Abstract
Objectives
We investigated the changes in adherens-junction-proteins, such as vascular endothelial (VE)-cadherin and β-catenin of skeletal muscle and vessels in patients with or without diabetes in the setting of CPB and cardiac surgery.
Methods
Skeletal muscle tissue samples were harvested pre- and post-CPB from non-diabetic (ND) (HgbA1c: 5.4 ± 0.1), controlled diabetic (CDM) (HgbA1c: 6.3 ± 0.1), and uncontrolled diabetic patients (UDM) (HgbA1c: 9.6 ± 0.3) undergoing CABG surgery (n = 8/group). The expression/phosphorylation of adherens-junction-proteins VE-cadherin and β-catenin were assessed by immunoblotting and immuno-histochemistry. Endothelial function of skeletal muscle arterioles was determined by videomicroscopy in response to the vasodilator substance P.
Results
The protein expression of total VE-cadherin was not changed at baseline or between pre- and post-CPB among groups. The pre-CPB level of phospho-VE-cadherin was found to be significantly increased in the UDM group compared with the ND or CDM groups (P <0.05). The post-CPB levels of phospho-VE-cadherin were significantly increased as compared with pre-CPB in all groups (P <0.05 each), and this increase was greater in the UDM group than that of the ND or CDM groups (P<0.05). Expression of basal β-catenin protein in UDM group was decreased as compared with ND or CDM (P<0.05). There were significant decreases in the β-catenin protein expression between pre- and post-CPB in all three groups (P<0.05 each) and this decrease was greater in the UDM group than the ND group (P<0.05). There were decreases in the relaxation response of skeletal muscle arterioles to substance P after CPB in all three groups (P<0.05), and this alteration was more pronounced in the UDM patients (P<0.05).
Conclusion
Uncontrolled diabetes causes inactivation and reduction in the expression of endothelial adherens-junction-protein in the arterioles of skeletal muscle early after CPB. The enhanced phosphorylation of VE-cadherin and degradation of β-catenin indicate deterioration of these proteins and damage of the cell-cell endothelial junctions, specifically in the diabetic peripheral vessels. These alterations may contribute to the increases in peripheral vascular permeability and endothelial dysfunction.
Keywords: Cardiopulmonary Bypass, Coronary artery bypass grafting, Adherens-Junction-Proteins, Diabetes, Vascular Permeability, Microvascular Endothelial Function
INTRODUCTION
Cardiopulmonary bypass (CPB) is often associated with increased vascular permeability, microvascular endothelial cell injury/dysfunction, and decreased peripheral vasomotor tone, manifested as postoperative systemic hypotension and tissue edema.1–5 In particular, these disturbances are more pronounced in the uncontrolled diabetic patients.1–5 These effects can attribute to CPB-induced systemic inflammation, and increased expression of vascular endothelial growth factor (VEGF) and vascular permeability factor (VPF).1,6,7 These alterations may contribute to an increased duration of stay and worse outcomes in uncontrolled diabetic patients after cardiac surgery.1,6–8 The mechanisms responsible for CPB-initiated peripheral vascular permeability need to be further defined. Recently, we demonstrated that the increased permeability after cardioplegic ischemia/reperfusion is associated with changes in the expression/phosphorylation of adherens-junction-proteins of the coronary microvasculature in uncontrolled diabetic patients.8 Therefore, we hypothesized that diabetes may also cause down-regulation of adherence-junction-proteins in peripheral tissues such as the arterioles of skeletal muscle early after CPB. Thus, the aims of the current study were to determine the role of diabetes in expression of selected proteins [vascular endothelial (VE)-cadherin and β-catenin] in adherens-junctions in human skeletal muscle and peripheral microvasculature, and to investigate the effects of diabetes on arteriolar endothelial function in the setting of CPB and cardiac surgery.
