from taking over (Miyake & Friedman, 2012; Young et al., 2009). Furthermore, Common EF has been linked with everyday self-regulatory and impulse-control abilities such as less procrastination and stronger maintenance of long-term goals in everyday life (Gustavson, Miyake, Hewitt, & Friedman, 2015). Thus, Common EF may be especially relevant to engaging in risky behavior with short-term benefits but long-term consequences, such as substance use (e.g., Day et al., 2015).
In contrast, we did not expect to see the same patterns with the Updating-Specific and Shifting-Specific factors. The Updating-Specific factor is thought to relate to the accuracy of gating information into working memory by the basal ganglia and memory retrieval, whereas the Shifting-Specific factor is thought to reflect the speed of replacing goals once they are no longer necessary (Friedman & Miyake, 2016; Miyake & Friedman, 2012). As such, we did not have any reasons to expect that these processes are systematically related to general substance use behaviors.
Importantly, we expected the associations between Common EF and substance use to be driven by shared genetic influences. As reported by Friedman et al. (2016), the Common EF factor was highly heritable at the two ages we examined (96% in late adolescence and 81% in early adulthood). Although the genetic influences on Common EF was the same in late adolescence and adulthood (rg=1.0), there was evidence for new nonshared environmental influences in adulthood (e2=15%). The nature of this new nonshared environmental variance is unclear, but may in part reflect the substantial environmental changes in emerging adulthood, such as moving out of the home and entering the workplace (Friedman et al., 2016). Therefore, although Common EF would unlikely be environmentally related to substance use in adolescence (when these factor play little to no role in Common EF), environmental factors may explain some covariation between Common EF and substance use in early adulthood. Such a pattern could lead to comparable or larger phenotypic correlations in adulthood compared to adolescence. However, if this new environmental variance on Common EF is unrelated to substance use, or if new genetic variation on substance use in adulthood is unrelated to EF, it would lead to smaller phenotypic correlations with Common EF in adulthood.
Method
Subjects
Analyses were based on 846 individuals (435 females, 411 males) from 429 same-sex twin pairs (232 monozygotic [MZ] and 197 dizygotic [DZ]) who completed EF or substance use assessments at either wave of testing. The LTS sample was recruited from Colorado Department of Health records of twin births between 1986 and 1990 in Colorado, and is representative of the population in Colorado at that time. Of the 846 subjects, 91.8% identified as White, 5.6% as more than one race, 1.2% as American Indian, 0.2% as Native Hawaiian/Pacific Islander, and 1.2% as unknown/not reported; 9.3% identified as Hispanic. Data collection for wave one occurred between 2003 and 2008, and data collection for wave two occurred between 2009 and 2013 (for more information on the sample, see Rhea, Gross, Haberstick, & Corley, 2013).
At wave one (late adolescence), 797 individuals completed the EF and the substance use assessments on the same day, at about age 17 (M=17.25 years, SD=0.64, Range=16.51–20.08). At wave two (early adulthood; N=751) the substance use and EF measures were not assessed on the same day (M=22.84 years, SD=1.29, Range=21.11–28.03 for EF; M=22.80 years, SD=1.27, N=757, Range=21.10–28.00 for substance use), but were generally assessed within the same week (M=0.05 years, SD=0.22 between EF and substance use assessments). The majority of individuals (N=713) completed measures at both waves (see supplement Section 1 for more information).
Measures
EF tasks
The EF battery included nine measures of three widely studied EFs: response inhibition, working memory updating, and set shifting. Here we briefly describe the basic task requirements; full task details and scoring procedures are described in Friedman et al. (2016).
The three inhibition tasks required subjects to stop a dominant or prepotent response (i.e., eye movements for antisaccade, categorization for stop-signal, and word reading for Stroop). Dependent measures were accuracy for antisaccade, stop-signal reaction time for stop-signal, and response time interference (to name colors of incongruent words vs. asterisks) for Stroop. The three updating tasks required subjects to continuously update the contents of working memory (with category exemplars for keep track, letter series for letter memory, and spatial locations for spatial n-back). Dependent measures were recall accuracy for keep track and letter memory, and target judgment accuracy for n-back. The three shifting tasks required subjects to switch between categorization dimensions (odd/even vs. vowel/consonant for number-letter, red/green vs. circle/triangle for color-shape, and big/small vs. living/nonliving for category-switch), according to a cue stimulus. Dependent measures were local switch costs, or average RT on trials that required a switch of categorization rule from the immediately prior trial minus average RT on trials that required a repeat of the same categorization rule (in “mixed” blocks of trials in which both rules were used).
Substance use
The substance use measures were based on responses to the Substance Abuse Module of the Composite International Diagnostic Interview (CIDI-SAM; Cottler, Robins, & Helzer, 1989) or supplemental questions that were added to this module to assess substance use that did not meet DSM criteria (see Salomonsen-Sauten, Sakai, Thurstone, Corley, & Hopfer, 2012). Each subject answered questions for 15 categories of substances: tobacco, alcohol, marijuana, cocaine, amphetamines, sedatives, inhalants, hallucinogens, opiates, PCP, MDMA, ketamine, GHB, Rohypnol, and a set of questions for “other drugs.” For dependence/abuse vulnerability only, this list was restricted to 11 categories: Subjects were not asked about dependence and abuse symptoms for “other drugs,” and they were only asked about dependence/abuse symptoms for the most used out of the four club drugs (MDMA, ketamine, GHB, and Rohypnol).
For each measure of substance use (lifetime number of substances used, frequency of use, and dependence/abuse vulnerability), we created a quasi-continuous variable based on responses to the 11 or 15 respective questionnaire items about each category (see below). However, because these variables were not normally distributed, we binned responses and analyzed them as ordinal variables, assuming an underlying normal liability distribution using threshold models. Such categorical data analysis has been shown to produce unbiased estimates in twin models of censored data, compared to transformations (Derks, Dolan, & Boomsma, 2004). We selected the bin cutoffs prior to analyses to avoid empty cells when participants were split into twin and zygosity groups for the genetic analyses. The scoring and binning procedure are described here, and more information on the unbinned variables can be found in the supplement (Table S1). The supplement also displays the prevalence of substance use at each age and how many subjects met criteria to be asked about dependence and abuse, separated by substance (Table S2).
Lifetime number of substances used
The number of substances ever used was scored based on responses to the item, “Have you ever used [X]?”, where X is each of the categories listed above. Scores were summed across the 15 responses, then binned into four levels: None, 1-2, 3-4, and 5 or more drug categories ever used.
Because this primary measure of lifetime substance use was cumulative, we also conducted and report below some post-hoc analyses using an alternative measure of the number of substances ever used (in early adulthood only) that controls for adolescent use. Namely, we created a difference score for the number of substances endorsed in early adulthood minus the number endorsed in late adolescence, binned into four levels: zero (n=148), one (n=195), 2-3 (n=156), or 4 or more substances (n=114). For more information, see supplement Section 3.2
Frequency of use
Frequency was calculated based on responses to the item, “How many days have you used [X] in the past six months (180 Days)?”, again where X is each of the categories listed above. Frequency scores were summed across the 15 responses, then binned into five levels: zero days (including subjects who never used any substance), 1 to 6, 7 to 25, 26 to 179, and 180+ days. These bins were chosen because they correspond to the following levels: no substance use, using substances less than once per month, using substances more than once per month (but less than twice per week), using substances more than twice per week (but less than everyday), and using at least one substance per day (on average).