METHODS
Human Subjects and Tissue Harvesting
Samples of skeletal muscle from the left intercostal muscle bed were harvested pre- and post-CPB from 100 patients undergoing coronary artery bypass grafting (CABG). Hemoglobin A1C (HgbA1c) was measured in all patients. The patients were divided into the following three groups: 1) patients with a normal HgbA1c and no history or treatment for diabetes were considered non-diabetic (ND); 2) patients with a history of diabetes with a HgbA1c >5.5 but ≤ 7 were considered well-controlled (CDM); and 3) diabetic patients with a HgbA1c ≥ 8.5 were considered uncontrolled (UDM). Patients who also underwent valve surgery were excluded from the study. Although there is no definitive, HgbA1c level that is universally accepted as a marker for poorly controlled diabetes, an HgbA1C of 7 or less is regarded generally as a marker of well-controlled diabetes, and HgbA1C greater than 7 is generally regarded as less well-controlled; an HgbA1C of 8.5 is indicative of poorly controlled diabetes. Eight randomly chosen patients in each of 3 groups from 100 cases were included for analysis in this study. The first sample of skeletal muscle in the left intercostal muscle bed was harvested after cannulation before CPB (pre-CPB), and the second sample of skeletal muscle was obtained after removal of the aortic cross-clamp and weaning from CPB (post-CPB).2 Tissue samples were frozen immediately in liquid nitrogen or stored immediately in 10% formalin for immunoblot analysis and immunofluorescent staining. Tissue samples for microvascular reactivity were stored immediately in cold (5° to 10°C) Krebs buffer solution.2 All procedures were approved by the Institutional Review Board of Rhode Island Hospital, Alpert Medical School of Brown University, and informed consent was obtained from all enrolled patients.
Immunoblot
The methods for tissue protein purification, Western blotting and imaging quantification have been described previously.2–5,8 Membranes were incubated overnight at 4°C with primary antibodies against VE-cadherin (Cell Signaling, Danvers, MA), phospho-VE-cadherin (Y685) and β-catenin (ABCAM, Cambridge, MA). After washing with TBST, membranes were incubated with the appropriate secondary antibody conjugated to horseradish peroxidase. All membranes were also incubated with GAPDH (glyceraldehyde-3-phosphate, Cell Signaling) for loading controls.
Immunofluorescence Microscopy
The detailed methods have been described previously.2–4,8 After PBS wash, tissue sections of skeletal muscle were incubated overnight with anti-phospho-VE-cadherin antibody (LifeSpan BioScience, Inc. Seattle, WA) and /or anti- smooth muscle α-actin antibody at 4°C (Cell Signaling). The tissue sections were finally mounted with VECTASHIELD Mounting Medium with DAPI (4',6-diamidino-2-phenylindole) (Vector Laboratories, Burlingame, CA).
Microvessel Reactivity
Arterial microvessels (100–180 µm internal diameters, n = 8/group) were dissected from skeletal muscle samples taken pre- and post-CPB. Microvessel studies were performed in vitro in a pressurized (40 mmHg) no-flow state using video-microscopy as previously described.1,12,13 The vessel was pre-contracted with thromboxane analog U46619 (3×10−6 M to 10−7 M) to achieve 30–40% of baseline diameter. Substance P (10−12 M to 10−7 M) was added to the organ bath and diameter measurements were taken. We have determined previously that the response of human coronary and peripheral arterioles to substance P is endothelium-dependent2–4,8
Chemicals
U46619 and substance P were purchased from Sigma-Aldrich (St. Louis, MO).
Data Analysis
All statistical analysis were performed with GraphPad Software (GraphPad Software, Inc, San Diego, CA). Data are expressed as the mean ± SEM. For analysis of data from patient characteristics, protein expression and other imaging, Kruskal-Wallis tests with Dunn's multiple test were performed. For analysis of categorical data, Fisher’s exact test was employed. For analysis of microvessel data, two way repeated-measures ANOVA with a post hoc Bonferroni test were performed. P values less than 0.05 were considered significant.
RESULTS
Patient Characteristics
The characteristics of the 24 enrolled patients are shown in Table 1. All patients with pre-operative hypertension were treated with anti-hypertensive medications (β-blocker, or calcium channel blocker, or angiotensin-converting enzyme inhibitor). The levels of pre-operative blood HgbA1c were 5.4 ± 0.1 in the ND patients, 6.3 ± 0.1 in the CDM patients, and 9.3 ± 0.3 in the UDM patients (P<0.05).
Table 1.
Patient Characteristics
| Patient Characteristics | Non- diabetes |
Controlled Diabetes |
Uncontrolled Diabetes |
P values |
|---|---|---|---|---|
| HgbA1c (%)* | 5.4 ± 0.1 | 6.3 ± 0.1 | 9.6 ± 0.3# | 0.0001 |
| Age (y)* | 66 ± 8 | 68 ± 9 | 65± 9 | 0.96 |
| Male/Female (n) | 6/2 | 6/2 | 7/1 | 1.0 |
| Hypertension (n) | 8 | 8 | 8 | 1.0 |
|
Hypercholestesterolemia (n) |
8 | 8 | 8 | 1.0 |
| Obesity (BMI>30) | 0 | 2 | 2 | 0.96 |
| Atrial fibrillation (n) | 0 | 0 | 0 | 1.0 |
|
Patient Blood Glucose (mg/dL, Pre-CPB)* |
107 ± 4 | 130 ± 11 | 214 ± 16# | 0.0007 |
|
Patient Blood Glucose (mg/dL, During-CPB)* |
148 ± 8.0 | 150 ± 10 | 199 ± 15# | 0.004 |
|
Pre-operative Insulin (n) (u/h*) |
0 | 4(1.4 ± 1.2) | 8 (5.5± 1.0) # | 0.001 |
|
Intra-operative Insulin (n) (u/h*) |
2 (1.33 ± 1.21) |
5 (5.5 ± 1.9) |
8 (21.7 ± 7)# | 0.007 |
| Duration of CPB (min)* | 125 ± 12 | 128 ± 15 | 130 ± 20 | 0.98 |
| Cross-clamp time (min)* | 108 ± 11 | 105 ± 16 | 110 ± 18 | 0.9 |
| CABG only (n) | 8 | 8 | 8 | 1.0 |
|
No. of Grafts Performed (n) |
3 | 3 | 3 | 1.0 |
BMI: body mass index; HgbA1c: hemoglobin A1c; CABG: coronary artery bypass grafting;
Data expressed as mean ± SD.
vs. non-diabetics.
Effect of CPB on Levels of VE-cadherin, Phospho-VE-cadherin, and β-catenins
The protein expression of total VE-cadherin was not changed at baseline or between pre- and post-CPB among groups (Figure 1A and Figure 1B). The pre-CPB level of phospho-VE-cadherin was found to be significantly increased in the UDM group compared with the ND or CDM groups (P = 0.001, Figure 1C and Figure 1D). The post-CPB levels of phospho-VE-cadherin were significantly increased as compared with pre-CPB in all groups (ND: P ≤0.03 each). This increase was greater in the UDM group than that of the ND or CDM groups (P < 0.05, Figure 1C and Figure D). Before CPB, the total amount of β-catenin protein was found to be unchanged between ND and CDM groups. (Figure 2A and Figure 2B). Expression of basal β-catenin protein in UDM group was decreased as compared with ND or CDM (P<0.05). There were significant decreases in the β-catenin protein expression between pre- and post-CPB in all three groups (P<0.05 each). This decrease was greater in the UDM group than the ND group (P<0.05, Figure 2).
Figure 1.
Expression of total VE-cadherin and phospho-VE-cadherin (Y685) in human skeletal muscle pre- and post-cardiopulmonary bypass (CPB). A and B: There were no differences in total VE-cadherin protein expression in the human skeletal muscle harvested pre- and post-CPB; C and D: There were significant increases in expression of phospho-VE-cadherin (Y685) after CPB among three groups; these effects were more pronounced in UDM group compared with ND or CDM, *P < 0.05 vs. pre-CPB; @P < 0.05 vs. ND- or CDM-pre-CPB ; #P < 0.05 vs. ND- or CDM-post-CPB; Mean ± SEM, n = 8/group.
Figure 2.
Expression of β-catenin protein in harvested human skeletal muscle. There were significant decreases in β-catenin post-CPB among three groups. *P<0.05 vs. pre-CPB; @P < 0.05 vs. ND- or CDM-pre-CPB; #P < 0.05 vs. ND- or CDM-post-CPB; Mean ± SEM, n = 8/group.