Note that a score of 10, for example, does not necessarily mean 10 days of use total, because subjects could have used tobacco and alcohol five times each, but on the same five days (which is also why it is possible to score higher than 180). Thus, although the term “days of use” is not necessarily accurate for all subjects (and may be better conceived of as “substance-days,” akin to “man-hours”), this measure should still capture the frequency of overall substance use within a given individual.
Dependence/abuse vulnerability
To assess substance dependence and abuse across multiple substances, we used dependence/abuse vulnerability, modified from a measure that has previously been shown to be heritable and related to other externalizing behaviors (Button et al., 2006; Stallings et al., 2003). Dependence/abuse vulnerability was calculated based on endorsement of seven dependence symptoms and four abuse symptoms for each of the categories (except tobacco, which does not have abuse symptoms), based on the DSM-IV criteria (supplement Table S4). To be asked questions about dependence/abuse for illicit substances on the CIDI-SAM, subjects must have endorsed using that substance more than five times. For tobacco, subjects must have met one of the following criteria: smoked 20 or more cigarettes, or had more than five instances of cigar smoking, pipe tobacco smoking, or chewing tobacco use. For alcohol, subjects must have indicated that they had used alcohol regularly, gotten drunk, or had problems due to alcohol.
The dependence/abuse vulnerability score was the sum of dependence and abuse symptoms endorsed across all substances, divided by the total number of substances that had been used to criterion. These scores were sorted into four bins: zero, up to 1, up to 2.5, and greater than 2.5 dependence symptoms per substance. Unlike frequency of use, subjects who did not meet the criterion for any substance (including subjects who had never used any substance) were not included in this measure, because their dependence/abuse vulnerabilities were unknown (i.e., censored). We treated these subjects as missing rather than assigning them zeros to keep the dependence and ever used/frequency of use variables conceptually separate.3 This resulted in a smaller total n for this variable compared to the lifetime use and frequency of use variables (see Table 1).
Table 1.
Frequencies in Substance Use Measure Bins and Twin Correlations
| Late Adolescence (Mean Age 17) |
Early Adulthood (Mean Age 23) |
|
|---|---|---|
| Number of Substances Ever Used | rMZ=.84, rDZ=.71 | rMZ=.72, rDZ=.56 |
| None | 294 | 18 |
| 1-2 substances | 268 | 273 |
| 3-4 substances | 175 | 265 |
| 5 or more substances | 55 | 203 |
| Frequency of Use | rMZ=.79, rDZ=.63 | rMZ=.52, rDZ=.48 |
| None | 374 | 53 |
| 1-6 days | 172 | 100 |
| 7-25 days | 85 | 181 |
| 26-179 days | 74 | 239 |
| 180 or more days | 86 | 186 |
| Dependence/Abuse Vulnerability | rMZ=.69, rDZ=.43 | rMZ=.56, rDZ=.36 |
| None | 124 | 213 |
| 1 symptom or less (per drug) | 98 | 191 |
| > 1 & < 2.5 symptoms (per drug) | 72 | 178 |
| 2.5 or more symptoms (per drug) | 72 | 132 |
Note: For dependence/abuse vulnerability, subjects were only included if they met the criterion for repeated use for at least one substance. rMZ and rDZ are polychoric correlations between twin 1 and twin 2 for monozygotic (MZ) and dizygotic (DZ) twins. All correlation p<.05.
To confirm that our results regarding dependence/abuse vulnerability were not affected by this operationalization of vulnerability, we performed alternative analyses comparing this vulnerability measure with the maximum symptom count (i.e., focusing on the substance with the most dependence/abuse symptoms), and the total symptom count (i.e., across all substances, but not divided by the number of substances used to criterion). These analyses revealed that the mean dependence/abuse vulnerability score used here had the largest heritability estimate (a2=.53 in adolescence, a2=.38 in adulthood), and there were only small differences in the correlations between EFs and these alternative measures of dependence/abuse vulnerability (for more information, see supplement Table S5 and Figure S2).
Data Analysis
All analyses were conducted with Mplus 7.2 (Muthén & Muthén, 1998-2012), which accounts for missing observations using full-information maximum likelihood (for models of EFs alone) or weighted least squares, mean and variance adjusted (WLSMV, delta parameterization, for models that include ordinal substance use variables). Because the overall chi-square (χ2) test is sensitive to sample size, we supplemented with the root-mean-square error of approximation (RMSEA) and comparative fit index (CFI), with χ2<2*df, RMSEA<.06, and CFI>.95 considered good fit (Hu & Bentler, 1998). For each twin pair, twins were assigned to twin 1 or twin 2 according to the same random assignments used in prior work (e.g., Friedman et al., 2008, 2016). To adjust for non-independence of twin pairs in the phenotypic analysis, we used the type=complex option in Mplus, which provides chi-squares and standard errors adjusted for clustering. Significance of parameters was evaluated with χ2 difference tests (χ2diff), using the difftest option for models with ordinal variables.
To ensure that our results were not affected by associations with sex and age, we removed age and sex variance from the EF and substance use measures as follows. For EFs, the dependent measures for the nine EF tasks were residuals saved from phenotypic regressions on age and sex. For substance use, sex and age were included as covariates in all of the models presented here (i.e., by regressing the ordinal substance use measures on sex and age within each model). For more information about the effects of sex and age on each dependent measure, see the supplement (Table S6).
Twin models
The twin models were based on the following standard assumptions. Additive genetic influences (A) correlate 1.0 in MZ twin pairs, because they share 100% of their alleles, but this correlation is 0.5 for DZ twins because on average they share 50% of their alleles identical by descent. Shared environmental influences (C) correlate 1.0 for both MZ and DZ twins, because both types are reared together. Nonshared environmental influences (E) do not correlate across twins, by definition. The genetic/environmental correlations presented in the Results were computed from Cholesky decompositions, which are statistically equivalent models to the correlated ACE models depicted in the figures.
Preliminary analyses
Full descriptive statistics for the EF measures were presented in earlier work (Friedman et al., 2016). For substance use, the number of observations in each of the binned variables are presented in Table 1, but more information about the unbinned variables can be found in the supplement (Table S1) along with the full phenotypic correlation matrix between all individual EF tasks and substance use measures (Table S9).
Before completing our primary analyses, we fit univariate biometric models for each of the substance use measures (because the univariate models for each EF task were reported by Friedman et al., 2016, we do not report them here). The results for the univariate models of substance use revealed no substantial differences from the multivariate models, so they are also not reported here.
Finally, to ensure that our findings were not driven by the effects of tobacco and/or alcohol, the two most commonly used substances in this sample, we also conducted our key phenotypic analyses (a) after removing the items related to tobacco use, and (b) after removing the items related to both tobacco and alcohol use. Because these models did not reveal any substantial differences in the pattern of results, we report the models that include both tobacco and alcohol use here, but also report these two alternative phenotypic models in the supplement (Table S3).
Results
Phenotypic Analyses
Phenotypic analyses were conducted in two parts. First, we examined the stability of each construct across waves. Second, we examined the association between EFs and substance use by fitting a full correlational model of both ages.