Vascular Distribution of Phospho-VE-cadherin
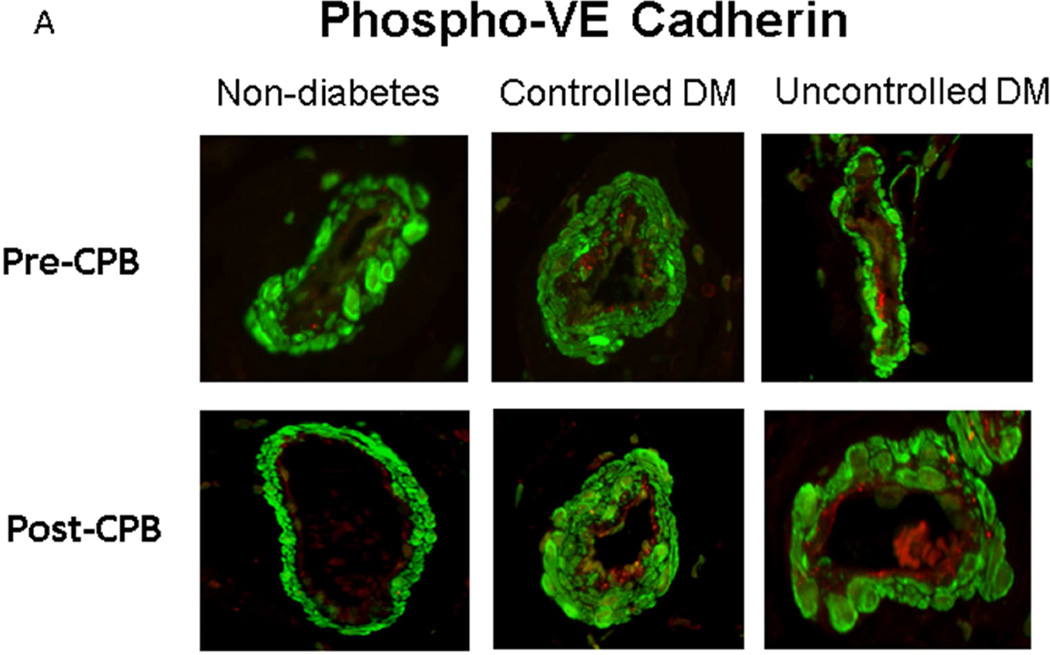
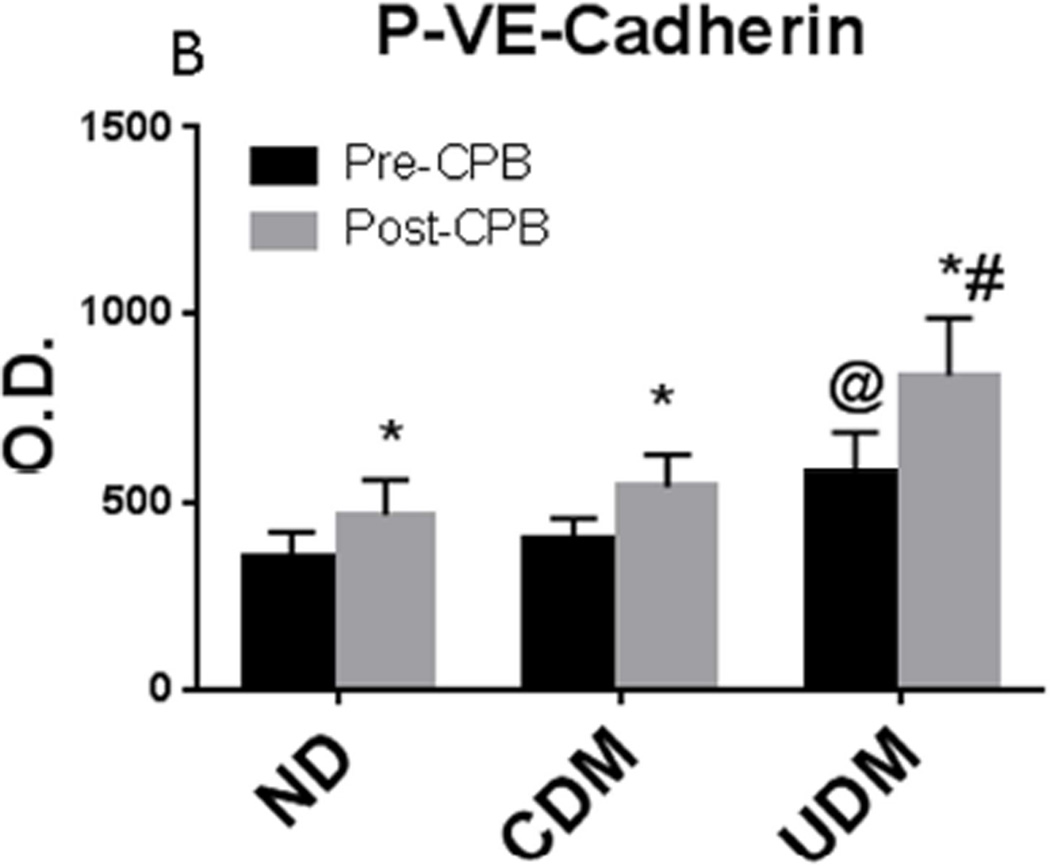
Immunofluorescent staining of phospho-VE-cadherin was observed in the arteriolar endothelial cells (red, Figure 3A and Figure 3B). At baseline (pre-CPB), phospho-VE-cadherin immunofluorescence was significantly increased in the UDM vessels compared with the ND and CDM groups. There were no significant differences in the pre-CPB levels of phospho-VE-cadherin immunofluorescence in vessels between ND and CDM. After CPB, phospho-VE-cadherin immunofluorescence were significantly increased compared with pre-CPB in all three groups, but this increase was greater in the UDM group than that of ND or CDM groups (P<0.05 each).
Figure 3.
Immunohistochemistry of phospho-VE-cadherin in human skeletal muscle pre- and post-CPB. A: Immuno-localization of phospho-VE-cadherin in peripheral vessels (magnification, 200×). Tissue slices were counter-stained for phospho-VE-cadherin (red), α-smooth muscle actin (green) and nucleus (blue) by incubating the slices with anti-phospho-VE-cadherin antibody and anti-α-actin antibody and DAPI. Negative controls documented a low level of background fluorescence (red), a strong signal of smooth muscle α-actin staining (green), and a signal of nuclear staining (blue); B: phospho-VE-cadherin density analysis; *P < 0.05 vs. pre-CPB; @P < 0.05 vs. ND- or CDM-pre-CPB; #P < 0.05 vs. ND- or CDM-post-CPB; Mean ± SEM, n = 8/group.
Microvascular Reactivity
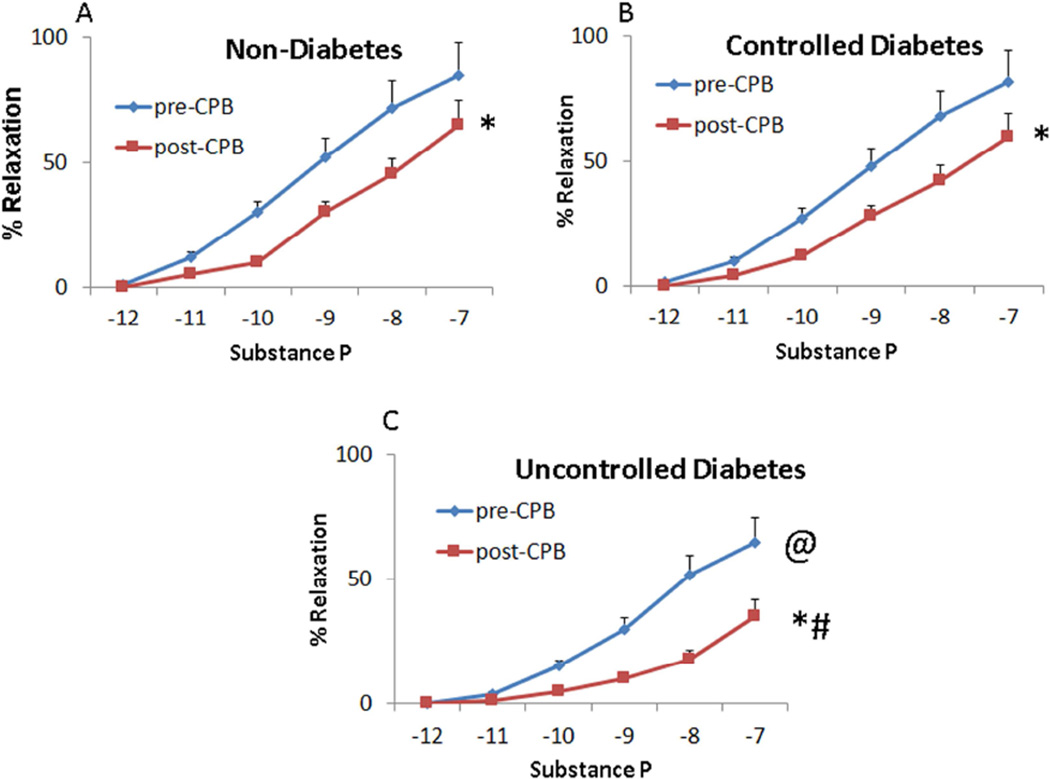
Table 2 shows no significant differences in the arteriolar baseline diameters between groups. The percentage of pre-contraction were 33 ± 4, 31 ± 3, and 30 ± 3 in the ND, CDM and UDM groups, respectively (P > 0.05). At baseline (pre-CPB), the difference in relaxation responses to the endothelium-dependent vasodilator substance P was insignificaant between the ND and CDM groups (Figure 4A and Figure 4B, respectively), However, the relaxation response of the UDM vessels were significantly diminished compared with that of ND or CDM groups (P < 0.05, Figure 4A–C). The post-CPB responses to substance P were significantly diminished in all three groups (P < 0.05 vs. their pre-CPB), but the reduction was greater in UDM group than the ND or CDP groups (P < 0.05, Figure 4).