Stability of EFs and substance use measures
As described earlier, recent research on this sample has revealed that individual differences in latent variable EFs are quite stable from late adolescence to early adulthood (Friedman et al., 2016). In this phenotypic model (on the age- and sex-residualized measures), χ2(110)=206.15, p<.001, RMSEA=.032, CFI = .970, all three EF factors in adolescence were highly correlated with the same construct in adulthood, r=.86 for Common EF, r=1.0 for Updating-Specific, and r=.91 for Shifting-Specific. Because the Updating- and Shifting-Specific factors are essentially residuals after the Common EF factor variance is removed, they are orthogonal with Common EF and each other within and across age.
The phenotypic relations among substance use measures are described in Table 2. Within each age, the lifetime number of substances ever used and the frequency of substance use (in the past six months) were more highly correlated with each other (r=.91 in late adolescence, r=.69 in early adulthood) than either measure was with dependence/abuse vulnerability (r=.37 to .42 in adolescence, r=.49 to .56 in adulthood). Furthermore, the number of substances used and frequency of use in late adolescence were strongly predictive of the number of substances used (r=.70) and frequency of use in young adulthood (r=.54), respectively. Adolescent dependence/abuse vulnerability was also positively correlated with early adulthood dependence/abuse vulnerability (r=.34).
Table 2.
Polychoric Correlations For Substance Use Measures
|
Late Adolescence
(Mean Age 17) |
Early Adulthood
(Mean Age 23) |
|||||
|---|---|---|---|---|---|---|
| Ever Used | Frequency | DAV | Ever Used | Frequency | DAV | |
| Late Adolescence | ||||||
| Ever Used | 1 | |||||
| Frequency | .91 | 1 | ||||
| DAV | .37 | .42 | 1 | |||
| Early Adulthood | ||||||
| Ever Used | .70 | .68 | .30 | 1 | ||
| Frequency | .50 | .54 | .18 | .69 | 1 | |
| DAV | .45 | .44 | .34 | .56 | .49 | 1 |
Note: Partial correlations, controlling for age and sex. Ever Used = lifetime number of substances ever tried, Frequency = Frequency of substance use in the past six months, DAV = Dependence/abuse vulnerability: the number of dependence and abuse symptoms per substance used to criterion. All p<.05.
In summary, these preliminary results suggest that we assessed correlated but unique aspects of substance use. Moreover, individual differences in both EF and substance use showed some stability over this six-year window.
Relations between EFs and substance use measures
Next, we examined the phenotypic correlations between EFs and substance use measures at both ages. The full correlational model fit the data well, χ2(244)=308.42, p<.001, RMSEA=.018, CFI=.991, and is displayed in Table 3. This full model displays both within-wave and between-wave correlations between EFs and substance use, but we focus here on within-wave correlations, as we were most interested in quantifying the associations between EFs and substance use at each wave.
Table 3.
Correlations Between EFs and Substance Use Measures
| Common EF | Updating-Specific | Shifting-Specific | ||||
|---|---|---|---|---|---|---|
| Late Adolescence |
Early Adulthood |
Late Adolescence |
Early Adulthood |
Late Adolescence |
Early Adulthood |
|
| Late Adolescence (Mean Age 17) | ||||||
| Ever Used | − .31 | − .21 | −.02 | −.01 | .08 | .11 |
| Frequency | − .25 | − .15 | −.02 | −.04 | .03 | .05 |
| DAV | .02 | −.02 | −.06 | .03 | − .17 | −.06 |
| Early Adulthood (Mean Age 23) | ||||||
| Ever Used | − .25 | − .18 | .14 | .03 | .20 | .07 |
| Frequency | − .13 | − .10 | .06 | −.03 | .17 | .05 |
| DAV | −.10 | −.09 | −.03 | − .12 | .05 | .02 |
Note: Displayed are partial correlations between substance use measures and EF latent variables after controlling for the effects of age and sex on all variables. EF latent variables were based on residualized scores after removing the effects of sex and age (before fitting this model). Binned substance use measures were modeled as ordinal variables with an underlying normal liability using threshold models (delta parameterization), and regressed on age and sex within the model. Ever Used = lifetime number of substances ever tried, Frequency = Frequency of substance use in the past six months, DAV = Dependence/abuse vulnerability: the number of dependence and abuse symptoms per substance used to criterion. Bold indicates p< .05; italics indicate p< .10.
As hypothesized, in late adolescence lower Common EF was significantly associated with more substances used, r= −.31, χ2diff(1)=23.82, p<.001, and more frequent use, r= −.25, χ2diff(1)=14.79, p<.001, but not with dependence/abuse vulnerability, r=.02, χ2diff(1)=04, p=.837. The correlation between Common EF and dependence/abuse vulnerability was significantly smaller than Common EF's correlations with the number of substances used and frequency of use, both χ2diff(1)>9.10, p<.003, but the correlations with the number of substances used and frequency of use were not significantly different from each other, χ2diff(1)=2.95, p=.086. The small correlation between Common EF and dependence/abuse vulnerability suggests that Common EF may play little or no role in dependence/abuse in adolescence. However, only about half of the subjects in this sample had vulnerability scores at this age, because they needed to have used any given substance multiple times to be asked the dependence/abuse questions.
In early adulthood, Common EF was again associated with more substances used, r= −.18, χ2diff(1)=8.33, p=.004. Although smaller in magnitude, this correlation was not significantly smaller than the corresponding correlation between Common EF and the number of substances used in adolescence, χ2diff(1)=2.68, p=.101. The correlation between Common EF and frequency of substance use was only marginally significant in adulthood, r= −.10, χ2diff(1)=2.76, p=.097, but this correlation was also not significantly smaller than the corresponding correlation in adolescence, χ2diff(1)=3.65, p=.056. As in adolescence, Common EF was not significantly associated with dependence/abuse vulnerability in adulthood, r= −.09, χ2diff(1)=1.91, p=.167.
We also examined the association between Common EF and the difference in number of substances used (i.e., the number of substances newly tried) between adulthood and adolescence. This difference score correlated negatively with the number of substances ever used in adolescence, r= −.21, χ2diff(1)=20.48, p<.001, suggesting that individuals who had tried the most substances during adolescence tried fewer new substances by early adulthood. Moreover, Common EF was not associated with the number of new substances used since adulthood, r=.02, χ2diff(1)=.10, p=.752. Therefore, although the association between Common EF and the number of substances used was not significantly smaller in early adulthood than late adolescence, the association in early adulthood was driven primarily by the substances already used by late adolescence.
The correlations shown in Table 3 suggest that adolescent Common EF is related most strongly to the number of substances used and not to dependence/abuse symptoms, confirming our hypothesis that Common EF would be primarily related to early rather than later stages of substance use. We also estimated multiple regression models in which Common EF was predicted by multiple substance use measures to examine what relations would remain significant when controlling for the shared variance across the different substance use measures. The results of these models, reported in the supplement (Section 6), suggest that, even after accounting for variation in either adolescent or adult dependence/abuse, the variance in ever using substances in adolescence is still significantly related to Common EF.
As expected, the Shifting-Specific and Updating-Specific factors were not significantly associated with any of the drug measures in adolescence or in early adulthood.4 Because these other EFs did not reveal any within-wave associations with substance use, and the hypotheses of this study primarily concerned the associations with Common EF, these associations with Shifting-Specific and Updating-Specific are not discussed further and are not described at the genetic level. For more information about results with these other EFs, see the supplement (Table S8 and Figure S3).
Finally, given moderate associations between EFs and intelligence (reported for this sample by Friedman et al., 2008), we performed follow-up analyses to examine whether the key associations between Common EF and substance use remained after controlling for intelligence. In adolescence, the associations between Common EF and the number of substances used and frequency of use remained significant after controlling for scores on the Wechsler Adult Intelligence Scale (administered at age 16), both χ2diff(1)>5.68, p<.017. In early adulthood, the association between Common EF and the number of substances used also remained significant after controlling for scores on a short version of Raven’s Advanced Progressive Matrices (administered during the same testing session as the EF tasks), χ2diff(1)=6.17, p=.013. Thus, the associations between Common EF and substance use were not driven by individual differences in intelligence.
In summary, lower Common EF was associated with more substances ever used and more frequent use in adolescence (mean age 17 years). By early adulthood (mean age 23 years), the correlation with the number of substances used remained significant but the correlation with frequency became nonsignificant. Because Common EF was not predictive of the number of substances newly tried since adolescence, this association between Common EF and the number of substances used in adulthood was due to the number of substances subjects had already used by late adolescence. Moreover, Common EF was not associated with dependence/abuse vulnerability at either age. Taken together, these findings suggest that the association between EF and substance use may be specific to earlier stages of substance use (ever using and frequently using substances vs. dependence/abuse vulnerability), and may be stronger in adolescence than in early adulthood.
Genetic Analyses
Before examining the genetic and environmental etiologies of the phenotypic relationships described above, we first fit models to the longitudinal relations within each individual construct. These models, shown in Figure 2, reveal the magnitude of the genetic (A), shared environment (C), and nonshared environmental (E) influences on each trait at each wave of assessment, and quantify the stability of these genetic and environmental factors over time (i.e., genetic and environmental correlations between the adolescent and adult measures).
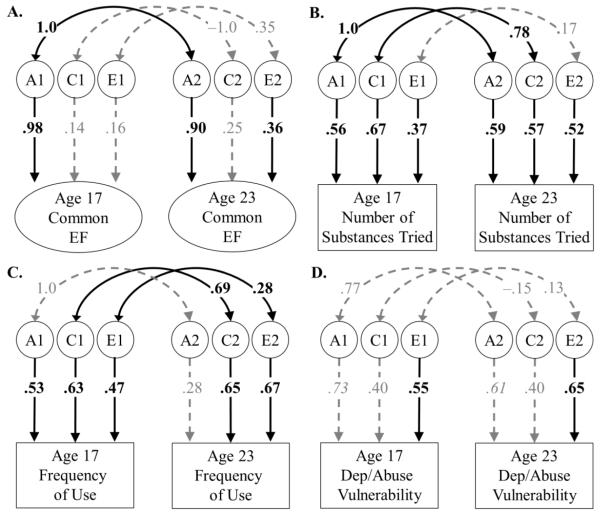
Figure 2.
Correlations between the genetic (A1 and A2), shared environmental (C1 and C2) and nonshared environmental (E1 and E2) variances for Common EF and substance use between late adolescence (mean age 17 years) and early adulthood (mean age 23 years). Each panel displays a separate analysis. In all models, circles indicate latent variables, rectangles indicate observed variables, and significant correlations or factor loadings are displayed in bold and in black text (p<.05). Factor loadings for EF tasks are not displayed, but were all statistically significant. For substance use models (B-D), sex and age were also allowed to predict substance use variables (paths not shown), so the ACE factors do not add up to 1.0 when squared and summed (i.e., the remaining variance is explained by sex and age). Heritability for the age- and sex- regressed measures can be obtained with the following formula: a2/(a2 + c2 + e2). Percent variation explained in each variable can be computed by squaring the factor loadings on their respective genetic/environmental latent variables.
Genetic and environmental influences on each construct individually
As discussed in previous work (Friedman et al., 2016), individual differences in EF abilities were primarily explained by genetic influences at both time points. In late adolescence, genetic factors were the only significant influences on individual differences in Common EF (a2=.96, computed by squaring the factor loading of .98 in Figure 2). In early adulthood, the genetic influences on Common EF were the same (rg=1.0), but Common EF had new significant nonshared environmental influences (e2=.13) and non-significant shared environmental influences (c2=.06), reducing its heritability to .81.5
Unlike the EF latent variables, each of the three aspects of substance use was explained by genetic, shared environmental, and nonshared environmental influences relatively equally, at both time points. As shown in Figure 2, heritability estimates (a2/(a2 + c2 + e2)) were .08 to .53 for measures of substance use. Substance use was also explained by both shared environmental influences (c2=.16−.48) and nonshared environmental influences (e2=.15−.46).
Although substance use behaviors were explained much more by environmental factors than EFs, the genetic variances in the number of substances ever used and frequency of use in adolescence were perfectly correlated with the genetic variances in the same measures in adulthood (rg=1.0). The genetic correlation for dependence/abuse vulnerability was also large, but not statistically significant, rg=.77, χ2diff(1)=2.42, p=.120, likely because of the much smaller sample for this measure in late adolescence. These findings suggest that, as was the case for EFs, the genetic influences on substance use remained highly stable between adolescence and adulthood.
Shared environmental influences were also fairly stable over time for number of substances used (rc=.78) and frequency (rc=.69), but not for dependence/abuse vulnerability (rc=−.15). In contrast, the nonshared environmental influences appeared to be largely specific to each age for all measures of substance use. The only nonshared environmental influence on adolescent substance use that was significantly correlated with its counterpart in adulthood was that for frequency of use (re=.28). Together, these results suggest that the genetic and shared environmental influences on substance use are generally stable over this age range, but the nonshared environmental influences (which include measurement error) are largely transitory across this six-year interval.
Finally, the genetic analyses involving our difference score measure of the number of substances newly tried since adolescence are reported in the supplement (Section 3). Although genetic influences on this difference score were identical to those on the number of substances ever used in adolescence (rg=1.0), the heritability was low and nonsignificant (a2=.14). Because the phenotypic association between this difference score and Common EF was nonsignificant, and because there was no evidence for significant genetic or shared environmental influences on this measure, we did not include this variable in our following genetic analyses.
EFs and substance use
To examine how the genetic and environmental variances for EFs are associated with those for substance use, we fit multivariate twin models. We estimated a separate model for each substance use measure at each age, for a total of six models (e.g., EFs and frequency of use in adolescence, EFs and frequency of use in adulthood, etc.). All six models had acceptable fit; χ2(484–489)<631.51, p>.001, RMSEA<.038, CFI>.914.
The genetic and environmental correlations estimated from these models are shown in Figure 3. The Shifting-Specific and Updating-Specific factors were included because they must be estimated to fit the Common EF factor, but are not pictured because they were not associated with any of the substance use measures at the phenotypic level (see supplement Table S8 for the genetic/environmental correlations involving these factors).6
Figure 3.
Correlations between the genetic (A), shared environmental (C), and nonshared environmental (E) variance components of Common EF and substance use measures in late adolescence (A-C) and early adulthood (D-F). In all models, circles indicate latent variables, rectangles indicate observed variables, and significant correlations or factor loadings are displayed in bold and in black text (p < .05). Each panel displays a separate analysis. Factor loadings on Common EF are not displayed, but were all statistically significant. Sex and age were also allowed to predict substance use variables (paths not shown), so the ACE factors do not add up to 1.0 when squared and summed (i.e., the remaining variance is explained by sex and age). Heritability for the age- and sex-regressed measures can be obtained with the following formula: a2/(a2 + c2 + e2). Genetic/environmental associations between substance use and the Updating- and Shifting-Specific abilities were also estimated in each model, but are also not displayed here (see supplement Table S8). Percent variation explained in each variable can be computed by squaring the factor loadings on their respective genetic/environmental latent variables.