Table 2.
Microvessel Diameter (micrometers)
| Pre-CP/CPB | Post-CP/CPB | |
|---|---|---|
| Non-Diabetics | 132 ± 7 | 141 ± 6 |
| Controlled Diabetics | 130± 5 | 127± 7 |
| Un-controlled Diabetics | 129 ± 7 | 135 ± 5 |
Mean ± SEM; n = 8/group
Figure 4.
Microvascular vasodilation in response to the endothelium-dependent vasodilator substance P (10−12 M–10−7 M): (A) pre-CPB vs. post-CPB (ND); (B) pre-CPB vs. post-CPB (CDM); (C) pre-CPB vs. post-CPB (UDM); *P < 0.05 vs. pre-CPB; @P < 0.05 vs. ND- or CDM-pre-CPB; # P < 0.05 vs. ND- or CDM-post-CPB; Mean ± SEM, n = 8/group.
DISCUSSION
Cellular junctions, particularly the adherens junctions play important roles in vascular permeability and endothelial barrier function.9–15 Adherens junctions are key components in cellular adhesion between endothelial cells and are composed of transmembrane vascular endothelial (VE)-cadherins that form the extracellular connections between vascular endothelial cells and catenins that link the intracellular portion of the cadherin proteins to the cytoskeleton.9–15 Alterations in the VE-cadherin complex have been demonstrated to result in increased vascular permeability and edema formation.9–15 We have shown previously that in a porcine model, CPB causes downregulation of adherens junction proteins resulting in the loss of the integrity of coronary endothelium.14,15 The present study demonstrated that CPB induced phosphorylation of VE-cadherin and degradation of β-catenins in peripheral skeletal muscle and arterioles in patients early after CPB. These findings are consistent with previous studies, where CPB resulted in downregulation of adherens junction proteins in porcine and human myocardium and coronary arterioles.8,14,15 These findings suggest that CPB has an important effect on the assembly of endothelial cadherin and thus the integrity of endothelial cells in human skeletal muscle.
Diabetes is associated with increased microvascular dysfunction as well as with increased morbidity and mortality after CPB and heart surgery.1–5,8,16,17 Recently, we demonstrated that uncontrolled diabetes induced inactivation and reduction in adherens junction proteins in human a trial myocardium and coronary arteriolar endothelium.8 Uncontrolled diabetes caused more VE-cadherin phosphorylation and β/γ-catenin degradation of coronary vasculature early after cardioplegic ischemia and reperfusion. In this study, we observed that uncontrolled diabetes also downregulated expression of endothelial adherens-junction-proteins in the arterioles of skeletal muscle early after CPB. Although no significant changes in total VE-cadherin protein were observed between poorly controlled and well controlled diabetics, the enhanced phosphorylation of VE-cadherin and degradation of β-catenin suggest more protein deterioration and damage of endothelial junctions in the uncontrolled diabetic vessels.8 These alterations may contribute to the increases in peripheral vascular permeability, tissue edema and endothelial dysfunction of peripheral arterioles in uncontrolled diabetic patients. In addition, no differences in phosphorylation/degradation of VE-cadherin and β-catenin between well-controlled diabetes and non-diabetics post-CPB suggest that relatively long-term control of hyperglycemia might be beneficial for preserving microvascular endothelial integrity.