As hypothesized, the genetic influences on Common EF at both ages were negatively correlated with those on substance use behaviors. There were strong negative correlations between the number of substances ever used and Common EF in late adolescence (rg= −.98) and early adulthood (rg= −.95). Similarly large genetic correlations were observed between Common EF and frequency in both adolescence, rg= −.80, χ2diff(1)=4.80, p=.028, and adulthood, rg= −.74, χ2diff(1)=1.55, p=.213, though the estimate was only statistically significant in late adolescence. The genetic influences on dependence/abuse vulnerability were not significantly correlated with Common EF in adolescence (rg= −.22) nor in adulthood (rg= −.61), consistent with the non-significant phenotypic correlations, although the genetic correlation between Common EF and dependence/abuse vulnerability was marginally significant in adulthood, rg= −.61, χ2diff(1)=2.74, p=.097.
In contrast to the genetic correlations between EFs and substance use, none of the environmental correlations between Common EF and the substance use variables were significant. This is not surprising for shared environmental influences, because such influences explain little to no variation in Common EF ability at either time point (see Figure 3). However, even though nonshared environmental influences did significantly predict Common EF in adulthood, those influences did not predict any of the substance use measures at that age.
The total phenotypic relationship between Common EF and each substance use measure can be decomposed into covariance predicted by the genetic and environmental correlations. In adolescence, the genetic correlation of rg= −.98 between Common EF and the number of substances used predicts a phenotypic correlation of r= −.46 when multiplied by the A paths for each trait. However, when that negative phenotypic correlation predicted by the genetic correlation is summed with the small positive phenotypic correlations predicted by the shared environmental (r=.08) and nonshared environmental correlations (r=.07) in the model, the total predicted phenotypic correlation drops to r= −.31, the same estimate as in the phenotypic model (Table 3). In adulthood, the genetic correlation (rg= −.95) and predicted phenotypic correlation due to that genetic correlation (r= −.44) do not change much compared to adolescence (−.98 and −.46, respectively), but the total predicted phenotypic correlation drops to r= −.15 (similar to the estimate of −.18 in the phenotypic model in Table 3) when added to the predicted correlations based on the environmental correlations in adulthood. These decompositions suggest that, across measures of substance use, the negative genetic correlations are entirely responsible for the negative association between Common EF and substance use in the phenotypic analyses presented in Table 3.
Discussion
The current study examined the relationship between EFs and substance use in adolescence and early adulthood. We tested the hypothesis that Common EF is genetically related to polysubstance use, particularly ever using multiple substances in adolescence. Confirming this hypothesis, we found that lower Common EF was associated with the number of substances ever used in late adolescence, and that this association was entirely driven by shared genetic influences. However, there was little evidence that Common EF was associated with dependence and abuse symptoms at either age. These results suggest that individual differences in goal maintenance and management are genetically associated with tendencies to ever use substances by late adolescence, but not with later stages of substance dependence and abuse, at least in this sample not selected for substance dependence and abuse.
This study is the first to investigate how different aspects of polysubstance use behaviors are related to EFs, particularly the common variance across multiple EFs. This study extends prior work by distinguishing dependence/abuse from the number of substances used (in the lifetime) and the frequency of use (in the past six months), which helped reveal what stages of substance use may be most related to Common EF. This is also the first study to examine these associations between EFs and general substance use behaviors using a multiwave design, focusing on these two key time points in the development of EFs and substance use (i.e., late adolescence and early adulthood). Finally, the use of a genetically informative design in this context provides unique information about the nature of phenotypic associations (i.e., genetic and environmental) between substance use and EFs across time.
In addition to showing that the association between EFs and substance use was driven by genetic influences shared between the Common EF factor and the number of substances ever used by late adolescence, these results are relevant to the understanding of the interplay between EF abilities and substance use behaviors more broadly. We focus here on two sets of theoretical implications: (1) those related to the different aspects of substance use genetically associated with EFs (ever using substances, frequency of use, and dependence/abuse vulnerability), and (2) those concerning the lack of strong environmental associations between EFs and substance use at either wave.
EFs and Substances Ever Used vs. Dependence/Abuse Vulnerability
Our findings suggest that the association between EFs and substance use may depend on the aspects of EFs and substance use that are measured. Specifically, we found that Common EF was primarily associated with the number of substances ever used at both ages, and frequency of use in adolescence, but that Common EF was not significantly related to dependence/abuse vulnerability at either wave (Table 3). Further, the association between Common EF and the number of substances used in adulthood appeared to be largely driven by the number of substances tried by late adolescence, rather than new substances tried between late adolescence and adulthood. These results suggest that Common EF may play a larger role in the early aspects of substance use, such as which individuals start using substances in adolescence. The development of problem use, beyond its relation to trying multiple substances, does not appear to be related to Common EF.
These results are consistent with existing theoretical perspectives on the association between EFs and substance use, such as the view of low EFs as a genetic risk for substance use (Giancola & Tarter, 1999), particularly trying substances. More broadly, these results are consistent with the view that that EFs are related to a general tendency to engage in externalizing behaviors (Caspi et al., 2014; Young et al., 2009), rather than a specific risk for substance use. For example, prior research on this sample (using the data in late adolescence) has revealed that prepotent response inhibition, which is isomorphic with the Common EF factor, was associated with a general latent Behavioral Disinhibition factor comprised of the number of substances used, conduct disorder, attention-deficit/hyperactivity disorder, and novelty seeking (Young et al., 2009). The results presented here extend this work by suggesting that the role of Common EF is strongest for the number of substances ever used during adolescence.
We included the measure of the lifetime number of substances ever used as a rough measure of an early stage of use, but we acknowledge that it does not capture all aspects of early use. In particular, it does not capture the extent of use (i.e., frequency and quantity). However, we believe that in the context of the other substance use measures we included (past six-month frequency of use and dependence/abuse symptoms), it provides information about the specificity of the relation between Common EF and early stages of use. That is, the number of substances ever used could be related to Common EF because it captures a tendency to try more substances or because individuals who try more substances also use them more frequently and eventually become dependent. But when we examined measures of dependence/abuse, they were less related to Common EF. That pattern suggests that it is the variance in the number of substances ever used in adolescence that is independent of dependence/abuse that is related to Common EF, a possibility confirmed with multiple regression.
The lack of association between Common EF and dependence/abuse is particularly interesting given that substance dependence and abuse is characterized by a loss of control and compulsive drug-seeking (George & Koob, 2010). Common EF is thought to draw on important all-purpose control mechanisms that help guide and regulate behavior (Friedman & Miyake, 2016; Miyake & Friedman, 2012). Given these findings, however, the aspects of self-control captured by Common EF may be different from those involved in the development of dependence. In particular, George and Koob (2010) discuss a number of aspects of prefrontal cortical control (over stress, anxiety, reward, pain, habits, and decision making) that could lead to the progression from initiation to addiction. Moreover, they suggest that dysfunction in different brain modules may be specific to particular stages of addiction. Our results suggest that Common EF, which may correspond most closely to their concept of “loss of control over habits and decision making” (p. 242), is most related to the choice to begin substance use rather than the tendency to escalate use. The loss of control related to the transition to dependence/abuse seems to be related to other non-EF neurobiological and environmental changes.