Several factors are found to induce vascular permeability via a VE-cadherin-dependent mechanism, such as tumor necrosis factor, VEGF, histamine, neutrophil-associated proteases, oxygen radicals, and thrombin.1,18–20 CPB and cardiac surgery cause a different regulation of specific genes in the myocardium of non-diabetic and diabetic patients.1, 21 Importantly, we have also demonstrated that the expression of permeability-modulating proteins, such as VEGF and VPF are enhanced in diabetic patients early after CPB and cardiac surgery.1,6 These alterations may contribute to diabetes-induced downregulation of adherens junction proteins. These findings may explain in part why diabetes increased vascular permeability, endothelial dysfunction, and edema formation in peripheral tissue and delayed recovery in diabetic patients from CPB and cardiac surgery.1,16,17,21
In conclusion, our results suggest that uncontrolled diabetes causes inactivation and reduction in the expression of endothelial adherens-junction-protein in the arterioles of skeletal muscle early after CPB. These alterations may contribute to the increases in peripheral vascular permeability and microvascular endothelial dysfunction.
Acknowledgments
We would like to thank all the nurses, physician assistants, and perfusionists at cardiac surgery operating room, and Division of Cardiac Surgery, Lifespan Hospitals for collecting patient consent forms, tissue samples and the data of patient characteristics.
FUNDING
This research project was supported by the National Institute of Health (NIH) R01-HL46716 and RO1HL128831 to F.W.S. This work was supported in part by the Institutional Development Award (IDeA) from the National Institute of General Medical Science (NIGMS) of the NIH [5P20-GM103652(Pilot Project)], NIH-1R01HL127072-01A1 and AHA-Grant-in-Aid (#15GRNT25710105) to J.F.; Summer Assistantship Award through Basic and Translational Research (BTR) Program (NIH T35HL094308) to N.S; and Summer Research Assistantship (SRA) Award of Brown University’s Program in Liberal Medical Education (PLME) to J.L.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 11th Annual Academic Surgical Congress (ASC), Feb. 2–4, 2016 Jacksonville, Florida
Confliction of interest
None declared
REFERENCES
- 1.Emani S, Ramlawi B, Sodha NR, Li J, Bianchi C, Sellke FW. Increased vascular permeability after cardiopulmonary bypass in patients with diabetes is associated with increased expression of vascular growth factor and hepatocyte growth factor. J Thorac Cardiovasc Surg. 2009;138:185–191. doi: 10.1016/j.jtcvs.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feng J, Liu YH, Chu LM, Singh AK, Dobrilovic N, Fingleton JG, et al. Changes in microvascular reactivity after cardiopulmonary bypass in patients with poorly controlled versus controlled diabetes. Circulation. 2012;126:S73–S80. doi: 10.1161/CIRCULATIONAHA.111.084590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feng J, Chu LM, Dobrilovic N, Liu YH, Singh AK, Sellke FW. Decreased coronary microvascular reactivity after cardioplegic arrest in patients with uncontrolled diabetes mellitus. Surgery. 2012;152:282–289. doi: 10.1016/j.surg.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng J, Liu YH, Khabbaz K, Hagberg R, Robich MP, Clements RT, et al. Decreased contractile response to endothelin-1 of peripheral microvasculature from diabetic patients. Surgery. 2011;149:247–252. doi: 10.1016/j.surg.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng J, Liu YH, Dobrilovic N, Chu LM, Bianchi C, Singh AK, et al. Altered apoptosis-related signaling after cardioplegic arrest in patients with uncontrolled type 2 diabetes. Circulation. 2013;128:S144–S151. doi: 10.1161/CIRCULATIONAHA.112.000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tofukuji M, Metais C, Li J, Franklin A, Simons M, Sellke FW. Myocardial VEGF expression after cardiopulmonary bypass and cardioplegia. Circulation. 1998;98(suppl II):II-242–II-246. discussion II-247–48. [PubMed] [Google Scholar]