This possibility is consistent with Hines et al.’s (2015) hypothesis that substance experimentation is genetically linked with novelty seeking and risk taking, but that the progression to heavy use and dependence/abuse is related to other genetic factors that may be more substance-specific (e.g., those that influence the subjective effects of substances or the metabolic pathways involved in responses to drugs). In fact, Incentive Sensitization Theory also suggests that hypersensitization to drugs and drug cues (e.g., in the dopamine system) can also explain use in addiction (Robinson & Berridge, 1993). Finally, these effects may also be consistent with the allostatic model of addiction, which highlights the role of withdrawal-based negative affect and the dysregulation of the reward system in dependence and addiction (Koob & Le Moal, 1997). Nevertheless, while the biological and environmental mechanisms associated with addiction in adolescence and early adulthood appear to be unique from the cognitive EFs we assessed here, it is possible that these changes affect EF neural systems later in adulthood, after individuals have been addicted for a longer time.
We were able to see these patterns because our methods differed from previous work on dependence vulnerability (Button et al., 2006; Stallings et al., 2003) by excluding individuals who had never used any substances, thereby distinguishing ever use from dependence/abuse. Had we assigned a score of zero to these individuals, we would have observed a correlation between Common EF and dependence/abuse vulnerability due to the number of substances ever used. Therefore, these methods and results serve to inform the general genetic/environmental associations between EFs and substance use at multiple waves, and may be useful in future work designed to disentangle the cognitive (and/or social and personality) factors involved in the transition from ever using substances to dependence.
Finally, although our primary hypotheses concerned Common EF, we also examined the associations between Shifting-Specific, Updating-Specific, and substance use. As expected, these aspects of EF were largely unrelated to general substance use behaviors, though we observed significant positive associations between Shifting-Specific in late adolescence and the number of substances used (r=.20) and frequency of use in early adulthood (r=.17). It is unclear if these cross-wave associations reflect real effects as they were not observed within-wave, but they are consistent with recent work linking better Shifting-Specific with other negative outcomes including higher Behavioral Disinhibition, more procrastination, and lower intelligence (Gustavson et al., 2015; Herd et al., 2014; Miyake & Friedman, 2012). Such patterns have been explained as reflecting a stability-flexibility tradeoff between Common EF, hypothesized to reflect the strength of goal maintenance, and Shifting-Specific abilities, hypothesized to reflect the speed of goal disengagement or replacement (Friedman & Miyake, 2016; Herd et al., 2014).
Furthermore, we observed that better Updating-Specific in adolescence weakly predicted more substances ever used by in early adulthood (r=.14). This association was also not expected, but is consistent with one recent study in which better working memory updating was associated with more alcohol use in individuals who also had greater approach tendencies (Patrick, Blair, & Maggs, 2008).
Absence of Environmental Association Between Common EF and Substance Use
We hypothesized that the associations between Common EF and substance use would be primarily genetic in origin, given the high heritability of Common EF at both waves (Friedman et al., 2008, 2016), and recent work on the general genetic liability to use substances (Hopfer et al., 2003; Kendler et al., 2003; McGue et al., 2000; Palmer et al., 2009; Rhee et al., 2003). However, given that nonshared environmental influences on Common EF emerge in adulthood (Friedman et al., 2016), these environmental influences could have correlated with those environmental influences on the number of substances used, the frequency of substance use, or dependence/abuse vulnerability. However, we did not observe any such environmental associations; instead, the environmental correlations between Common EF and substance use measures were not significant, and were nominally positive rather than negative. Though this is a null result, we believe that it has some important implications for the link between EFs and substance use and misuse.
Though these correlational data cannot be used to draw strong causal inferences, the lack of significant negative environmental correlations between Common EF and substance use is inconsistent with a simple causal model that assumes that substance use causes lower EFs. As proposed by Plomin and Haworth (2010), twin models allow one to examine whether an association persists after controlling for unmeasured confounds (specifically, genetic and shared environmental influences). More specifically, the absence of within-wave E correlations in our data suggests that, at both ages, twin differences in histories of substance use until that point (lifetime in the case of ever used and past 6 months in the case of frequency of use) were not associated with twin differences in Common EF at that point.7 Although measurement error can limit the power to detect these E correlations (our measures of substance use were not free from measurement error because they were not estimated at the latent variable level), all of the nonshared environmental correlations between Common EF and substance use were not only nonsignificant, but, if anything, were estimated in the positive direction. Such findings seem inconsistent with the causal pattern that one would expect for the hypothesis that substance use causes lower Common EF.
Moreover, the phenotypic correlation between Common EF and substance use actually decreased over time despite the fact that individuals were using substances more frequently in adulthood (although this decrease in phenotypic correlation was not significant). Of course, we may not have sampled enough heavy drug users to see strong cognitive effects here. Nevertheless, these data are more consistent with a model in which genetic influences predispose individuals for both low EFs and more substance experimentation (Giancola & Tarter, 1999), or externalizing behaviors more generally (Caspi et al., 2014; Young et al., 2009), rather than one in which there is a causal effect of substance use on EFs (or vice versa).
We were able to better understand these nonshared environmental relations between EFs and substance use because we examined EFs at the level of latent variables. Choosing a single EF task would have reduced our ability to differentiate Common EF from processes specific to other EFs (e.g., Updating-Specific and Shifting-Specific), and would have inflated our estimate of nonshared environmental influences on EFs (due to measurement error), masking the genetic correlation and further reducing the phenotyptic correlation. Therefore, future work on the associations between EFs and substance use would continue to benefit from modeling these associations at the level of latent variables, in genetically informative samples, and using longitudinal data that may further inform these associations at other times in the lifespan.
Limitations
Individual substances likely have their own unique associations with EFs above and beyond a common liability to use many substances. We could not investigate substance-specific effects due to low endorsement for most categories in this population sample. However, the phenotypic analyses described in the supplement (Table S3) suggest that the associations were at least not driven strongly by the most frequently used substances (tobacco and alcohol), because relationships remained the same when those substances were removed. Future work using larger samples of specific substance users will be fruitful in further elucidating the cognitive control processes related to use of particular substances.
The conclusions are also limited by the sample and measures available. This sample was drawn from the general population, but associations between EFs and substance use may be different in a sample with more individuals above a clinical threshold. This may be especially true for dependence/abuse vulnerability, as a clinical sample would have a larger range of dependence and abuse symptoms. Moreover, in adolescence, the number of substances ever used was empirically indistinguishable from frequency of use because the two measures were so highly correlated. In addition, although we measured EFs with multiple tasks and examined EFs at the level of latent variables (thereby reducing measurement error), substance use was measured with manifest variables binned into three to five categories because of non-normality. These measures still captured substance use across many substances, but also included measurement error. It is therefore likely that we underestimated some of the associations between EFs and substance use described here, perhaps particularly with respect to the nonshared environmental influences, which in twin models include such measurement error.
Finally, we were able to explore the dynamic relationship between EFs and substance use across time, but only two time points is not enough to model individual differences in trajectories (e.g., growth curves). Future research on the association between EFs and substance use in the general population will benefit from growth and transition models, which can more directly address questions related to the time course of this association (e.g., how do EFs influence individual differences in transitions from ever trying substances to frequent use and from frequent use to dependence?).
Concluding Remarks
This study is the first to examine the relations between EFs and substance use behaviors across multiple aspects of substance use (i.e., number of substances used, frequency of substance use, and dependence/abuse vulnerability), and at two key stages in life for both EFs and substance use (i.e., late adolescence and early adulthood). Results indicated that Common EF was most strongly associated with the number of substances used and frequency of use in adolescence. Moreover, these relations are explained by genetic factors. These findings lead us to conclude that low Common EF is a genetic risk factor for early aspects of substance use in adolescence (i.e., ever using substances) rather than the subsequent development of dependence and abuse in adulthood.
Supplementary Material
General Scientific Summary.
This research examines how multiple aspects of substances use behaviors (ever using substances, frequency of use, and dependence/abuse vulnerability) in late adolescence and early adulthood are associated with executive functions, important goal-related cognitive abilities that control and regulate behavior. We found that lower general executive function ability was associated primarily with the number of substances ever used but not with dependence/abuse, and this association was strongest in late adolescence. Moreover, this association was entirely due to shared genetic influences. Lower executive function abilities may be a genetic risk factor for increased polysubstance use in late adolescence, but non-executive factors may play a larger role in the progression to substance dependence/abuse.
Acknowledgments
This research was supported by Grants MH063207, MH016880, DA035804, DA011015, and AG046938 from the National Institutes of Health.
The authors would like to thank Sally Ann Rhea for her assistance with data collection and study coordination.
Footnotes
“Bifactor” does not refer to the number of factors, but to the structure of the model (i.e., a common factor and specific factors at the same level of hierarchy).
The bin for zero included 34 individuals who reported ever using fewer substances in adulthood than adolescence, likely due to errors in self-reported memory of early use. Removing these individuals from this difference measure altogether did not affect the results, so they are included here. Furthermore, this difference score measure excludes 64 subjects who participated in adulthood but not adolescence, because their difference score could not be computed (see supplement Section 1 for information about these cases).
Button et al. (2006) and Stallings et al. (2003) assigned dependence vulnerability scores of zero to people who did not use any substance to criterion. We diverge from this prior work because we are also examining the number of substances ever used (see supplement Section 4 for more information about the effects of including these censored cases). Although these data were collected using DSM-IV criteria, this dependence/abuse vulnerability score should reflect more recent collapsing of dependence and abuse symptoms in the DSM-V.
We focus on the within-wave correlations, but Table 3 also shows cross-wave correlations, some of which were significant: Of note, Shifting-Specific ability in adolescence was positively correlated with the number of substances ever used and frequency of use in adulthood and Updating-Specific in adolescence was positively correlated with the number of substances ever used in adulthood.
Although the shared environmental influences on Common EF in young adulthood were perfectly negatively correlated with the shared environmental influences on Common EF in adolescence (rc= –1.0), this nonsignificant correlation, χ2diff(1)=.23, p=.631 should not be interpreted given that shared environmental influences accounted for so little variance in Common EF, particularly in adolescence (c2= .02 to .06).
In all decompositions, shared environmental variances (C) for Shifting-Specific and Updating-Specific factors were estimated at zero, so were fixed to zero in these models to aid convergence, as were all of the EF task-specific shared environmental variances. The shared environmental variances for Common EF, though not significant, were not zero in the longitudinal model presented in Friedman et al. (2016), so these were freely estimated and allowed to predict the substance use measures to obtain unbiased estimates of the genetic correlations. Because the Updating-Specific E variance was also estimated at zero in adolescence, we left this variance component in the model, but did not allow it to predict substance use measures (in adolescence only).
Discordant twin designs have been used to test whether differences in exposure within twin pairs are related to differences in outcome, with the non-exposed twin being a matched control (in terms of genetic and shared environmental influences) for the exposed twin (McGue, Osler, & Christensen, 2010). When this procedure is applied to cases in which exposure is a continuous variable, as it is in the current study, Plomin and Haworth (2010) recommended using a full multivariate twin model of the exposure (substance use) and outcome (Common EF). In such a model, the differences between twins are captured by the nonshared environmental (E) components and the relations between these differences with the nonshared environmental correlation (re)
Prior dissemination of the data: These findings were presented by Naomi P. Friedman as an oral presentation at the 45th annual meeting of the Behavioral Genetics Association in June of 2015.
References
- Button TM, Hewitt JK, Rhee SH, Young SE, Corley RP, Stallings MC. Examination of the causes of covariation between conduct disorder symptoms and vulnerability to drug dependence. Twin Research and Human Genetics. 2006;9:38–45. doi: 10.1375/twin.9.1.38. [DOI] [PubMed] [Google Scholar]
- Caspi A, Houts RM, Belsky DW, Goldman-Mellor SJ, Harrington H, Israel S, Moffitt TE. The p factor: One general psychopathology factor in the structure of psychiatric disorders? Clinical Psychological Science. 2014;2:119–137. doi: 10.1177/2167702613497473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality Behavioral health trends in the United States: Results from the 2014 National Survey on Drug Use and Health. 2015 (HHS Publication No. SMA 15-4927, NSDUH Series H-50). Retrieved from http://www.samhsa.gov/data/
- Coolidge FL, Thede L,L, Young SE. Heritability and the comorbidity of attention deficit hyperactivity disorder with behavioral disorders and executive function deficits: A preliminary investigation. Developmental Neuropsychology. 2000;17:273–287. doi: 10.1207/S15326942DN1703_1. [DOI] [PubMed] [Google Scholar]
- Cottler LB, Robins LN, Helzer JE. The reliability of the CIDI - SAM: A comprehensive substance abuse interview. British Journal of Addiction. 1989;84:801–814. doi: 10.1111/j.1360-0443.1989.tb03060.x. [DOI] [PubMed] [Google Scholar]
- Day AM, Kahler CW, Ahern DC, Clark US. Executive functioning in alcohol use studies: A brief review of findings and challenges in assessment. Current Drug Abuse Reviews. 2015;8:26–40. doi: 10.2174/1874473708666150416110515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derks EM, Dolan CV, Boomsma DI. Effects of censoring on parameter estimates and power in genetic modeling. Twin Research. 2004;7:659–669. doi: 10.1375/1369052042663832. doi:10/1375/twin.7.6.659. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Meyerhoff DJ. The neurobiological and neurocognitive consequences of chronic cigarette smoking in alcohol use disorders. Alcohol and Alcoholism. 2007;42:174–185. doi: 10.1093/alcalc/agm020. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Clark L, London M, Robbins TW, Sahakian B. Profile of executive and memory function associated with amphetamine and opiate dependence. Neuropsychopharmacology. 2006;31:1036–1047. doi: 10.1038/sj.npp.1300889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP, Miyake A. Unity and diversity of executive functions: Individual differences as a window on cognitive structure. Cortex. 2016 doi: 10.1016/j.cortex.2016.04.023. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Altamirano LJ, Corley RP, Young SE, Rhea SA, Hewitt JK. Stability and change in executive function abilities from late adolescence to early adulthood: A longitudinal twin study. Developmental Psychology. 