- 7.Ruel M, Khan TA, Voisine P, Bianchi C, Sellke FW. Vasomotor dysfunction after cardiac surgery. Eur J Cardiothorac Surg. 2004;26:1002–1014. doi: 10.1016/j.ejcts.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 8.Feng J, Liu Y, Sabe AA, Sadek AA, Singh AK, Sodha NR, Sellke FW. Differential impairment of adherens-junction expression/phosphorylation after cardioplegia in diabetic versus non-diabetic patients. European Journal of Cardiothoracic Surgery. 2016;49:937–943. doi: 10.1093/ejcts/ezv202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dejana E. Endothelial adherens junctions: implications in the control of vascular permeability and angiogenesis. J Clin Invest. 1996;98:1949–1953. doi: 10.1172/JCI118997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao K, Allison DF, Kottke MD, Summers S, Sorescu GP, Faundez V, et al. Mechanisms of VE-cadherin processing and degradation in microvascular endothelial cells. J Biol Chem. 2003;278:19199–19208. doi: 10.1074/jbc.M211746200. [DOI] [PubMed] [Google Scholar]
- 11.Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84:869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- 12.Lampugnani MG, Corada M, Caveda L, Breviario F, Ayalon O, Geiger B, et al. The molecular organization of endothelial cell to cell junctions: differential association of plakoglobin, beta-catenin, and alpha-catenin with vascular endothelial cadherin (VE-cadherin) J Cell Biol. 1995;129:203–217. doi: 10.1083/jcb.129.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan Y, Meng FY, Huang Q, Hawker J, Wu HM. Tyrosine phosphorylation of paxillin / pp125FAK and microvascular endothelial barrier function. Am J Physiol. 1998;275:H84–H93. doi: 10.1152/ajpheart.1998.275.1.H84. [DOI] [PubMed] [Google Scholar]
- 14.Bianchi C, Araujo EG, Sato K, Sellke FW. Biochemical and structural evidence for pig myocardium adherens junction disruption by cardiopulmonary bypass. Circulation. 2001;104:I319–I324. doi: 10.1161/hc37t1.094519. [DOI] [PubMed] [Google Scholar]
- 15.Khan TA, Bianchi C, Araujo E, Voisine P, Xu SH, Feng J, et al. Aprotinin preserves coronary cellular junctions and reduces myocardial edema after regional ischemia and cardioplegic arrest. Circulation. 2005;112(Suppl I):I196–I201. doi: 10.1161/CIRCULATIONAHA.104.526053. [DOI] [PubMed] [Google Scholar]
- 16.Thourani VH, Weintraub WS, Stein B, Gebhart SS, Craver JM, Jones EL, et al. Influence of diabetes mellitus on early and late outcome after coronary artery bypass grafting. Ann Thorac Surg. 1999;67:1045–1052. doi: 10.1016/s0003-4975(99)00143-5. [DOI] [PubMed] [Google Scholar]
- 17.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 18.Wong RK, Baldwin AL, Heimark RL. Cadherin-5 redistribution at sites of TNF-alpha and IFN-gamma induced permeability in mesenteric venules. Am J Physiol. 1999;276:H736–H748. doi: 10.1152/ajpheart.1999.276.2.H736. [DOI] [PubMed] [Google Scholar]
- 19.Murohara T, Horowitz JR, Silver M, Tsurumi Y, Chen D, Sullivan A, et al. Vascular endothelial growth factor/vascular permeability factor enhances vascular permeability via nitric oxide and prostacyclin. Circulation. 1998;97:99–107. doi: 10.1161/01.cir.97.1.99. [DOI] [PubMed] [Google Scholar]
- 20.Bannerman DD, Sathyamoorthy M, Goldblum SE. Bacterial lipopolysaccharide disrupts endothelial monolayer integrity and survival signaling events through caspase cleavage of adherens junction proteins. J Biol Chem. 1998;273:35371–35380. doi: 10.1074/jbc.273.52.35371. [DOI] [PubMed] [Google Scholar]
- 21.Voisine P, Ruel M, Khan TA, Bianchi C, Xu S, Kohane I, et al. Differences in gene expression profiles of diabetic and non-diabetic patients undergoing cardiopulmonary bypass and cardioplegic arrest. Circulation. 2004;110:II280–II286. doi: 10.1161/01.CIR.0000138974.18839.02. [DOI] [PubMed] [Google Scholar]