2016;52:326–340. doi: 10.1037/dev0000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Young SE, DeFries JC, Corley RP, Hewitt J. Individual differences in executive function are almost entirely genetic in origin. Journal of Experimental Psychology: General. 2008;137:201–225. doi: 10.1037/0096-3445.137.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Koob GF. Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neuroscience and Biobehavioral Reviews. 2010;35:232–247. doi: 10.1016/j.neubiorev.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancola PR, Tarter RE. Executive cognitive functioning and risk for substance abuse. Psychological Science. 1999;10:203–205. [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Rapoport JL. Brain development during childhood and adolescence: A longitudinal MRI study. Nature Neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gustavson DE, Miyake A, Hewitt JK, Friedman NP. Understanding the cognitive and genetic underpinnings of procrastination: Evidence for shared genetic influences with goal-management and executive function abilities. Journal of Experimental Psychology: General. 2015;144:1063–1079. doi: 10.1037/xge0000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Garavan H. Executive dysfunction in cocaine addiction: Evidence for discordant frontal, cingulate, and cerebellar activity. The Journal of Neuroscience. 2004;24:11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herd SA, O’Reilly RC, Hazy TE, Chatham CH, Brant AM, Friedman NP. A neural network model of individual differences in task switching abilities. Neuropsychologia. 2014;62:375–389. doi: 10.1016/j.neuropsychologia.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines LA, Morley KI, Mackie C, Lynskey M. Genetic and environmental interplay in adolescent substance use disorders. Current Addiction Reports. 2015;2:122–129. doi: 10.1007/s40429-015-0049-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfer CJ, Crowley TJ, Hewitt JK. Review of twin and adoption studies of adolescent substance use. Journal of the American Academy of Child & Adolescent Psychiatry. 2003;42:710–719. doi: 10.1097/01.CHI.0000046848.56865.54. [DOI] [PubMed] [Google Scholar]
- Hu L, Bentler PM. Fit indices in covariance structure modeling: Sensitivity to underparameterized model misspecification. Psychological Methods. 1998;3:424–453. doi: 10.1037/1082-989X.3.4.424. [DOI] [Google Scholar]
- Ito TA, Friedman NP, Bartholow BD, Correll J, Loersch C, Altamirano LJ, Miyake A. Toward a comprehensive understanding of executive cognitive function in implicit racial bias. Journal of Personality and Social Psychology. 2015;108:187. doi: 10.1037/a0038557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Jacobson KC, Prescott CA, Neale MC. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. American Journal of Psychiatry. 2003;160:687–695. doi: 10.1176/appi.ajp.160.4.687. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: Hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Luciana M, Conklin HM, Hooper CJ, Yarger RS. The development of nonverbal working memory and executive control processes in adolescents. Child Development. 2005;76:697–712. doi: 10.1111/j.1467-8624.2005.00872.x. [DOI] [PubMed] [Google Scholar]
- McGue M, Elkins I, Iacono WG. Genetic and environmental influences on adolescent substance use and abuse. American Journal of Medical Genetics. 2000;96:671–677. doi: 10.1002/1096-8628(20001009)96:5<671::AID-AJMG14>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- McGue M, Osler M, Christensen K. Causal inference and observational research: The utility of twins. Perspectives on Psychological Science. 2010;5:546–556. doi: 10.1177/1745691610383511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP. The nature and organization of individual differences in executive functions: Four general conclusions. Current Directions in Psychological Science. 2012;21:8–14. doi: 10.1177/0963721411429458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss HB, Chen CM, Yi HY. Early adolescent patterns of alcohol, cigarettes, and marijuana polysubstance use and young adult substance use outcomes in a nationally representative sample. Drug and Alcohol Dependence. 2014;136:51–62. doi: 10.1016/j.drugalcdep.2013.12.011. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. 7th ed Muthén & Muthén; Los Angeles, CA: 1998-2012. [Google Scholar]
- Nigg JT, Wong MM, Martel MM, Jester JM, Puttler LI, Glass JM, Zucker RA. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. Journal of the American Academy of Child & Adolescent Psychiatry. 2006;45:468–475. doi: 10.1097/01.chi.0000199028.76452.a9. [DOI] [PubMed] [Google Scholar]
- Palmer RHC, Young SE, Hopfer CJ, Corley RP, Stallings MC, Crowley TJ, Hewitt JK. Developmental epidemiology of drug use and abuse in adolescence and young adulthood: Evidence of generalized risk. Drug and Alcohol Dependence. 2009;102:78–87. doi: 10.1016/j.drugalcdep.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick ME, Blair C, Maggs JL. Executive function, approach sensitivity, and emotional decision making as influences on risk behaviors in young adults. Journal of Clinical and Experimental Neuropsychology. 2008;30:449–462. doi: 10.1080/13803390701523109. [DOI] [PubMed] [Google Scholar]
- Pharo H, Sim C, Graham M, Gross J, Hayne H. Risky business: Executive function, personality, and reckless behavior during adolescence and emerging adulthood. Behavioral Neuroscience. 2011;125:970. doi: 10.1037/a0025768. [DOI] [PubMed] [Google Scholar]
- Plomin R, Haworth CM. Genetics and intervention research. Perspectives on Psychological Science. 2010;5:557–563. doi: 10.1177/1745691610383513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhea SA, Gross A, Haberstick BC, Corley RP. Colorado Twin Registry: An update. Twin Research and Human Genetics. 2013;16:351–357. doi: 10.1017/thg.2012.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SH, Hewitt JK, Young SE, Corley RP, Crowley TJ, Stallings MC. Genetic and environmental influences on substance initiation, use, and problem use in adolescents. Archives of General Psychiatry. 2003;60:1256–1264. doi: 10.1001/archpsyc.60.12.1256. [DOI] [PubMed] [Google Scholar]
- Roberts CA, Jones A, Montgomery C. Meta-analysis of executive functioning in ecstasy/polydrug users. Psychological Medicine. 2016;46:1581–1596. doi: 10.1017/S0033291716000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Research Reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-P. [DOI] [PubMed] [Google Scholar]
- Salomonsen-Sautel S, Sakai JT, Thurstone C, Corley R, Hopfer C. Medical marijuana use among adolescents in substance abuse treatment. Journal of the American Academy of Child & Adolescent Psychiatry. 2012;51:694–702. doi: 10.1016/j.jaac.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR, Miyake A, Hankin BL. Advancing understanding of executive function impairments and psychopathology: Bridging the gap between clinical and cognitive approaches. Frontiers in Psychology. 2015;6:328. doi: 10.3389/fpsyg.2015.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, Vendetti J. Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA. 2002;287:1123–1131. doi: 10.1001/jama.287.9.1123. [DOI] [PubMed] [Google Scholar]
- Stallings MC, Corley RP, Hewitt JK, Krauter KS, Lessem JM, Mikulich SK, Crowley TJ. A genome-wide search for quantitative trait loci influencing substance dependence vulnerability in adolescence. Drug and Alcohol Dependence. 2003;70:295–307. doi: 10.1016/S0376-8716(03)00031-0. [DOI] [PubMed] [Google Scholar]
- Verdejo-García A, Bechara A, Recknor EC, Perez-Garcia M. Executive dysfunction in substance dependent individuals during drug use and abstinence: An examination of the behavioral, cognitive and emotional correlates of addiction. Journal of the International Neuropsychological Society. 2006;12:405–415. doi: 10.1017/s1355617706060486. doi:10/1017/S1355617706060486. [DOI] [PubMed] [Google Scholar]
- Young SE, Friedman NP, Miyake A, Willcutt EG, Corley RP, Haberstick BC, Hewitt JK. Behavioral disinhibition: Liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. Journal of Abnormal Psychology. 2009;118:117–130. doi: 10.1037/a0014657. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.